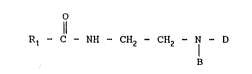Note: Descriptions are shown in the official language in which they were submitted.
Z00~5~ ~
, , ! , '
`.'. PATENT
:.l Cas~ D 8599
... .
,. . . .
-:J
.:,
.!.,,
::`,'`
,. .'!
."`'~ ~.
'"`',
'''1, ~
: ?:'
~:.1
, " ~ .
. :i.~
'~' A PROCESS FOR THE PRODUCTION OF AMPHOTERIC
';',;,4 SURFACE-ACTIVE IMIDAZOLINE DERIVATIVES
., ~ . .
~ BACKGROUND OF THE INVENTION
.; ~1
:~1 Field of the Invention:
This invention relates to a new process for the
production of amphoteric surfactants corresponding to formula
.i:? (I)
,;~ .
R1 C - NH - CH2 - CH2 - I - D ~I)
in which R1 is a Cs21 alkyl radical, B is a C24 aminoalkyl or
~ i il
hydroxyalkyl radical and D is an -R2-COOM group, where R2 is
a C14 alkylene radical or a C24 alkenylene radical and M is
hydrogen, ammonium or an alkali metal. These surfactants may
be used in admixture with anionic surfactants for the
production of ready-to-use body-care and personal hygiene
preparations characterized by high compatibility with the
skin. With small quantities of conventional thickeners, they
.. .~
t
~ ?.:
;C",'~
;: ~;1
:.:,.
`- ~ Z~1055~il3
enable the viscosity required for commercial products of this
:l type to be readily established.
Discussion of Related Art:
The process generally comprises hydrolyzing an
i 5 imidazoline corresponding to formula II
:~; ~N CH2
Rl - C/\ (II)
. N ~ CH2
. B
. in which Rl and B are as defined above,
under controlled conditions to form an intermediate amidoamine
corresponding to formula III
:, o :
R1 - C - N - CH2 - CH2 - NH (III)
H B
in which Rl and B are as defined above, ~`~
;~ and subsequently alkylating this amidoamine with an alkylating
, agent corresponding to formula V
:
:I X - D (V) :: ::
.j ~
, 20 in which X is a halogen atom or a hydrogen atom and D is as
defined above, ~; ;
in the presence of a base to shift the alkylation equilibrium
in the req~lired direction by neutralization of the hydrohalic :
acid formed. The object of all this is, primarily, the .~;~
; 25 formation o* the end compound corresponding to formula I.
The imidazoline compounds corresponding to formula II,
..
~ 2 ~
~ ,', .
'' ' "~
,' "'`"'
which may be used as starting material for the process
according to the invention, are known compounds which may ~e
prepared by known methods of organic synthesis, for example
in accordance with European Patent Specifications 2943 and
540, 640 by reaction of a fatty acid having the formula R1 -
COOH or a fatty acid methyl ester having the formula R1 -
COOCH3 with a diamine having the formula H2N - CH2 - CH2 - NH ~
B.
These fatty acids and the fatty acid residues of the
~n methyl esters in question may be of natural or synthetic
origin. Sui~able starting materials are, for example, caproic
acid, heptanoic acid, caprylic acid, undecylic acid, lauric
acid, myristic acid, palmitic acid, stearic acid, behenic
acid, arachic acid, oleic acid, linoleic acid, ricinoleic
acid, ethyl hexanoic acid and isostearic acid and esters
thereof. The individual fatty acids or esters of individual
fatty acids may be. used for the synthesis of the imidazolines.
However, it is also possible to use fatty acid and fatty acid
ester mixtures of the type directly obtainable from naturally
occurring fats and oils, for example from coconut oil, by
lipolysis or transesterification with methanol.
Suitable alkylating agents corresponding to formula V
are, in particular, haloalkane acids (X = halogen; R2 = C, 4
alkylene radical), preferably monochloroacetic acid, and
alkenoic acids (X = halogen; R2 = C24 alkenyl radical),
preferably acrylic acid, and salts thereof, particularly
200556~
alkali metal salts. Sodium monochloroacetate i5 a
particularly preferred alkylating agent.
The diamines corresponding to the above formula are those
in which B is a hydroxyalkyl raclical or an aminoalkyl radical,
B preferably being a C~ 4 hydroxyalkyl radical, more especially
a hydroxyethyl radical.
In the processes described in European Patent
Specifications 1006 and 540,508 for the production of
amphoteric surfactants of the type in question here, an
imidazoline corresponding to formula II is directly reacted
with an aqueous solution of an alkylating agent ~V) by means
c.f a base. In these processes, the imidazoline is
simultaneously hydrolyzed and alkylated. A mixture of mono~
and dialkylamidoamines is formed and a product of low
viscosity is generally obtained. As mentioned above, the
imidazoline is already partly alkylated before the formation,
by its hydrolysis, of a mixture of amidoamines having linear
chains at which the alkylation then continues.
The end product consists of a complex mixture of
alkylated compounds formed partly by alkylation of the
imidazoline, subsequent hydrolysis and further alkylation of
the linear hydrolysis products and partly by direct hydrolysis
of the imidazoline and alkylation of the linear amidoamines.
The composition of the end product depends on the temperature
and pH conditions under which both the imidazoline and the
alkali metal hydroxide are added. At a high pH value,
:.
, . ~
3?:: C . - . ~ , " " ~ " ; , ~ ~ ,
0~6~3
:
hydrolysis of the imidazoline has precedence over the direct
alkylation and products corresponding to formula I
O
: ':', . .
Rl ~ C - NH - CH2 - CH2 - N - D (I)
B
in which B and D are as defined above, are formed. On an ~ -~
industrial scale, however, it is extremely difficult under the
conditions mentioned above consistently to obtain end products
having viscosities which lie within an acceptable narrow range
of variation.
If, on the other hand, the pH value is near the neutral `
point or in the moderately alkaline range, for example in the
range from pH 7 to ll, the rate of alkylation of the
imidazoline is greater than its hydrolysis rate. A highly
15alkylated, low-viscosity end product is formed, consisting
essentially of a mixture of compounds corresponding to
formulae VI to VIII
O
Rl - C - I - CHz - CH2 - NH (VI) ;~
B D
1l ' : .
Rl ~ C - I - CH2 - CH2 - N - D (VII)
B D
1l :
R1 - C - Nl- CH2 - CH2 - N - D (VIII)
.. . . . . ... . .. . . . . . . . . . . . . .. . .
,". ~ :
2~5t~68
in which R1, B and D are again as defined above. After the
reaction, any excess of alkylating agent present must be
hydrolyzed so that no unwanted effects, for example serious
skin irritation, occur in the use of the end product. If, for
example, sodium monochloroacetate is used as the alkylating
agent, sodium chloride and sodium glycolate are formed during
its hydrolysis. In this hydrolysis reaction, the tertiary
amides corresponding to formulae (VI) to (VIII) are also
attacked, resulting in the formation of unwanted alkali metal
l~ soaps which lead to an increase in the viscosity of the end
product and, even more seriously, greatly impair the
compatibility of the product with hard water.
Another disadvantage of the products obtained in this way
is that they require large quantities of thickener, for
example polyethylene glycol distearate 6,000, when they are
formulated together with alkyl sulfates corresponding to
formula IX or alkyl ether sulfates corresponding to formula ;
X " . ::
R2 ~ 0 - S03 - M tIX)
R2 ~ (OCH2CH2)n - 0 - S03M (X)
in which R2 is a Cl0,8 alkyl radical, n is a number of 1 to lO
and M is an alkali metal, preferably sodium, to ensure that
the end products have the viscosities which the market demands
for such products. -~
If the disadvantages of the prior art are summarized, it
can be seen that the known processes either give products of
6 ;~-~
. . '.' ;
... . . ... . . ..
Z~0556~ :
high, but poorly reproducible viscosity or products of low
viscosity which, on the one hand, have to be formulated with
considerable quantities of thickeners to obtain adequate
viscosities in the end products and which, on the other hand,
lead to losses of stability towards hard water through their
considerable content of alkali metal soaps.
DESCRIPTION OF THE INVENTION
Other than in the operating examples, or where otherwise
indicated, all numbers expressing quantities of ingredients
'~ or reaction conditions used herein are to be understood as : :
modified in all instances by the term "about". :;
The present invention provides a process for the
production of a mixture of amphoteric surface-active
imidazoline derivatives having a medium to high viscosity
which primarily contains compounds corresponding to formula
I
O
R1 ~ l - NH - CH2 - CH2 - 7 D (I) :
B
in which Rl is a Cs21 alkyl radical, B is a C24 aminoalkyl or
hydroxyalkyl radical, preferably a hydroxyethyl radical, and
D is an -R2-COOM group wherein R2 is a Cl4 alkylene radical or
a C24 alkenylene radical and M is hydrogen, ammonium or an
alkali metal, preferably sodium. The new process comprises
A) hydrolyzing an imidazoline corresponding to formula II ~-
,: ,:, .; . , .. ::: : .,.. ,. .. :...... . - : ~. : :. : . . : , :: : . : : . .
---` 2C~ 6~
N - CH
R1 ~ C D 1 2 (II)
CH2 , ,
B
in which R1 and B are as defined above,
with wa-ter in a molar ratio o~ 1:8 to 1:20 over a period
of 1 to 24 hours at a temperature in the range from 50 ~:
to 130 C under atmospheric pressure or a slight excess
pressure to form a mixture of amidoamines corresponding
to formulae III and IV ~
Rl ~ C - I - CH2 - CH2 - NH (III) ~ .
H B
R1 - C - N - CH2 - CH2 - NH2 (IV)
in which Rl and B are as defined above,
B) alkylating the mixture of the amidoamines obtained with
an alkylating agent corresponding to formula V
X - D (V)
in which X is a halogen atom, preferably a chlorine atom, "~
or a hydrogen atom and D is as defined above, in a molar
ratio of alkylating agent (V) to amidoamines (III + IV)
of from 1:1 to 4:1 by means of a base at 40 to 70 C and ~-
at a pH value in the range from 10 to 11.5, as measured
in a 10% by weight aqueous solution of the reaction ; ~
8 . ~:
Z0055~ :
. - . .
mixture, so that, primarily, the end compound
corresponding to formula I and, to a lesser extent,
compounds corresponding to formulae VI and VII are formed
R1 ~ C - N - CH2 - CHz - NB (VI)
R1 ~ C - N - CH2 - CH2 - ~ - D (VII)
in which R1 and D are as defined above, and then
C) hydrolyzing the excess alkylating agent corresponding to
formula V at a temperature in the range from 80 to 95 C
and at a pH valu~ in the range from lO to ll.5, as
measured in a 10% by weight aqueous solution of the
reaction mixture.
The products obtained by the process according to the
invention naturally ha~e a high viscosity so that the
applicational disadvantages attending state-of-the-art
products are considerably reduced. Products having high, but
not excessive, viscosities in the range from l,000 to 5,000
Cps can be produced by the new process. These products may
readily be handled and transported in typical industrial
plants. In addition, the quantity of thickener needed to
éstablish the viscosities required by the market in end
products, for example shampoos, can be considerably reduced.
Finally, the content of troublesome metal soaps in the
`` 200~S68
products obtained by the process according to the invention
is considerably lower than in the products obtained by known
processes.
The controlled hydrolysis of the imidazoline before
alkylation surprisingly has the effect that the composition . -~
of the end product can be controlled and oriented in such a .
way that the above-mentioned properties are obtained.
The preparation of the intermediate imidazoline .:~
corresponding to formula II
N - CH2 ~::-
D ;~
R1 ~ C \ (II)
N - ( H2
,,,'',' '
in which R1 and B are as defined above, ;~ ~;
is sufficiently well-known and is described, for example, in ;;~
.. .. ..
European Patent Specifications 540 640 and 2943.
The hydrolysis of the imidazoline corresponding to
formula II can yield two different types of amidoamine. The
amidoamine corresponding to formula IIT
B
Rl - C - N - CH2 - CH2 - NH (III)
"';' '' ;'`
is formed when hydrolysis takes place between carbon atom 1 :~
and nitrogen atom 5 of the imidazoline ring. The amidoamine
corresponding to formula IV ;~
,,~
~, :' ' ':
'.~':' :'.
.. . . . ., . , . . . . . . , , , _ , .-, ~ .
.`-"` 2ÇDa~SS~
Il . ``.
R1 - C - I - CH2 CH2 ~ NH2 (IV)
B
is formed when hydrolysis take!; place between carbon atom 1
and nitrogen atom 2 of the imidazoline ring.
It has surprisingly been found that hydrolysis of the
imidazoline takes place with total cleavage of the -C=N bond
by a kinetic mechanism in which formation of the amidoamine
corresponding to formula IV takes pxecedence. A slow
1~ isomerization reaction also takes place in which the
~idoamine corresponding to formula IV disappears with
formation of the amidoamine corresponding to formula III which
is thermodynamically more stable. Accordingly, it is possible
during the hydrolysis to establish the desired ratio between
1~ the two types of amidoamine (III) and (IV) in the hydrolysis
product by control of the temperature and time conditions. The
properties of the end product are optimal when a proportion
of amidoamines corresponding to formula III of 50 to 95% by
weight and preferably 80 to 90~ by weight, based on the
hydrolysis product as a whole, is obtained during the
hydrolysis of the imidazoline.
If it is possible to control and determine the
composition of the intermediate hydrolysis product, a
completely defined end product can also be obtained.
Specifically, the compounds corresponding to formulae VI and
VII are obtainecl from the amidoamine corresponding to formula
)556~
IV where alkylation is carried out with an alkylating agent
corresponding ~o the formula X - D. By contrast, alkylation
of the amidoamine corresponding to formula III leads to the
alkylation product of formula I which generally makes up the
largest proportion of the end product.
The hydrolysis of the imidazoline corresponding to
formula II may be carried out at atmospheric pressure or under
a slight excess pressure at a temperature in the range from
50 to 130 C and preferably at a temperature in the range from
1.(-; 80 to 90 C with a molar ratio of imidazoline to water of from
1:8 to 1:20 and preferably from 1:8 to 1:15. It is important
in this regard to bear in mind that the formation of the
secondary amidoamine corresponding to formula III is promoted
over that of the tertiary amidoamine corresponding to formula
IV by long hydrolysis times, particularly by hydrolysis times
of 1 to 24 hours and preferably 6 to 15 hours, and high
temperatures, preferably in the range from 50 to 130 C
and more preferably in the range from 90 to 110 C.
As stated above, the hydrolysis products are alkylated
by known methods. To this end, the mixture of amidoamines
obtained in the preceding hydrolysis step is added to an
aqueous solution of the alkylating agent, preferably sodium
monochloroacetate, at a temperature in the range from 40 to
70 C and preferably at a temperature in the range from 45 to
55 C. The mola:r ratio of alkylating agents to amidoamines is
from 1:1 to 4:1 and preferably from l.S:l to 2.8:1. An
''~' ''; '~
. . ,
, , . . . - . . . . ..
2~05~,8
aqueous alkali metal hydroxide solution, preferably a sodium
hydroxide solution, is then gradually added at the
temperatures mentioned above at such a rate that the pH value,
as measured in a 10% by weight aqueous solution of the
5reaction mixture, is kept between 10 and 11.5 and preferably
between 10.8 and 11.2 and the alkylation takes place at the
amine nitrogen atoms present ovler a reasonable period of from
about 3 to 6 hours. The conditions mentioned above also
afford the possibility of adjusting the rate of hydrolysis of
the alkylating agent in accordance with the following equation
ClCH2COOONa + NaO~- ~ NaCl + HOCH2CQONa
to a low value in this phase of the process. When the desired
degree of alkylation is reached, the temperature of the
reaction mixture is increased to 80 - 95 C and preferably to
1585 - 90 C. The reaction mixture is then kept at that
temperature until the excess alkylating agent is destroyed,
sodium hydroxide solution being continuously added to maintain
a pH value within the limits indicated above.
20Examples
Example 1
165.6 g (0.62 mol) hydroxyethyl alkylimidazoline (R1 =
C11; imidazoline content ~ 95% by weight) were introduced into
and heated with stirring to 80 C in a glass laboratory
25reaction vessel equipped with a heating system and mechanical ~ -
stirrer. 128 g (7.1 mol) distilled water were then added over
13
'`~,.' ' ~ ' - :' - " ' . :
. . .
:, , . -, - . ' - ~ .,. - ` . . '" ,, '
i........................ - ~ . . .
:;` 20~5~;8
a period of 30 minutes. The mixture was then refluxed with
stirring for 15 hours so that an aqueous solution of a mixture
of amidoamines containing 83% by weight secondary amidoamine
(III), based on solids, was obtained.
In a second glass laboratory reaction vessel equipped in
the same way as the reaction vessel mentioned above, 143.6 g
(1.23 mol) sodium monochloroacetate were dissolved in 482.5
g distilled water. This solution was heated to 50 C. The
above-described mixture of amidoamines was then added with
stirring over a period of 30 minutes. After stirring for 3
hours at the temperature of 50 C, 64 g of a 50% by weight
aqueous sodium hydroxide solution were added so that the pH
value of a 10% by weight aqueous solution of the reaction
mixture was between 11.1 and 11.5. Finally, the temperature
was increased to 90 C and 16 g of a 50% by weight sodium
hydroxide solution were added over a period of 5 hours, so
that the pH value of a 10~ by weight aqueous solution of the
reaction mixture was between 10.0 and 10.5. The end product
had a water content of 65~ by weight and a viscosity at 20 C
of 1,500 Cps, as measured with a Brookfield viscosimeter.
Example 2
133 g ~0.5 mol) hydroxyethyl alkylimidazoline IRl = C11;
imidazoline content > 95% by weight) were introduced into and
heated to 105 C in the glass laboratory reaction vessel
described in Example 1. 103 g (5.7 mol) distilled water were
added with stirring over a period of 30 minutes. The mixture
',,'`',''~ ''
'.".'~:
'' `,'''' '
... , .. . . , . .. : :,. . : ~ , : , :. ... , - . :. .. ... . ... . . " : - - . -
~-- 200~6~3
, ~ .
was then refluxed with stirring for 2 hours so that an aqueous
solution of a mixture of amidoamines containing 73% by weight
amidoamine (III), based on solids, was obtained.
Alkylation was carried out under the same conditions as
described in Example 1 using 155.8 g (1.3 mol) sodium
monochloroacetate, 107 g 50% by weight aqueous sodium
hydroxide solution and 502 g distilled water.
The end product had a water content of 65% by weight and
a viscosity at 20 C of 1800 Cps, as determined with a
~0 Brookfield viscosimeter.
Example 3
Using the same reagents and quantities as in Example 2,
hydrolysis was carried out over a period of 6 hours at 105C
and gave an aqueous solution of amidoamines containing 96% by
weight secondary amidoamine (III), based on solids.
Using the same reagents in the same quantities as in
Example 2 and maintaining the same conditions, an end product
having a water content of 65% by weight and a viscosity at
20 C of 5,800 Cps, as measured with a Brookfield viscosimeter,
was obtained.
Example 4
With the same reagents in the same quantities and under
the same conditions as in Example 1, but using an alkyl
- hydroxyethyl imidazoline of formula I in which the substituent
R1 consisted of a mixture of C7l7 alkyl radicals derived from
the fatty acicl component of coconut oil, an end product having
,.,,. .. . - .. . . . : - ~ ,: - -
2~
a water content of 65% by weight and a viscosity at 20 C of : ~:
lr200 Cps, as measured with a Brookfield viscosimeter, was
obtained.
,.~' ' ,,
, - ,
16