Note: Descriptions are shown in the official language in which they were submitted.
2~!0~938
FIE~D OF THE I~VENTION
The present invention relates to additives for
improving the flow properties and viscometric properties of
certain oleaginous compositions and to oleaginous
compositions containing said additivec. More particularly,
the present invention relates to additives for improving
the low temperature flow properties and viscometric
properties such as viscosity index of lubricating oil
compositions and to lubricating oil compositions containing
said additives. Still more particularly, the present
invention relates to improved lubricating oil compositions
including such additives and exhibiting improved low
temperature flow properties and viscometric properties.
The present invention also relates to methods for improving
the flow properties and viscometric properties of oleagi-
nous composition, particularly engine crankcase lubricant
composition~.
BACKGROU~D OF THE INVENTION
A wide variety of compounds for use as lubricating
oil or fuel oil additives are known in this art. These
include compound~ variously referred to as pour point
depressant~, viscosity index improving compositions, wax
crystal modifiers, and the like. In particular, Cashman et
al., U.S. Patent No. 2,825,717, discloses the preparation
of certain lubricating oil additives by the copolymer-
ization of polycarboxylic acid esters with other polymer-
izable monomeric materials, including vinyl compounds such
as vinyl acetate. The preferred unsaturated polycarboxylic
acid esters therein are fumaric acid esters produced from
C1 through C18 aliphatic alcohols.
9 3 8
-- 2 --
Bartlett, U.S. Patent No. 2,618,602, discloses
pour point depressing and/or viscosity index improving
materials obtained by polymerizing certain specified alkyl
fumarate esters. In particular this patentee discloses the
use of polymerized fumarate esters of C12 to C14
alcohols for such purposes. This patent specifically
discloses that the C12 alcohol was more effective than
the C14 alcohol, although both polymerized esters
exhibited pour point depressing properties.
Rossi et al., U.S. Patent No. 4,089,589, discloses
the use of specified mixtures of lubricating oil pour point
depressants which include polyesters consisting of a
polymeric ester of acrylic acid or methacrylic acid and a
monohydric alcohol containing from 10 to 18 ca~bon atoms,
and/or interpolymers of a vinyl alcohol ester of a C2 to
C18 alkanoic acid (e.g., vinyl acetate) and a
di(C6 C18 alkyl) fumarate as one of the components
thereof for improving the viscosity index of high wax
content lubricating oils which also include viscosity index
improving ethylene copolymers. Also, Wyman, U.S. Patent
No. 3,250,715, discloses terpolymers of dialkyl fumarates,
vinyl esters, and alkyl vinyl ether~ for improving the pour
point of lubricating oils, and most particularly in which
the dialkyl f~marates are prepared for various C10
through C18 alcohols including tetradecyl alcohol alone
a~ well as alcohol mixtures averaging from 12 to 14 carbon
atom~.
There has also been disclosed in U.S.
Patent No. 4,713,088 and U.S. Patent 4,863,486
the use in various middle distillate fuel
compositions for lowering the pour point and
A
_ 3 _ ~ 9~8
controlling the size of wax crystals in these composition
additives which specifically include polymers and
copolymers of specific dialkyl fumarate vinyl acetate
copolymers. Most specifically, these patent applications
disclose the use of such additives in which the average
number of carbon atoms in the alkyl groups in the polymer
or copolymer must be from 12 to 14. In addition these
additive~ are also disclosed as being useful in combination
with the polyoxyalkylene esters, ethers, ester/ethers and
mixtures thereof, as well as with various other additives.
Furthermore, British Patent No. 2,023,645 discloses, for
use in treating distillate fuel oils, various three-compo-
nent systems which include as a first component flow
improvers having an ethylene backbone, such as various
ethylene polymers including ethylene polymerized with
various mono- or diesters (e.g., vinyl acetate; and C13
fumarates), as a second component a lube oil pour depres-
sant such as various oil soluble esters and/or higher
olefin polymers (e.g., dialkyl fumarate, vinyl acetate
copolymers), and as a third component various polar
oil-soluble compounds (e.g., phenates, sulfonates,
phosphates, and carboxylates).
It is also disclosed in Lewtas's U.S. Patent Nos.
4,661,121 and 4,661,122 that the size of wax crystals
forming in fuel~ boiling in the range of 120-C to 500 C can
be controlled by an additive which includes the polymers
and copolymer~ of mono- and di-n-alkyl esters of
mono-ethylenically unsaturated C4 to C8 mono- or
dicarboxylic acids, in which the average number of carbon
atoms in the n-alkyl groups is from 14 to 18. These
patents show a preference for copolymers of di-n-alkyl
fumarates and vinyl acetate, and specifically state that
the fumarates can be made from single alcohols or mixtures
of alcohols, and when mixture~ are used they are mixed
_ _ 4 -
prior to esterification. Furthermore, these patents
disclose the use of various ethylene unsaturated ester
copolymer flow improvers as co-additives therewith, but do
not specify that these additive~ are produced from alcohol
mixtures. In Canadian patent application Serial No.
- 474,621, now Canadian Patent 1,237,238, there is disclosed
as a dewaxing aid a copolymer of dialkyl fumarate and vinyl
acetate in which a large proportion of the alkyl groups are
C20 to C24 alkyl groups. In U.S. Patent 4,839,074 there is
disclosed a dual component flow improver additive
composition for oleaginous compositions which comprises (i)
low molecular weight polymers and interpolymers (e.g.,
copolymers) of unsaturated mono- or dicarboxy esters having
the formula
.
o
H C - OR
C -- C
R' H
in which R' is either hydrogen or a COOR radical, and R is
a C14 alkyl group; and (ii) low molecular weight
lubricating oil f;ow improver (LOFI) comprising
non-ethylene containin~ polymers which are soluble or
dispersable in these lubricating oils, preferably
interpolymers of dialkyl fumarates and vinyl esters in
which the fumarate~ are esterified with mixtures of C6
through C20 alcohols.
Various oil-soluble hydrocarbon polymeric
materials such a~ ethylene-alpha-olefin copolymers, e.g.,
ethylene-propylene copolymers, are known to be useful as
viscosity index improvers for oleaginous compositions such
as lubricating oils.
i.
- 5 - 2~ 9~B
While these various types of additive compositions
have met with various degrees of success in the particular
environments in which they are employed it has been
observed that various lubricating oil compositiOns, such as
thos~ containing certain viscosity improving additives such
as copolymers of ethylene and propylene, as well as those
lubricating oil compositions containing lubricating oil
flow improvers, nevertheless experience difficulty in
passing recently adopted, more stringent, low temperature,
slow cool performance tests designed to measure the low
temperature pumpability of crankcase lubricating oils. It
is therefore an object of the present invention to provide
oleaginous compositions, particularly lubricating oil
compositions, which exhibit enhanced low temperature
pumpability and viscometric properties.
SUMMARY OF THE INVENTION
In accordance with the present invention there is
provided an oleaginous composition, particularly a
lubricating oil composition, exhibiting improved low
temperature flow properties and viscometric properties
which comprises: (i) oleaginous material such as
lubricating oil: (ii) a first additive or component which
is a lubricating oil flow improver (LOFI) comprising low
molecular weight, e.g., low number average molecular weight
(~n)~ polymers and interpolymers (e.g., copolymers)
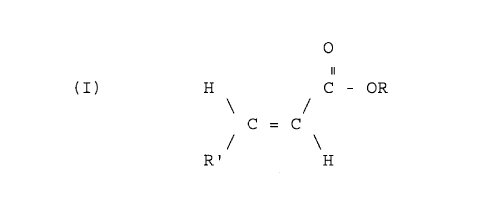
o~ unsaturated mono- or dicarboxy esters having the formula:
H C - OR (I)
C = C
R' H
in which R' is either hydrogen or a COOR radical, and R is
a C14 alkyl group: and (iii) a second additive or
2~39~B
component which is an oil soluble hydrocarbon polymeric
viscosity index improver, preferably an
ethylene-alpha-olefin copolymer.
In a preferred embodiment of the present
invention, the first additive, i.e., lubricating oil flow
improver, comprise~ a low molecular weight (~n)
interpolymer of at least one of the carboxy ester monomers
of formula (I) above interpolymerized with a variety of
different comonomers such as a polymerizable vinyl ester
monomeric compound having the formula:
H o
..
(II) CH2 = C - O - C - R1
in which Rl is an alkyl group containing from about 1 to
18 carbon atoms, preferably from about 1 to 6 carbon atoms,
and most preferably 1 carbon atom. The preferred ester
monomer of formula (II) is vinyl acetate.
The second additive, i.e., the viscosity index
improver, comprises an oil soluble hydrocarbon polymer,
preferably an ethylene-alpha-olefin copolymer, and more
preferably an ethylene-propylene copolymer.
D~TAILED DESCRIPTION
Th- oleaginous compositions of the present
invention compris- (i) oleaginou~ material, preferably
lubricating oil, generally in a major amount; (ii) first
additive comprised of poly~ers or interpoly~ers of
unsaturated carboxy esters; and (iii) second additive
comprised of a hydrocarbon polymeric viscosity index
improver.
The first additive of the present invention is a
lubricating oil flow improver and is comprised of a polymer
or interpolymer represented by the formula
- ~ 7 ~ ~ n Q 8 9
H C - OR (I)
C = C
R' H
in which R' is either hydrogen or the COoR radical, and in
which R i5 a C14 alkyl group. The production of these
ester and diester polymers include~ an esterification
reaction between unsaturated mono- or dicarboxylic ~cids or
their corresponding anhydrides, as well as the polymeri-
zation of the esterified monomers, and is well known in the
art, as specifically disclosed beginning at column 2, line
of Cashman et al., U.S. Patent No. 2,825,717.
While the alkyl group represented by R may be
straight chain or slightly branched, the straight chain
alkyl group is preferred.
Some illustrative examples of compounds of formula
I include
O
H C - o - C14H29 and
C = C
H H
H C - ~ ~ C14H29
C = C
C14H29 ~ O - C H
o
2~ 3~3
The first additive or component may be a homo-
polymer, e.g., a homopolymer derived from monomers of
formula I, or an interpolymer as defined hereinafter.
The first additive or component preferably
includes the interpolymers of the diester monomers of
formula (I), wherein R' is COOR, with certain specified
polymerizable monomeric compounds, namely vinyl esters,
alpha-olefins, or styrenes such as styrene itself. One of
these copolymerizable compounds is a monomer of formula
H O
..
(II) CH2 = C - O - C - Rl
in which Rl is an alkyl group containing from 1 to about
18 carbon atoms, preferably from 1 to about 6 carbon atoms,
and most preferably 1 carbon atom, preferably vinyl
acetate, which is interpolymerized with the diester of
formula I in a reaction which is carried out in the
presence of free radical initiators, such as peroxide
catalyst.
The first component is characterized by a low
molecular weight, i.e., a number average molecular weight
(~n) ~f not greater than about 40,000, and
typically ranging from about 1,500 to about 40,000, and
preferably from about 2,500 to about 15,000.
Alternatively, such molecular weights of the first
component lubricating oil flow improvers of the present
invention are more conveniently expressed by the specific
viscosity exhibited by such polymers. Accordingly, such
specific viscosities will typically range from about O.ll
to about 2.2, preferably from about 0.2 to about 0.9, and
most preferably from about 0.2 to about 0.7.
Such specific viscosities are determined in
accordance with the following equation:
X~938
SpeCific Visco~ity = K-vis of SolutiOn
K-vis of Solvent
wherein "K-vi~ of Solution" is the kinematic viscosity at
104-F (40-C) of a 2.0 mass/volume percent solution of the
polymer (a.i. basis) in mixed xylenes (solvent) available
commercially, using Ubbelohde-type viscometers with a
viscometer conqtant of about 0.003 cSt/second; and the
"K-vis of Solvent" is the corresponding kinematic viscosity
of the solvent alone at the same temperature. All specific
viscosities reported herein are determined ~y the above
method.
When interpolymers of monomer components depicted
by formulas (I) and (II) are employed as the first
component, the mole ratio employed for the polymerization
of such monomers can typically vary from about 1.3:1 to
about 0.5:1, preferably from about 1.2:1 to about 0.5:1,
and most preferably from about 1.2:1 to about 1:1.
Furthermore, the details with respect to
conditiona for esterification, homopolymerization, and
interpolymerization reactions are essentially the same as
set forth below with reference to the esterification and
interpolymerization Or the dicarboxylic acid esters
described below in connection with the vinyl-ester-
containing interpolymers Or the second component thereof.
Th~ particular dicarboxylic acid or anhydridemonom~r which is preferred will depend on the identity of
its comonomer. Thus, when the comonomer is a vinyl ester,
the preferred d~carboxylic acid i5 fumaric acid. When the
comonomer is an alpha-olefin or styrene, the preferred
dicarboxylic monomer is maleic anhydride.
Furthermore, whether it i~ preferable to esterify
the dicarboxylic acid or anhydride monomer first and then
interpolymerize, or to first interpolymerize the free acid
-- 10 --
or anhydride monomer and then esterify, depends on the
particular identify of the dicarboxylic monomer and its
comonomer.
Thus, for example, it is conventional to first
esterify the fumaric acid monomer or any other dicarboxyliC
monomer, prior to interpolymerization with a vinyl ester.
In contrast, it is also conventional to polymerize
maleic anhydride with styrene or the alpha-olefins, and to
then esterify.
Moreover, while it is preferred to achieve
complete esterification of all of the carboxyl groups of
the dicarboxylic monomer, it is permissible to achieve only
partial esterification, of typically not less than about
70, and preferably not less than about 80, mole % of the
available esterifiable carboxyl groups.
The lubricating oil flow improvers, i.e., first
additive of component, are preferably interpolymers,
preferably copolymers, of certain unsaturated dicarboxy
esters with certain specified polymerizable monomeric
compounds, namely, vinyl esters, alpha-olefins, or styrene.
Suitable ethylenically unsaturated dicarboxylic
acids or their anhydrides, which are eventually esterified,
have the carboxyl or anhydride groups located on vicinal
carbons, and have 4 to 10 carbons in the unesterified
monomer molecule. Suitable dicarboxylic acids or
anhydrides thus include fumaric acid, maleic anhydride,
mesaconic acid, citraconic acid and anhydride, and itaconic
acid and its anhydrides.
Accordingly, esterification is conducted with a
C14 alcohol, which alcohol can be slightly branched or
straight chain, preferably straight chains, and most
preferably straight chain alkyl. Thus, the alcohol used
for esterification is selected from the C14 aliphatic
alcohols. Primary alcohols are preferred over secondary
2~ 9~3
and tertiary alcohols, and the alcohols are preferably
saturated, although some degree of unsaturation (i.e., less
than about 2 mole %) i~ permissible. Straight and lightly
branched chain alcohola are preferred over hiqhly branched
alcohols.
As indicated hereinafore, the dicarboxylic monomer
of formula I can be interpolymerized with a variety of
different comonomerq. The first of these comonomers, as
indicated hereinafore, is a vinyl ester represented by
formula II, with the preferred ester monomer of formula II
being vinyl acetate. The preferred interpolymer of this
class of lubricating oil flow improvers is C14 dialkyl
fumarate/vinyl acetate copolymer.
The mole ratio of the unsaturated dicarboxyl
monomer to vinyl ester in the polymerization reaction
mixture can vary typically from about 1.3:1 to 0.5:1,
preferably from about 1.2:1 to 0.7:1, and most preferably
from about 1.2:1 to 1:1.
These interpolymers can be prepared by
conventional free radical polymerization techniques,
starting with a mixture of all of the constituent monomers
which is essentially free of polymer. Thus the polymers
are random interpolymers and are not graft or block
interpolymers. Conventional free radical polymerization
catalyst~, such a~ azobis-(isobutyronitrile), tert-butyl
hydroperoxide, and benzoyl peroxide, can be used. Such
polymerization techniques can be conducted neat in the
absence of solvent or in bulk.
Polymerization of the ester monomers is preferably
carried out in an inert hydrocarbon solvent, such as hexane
or heptane, or low viscosity lubricating oils. Polymeri-
zation is carried out in an oxygen-free reactor. The
desired atmosphere can be maintained by carrying out the
polymerization in a nitrogen atmosphere as i~ known in the
~ - 12 - 2~9~8
art. Temperature~ of about 65 to about 150 C, depending on
the choice of initiator, can be used. Polymerization is
carried out at either atmospheric or super-atmospheric
pressure and on either a batch or a continuous basis.
Polymerization can be stopped when t~e described degree of
polymerization is reached by known techniques, such as
adding inhibitors to the reaction mixture, or can be
allowed to go to completion.
~ he second type of comonomer employed for
interpolymerization with the unsaturated dicarboxyl monomer
i~ an alpha-olefin. Straight chain alpha-olefins are
preferred over branched chain alpha-olefins. Moreover, if
branching occurs, it is preferred that it occur at the
beta-carbon, and that such branching contain not more than
about 5, and preferably not more than about 2, carbons.
Suitable alpha-olefins typically contain between about 6
and 46, e.g., between about 10 and 22, and preferably about
18 carbon atoms per molecule. Mixtures of olefins may be
used~ e.g., a C10-C24 mixture.
Representative olefins include l-hexene,
l-heptene, l-nonene, l-decene, l-hexadecene, l-octadecene,
l-eicosene, l-heneicosene, 1-docosene, l-tricontene,
l-tetracontene, 2-methyloctadecene, 2-ethyleicosene, and
mixtures thereof.
The mole ratio of alpha-olefin to unsaturated
dicarboxyl monomer employed in the reaction mixture will
typically range from about 1.2:1 to about 0.8:1, preferably
from about 1.1:1 to about 0.9:1, and most preferably about
1 : 1 .
The preferred interpolymer of this class is an
interpolymer of l-octadecene and maleic anhydride
subsequently esterified with the aforedescribed C14
alcohol in the manner described hereinafter.
2~9~
The third preferred comonomer for interpolymeri-
zation with the unsaturated dicarboxy monomer is a styrene
compound such as styrene.
In forming this preferred unesterified
intermediate polymer, the molar ratio of styrene to
unsaturated dicarboxy-containing monomer (e.g., maleic
anhydride) can typically vary from about 3:1 to about 1:1,
preferably from about 2:1, to about 1:1, and most
preferably from about 1.5:1 to about 1:1.
Most preferably, equal molar amounts of styrene
and unsaturated carboxy containing monomer (e.g., maleic
anhydride) are employed. In addition, minor amounts of
other miscellaneou~ interpolymerizable comonomers can be
included in the reaction mixture. By minor amount is
typically meant less than about 1, preferably less than
about 0.3 mole of miscellaneous monomers per mole of
carboxy containinq monomer. Similar considerations,
vis-a-vis miscellaneous monomers, apply with respect to use
of the alpha-olefin~ as a comonomer for interpolymerization
with the dicarboxy monomer.
Various methods of polymerizing styrene or the
alpha-olefins and the dicarboxy-containing monomers are
known in the art and need not be discussed in detail
herein. Such methods include neat and bulk polymerization
technique~.
The polymerization reaction for use of either the
styrene or alpha-olefin comonomers with the dicarboxy
monomer is typically conducted to produce an unesterified
interpolymer having a number average molecular weight of
less than about 25,000, preferably less than about 15,000,
as determined by membrane osmometry. Upon esterification,
such molecular weights will be as described generally above
as well as the corresponding specific viscosities.
- 14 - 2~93~
The resulting interpolymer is then esterified with
the C14 alcohol of the type described above with respect
to esterification of the dicarboxy monomer.
The esterification reaction can be accomplished
simply by heating the dicarboxy-containing polymer and the
C14 alcohol under conditions typical for effecting
esterification. Such conditions usually include, for
example, a temperature of at least about 80 C, preferablY
from about 100-C to about l50 C, provided that the
temperature be below the decomposition point of the
reaction mixture, and the water of esterification is
removed as the reaction proceeds. Such conditions may
optionally include the use of an excess of the alcohol
reactant so as to facilitate esterification, the use of a
solvent or diluent such as mineral oil, toluene, benzene,
xylene or the like, and the use of an esterification
catalyst such as toluene sulfonic acid, sulfuric acid,
phosphoric acid, or the like. These conditions and
variations thereof are well known in the art.
The first additive or component compositions of
this invention are oil-soluble, dissolvable in oil with the
aid of a suitable solvent, or are stably dispersible
materials. Oil-soluble, dissolvable, or stably dispersible
as that terminology is used herein does not necessarily
indicate that the materials are soluble, dissolvable,
miscible, or capable of being suspended in oil in all
proportions. It does mean, however, that the first
additive composition, for instance, is soluble or stably
dispersible in oil to an extent sufficient to exert its
intended effect in the environment in which the oil is
employed. Moreover, the additional incorporation of other
additives may also permit incorporation of higher levels of
a particular first additive composition hereof, if desired.
-
- 15 -
2~938
The lubricating oil compositions of the present
invention contain an amount of said first additive or
component composition which is effective to improve the
flow properties, particularly low temperature flow
properties, of the lubricating oil composition, i.e., a
lubricating oil flow improving effective amount.
Generally, this effective amount may vary somewhat
depending on the particular type of oil. Accordingly,
while any effective amount of the first additive
composition can be incorporated into the final, e.g., fully
formulated, lubricating oil composition, it is contemplated
that such effective amount be sufficient to provide said
lube oil composition with an amount of the first additive
composition of typically from about 0.001 to about 1.5,
preferably from about 0.005 to about 1.0, and more
preferably from about 0.01 to about 0.5 wt. percent, based
on the weight of said lubricating composition.
The second additive or component of the instant
invention is a viscosity index improver or modifier
comprised of a hydrocarbon polymer.
These oil-soluble hydrocarbon polymeric viscosity
index (V. r. ) improver additives contemplated to be
compounded into the lubricating oil in accordance with this
invention are generally high molecular weight hydrocarbon
polymers. The V.I. improvers may also be derivatized to
include other properties of functions, such as the addition
of dispersancy properties.
Theso oil soluble V.I. polymers will generally
have number average molecular weights of from about 20,000
to 1,000,000, preferably from about 40,000 to about
300,000, as determined by gel permeation chromatography or
membrane osmometry.
Examples of suitable hydrocarbon polymers include
homopolymers and interpolymers of two or more monomers of
C2 to C30, e.g., C2 to C8 olefins, including both
alpha-olefins and internal olefins, particularly preferred
-
- 16 -
2~9~a
being the copolymers of ethylene and propylene. Other
polymers can be used such as polyisobutylenes, homopolymers
and interpolymers of C6 and higher alpha-olefins, atactic
polypropylene, hydrogenated polymers and copolymers and
terpolymers of styrene, e.g., with isoprene and/or
butadiene.
More specifically, other hydrocarbon polymers
suitable as viscosity index improvers in the present
invention include those which may be described as
hydrogenated or partially hydrogenated homopolymers, and
random, tapered, star or block interpolymers (including
terpolymers, tetrapolymers, etc.) o~ conjugated dienes
and/or monovinyl aromatic compounds with, optionally,
alpha-olefins or lower alkenes, e.g., C3 to C18 alpha-
olefins or lower alkenes. The conjugated dienes includeisoprene, butadiene, 2,3-dimethylbutadiene, piperylene
and/or mixtures thereof, such as isoprene and butadiene.
The monovinyl aromatic compounds include any of the
following, or mixture~ thereof, vinyl di- or polyaromatic
compounds, e.q., vinyl naphthalene, but are preferably
monovinyl monoaromatic compounds, such as styrene or
alkylated styrenes substituted at the alpha-carbon atoms of
the styrene, such as alpha-methylstyrene, or at ring
carbons, such a~ o-, m-, p-methylstyrene, ethylstyrene,
propylstyrene, isopropyl-styrene, butylstyrene,
isobutylene, tert-butylstyrene (e.g.,
p-tert-butylstyrene). Also included are vinylxylenes,
methylethyl styrenes and ethylvinylstyrenes. Alpha-olefins
and lower alkenes optionally included in these random,
tapered and block copolymer~ preferably include ethylene,
propylene, butene, ethylene-propylene copolymers, iso-
butylene, and polymers and copolymers thereof. As is also
known in the art, these random, tapered and block copoly-
mers may include relatively small amounts, that is less
than about S moles, of other copolymerizable monomers such
CA 02008938 1997-11-0~
as vinyl pyridines, vinyl lactams, methacrylates, vinyl
chloride, vinylidene chloride, vinyl acetate, vinyl
stearate, and the like.
Specific examples include random polymers of
butadiene and/or isoprene and polymers of isoprene and/or
butadiene and styrene. Typical block copolymers include
polystyrene-polyisoprene, polystyrene-polybutadiene~
polystyrene-polyethylene, polystyrene-ethylene propylene
copolymer, polyvinyl cyclohexane-hydrogenated polyisoprene,
and polyvinyl cyclohexane-hydrogenated polyb~adiene.
Tapered polymers include those of the foregoing monomers
prepared by methods ~nown in the art. Star-shaped polymers
typically comprise a nucleus and polymeric arms linked to
said nucleus, the arms being comprised of homopolymer of
interpolymer of said conjugated diene and/or monovinyl
aromatic monomers. Typically, at least about 80% of the
aliphatic unsaturation and about 20% of the aromatic
unsaturation of the star-shaped polymer is reduced by
hydrogenation.
Representative examples of patents which disclose
such hydrogenated polymers or interpolymers include U.S.
Patent Nos. 3,312,621: 3,31~,813: 3,630,90S: 3,668,12S:
3,763,044: 3~?-9s, 615; 3,835,053: 3,838,049; 3,96,019;
4,358,565; and 4,557,849.
The polymer may be degraded in molecular weight,
for example by mastication, extrusion, oxidation or thermal
degradation, and it may be oxidized and contain oxygen.
Also included are derivatized polymers such as post-grafted
- interpolymers of ethylene-propylene with an active monomer
such a~ maleic anhydride which may be further reacted with
an alcohol, of amine, e.q., an alkylene polyamine or
hydroxy amine, e.~., see U.S. Patent Nos. 4,089,794;
4,160,739: 4~l37~las; or copolymers of ethylene and
~ .
- - 18 -
2~ 93B
propylene reacted or grafted with nitrogen compounds such
as shown in U.S. Patent Nos. 4,068,056; 4,068,058;
4,146,489: and 4,149,984.
Suitable hydrocarbon polymers are ethylene
interpolymers containing from 15 to 90 wt. % ethylene,
preferably 30 to 80 wt. ~ of ethylene and 10 to 85 wt. %,
preferably 20 to 70 wt. % of one or more C3 to C8,
alpha-olefins. While not essential, such interpolymers
preferably have a degree of crystallinity of less than 10
wt. %, as determined by X-ray and differential scanning
calorimetry. Copolymers of ethylene and propylene are most
preferred. Other alpha-olefins suitable in place of
propylene to form the copolymer, or to be used in
combination with ethylene and propylene, to form a
terpolymer, tetrapolymer, etc., include l-butene,
l-pentene, 1-hexene, l-heptene, l-octene, etc.; also
branched chain alpha-olefins, such as 4-methyl-1-pentene,
4-methyl-1-hexene, 5-methylpentene-1, 4,4-dimethyl-1-
pentene, and 6-methyl-heptene-1, etc., and mixtures
thereof.
Terpolymers, tetrapolymers, etc., of ethylene,
said C3-8 alpha-olefin, and a non-conjugated diolefin or
mixtures of such diolefins may also be used. The amount of
the non-conjugated diolefin generally ranges from about 0.5
to 20 mole percent, preferably from about 1 to about 7 mole
percent, based on the total amount of ethylene and
alpha-olefin present.
The second additive or component compositions of
this invention are oil-soluble, dissolvable in oil with the
aid of a suitable solvent, or are stably dispersible
materials. Oil-soluble, dissolvable, or stably dispersible
as that terminolo~y is used herein does not necessarily
indicate that the materials are soluble, dissolvable,
miscible, or capable of being suspended in oil in all
- - 19 -
2~
proportions. It does mean, however, that the second
additive composition, for instance, is soluble or stably
dispersible in oil to an extent sufficient to exert its
intended effect in the environment in which the oil is
employed. Moreover, the additional incorporation of other
additives may also permit incorporation of higher levels of
a particular first additive composition hereof, if desired.
The lubricating oil compositions of the present
invention contain an amount of said second additive or
component composition which is effective to improve the
viscometric properties, particularly viscosity index of the
lubricating oil composition, e.g., a viscosity index
improving effective amount. Generally, this effectiVe
amount may vary somewhat depending upon the particular type
of oil. Accordingly, while any effective amount of the
second additive composition can be incorporated into the
final, e.g., fully formulated, lubricating oil composition,
it is contemplated that such effective amount be sufficient
to provide said lube oil composition ~ith an amount of the
second additive composition of typically from about 0.01 to
about 10, preferably from about 0.05 to about 5, and more
preferably from about 0.1 to about 3.0 wt. percent, based
on the weight of said lubricating composition.
The additive compositions of the present invention
can be incorporated into the lubricating oil in any
convenient way. Thus, they can be added directly to the
oil by dispersin~, or dissolving the same in the oil at the
desired level of concentration. Such blending can occur at
elevated temperatures. Alternatively, the additive
compositions may be blended with a base oil to form a
concentrate, and the concentrate then blended with
lubricating oil base stoc~ to obtain the final compo-
sition. Such concentrates will typically contain the first
additive composition in amounts of from about 0.5 to about
6, preferably from about 0.5 to about 5 percent by weight,
-- - 20 -
938
based on the concentrate weight, and the second additive
composition in amount~ of from about 0.5 to about Z0,
preferably from about 0.5 to a~out 12 percent by weight,
based on the concentrate weight.
It is to be noted that the amounts of the additive
compositions of this invention present in the fully
formulated oil compositions or concentrates are on an
active ingredient basis l.i.).
The lubricating oil base stock for the additive
compositions of the present invention typically is adapted
to perfor~ a selected function by the incorporation of
other additives therein to form lubricating oil composi-
tions designated as formulations.
Representative other additives typically present
in such formulations include corrosion inhibitors,
oxidation inhibitors, friction modifiers, dispersants,
anti-foaming agents, anti-wear agents, detergents, rust
inhibitors and the like.
Corrosion inhibitors, also known as anti-corrosive
agents, reduce the degradation of the metallic parts
contacted by the lubricating oil composition. Illustrative
of corrosion inhibitors are phosphosulfurized hydrocarbons
and the products obtained by reaction of a phosphosul-
furized hydrocarbon with an alkaline earth metal oxide or
hydroxide, preferably in the presence of an alkylated
phenol or of an alkylphenol thioester, and also preferably
in the presence of carbon dioxide. Phosphosulfurized
hydrocarbons are prepared by reacting a suitable hydro-
carbon such as a terpene, a heavy petroleum fraction of a
C2 to C6 olefin polymer such as polyisobutylene, with
from 5 to 30 wt. percent of a sulfide of phosphorus for 1/2
to 15 hours, at a temperature in the range of 150 to
600'F. Neutralization of the-phosphosulfurized hydrocarbon
may be effected in the manner taught in U.S. Patent No.
1,969,324.
- 21 - ~ n ~
Oxidation inhibitors reduce the tendency Of
mineral oils to deteriorate in service which deterioration
can be evidenced by the products of oxidation such as
sludge and varnish-like deposits on the metal surfaces, and
by viscosity growth. Such oxidation inhibitors include
alkaline earth metal salts of alkyl phenolthioesters havin~
preferably c5 to C12 alkyl side chains, e.g.,
calcium nonylphenol sulfide, barium t-octylphenyl sulfide,
dioctylphenylamine, phenylalpha-naphthylamine, phospho-
sulfurized or sulfurized hydrocarbons, etc.
Friction modifiers serve to impart the proper
friction characteristics to lubricating oil compositionS
such as automatic transmission fluids.
Representative examples of suitable friction
modifiers are found in U.S. Patent No. 3,933,659 which
discloses fatty acid esters and amides; U.S. Patent No.
4,176,074 which describes molybdenum complexes of
polyisobutenyl succinic anhydride-amino alkanols; U.S.
Patent No. 4,105,571 which discloses qlycerol esters of
dimerized fatty acid~; U.S. Patent No. 3,779,928 which
discloses alkano phosphonic acid salts; U.S. Patent No.
3,778,375 which discloses reaction products of a
phosphonate with an oleamide; U.S. Patent No. 3,852,205
which discloses S-carboxyalkylene hydrocarbyl succinimide,
S-carboxyalkylene hydrocarbyl succinamic acid and mixtures
thereof; U.S. Patent No. 3,879,306 which discloses
N-(hydroxyalkyl)alkenyl-succinamic acids or succinimides;
U.S. Patent No. 3,932,290 which discloses reaction products
of di- (lower alkyl) phosphites and epoxides; and U.S.
Patent No. 4,028,258 which discloses the alkylene oxide
adduct of phosphosulfurized N-(hydroxyalkyl) alkenyl
succinimides. The most preferred friction modifiers are
succinate esters, or metal salts thereof, of hydrocarbyl
substituted succinic acids or
~6, ~
iJ ~
anhydrides and thiobisalkanols such as described in U.S.
Patent No. 4,344,853.
-- Dispersants maintain oil insolubles, resulting
from oxidation during use, in suspension in the fluid thus
preventing sludge flocculation and precipitation or
deposition on metal parts. Suitable dispersants include
high molecular weight alkyl succinates, the reaction
product of oil-soluble polyisobutylene succinic anhydride
with ethylene amines such as tetraethylene pentamine and
borated salts thereof.
Foam control can be provided by an antifoamant of
the polysiloxane type, e.g., silicone oil and polydimethyl
siloxane.
Anti-wear agents, as their name implies, reduce
wear of metal parts. Representative~ of conventional
anti-wear aqents are zinc dialkyldithiophosphate and zinc
diaryldithiosphate.
Detergents and metal rust inhibitors include the
metal salts of sulphonic acids, alkyl phenols, sulfurized
alkyl phenols, alkyl salicylates, naphthenates and other
oil soluble mono- and di-carboxylic acids. Highly basic
(viz, overbased) metal salts, such as highly basic alkaline
earth metal sulfonates (especially Ca and Mg salts) are
frequently used as detergents. Representative examples of
such materials, and their methods of preparation, are found
in Canadian Patent 1,262,721.
Some of these numerous additives can provide a
multiplicity o~ effects, e.g., a dispersant-oxidation
inhibitor. This approach is well known and need not be
further elaborated herein.
Compositions when containing these conventional
additives are typically blended into the base oi1 in
amounts which are effective to provide their normal
- 23 - 2~938
attendant function. Representative effective amounts of
such additives are illustrated as follows:
% Active Inqredient By
Additive Volume Weight
Corrosion Inhibitor 0.01-1 0.01-1.5
oxidation Inhibitor 0.01-1 0.01-l.S
Dispersant 0.1-7 0.1-8
Anti-Foaming Agents 0.001-0.1 0.001-0.15
Anti-Wear Aqents 0.001-1 0.001-1.5
Friction Modifiers 0.01-1 0.01-1.5
Detergents/Rust Inhibitors 0.01-2.5 0.01-3
Mineral Oil Base Balance Balance
When other additives are employed, it may be
desirable, although not necessary, to prepare additive
concentrates comprising concentrated solutions or
dispersions of the dual additive composition (in
concentrate amounts hereinabove described), together with
one or more of said other additives (said concentrate when
constituting an additive mixture being referred to herein
as an additive-package) whereby several additives can be
added simultaneously to the base oil to form the
lubricating oil composition. Dissolution of the additive
concentrate into the lubricating oil may be facilitated by
solvents and by mixing accompanied with mild heating, but
this is not essential. The concentrate or additive-package
will typically be formulated to contain the first and
second additive compositions of -he instant invention and
optional additional additives in proper amounts to provide
the desired concentration in the final formulation when the
additive-package is combined with a predetermined amount of
base lubricant. Thus, the additive composition of the
present invention can be added to small amounts of base oil
or other compatible solvents along with other desirable
additives to form additive-packages containing active
ingredients in collective amounts of typically from about
2.5 to about 90~, and preferably from about 5 to about 75%,
- 24 - ~ ~Q~3~ ~
and most preferably from about 8 to about 50% by weight
additives in the appropriate proport~ons with the remainder
being base oil.
The final formulations may employ typically about
wt. % of the additive-package with the remainder being
base oil.
All of said weight and-volume percents expressed
herein are based on active ingredient (a.i.) content of the
additive, and/or upon the total weight of any additive-
package, or formulation which will be the sum of the a.i.weight of each additive plus the weight of total oil or
diluent.
Neither the oleaginou~ compositions nor the
additive concentrates of the instant invention contain,
i.e., are free of, the second component lubricatinq oil
flow improvers described in copending U.S. Patent
4,839,874. These second component lubricating oil flow
improvers are comprised of:
(i) polymers of ethylenically unsaturated
dicarboxylic acids or their anhydrides having the
carboxyl or anhydride qroups located on vicinal
carbons and having 4 to 10 carbons in the
unesterified monomer molecule, esterified with a
mixture of Cl to C20 aliphatic alcohols,
preferably mixtures of alcohols in the C4 to
C20 average carbon number range, more preferably
in the C8 to C18 carbon range;
(ii) interpolymers ~f (a) ethylenically
unsaturated dicarboxylic acids or their anhydrides
having the carboxyl or anhydride groups located on
vicinal carbon~ and having 4 to lO carbons in the
unesterified monomer molecule, esterified with a
mixture of Cl to C20 aliphatic alcohols,
preferably mixtures of alcohols in the C4 to
C20 average carbon number range, more preferably
.~ :
- 25
'Z~39~3
in the C8 to C18 carbon range, and (b) vinyl
esters, alpha-olefins or styrene;
(iii) polymers of unsaturated monoesters,
preferably polymers of long side chain unsaturated
mono- esters, and interpolymers of long and short
side chain unsaturated monoesters. The
unsaturated esters are generally acrylate or
2-alkylacrylate mono-esters represented by the
formula:
R2
I
(III) C = CH2
I
R3
wherein R2 is hydrogen or a C1 to C5 alkyl
group; and R3 is a COOR4 group wherein R4 is
a Cl to C20, preferably a C10 to C18 alkyl
group. A 2-alkylacrylate is one wherein R2 is
alkyl. The hydrocarbyl groups constituting R4
represent the hydrocarbyl residues of mixtures of
alcohol~ from which the same are prepared, which
alcohols are preferably saturated, although some
degree of unsaturation is permissible when
mixtures of alcohols are employed, e.g., less than
about 2 mole % of the alcohols in the mixture can
be unsaturated. Straight chain or lightly
branched alcohols are preferred over highly
branched alcohol~. The mixture of alcohols
employed are those containing from Cl to about
C20 carbons which can be employed in such
proportions that the average number of carbons in
the alcohol residue of the monomer molecule is
preferably between about 10 and about 18.
Furthermore, it is preferred that at least 60 mole
26 -
Z 6~ ,3 ~3
%, most preferably at least 80 mole % of the
alcohols present in such mixture contain between
10 and 18 carbon atoms.
Illustrative non-limiting examples of (i) are polymers of
dialkyl fumarates wherein the fumarates are esterified with
mixtures of C6 through C20 alcohols. An illustrative
non-limiting example of (ii) are interpolymers of dialkyl
fumarates and vinyl esters, preferably vinyl acetate, in
which the fumarates are esterified with mixtures of C6
through C20 alcohols.
The following examples are given as specific
illustrations of the claimed invention. It should be
understood, however, that the invention is not limited to
the specific details set forth in the examples. All parts
and percentages in the examples, as well as in the
remainder of the specification, are by weight unless
otherwise specified.
- 27 -
9~38
The following Comparative Examples fall outside
the scope of the instant invention and are presented for
comparative purposes only.
COMPARATIVE EXAMPLE 1
A fully formulated 15W-40 lubricating base oil
(designated Base Oil A) was prepared containing mineral oil
base stock oil (i.e., a mixture of 150N and 750N); no
lubricating oil flow improver (LOFI); a conventional
detergent/inhibitor package containing ashless dispersant,
anti-oxidant, anti-wear additive, and overbased sulfonate;
and 0.85 wt. % (a.i.) of a V.I. improver comprised of
ethylene-propylene copolymer having a Thickening Efficiency
of about 2.0, a ratio of weight average molecular weight to
number average molecular weight greater than 2, and an
ethylene content of about 48 wt. %.
COMPARATIVE EXAMPLE 2
Comparative Example 1 was repeated except that the
base oil contained 0.19 wt. % (a.i.) of lubricating oil
flow improver composition falling outside the scope of the
instant invention - LOFI B (di-Clo alkyl fumarate-vinyl
acetate copolymer wherein the fumarate was derived from a
C10 alkanol and the fumarate: vinyl acetate mole ratio
employed in the synthesis of said LOFI B composition was
1:0.8). With the exception of the presence of LOFI B, the
types and amounts of other additives were the same as in
Comparative Example 1.
COMPARATIVE EXAMPLE 3
Comparative Example 2 was repeated except that the
lubricating oil flow improver, LOFI B, of Comparative
Example 2 was replaced with 0.19 wt. % (a.i.) of another
lubricating oil flow improver fallin~ outside the scope of
the instant invention - LOFI C (di-C12 alkyl fumarate-
vinyl acetate copolymer wherein the fumarate: vinyl
acetate mole ratio employed in the synthesis o~ LOFI C
- 28 -
X~938
composition was 1:0.8). With the exception of the
lubricating oil flow improver, the types and amounts of
other additives were the same as in Comparative Example 2.
COMPARATIVE EXAMPLE 4
Comparative Example 2 was repeated except that the
lubricating oil flow improver, LOFI B, of Comparative
Example 2 was replaced with 0.19 wt. % (a.i.) of another
lubricating oil flow improver falling outside the scope of
the instant invention - LOFI D (di-C16 alkyl fumarate-
vinyl acetate copolymer wherein the fumarate: vinyl
acetate mole ratio employed in the synthesis of said LOFI Dcomposition was 1:0.8). With the exception of the
lubricating oil flow improver, the types and amounts of
other additives were the same as in Comparative Example 2.
COMPARATIVE EXAMPLE 5
Comp~ratiYe Example 2 was repeated except that the
lu~ricating oil flow improver, LOFI B, of Comparative
Example 2 was replaced with 0.19 wt. % (a.i.) of another
lubricatinq oil flow improver falling outside the scope of
the instant invention - L0FI E (di-C18 alkyl fumarate-
vinyl acetate copolymer wherein the fumarate: vinylacetate mole ratio employed in the synthesis of said LOFI E
composition was 1:0.8). With the exception of the
lubricating oil flow improver, the types and amounts of
other additives were the same as in Comparative Example 2.
The followinq Examples illustrate the compositions
of the instant invention.
EXAMPLE 6
A fully formulated 15W-40 lubricating base oil
(designated Base Oil A) was prepared containing mineral oil
_ - 29 ~
base stock oil (i.e., a mixture of 150N and 750N); about
0.19 wt. % (a.i.) of first additive composition of the
instant invention - LOFI A (di-C14 alkyl fumarate - vinyl
acetate copolymer wherein the di-alky fumarate was derived
from C14 alcohol and wherein the fumarate: vinyl acetate
mole ratio employed in the synthesis of said first additive
composition was 1:0.8); about 0.85 wt. % (a.i.) of the
second additive composition comprised of V.I. improver
comprised of ethylene-propylene copolymer having a
Thickeninq Efficiency of about Z.0, a ratio of weiqht
average molecular weight to number avera~e molecular weight
greater than 2, and an ethylene content of about 48 wt. %;
and a conventional detergent/inhibitor package containing
ashless dispersant, anti-oxidant, anti-wear additive, and
overbased sulfonate.
Thickenin~ Efficiency (T.E.) is defined as the
ratio of the weight percent of a polyisobutylene (sold as
an oil solution by Exxon Chemical Co. as Paratone N),
having a Staudinger Molecular Weight of 20,000, required to
thicken a solvent-extracted neutral mineral lubricating
oil, having a viscosity of lS0 SUS at 37.8 C, a viscosity
index of 105 and an ASTM pour point of 0-F, (Solvent 150
Neutral) to a viscosity of 12.4 centistokes at 98.9'C, to
the weight percent of a test copolymer required to thicken
the same oil to the same viscosity at the same tempera-
ture. T.E. is related to ~n and is a convenient,
useful measurement for formulation of lubricating oils of
various grades.
EXAMPLE 7
Example 6 was repeated, except that a different
15W-40 mineral oil base stock was employed. The base oil,
fully formulated in accordance with Example 6, was
designated Base Oil B and contained the same types and
amounts of additives as Base Oil A of Example 6.
- 30 -
39~
EXAMPL~ 8
Example 6 was repeated except that a different
15W-40 mineral oil base stock was employed. The base oil,
fully formulated in accordance with Example 6, was
designated Base Oil C and contained the same types and
amounts of additives as Base Oil A of Example 6.
EXAMPLE 9
Example 6 was repeated except that a different
15W-40 mineral oil base stock was employed. The base oil,
fully formulated in accordance with Example 6, was
designated Base Oil D, and contained the same types and
amounts of additives as Base Oil A of Example 6.
EXAMPLE 10
Example 6 was repeated, except that a different
15W-40 mineral oil basé stock was employed. The base oil,
fully formulated in accordance with Example 6, was
designated Base Oil E and contained the same types and
amounts of additives as Base Oil A of Example 6.
EXAMPLE 11
Example 6 was repeated, except that a different
15W-40 mineral oil base stock was employed. The base oil,
fully formulated in accordance with Example 6, was
designated Base Oil F and contained the same types and
amounts of additives as Base Oil A of Example 6.
EXAMPLE 12
Example 6 was repeated, except that a different
15W-40 mineral oil base stock was employed. The base oil,
fully formulated in accordance with Example 6, was
designated Base Oil G and contained the same types and
amounts of additives as Base Oil A of Example 6.
- 31 -
2~;9~8
The flow properties of Comparative Examples 1-5
and of Examples 6-12 were tested by the Mini Rotory
Viscometer (MRV) procedure, and the results are summarized
in TABLE I. The analysiq of the flow properties was
conducted by testing the lubricating oil formulations in a
Mini Rotory Viscometer after subjecting each sample to a
temperature profile controlled in accordance with ASTM
D4684 over about a 40 to 44 hour cooling cycle. More
specifically, this test is used by the SAE (J300
Specification-JUN87) for determining the low temperature
pumpability of a crankcase oil. In the test procedure
itself, the temperature is gradually lowered to -20'C, and
then at that temperature the yield stress (YS) is measured
in pascals, and the apparent viscosity (VIS) is measured in
pascal seconds. The latter is required because this is a
two-phase system, so that a true viscosity measurement
cannot be made. Thus, in accordance with SAE requirements
for 15W-40 oils, the target values of less than 35 pascals
(YS) and not qreater than 300 pascal seconds (VIS) are
considered acceptable in order to provide a pumpable
composition at -20 C, i.e., to maintain fluidity. For
purposes of ths instant application a sample is considered
to "fail" if either the YS is greater than 35 pascals or
the viscosity i~ qreater than 300 pascal seconds.
- 32 - 2~
v ~ r
,~ _
~ ~ ~~ ~ U~
~ ~ ~v ~ v v v v
~ S ~~ ~~~~~~~~~
,~ o o o o o c o o o
¢
E ~ ~ I o ~
aJ
4 5J O
O " Z ~ 0
~ ~ Q Q Q Q ~
~ ~ , '
TAELE I (ClNnFn~JED)
LDFI Test Results
V.I.
Example or Lmprover VIS
Comparative Base Carbon No. AmcuntAmountYS (Pascal
Example ~o. Oil IYL~ Of Aloohol ~wt. ~~wt. ~~Pascals~ SeoQnds~
Example 10 A A C14 0.19 0.85 < 35 206
Example 11 B A C14 0.19 0.85 < 35 183 w
Example 12 C A C14 0.19 0.85 < 35 201
Targets for 5AE 15W-40 oil < 35 300 max.
~3
~ 34 ~ 2~938
As illustrated by the data in TABLE I, the
combination of the first and second additive compositions
of the present invention provide lube oil formulations
(Examples 6-12) which meet, with the exception of the
composition of Example 9, the target for SAE 15W-40 oil
with a varie y of different base oils. As discussed
hereinafore, different types of oils may generally require
different amounts of first and/or second additives of the
instant invention, i.e., lubricating oil flow improvers and
viscosity index improvers or modifiers. Thus, while the
amounts of the instant additives utilized in the
compositions of Examples 6-12 were effective in improving
the low temperature flow properties and viscometric
properties of oils A-C and E-G, they were not adequate to
improve the viscometric properties of oil D (Example 9)
sufficiently to meet the SAE requirements for 15W-40 oil.
In contrast, using lubricating oil flow improvers other
than those of the instant invention (Comparative Examples
2-8), or using no lubricating oil flow improvers at all
(Comparative Example 1) results in lube oil formulations
which fail to meet the target for SAE 15W-40 oil. rt is to
be noted that LOFrs 8-E of Comparative Examples 2-5 differ
from LOFI A of Examples 6-12 in that the di-alkyl fumarate
is derived from an alcohol different from the C14 alcohol
of LOFI A.
Although the invention herein has been described
with reference to particular embodiments, it is to be
understood that these embodiments are merely illustrative
of the principles and applications of the present
invention. It is therefore to be understood that numerous
modifications may be made to the illustrative embodiments
and that other arranqements may be devised without
departing from the spirit and scope of the present
invention as defined by the appended claims.