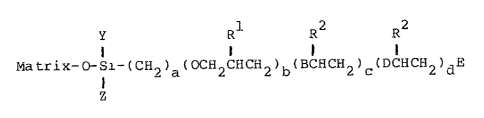Note: Descriptions are shown in the official language in which they were submitted.
PRESS OF RE~10VING AND CONCENTRATING DESIRED IONS FROM
SOLUTIONS THEREOF
INTRODUCTI ON
The process of the present invention comprises removing
and concentrating certain ions, such as the transition metal
ions, from solutions thereof admixed with other ions which
may be present in much higher concentrations. A complex of
the desired ion: is formed with a compound comprising an
amine-containing hydrocarbon covalently bonded to an
inorganic ma tri.x;. The complex can be formed by flowing such
solutions through a chromatography column packed with the
compound. The complex, is then broken to recover the desired
ions. This can be done by flowing a receiving liquid in much
smaller volume irhan the: volume of solution passed through the
column to remove' and concentrate the desired ions in solution
in the receivinc; liquid. The receiving liquid may then be
analyzed by knovJn methods, and the desired ions may be
recovered from .Lt.
The amine-containing intermediates covalently bonded to
an inorganic :mai~rix, e.g., sand, silica gel, glass, glass
fibers, alumina" nickel oxide, zirconia, titania and the
like, which are used to separate the desired ions are shown
by the structural formula (1>.
69912-188
2007311
2
i 2 z
Matrix-O Si-(CHZ)a(OCHZCHCHZ)b(BCHCH2)~(D HCHZ)dE (1)
I
Z
In formula ( 1 ) ,
B and D are each a radical selected from the group
consisting of N (R3) , N (R3) CHz, O and OCH2, with the proviso that
at least one of B anal D must be selected from the group
consisting of N (R3) .and N (R3) CHZ;
E is a radical selected from the group consisting of
H, NH (R3) , SH, OH, lower alkyl, and
N (R3) [CH2CH (Rl) CH20] b (CHZ) aSiYZ (O-matrix) ] ;
Y and Z are each a radical selected from the group of
C1, OCH3, OCZHS, metY;uyl, ethyl and halogenated substituents
thereof, and 0-matrix;
Rl is a radical selected from the group consisting of
H, SH, OH, lower alkyl and aryl such as phenyl, naphthyl and
pyridyl;
RZ is a radical selected from the group consisting of
H and lower alkyl;
R3 is a radical selected from the group consisting of
H, lower alkyl and aryl such as phenyl, naphthyl and pyridyl;
a is 2 to about 10;
b is 0 or 1;
c is 1 to about 2000;
69912-188
3 pp~11
d is 0 to about 2000; and
Matrix is selected from the group consisting of sand,
silica gel, glass, glass fibers, alumina, nickel oxide,
zirconia, and titania.
Preferred values are: Matrix is silica gel or
titanized silica gel; a is 3; b is 1; c is 1 to 5; d is 0; R1 is
OH; R2 is H; R3 is H,; B is NH; E is NHCH2CH (OH) CH20 (CH2) 3Si (0-
silica gel) 3 or NHCHZCH (OH) CH20 (CHz) 3Si (O-titanized silica gel) 3,
provided that when Matrix is silica gel, then E is
NHCH2CH (OH) CH20 (CH2) 3Si (0-silica gel) 3 and when Matrix is
titanized silica gel., then E is NHCHzCH (OH) CHZO (CHZ) 3Si (O-
titanized silica gel.); and Y and Z are each selected from O-
silica gel, O-titani.zed silica gel and OCH3, provided that when
Matrix is silica gel., then Y and Z are each O-silica gel or
OCH3 and when Matrix is titanized silica gel, then Y and Z are
each O-titanized silica gel or OCH3.
The compounds of formula (1) can be used in a~novel
process of selectively and quantitively removing and
concentrating a selE:cted ion or group of ions of the transition
metal type, e.g., copper, silver, mercury, lead, zinc, and
other transition metals, present at low concentrations from a
plurality of other ions in a multiple ion solution in which the
other ions may be present at much higher concentrations.
Preferably, those transition metal ions are Cu(II), Pb(II),
Ag(I) , Ru(III) , Pd(II) , Ir(III) , Zn(II) , Rh(II) , Cd(II) ,
Hg (II) , Os (II) , Mn (.CI) , Au (I) , Au (II) , Pt (II) , Pt (IV) , Co (III)
,
Co(II), Cr(II) and Cr(III). The process comprises bringing the
multiple ion solution into contact with a compound of formula
(1) to complex the desired ions) with the compound and
breaking the comple:~ with a. receiving liquid to render the
ions) soluble in the receiving solution in a concentrated
from. The receiving liquid may then be analyzed by known
A
69912-188 ~ ~ 7-1 1 g
3a
methods, or the ions) may be recovered therefrom by known
methods. The preferred embadiment disclosed herein involves
carrying out the process by bringing a large volume of the
multiple ion solution into contact with a compound of formula
(1) of the invention in a separation column. The multiple ion
solution flows through the column and the desired ion or ions
form a complex with the compound of formula (1). A smaller
volume of a receiving liquid such as dilute aqueous
hydrochloric or nitric acids for example, is then passed
through the column to break the complex by chemical or thermal
means. The receiving liquid further dissolves the desired ions
and carries them out of the column in a concentrated form.
Instead of using a column, the compound of formula (1) may be
slurries in a suitable liquid such as water. The multiple ion
mixture can be present in the slurrying liquid or subsequently
added to the slurry, the
A
~,.
4
desired ions) complex with the compound of formula (1> in
the slurry, and t:he slurry is then filtered. The resulting
solids are washed with a receiving solution to break the
complex and recover the desired ions) in the receiving
liquid. The desired ions may then be analyzed by known
methods, or recovered from the receiving phase by well known
procedures.
In a particularly preferred embodiment of the pracess the
bonded matrix-amine compound of formula (1) is placed in a
contacting device' such as a tall column. The multiple ion
mixture is passed th rough the column with the desired ions
from the multiple ion mixture forming a complex with the
bonded matrix to separate the desired ions from the rest of
the mixture which flows out of the column . A small volume of
the receiving liquid is thereafter passed through the column
to break the complex as well as to dissolve and carry out of
the column the dEaired i.on(s) . The desired ions may then be
analyzed by known methods such as atomic absorption
spectroscopy. In addition, the ions) can be recovered from
the receiving liquid by well known procedures.
BACKGROIJ~ND OF THE IN(iENTION
The fact is known that amine-containing hydrocarbon
ligands present as solutes in a solvent such as water are
characterized by their ability to selectivity form strong
bonds with the transition metal ions or groups of these ions
present as solutes in the same solvent as described in a book
5
by R.M. Smith and A.E. Martell, CRITICAL STABILITY CONSTANTS
VOL. 2: AMINES, Plenum Press, New York, 1975, pp. 1-401.
However, researchers have not previously been able to
effectively incorporate amine-containing hydrocarbon ligands
into separation ~;ystems where the behavior of the amine-
containing ligands in the separation systems in comparison to
that of the amine-containing ligand as a solute is unchanged
and/or the amine-containing ligand will remain in the
separation system for repeated separations of cations.
Articles such as those entitled SILANE COMPOUNDS FOR
SILYLATING SURFACES by E.P. Plueddemann, in "Silanes,
Surfaces and Interfaces Symposium, Snowmass, 1985,'° Ed. by
D.E. Leyden, Gor~ion and Breach, Publishers, 1986, pp. 1-25
and SILANE COUPL~CNG AGENTS by E. P. Plueddemann, Plenum Press,
1982, pp. 1-235 :gist many different types of organic
materials which raave been attached to silane compounds and
discusses some oi: their properties. The preparation and uses
of amine-containLng hydrocarbons attached to silane or silica
through a hydrocarbon linkage is discussed. The structures
reported in those publications contained only aminopropyl and
ethylene diamino~?ropyl groups [ formula ( 1 > where a is 3 , b is
0, c is 0 or l, d is 0, B is NH, D is not present, E is H or
NH2 and R2 is H]. These: latter compounds were used to
complex copper ions.
E.P. Plueddemann in METAL EXTRACTION FROM SOLUTION AND
IMMOBILIZED CHELATING At~ENTS USED THEREFORE, Canadian Patent
number 1,196,618 of November 12, 1985 reported th a
6
preparation of a variety of amine-containing silica gel
materials. These materials were made by ffirst reacting
chloropropyltrime:thoxysilane with the amine forming a
trimethoxysilane containing the amine function which was
coated onto silica gel and heated to effect a covalent
attachment of the amine to the silica gel. The resulting
compound had the amine function three carbon atoms removed
f rom sil ica . These materials do complex and thus remove
heavy metals. Hc~aever, these types of aminopropyl functions
are not completely stable as discussed in the next paragraph.
The Plueddeman:n Canadian patent lists other references
concerning the same type of silica gel-bound amine complexing
materials.
It is a known fact that amine functional groups attached
to silica gel, where the amine function is three carbon atoms
removed from th a sil ica gel , are not completely stable . E. P.
Plueddemann, in t:he above mentioned article in the book
edited by D.E. Leyden, reported that his amine materials
(amine group three atoms removed from silane) slowly lost
their ability to complex. copper II. D.~i. Wonnacott and E.V.
Patton in HYDROL'tTIC ST1~,BILITY OF AMINOPROPYL STATIONARY
PHASES USED IN TFiE SIZ E-EXCLUSI ON CHR~lATOGRAPHY OF CATIONIC
POLYi~tERS, Journal of Chromatography, vol. 389, pp. 103-113
(1987) and T.G. Z~addell, D.E. Leyden and M.T. DeBello in THE
NATURE OF ORGANOSILANE TO SILICA-SURFACE BONDING, Journal of
Americal Chemica:L Society, vol. 103, pp. 5303-5307 (1981)
discuss the stability of the aminopropyl-silica gel types of
7
materials . In the conclusion to the ~lonnacott and Patton
article it is stated that "aminoalkyl silanes which have been
used extensively in the .synthesis of silica-based, weak ion
exchangers do not lend tizemselves to this type of
chromatography du'e to their hydrolytic instability."
Bonded silica gel phase supports containing amine
functions have been prepared by reacting the amine with
3-glycidoxypropylsilane :bonded to silica gel. S.H. Chang,
K.M. Gooding and F.E. Regnier in USE OF OXIRANES IN THE
PREPARA'I°ION OF Bt>NDED PHASE SUPPORTS, Journal of
Chromatography, v~ol. 120, pp. 321-333 (i976) and M-A Bagnoud,
J-L Veuthey and Ht. Haerdi in INTERACTIONS SILICE METALLIQUE-
SOLUTE: POSSIBII'ITE d'APPLICATIONS en PRECONCENTRATION et en
CiHROMAOGRAPHIE d'ECHANGE de LIGANDS (LEC)r ChimiCa, VOl. 40,
pp. 432-434 (190E~) have reacted amines with 3-
glycidoxypropyl-bonded silica gel. Chang, Gooding and
Regnier reported on four such compounds prepared from
diethylamine [farmula (1>, where a is 3, b is l, c and d is
0, R1 is OH, and E is N (ethyl)2], dimethylaminoethanol
[formula (1), where a is 3, b is 1, c is 1, d is 0, B is O,
R1 is OH, R2 is H, E is N (methyl)2], diethylaminoethanol
[same as the previous structure except N(ethyl)2 at the end],
and polyethyleneamine [formula (1>, same as previous formula
except c is a lai.-ge number and E is NH2]. These materials
were used to separate proteins but not metal cations.
Bagnoud, Veuthey and Haerdi prepared a compound from a
cyclic tetraamine (cyclam) which does not have a structure
8
similar to the si:ructurea of f figure ( 1 ) . Thi s material was
used to bind metal ions and the bound metallic material was
used in liquid exchange chromatography to separate certain
organic compound:. In neither of these studies were metal
ions separated and recovered. 'There is a particular need in
modern society to (1) measure the concentrations of heavy
metal rations in the low part per billion (PPB)
concentration, (;2> remove low levels of toxic heavy metal
ions from solut ions such as potable water, and (3) recover
valuable metal ions pre~~ent in solution at low levels. For
example the allowable amounts of lead, mercury, cadmium and
silver ions in d:rin~ing water are in th a low PPB levels.
Present methods for ana7_ysis of these rations are not
accurate at those levels without time consuming methods to
concentrate the ~~ations up to the low part per million level.
Furthermore, removal of the metals is not selective, but is
expensive and equipment intensive using present methods.
Thus, the comple:xing properties of hydrolytically stable
amine-containing hydrocarbon ligands attached to an inorganic
support such as silica c;el or titanized silica gel are of the
utmost importance for the repeated separation and
concentration of certain heavy metal rations for analysis
and/or recovery purposes . The process of the present
invention using the amine-containing materials of formula (1)
accomplish this fea t.
9
SUMMARY' OF TfiE IIIe11~7ENTION
The process of the present invention uses compounds of
formula (1) having the amine-containing hydrocarbon ligand
covalently bonded to an inorganic support, e.g. sand, or
silica gel, glasa, glass fibers, alumina, nickel oxide,
zirconia or titanic. The compounds of formula (1), are
characterized by high selectivity for and removal of desired
metal ions or groups of metal ions such as the transition
metal ions present at low concentrations from the source
phase containing a mixture of these metal ions with other
ions. The ions which are not desired to be removed may be
present in much greater concentrations in the solution than
the metal ions which area to be removed. The process of the
present invention comprises selectively removing and
concentrating ths~ desired ions) and is characterized by the
abil ity to quantitatively complex from a large volume of
solution the desired ions) when they are present at low
concentrations. The desired ions are complexed with a
compound of formvala (1) when brought into contact with such
compound. For dais purpose, the solution from which the
desired ions are to be i:emoved is passed th rough a column
containing the compound of formula (1) . The desired ions are
then recovered from the separation column by flowing through
it a small volume of a receiving phase which contains a
solubilized reagent which need not be selective, but which
will strip the ions from the ligand quantitatively. The
analysis of the desired metal ions in the concentrated
IO
solution is accomplished by known methods such as atomic
absorption spectroscopy. The recovery of the desired metal
ions from the re<:eiving phase is easily accomplished by well
known procedures" The process for producing the compounds of
formula (1) will be mentioned but is not a part of the
present invention.
BR:CEF DESC',RIPTION OF THE DRAWINGS
The invention will be described and illustrated by
reference to a drawing in which
Fig. 1 represents schematically a suitable column for
holding the matrix bonded amine-containing hydrocarbon ligand
material through which a solution of metal ions can be flowed
to complex selectively with a desired ion or group of ions in
accordance with 'the invention.
DESCRIPTION OE' THE PREFERRED EMBODIMENT OF THE INVENTION
The preferred embodinnent of the ion-recovery process of
the invention utilizes t_he compounds represented by f~rmula
(1). The process of producing these new compounds is not an
aspect of the present invention but will be mentioned briefly
here.
Amine-containing hydrocarbon ligands are covalently
bonded to the inorganic support. For example, the inorganic
support such as silica gel is first heated with 3-glycidoxy-
propyltrimethoxysilane to produce a 3-glycidoxypropyl-bound
silica gel. 'his gel i s then heated with the appropriate
11
amine to effect a covalent bond as shown in equation (2).
The nature of thE~ amine will determine what B, D, E and R2
are in formula (1).
O
\ Sil ica Gel
(CH30) 3Si (CHZ) 30CH2---CH--CH2
heat
O R1~H or R ~1H
/ \ 2 2 (2)
(Silica Gel-O) 3S:i (CI~2 ) 3C~H~-CH~--CH2
heat
(Silica Gel-O)3S:i(CH2>3C~H~CH(OH>CHZ-L~HR or NRZ
The following examples are given to illustrate two
representative c~~mpound~~ which have been made in accordance
with formula (1) of the present invention. Other amine-
containing hydrocarbons bonded to an inorganic support were
and can be made in the Name manner.
3a
Example 1
In this example, an amine-containing hydrocarbon bonded
to a silica gel Haas made corresponding to a compound of
formula (1) wherein a is 3, b is 1, c is 5, d is 0, R1 is
hydroxy, R2 is hydrogen,, B is 1VH, D is not present since d i~
J, E is NHCH2CH(OH)CH20(CH2)3Si(O-silica gel)3, and Y and Z
are ~-silica gel groups.
12
Silica gel ( 60-200 mesh ) ( 1. 6 kilograms ) was suspended in
7 liters of toluene which contained 304 grams of 3-glycidoxy-
propyltrimethoxy~~ilane. The gel was stirred slowly to
insure that the c~el was not physically damaged, and the
mixture was heatE:d at 100oC for 8 to 18 hours. Then, 175 to
225 grams of pent.aetnylenehexaamine was slowly added and the
mixture was slowly stirred for 5-10 hours while maintaining a
temperature of lCiO°C. The solvent was filtered and the solid
amine-bound silk°a gel was dried in air in a well ventilated
hood.
Example 2
In this example, an amine-containing hydrocarbon bonded
to titanized sil~_ca gel was made corresponding to a compound
of formula (1) wherein a is 3, b is 1, c is 5, d is 0, R1 is
hydroxy, R2 is hydrogen, B is NH, D is not present, E is
NHCH2CH(OH)CH20(CH2)3Si(O-Titanized Silica gel)3, and Y and Z
are O-Titani zed Sil ica gel groups .
The titanized silica gel was first prepared by suspending
60-200 Mesh sili<:a gel (50g) in 200 ml of dry toluene.
TetraisopropoxytLtanium ( 20 ml ) was slowly added to the
stirred reaction mixture. Heat wa s evolved. The resulting
mixture was allovued to ~>tand for 16 hours and f filtered . The
residue was washed successively with 100 ml of toluene, 100
ml of methanol and 100 ml. of water and allowed to air dry at
room temperature. The overall weight of the silica gel
~~~c~~~
13
increased to 52.5 g resulting in 1.25 mmoles of Ti02 per gram
of material.
Pentaethylenekiexaamin.e was attached to the titanized
silica gel in they same manner as in Example 1 using 20 g of
titanized silica gel and 4 ml of 3-glycidoxypropyl-
trimethoxysilane in 50 ml of toluene to give the 3-glycidoxy-
propyl-titanized silica gel material, and then 10 g of this
latter titanized silica gel was reacted with 3 ml of
pentaethylenehex<~amine i.n 50 ml of refluxing methanol for 1
hour .
I~iETAL ICDN RECOVERY AND CONCENTRATION PROCESS
The metal ion recovery and concentration process of the
invention relates to the selective recovery of desired metal
ions from mixtures thereof with other metal ions using the
compounds of formula (la of the invention as defined above.
Effective method of recovery and/or separation of metal
ions, particular:Ly the transition metal ions, from other
metal ions in water supplies, waste solutions, deposits and
industrial solutions and silver recovery from waste
solutions, e.g., from emulsions on photographic and X-ray
film, represent a real need in modern technology. These ions
are typically present at low concentrations in solutions
containing other ions at much greater concentrations.
Likewise there is a need to concentrate these metal ions so
that an effective analysis using well known methods such as
atomic absorption spectroscopy can be carried out. Hence,
~~~a~~.~
14
there is a real need for a process to selectively recover and
concentrate these metal ions. The present invention
accomplishes thi:> separation and concentration effectively
and ef f is iently by th a use of compounds selected f rom th a
families represented by formula (1>.
The amine-containing inorganic matrix material of formula
(1) is placed in a column as shown in Figure 1. An aqueous
solution contain~_ng the desired ion or ions, in a mixture of
other ions which may be in a much greater concentration, is
passed through the column. The flow rate for the solution
may be increased by applying pressure (with a pump) on the
top of the column or ap~)lying a vacuum in the receiving
vessel. After the solution has passed through th a column, a
much smaller volume of a. recovery solution, i.e. an aqueous
acid solution, which will protonate the amine groups of the
ligands thereby _celeasir.~g the metal ions, i.s passed through
the column. This receiving solution contains only the
desired metal ions in a concentrated form for subsequent
ana lys is and/or :recovery .
2(l The following examplEa of separations and recoveries of
transition metal ions by the inorganic support-bound amine-
containing materials of Examples 1 and 2 and materials
prepared in a similar manner are given as illustrations.
These examples are illu:atrative only, and are not
comprehensive of th a many separations of transition metal
ions that are possible using the materials of formula (1) .
15
Example 3
In this example, 2 grams of the silica gel-bound arnine-
containing hydrocarbon of Example 1 was placed in a column as
shown in Figure 1.. A 500 ml solution of about 10 ppm of Cu2+
in 0.1 M aqueous MgCl2 was passed through the column using a
vacuum pump at 6~I0 torr to increase the flow rate. A 10 ml
aqueous solution of 1 M HC1 was passed through the column.
An analysis of the recovery solution by atomic absorption
spectroscopy (AA,'~ snowed that greater than 99~ of the copper
II ions original7_y in the 500 ml copper II solution was in
the 10 ml recovery solution.
Example 4
The experiment: of Example 3 was repeated with about 1 ppm
of Cd2+ in an aqueous solution of 0.1 M MgCl2. Again,
greater than 99~ of the Cd2+ ion in the original solution was
found in the recovery solution.
Example 5
The experimeni~ of Example 3 was repeated with about 13
ppm of Hg2+ in an aqueous solution of 0.1 M Mg3r2. In this
case, 42~ of the Hg2+ ion in the original solution was found
in the recovery :~olutior~.
Example 6
In this example, the titanized silica gel-amine material
of Example 2 was used to separate 10 ppm Cu2+ in an aqueous
~~~~! a~~.~
16
solution of 0.1 M MgCl2 as in Example 3. The Cu2+ was
removed and concE:ntrated in the same manner and with th a same
results as the E~aample 3.
Example 7
'The amine matE~rial of Example 1 has also been used to
make separations among the transition metals and the common
salt ions in solution. An example of this is the separation
and concentration of Cd2+, Pb2+ and Cu2+ from Na+, ~+, Mg2+
and Ca2+ solutions normally found in potable water. The
solution (1000 ml) was passed through the column, then a 10
ml of 1 M aqueou:~ HCl recovery solution. The lU ml recovery
solution containESd Cd2+, pd2+ and Cu2+ in concentrated form.
The concentrated solution was analyzed by atomic absorption
spectroscopy and found t:o contain the expected amounts of the
three ca tions.
Example 8
The experiment of Example 7 was repeated with trace
concentrations o:E Zn2+, ~,gn2+ and Ni2+ in an aqueous solution
containing the common salts found in ocean water at the
concentrations found in ocean water. The 10 m1 recovery
solution was found to contain only Zn2+, Mn2+ and ~Ti2+ in the
expected concentrations,.
17
Example 9
In this example, 2 grams of th a silica gel-bound amine-
containing hydrocarbon was prepared following th a procedure
of Example 1, except that ethylene diamine was used rather
than pentaethyleneh exaam.ine of Example 1. The amine-
containing silica gel used in Example 9 was that shown in
formula ( 1 ) where a is 3, b is 1, c is l, d is 0, B is NH, E
is NHCH2CH(Oz~)CH,yO(CH2)3 Si(O-silica gel), Rl is OH and R2
s
is H. Solutions (500 ml) of Cu2+ and Pd2+ ions in aqueous
0.1 M Mg(N 03)2 solutions were each separated as in Example 3.
In both cases, o~rer 99~ of the Cu2~ or Pd2+ was recovered in
the recovery solutions.
From the foregoing, it will be appreciated that the
inorganic matrix--bound amine-containing hydrocarbon ligands
of formula (1) oj_ the present invention provide a material
useful for the separation and concentration of the transition
metal rations from mixtures of those rations with other metal
rations. The transition metals can th en be analyzed and/or
recovered from the concentrated recovery solution by standard
techniques ~.nown in the science of these materials.
Although the .c~rocess of separating and concentrating
certain metal ions in this invention has been described and
illustrated by reference to certain specific silica gel-bound
amine-containing hydrocarbon ligands of formula (1),
processes using analogs of these amine-containing hydrocarbon
ligands are within the scope of the processes of the
invention as defined in th a following claims.