Note: Descriptions are shown in the official language in which they were submitted.
~009~'7S
FIELD OF THE INVENTION
This invention relates to a novel enzymatic cellulose
derivative hydrolysate and fractions thereof. The invention
also provides methods for producing the same, as well as novel
uses for the enzymatic hydroll-sate and its fractions.
BACKGROUND OF THE INVENTION
Cellulose derivatives such as carboxymethylcellulose,
methylcellulose, hydroxypropylmethylcellulose, ~t~,lethylcellulose and
hydroxypropylcellulose are non-caloric (non-metabolizable by humans or
intestinal flora in human beings), odorless, tasteless water-soluble or
water-suspendable polymers derived from cellulose. These
cellulose derivatives may act as thickeners, binders,
stabilizers, suspending agents or flow control agents. They
form films resistant to oils, greases and organic solvents.
They dissolve rapidly in cold and hot water and are
physiologically inert. These functions make them suitable for
use in a broad range of applications in food, pharmaceutical,
cosmetic, paper and other industries.
For such applications, degradation of cellulose derivatives
is normally considered undesirable and to be avoided.
Cellulolytic and viscosity reducing enzymes have been
deliberately avoided in the past.
Enzymatic hydrolysis of cellulose derivatives have been
studied in the past in the context of synergism studies among
- 1 -
2i)096'~~
combinations of enzymes, the possible indexing of substituent
distribution patterns, the effect of various substitutents on
enzymatic hydrolysis and the like. Such studies have been
ptblished in the following: Chouchon et. al., Biotech.
BioenQ., Vol. 26, pp. 988-991 (1984); Henrissat et. al.,
Biotechnology. Vol. 3, pp. 722-726 (1985); Chetkarov et. al.,
Monatshelte Fur Chemie, Vol. 116, pp. 1433-45(1985); Chetkarov
et. al., Monatshefte Fur Chemie, Vol. 117, pp. 1021-1026
(1986); Wirick, J. Polym. Sci., Part A-1, Vol. 6, pp. 1195-1974
(1968); Bh~.ttacharjee, J. Polym. Sci., Part C, Vol. 36, pp,
509-521 (1971). Reduction of chain length determinations have
also been studied. Almin et. al., Arch. Biochem. Biophys., pp.
I5 124, 129 (1968); Ghose, Biotech. Bioenq., Vol. 11,- pp. 239
(I969).
The present invention deals with a novel enzymatic
hydrolysis of cellulose derivatives and products incorporating
such hydrolysates. We have also found that some of the
properties of these cellulose derivatives can be further
improved by enzymatic hydrolysis into low molecular weight polymers
or oligorr~ric mixtures. These enzymatic hydrolysates and fractions
(of the total hydrolysate mixture of oligomers into further separated
mixtures of oligomers of varying chain length) thereof are more
advantageous for several different applications in food,
pharmaceutical. paper, cosmetic and textile industries than
their unhydrolysed counterparts.
- 2 -
CA 02009675 1999-09-02
SL11~ARY OF THE INVENTION
The invention discloses a novel water-soluble or
water-suspendable enzymatic hydrolysate of different
cellulose derivatives as well as its fractions.
The hydrolysate can be made from several
different cellulose derivatives, the most preferred raw
material being carboxymethylcellulose. The hydrolysate can
be prepared by different modified and unmodified
cellulolytic enzymes, the most preferred sources of the
enzyme being strains of Trichoderma reesei and Aspergillus.
The enzymatic hydrolysate and its fractions have
several applications in food, paper, pharmaceutical and
cosmetic industry, but they are especially useful as
calorie saving or low caloric substitutes in a wide range
of food stuffs.
In accordance with the invention there is
provided a water soluble enzymatic hydrolysate of a
cellulose derivative comprising a cellulose derivative
hydrolyzed with an enzyme preparation to form a mixture of
oligomers having an average degree of polymerization in the
range of 3 to 300 and a molecular weight of 500 to 100,000.
The soluble cellulose derivative is preferably selected
from the group of carboxymethylcellulose, methylcellulose,
methylethylcellulose hydroxypropylmethylcellulose and
carboxymethylcellulose and mixtures thereof.
Since the preferred cellulose derivative is
carboxymethylcellulose, the invention further provides a
water soluble enzymatic hydrolysate of carboxymethyl-
cellulose comprising a carboxymethylcellulose hydrolyzed
3
CA 02009675 1999-09-02
with an enzyme preparation to form a low molecular weight
polymer or a mixture of oligomers having an average degree
of polymerization between 3 and 300, a molecular weight of
3a
500 to 60,000 and a viscosity lower than 20 mPa.s at 25°C
at a concentration of 20$ by weight. The enzyme preparation
is tvpicallv selected
z~~oss~s
from the group of cellulases, modified cellulases and mixtures
thereof.
The enzyme preparation is a cellulase or modified cellulase
(i.e., modified to remove or prevent the formation of
saccharide producing enzymes in the cellulase preparation in
the first instance. e.g., by genetic alteration of the
microorganism from which the cellulase preparation is prepared) .
preferably produced from microorganisms selected from the group
of Trichoderma, Asp_er illus and Penicillium. Most preferably
the cellulase preparation is derived from Trichoderma reesei
from which at least one of beta-glucosidase and
cellobiohydrolase activities have been removed. The enzyme
preparation most preferably comprises endo- 1, 4-
beta-glucanase.
The invention also provides a method for producing a
mixture of oligomers from cellulose derivatives comprising the
steps of: selecting a cellulose derivative; selecting a
cellulolytic material including an enzyme which hydrolyzes the
selected cellulo.:e derivative into a mixture of oligomers
having an average degree of polymerization in the range of 3 to
300 and a molecular weight in the range of 500 to 100,000; and
reacting the selected cellulolytic material with the selected
cellulose derivative for a time and at a temperature sufficient
to pcoduce the mixture of oligomers. The cellulose derivative
is preferably selected from the group of carboxymethyl-
- 4 -
CA 02009675 1999-09-02
cellulose, methylcellulose, hydroxypropylcellulose, hydroxy-
methylcellulose and mixtures thereof.
Since the preferred cellulose derivative is
carboxymethylcellulose, the invention further provides a
method for producing a mixture of oligomers from
carboxymethylcellulose comprising the steps of:
selecting a carboxymethylcellulose~
selecting a cellulolytic material including an
enzyme which hydrolyzes the selected carboxymethyl
cellulose into a low molecular weight polymer or mixture of
oligomers having an average degree of polymerization
between 3 and 300, a molecular weight in the range of 500
to 60, 000 and a viscosity lower than 20 mPa. s at 25°C at a
concentration of 20~ by weight; and
reacting the selected cellulolytic material with
the selected cellulose derivative for a time and at a
temperature sufficient to produce the mixture of oligomers.
The step of selecting the cellulolytic material
typically comprises selecting a microorganism which
produces the cellulolytic material and preparing the
cellulolytic material from a culture of the microorganism.
The cellulolytic material produced by the microorganism may
be purified to remove enzymes which will react with the
cellulose derivative to produce saccharides. The
microorganism is preferably selected from the group of
Trichoderma, Aspergillus and Penicillium.
In order to prevent hydrolysis of the cellulose
derivative into saccharides the selected microorganism may
alternatively be treated to alter the genes of the
microorganism such that production of saccharide generating
enzymes by the genes is disenabled.
5
CA 02009675 1999-09-02
The invention further contemplates removing all
or a portion up to 505 by weight of a selected fat
contained in a foodstuff and substituting a mixture of
oligomers produced according to the invention for the
removed fat; and/or, removing up to about 40~ of a selected
carbohydrate contained in a foodstuff and substituting a
mixture of oligomers produced according to the invention
for the removed carbohydrate.
The invention also contemplates separating a
mixture of oligomers produced according to the invention
into fractions of oligomers of different average molecular
weight, removing up to
5a
2~)09~'~S
503 by weight of a selected fat contained in a foodstuff, and
substituting one or more of the fractions for the removed fat:
and/or, separating the mixture of oligomers produced according
to the invention into fractions of oligomers different average
molecular weight, removing up to 40~ by weight of a selected
carbohydrate contained in a foodstuff and substituting one or
more of the fractions for the removed carbohydrate.
BRIEF DESCRIPTION OF THE DRAWINGS
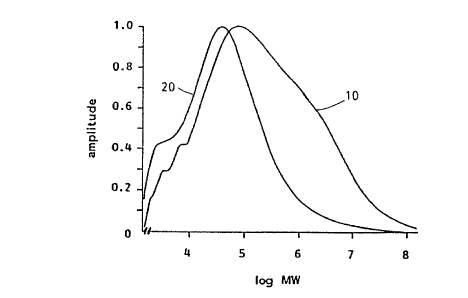
FIG. 1 shows molecular. weight distribution patterns of a
methylcellulose and its hydrolysate as described in Example 2a
herein;
FIG~ 2 shows molecular weight distribution patterns of
hydroxypropylmethylcellulose and its hydrolysate as described
in Example 2b herein;
FIG. 3 shows molecular weight distribution patterns of a
carboxymethylcellulose and its hydrolysate as described in
Example 2c(i) herein:
FIG. 4 shows molecular weight distribution patterns of a
carboxymethylcellulose and its hydrolysate as described in
Example 3 herein:
FIG. 5 shows molecular wei ht distribution
g patterns of
selected fractions of the carboxymethylcellulose hydrolysate as
described in EXample 3 herein:
- 6 -
~tlU~6'~S
DETAILED DESCRIPTION OF THE INVENTION
This invention relates to a soluble hydrolysate of a
cellulose derivative and its fractions. The hydrolysates are
characterized by having an average degree of polymerization
(DP) in the range of 3-3~0 and a molecular weight in the range
of 5oo- 100,000.
Enzyme Preparation
Enzymes used in this invention are various food-grade
cellulase preparations. They can be produced from a multitude
of different microorganisms such as strains of Trichoderma,
Asnergillus, Penicillium, etc. The selected microorganism
strain is grown by conventional means in a medium containing
food grade materials such that the cellulases are produced, the
microorganism is separated from the medium, the medium is
collected and typically concentrated and dried. These enzymes
can be used as such or in mixtures and they can be modified in
many different ways known to the man skilled in the art. The
most preferred enzyme preparation is produced from Trichoderma
reesei, from which preparations the beta-glucosidase and/or the
cellobiohydrolase activities are removed chromatographically or
genetically. Beta-glucosidase and/or cellobiohydrolase
mctivities are removed from the selected cellulase preparation
so as to prevent the degradation of the cellulose derivative
into cellobiose and/or glucose. Genetic alteration of the
appropriate enzyme producing microorganism may be effected with
24)C~9fi'75
radiation or mutageaic chemical agents (or by gene inactivation
~r rtcombinant DNA methods) so as to ilisenable production of
pasta-glucosidase and cellobiohydrolase by the microorganism.
Gllulase preparations suitable for use herein are, e.g., the
commercially available cellulase preparations designated as the
Ecoaase CE series as produced by Alko Ltd., Helsinki Finland.
Starting Materials
According to the invention the cellulose derivative
hydrolysate and its fractions can be produced by enzymatic
hydrolysis of a soluble cellulose derivative. Preferred
cellulose derivatives for use herein are carboxymethyl-,
methyl-, hydro:cypropylmethyl- or hydroxypropylcellulose and any
co'~inations thereof. The invention is not limited to the use
c: these cellulose derivatives.
General Dreoaration of the hydrolysate
Cellulose derivative hydrolysates are prepared from soluble
cellulose derivatives as defined above by an enzymatic
hy3rolysis utilizing a cellulase preparation having endo- 1, Q-
ba;.a-glucanase as the sole active hydrolytic anent such that
o~ly insignificant amount of saccharides (e.g., glucose and
ceilobiose) :rhich are absorbed in human intestine (e. g.,
glucose) or hydrolyzed by the intestinal bacterial flora (e. g.,
cellobiose)), are produced. On the other hand the average
degree of polymerization (DP) of the oligomers formed by such a
hydrolysis is lower than 300, and thus the viscosity of
_ g _
CA 02009675 1999-09-02
solutions of the hydrolysate is reduced significantly
compared to the viscosity of solutions of the unhydrolysed
cellulose derivatives. Using carboxymethylcellulose as a
cellulose derivative, the viscosity of the obtained
hydrolysate is lower than 20 mPa.s at 25°C at a
concentration of 20~ by weight. The specific conditions
suitable for and the specific time sufficient to secure the
desired hydrolysis may be readily determined for each
selected cellulose derivative and each selected enzyme
preparation.
Use of the cellulose derivative hydrolysates
The cellulose derivatives used as starting
materials in the present invention are as such non-caloric.
Because the hydrolysis according to the present invention
does not produce significant amounts of metabolizable
sugars, the hydrolysates according to this invention with
their improved properties, are especially useful as low-
caloric substitutes in food stuffs.
The cellulose derivative hydrolysates and
fractions thereof produced according to this invention can
be used for example as new low-caloric fat sparing agents
or bulking agents. These hydrolysates can be used to
replace fat in varying food stuffs, like baked goods,
butter icing and custard. Fat can be replaced entirely or
in part, typically up to a level of at least 50~, by these
hydrolysates but a level of 40~ is most preferred at least
when carboxymethyl hydrolysates are use. The amount which
can be replaced depends on the application. The texture of
the food stuff and the eating quality of the new product
should be improved or remain unchanges.
9
~oogs~~
~ cellulose derivative hydrolysates and fractions
thereof
d accordinq to this invention can be used also
as new
~uce
l~-calocic bulking agents. These hydrolysates
can be used to
replace cnrbohydrates such as sugar in different
kinds of baked
products or in other food stuffs. The amount
of carbohydrate
replaced with these hydrolysates depends on
the application and
average chain length of the hydrolysate oligomers.
By
conventional means hydroiysate mixture may be
further separated
into fractions of oligomers of differing average
chain
lengths. The viscosity of the various fractions
will vary with
the degree of average chain length of the oligomers
contained
within in a fraction. Depending on the particular
food stuff
application, the invention contemplates selecting
a fraction
from a hydrolysate mixture having a viscosity
(average chain
length) which is r"ost appropriate for the particular
food stuff
application. The selection of a particular average
chain
length fraction and the amount of such a fraction
to be used in
ary given food stuff application may vary according
to the
mount of fat or carbohydrate to be replaced,
it being
recognized that the higher the absolute amount
of substitution
agent desired to be used in a particular foodstuff,
the lower
the viscosity (average molecular weight) of
the fraction of
mixture of oligomers should be used.
The invention ie described in great8r detail
in the
following exacaples.
- 10 -
2t~U 96°7; ~
Example 1 - Cellulase Preparation
The beta-glucosidase activity was removed by ion exchange
chromatography from the cellulase preparation, Econase CE, Alko
Ltd., Helsinki, Finland which was produced from a strain of
Trichoderma reesei. The cellulase preparation (column~A, Table
1) was passed through a cation exchange column (S-Sepharose FF,
Pharmacia, LKB Biotechnalogy AB, Uppsala, Sweden) and
equilibrated with 50 mM sodium acetate pH 3.8 equilibrium
buffer. The unbound protein (including oligomer producing
endoglucanases) was washed out with the equilitration buffer
(column B, Table 1). Beta-glucosidase activity remained bound
to the column and could be separately eluted with 1M NaCl.
TABLE 1
EZZyme Relative Enzyme Activity (%)
A B
before ion exchange after ion exchange
procedure procedure
Beta-glucosidase 100 ~ 1
endo-1, 4, -beta- 100 70
glucanase
g~do_ 1, d- beta-giucanase and beta-glucosidase activities were
measured as described by Bailey & Nevalainen (1981): Enzyme
Microb. Technol. 3: 153-157. The relative enzyme activities
reported in Table 1 of the Econase preparations before and
- 11 -
2009f'~~
after passage through an ion exchange column demonstrate the
results of a typical means according to the invention of
preparing an essentially beta-glucosidase free preparation for
use in producing the oligomeric hydrolysates contemplated by
the invention.
Although Table 1 reports relative enzyme activities, the
absolute amount~of enzyme'used in any particular example is
hereafter reported in terms of the amount of enzyme activity of
the enzyme employed according to the universal activity unit of
nano-katal (nkat) which stands, for that amount of enzyme which
produces one nanomoie of reaction product in one second. (In
the context of this application a hydrolysate reaction product
such as an oligomer or glucose which is capable of reducing an
agent such as dinitrosalicylic acid which is reduce;' by the
hydrolysate reaction product and subsequently measured.) The
method of Bailey et. al., Enzyme Microb. Technol_, 401. 9, pp.
153-157 describes how such measurements of enzyme activity can
be made using glucose as a standard.
Example 2 -- Cellulose Derivative F? drol ses
a. Methylcellulose hydrolysate
30 g of methylcellulose (MC, Methocel MC, 64630, Fluka
Chemie AG, CH-9470 Buchs, Switzerland) was mixed in 3 1 of
s~ater and the pH of the solution was adjusted to 5.5 with 15%
phosphoric acid and the temperature was raised to 40oC. 0.3
- 12 -
c,(~096'~~
ml of the enzyme- -preparation having an end~r-1, 4 beta-glucarrase
activity of 1680 n~cat from which the ber_a~glucr>sidase activity
was ren~c:~:c:c'. Chr~r~aT_ograpiriCally (as descriLed in Exdn~l~le 1) was
ad~:e~: to the solution. After hydrolysis for 24 hours the
eniy:::e was :nact?vated by heating (90oC, 15 min.). ThE
hydrolysate solution was saYzsenue:~tiy cooled and freeze dried.
The hydrolysata product contained less that: 0.5% by weight
cf ciucose and cellcbiose.
mre ;l;olecular ~.re'_grt dlstrihution pasterns of
met::ylcellulose, curve 10, ar_d its hydrolysate, curve 20, are
s'.~.~~w:~ in FIG. 1.
':'he molecular :re-ght distributions of .he cellulose
de=_vatives and r_hF:r hydroiysates ~.~c_~e deter:nir:c:d by H2hC
Ls:nc a gel filtration colL!?~t1 (TSY gel G25001~~:, Toyo Soda
h:~.~.nfacturina Co. , Ltd. , Japau) wit?r a reractivE. finder
detector (rP 1037 A) and Pharmacosmns Dert:~a:~ Standards
(?haraacra:rr,s, DY-X130, Viby Sj . , Dennar.k) . The eluent was 0. 5
M SCd~i.:fli Chi9rlde.
b. ryccory~rppy~methylcellul~se,_~ycro_ysate
20 g of hydro~ypropylmethylcellulose (:?Pt~C, H-9262, Sigma
Chemical Company. St. Louis. MO, U.S.A.) was mixed in 1 1 of
,,ater and the pH of the solution was adjusted to 5.5 with 15%
phosphoric acid a.nd the temperature was raised to 40o C~
0.24 ml of the enzl:re preparation having an endo-1, 4
beta-glucanase activity of 1340 nkat from which the
- 13 -
zao~s~s
be::a-glucosidase activity was removed chromatographically (as
described in Example 1) was added to the solution. After two
hours another 20g of hydroxypropylmethylcellulose was added to
the solution. After the hydrolysis of 22 hours the enzyme was
inactivated by heating (90°C. 15 min.). Finally the
hydrolysate solution was cooled and freeze-dried.
The product contained less th~a 0.05% by weight of glucose
and cellobiose.
The molecular weight distribution patterns of the
hydroxypropylmethylcellulose, curve 30, and its hydrolysate,
curve 40, are shown in FIG. 2. The molecular weight
distribution pattern was determined as described in Example 2A.
c. Carboxymethylcellulose hydrolysate
(i) ~drolysis with Trichoderma reesei derived enzyme
Dre°aration
kg of carboxymethylcellulose (CMC 7.~IFD-type, a cellulose
20 gum, also designated by the tradename Blanose and available
~rom Hercules Chemical Company, 92507, Rueil-Malmaison Ceder,
France: 7MFD designating a medium viscosity, food grade sodium
carboxymethylceilulose having 7 out of l0 glucose units
s~stituted with carboxymethyl) was mixed in 320 1 of Water and
the pH of the solution was adjusted to 5.5 With 15% phosphoric
acid and the temperature was raised to 40°C. 0.27 1 of the
enzyme preparation having an endo-1, 4 beta-glucanase activity
- 14 -
2~~0~~'7~
of 1.780,000 nkat from which the beta-glucosidase act~'_vity was
removed chromatographically (as described in Example 1) was
added to the CMC solution. After one hour another 20 kg of CMC
was added to the solution. After hydrolysis of 23 hours the
enzyme was inactivated by heating (90°C, 15 man.). finally.
the hydrolysis solution was concentrated by conventional
evaporating and spray-drying.
.The product contained less than 2% by weight of glucose and
cellobiose. Wheri the same hydrolysis was carried out with the
original cellulase enzyme preparation of Trichoderma
reesei-fungus, the amount of produced glucose and cellobiose
was above 5% by weight.
The molecular weight distribution patterns of
carboxymethylcellulose, curve 50, and its hydrolysate, curve
60, are shown in FIG. 3.
The molecular wezght distribution pattern was determined as
described in Example 2a.
(ii) Hydrolysis with Asperqillus and Penicillium derived
enzyme preparations
The enzyme preparations selected were commercially
available Cellulase AP 3 (Rmano Pharmaceutical Co., Ltd.,
2 5 Nagoya. Japan) produced using an Asperqillus strain and
Cellulase CP (Sturge Enzymes. North Yorkshire. England)
produced using a Penicillium strain. Carboxymethylcellulose
hydrolysates were prepared as described in Example 2c(i).
- 15 -
2s~~~9~'~~
except that 30g of CMC-7MFD was used in 1 1 of water, and the
amounts of enzymes added were 0.028 g of Cellulase A~ 3 (having
an endo-1, 4 beta-glucanase activity of 1350 nkat) and 0.048 g
of Cellulase CP (having an endo-1, 4 beta-glucanase activity of
1350 nkat). The viscosities and molecular weight distributions
of the hydrolysates produced by either cellulase were similar
(FIG. 3) to the hydrolysate produced with enzymes derived from
Trichoderma reesei.
The viscosities of the various cellulose derivatives and
their hydrolysates as described and prepared in Example 2 wera
measured using a Haake-Rotovisco viscometer with sensor systems
NV (Karlsruhe, Federal Republic of Germany) (Table 2). The
viscosities were measured in water solutions at 25°C. Table
2 set forth the concentrations (by weight) of a variety of
solutions all having the same viscosity.
2 0 TABLE 2
Concentrations of cellulose derivatives and their
respective hydrolysates in solution all having a viscosity
of 20 mPa.s (milli-Pascals-second) at 25oC.
Cellulose-Derivative------------------Concentration (by weight)
Methylcellulose 2%
Methylcellulose 5%
hydrolysate
Hydroxypropylmethylcellulose 3%
- 16 -
2n~~~'~:~
Hydroxypropylmethylcellulose 10%
hydrolysate
Carboxymetaylcellulose 2%
Carboxymethylcellulose 20%
hydrolysate
10
As the data in Table 2 indicates, the hydrolysate of a
cellulose derivative has a substantially lower viscosity than
an equal amount by weight in aqueous solution of the cellulose
derivative itself and can be incorporated into a foodstuff in
substantially higher quantity as a fat or sugar substitute
than the cellulose derivative itself without compromising the
texture, volume, density or the like of the foodstuff.
Example 3 -- The fractionation of carboxymethylcellulose
hvdrolysate
The carboxymethylcellulose hydrolysate was prepared as
described in Example 2c, excegt that the raw material was CMC
7LFD designating a low viscosity, food grade cellulose gum
having 7 out of l0 glucose units substituted with
carboxymethyl, designated under the tradename Blanose and
available from Hercules Chemical Co., France) 1.6 kg CMC was
used in 8 1 of water and that the amount of enzyme added was
13.2 ml having an endo- 1, 4 beta-glucanase activity of 87,000
nkat. S ml of the hydrolysate (0.5 g of dry matter) was
further fractionated into three fractions by gel permeation
chromatography (Pharmacia K 26/100 -column, Sephacryl S-200
-gel. Pharmacia LRB Biotechnology AS, S-75182 Uppsala,
_ ly _
2UU96'~~
Sweden). The eluent was distilled water, the flow rate was 14
ml/hour, and the fractionation process was carried out for 45
hours and Fractions collected at intervals of 0.5 hours and
pooled into three fractions (18 hours - 26 hours, curve 90, 26
hours - 32 hours. curve 100, and 32 hours - 38 hours, curve
110, FIG. 5, respectively). The molecular weight distributions
of carboxymethylcellulose, curve 70, carboxymethylcellulose
hydrolysate, curve 80, and the three further fractions,-curves
90, 100, 110, FIGS. 4, 5 were determined by HPLC as described
in Example 2.
Example 4 -- the evaluation of the fat sparing aqent_
according to the invention in Madeira cake
Standard Madeira cakes were prepared by mixing 200 g of
high ratio cake flour, 250 g of sugar-caster, 130 g of high
ratio shorteninq,~l6 g of skimmed milk powder, 3 g of salt. 12
g of baking powder. 180 g of water and 176 g of egg
(defrosted). 180 g of the mix was scaled into greased tins and
baked at 170oC 30 min. Madiera cakes, in which 40% of the
fat was substituted (i.e., 130 g of shortening was reduced by
40% to 78 g) with 1) carboxymethylcellulose hydrolysate (fat
sparing agent according to the invention), 2)
carboxymethylcellulose or 3) potato maltodextrin (a
conventional fat sparing agent) were compared to the standard
Madiera cakes and to each other.
- 18 -
2~1C)9E~'~S
According to a trained panel the results were as described
in Table 3.
TABLE 3
Fat Sparing
Agent Concen-
Level of Fat tration in
the
Product Substitution Product TestedSummary of
tested (%) (% by weight)Performance
________________________________________________________________
Standard -- -- A good
volume,
open-textured
cake was
produced.
It
gave a light
eat with a
'melt-in-the-
mouth' eating
quality.
Same as Standard 40.0 2.2 A good
with Carboxymsthyl- volumed.
cellulose open-textured
hydrolysate cake was
used as fat produced.
It
substitute gave an
eating
qualitx close
to that of
standard,
but
not quite
as
good.
Same as Standard 40.0 0.3 A lower
with Carboxymethyl- -volumed,
cellulose was denser cake
as fat substitute was
produced.
The eating
quality was
vary poor
-
a pronounced
gumminess
was
gresent and
there was
no
'melt-in-the-
- 19 -
2c)~J96'7~
mouth'
characteristic.
Same as Standard 40.0 0.3 A good
with Potato volumed,
maltodextria open-textured
used as fat substitute cake was
produced.
The eating
quality was
close to
that
of standard
and carboxy-
methyl-
cellulose
~ hydrolysate.
Again it
was
slightly
poorer than
the standard.
Example S - Evaluation of the fat gent according
sparing a
to the invention in butter icing
A standard butter icing was prepared ng 179 g
by mixi of
butter (unsalted), 225 g of icing of water.
sugar and 96 g The
butter icings, in which 30% of fat ed (i.e.,
was substitut 179 g
of butter was reduced by 39% to 125.3
g) with 1)
carboxymethylcellulose hydrolysate agent according
(fat sparing
to this invention), 2) carboxymethylcellulose3) potato
or
maltodextrin-and all were compared
to the standard butter icing
and to each other.
According to a trained panel the results
were as described
in Table 4.
- 20 -
2()0~6'~~
Exam le 6 -- Evaluation _of the bulking agent according to
the nvention in marzipan
A standard marzipan product was prepared by mixing
21.8 g of icing sugar, 21.8 g of castes sugar, 4~.7 g of ground
almonds, 0.8 g of vanilla flavoring, 10.9 g of eggs (lightly
beaten) and 1 g of lemon juice for every 100 g of product. The
mixture was formed into a ball, kneaded lightly, rolled out and
cut out. Marzipan, in which l0% of sugar was substituted with
carboxymethylcellulose hydrolysate (bulking agent according to
the invention), had good almond flavor and same color as the
standard marzipan product. It was also easy to roll and cut
out. Higher percentage sugar replacement maybe required to
reduce calories significantly. Appropriate lower viscosity
DP-fractions of carboxymethylcellulose hydrolysate may be
employed in greater than l0% by weight amounts, preferably up
. to as much as about 40% while still maintaining normal
texture.
TABLE 4
Fat Sparing
Agent Concen-
Level of Fat tration in the
Product Substitution Product Tested Summary of
tested (~) (~ by weight) Performance
Standard - - An acceptable
icing was
produced. It
was firm,
smooth textured
and held onto
- 21 -
2~~a9s~~
water extremely
well. It was
much better
than the icing
made with
carboxymethyl-
cellulose and
potato
maltodextrin.
Same as Standard 30.0 4.3 A very accept-
with Carboxy- able icing was
methylcellulose produced. It
hydrolysate used as was firm,
fat substitute smooth textured
and held onto
the Water
extremely
well. It Was
better than the
standard icing.
Same as Standard 30.0 0.5 A very poor,
with Carboxy- unacceptable
methylcellulose icing Was
used as fat substitute produced. The
system did not
hold onto the
water at all
well.
Same as Standard 30.0 2.7 Again a poor,
with Carboxy- unacceptable
methylcellulose icing was
used as fat substitute produced. The
water was not
held within t ~e
system.
It will now be apparent to those skilled in the art that
2 5 other embodiments, improvements, details, and uses can be made
consistent with the letter and spirit of the foregoing
disclosure and within the scope of this patent. which is
limited only by the following claims, construed in accordance
3 0 pith the patent law, including the doctrine of equivalents.
- 22 -