Note: Descriptions are shown in the official language in which they were submitted.
2a~~49fl
2
;~i,
The invention relates to a process for producing
solid pharmaceutical preparations such as granules,
powders, pellets and crystalline active substances,
which may optionally be coated.
Of the processes used in the pharmaceutical
industry, the production of solid preparations of this
kind for sale in this form (e.g. edible granules, dry
syrup granules) and as precursors for the production of
tablets is one of the most important technologies.
The process which is most frequently used in terms
of its basic principle is the fluidised bed granulation
process, in which all the individual production steps
(mixing, wetting, granulation, drying and final mixing)
can be carried out in one apparatus. In spite of this
advantage, this method of production has not produced
satisfactory results in a number of cases.
In addition to the problems connected with the
particular recipe or with the substances used, there are
major disadvantages, namely a broad spectrum of particle
sizes and high contents of dust or fine dust.
These disadvantages make it particularly difficult
to achieve a reproducible quality and validation.
Furthermore, certain pharmaceutical preparations
require special procedures (e. g. maximum possible
coating of the individual granules) in order to achieve
better stability for example in the presence of heat and
moisture and resistance to mechanical stresses (mixing,
pneumatic conveying, compression), so that even after
lengthy periods of storage under unfavourable conditions
the pharmacological activity of the drug is maintained.
In addition to so-called coating, the process of
granulation by the building-up method (agglomeration)
has always presented problems with the processes known
hitherto.
The aim of the present invention is to provide a
process which makes it possible to produce solid
pharmaceutical preparations, particularly for human
- 3 - 27400-115
pharmacological applications, with a narrow range of particle size
distribution and a low dust content using simple methods.
According to the invention, this aim is achieved with
the so-called air controlled granulating system (SKD granulation
method). In the SKD method the powdered starting material is
placed in an air controlled granulating system (e. g. HSP-5/10,
250 or 750 made by Huttlin, Steinen) and completely coated with
a suitable costing agent. The technical apparatus is disclosed
in German Patent 29 32 803. European Patent 146 680 discloses a
filter arrangement which can be used for this purpose.
Using this process it is possible to produce coated
particles which contain active substance and which are used as
the starting material for the production of conventional pharma-
ceutical preparations, e.g. for the preparation of tablets,
capsules and the like, or may be used directly as a pharmaceutical
preparation. The coated particles may be obtained in the form of
powders, granules or pellets, depending on the starting material
used. Depending on the coating material used, the coating
obtained may have the function of controlling the release of active
substance or may simply have flavouring or protective functions.
The process according to the invention can be used for
build-up granulation. In this process a substrate is used, e.g.
small sugar pellets, which are then sprayed with a solution of
active substance.
Surprisingly, it has been found that using the apparatus
described in the patents referred to, solid pharmaceutical prepara-
2~Q~~~~
- 3a - 27400-115
tions, e.g. including those in the form of a substrate material
which contains active substance, can be prepared which have a
narrower particle size distribution and a smaller dust content
than similar preparations produced and coated in one of the con-
ventional fluidised bed granulators.
2Qn~~~~
4
Other advantages over the preparations made in the
conventional manner are the fact that the preparation
produced by the process according to the invention has a
very narrow particle size distribution which can be
adjusted within wide limits, the preparation has better
flow properties and the odour of strong-smelling
starting products is largely suppressed.
Both solid and liquid active substances are
suitable for use in the process according to the
invention. Liquid pharmaceutical active substances may
be processed by spraying them onto a suitable substrate
and then coating them as required.
Suitable active substances include inter alia
pharmaceutical active substances, particularly those
which are used in human drugs such as ranitidine,
cimetidine, atenolol, enalapril, captopril, nifedipine,
naproxene, diclofenac, sodium diclofenac, piroxicam,
cefaclor, diltiazem, ketotifen, ketofifen-hydrogen
fumarate, salbutamol, propranolol, amoxicillin,
triamteren, norethisterone, mestranol, cefotoxamine,
sodium cefotoxamine, ceftriaxon, disodium ceftriaxon,
cefalexin, dipyridamole, alprazolam, cefoxitine,
cyclosporin, metoprololtartrate, acyclovir, sulindac,
clavulanic acid, methyldopa, nicardipin, pentoxifyllin,
glyceroltrinitrate, timolol, idebenone, terfenadine,
tamoxifen dihydrogen citrate, prazosine, doxorubicin,
amiloride, amiloride HCl, hydrochlorothiazide,
dihydroergocornin, dihydroergocornin methanesulphonate,
erythromycin, erythromycinstearate, triazolam,
latamoxef, cromoglicinic acid, ceftazidim, clenbuterol,
bromhexine oxytetracycline, dexamethasone-21-
isonicotinate, sulphadiazine, cimaterole, aditoprim,
mederantil, climazolam, carprofen, caffeine, catapresan
and acetylsalicyclic acid - or all vitamins permitted as
drugs or foods such as Vitamin A, A1, A2, B1, B2, Ba. Bs,
B1?, C (ascorbic acid), ascorbylpalmitate and other
pharmacologically acceptable derivatives of ascorbic
X009090
acid, D, Dl, DZ, D3, D4, E, H, K, Kl, K2, P arid Q - or
active substances - such as avoparcin, flavopholipol,
monensin, sodium monensin, salinomycin, carbadox,
nitrovin and olaquindox, and active substances listed in
the Red List 1989 (Editio Cantor Verlag fur Medizin and
Naturwissenschaften GmbH and Co. KG,
Aulendorf/Wurttemb.), the contents of which are hereby
referred to.
Suitable coating materials include compounds such
as those used as coating agents in galenic
pharmaceuticals, such as polyacrylate, polysaccharides,
inorganic coating agents such as silicates or carbonates
provided that they are soluble or readily suspendable in
water or volatile organic solvents or mixtures of
solvents. Depending on the active substance involved,
it is also possible to use fats, lipids, lecithin, waxes
and surfactants as coating agents. The following
compounds are also suitable as inorganic coating
materials: bentonite, montmorillonite, calcium
silicate, kaolinite clays, kieselguhr, silicic acids
(precipitated and dried), sodium aluminium silicate,
silicon dioxide, perlite, vermiculite. Coating agents
of a basic nature which may be used include, for
example, calcium hydrogen orthophosphate, calcium oxide,
calcium tetrahydroorthophosphate, diammonium hydrogen
orthophosphate, dicalcium diphosphate, disodium
dihydrogen diphosphate, disodium hydrogen
orthophosphate, potassium dihydrogen orthophosphate
and/or sodium dihydrogen orthophosphate. Other coating
materials include celluloses, particularly hydroxy-
propylmethylcellulose, sodium carboxymethylcellulose,
hydroxyethylcellulose and methylcellulose.
Ethylcellulose, cellulose acetate phthalate, cellulose
acetate succinate, hydroxypropylmethylcellulose
phthalate, polyvinylacetate, polyvinylpyrrolidones,
alg.inic acids, polyethylene glycols, hexadecyl alcohols
and hydroxypropylcellu~.ose are also suitable. The
- 2009090
6
coating material may also be made up of a mixture of the
coating materials listed.
Other suitable coating materials are known
compounds which bring about delayed release of the
active substance. These include, for example, coatings
of polyacrylates (Eudragit E 30 D, E 100 etc. made by
Rohm of Darmstadt) and polyethyleneglycols.
Suitable carriers include, for example, lactose,
saccharose, glucose, sugar, corn starch, calcium
carbonate and all the substances listed as suitable
coating materials.
The minimum possible size for the particles used as
carrier material is about 0.5~ttm whilst the upper limit
is about 1000~tem. The preferred size range is from 10
to 300 Vim.
Suitable solvents include all the low-boiling and
pharmaceutically acceptable solvents or mixtures of
solvents which are inert in the presence of the carriers
and coating materials and the active substances. The
preferred solvent is water.
The proportion of coating material ranges from 2.5
to 30% by weight, preferably from 4 to 20% by weight,
more particularly from 5 to 7% by weight, based on the
starting material used, including any carrier material
present. It goes without saying that larger amounts of
coating material may be used, although within the scope
of the invention every effort is made to minimise the
quantity of coating material. The process according to
the invention proceeds as follows:
The powdered starting material, for example having
a particle size of between 0.5 and 60 ~tZn, is placed in
the slotted gyrodynamic filter granulating apparatus
mentioned hereinbefore. The air flowing into the bed of
powder through the rotating slotted gyro slots fluidises
the product. Then a 3 to 10%, preferably'S to 7%
solution or suspension of the coating material, which is
used as fre granulation or coating fluid, is sprayed
Trade-Mark
25771-555
2009090
into this fluidising zone in the apparatus from below.
The individual process parameters such as the quantity
of air supplied, the temperature of the air supplied,
the temperature of the exhaust air and the humidity of
the exhaust air, the spray nozzle diameter, spray rate,
speed of slot/nozzle are dependent inter alia on the
size of the apparatus used. More specific information
on these parameters is given in the Examples. If
desired, the process can be carried out using an inert
gas such as nitrogen.
Compared with conventional granulation processes,
( e.g. fluidised bed granulation, the entire process time
is much reduced. There is no need for subsequent
screening in order to eliminate secondary agglomerates.
Another advantage is that the preparation is not only
granulated by the build-up method but the coating
completely envelopes the starting material. The
difference from conventional granulation methods in
which the coating is incomplete can be demonstrated by
images taken with a scanning electron microscope.
Because of its very smooth surface the product
according to the invention has extremely good flow
properties. The granules produced by the process
according to the invention exhibit a typical, relatively
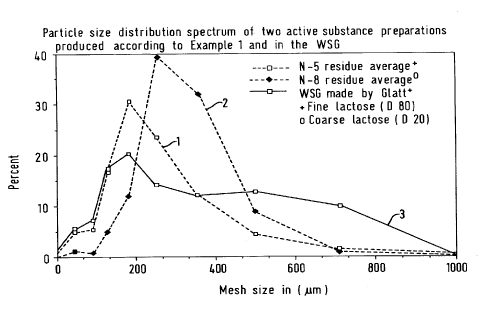
narrow particle size distribution. In Figure 1 the
particle size distribution is illustrated using
clenbuterol as an example. With conventional fluidised
bed granulation, such a spectrum can only be achieved
after repeated screening and mixing.
By contrast with products produced in the
conventional manner the product according to the
invention contains virtually no fine dust and shows no
abrasion. The pharmaceutical preparations produced by
means of the SKD process show no demonstrable abrasion
of active substance in the Stauber-Heubach test
(Fresenius; Z. Anal. Chem. 318 (1984) 522-524). This
rules out the possibility of a health risk caused by
2009090
8
fine dust containing the active substance during proper
handling of the preparations produced by the process
according to the invention.
A further advantage of the granules produced
according to the invention is the prevention of dust
explosions, which often constitute a risk when handling
finely powdered substances.
The properties enumerated have major advantages in
the further processing of pharmaceutical preparations,
e.g. in mixing with other substances and further
processing to form tablets and filling capsules.
Another advantage consists of the very homogeneous
distribution of active substance in the products
prepared according to the invention, thus ensuring a
high degree of accuracy in dosing.
A further advantage results from the very good flow
properties of the products prepared according to the
invention, thus permitting problem-free packaging.
Furthermore, there is no risk of the product
becoming unmixed during transportation and further
processing.
A further advantage of the process according to the
invention is the fact that, by contrast with the
fluidised bed granulation method, micronised starting
materials with particle sizes of up to 0.5 ~,cm can be
used, whilst the yields achieved with the aid of a fine
dust recycling system may reach almost 100%.
Furthermore, using the process according to the
invention it is possible to obtain substrates having a
predetermined particle size spectrum.
In addition, the process according to the invention
is suitable for masking flavours.
2009090
9
Examt~le 1
Composition
Ingredients: g/100 g g/10 kg
(O1)Clenbuterol 0.0016 0.160
(02)Mannitol 0.3184 31.840
(03)Fine lactose (D 80) 60.000 6 000.000
(04)Corn starch, dried* 33.680 3 368.000
(05)Collidone*25 (PVP**) 1.000 100.000
(06)Soluble starch 5.000 500.000
100.000 10.000.000
* If undried corn starch is used, an additional weight
of 7% must be included in the calculation
** PVP = polyvinylpyrrolidone
Starting material in the SKD process
6.000 g lactose, coarse (specification D 20)
3.368 g corn starch, dried
Preparation of the granulating solution for a 10 ks
batch
Solution 1:
0.160 g of clenbuterol are dissolved in 50 ml of
H20 .
Solution 2:
800 ml of HZO are placed in a thermostatically
controlled vessel at ambient temperature and 500 g of
soluble starch are suspended therein with stirring.
Trade-Mark
25771-555
20ososo
Solution 1 is stirred into this suspension.
Solution 3:
2.500 ml of H20 are placed in a 5 litre beaker.
31.84 g of mannitol are dissolved therein with stirring
and the solution is heated to boiling point.
Solution 4:
The hot mannitol solution is added to solution 2
with stirring, whereupon the soluble starch immediately
clumps together visibly (swells).
Solution 5:
650 ml of H20 are heated in a glass beaker to
boiling point and 100 g of collidone 25 are dissolved
therein with stirring.
Granulating solution:
Solution 5 is then stirred into Solution 4 (in the
thermostatically controlled vessel). The temperature of
50°C required for spraying is adjusted.
Granulation in the SKD*:
Preparation: The SKD is preheated to about 60°C. Then
the previously prepared fillers,
unscreened, are put in and mixed together
for about 2 to 3 minutes (about
50 Nm3Nz/h). In the meantime the SKD is
heated further (temperature for air
supplied is adjusted to 100°C). After
mixing, the granulating solution is
sprayed on at a temperature of about 50°C.
After all the granulating solution has
been sprayed in, the thermostatically
controlled vessel is rinsed out twice with
ml. of H;O, which is also sprayed in.
11
27400-115
The granules are dried until the product
temperature is 60°C. (The residual moisture
is then about 4.5 to 6% according to the Karl
Fischer Method.)
*SKD = air controlled granulating system.
Example 2
Composition: 95.0 g active substance
5.0 g methylcellulose
100.0 g active substance granules
Method: The powdered starting material, the active
substance, with a particle size of 1 to 40
um (main fraction: 10 to 15 um) is placed
in the SKD. A 5o methylcellulose solution
is prepared, which is used as the granulat-
ing liquid. The air flowing into the
powder bed through the rotating slotted
gyro slots fluidises the product and the
granulating liquid is then sprayed into
these fluidising zones from below.
Process
parameters: Quantity of air fed in: 150 to 250 m3/h
Temperature of incoming air: 60 to 70°C
Spray nozzle diameter: 1.2 mm
Average spray rate: 18 ml/min
Speed of slot/nozzle: 3 rpm
~ lla 2 0 0 9 0 9 0
27400-115
The total spraying time is about 155
minutes and the drying phase about 25
minutes. The entire process time is
therefore about 180 minutes. Compared
with this, the total process time for
_u ~ X009090
12
granules of active substance produced in a
conventional fluidised bed granulator
(e. g. WSG 200 made by Glatt) is more than
8 hours, whilst additional screening and
mixing are also required because of the
large secondary agglomerates and the broad
spectrum of particle size distribution.
After only 15 minutes, the formation of
granules is apparent. The granulation
phase merges, apparently smoothly, into a
coating phase, in which the granules
initially formed are gradually coated with
methylcellulose. Images taken using a
scanning electron microscope 600/1800 x
magnification show different smooth
surfaces in two samples which were coated
with 5.14 and 9.48% of methylcellulose,
respectively, the higher coating
concentration producing the smoother
surface.
The end product produced by means of the
SKD contains virtually no fine dust
(particles smaller than 45 ~tm = 0%) and
has a narrow particle size distribution
spectrum (particles 200 to 400 ~Gm = 92%).
This is accompanied by good flow
properties of the granules (angle of
slope: 33.6°, nozzle opening 6 mm, flow
time for 200 ml: about 75 seconds).