Note: Descriptions are shown in the official language in which they were submitted.
tr," ~ .
f~ ~ DR-5186
20~)7~1
SYhln~llC DETERGENT aARS
by
Frank J. Steer
and
Edmund D. George
Field o~ Invention
The present invention concerns slowly dissolving synthetic
detergent bar8. In particular, the present invention concerns
the incorporation of sodium bicarbonate as a dissolution
retarding agent in sUCh synthetiC detergent bar articles. The
present invention especially concerns a scrubbing article
comprising a porous 5ubstrate containing the synthetic detergent
bar of the present invention.
Bac~LG~I~ o~ Invention
Synthetic detergent bars (i.e., Nsyndet" bars) are well
known. For example, U.S. 3,376,229 to Haass, et al. discloses
syndet bars containing acyl isethionate as the principal deter-
gent component together with supplemental detergent, binder and
. _~
7911
plastizer components. Up to about 17% unesterified watersoluble
alkali metal isethlonate i8 incorporated in the bar of Haass, et
al., apparently to improve firmne88. The supplemental
detergents include the higher aliphatie alcohol sulfates, the
alkylaryl sulfonate8, and the higher aliphatic fatty acid
taurides. Binder plasticizers include aliphatic carboxylic
acids having 12-25 carbons and polyethylene glycols Bar
hardness may be increased by incorporating sodium chloride.
U.S. 2,177,055 to Cranor discloses a toilet soap composition
containing, in addition to the soap constituent, a relatively
large quantity of biearbonate of soda, the resulting composition
being effective in neutralizing odors of perspiration. Although
as little as 5~ bicarbonate is 8tated as having a deodorant
effect, Cranor indicate8 that for praetical purposes 10 to 90
parts bicarbonate shoUld be incorporated into the soap
composition.
U.S. 3,956,160 to Watanabe, et al. discloses a detergent
powder containing a powdered hydrous sodium carbonate and a
fatty acid at a mole ratio of 1-1.5 mol carbonate per mol fatty
acid. The powdered sodium carbonate includes sodium
sesquicarbonate (Na2C03 NaHC03 2H20) and mixtures of
sodium carbonate and sodium bicarbonate. The carbonate and
zoa7sl~.
- 3 -
fatty acid react when at a temperature above the meltlng point
of the acld, to produce a coarge soap that may be disintegrated
into a detergent powder.
skin-conditioning toilet 80ap bars are disclosed in U.S.
4,198,311 to France, et al., wherein the soap is a coco/tallow
blend and the bar also includes an anionic surfactant such as an
olefin sulfonate and fatty acid ester of sodium isethionate.
Soap composition~ containing an alkali metal C8-~18 acyl
isethionate are disclosed in U.S- 4,695,395 to Caswell, et al.
Antimicrobial toilet bars are disclosed in U.S. 4,714,563 to
Ra~s, et al.
A long-lastlng detergent bar i8 disclosed in U.S. 4,735,746
to Speranza, et al- wherein a water-soluble polyamide or
polyester i8 prepared or melted in the presence of a
surface-actiVe agent- Solid transparent cleansers are disclosed
in Gordon, U.S. 4,165,293.
Soaps capable of dispensing lime soap are disclosed in U.S.
3,850,834 to Hellsten, et al. See also U.S. 3,630,927 to Yo
Shen; U.S. 3,640,882 to Groves; 3,767,584 to Hirst: 4,000,081 to
Woo, et al; 3,793,215 to Smith and 3,988,255 to Seiden, each of
these patents being incorporated herein by reference thereto.
~ 2~)7911
Although the solubillty characteric~ of syndQt bars such as
disclosed by Haa88 are generally ~uitable, 8yndet bars for some
uses tend to be too quickly di9solving. Thu8, where water con-
tact with the bar tends to be prolonged, it i8 necessary to have
a bar that re~ists rapid dls801ution. Also, syndet bars which
~ay lie in water have a tendency to form a gel-like scum at the
bar surface that i5 in contact with water, which is not par-
ticularly attractive to consumers.
It has been found that the dissolution characteristics of
syndet bars can be improved by incorporating therein from above
about 5 to about 30% sodium bicarbonate. The synthetic deter-
gent or syndet bars of the present invention advantageously have
a ~low diRsolution rate when in contact with water. Moreover,
the bars herein have good foam generation characterictics and
good resistance to hard water film formation. Further, the
syndet bars of the present invention are mild to the skin,
especially important for hard and soft surface cleaning
applications where skin contact is likely. The syndet bars are
easily and inexpensively manufactured utilizing conventional
soap bar manufacturing apparatus and processes.
Z(~ 911
~?
Summary of the Invention
It is an ob~ect of the present invention to provide a syn-
thetic detergent or 8yndet bar that resi8t8 rapid dissolution,
yet forms a suitable amount of suds when immersed in water
~ t is another ob~ect of the invention to provide a syndet
bar that has a long length of life.
It is yet another ob~ect of the present invention to provide
a syndet bar that resists cracking.
Another aspect of the present invention i8 to incorporate
the syndet bar of the present invention into a scrubbing device.
The syndet bar of the present invention comprises from about
20 to about 90% of a particular synthetic detergent known in the
art as a lime 80ap disper8ant and up to about 30% sodium
bicarbonate, the amount of bicarbonate present in the bar being
effective to retard the dissolution rate of the bar and
preferably being from about 10 to about 20% by weight of the
bar. Preferably, the lime soap dispersant detergent is from
about 30 to about 50% by weight of the bar, and is selected from
the group consi8ting of sodium cocoyl isethionate and disodium
lauryl sulfosuccinate. The moisture content of the bars is from
2Q~)7911.
about 3.5 to about 8%. Other con8tituents may be lncorporated
a~ hereinafter described.
The syndet bar8 of the present invention may also contaln up
to about 40% of a fatty acid; other detergents that are not lime
soap dispersant8, typically in amounts of less than 50%; up to
about 15% alXali metal salt of a fatty acid, i.e., soap, and one
or more ad~uvant8 in amount8 effective to achieve their
~unctional purpose.
The bars of the present invention may be prepared by
extrusion and melt casting methods.
Brief Description of the Drawings
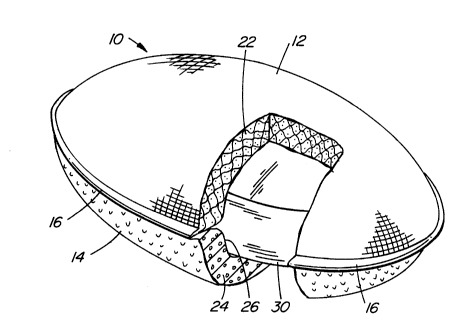
Figure 1 is a 8crubbing article in accordance with the
present invention, a portion of which has been broken away to
illustrate its interior structure.
Figure 2 is an alternate embodiment of the scrubbing article
of the present invention, in broken-away perspective view.
2Q3791~.
~? ~
Detailed Description of
The Preferred Embodiment
The synthetic detergent or 8yndet bar of the present inven-
tion advantageOU81Y has a very slow rate of dissolution in
water. On the other hand, the syndet bars herein have good foam
generatiOn characteristics, offer good resistance to the
formation of scum in the presence of hard water, and are mild to
the skin. Thus, the 8yndet bars are useful as skin cleanslng
bars. In such utility the tendency of syndet bars to form a
gel-like film or 8cum at the water-soap interface when in
contact with water i5 greatly reduced
The syndet bar8 of the present invention, however, are
particularly useful for incorporation in scrubbing articles,
such as ~crubbing articles di8closed in U.S. Patents 4,240,760
to Levine; 3,581,447 to Falivene: 4,665,580 to Morris; 4,510,641
to Morris; 4,674,237 to Sullivan; 4,190,550 to Campbell;
4,457,640 to Anderson, 4,659,496 to Klemm, et al. and 4,062,792
to ~cNabb, each of which is incorporated herein by reference
thereto.
A particularly preferred substrate for use ~n accordance
with the present invention ig the product LEM-METM sold by
swiss-Tex~ Inc. of Greenville, South Carolina. The LEM-METM
- - - - - -
~ ~ 20~79~ ~
- 8 -
article is of the type 8hown in Figure 2, hereinafter
described. The bar contained in the LEM-MET~ substrate is a
soap bar of conventional composition and doe~ not contain sodium
bicarbonate. The use-up rate of the soap bar contained in the
LEM-METM article was faster than desirable.
Such scrubbing articles are useful, for example, for washing
hard surfaces 8uch a8 countertops and dishes and soft surfaces,
e.g., as abrasive skin cleansers. These articles generally
comprise reticulated foam 8ub8trates of the same or different
texture ~oined at their edges in 8uch manner as to form an
interior pocket in which the 8yndet bar lies. These articles
are immersed periodically in water and may remain in water
contact, for example, in the 8ink or bathtub, for some time.
Even after u8e, thQ 80ap bar contained in the article is wetted
by the residual water trapped in the pores of the sponge-like
substrate. Accordingly, it i8 very important for the syndet bar
contained within the substrate to have a reasonable life. In
this regard, syndet bars of the present invention are quite
suitable. In addition, when the surface of the bar is in
contact with water, a8 when the scrubbing article is placed in
water or on a sinktop after use, there i8 reduced tendency for
the bar to become 80ft and mushy- Of course, these properties
are also advantageous in other products.
, ~ f~ 2Q~)7911.
g
Merely by way o~ illu8tratlon, a Bcrubbing article in accord-
ance with the present lnvention iQ illustrated in Flgure 1.
The ~crubbing article 10 compri8es a first reticulated
laminate 12 and a 8econd reticulated laminate 14, which lami-
nates 12 and 14 are affixed at seam 16, for example, by heat
sealing or by other conventional means. As seen in the
broken-aWay Bection, the interior of the article 10 is hollow, a
pocket 26 being formed, which pocket 26 accommodates the syndet
bar 30 of the present invention.
As indicated by the cros8-hatching or side walls 22 and 24
of the laminates 12 and 14, respectively, the laminates may be
~ade from different materials having different textures. Thus,
laminate 12 i8 Bmooth, which laminate 14 is more abrasive. The
thickness of the wallB 22 and 24 may be the same or different.
Suitable materialB for the lamlnate8 12 and 14 are cellular
porous materials that can be glued, sewn or heat sealed, such as
polyester, polyester-polyurethane, polyurethane esters, cellu-
lose foam, paper, rayon, nylon, cotton, wool, polyolefins and
t`he like.
Preferred laminate material8 are polyester-polyurethane and
polyurethane esters.
2~)791~.
-- 10 --
In another embodlment, shown in Figure 2, the article 100
nay comprise a third laminate 118 positioned within the article
100, to form a first pocket 126 and a seCond pocket 128, the
detergent bar 130 being placed within the pocket 126. In this
embodiment, the laminate 114 is quite porous. It is also very
abrasive, and eminently suitable for hard-to-clean surfaces.
The laminate 112 in this embodiment is smoother and tends to be
less porous. Because it i8 porous, there is a tendency for the
detergent solUtiOn to pass through the laminate 114 easily.
Accordingly, a barrier laminate 118 iB provided. The laminate
118 is typically of the same material as the laminate 112,
although usually thinner- In this manner the rapid diffusion of
detergent solution through the laminate 114 is avoided.
The syndet bar of the present invention comprises by weight
of the bar at least one synthetic detergent constituent that is
a lime soap dispersant and which i8 present in an amount
effective to provide cleaning, broadly from about 20 to about
90%, preferably from about 30 to about 50%, and preferably from
about 10 to about 20% ~odium bicarbonate, although up to about
30% can be incorporated. Although the reason is not well
understood, the presence of the bicarbonate in the syndet bar
reduces its rate of dissolution- Moreover, there is less of a
X(~)791~.
11 --
tendency of the bar to become soft, e8pecially where the surface
of the bar ls in contact with water. These attrlbutes might be
realized wlth as little as about 5% sodium bicarbonate, by
weight of the bar. However, preferably about 10% sodium
bicarbonate should be incorporated ln the bars of the present
invention.
Among the lime soap dispersant that may be incorporated in
the soap bars of the present invention are the neutrali2ed fatty
acid esters of isethionic acid and fatty acid half esters of
succinic acid. Preferred lime soap dispersants include 60dium
cocoyl isethionate and di80dium lauryl sulfosuccinate.
IllustratiVe of the isethionate are Jordapon CI, an 83% active
sodium cocoyl isethionate manufactured by PPG/Mazur Chemical
Co., and Isepon AC-78 manufactured by GAF Chemicals Corp.
Representative of the sulfo8uccinate half e8ters are Emcol 4300
manufactured by Witco Chemical Co. and Monamate LA-lO0 made by
Mona Industries, Inc.
Lime soap dispersants have superior tolerance to hard water,
and therefore are less likely to form a soap scum or residue.
These surfactants are good grease cutters, and hence the bars of
the present invention may be employed in scrubbing articles
intended for hard surface cleaning, especially dishes. These
7~911
(~ ~
- 12 -
surfactants are al80 mlld to the 8kin, and hence are eminently
suitable for ~uch utilities as dish cleaner~, loofahs~ and the
like. Finally, the8e material8 are available in solid form, and
hence are usable in extru8ion processe8, which is the preferred
manufacturing method.
Other synthetic detergent may be incorporated in the syndet
bar of the present invention to improve detergency and increase
foaming.
The anionic surfactants are typically water-soluble alkyl or
alkylaryl compounds, the alkyl having from about 8 to about 22
carbons, including generally a sulfate or sulfonate substituent
group that has been base-neutralized, typically to provide an
alkali metal, e.g., sodium or potassium, or an ammonium anion,
including, for example: (1) alkyl and alkylaryl sulfates and
sulfonates having preferably 10 to 18 carbon~ in the alkyl group,
which may be straight or branched chain, e.g., sodium lauryl
sulfate and sodium dodecylbenzene 8ulfonate; (2) alphaolefin aryl
sulfonates preferably having from about 10 to 18 carbons in the
olefin, e.g., 50dium C14_16 olefin 8ulfonate, which is a
~ixture of long-chain sulfonate 8alt8 prepared by sulfonation of
C14-C16 alpha-olefin9 and chiefly comprlsing sodium alkene
sulfonate9 and sodium hydroxyalkane 8ulfonates; (3) sulfated and
- - .
~ ~Q~79~
- 13 -
sulfonated monoglycerides, e8pecially tho8e derived from coconut
oil fatty acid8; (4) sul~ate e8ter8 of (a) ethoxylated fatty
alcohols having 1-10 mol~ ethylene oxide, e.g., sodium
polyoxyethylene (7 mols E0) lauryl ether 8ulfate, and (b) of
ethoxylated alkyl phenol8 having 10 mol8 ethylene oxide and 8 to
12 carbons in the alkyl, e-g-, ammonium polyoxyethylene (4 mols
E0) nonyl phenol ether 8ulfate; (5) fatty acid amides of a
methyl tauride, e.g., 60dium methyl cocoyl taurate, (6)
B-acetoxy- or B-acetamido-alkane sulfonates where the alkane has
from 8 to 22 carbon8, and (7) C8-C13 ~arCOsinates~ e.g.,
sodium lauroyl sarcosinate.
Nonionics such a8 (1) fatty alcohol alkoxylates, especially
the ethoxylates, wherein the alkyl group has from 8 to 22,
preferably 12 to 18, carbon8, and typically 6 to 15 mols alkoxide
per molecule, e.g., coconut alcohol condensed with about 9 mols
ethylene oxide; (2) fatty acid alkoxylate8 having from about 6 to
about 15 mols alkoxylate, especially the ethoxylate; (3) alkyl-
phenoxy alkoxylates, especially the ethoxylates, preferably the
octyl or nonyl ethoxylates containing 6 to 12 carbons in the
alkyl, and having about 5 to 25, preferably 5 to 15 mols alkylene
oxide per molecule, e.q., nonyl phenol ethoxylated with about 9.5
mols ethylene oxide (Igepal C0-630); (4) condensates of ethylene
oxide with a hydrophobic base formed by condensation of
ZQ~9~
propyleneoxide with propylenQ glycol, e.g., nonionic surfactants
of the Pluronic 8erie8 manufactured by BASF Wyandotte, (5)
condensates of ethylene oxide with an amine or amide; (6) fatty
amine oxides, e.g., stearyl dimethyl amine oxlde, and (7)
alkylolamides.
Preferred anionics are the alkyl and alkylaryl sulfates and
the alpha-olefin aryl sulfonate8, while preferred nonionics are
the fatty alcohol ethoxylates and the alkyl phenoxy ethoxylates.
These other synthetiC deter~ents are included in an amount of
up to about 50% by weight of the 8yndet bar, preferably from
about 1 to about 20% of the bar, most especially from about 1 to
about 10% by weight of the bar.
The syndet bar of the present invention ~ay also include
other constituent8. For example, the syndet bar may include
nonsyndet surface-active agents in minor amounts- Most important
of these constituents are the alkali metal soaps, especially
soaps from fatty acids having from about 12 to about 18 carbons.
Usually this component is present in an amount of less than 25%,
preferably between about 5 to about 15%. More than one soap
constituent may be present. Suitable is a mixture of 0-50% coco
and 50-100% tallow soapg, especially mixture5 of 10-25% coco and
~, ~) 2~ 9~1
- 15 -
75-90% tallow ~oaps. Soaps are employed to modify the physical
properties of the bar, without detracting from its intended
function. Thus, increa51ng the level of tallow 80ap present ln
the bar will increase the bar's hardness resulting in a more
slowly dissolving bar- Analogously, increasing the coco soap
level provides a softer, more easily dissolved bar. A 15/85
coco/tallow mixture represent8 a preferred blend to provide an
optimum balance among the several important physical properties.
Another optional constituent is a binder in the form of a
waxy and generally water-insoluble material. Preferably, the
binder is extrudable and present in an amount of from O to about
50% by weight of the bar. The preferred level is from about 10
to about 40% by weight- The binder is for example a fatty acid,
especiallY stearic acid- Because the fatty acid ig a weak acid,
it does not react with the bicarbonate, a weak base, at the
moisture level present in the bar. Other suitable binders
include glycol esters of fatty acids and amides of fatty acids.
HydroxypropylmethYl cellulose and microcrystalline cellulose may
be used as binders and/or solubility control agents.
The bar may also contain one or more inorganic fillers, for
example, sodium chloride, in an amount of from O to about 5% by
weight, preferably between 0.1-1% by weight. It is believed that
-
~ ~ 2~7911.
- 16 -
sodium chloride, pos8ibility because it i8 an electrolyte, plays
a role in the con8istency and/or proce8sabillty of the syndet
bar.
Another optional constituent i8 sodium i5ethionate in an
amount under about 5%. Thi8 material ig believed to aid in bar
firmness as indicated in the aforementioned ~aass patent.
The bar of the present invention may also contain a chelating
of sequestering agent, for example, ethylene diamine-tetraacetic
acid (EDTA). The chelating/sequestering agent is present in an
amount effective to tie up hardness ions -- calcium and
magnesium. A polyacrylic re8in may also be incorporated for this
purpose. Usually, the amount contained in the bar i6 from 0.1 to
about 5% by weight of the bar, preferably from 0.1 to 2% by
weight.
The bar may al80 contain an emollient especially where the
bar is intended for use as a skin cleanser. Suitable emollients
are PEG-75 Lanolin, isopropyl palmitate, and the like. The
emollient may be present in the bar in an amount of up to about
5%. See generally, McCutcheon'8 Functional Materials, p. 140, et
seq. (N. Amer. Ed. 1987).
~ r~~ ZQ3791~.
A fragranCQ may be included ln an amount o~ up to about 5%,
preferably about 1%, while a dye might be incorporated in small
quantity to provide a plea8ant color. Usually, the dye is
present in an amount of le88 than 1%, often les~ than 0.1~. A
preservative, e.g., fungicide, germicide, may be present in an
amount typically under about 0.5%. An antimicrobial might also
be included, especially when the bar is to be employed, say, as
a surgical scrub- Mention may be made of hexachlorophene,
3,4,4'-trichlorocarbanilide 801d a8 TCC by Monsanto Co. and
substituted diphenyl ethers 8uch a8 Irga6an DP 300 made by
Ciba-Geigy Corp. Suitable preservative8 and antimicrobials are
well-known in the art. See McCutcheon's, p.11-17.
Water is an e8sential ingredient for several reasons.
First, it has been found that between about 3.5 and about 8%
water content will provide a product consistent with the goals
of the present invention. At les8 than about 3.5% water the
syndet bar will crumble- At greater than about 8%, the 6yndet
bar will lose length of life- Moreover, extrudability of the
syndet bar become8 more problematic at the higher water levels.
The bars of the present invention preferably contain from about
5 to about 7% water.
This water content i8 mea8ured a~ free water, i. Q.,
moisture, preqent in the bar from all source8. Usually, water
2Q~)~911
- 18 -
has to bs added to reach the de81red level. Water content may
be measured by a Xarl Fi8her tltration modified ln that the
sample is first disper8ed in methanol to extract the water, the
filtrate then being titrated- This modification is necessary to
remove bicarbonate from the sample, which may cause inaccurate
results.
The bar of the present invention may be manufactured in
several ways. First, a cast melt proces8 may be used, wherein
the ingredients are blended together, heated as to liquefy them,
with gentle stirring to provide homogeneity to the mixture, and
then poured into a mold- The mold i8 then cooled and the bar
released. The variou8 8pecific molding techniques that may be
used in the manufacture of the bar8 are well known in the art.
Preferably, however, an extrusion process i8 used. The
various ingredient8 except the fragrance and the sodium
bicarbonate are preferably admixed to form a dough-like
consistency and then flaked in a hot nipped chill roll. The
flakes are thereafter combined with the bicarbonate (which may
be sensitive to the heat of the flaking operation) and the
fragrance oil (which is volatile and preferably in added just
prior to extrusion) to form a homogenous extrudable mixture.
~)
-- ~ 2(~)79~1.
-- 19 --
The homogeneou~ mixture i8 then introduced into the feed hopper
o~ an extruder. The barrel temperature varieg usually between
55 and lOO-F. The pre88ure through the forming plate i~ less
than 500 psig, generally from about 100 to about 300 psig. The
extrudate generally referred to as a billet is cut to the
desired size as it leaves the finiæh extruder. As a further
step, larger billet8 may be stamped to obtain a plurality of
individual syndet bars.
The present invention i8 illustrated further by the
following examples.
Era~ple 1
39.13 parts by weight sodium cocoyl isethionate and 32.02
parts stearic acid were melted together at 180-F, and the
resulting mixture wa8 then chilled and flaked. Thereafter, 10.0
parts sodium bicarbonate, 6.71 parts sodium tallow soap, 1.18
parts sodium coco 80ap, 3-95 parts sodium alpha olefin
sulfonate, 1.58 parts perfume, and 5.43 parts water were added
to the isethionate-stearic acid mixture and blended to
homogeneity using an amalgamator- This mixture was then refined
and extruded, and individual bar8 cut from the billet.
~ 79~
- 20 -
Bxample 2
The syndet bar compositions in Table II were obtained by
admixing sodium bicarbonate and fragrance with base compositions
having a nominal analysi8 as shown in Table I. Sodium
bicarbonate, fragrance and varioua base compositions having
different moisture levelg (a difficult parameter to control)
were blended together to obtain composite Compositions 2A
through 2E shown in Table II- The actual bicarbonate and water
levels are indicated in the Table II.
TABLE I
Base Composition
Constituent Concentration, Wt. %
Jordapon CI (1) 46.0
Stearic Acid 30.9
Bio Terge AS90 (2) 4.7
Toilet Soap (3) 9.4
Sodium Isethionat 1.63
Sodium Chloride 0.44
Preservative 0.03
Water 6.93
Total 100.0
79~1.
f~
(1) Sodlum cocoyl i8ethionate sold by PPG/Mazur Chemical Co.
(80% Active).
(2) Sodium alpha olefin 8ulfonate 801d by Stepan Co. (90
Active).
(3) 15/85 coco/tallow soap.
TABI~ II
Syndet Bar Com~ositions
Constituent Concentration, Wt. %
2A2B 2C 2D 2E
Base Composition 98.393.3 88.3 83.3 78.3
Sodium Bicarbonate 0 5.0 10.0 15.0 20.0
Fragrance 1.71.7 1.7 1.7 1.7
Total 100.0100.0100.0 100.0 100.0
~aHC03 Analysis (wt. %) 05.56 10.25 15.56 21.13
~oisture Analysis (wt. %) 5.7 5.7 5.3 6.5 6.1
~ 2(~9~
- 22 -
A homogeneous mixture of the constituents of Compositions
2A through 2E wa8 obtained in an amalagamator. The homogeneous
mixture was passed through a refiner and an extruder to obtain
20 gram billets of essentially constant moisture (6.1+0.4%),
which were evaluated as outlined below.
Commercially available dish cleaning articles of the type
shown in Fig. 2 and sold under the trade name LEM-METM were
cut open and the soap bar8 contained therein removed. Ten (10)
syndet bars of each Composition 2A through 2E were placed inside
the now empty pocket8 of the LEM-ME scrubbers, and the scrubbers
were resealed. Referring to Fig- 2, the laminate9 112 and 118
are of a closed cell polyurethane ester having a density of 1.7
lbs/ft3 and a porosity of about 50-60 pores/in2. The
laminate 112 thickness i8 about 0-5 inch, while the laminate 118
thickness is about 0.1 inch. Laminate 114 is comprised of an
open cell polyurethane ester and has a variable thickness
ranging between 0.25 and 0.75 inch. Its density is about 2.3
lbs/ft3 and has a porosity of from 10-50 pores/in2.
These articleg were tested over a five week period as
follow~. An article was secured in an apparatus eguipped with a
roller which was adapted to uniformly s~ueeze each of the
2~t:)7~
articles. The roller then made 2S stroke8 ln 2 1/2 minutes and
operated in this manner while 40-C water was sprayed onto the
article. Thereafter, the article was inverted and another cycle
of 25 strokes for 2 1/2 minuteg was conducted. This procedure
~as repeated for each article, and was repeated for five days.
After the fifth run, the articles were permitted to dry
thoroughly in air for about 48 hours, except that two of the ten
articles were oven-dried to constant weight and the weight of
syndet delivered was determined. The remaining articles were
sub~ected to the same procedure outlined above. The test was
carried out for a total of five weeks (after which all ten
articles for each Compositions 2A through 2E had been oven-dried
and the amount of syndet delivered measured). The results are
repeated in Table III below.
TABLE III
Average Weight of Syndet
Composition Delivered From Bar (grams)
$NaHC03 Week 1 Week 2 Week 3 Week 4 Week 5
2A 0 8.4 17.8 19.7 19.1 19.7
2B 5 15.7 20.5 19.8 19.8 20.1
2C 10 9.4 14.8 19.0 19.4 19.9
2D 15 6.3 10.3 14.0 16.1 19.3
2E 20 6.9 10.5 13.8 15.6 19.1
2(~3~79~ ~
It is seen that the bicarbonate level had a direct
influence on length of life of the 8yndet bar. Thu8, after two
weeks the control of Compo8ition 2A having 0~ bicarbonate and
the bar of Compo8ition 2B having 5% bicarbonate had been
substantially used up, while the remaining bars had
significantly greater (with a 95% confidence limit) mass
indicating a slower rate of dissolution.
ExamPle 3
Similar tests were conducted to ascertain the importance of
~oisture level on length of life- The Compositions 3A through
3J were prepared by ad~usting the moisture level in a base
composition similar to the Ba8e Compo8ition of Table I and then
adding sodium bicarbonate and fragrance to the desired level.
These composition~ were tested a8 previou81y indicated except
the test was terminated after three day8 in the second week.
The residual weight of the syndet bar wa8 then determined. The
results are indicted below in Table IV. In Table IV the initial
bicarbonate and water moi8ture level8 of the bar were
analytically determined. The 8yndet bar had an initial weight
of 20 grams.
~ ¢ Z(~7911.
- 25 -
TABLE rv
Weight of Bar
Wt. % (Actual) (gm8) Remalning
Composition H20 NaHC03A~ter E~ght Days
3A 3.54 0 5.22
3B 3.65 0 S,30
3C 3.589.05 4.70
3D 3.5120.01 12.39
3E 4.499.04 7.18
3F 4.709.11 7.30
3G 4.0019.32 13.23
3H 5.4119.98 14.23
3I 6.18 0 7,40
3J 6.268.98 8.86
AnalysiS on thi8 data indicates that the increases in the
length of life of the 8yndet bar8 of the present invention is
linear with regard to water content and parabolic with respect
to bicarbonate level.
Example 4
Base Composition of Table I was used to make bars of the
following compositions:
(~? Z(~79~1
- 26 -
TABLE V
Constituent Concentration, Wt. %
(6)
4A 4B 4C 4D 4E 4F
Base Composition 82.3 80.3 78.3 82.3 78.3 73.3
Sodium Bicarbonate 15.0 15.0 15.0 15.0 15.0 15.0
Fragrance 1.7 1.7 1.7 1.7 1.7 1.7
Stepanol WA100(4) 1.0 3.0 5.0
Nacconal 90G (5) - _ _ 1.0 5.0 10.0
(4) Sodium lauryl sUlfate sold by Stepan Chemical Co. (99% Active).
(5) Sodium alkylaryl sulfonate ~old by Stepan Chemical Co. (90%
Active).
(6) Extruded bars were not acceptable.