Note: Descriptions are shown in the official language in which they were submitted.
2006413
Merck Patent Gesellschaft
mit beschrankter Haftung
6100 D a r m s t a d.t
Nitrogen-containing ring compounds
S The invention relates to novel nitrogen- containing ring
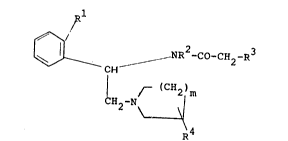
compounds of formula I
,~,/ 3
NR -CO-CH2-R 1 ~: ~
r ~ CH2 ~ m , - ,
H2 -N~ ., .
\4
wherein
R is H,
R2 is A, ; .
R1 and R2 can also be -(CH2)n-,
R3 is a phenyl, thienyl, naphthyl or
benzothienyl group,
R is H or E-Q,
m and n ~are each 1,2 or 3,
2006413
_ 2 :
wherein, furthermore, independently from each other,
one CH group each in the benzene ring and/or in the
radical R3 can be replaced by N, and/or wherein, inde-
pendently from each other, the benzene ring and/or the
radical R3 can be substituted each one, two or three
times by A, OA, F, Cl, Br, I, CF3, N02, NH2, NHA, NA2
and/or by one methylenedioxy group and /or by one ~:
or two of the groups -NZ-CY-NZ- and/or -E-Q , ~:
';,
wherein
.. ~ .~,
10 E is missing or is -alkyl-, -O-alkyl-, -NZ-alkyl-, ~ ..
-CY~alkyl-, -NZ-CY-alkyl- or -CY-NZ-alkyl-, ::.
Q is -OH, -NZ-CY-Z, -NZ-(CY)r-NHZ, -NZ-S02-A, ~ -
-COOR , -CO-NH-Z, Y
-(NZ)p-CY-NZ ~ ~ y -(NZ)p-CY-NZ ~
N N ~ -
Z Z .,
~(NZ)p(CY)q~NW or -S02-NHZ,
W is -CH2CH2-NZ-CY- or -CTl-CT2-NZ-CT3-CT4-, ~ :
Y is =O or =NZ,
20 Z is H or A,
Tl, T2,
T3 and T4 are each =0 or (H, H),
: R5 is H! 6A,!AO-7alkyl, A-CO-O-alkyl, AO-CO-O-alkyl
or 2-R 0-3-R O-propyl,
R6 and R7 are each A or are together alkylidene or cyclo-
alkylidene each containing up to 6 C atoms,
p und q are each O or 1,
r is 1 or 2
A is alkyl containing 1-6 C atoms and -
: .,,
. .
, .: .: .,: . .
PAT LOG 19-B 241089/0001Ø0 ~ ~ -
-' '.' `" '', :'",'''.~
`- Z006413
- 3 - ~ ~ ~
~ ,
-alkyl is alkylene containing 1-4 atoms,
wherein, furthermore, in case there are several groups
A E, Q, Y and/or z, these can be the same or different
from each other
and to salts thereof.
_ Similar compounds are disclo~ed in German patent
application A-27 49 950.
The ob~ect of the invention was to find novel
compounds wLth valuable propertiQs, especially those
which can be used for the preparation of drugs. ;~
It has been found that the compounds of formula
I and their physiologically biocompatible salts po~sess
valuable pharmacological properties. In particular, they
exhibit analgesic properties. Thus the compounds have a
particularly strong action in the writhing test on mice
or rats (for method q.v. Siegmund et al., Proc. Soc. Exp.
aiol. 95, (1957), 729-731). The analgesic action can
also be demonstrated in the tail flick te~t on mice or
rats (for methodology q.v. d'Amour and Smith, J. Phar-
macol. Exp. Ther. 72, (1941), 74-79) and in the hot plate
test (q.v. Schmauss and Yaksh, J. Pharmacol. Exp. Ther.
228, (1984), 1-12 and the literature cited therein). In
these tests, the compounds show little or no tendency to
. _ , .. ..
cause physical dependence. Furthermore, anti-
inflammatoric, antiasthmatic, diuretic,
~; anticonvulsant and/or antitussive actions are apparent
`~ which can also be demonstrated by methods commonly used
for thi~ purpose. The compounds are moreover suitable
for protecting against and treating cerebral oedemas and
-~ ~ 30 supply deficiencies of the central nervous system,
especially hypoxia. -~
The compounds can therefore be u~ed as pharma-
cological -active ingredients in human and veterinary
~ , .
medicine. They are also suitable as intermediates for
the preparation of other compounds with valuable proper-
ties.
.
:.: ..."~: ~,
. :
Z006413
_ ~ 4 ~
The invention relates to compounds of formula I
and to their salts.
The group A is preferably alkyl containing 1, 2,
3 or 4 C atoms, especially methyl or ethyl, but also ~ ~ ~
propyl, isopropyl, butyl, sec-butyl, tert-butyl, pentyl ~;
or hexyl. Accordingly the group OA is preferably methoxy
or ethoxy or also propoxy, isopropoxy, butoxy, isobutoxy,
sec-~futoxy, tert-butoxy, pentoxy or hexyloxy. ~ ~
The group "-alkyl" is preferably -(CH2)t- :
(t = 1,2,3,4,5 or 6), particularly -CH2- or ~CH?)2-,but also ; :
-(CH2)3-~ -(CH2)4-~ -(CH2)5_, (C 2 6 ' 3
~C(CH ) - -cH(cH3)-cH2-~ -CH2-CH(CH3) or -cH2_c(cH3)2_~ ~.
The compounds of formula I wherein R and R
are together -(CH2)n- and wherein there is no CH proup ~ :
replaced by N in the benzene ring specifically include
the corresponding compounds of partial formulae Ia to Ii -.
(wherein the radicals R3 and R4 are as defined for
formula I):
Ia pyrrolidino-l-methylisoindolineY (I, m - n = l);
Ib piperidino-1-methylisoindolines (I, m - 2, n = 1);
Ic hexahydroazepino-l-methylisoindolines (I, m - 3,
n - l);
~` Id pyrrolidino-1-methyl-1,2,3,4-tetrahydroisoquinolines
(I, m - 1, n - 2);
Ie piperidinoll-methyl~ 2~3~4-tetrahydroisoquinolines
(I, m = n = 2);
If hexahydroazepino-l-methyl-1,2,3,4-tetrahydroiso ~ ;--
quinolines (I, m - 3, n = 2);
Ig pyrrolidino-l-methyl-2,3,4,5-tetrahydro-lN-2-benz
azepines (I, m = 1, n = 3); ;~
Ih piperidino-l-methyl-2,3,4,5-tetrahydro-lH-2-benzaze
pines (I, m - 2, n - 3); ~ --
Ii hexahydroazepino-l-methyl-2,3,4,5-tetrahydro-lH-2-
benzazepines (I, m = n = 3).
2006413
:, ,.", ~:
_ 5 _
The compounds of formula I wherein Rl is H and R2 is A
specifically include the corresponding compounds of
partial formulae Ij to Io (wherein the radicals R3 and
R are as defined for formula I):
Ij N-[2-phenyl-2-(NR2-COCH2R3)-ethyl]-pyrrolidines
(m = l),
Ik N-[2-(pyridyl)-2-(NR2-COCH2R3)-ethyl~-pyrrolidines
(m = 1),
Il N-[2-phenyl-2-(NR2-COCH2R3)-ethyl]-piperidines
(m = 2),
Im N-[2-(pyridyl)-2-(NR2-COCH2R3)-ethyl]-piperidines
(m = 2),
In N-[2-phenyl-2-(NR2-COCH2R3)-ethyl]-hexahydro-
azepines (m = 3),
Io N-t2-(pyridyl)-2-(NR2-COCH2R3)-ethyl]-hexahydro-
azepines (m = 3).
In the compounds Ij to Io R2 is preferably methyl. -
.
The preferred compounds are those of formulae Id, Ij and ~-~
Ik, furthermore those of formulae Ie, Il and Im; thus m
is preferably 1, furthermore 2, and n is preferably 2.
,' .~ ~ . . .
...
Z is preferably H, or also CH3.
,',. :-
Y is preferably =O, or also =NH or =NCH3. ~ ~
, :,
Preferably none, one or two of the four groups T1, T2,
T3 and T4 is (are) =O, the others are (H,H). --~;~
W is preferably -CH2CH2-NH-CO-, or also -CH2CH2-N(CH3)- -;
CO-, -CH2CH2-NH-CH2CH2-, -CH2CH2-N(CH3)--
-~O-CH2-NH-CH2CH2- or -CO-CH2-NH-CO-CH2--
PAT LOG 19-B 241089/0002Ø0
` 2006413 ~
- 6 - -
. ', '~:
E is preferably missing or is preferably -alkyl-, fur-
thermore preferably--O-alkyl-, -NH-alkyl-, -CO-alkyl-,
-NH-CO-alkyl- or -CO-NH-alkyl-.
Q is preferably -OH, -NH-CO-Z such as -NH-CO-H or
-NH-CO-CH3, -NH-CO-NH2, -NH-CO-NHCH3, -NH-CO-CO-NH2,
-NH-SO2A such as -NH-SO2CH3, -CooR5 such as COOH or
COOCH3, -CONH2 or -CO-NH-CH3.
The group E-Q is preferably -NH-CO-NH2, -CONH2 or
-CH2CONH2, furthermore preferably -O-alkyl-CONHZ such
as -O-CH2CONH2 or -O-CH2CONHCH3, -O-alkyl-COOZ such as
-O-CH2COOH, -O-CH2-COOCH3 or -O-CH2-COOC2H5, -NZCO-Z
such as -NH-CO-H, -NH-CO-CH3, -N(CH3)-CO-H or -N(CH3)-
CO-CH3, -NHSO2A such as -NHSO2CH3, -NH-CO-NH-A such as
-NH-CO-NH-CH3, -NH-CO-CO-NH2, -COOZ such as -COOH,
-COOCH3 or -COOC2H5, -alkyl-COOZ such as -CH2-COOH,
-CH2-COOCH3 or -CH2-COOC2H5 or CONHA such a6 -CO-NH-
CH3, furthermore preferably -OH; -alkyl-OH such as
CH OH CH(CH )-DH, -CH2CH2OH, -C(CH3)2 , 2
(CH3)-OH, -CH2-C(CH3)2OH; -O-alkyl-OH such as
-O-CH2CH2-OH; -NZ-alkyl-OH such as -NH-CH2CH2-OH;
-NZ-CO-alkyl-OH such as -NH-CO-CH2CH2-OH or -N(CH3)- ~ .
CO-CH2CH2-OH; -CO-NZ-alkyl-OH such as CO-NH-CH2CH2-
OH-; -alkyl-NZ-CO-Z such as -CH2-NH-CO-H, -CH2NH-COCH3,
-CH2CH2-NH-CO-H or -CH2CH2-NH-COCH3; -O-alkyl-NZ-CO-Z : .
sush as -O-CH2CH2-NH-CO-H or -O-CH2CH2 NH COCH3;
-NZ-alkyl-NZ-CO-Z such as -NH-CH2CH2-NH-CO-H, . . .
-NH_CH2cH2_NH-cocH3~ -N(CH3)-CH2cH2-NH-cO-H or
-N(CH3)-CH2cH2-NH-cOcH3; :-~`
: .
PAT LOG 19-B 241089/0003Ø0
': '~ :' '- :: : ,
.
-CO-alkyl-NZ-CO-Z such as -CO-CH2-NH-CO-H, -CO-CH2-NH-
COCH3, -CO-CH2CH2-N~-CO-H or -CO-CH2CH2-NH-COCH3; -NZ-CO-
alkyl-NZ-CO-Z such as -NH-CO-CH2-NH-CO-H, -NH-CO-CH2-NH-
COCH3, -N(CH3)-CO-CH2-NH-CO-H, -N(CH3)-CO-CH2-N~-COCH3,
5 -NH-CO-CH2CH2-NH-CO-H, -NH-CO-CH2CH2-NH-COCH3,
-N(CH3)-CO-CH2CH2-NH-CO-H or -N(CH3)-CO-CH2-CH2-NH-COCH3;
-CO-NZ-alkyl-NZ-CO-Z such as -CO-NH-CH2CH2-NH-CO-H or
-co-NH-cH2cH2-NH-cocH3 i
-alkyl-NZ-CO-NHZ such as -CH2-NH-CO-NH2 or -CH2CH2-NH-
CO-NH2; -O-alkyl-NZ-CO-NHZ such as -O-CH2CH2-NH-CO-NH2
or -O-CH2CH2-NH-CO-NH-CH3;
-NZ-alkyl-NZ-CO-NHZ such as -NH-CH2CH2-NH-CO-NH2,
-NH-CH2CH2-NH-CO-NH-CH3, -N(CH3)-CH2CH2-NH-CO-NH2 ~ :-
or -N(CH3)-CH2cH2-NH-cO-NH-cH3-;
15 -CO-alkyl-NZ-CO-NHZ such as -CO-CH2-NH-CO-NH2, .~ :
-CO-CH2-NH-CO-NH-CH3, CO-CH2CH2-NH-CO-NH2 or ,:
-CO-CH2CH2-NH-CO-NH-CH3; . :
-NZ-CO-alkyl-NZ-CO-NHZ such as -NH-CO-CH2-NH-CO-NH2,
-NH-co-cH2cH2-NH-co-NH2~ -N(CH3)~co-cH2-NH-co-NH2 ~ .-
or -N(CH3)-CO-CH2cH2-NH-cO-NH2;
-CO-NZ-alkyl-NZ-CO-NHZ such as -CO-NH-CH2CH2-NH-CO-NH2 : -
or -CO-NH-CH2CH2-NH-CO-NH-CH3;
-alkyl-NH-SO2-A such as -CH2-NH-SO2-CE3 or -CH2CH2-NH-
S2 CH3; . . ;
-O-alk~l-NH-SO2-A such as -O-CH2CH2-NH-SO2-CH3 ;
-NZ-alkyl-NH-SO2-A such as -NH-CH2CH2-NH-SO2-CH3 or :~
-N(CH33-cH2cH2-NH-so2-cH3; ~ :
-Nz-co-alkyl-NH-so2-A such as -NH-CO-CH2cH2-NH-sO2cH3;
-CO-NZ-alkyl-NH-SO2-A such as -CO-NH-CH2CH2-NH-SO2-CH3;
-NZ-alkyl-COOZ such as -NH-CH2-COOH, -NH-CH2-COOCH3,
-NH-CH2CH2-COOH or -NH-CH2CH2-COOCH3~
-CO-alkyl-COOZ such as -CO-CH2CH2-COOH or -CO-CH2CH2-
COOCH3; -NZ-CO-alkyl-COOZ such as -NH-CO-CH2CH2-COOH :
or -NH-CO-CH2cH2-cOOc2H5;
-CO-NZ-alkyl-COOZ such as -CO-NH-CH2-COOH or
-CO-NH-CH2CH2-COOCH3;
:3
P~T LOG 19-B 241089/0004Ø0 . ,:
' ''' ,'.'~'
. - . . . .. ,, .: ., , ~ ',, . :. : ` : :: ., , . ` :
Z00~413
-alkyl-CO-NHZ such as -CH2-CO-NHCH3, -CH2CH2-CONH2 or
-CH2CH2-CO-NHCH3;
-O-CH2CH2-CO-NHZ such as -O-CH2CH2-CONH2 or -O-CH2CH2-
CO-NHCH3;
-NZ-alkyl-CO-NHZ such as -NH-CH2-CONH2, -NH-CH2-CO-
NHCH3, -N(CH3)-CH2-CONH2' -N(CH3)-CH2-CO-NHCH3'
-NH-CH2CH2-CONH2, -NH-CH2CH2-CO-NHCH3, -N(CH3)-CH2CH2~
CONH2 or ~N(CH3)-CH2CH2-CO-NHCH3;
-CO-alkyl-CO-NHZ such as -CO-CH2-CONH2, -CO-CH2-CO-
NHCH3, -CO-CH2CH2-CONH2 or -CO-CH2CH2-CO-NHCH3;
-NZ-CO-alkyl-CO-NHZ such as -NH-CO-CH2CH2-CONH2;
-CO-NZ-alkyl-CO-NHZ such as -CO-NH-CH2-CONH2 or
-co-NH-cH2-co-NHcH3;
-CO-NH-(2-pyridon-3-, -4 , -5- or -6-yl); :.
-CH2-CO-NH-(2-pyridon-3-, -4-, -5- or -6-yl);
-NH-CO-NH-(2-pyridon-3-, -4-, -5- or -6-yl);
-O-CH2-CO-NH-(2-pyridon-3-, -4-, -5- or -6-yl);
-CO-NH-(4-pyridon-2- or -3-yl);
-CH2-CO-NH-(4-pyridon-2- or -3-yl);
20 -NH-CO-NH-(4-pyridon-2- or -3-yl); ;~
-CH2-CO-NH-(4-pyridon-2-or-3-yl);
2-oxo-imidazolidin-1-yl; 3-methyl-2-oxo-imidazolidin-1-yl; :.
2-oxo-imidazolidin-1-yl-carbonyl; 3-methyl-2-oxo-imida-
zolidin-l-yl-carbonyl;
2-oxo-imidazolidin-1-yl-carbonylmethyl; 3-methyl-2-oxo-1-
~: imidazolidin-l-yl-carbonylmethyl; 2-oxo-imidazolidin-1-yl- . ~:
carboxamido; 3-methyl-2-oxo-imidazolidin-1-yl-carboxamido; ~:;
2-oxo-imidazolidin-1-yl.-carbonylmethoxy; 3-methyl-2,oxo-. ~:
imidazolidin-l-yl-carbonylmethoxy;
-SO2-NHZ such as SO2NH2 or SO2NHCH3;
-alkYl-so2-NHz such as -CH2-SO2NH2 or -CH2CH2SO2NHCH3;
-O-alkyl-SO2-NHZ such as -O-CH2-SO2NH2;
-NZ-alkyl-SO2-NHZ such as -NH-CH2-SO2NH2, -NH-CH2-SO2-
3 (CH3) CH2-SO2NH2, -N(CH3)-CH -SO NHCH
-NH-CH2CH2-SO2NH2~ -NH-CH2CH2-SO2 NHCH3~ :
-N(CH3)-CH2CH2-SO2NH2 or -N(CH3)-CH2CH2-SO2NHCH3; ~-
PAT LOG l9-B 241089/0005Ø0
: ~.
Z0064~3
-CO-alkyl-SO2NHZ such as -CO-CH2CH2-SO2NH2;
-NZ-CO-alkyl-SO2NHZ~such as -NH-C0-CH2CH2-SO2NH2;
-CO-NZ-alkyl-SO2NHZ such as -C0-NH-CH2-SO2NH2
In the compounds of formula I, the benzene ring prefer-
ably does not carry further substituents (except R1 and
the group which is in o-position to Rl and which carries
the radicals R2, R3 und R4). How~ver, if the benzene ring
is substitutend additionally, it is preferably substi-
tuted once. This substitution is preferably in p-position
relative to the radical R1, i.e. in the 6-position in the
isoindolines Ia, Ib or Ic, in 7-position in the tetra-
hydroisoquinolines Id, Ie or If etc. Preferred substi-
tuents are here NO2, NH2 or the group E-Q, which, in
turn, is here preferably -NH-CO-NH2, -N-CO-Z such as
15 -NH-CO-CH3, -NH-CO-alkyl-COOR5 such as -NH-CO-CH2CH2- ~ ;-
COOC2H5, -NH-CO-alkyl-CONHZ such as -NH-CO-CH2CH2-CONH2
or 2-oxo-imidazolidin-1-yl . ~ ~
;~ Especially preferred radicals R3 are 3,4-dichloro- - phenyl, o-, m- or p- nitrophenyl, o-, m- or p-amino-
phenyl, o-, m- or p-ureidophenyl, o-, m- or p-carbamoyl-
methylphenyl, 1-naphthyl and 4-benzothienyl. However,
other preferred meaning~ of the radical R3 are phenyl,
o-, m- or p-tolyl, o-, m- or p-ethylphenyl, o-, m- or p-
methoxyphenyl, o-, m- or p-ethoxyphenyl, o-, m- or p-
hydrox~phenyl, o-, m- or p-fluorophenyl, o-, m- or p-
chlorophenyl~ q-, m-l or p-bromophenyl, o-,j m- or p-
carboxyphenyl, o-, m- or p-methoxycarbonylphenyl, o-, m-
or p-ethoxycarbonylphenyl, o-, m- or p-methoxymethoxy-
carbonylphenyl, o-,, m- or p-ethoxymethoxycarbonylphenyl,
~` 30 o-, m- or p-acetox~methoxycarbonylphenyl, o-, m- or p-(l-~ ;l``;
acetoxyethoxycarbonyl)phenyl~ o-, m- or p-trimethyl- ~;-
`~ aeetoxymethoxycarbonyl, o-, m- or p-(l-trimethylaeetoxy-~ ;
ethoxycarbonyi)phenyl, o-, m- or p-methoxyearbonyloxy- -
i methoxyearbonylphenyl, o-, m- or p-ethoxyearbonyloxy- ~
;~ . '
i,
~i PAT LOG 19-B 241089/0006ØO
2006413
1 0 -
methoxycarbonylphenyl, o-, m- or p-(l-methoxycarbonyloxy-
ethoxycarbonyl)phenyl, o-, m- or p-(l-ethoxycarbonyloxy-
ethoxycarbonyl)phenyl, o-, m- or p-(2,3-isopropylidene-
dioxypropyloxycarbonyl)phenyl, o-, m- or p-carboxymethyl-
phenyl, o-, m- or p-methoxycarbonylmethylphenyl, o-, m-
or p-ethoxycarbonylmethylphenyl, o-, m- or p-acetoxy-
methoxycarbonylmethylphenyl, o-, m- or p-(l-acetoxy-
ethoxycarbonylmethyl)phenyl, o-, m- or p-trimethylace-
toxymethoxycarbonylmethylphenyl, o-, m- or p-(l- tri-
methylacetoxyethoxycarbonylmethyl)phenyl~ o-, m- or p-
methoxycarbonyloxymethoxycarbonylmethylphenyl, o-, m- or
p-ethoxycarbonyloxymethoxycarbonylmethylphenyl, o-, m- or
p-(1-methoxycarbonyloxyethoxycarbonylmethyl)phenyl, o-,
m- or p-(l-ethoxycarbonyloxyethoxycarbonylmethyl)phenyl,
o-, m- or p-(2,3-isopropylidenedioxypropyloxycarbonyl-
methyl)phenyl, o-, m- or p-carboxymethoxyphenyl, o-, m-
or p-methoxycarbonylmethoxyphenyl, o-, m- or p-ethoxy-
carbonylmethoxyphenyl, o-, m- or p-acetoxymethoxycarbony-
lmethoxyphenyl, o-, m- or p-(1-ncetoxyethoxycarbonyl-
methoxy)phenyl, o-, m- or p-trimethylacetoxymethoxy-
carbonylmethoxyphenyl, o-, m- or p-(1-trimethylacetoxy-
ethoxycarbonylmethoxy)phenyl, o-, m- or p-methoxycarbony-
loxymethoxycarbonylmethoxyphenyl, o-, m- or p- ethoxy-
carbonyloxymethoxycarbonylmethoxyphenyl, o-, m- or
p-(l-methoxyrcarbonyloxyethoxycarbonylmethoxy)phenyl~ o-,
m-orp-(l-ethoxycarbonyloxyethoxycarbonyLmethoxy)phenyl,
... _ _ . .. _ ... . .
o-, -m- or p-(2,3-i~opropylidenedioxypropyloxycarbonyl- : .
methoxy)phenyl, 2,3-, 2,4-, 2,5-, 2,6-, 3,4- or 3,5-
dimethylphenyl,, 2!,3-,i 2,4-, 2,5-, 2,6-, 3,4- or 3,5-
dimethoxyphenyl, 2,3-, 2,4-, 2,5-, 2,6-, 3,4- or 3,5-
difluorophenyl, 2,3-, 2,4-, 2,5-, 2,6- or 3,5-dichloro-
: phenyl, 2,3-, 2,4-, 2,5-, 2,6-, 3,4- or 3,5-dibromo-
phenyl, 2-chloro-4-hydroxyphenyl,
'; . ' ~ '-
. :,:
2006413
~-'',,.
3-chloro-4-hydroxyphenyl, 2-hydroxy-4-chlorophenyl,
3-hydroxy-4-chlorophenyl, 3-chloro-4-carboxymethoxy- :
phenyl, 3-chloro-4-methoxycarbonylmethoxyphenyl, 3-chloro-
4-ethoxycarbonylmethoxyphenyl, 2- or 3-thienyl, 3-, 4-, ::
or 5-chloro-2-thienyl, 3-, 4-, or 5-bromo-2-thienyl, 2-,
4-, or 5-chloro-3-thienyl, 2-, 4-, oder 5-bromo-3-thienyl, . .
3,4-, 3,5- or 4,5-dichloro-2-thienyl, 2-naphthyl, 2-, 3-, :
4-, 5-, 6-, 7- or 8-chloro-1-naphthyl, 2-, 3-, 5-, 6- or
7-benzothienyl, o-, m- or p-carbamoylphenyl, o-, m- or : :
p-carbamoylmethoxyphenyl, o-, m- or p-formamidophenyl, :
o-, m- or pLac$tamidophenyl, o-, m- or p-methylsulfon- :~.
amidophenyl, o-, m- or p-N'-methylureidophenyl, o-, m-
or p-N-methylcarbamoylphenyl, o-, m- or p-aminooxalyl- ~: ~
aminophenyl ~H2N-CO-CO-NH-C6H4), 2-, 3- or 4-pyridyl. . ~ .-
R4 is preferably H, furthermore preferably E-Q, particu-
larly -NH-CO-NH2.
R5 is preferably H, A (especLally methyl or ::
ethyl)~ AO-alkyl ~especially methoxymethyl, ethoxymethyl,
; 1- or 2-methoxyethyl or 1- or 2-ethoxyethyl)~ A-CO-O- i : -:
alkyl (especially acetoxymethyl, 1- or 2-acetoxyethyl, . :
. trimethylacetoxymethyl or 1- or 2-trimethylacetoxyethyl) ...
;: or AO-CO-O-alkyl (especially methoxycarbonyloxymethyl, . .
ethoxycarbonyloxymethyl, 1- or 2-methoxycarbonyloxyethyl : s .
or 1- or 2-ethoxycarbonyloxyethyl).
Other preferred compounds are those in which R5 ~: :
is a group R~CH2-CH(oR7)-CH2-, wherein RS and R7are each .
~; ~ . A (preferably methyl or ethyl) or together alkylidenç or
cycloalkylidene each containing up to 6 C atoms (prefer-
ably isopropylidene or also 2-butylidene, cyclopentyl-
~ 30 idene or cyclohexylidene).
`~ Accordingly the Lnvention relates in particular
~ to those compounds of formula I in which at lea~t one of
: said radicals has one of the preferred meanings indicated
:~ above. Some preferred groups of compound~ can be ex-
,' ;
~006413
- 12 -
pressed by partial formulae I' and Ia' to Io', which have - -
formulae I (and Ia 'to Io) and wherein the radical~ not -~
described more precisely are as defined for formula I, ~-:
but wherein R3 is phenyl, tolyl, methoxyphenyl, hydroxy~
phenyl, fluorophenyl, cS~lorophenyl, bromophenyl, carboxy-
._ . .. .
phenyl, methoxycarbonylphenyl, ethoxycarbonylphenyl,
carboxymeth~lphenyl,methoxycarbonylmethylphenyl,ethoxy-
carbonylmethylphenyl, carboxymethoxyphenyl, methoxy-
carbonylme;:hoxyphenyl, ethoxycarbonylmethoxyphenyl,
dichlorophenyl, chlorohydroxyphenyl, thienyl, naphthyl or -
benzothienyl. nitrophenyl, aminophenyl. ureidophenyl,
carbamoylmethylphenyl, carbamoylphenyl, carbamoylmethoxy-
phenyl, formamidophenyl, acetamidophenyl,methylsulfonamido-
phenyl, N'-methylureidophenyl N-methylcarbamoylphenyl
aminooxalylaminophenyl or 2-, 3- or 4-pyridyl.
Other preferred compounds are those of formulae
I'' and Ia'' to Io'', which have formulae I and Ia to Io
but wherein
R3 i~ phenyl, tolyl, methoxyphenyl, chlorophenyl, bromo~
phenyl, dichlorophenyl, chlorohydroxyphenyl, thienyl,
naphthyl, benzothienyl, nitrophenyl, aminophenyl,ureido-
phenyl or carbamoylmethylphenyl.
Other preferred compounds are those of formulae
;~ I''' and Ia''' to Io''', which have formulae I and Ia to -
25 Io, but wherein
R3 is 3,4-dichlorophenyl, l-naphthyl, 4-benzothienyl,
o-, m- or p-ureidophenyl or o-, m- or p-carbamoylmethylphenyl. ~
~ Especially preferred compounds of formulae I Ia ; - ~;
to Io, I', Ia' to Io', I'' Ia'' to Io'', I''' and Ia'''
; 30 to Io''' are those in which the benzene ring does not carry ;~
additional substituents. -
Furthermore, among all compounds mentioned,those
are preferred wherein R is H.
~' -: ,' ,.,',. '":
;~006413
- 13 -
'' ' ''''
The invention further relates to a process for -.
the preparation of the compounds of formula I and their : ;
salts, characterized in that a compound of formula II
Rl
NR2-H II
CH
~ (CH2)m
CH2 N ¦ R4 ~ ;
wherein
in the benzene ring one CH group can be replaced by N
and/or the benzene ring can be substituted as defined and .
wherein R , R2, R and m are as defined, . ;~:
is reacted with a compound of formula III .~ ,;
.~ lO Hooc-cH~-R3 III :;
~ wherein R is as defined and can be substituted as : ;.-
,.? ~ ~ defined,
or with one of its functional derivatives
: or in that a compound of formula IV - . ::.
l 2 ~:
1S OE CH ~ NR -Co-CH2-R3 IV
CEI2-X
~'``~ - ,:
~, wherein independently from each other, one CH group
each in the benzene ring and/or in the radical R can be
replaced by N, and/or wherein, independently from each .~
. ~ ~ other, the benzene ring and/or the radical R3 can be : -
substituted as defined, : ~
~' .
~0064~3
- 14 - ~
' ' :
X i8 Xl or NH2,
Xl i8 Cl, Br, I or a free or functionally modified OH
group, and
R , R2 and R3 are as defined,
is reacted with a compound of formula V
X2-CH2-(CH2)m-CH2-CH2-X3 V
wherein
one H atom in one of the CH2 groups is replaced by the
radical R4,
;
x2 and X3 are together NH or, if X is NH2, are each Xl,
and
m is as defined,
or in that a compound which has formula I, except that
instead of one or more H atoms it contains one or more
~; 15 reducible or hydrogenolytically cleavable groups and/or
C-C and/or C-N bonds, is treated with a reducing agent,
and/or in that one or more of the radical8 R3, R4, E and/or ;
~, Q, in a compound of formula I, are exchanged for one or
more different radicals R3, R , E and/or Q,
and/or in that a base or acid of formula I is converted
into one of its salts by treatment with an acid or base
respectively.
i The compounds of formula I are normally prepared
by methods known per se, as described in the literature
-~ 25 (e.g. in the standard works such as Houben-Weyl, Methoden
der Organischen Che~mie (Methods of Organic Chemistry),
Georg-Thieme-Verlag, Stuttgart), i.e. under reaction
`! ::
: . :: :"
'' ''',-::
i '~' '', ~'.:
2006413
conditions which are known and suitable for said
reactions. It i~ also possible to use variants which are
known per se and are not mentioned in further detail
here.
The starting materlalq are generally known or can
be prepared analogously to known substances by processes
known per se. If desired, they can also be formed in
~itu in a manner such that they are not isolated from the
reaction mixture, but immediately reacted further to give
the compounds of formula I. On the other hand, the re-
action can be carried out in step~, in which case it is
- possible to isolate other intermediates.
The individual process variants are illustrated
in further detail below.
The compounds of formula I can preferably be pre-
pared by reacting the compounds of formula II with car-
boxylic acids of formula III or their reactive functional
derivatives. Suitable reactive functional derivatives of
the compounds of formula III are especially the corres-
ponding esters, in particular the methyl or ethyl esters,
and the halides, anhydrides, azides or nitriles.
Compounds of formula II c~n be obtained for example by
; reducing compounds of formula VI
~Rl
~ .
\ NR2'
C ~ VI
I
R ~-NR
wherein
one CH group in the benzene ring may be replaced by N
and/or the benzene ring may be substituted as defined
above, ~- ;
;:
,.
2006413
- 16 -
R and R are (a) as R and R as defined above
or (b) together -CH=CH- -CH2-CH=CH- or
--CH=CH-CH2-,
R8 is (a) -CH2- or (b) -C0- and
R i~ (a) a chain -(CH2)m~3-
wherein one H atom is replaced by the radical R
or (b) a chain -(CH2)m+3-
wherein one H atom is replaced by the radical R4
but which contains at least one C-C double bond
and
m is as defined, but wherein the radicals R
R2 R8 and R9 cannaot simultaneously have the
meanings indicated in each case under (a).
Typical compounds of formula II are e.g. pyrroli-
dino-l-methyl-, piperidino-l-methyl- and hexahydroaze-
pino-l-methyl-isoindoline,pyrrolidino-l-methyl-,piperi-
dino-l-methyl- and hexahydroazepino-l-methyl-l,2,3,4-
tetrahydroi~oquinoline and pyrrolidino-l-methyl-, piperi-
~i dino-l-methyl- and hexahydroazepino-l-methyl-2,3,4,5- ~ ~.
tetrahy~ro-1H-2-benzazepine, 2-methylamino-2-phenyl- ;
ethyl-pyrrolidine, 2-, 3- or 4-(1-methylamino-2-pyrrolidino-
ethyl)-pyridine.
Typical compounds of formula III are e.g. phenyl-
acetyl chloride, bromide and azide, methyl and ethyl
~- 25 phenylacetate, phenylacetic anhydride, phenylacetonitrile
and the corres~onding derivative~ of 3,4-dichlorophenyl-;
acetic acid (e.g. 3,4-dichlorophenylacetyl chloride)~ of -~
l-naphthylacetic acid (e.g. l-naphthylacetyl chloride)~
;` of 4-benzothienylacetic acid (e.g. 4-benzothienylacetyl `
~; 30 choride) and of o-, m- or p-nitrophenylacetic acid (e.g.
~;~ o-, ~- or p-nitrophenylacetyl chloride)
: .~: :
.~ . , ,
;~006413
_ 17-
The reaction of II with III or derivatives of III
is conveniently carried out in the presence or absence of
an inert organic solvent, e.g. a halogenated hydrocarbon
such as methylene chloride, chloroform or trichloro- -
ethene, an alcohol such as methanol, ethanol or butanol,
an ether such as tetrahydrofuran (THF) or dioxane, an
amide such as dimethylformamide (DMF), or a sulphoxide
such as dimethyl sulphoxide (DMSO), and/or in the pre-
sence or absence of a condensation agent, e.g. a base, at
temperatures in the range from -20 to 200, preferably
from O to 100~. Example~ of suitable bases are alkali
metal hydroxides such as NaOH or ~OH, alkali metal car-
bonates such as Na2CO3 or K2C03, and tertiary amines such
as triethylamine or pyridine. Methylene chloride and
triethylamine are especially preferred as the solvent and
base respectively.
Reaction of the compounds of formulae IV and V
also yields compounds of formula I. This reaction is
conveniently carried out in one of the inert solvents
indicated above, at temperatures in the range from about
O to about 200-, preferably from 10 to 120, the addition
of a base possibly being advantageous.
- '
- Compounds of formula IV (X = OH) can be obtained ~ ;~
e.g. by acylating corresponding 1-hydroxymethyl-iso_ ~ ~-
indolines, -1,2,3,4-tetrahydroisoquinolines or -2,3,4,5-
; tetrahydro-lH-2-benzazepines or corresponding 2-~2NH-2-
phenylethanols or 2-R2NH-2-(pyridyl)-ethanols with compouds -
of formula III or,their derivatives. The other compounds of for ~ a
IV can be prepared therefrom in conventional manner: IV
(X = Br) e.g. with HBr in acetic acid, and IV (X = NH2)
from the bromo compo~md with potassium phthalimide and
;; sub~equent hydrolysis. Compounds of formula IV (X = NH2) ---
can also be prepared by reducing ~eis~ert compounds (of ~;
formula IV, but with CN instead of CH2-X).
: ~ ' ,'',-,''' ,,
. .- .: .
-;"';'' ',''.'`,`
:. . - .. - .
. . . -, ~:
'~ '. ' :' . ' ' . ' : :: ~ ' . . .` ' ` :,: ' ' ' . ', ': ' ' ' . ' ::
2006413
- 18 -
Compound~ of formiula V (XZ = X3 = OH) can be
obtained e.g. by reducing esters of the formula AOOC-
(CH2)m-CH2-COOA. The ot~her compound~ of formula V can be
prepared therefrom in conventional manner: V ( X2 = X3 =
Br) e.g. wlth SOBr2.
Suitable startinq materials for the preparation
of compounds of formiula I by reducing corresponding com-
pounds which instead of H atom~ contain one or more
additional reducible or hydrogenolytically cleavable
groups and/or C-C bonds and/or C-N bonds are, in particu-
lar, compounds of formula VII
1' R21 :::
-CO-CH2-RlO VII
IH .;:~ ;
R8-NR9 ~
,., ~ :,' ' .'
wherein
R10is ~a) R3 or (b) a radical which corresponds to R3.;:~
except that instead of the H atom of
an OH group it contains a hydrogeno-
~: lytically cleavable radical, :~
and
R , R , R , R and R are as defined;
howev~r, VII must differ from I, i.e. the radicals
R1 , R2~, R , R9~ and R~0 cannot simultaneously have the ~;~
meanings indicated in each case under (a). ~ ~
The radical R10 can preferably be o-, m- or p- ~;
benzyloxyphenylacetyl.
r~
, , ~ ...... .. : .. ,-,: ~ -::.. ": .. . - ::: : .. ., ....... ~ . ,
X006413
. ' ' : :.
- 19- i
A cuitable reducing agent i~ preferably hydrogen
in the presence of a càtalyst, especially a noble metal,
nickel or cobalt catalyst. Typical noble metals are, in
particular, platinum and palladium, which can be present
on support~ (e.g. on charcoal, calcium carbonate or
strontium carbonate), as oxides ~e.g. platinum oxide) or
in finely divided form. Nickel and cobalt catalysts are
conveniently used as Raney metals. Hydrogenation is
conveniently carried out at pressures in the range from
about 1 to about 200 bar and at temperatures in the range
from about -80 to +150, preferably from 20 to 100, in
the presence of an inert solvent, e.g. an alcohol such as
methanol, ethanol or isopropanol, a carboxylic acid such
as acetic acid, an ester such as ethyl acetate, or an
ether such as THF or dioxane.
If desired, one or more of the radicals R3, R4, E :
and/or Q , in a compound of formula I, can be exchanged
for one or more different radicals R3, ~, E and/or Q.
$hus it is possible to cleave ether groups (e.g.
OA groups) to form OH groups, e.g. by treatment with di-
~ethyl sulphide/boron tribromide complex, e.g. in tolu- ~ -
ene, $HF or DNSO, or by fusion with pyridine or aniline
hydrohalides, preferably pyridine hydrochloride, at about
150-250-, or by treatment with diisobutyl aluminium
hydride in toluene at about 0-110-. -
A further possibility is to hydrolyze ester ~ -
groups (e.g. COOA groups) to form COOH groups, this
reaction conveniently beijng carried out with NaOH or KOH
in an alcohol such as methanol or ethanoi or mixtures
thereof with water, at temperatures in the range from O -
to 100-. -
It is also po3sible to etherify or esterify OH "'' '~'!~'~
group~, e.g. by first preparing the corresponding alkali
metal (e.g. Na or K) alcoholates, phenates or salts and
then reacting these with appropriate halogen compounds,
2~06413
- 20 -
':
e.g.-with alkyl halides~such ai~ methyl chloride, bromide
or iodide, methyl or ethyl chloroacetate or bromoacetate,
methoxymethyl chloride or 2,3-isopropylidenedioxypropyl
chloride, conveniently in the presence of one of the
S solvents indicated above, at temperatures in the range
from 0 to 100.
Nitro groups can be reduced to amino groups, preferably by
catalytic hydrogenation under the conditions given above,
e.g. with Raney nickel in methanol or ethanol at 15 -40
and normal pressure. ~ ~;
Amino groups can be acylated, e.g. with acid chlorides such ;
as acetyl, methanesulfonyl, oxalic acid half ester or
succinic acid half ester chloride, preferably in inert solvents
such as dichloromethane at 15 - 40. A formylation of
lS amino groupscan also be achieved by reaction with an excess ~;
of formic acid at 80 - 100 for several hours. Reaction of
primary amino compounds with cyanates, e.g. KCN0 in water
at 15 - 40, gives the corresponding ureido compounds;
- with alkyl isocyanates, e.g. in inert solvents such as THF -
at lS - 40, N'-alkylureido compounds are obtained. -~ ~ -
Carboxylic acids can be transferred into carboxamides, ~ ~ ~
preferably by reaction with ammonia or alkylamines in presence ~ ;
` of a dehydration agent such as dicyclohexylcarbodiimide or
carbonyldiimidazol in an inert solvent such as DMF at ;~
lS- 40.
.. :.: . :.
, :,;;. ':' .'; :'.
. . . : . :,
' ' ' ':
'' ''''"'
'':
2~)06~13
- 21 -
A baqe of formula I can be converted into the
corresponding acid addition salt with an acid. Acids
which can be used for this reaction are those producing
physiologically biocompatible salt~. Thus it is po~sible
to use inorganic acids, e.g. sulphuric acid, nitric acid,
hydrohalic acids such as hydrochloric acid or hydrobromic
acid, phosphoric acids such as orthophosphoric acid, and
sulphamic acid, or organic acids, in particular ali-
phatic, alicyclic, araliphatic, aromatic or heterocyclic
monobasic or polybasic carboxylic, sulphonic or sulphuric
acids, e.g. formic acid, acetic acid, propionic acid,
pivalic acid, diethylacetic acid, malonic acid, succinic
acid, pimelic acid, fumaric acid, maleic acid, lactic
acid, tartaric acid, malic acid, benzoic acid, salicylic
acid, 2- or 3-phenylpropionic acid, citric acid, gluconic
acid, ascorbic acid, nicotinic acid, isonicotinic acid,
methane- or ethane-sulphonic acid, ethanedisulphonic
acid, 2-hydroxyethanesulphonic acid, benzenesulphonic
acid, p-toluenesulphonic acid, naphthalenemonosulphonic
and naphthalenedisulphonic acids and laurylsulphuric
acid. Salts with physiologically bioincompatible acids,
e.g. picrates, can be used to purify the compounds of
formula I.
Conversely, an acid of formula I (e.g. R3 = COOH)
can be converted into one of its metal or ammonium salts
by treatment with a base. Typical salts are, in par-
ticular, the ~odium, potassium, magnesium, calcium and
ammonium salts, as well as substituted ammonium salts.
If desired, the frèe bases of formula I can be liberated
from their salts by treatment with strong bases such as
sodium or potassium hydroxide or sodium or potassium
carbonate.
The compounds of for~ula I contain at least one
2006413
- 22 -
. ~:
chiral centre and can therefore exist in racemic or
optically active form. Racemates obtained can be
mechanically or chemically resolved into the enantiomers
by methods known per sè. Preferably, diastereoisomers
are formed from the racemic mixture by reaction with an
optically active resolving agent. Examples of suitable
resolving agents are optically active acids such as the
D and L forms of tartaric acid, diacetyltartaric acid,
dibenzoyltartaric acid, mandelic acid, malic acid or
lactic acid, or the various optically active camphor-
sulphonic acids such as ~-camphorsulphonic acid. It is
also advantageous to resolve enantiomers with the help of
a column packed with an optically active resolving agent
(e.g. dinitrobenzoylphenylglycine); a suitable eluent is
e.g. a mixture of hexane/isopropanol/acetonitrile, e.g.
in a volume ratio of 82s15s3.
Naturally it is also possible to obtain optically
active compound~ of formula I by the methods described
above using starting materials (e.g. those of formula II)
which are already optically active.
The invention further relates to the use of the -~
compounds of formula I and their physiologically bio-
compatible salts for making pharmaceutical preparations, --
especially by a non-chemical method. This can be done by
converting them into a suitable dosage form, together
with at leaat one solid, liquid and/or semiliquid carrier
or ad~unct and, if necessary, in combination with one or
more additional active ingredients.
The invention further relates to compositions,
especially pharmaceutical preparatiGns, comprising at
least one compound of formula I and/or one of its physio-
logically biocompatible salts.
These preparations can be used as drugs in human
or veterinary medicine. Possible carriers are organic or
inorganic substances which are suitable for enteral (e.g.
oral), parenteral or topical administration and which do
not react with the novel compounds, for example water,
vegetable oils, benzyl alcohols, alkylene glycols, poly- ~ ;
ethylene glycols, glycerol triacetate, gelatin, carbo- ~
:'.-'"
ZOOG413
- 23 -
hydrate~ such as lactose or starch, magnesium stearate,
talc and petroleum ~elly. Forms used for oral adminis-
tration are, ln particular, tablets, pills, coated
tablets, capsules, powders, granule~, syrups, ~uice~ or
drops, forms used for rectal administration are suppoi~i-
tories, forms used for parenteral administration are
solutions, preferably oily or aqueous solutions, as well
as suspensions, emulsions or implants, and forms used for
topical administration are ointments, creams or powders.
The novel compounds can also be lyophilized and
the resulting lyophilizates used e.g. to make in~ectable
preparations. The preparations indicated can be steril-
ized and/or can contain ad~uncts such as lubricant~
preservatives, stabilizers and/or wetting agents, emulsi-
lS fiers, salts for modifying the osmotic pressure, buffer
substances, colourants, taste improvers and/or flavour-
ings. If desired, they can also contain one or more
additional active ingredients, e.g. one or more vitamins.
The compounds of formula I and their physio-
logically biocompatible salts can be used for combating
diseases, especially conditions of pain.
Here the substances of the invention are normally
administered analogously to known analgesics, preferably
in dosages of between about 1 and 500 mg, especially of
between 5 and 100 mg, per dosage unit. The daily dosage
is preferably between about 0.02 and 10 mg/kg of body
weight. However, the particular dose for each individual
patient depends on a very wide variety of factors, for
example efficacy of the particular compound used, age,
body weight, general state df health, sex, diet, time and
route of administration, rate of excretion, drug com-
bination and severity of the particular disease for which
the therapy is intended. Oral administration is pre
ferred.
All temperatures in the present specification are
given in C. In the following Examples, "conventional
working-up" meanss Water or dilute sodium hydroxide
solution is added, if necessary, the mixture is extracted
with methylene chloride, the phases are separated, the
~ ~: ."
2006413
- 24 -
organic phase is dried with sodium sulphate and filtered,
the filtrate is evaporated and the residue i8 purified by
chromatography on silica gel and/or by crystallization.
Rf = Rf value on thin layer silica gel 60 F254 (E. Merck,
S article no. 5715), dichloromethane/methanol 9:1 with 0.1%
of triethylamine. L~ i = L~ ~20, c = 1 in methanol.
Example 1
' '
30 g of triethylamine are added to a solution of
21.6 g of pyrrolidino-1-methyl-1,2,3,4-tetrahydroiso-
quinoline (obtainable by reducing pyrrolidino-l-methyl-
isoquinoline with Na in ethanol) in 250 ml of methylene
chloride. ~ solution of 22.4 g of 3,4-dichlorophenyl-
acetyl chloride in 250 ml of methylene chloride is then
added dropwise, with stirring, and the mixture is stirred
for a further 2 hours at 20 to give pyrrolidino-l-
methyl-2-~3,4-dichlorophenylacetyl)-1~2~3~4-tetrahydro-
isoquinoline ("Pl') after conventional working-up. Hydro-
chloride: m.p. 264.
If theenantiomeric pyrrolidino-l-methyl-1,2,3,4-
tetrahydroisoquinolines are used as the starting mater-
ial, the two enantiomeric forms of pyrrolidino-l- methyl-
-2-(3,4-dichlorophenylacetyl)-1,2,3,4-tetrahydroisoquino-
line are obtained analogously.
The following 1,2,3,4-tetrahydroi~oquinolines are
obtained in analogous manner:
pyrrolidino-1-methyl-2-phenylacetyl-,hydrochloride:m.p.
277
pyrrol~dino-l-methyl-2--o-tolylacetyl-
pyrrolidino-l-methyl-2-m-tolylacetyl-, hydrochloride:
m.p. 242
pyrrolidino-l-methyl-2-p-tolylacetyl- -
pyrrolidino-l-methyl-2-o-methoxyphenylacetyl-
pyrrolidino-l-methyl-2-m-methoxyphenylacetyl-, hydro-
chloride: m.p. 219 ;~
. .. ~"
2006413
- 25 -
pyrrolidino-l-methyl-2-p-methoxyphenylacetyl-
pyrrolidino-1-methyl-2-o-ethoxyphenylacetyl-
pyrrolidino-l-methyl-2-m-ethoxyphenylacetyl-
pyrrolidino-l-methyl-2-p-ethoxyphenylacetyl-
pyrrolidino-1-methyl-2-o-fluorophenylacetyl-
pyrrolidino-l-methyl-2-m-fluorophenylacetyl-
pyrrolidino-l-methyl-2-p-fluorophenylacetyl-
pyrrolidinc-l-methyl-2-o-chlorophenylacetyl-
pyrrolidino-l-methyl-2-m-chlorophenylacetyl-, hydro-
chloride: m.p. 219 -
pyrrolidino-l-methyl-2-p-chlorophenylacetyl-hydrochloride: m.p.259
pyrrolidino-l-methyl-2-o-bromophenylacetyl-
pyrrolidino-l-Methyl-2-m-bromophenylacetyl-
pyrrolidino-l-methyl-2-p-bromophenylacetyl-, hydrochlor-
ide: m.p.261
pyrrolidino-l-methyl-2-o-methoxycarbonylphenylacetyl-
pyrrolidino-l-methyl-2-m-methoxycarbonylphenylacetyl-
pyrrolidino-l-methyl-2-p-methoxycarbonylphenylacetyl-
hydrochloride: m.p. 233
pyrrolidino-1-methyl-2-o-ethoxycarbonylphenylacetyl-
pyrrolidino-l-methyl-2-m-ethoxycarbonylphenylacetyl-
pyrrolidino-l-methyl-2-p-ethoxycarbonylphenylacetyl-
pyrrolidino-l-methyl-2-o-methoxycarbonylmethylphenyl-
acetyl-
pyrrolidino-1-methyl-2-m-methoxycarbonylmethylphenyl-
acetyl_, hydrochloride: m.p. 199O
pyrrolidino-l-methyl-2-p-methoxycarbonylmethylphenyl-
acetyl-, hydrochlsoride: m.p. 239
pyrrolidino-l-methyl-2-o-ethoxycarbonylmethylphenyl-
acetyl-
pyrrolidino-l-methyl-2-m-ethoxycarbonylmethylphenyl-
acetyl-
pyrrolidino-1-methyl-2-p-ethoxycarbonylmethylphenyl-
acetyl-
pyrrolidino-1-methyl-2-o-methoxycarbonylmethoxyphenyl- ~ ;
acetyl- . ~ ~,.,","
pyrrolidino-1-methyl-2-m-methoxycarbonylmethoxyphenyl- -- ~'~
acetyl-, hydrochloride: m.p. 188
:, . ,;
',',~''
;2006413
- 26 -
py~rolidino-1-methyl-2-p-methoxycarbonylmethoxyphenyl-
acetyl-
pyrrolidino-1-methyl-2-o-ethoxycarbonylmethoxyphenyl-
acetyl-
S pyrrolidino-l-methyl-2-m-ethoxycarbonylmethoxyphenyl-
acetyl-
pyrrolidino-1-methyl-2-p-ethoxycarbonylmethoxyphenyl-
acetyl-
pyrrolidino-1-methyl-2-(3,4-dimethoxyphenylacetyl)-,
hydrochloride: m.p. 232
pyrrolidino-1-methyl-2-(3,4-difluorophenylacetyl)-
pyrrolidino-1-methyl-2-(2,3-dichlorophenylacetyl)-
pyrrolidino-1-rnethyl-2-(2,4-dichlorophenylacetyl)-
pyrrolidino-1-methyl-2-(2,5-dichlorophenylacetyl)-
pyrrolidino-1-methyl-2-(2,6-dichlorophenylacetyl)-
pyrrolidino-1-methyl-2-(3,5-dichlorophenylacetyl)-
pyrrolidino-1-methyl-2-(3,4-dibromophenylacetyl)-
pyrrolidino-1-methyl-2-(3-chloro-4-metho~ycarbonylmethox-
yphenylacetyl)-
pyrrolidino-1-methyl-2-(3-chloro-4-ethoxycarbonylmethoxy-
phenylacetyl)-
pyrrolidino-1-methyl-2-(2-thienylacetyl)-, hydrochloride:
m.p. 275
pyrrolidino-1-methyl-2-(1-naphthylacetyl)-, hydrochlor-
ide: m.p. 262
pyrrolidino-1-methyl-2-(2-naphthylacetyl)-, hydrochlor- -
- ide: m.p. 255 -
pyrrolidino-1-methyl-2-(2-benzothienylacetyl)- -
pyrrolidino-1-methyl-2-(3-benzothienylacetyl)-, hydro-
chloride: m.p. 208
pyrrolidino-1-methyl-2-(4-benzothienylacetyl)-, hydro-
chloride: m.p. 269 -~
pyrrolidino-1-methyl-2-(5-benzothienylacetyl)- ~-
pyrrolidino-l-methyl-2-(6-benzothienylacetyl)- -
pyrrolidino-1-methyl-2-(7-benzothienylacetyl)-
pyrrolidino-l-methyl-2-(3,4-dichlorophenylacetyl)-6,7- ;
- dimethoxy-
' :~,,,~,
~06413
- 27 -
pyrrolidino-l-methyl-2-(1-naphthylacetyl)-6,7-dimethoxy-
pyrrolidino-l-methy1-2-(4-benzothienylacetyl)-6,7-
dimethoxy-
pyrrolidino-l-methyl-2-(3,4-dichlorophenylacetyl)-6,7-
methylenedioxy-
pyrrolidino-l-methyl-2-(1-naphthylacetyl)-6,7-methylene-
dioxy-
pyrrolidino-l-methyl-2-(4-benzothienylacetyl)-6,7-
me~hylenedioxy-
1~ piperidino-1-methyl-2-(3,4-dichlorophenylacetyl)-, hydro-
chloride hemihydrate hemiacetone solvate: m.p. 102
piperidino-l-methyl-2-(l-naphthylacetyl)-~ hydrochloride:
m.p. 288
piperidino-l-methyl-2-(4-benzothienylacetyl)-, hydro-
chloride: m.p. 278
hexahydroazepino-l-methyl-2-(3,4-dichlorophenylacetyl)-
hydrochloride: m.p. 184~
hexahydroazepino-1-methyl-2-(1-naphthylacetyl~-, hydro-
chloride; m.p. 274~
hexahydroazepino-1-methyl-2-(4-benzothienylacetyl)-.
Example 2
Pyrrolidino-l-methyl-2-(3,4-dichlorophenylacety~
isoindoline is obtained with pyrrolidino-l-methyli~o-
indoline analogously to Example 1.
The following isoindolines are obtained in
analogous manner:
' '~.
pyrrolidino-l-methyl-2-(1-naphthylacetyl)-
pyrrolidino-l-methyl-:2-(4-benzothienylacetyl)- -~
. .:
piperidino-l-methyl-2-(3,4-dichlorophenylacetyl)-
piperidino-1-methyl-2-(1-naphthylacetyl)-
piperidino-l-methyl-2-(4-benzothienylacetyl)-
-, , .
': ~..'' ''
~006413
, .. . . .
. . . ..
- 28 - -
.: . - " :- .
~,
hexahydroazepino-l-methyl-2_(3,4-dichlorophenylacetyl)-
hexahydroazepino-l-methyl-2-(l-naphthylacetyl)
hexahydroazepino-l-methyl-2-(4-benzothienylacetyl)-
and the following 2,3,4,5-tetrahyd~Q-lH-2-benzazspines
are also obtained in analogous mamler:
pyrrolidino-l-methyl-2-(3,4-dichlorophenylacetyl)-
pyrrolidino-l-methyl-2-(1-naphthylacetyl)-
pyrrolidino-l-methyl-2-(4-benzothienylacetyl)-
piperidino-l-methyl-2-(3,4-dichlorophenylacetyl)-
piperidino-1-methyl-2-(1-naphthylacetyl)-
piperidino-1-methyl-2-(4-benzothienylacetyl)-
hexahydroazepino-l-methyl-2-(3~4-dichlorophenylacetyl)
hexahydroazepino-l-methyl-2-(l-naphthylacetyl)-
hexahydroazepino-1-methyl-2-(4-benzothienylacetyl)-. - ;
.. : . .
Example 3 ~;
. .: . ,
6 g of 3-chloro-4-hydroxyphenylacetic acid
hydrazide (obtainable from the ester with hydrazine
hydrate) are dissolved in 200 ml of water and 40 ml of
1 N hydrochloric acid, a solution of 2.4 g of NaN02 in 40
ml of water is added dropwise at 0-3, with stirring, the
mixture is stirred for a further 30 min and the azide
formed iq extracted with methylene chloride. After
drying over MgS~ and concentration to 50 ml, the solu-
tion is added dropwise, with stirring, to a solution of
9 g ofpyrrolidino-1-methyl-1,2,3,4-tetrahydroisoquinoli-
ne and 4.4 ml of tr:Lethylamine in 100 ml of methylene
chloride. The mixture is stirred for a further 2 hour~
.: .. :: . : .:.:
'~, -,,
'' " :
~ .
200~413
- 29 -
at 20 and worked up in conventional manner to give
pyrrolidino-1-methyl-2~(3-chloro-4-hydroxyphenylacetyl)--
1,2,3,4-tetrahydroisoquinoline. Hydrochloride: m.p.
178.
S The following 1,2,3,4-tetrahydroisoquinolines are
obtained in analogous manner:
pyrrolidino-l-methyl-2-o-hydroxyphenylacetyl-
pyrrolidino-l-methyl-2-m-hydroxyphenylacetyl-
pyrrolidino-l-methyl-2-p-hydroxyphenylacetyl-.
Example 4
A solution of 41.3 g of 1-bromomethyl-2-(3,4-
dichlorophenylacetyl)-1,2,3,4-tetrahydroisoquinoline
(obtainable from l-bromomethyl-1,2,3,4-tetrahydroiso-
quinoline and 3,4-dichlorophenylacetyl chloride) and 22
g of pyrrolidine in 600 ml of THF is boiled for 6 hours
and evaporated and the residue is worked up in conven-
tional manner to give "P". Hydrochloride: m.p. 264~
~ "~
Example 5 ;
A mixture of 34.9 g of l-aminomethyl-2-(3~4~
20 dichlorophenylacetyl)-1,2,3,4-tetrahydroisoquinoline, ~ ~:
21.6 g of 1,4-dibromobutane, 15 g of K2C03 and 800 ml of ~ ~
ethanol is boiled for 8 hour~ and evaporated and the ~ -
residue i~ worked up in conventional manner to give ~P .
Hydrochloride: m.p. 264.
, -,: . ::
.
i . ,.- , . . . ., ~
~00~13
- 30 -
Example 6
A solution of 1 g of pyrrolidino-1-methyl-2-tl-
naphthylacetyl)-l~2-dihydroisoquinoline [obtainable by
reaction of isoqu.inoline with l-naphthylacetyl chloride/
KCN to give l-cyano-2-(1-naphthylacetyl)-1,2-dihydroiso-
quinoline, reduction to the l-aminomethyl compound and
reaction with 1,4-dibromobutane] in 20 ml of THF is
hydrogenated on 0.5 g of 5% Pd/C at 20 and 1 bar until
1 eq~..ivalent of H2 has been absorbed, the mixture is
filtered, the filtrate is evaporated and the residue is
worked up in conventional manner to give pyrrolidino-l- .. -.
methyl-2-(1-naphthylacetyl)-1,2,3,4-tetrahydroisoquinoli-
ne. Hydrochloride: m.p. 262. :
Example 7
lS A solution of 1 g of piperidino-1-methyl-2-p-
benzyloxyphenylacetyl-1,2,3,4-tetrahydroisoqu.inoline in ;.~.
25 ml of ethyl acetate is hydrogenated on 0.5 g of 5
Pd/C at 20 and 1 bar until no more hydrogen is absorbed, . ........ ... -~
the mixture is filtered and the filtrate is evaporated to :.
give piperidino-1-methyl-2-p-hydroxyphenylacetyl-1,2,3,4-
tetrahydroi.oquinoline.
Example 8 -
: ~ ,:
A mixture of 3.64 g of pyrrolidino-1-methyl-2-m-
methoxyphenylacetyl-1,2,3,4-tetrahydroisoquinoline and
3.5 g of pyridine hydrochloride is heated for 3 hours at . .
160, cooled and worked up in conventional manner to give : .
pyrrolidino-l-methyl-2-m-hydroxyphenylacetyl-1,2,3,4- .
tetrahydroisoquinoline.
;~006413
- 31 -
Example 9
2.3 g of Na are dissolved in 600 ml of methanol,
38.5 g of pyrrolidino-1-methyl-2-(3-chloro-4-hydroxy-
phenylacetyl)-ll2~3~4-tetrahydroisoquinoline are added,
the mixture is stirred for 2 hours at 20 and 10.9 g of
methyl chloroacetate are added dropwise. After boiling
for 1 hour, the mixture i8 cooled and worked up in con-
ventional manner to give pyrrolidino-1-methyl-2-(3-
chloro-4-methoxycarbonylmethoxyphenylacetyl)-l~2~3~4
tetrahydroisoquinoline.
Example 10 -~
A mixture of 10 g of pyrrolidino-1-methyl-2-(3-
chloro-4-methoxycarbonylmethoxyphenylacetyl)-1,2,3,4- ~.
tetrahydroisoquinoline, 2 g of KOH and 150 ml of ethanol ` ;.
lS is boiled for 2 hours. It is evaporated, the residue is `~ `
taken up in water, the calculated amount of dilute hydro-
chloric acid is added and the precipitate of pyrrolidino-
l-methyl-2-(3-chloro-4-carboxymethoxyphenylacetyl)- ; ; ... :
1,2,3,4-tetrahydroisoquinoline is filtered off.
Dihydrate: m.p. 136. ;~
The following 1,2,3,4-tetrahydroisoquinolines are ob- . -~
tained in analogous manner by saponification of the
corresponding esters:
pyrrolidino-l-methyl-2-o-carboxyphenylacetyl-
pyrrolidino-1-methyl-2-m- carboxyphenylacetyl-, -~
hydrochloride, monohydrate: m.p. 154
pyrrolidino-1-methyl-2-p-carboxyphenylacetyl-,
hydrochloride, dihydrate m.p. 167-
~ - " '.'~
.......................................................................... ' ;` ' .
2006413
- 32 -
pyrrolidino-l-methyl-2-o-carboxymethylphenylacetyl-
pyrrolidino-1-methyl-2-m-carboxymethylphenylacetyl-
pyrrolidino-l-methyl-2-p-carboxymethylphenylacetyl-
pyrrolidino-l_methyl-2-o-carboxymethoxyphenylacet
pyrrolidino-l_methyl-2-m-carboxymethoxyphenylacety~
hydrochloride, m.p.~ 240 .
pyrrolidino-l_methyl-2-p-carboxymethoxyphenylacetyl~
Example 11 ~ ;
A mixture of 46.5 g of the Na salt of pyrroli~
dino-1-methyl-2-(3-chloro-4-carboxymethoxyphenylacetyl)- `:
1,2,3,4-tetrahydroi~oquinoline, 15.1 g of trimethyl-
acetoxymethyl chloride and 600 ml of DMF is stirred for .
3 hours at 20. It is evaporated and the residue is ~-
worked up in conventional manner to give pyrrolidino-l--
methyl-2- (3-chloro-4-trimethylacetoxymethoxycarbonyl- .
mç~hox~?henylacetyl~-1,2,3,4-tetrahydroisoguinoline.
The followins 1,2,3,4-tetrahydroisoquinolines are ob-
tained in analogous manner~
pyrrolidino-l-methyl-2-(3-chloro-4-methoxymethoxycar-
bonylmethoxyphenylacetyl)-
pyrrolidino-l-methyl-2-(3-chloro-4-acetoxymethoxyc- ~ :
arbonylmethoxyphenylacetyl)-
pyrrolidino-l-methyl-2-[3-chloro-4-(1-acetoxyethoxy- `~
carbonylmethoxy)phenylacetyl]
pyrrolidino-1-methyl-2-~3-chloro-4-(1-trimethylacetoxy- .:. :
ethoxycarbonylmethoxy)phenylacetyl]- , - : .'
pyrrolidino-l-methyl-2-(3-chloro-4-methoxycarbonyloxy-
methoxycarbonylmethoxyphenylacetyl)-
pyrrolidino-l-methyl-2--[3-chloro-4-(1-methoxycarbonyloxy- ~
ethoxycarbonylmethoxyphenylacetyl]- ~ ~ ;
pyrrolidino-l-methyl-2-[3-chloro-4-(2,3-isopropylidene-
dioxypropoxycarbonylmethoxy)phenylacetyl]-.
- Z006413
- 33 -
Example 12
l-Pyrrolidinomethyl-2-o-nitrophenylacetyl-1,2,3,4-tetra-
hydroisoquinoline, Rf 0.45, is obtained analogously to
Example 1 from o-nitrophenylacetyl chloride.
~.,,
Analogously, the following 1,2,3,4-tetrahydroisoquino-
lines are obtained:
..
1-pyrrolidinomethyl-2-m-nitrophenylacetyl-, Rf 0.72
1-pyrrolidinomethyl-2-p-nitrophenylacetyl-
(-)-1-pyrrolidinomethyl-2-m-nitrophenylacetyl- [by opti-
cal resolution of 1-pyrrolidinomethyl-1,2,3,4-tetrahydro-
isoquinoline with benzoyl tartaric acid and reaction of `
the (+)-enantiomer (~a] + 58; hydrochloride, m.p. 278) ~-
with m-nitrophenylacetyl chloride]
(+)-l-pyrrolidinomethyl-2-m-nitrophenylacetyl-
(-)-1-pyrrolidinomethyl-2-p-nitrophenylacetyl-, Rf 0.76
(+)-l-pyrrolidinomethyl-2-p-nitrophenylacetyl-
l-pyrrolidinomethyl-2-(2-pyridyl-acetyl)-
l-pyr~rolidinomethyl-2-(3-pyridyl-acetyl)-
l-pyrrolidinomethyl-2-(4-pyridyl-acetyl)- `
1-pyrrolidinomethyl-2-o-(2-oxo-1-imidazolidinyl)-phenyl-
acetyl-
1-pyrrolidinomethyl-2-m-(2-oxo-1-imidazolidinyl)-phenyl-
acetyl-
l-pyrrolidinomethyl-2-p-(2-oxo-1-imidazolidinyl)-phenyl-
acetyl- i~
l-piperidinomethyl-2-phenylacetyl-, hydrochloride,
m.p. 217
l-piperidinomethyl-2-p-bromphenylacetyl-, hydrochloride,
m.p. 154 ~ -
1-piperidinomethyl-2-m-tolylacetyl-, hydrochloride,
m.p~ 151 `;
1-piperidinomethyl-2-o-nitrophenylacetyl-
l-piperidinomethyl-2-m-nitrophenylacetyl-
l-piperidinomethyl-2-p-nitrophenylacetyl-.
, ,
."", ~`i
PAT LOG l9-B 241089/0008Ø0
,: . : :.. . ..
2006413 :
- 34 -
Example 13
Analogously to Example 1!3-[l-(N-methyl-N-3,4-dichloro-
phenylacetyl-amino)-2-pyrrolidino-ethyl]-pyridine,
Rf 0.45, is obtained ~rom 3-(1-methylamino-2-pyrrolidino-
ethyl)-pyridine [obtainable by reaction of 2-amino-2-(3-
pyridyl)-acetic acid with pyrrolidine to the pyrrolidide,
N-formyliation to 2-formamido-2-(3-pyridyl)-acetic acid
pyrrolidide and reduction with LiAlH4]. ;
Analogously, there are obtained from 2-, 3- or 4-(1- ~ ;~
methylamino-2-pyrrolidino-ethyl)-pyridine~
:
2-[l-(N-methyl-N-o-nitrophenylacetyl-amino)-2-pyrrolidino-
ethyl]-pyridine
2-~1-(N-methyl-N-m-nitrophenylacetyl-amino)-2-pyrrolidino-
ethyl]-pyridine
2-ll-(N-methyl-N-p-nitrophenylacetyl-amino)-2-pyrrolidino-
ethyl]-pyridine
3-[1-(N-methyl-N-o-nitrophenylacetyl-amino)-2-pyrrolidino- ~
ethyl]-pyridine - '
3-~1-(N-methyl-N-m-nitrophenylacetyl-amino)-2-pyrrolidino-
ethyl]-pyridine
3-[1-(N-methyl-N-p-nitrophenylacetyl-amino)-2-pyrrolidino-
ethyl]-pyridine
4-[1-(N-methyl-N-o-nitrophenylacetyl-amino)-2-pyrrolidino-
ethyll-pyridine
4-[l-(N-methyl-N-m-nitrophenylacetyl-amino)-2-pyrrolidino-
ethyl]-pyridine
4-[1-(N-methyl-N-p-nitrophenylacetyl-amino)-2-pyrrolidino-
ethyl]-pyridine.
PAT LOG l9-B 241089/0009Ø0 ~;
2006413 -
Analogously, l-(N-methyl-N-3,4-dichlorophenylacetylamino)-
l-p-nitrophenyl-2-pyrrolidino-ethane is obtained from
l-methylamino-l-p-nitrophenyl-2-pyrrolidino-ethane [di-
hydrochloride: m.p. 212; obtainable from l-methylamino-
1-phenyl-2-pyrrolidino-ethane via l-N-methylacetamido-1-
phenyl-2-pyrrolidino-ethane (hydrochloride: m.p. 240)
and l-N-methylacetamido-l-p-nitrophenyl-2-pyrrolidino- ~-~
ethane (hydrochloride: m.p. 209)].
Example 14
Analogously to Example 1, there are obtained the follow-
ing 1-pyrrolidinomethyl-7-nitro-1,2,3,4-tetrahydroiso-
quinolines from l-pyrrolidinomethyl-7-nitro-1,2,3,4-
tetrahydroisoquinoline (obtainable by acetylation of
l-pyrrolidinomethyl-1,2,3,4-tetrahydroisoquinoline,
nitration with KN03/H2S04 to 1-pyrrolidinomethyl-2- - -
acetyl-7-nitro-1,2,3,4-tetrahydroisoguinoline and remo-
val of the acetyl group with 37 % hydrochloric acid):
2-(3,4-dichlorophenylacetyl)-, Rf 0.60
2-m-methoxyphenylacetyl-, Rf 0.70
2-o-nitrophenylacetyl-
2-m-nitrophenylacetyl-
2-p-nitrophenylacetyl-, Rf 0.63 -~
(-)~2-(3,4-dichlorophenylacetyl)-, Rf 0.60.
.; ' , ! '
Example 15
25 A solution of 10 g of l-pyrrolidinomethyl-2-o-nitro- ~ -
phenylacetyl-1,2,3,4-tetrahydroisoquinoline in 200 ml of
methanol is hydrogenated at 20~ and normal pressure in
the presence of 5 g of Raney nickel, until the calculated ~
amount of hydrogen is taken up. The mixture is filtered, ~ ;
30 the filtrate is evaporated, and l-pyrrolidinomethyl-2-o- ~
aminophenylacetyl-1,2,3,4-tetrahydroisoquinoline, Rf 0.23, ~;
is obtained.
PAT LOG l9-B 241089/0010Ø0
2006413
- 36 - .
, :,
Analogously, the following 1,2,3,4-tetrahydroisoquino-
lines are obtained by ~eduction of the corresponding nitro
compounds:
l-pyrrolidinomethyl-2-m-aminophenylacetyl-, Rf 0.24
1-pyrrolidinomethyl-2-p-aminophenylacetyl-
(-)-l-pyrrolidinomethyl-2-o-aminophenylacetyl-
(+)-l-pyrrolidinomethyl-2-o-aminophenylacetyl-
(-)-l-pyrrolidinomethyl-2-m-aminophenylacetyl-
(~)-l-pyrrolidinomethyl-2-m-aminophenylacetyl-
(-)-1-pyrrolidinomethyl-2-p-aminophenylacetyl-, Rf 0.53
(+)-l-pyrrolidinomethyl-2-p-aminophanylacetyl-
l-pyrrolidinomethyl-2-(3,4-dichlorophenylacetyl)-7- .;.
amino-, Rf 0.25 - ;:~:
1-pyrrolidinomethyl-2-m-methoxyphenylacetyl-7-amino-, .i-.
Rf 0.30 ~ .
l-pyrrolidinomethyl-2-o-aminophenylacetyl-7-amino-
1-pyrrolidinomethyl-2-m-aminophenylacetyl-7-amino-
l-pyrrolidinomethyl-2-p-aminophenylacetyl-7-amino_,
Rf 0.07
(-)-1-pyrrolidinomethyl-2-(3,4-dichlorophenylacetyl)-
7-amino-
l-piperidinomethyl-2-o-aminophenylacetyl-
1-piperidinomethyl-2-m-aminophenylacetyl-
l-piperidinomethyl-2-p-aminophenylacetyl-
as well as
l-p-aminophenyl-~-(N-methyl-N-3,4-dichlorophenylacetyl-
amino)-2-pyrrolidino-ethane, Rf 0.16
. ~.
PAT LOG 19-B 241089/0011Ø0 ¦
~0064~3
- 37 -
2-[1-(N-methyl-N-o-aminophenylacetyl-amino)-2-pyrrolidino-
ethyl]-pyridine
2-[1-(N-methyl-N-m-aminophenylacetyl-amino)-2-pyrrolidino-
ethyl]-pyridine
2-[1-(N-methyl-N-p-aminophenylacetyl-amino)-2-pyrrolidino-
ethyl~-pyridine
3-[1-(N-methyl-N-o-aminophenylacetyl-amino)-2-pyrrolidino-
ethyl]-pyridine
3-[1-(N-methyl-N-m-aminophenylacetyl-amino)-2-pyrrolidino-
ethyl]-pyridine
3-[1-(N-methyl-N-p-aminophenylacetyl-amino)-2-pyrrolidino-
ethyl~-pyridine
4-[1-(N-methyl-N-o-aminophenylacetyl-amino)-2-pyrrolidino-
ethyl]-pyridine
4-[1-(N-methyl-N-m-aminophenylacetyl-amino)-2-pyrrolidino-
ethyl]-pyridine - ::
4-[1-(N-methyl-N-p-aminophenylacetyl-amino)-2-pyrrolidino-
ethyl]-pyridine.
Example 16
A solution of 0.79 g of acetyl chloride in 10 ml of ~:.
. dichloromethane is added dropwise to a solution of 3.49 g
l-pyrrolidinomethyl-2-o-aminophenylacetyl-1,2,3,4-tetra-
:- : hydroisoquinoline in 175 ml of dichloromethane with stirr-
ing. Stirring is continued for 10 minutes, the solution : ::
is concentrated, and the obtained 1-pyrrolidinomethyl-2-
o-acetamidophenyiacetyi-1,2,3,4-tetrahydroisoquinoline is ..
filtered off. Rf 0.22. :~
: ; ' ~'
Analogously, the following 1,2,3,4-tetrahydroisoquino- ;~. ;-.
lines are obtained by acylation of the corresponding pri~
; 30 mary amino compounds: : : :
'~
: :~
: . ':~:
PAT LOG l9-B 241089/0012ØO
2006413
- 38 -
l-pyrrolidinomethyl-2-m-acetamido-phenylacetyl-, Rf 0.29
1-pyrrolidinomethyl-2-p-acetamido-phenylacetyl-
~ l-pyrrolidinomethyl-2-m-acetamido-phenylacetyl-
(+)-1-pyrrolidinomethyl-2-m-acetamido-phenylacetyl-
5 (-)-1-pyrrolidinomethyl-2-p-acetamido-phenylacetyl- ~ ':
(+)-l-pyrrolidinomethyl-2-p-acetamido-phenylacetyl-
1-pyrrolidinomethyl-2-(3,4-dichlorophenylacetyl)-7-
acetamido-, Rf 0.22
1-pyrrolidinomethyl-2-m-methoxyphenylacetyl-7-acetamido-
1-pyrrolidinomethyl-2-o-acetamidophenylacetyl-7-acetamido-
l-pyrrolidinomethyl-2-m-acetamidophenylacetyl-7-acetamido-
l-pyrrolidinomethyl-2-p-acetamidophenylacetyl-7-acetamido-
(-)-l-pyrrolidinomethyl-2-(3,4-dichlorophenylacetyl)-7-
acetamido-
1-piperidinomethyl-2-o-acetamido-phenylacetyl-
1-piperidinomethyl-2-m-acetamido-phenylacetyl-
l-piperidinomethyl-2-p-acetamido-phenylacetyl-
1-pyrrolidinomethyl-2-o-methylsulfonamido-phenylacetyl-,
Rf 0.40
1-pyrrolidinomethyl-2-m-methylsulfonamido-phenylacetyl-,
Rf 0.32
1-pyrrolidinomethyl-2-p-methylsulfonamido-phenylacetyl-
1-piperidinomethyl-2-o-methylsulfonamido-phenylacetyl-
l-piperidinomethyl-2-m-methylsulfonamido-phenylacetyl-
25 1-piperidinomethyl-2-p-methylsu~:fonamido-phenylacetyl- :~
as well as
l-p-acetamidophenyl-1-(N-methyl-N-3,4-dichlorophenyl-
acetyl-amino)-2-pyrrolidino-ethane, Rf 0.15. ~ .
PAT LOG 19-B 241089/0013Ø0
2006413
- 39 -
Example 17
A mixture of 1 g of 1-pyrrolidinomethyl-2-o-amino-
phenylacetyl-1,2,3,4-tetrahydroisoquinoline and 10 ml
of formic acid is boiled for 2 hours and then evaporated.
After conventional working-up, l-pyrrolidinomethyl-2-o-
formamido-phenylacetyl-1,2,3,4-tetrahydroisoquinoline,
Rf 0.30, is obtained.
Analogously, the following 1,2,3,4-tetrahydroisoquino-
lines are obtained by formylation of the corresponding
primary amines:
l-pyrrolidinomethyl-2-m-formamido-phenylacetyl-, Rf 0.30
l-pyrrolidinomethyl-2-p-formamido-phenylacetyl-
1-piperidinomethyl-2-o-formamido-phenylacetyl-
1-piperidinomethyl-2-m-formamido-phenylacetyl-
1-piperidinomethyl-2-p-formamido-phenylacetyl-.
- :' ~
Example 18 ; ;~
., : .. ' , -,~
KCNO (0.81 g) is added to a solution of 4.22 g of
l-pyrrolidinomethyl-2-o-aminophenylacetyl-1,2,3,4-tetra-
hydroisoquinoline-dihydrochloride in 50 ml of water.
After 3 hours, the mixture is concentrated, and 1-pyrro-
lidinomethyl-2-o-ureido-phenylacetyl-1,2,3,4-tetrahydro-
isoquinoline-hydrochloride is obtained. Rf 0.11. ~ ~
- ',,.
Analogously, the following 1~2~3~4-tetrahydroisoquinoline
are obtained from the c:orresponding primary amines:
1-pyrrolidinomethyl-2-m-ureido-phenylacetyl-, Rf 0.11 ,~
l-pyrrolidinomethyl-2-p-ureido-phenylacetyl-
(-)-l-pyrrolidinomethyl-2-o-ureido-phenylacetyl-, Rf 0.11; j~
hydrochloride, [a]-2 ~ ; ;
(+)-l-pyrrolidinomethyl-2-o-ureido-phenylacetyl- ~ ~
:'' .'."..
'': ;''':
PAT LOG 19-B 241089/0014Ø0 ~ ~
2006413
:... `.
- 40 -
(-)-l-pyrrolidinomethyi-2-m-ureido-phenylacetyl-,
Rf 0.11; hydrochloride, [~]-77
(+)-l-pyrrolidinomethyl-2-m-ureido-phenylacetyl-
(-)-l-pyrrolidinomethyl-2-p-ureido-phenylacetyl-, Rf 0.17;
hydrochloride, [a]-91
(+)-1-pyrrolidinomethyl-2-p-ureido-phenylacetyl-
1-pyrrolidinomethyl-2-(3,4-dichlorophenylacetyl)-7-ureido-,
Rf 0.07
l-pyrrolidinomethyl-2-m-methoxyphenylacetyl-7-ureido-,
Rf 0.06
l-pyrrolidinomethyl-2-o-ureidophenylacetyl-7-ureido-
l-pyrrolidinomethyl-2-m-ureidophenylacetyl-7-ureido-
l-pyrrolidinomethyl-2-p-ureidophenylacetyl-7-ureido-
(-)-1-pyrrolidinomethyl-2-(3,4-dichlorophenylacetyl)-7-
ureido-
l-piperidinomethyl-2-o-ureido-phenylacetyl-
l-piperidinomethyl-2-o-ureido-phenylacetyl- ::
l-piperidinomethyl-2-p-ureido-phenylacetyl-
as well as
1-(p-ureido-phenyl)-1-(N-methyl-N-3,4-dichlorophenyl-
acetyl-amino)-2-pyrrolidino-ethane, Rf 0.06 ;~
2-[1-N-methyl-N-o-ureidophenylacetyl-amino)-2-pyrrolidino-
ethyl~-pyridine
2-~1-N-methyl-N-m-ureidophenylacetyl-amino?-2-pyrrolidino-
ethyl]-pyridine
2-[1-N-methyl-N-p-ureidophenylacetyl-amino)-2-pyrrolidino- ~ : :
ethyl]-pyridine
3-[1-N-methyl-N-o-ureidophenylacetyl-amino)-2-pyrrolidino- ~.
ethyl]-pyridine ~. .`.
3-11-N-methyl-N-m-ureidophenylacetyl-amino)-2-pyrrolidino-
ethyl]-pyridine ~ ........... .
3-[1-N-methyl-N-p-ureidophenylacetyl-amino)-2-pyrrolidino-
ethyl]-pyrid.ine ~ -
: ~ -
PAT LOG 19-B 241089/0015Ø0
- 2006413
- 41 -
4-[1-N-methyl-N-o-ureidophenylacetyl-amino)-2-pyrrolidino-
ethyl]-pyridine
4-[1-N-methyl-N-m-ureidophenylacetyl-amino)-2-pyrrolidino-
ethyl]-pyridine
4-[1-N-methyl-N-p-ureidophenylacetyl-amino)-2-pyrrolidino-
ethyl]-pyridine.
Example 19
.. ,
A mixture of 3.49 g of 1-pyrrolidinomethyl-2-o-amino-
phenylacetyl-1,2,3,4-tetrahydroisoquinoline, 0.57 g of
10 methyl isocyanate and 50 ml of THF is stirred at 20 for ~i
30 minutes. The mixture is concentrated. After conven- - ~ ~;
tial working-up, 1-pyrrolidinomethyl-2-o-N'-methyl-
ureido-phenylacetyl-1,2,3,4-tetrahydroisoquinoline is
obtained. Rf 0.28.
15 Analogously, the following 1,2,3,4-tetrahydroisoguino- ~
lines are obtained from the corresponding primary amines; -~ - ;
l-pyrrolidinomethyl-2-m-N'-methyl-ureido-phenylacetyl-, ~ -
Rf 0.21
l-pyrrolidinomethyl-2-p-N'-methyl-ureido-phenylacetyl-
1-piperidinomethyl-2-o-N'-methyl-ureido-phenylacetyl-
l-piperidinomethyl-2-m-N'-methyl-ureido-phenylacetyl-
l-piperidinometyhl-2-p-N'-methyl-ureido-phenylacetyl-.
Example 20
,. .,. :,
A mixture of 4.43 g of 1-pyrrolidinomethyl-2-(3-chloro-
4-carboxymethoxyphenylacetyl)-1,2,3,4-tetrahydroisoquino- -
line, 1.62 g of carbonyldiimidazol and 140 ml of DMF is ~ -~
stirred 1 hour at 20. 15 ml of a 25% aqeous NH3 solution are
added, stirring is continued for further 12 hours, the
mixture is evaporated and taken up in 1 N aqueous hydro-
30 chloric acid. The solution is washed with ethyl acetate, :.
PAT LOG 19-B 241089/0016Ø0
` 20064~3
. .
- 42 -
the mixture is worked ~p conventionally with sodium
hydroxide solution/ethyl acetate, and l-pyrrolidinomethyl-
2-(3-chloro-4-carbamoylmethoxy-phenylacetyl)-1,2,3,4-
tetrahydroisoquinoline is obtained. Rf 0.19.
Analogously, the following 1,2,3,4-tetrahydroisoiquino-
lines are obtained from the corre~ponding carboxy com-
pounds with ammonia or methyl amine, respectively::
l-pyrrolidinomethyl-2-o-carbamoyl-phenylacetyl~
l-pyrrolidinomethyl-2-m-carbamoyl-phenylacetyl-, Rf 0.15 ~-
10 1-pyrrolidinomethyl-2-p-carbamoyl-phenylacetyl- ~ .
l-pyrrolidinomethyl-2-o-carbamoylmethyl-phenylacetyl-
l-pyrrolidinomethyl-2-m-carbamoylmethyl-phenylacetyl-,
Rf 0 13 -
l-pyrrolidinomethyl-2-p-carbamoylmethyl-phenylacetyl-,
Rf 0.12 ~ -~
l-pyrrolidinomethyl-2-o-carbamoylmethoxy-phenylacetyl-
l-pyrrolidinomethyl-2-m-carbamoylmethoxy~phenylacetyl- ~
l-pyrrolidinomethyl-2-p-carbamoylmethoxy-phenylacetyl- . ~.. - .
l-pyrrolidinomethyl-2-o-N-methyl-carbamoyl-phenylacetyl-
1-pyrrolidinomethyl-2-m-N-methyl-carbamoyl-phenylacetyl-,
: Rf 0.17
l-pyrrolidinomethyl-2-p-N-methyl-carbamoyl-phenylacetyl-
l-pyrrolidinomethyl-2-o-N-methyl-carbamoylmethyl-phenyl- . ,;.:,
acetyl- --~
25 1-pyrrolidinomethyl-2-m-N-methyl-carbamoylmethyl-phenyl- ~ .
acetyl- .- .
l-pyrrolidinomethyl-2-p-N-methyl-carbamoylmethyl-phenyl-
acetyl-
l-pyrrolidinomethyl-2-o-N-methyl-carbamoylmethoxy-phenyl-
acetyl~
l-pyrrolidinomethyl-2-m-N-methyl-carbamoylmethyl-phenyl- . ~-
acetyl-
l-pyrrolidinomethyl-2-p-N-methyl-carbamoylmethyl-phenyl- .
acetyl-
1-piperidinomethyl-2-o-carbamoyl-phenylacetyl-
PAT LO& l9-B 241089/0017Ø0
f,,;~
2006413
- 43 -
l-piperidinomethyl-2-m-carbamoyl-phenylacetyl-
l-piperidinomethyl-2-p-carbamoyl-phenylacetyl-
l-piperidinomethyl-2-o-carbamoylmethyl-phenylacetyl-
l-piperidinomethyl-2-m-carbamoylmethyl-phenylacetyl-
1-piperidinomethyl-2-p-carbamoylmethyl-phenylacetyl-
l-piperidinomethyl-2-o-carbamoylmethoxy-phenylacetyl-
l-piperidinomethyl-2-m-carbamoylmethoxy-phenylacetyl-
l-piperidinomethyl-2-p-carbamoylmethoxy-phenylacetyl-
l-piperidinomethyl-2-o-N-methyl-carbamoyl-phenylacetyl-
1-piperidinomethyl-2-m-N-methyl-carbamoyl-phenylacetyl-
l-piperidinomethyl-2-p-N-methyl-carbamoyl-phenylacetyl-
l-piperidinomethyl-2-o-N-methyl-carbamoylmethyl-phenyl-
acetyl-
l-piperidinomethyl-2-m-N-methyl-carbamoylmethyl-phenyl-
acetyl-
l-piperidinomethyl-2-p-N-methyl-carbamoylmethyl-phenyl-
acetyl-
l-piperidinomethyl-2-o-N-methyl-carbamoylmethoxy-phenyl- ~;
acetyl-
1-piperidinomethyl-2-m-N-methyl-carbamoylmethoxy-phenyl-
acetyl-
l-piperidinomethyl-2-p-N-methyl-carbamoylmethoxy-phenyl- -~acetyl-.
: - ;,
Example 21 ~
~, ' :.
To a solution of 3.49 g of 1-pyrrolidinomethyl-2-o-amino-
phenylacetyl-1,2~3,;4-tetrahydroisoquinoline in 180 ml of
dichloromethane,l.23 g of Cl-CO-COOCH3 are added dropwise
at 20 with stirring. Stirring is continued for 10 minutes,
l-pyrrolidinomethyl-2-o-methoxalylaminophenylacetyl-
1,2,3,4-tetrahydroisoquinoline obtained is dissolved in
450 ml of methanol. With stirring, ammonia gas is intro-
duced at 20 for 15 minutes, the mixture is left to stand
for 12 hours, evaporated and worked up conventionally.
l-Pyrrolidinomethyl-2-o-aminooxalylamino-phenylacetyl-
1,2,3,4-tetrahydroisoquinoline is obtained.
PAT LOG l9-B 241089/0018Ø0 ;-~
,
2006A13
- 44 -
Analogously the following 1,2,3,4-tetrahydroisoquinolines
are obtained:
l-pyrrolidinomethyl-2-m-methoxalylamino-phenylacetyl-
1-pyrrolidinomethyl-2-p-methoxalylamino-phenylacetyl-
1-piperidinomethyl-2-o-methoxalylamino-phenylacetyl-
l-piperidinomethyl-2-m-methoxalylamino-phenylacetyl-
1-piperidinomethyl-2-p-methoxalylamino-phenylacetyl-
as well as ~ ~;
1-p-methoxalylaminophenyl-1-(N-methyl-N-3,4-dichloro- -~
10 phenylacetyl-amino)-2-pyrrolidino-ethane, ` ~`
and therefrom with ammonia the following 1,2,3,4-tetra-
hydroisoguinolines~
'`' ~' ' ""..' ',''',,',
l-pyrrolidinomethyl-2-m-aminooxalylamino-phenylacetyl- :
l-pyrrolidinomethyl-2-p-aminooxalylamino-phenylacetyl-, ~ `:
Rf 0.47 -
l-piperidinomethyl-2-o-aminooxalylamino-phenylacetyl-
l-piperidinomethyl-2-m-aminooxalylamino-phenylacetyl- :
l-piperidinomethyl-2-p-aminooxalylamino-phenylacetyl-
as well as
1-p-aminooxalylamino-phenyl-1-(N-methyl-N-3,4-dichloro-
phenylacetyl-amino)-2-pyrrolidino-ethane, Rf 0.18.
Example 22 :
Analogously to Example 21, 1-pyrrolidinomethyl-2-(3,4-
dichlorophenylacetyl)-7-(3-ethoxycarbonyl-propionamido)-
1,2,3,4-tetrahydroisoquinoline (Rf 0.44) is obtained
from l-pyrrolidinomethyl-2-(3,4-dichloropherlylacetyl)-
7-amino-1,2,3,4-tetrahydroisoquinoline and succinic acid
monoethylester monochloride,and therefrom with NH3 in
PAT LOG 19-B 241089/0019Ø0
:
-- 2006413 ~ ~
,, ,
. .
- 45 ~
methanol l-pyrrolidinomethyl-2-(3,4-dichlorophenyl-
acetyl)-7-(3-carbamoyl-propionamido)-1,2,3,4-tetrahydro-
isoguinoline, Rf 0.05, is obtained.
Example 23
In analogy to Example 1, 2-[1-(N-methyl-N-o-methoxy-
carbonyl-phenylacetyl-amino)-2-pyrrolidino-ethyl]-pyri-
dine is obtained from 2-(1-methylamino-2-pyrrolidino- : ~:
ethyl)-pyridine and o-methoxycarbonyl-phenylacetyl . ~
chloride. ~ :
. ";, ....
10 Analogously, there are obtained . :
- ~
2-[1-(N-methyl-N-m-methoxycarbonyl-phenylacetyl-amino)- :: ;
2-pyrrolidino-ethyl]-pyridine :~ . :
2-[1-(N-methyl-N-p-methoxycarbonyl-phenylacetyl-amino)-
2-pyrrolidino-ethyl]-pyridine
3-[1-(N-methyl-N-o-methoxycarbonyl-phenylacetyl-amino)-
2-pyrrolidino-ethyl]-pyridine . - .
: 3-[1-(N-methyl N-m-methoxycarbonyl-phenylacetyl-amino)- .~ :
2-pyrrolidino-ethyl]-pyridine
3-[1-(N-methyl-N-p-methoxycarbonyl-phenylacetyl-amino)-
2-pyrrolidino-ethyl]-pyridine
4-[1-(N-methyl-N-o-methoxycarbonyl-phenylacetyl-amino)-
2-pyrrolidino-ethyl]-pyridine
4-[1-(N-methyl-N-m-methoxycarbonyl-phenylacetyl-amino)-
2-pyrrolidino-e~hyl]-pyrldine ! , ! '
4-[1-(N-methyl-N-p-methoxycarbonyl-phenylacetyl-amino)-
2-pyrrolidino-ethyl]-pyridine.
"
Example 24
: ..., .~-
In analogy to Example 10, the methylester obtained accord- :
ing to Example 23 is saponified to yield 2-11-(N-methyl- .
N-o-carboxyphenylacetyl-amino)-2-pyrrolidino-ethyl]-pyri-
,.: .. .. ..
dine. .:.
,".~ :,
PAT LOG l9-B 241089/0020Ø0 .~ ~
,:
2006413
- 46 -
Analogously, there are obtained
2-[N-methyl-N-m-carboxyphenylacetyl-amino)-2-pyrroli-
dino-ethyl]-pyridine
2-[N-methyl-N-p-carboxyphenylacetyl-amino)-2-pyrroli-
dino-ethyl]-pyridine
3-[N-methyl-N-o-carboxyphenylacetyl-amino)-2-pyrroli-
dino-ethyl]-pyridine
3-[N-methyl-N-m-carboxyphenylacetyl-amino)-2-pyrroli-
dino-ethyl]-pyridine
3-[N-methyl-N p-carboxyphenylacetyl-amino)-2-pyrroli-
dino-ethyl]-pyridine
4-[N-methyl-N-o-carboxyphenylacetyl-amino)-2-pyrroli-
dino-ethyl]-pyridine
4-~N-methyl-N-m-carboxyphenylacetyl-amino)-2-pyrroli-
15 dino-ethyl]-pyridine ;
4-1N-methyl-N-p-carboxyphenylacetyl-amino)-2-pyrroli-
dino-ethyl]-pyridine.
Example 25
In analogy to Example 20 2-[1-N-methyl-N-o-carbamoyl-
phenylacetyl-amino)-2-pyrrolidino-ethyl]-pyridine is
obtained from the carboxylic acid obtained according
to Example 24.
Analogously, there are obtained
2-~1-(N-methyl-N-m-carbamoylphenylacetyl-amino)-2-
pyrrolidino-ethyl]-pyridine
2-[1-(N-methyl-N-p-carbamoylphenylacetyl-amino)-2-
pyrrolidino-ethyl]-pyridine
3-[1-(N-methyl-N-o-carbamoylphenylacetyl-amino)-2-
pyrrolidino-ethyl]-pyridine
3-[1-(N-methyl-N-m-carbamoylphenylacetyl-amino)-2-
pyrrolidino-ethyl]-pyridine ~ ~
.: , ,'", ,,~''
PAT LOG 19-B 241089/0021Ø0
:` Z006413
. - : ,- 47 - ~ :
3-[1-(N-methyl-N-p-carbamoylphenylacetyl-amino)-2-
pyrrolidino-ethyl]-pyridine
4-[1-(N-methyl-N-o-carbamoylphenylacetyl-amino)-2-
pyrrolidino-ethyl]-pyridine ::
5 4-[1-(N-methyl-N-m-carbamoylphenylacetyl-amino)-2- :~
pyrrolidino-ethyl]-pyridine ~ :
4-[1-(N-methyl-N-p-carbamoylphenylacetyl-amino)-2-
pyrrolidino-ethyl]-pyridine. :;:.:.
.. . ., ~ ...
: ., ,. ~:
. . ,
;' .';: :
: ','.'"'.~. ~
.
.:".""','.'' ;'"~'
.': : i'. ' ".:.
' ' ' , . ':
' :. `"'" ';'.'
.' -"",',',,'
PAT LOG l9-B 241089/0022Ø0 : ~ .
': ~: ':' ,
2006413
- 48 -
Example 26
In analogy to Example 1, 1-pyrrolidinomethyl-2-(3,4-
dichlorophenylacetyl)-7-(2-oxo-1-imidazolidinyl)-1,2,3,4-
tetrahydroisoquinoline is obtained from l-pyrrolidino- .
methyl-7-(2-oxo-1-imidazolidinyl)-1,2,3,4-tetrahydroiso-
quinoline [obtainable from l-pyrrolidinomethyl-7-nitro-
1,2,3,4-tetrahydroisoquinoline by means of N-benzoylation
and reduction to 1-pyrrolidinomethyl-2-benzoyl-7-amino-
1,2,3,4-tetrahydroisoguinoline, subsequent reactions with
chloroacetyl chloride and with NH3 to l-pyrrolidinomethyl-
2-benzoyl-7-aminoacetamido-1,2,3,4-tetrahydroisoquinoline,
LiAlH4 reduction to 1-pyrrolidinomethyl-2-benzyl-7-(2-
amino-ethylamino)-1,2,3,4-tetrahydroi~oquinoline, reac-
tion with ethyl chloroformate and hydrogenolytic removal
of the benzyl group].
Analogously the following 7-(2-oxo-1-imidazolidinyl)-
1,2,3,4-tetrahydroisoquinolines are obtained:
l-pyrrolidinomethyl-2-o-nitrophenylacetyl-
l-pyrrolidinomethyl-2-m-nitrophenylacetyl-
20 1-pyrrolidinomethyl-2-p-nitrophenylacetyl- :.
l-pyrrolidinomethyl-2-o-methoxyphenylacetyl-
1-pyrrolidinomethyl-2-m-methoxyphenylacetyl-
1-pyrrolidinomethyl-2-p-methoxyphenylacetyl-
1-pyrrolidinomethyl-2-o-(1-naphthyl-acetyl)-
1-pyrrolidinomethyl-2-o-(4-benzothienyl-acetyl)-.
~ .. ..
Example 27
In analogy to Example 4 1-(3-ureido-pyrrolidinomethyl)- -~.
2-(3,4-dichlorophenylacetyl)-1,2,3,4-tetrahydroisoquino- -~
line is obtained from l-p-toluolsulfonyloxymethyl-2- ;
(3,4-dichlorophenylacetyl)-1,2,3,4-tetrahydroisoguino-
line and 3-ureido-pyrrolidine.
PAT LOG 19-B 241089/0023Ø0 ¦ :
: 2006413 ~ ~
;: :,, . , -
;~., , , . ~,:
- 49 -
Analogously, there are obtained from 2-, 3- or 4~
(N-methyl-N-3,4-dichlorophenylacetyl-amino)-2-bromo- :. .:.
ethyl]-pyridine: :
2-[1-(N-methyl-N-3,4-dichlorophenylacetyl-amino)-2-(3-
ureido-pyrrolidino)-ethyl]-pyridine
3-[1-(N-methyl-N-3,4-dichlorophenylacetyl-amino)-2-(3-
ureido-pyrrolidino)-ethyl]-pyridine
4-[1-(N-methyl-N-3,4-dichlorophenylacetyl-amino)-2-(3-
ureido-pyrrolidino)-ethyl]-pyridine.
-- -- '' ' ', ` '.
, . . . ~ ~ ` .
` :~
PAT LOG l9-B 241089/0024Ø0 ~ ::
., ,~,.
2006413 :`
: ,
.. .. :
The following Examples relate to pharmaceutical
preparations containing amines of formula I or their acid
addition salts:
Example As Tablets
A mixture of 0.1 kg of "P" hydrochloride, 4 kg of
lactose, 1.2 kg of potato starch, 0.2 kg of talc and 0.1
kg of magnesium stearate i8 compressed to tablets in con-
ventional manner such that each tablet contains 10 mg of
active ingredient.
Example B: Coated tablets
Tablets are produced by compression analogously
to Example A and are then covered in conventional manner
with a coating of sucrose, potato starch, talc, traga-
canth and colourant.
Example Cs Capsules
2 kq ofpyrrolidino-1-methyl-2-(1-naphthylacetyl-
)-1,2,3,4-tetrahydroisoquinoline hydrochloride are filled
into hard gelatin capsules in conventional manner such
that each capsule contains 20 mq of active ingredient.
:. ' : " ' '.' ''.
,, . -: :.-. .
Example D~ Ampoules
A solution of 1 kg of pyrrolidino-1-methyl-2-(4-
benzothienylacetyl)-1,2,3,4-tetrahydroisoquinoline hydro-
chloridq in lS l of propane-1,2-diol and 15 1 of double-
distilled water is filtered under sterile conditions and
filled into ampoules, and the ampoules are sealed under
sterile conditions. Ea~ch ampoule contains 2 mg of active
ingredient.
Tablets, coated tablets, capsules and ampoule~
which contain one or more of the other active ingredients
of formula I and/or their physiologically biocompatible
salts can be obtained in analogous manner.