Note: Descriptions are shown in the official language in which they were submitted.
MJ 675
~r~~.~~J
UNIT nOSE PACKAGE
Field of the Invention
The present invention is directed generally to
a
. _ unit dose package. More particularly, the present
invention
is directed to a unit dose package for a reconstitutable
powder. Most specifically, the present invention
is
directed to a unit dose package for a reconstitutable
nutri-
tional infant or adult formula powder form. A
unit dose
container carries a pre-measured amount of the
nutritional
formula. The container has a plastic fitment
which is
provided with a mouth opening and with an annular
threaded
recess. The threaded recess is sized to cooperate
with a
threaded neck of a graduated bottle that contains
a measured
amount of a reconstituting liquid. In use, an
overcap and a
foil seal are removed from the unit dose package's
fitment.
Once this has been done, the dose package and
graduated
bottle are secured together and the contents
of the unit
dose package reconstituted by being mixed with
the liquid in
the graduated bottle. When secured together,
the dose
package and graduated bottle allow ample capacity
to
comp=ete mixing of the powder and liquid because
of the
extra head space provided by the dose package.
Description of the Prior Art
Various infant and adult nutritional formulas are
,. -_ generally well known in the art. These typically take the
form of powders or concentrated liquids which must be
reconstituted or suitably diluted prior to usage. Particu-
larly when using powdered nutritional formulas, a measured
amount of the powder must be combined and then mixed with a
corresponding volume of a reconstituting liquid prior to
use.
Powdered nutritional formulas are frequently
packaged in a bulk container which may be supplied to the
user with a measuring scoop or spoon of some type. The user
is then required to remove the appropriate dry measure of
the powdered formula from the bulk container and to add this
powdered formula to a volume of reconstituting liquid,
typically water, in a baby bottle or mixing container. This
procedure is apt to be less than ideal for several reasons.
In the process of removing powdered formula from a bulk
container with a scoop and transferring this powder to the
liquid container, there is a significant opportunity to
spill some of the formula. Such spillage obviously creates
a mess which must be cleaned up. More importantly, such
spillage is apt to adversely affect the accuracy of the
reconstituted formula.
An associated problem with manual mixing of a
powdered or concentrated nutritional formula with a
reconstituting liquid is one of incorrect formula strength
caused by inaccurate formula measurement. When a group of
_ 2 _
1
~2t~l~On 1
people were asked to prepare a formula using a certain
number of scoops of powdered formula, a wide range of
product concentrations was observed. People often have a
difficult time properly measuring the proper amount of the
powdered nutritional formula and properly mixing it with the
reconstituting liquid.
Another problem is obtaining thorough mixing of
powdered formula because of the lack of head space when the
nursing bottle containing the reconstituting liquid is full.
The unit dose package of the instant invention provides
extra head space when secured to the nursing bottle thereby
permitting complete mixing.
When using a bulk container of a powdered infant
or adult nutritional formula, there is clearly the possi-
bility that the bulk container, once it has been opened, may
become contaminated. In a home environment, such possible
contamination will typically be accidental, while in a
hospital or similar setting it may not be. The hospital is
apt to be quite reluctant to expose itself to the potential
liability which usage of a bulk powdered nutritional formula
may mean. Thus, a more costly alternative may be selected
in order to avoid and potential risk of contamination.
Measuring a mixing of a reconstitutable powdered
nutritional formula is apt to be more time consuming than
some parents are willing to spend. Similarly, hospital
nurseries and other institutional users of powdered
nutritional formulas cannot afford to spend a great deal of
- 3 -
X020005
time measuring and mixing the particular formulas required
by the various babies or persons being cared for. Thus, the
conventional arrangement of a bulk powdered reconstitutable
nutritional formula is unacceptable to these users.
The use of powdered infant and adult nutritional
'.. formulas which must be reconstituted by mixing a measured
portion of the formula with liquid has, as discussed above,
various disadvantages. The mixing process is apt to be
messy and may take more time than many parents and virtually
all hospital nurseries are willing to take. It is also
often difficult to obtain an accurate measure of the powder.
Bulk containers of formula are also possible targets of
product contamination or adulteration. Thus, it will be
clear that a need exists for a unit dose package which will
overcome these drawbacks of the prior art devices. The unit
dose package, in accordance with the present invention,
provides such a package and represents a significant advance
in the art.
Summary of the Invention
2p It is an object of the present invention to
provide a unit dose package.
Another object of the present invention is to
provide a unit dose package for mixing and dispensing liquid
and powdered nutritional formulas.
A further object of the invention is to provide a
unit dose package which is usable with a bottle to form a
mixing container.
- 4 -
~o~.oooJ
Yet another object of the present invention is to
provide a unit dose package which is hermetically sealed and
tamper resistant.
..
Still a further object of the present invention is
to provide a unit dose package that facilitates formula
reconstitution without mess or error.
Even yet another object of the present invention
is to provide a unit dose package that provides the user
with an accurate amount of formula for reconstitution.
Still even a further object of the present
invention is to provide a unit dose package which is quick
and easy to use.
Still another object of the present invention is
to provide a unit dose package that provides additional head
space for complete mixing of powder and reconstituting
liquid.
As will be diseussed in greater detail in the
description of the preferred embodiment which is set forth
subsequently, the unit dose package in accordance with the
present invention includes a unit dose container which has a
closed bottom and a plastic fitment having a mouth which is
bounded by a threaded recess. The threaded recess is sized
to be cooperative (compatible) with the neck of a plastic
bottle. This plastic fitment is sealed by a foil closure
seal and which, in turn is covered by an overlying
protective overcap.
The unit dose package is supplied to the user with
a measured amount of a nutritional formula, typically a
- 5 -
~01000~
powder. An appropriate amount of reconstituting liquid,
such as water, is placed in a graduated plastic nurser
bottle and the overcap and foil membrane seal are removed
from the plastic fitment of the unit dose package. The neck
of the bottle is screwed into the recess in the plastic
fitment, as the unit dose package is inverted. The contents
of the unit dose package are thus added to the reconsti-
tuting liquid. The combined unit dose package and plastic
bottle provide a closed system with sufficient space to
insure that the nutritional formula can be easily and
thoroughly mixed with the reconstituting liquid by shaking
the closed system.
In marked contrast to the prior art approaches
which required removal of an amount of powder from a bulk
container and addition of this powder to the liquid, the
unit dose package of the present invention eliminates the
spillage, mess, and possible inaccuracies which accompanied
the prior art. Each unit dose container is supplied to the
user with an appropriate quantity of nutritional formula
which has been pre-measured during packaging. The formula
is added to the reconstituting liquid once the unit does
package and plastic nursing bottle have been cooperatively
joined together. Thus, all of the formula is mixed with the
liquid and the correct amount of formula is utilized.
Fach unit dose package is intended to be used only
once. Further, various formula strengths and compositions
can be provided in appropriately labeled containers. Since
each package is a unit dose, no time is wasted in measuring
- 6 -
201000
and mixing. The proper unit dose package is selected,
opened, combined with a bottle, and mixed for use. This
convenience and time saving aspect of the present invention
makes it particularly attractive for busy parents and even
more attractive to hospital nurseries and similar
facilities.
The unit dose package is a hermetically sealed
package which may be filled and sealed under a nitrogen or
similar inert atmosphere to provide a product with a long
shelf life. The plastic overcap protects the metal foil
membrane seal which itself provides excellent tamper
evidence. When this foil seal is removed, it will take with
it a portion of the container itself. Thus, if the
container is washed for reuse, it will be apt to start to
decompose. Thus, it is a disposable package which has been
structured to prevent reuse.
The unit dose package in accordance with the
present invention provides a package through which a
reconstitutable nutritional formula can be accurately,
efficiently, and effectively mixed with a reconstituting
liquid for use. It eliminates formula spillage and
inaccurate measurements. At the same time, it reduces the
time required to prepare and mix the formula while also
minimizing the possibility of product contamination. Also,
it allows sufficient head space to thoroughly mix the
nutritional powder and reconstituting liquid. The unit dose
package of the present invention is clearly superior to
_ 7 _
~01(~00~
prior art devices and performs its desired functions in an
expeditious manner.
Brief Description of the Drawines
While the novel features of the unit dose package
in accordance with the present invention are set forth with
particularity in the appended claims, a full and complete
understanding of the invention may be had by referring to
the detailed description of the preferred embodiment which
is presented subsequently, and as illustrated in the
accompanying drawings, in which:
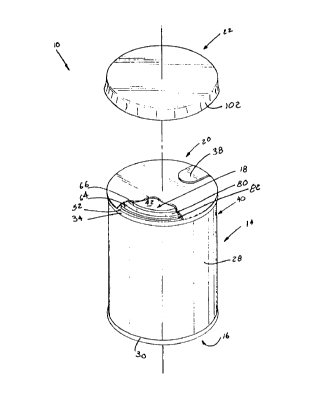
Fig. 1 is an exploded perspective view of the unit
dose package of the present invention.
Fig. 2 is a perspective view of a graduated
nursing bottle usable with the unit dose package of Fig. l;
Fig. 3 is an elevation view, partly in section and
showing the unit dose package and bottle in their assembled,
mixing position; and
Fig. 4 is an exploded perspective view of the unit
dose package and bottle complimentarily positioned.
Description of the Preferred Embodiment
Referring initially to Fig. 1 there may be seen,
generally at 10, a unit dose package in accordance with the
present invention. As will be discussed in greater detail
shortly, unit dose package 10 is usable with a cooperating
bottle, typically a plastic graduated nuxsing bottle,
generally at 12 in Fig. 2. Unit dose package IO and bottle
_ g _
CA 02010005 2000-03-20
12 are securable together in a manner as is shown in Fig. 3
to provide a closed system for mixing and reconstitution of
the contents of the unit dose package with a reconstituting
liquid which is in bottle 12. After such mixing and
S reconstitution, the now empty unit dose package 10 may be
separated from bottle 12, as shown in Fig. 4 and the
contents of bottle 12 may now be used in a generally
conventional and well known manner. While unit dose package
will be discussed hereinafter as containing a powdered
10 nutritional formulation, such as powdered baby formula, and
further while bottle 12 will be discussed as being a plastic
graduated nursing bottle in which'the reconstituting liquid
is water, it will be understood that this is for ease of
explanation and that the contents of unit dose package 10
are not to be construed as being so limited and that the
bottle and its reconstituting liquid also are not so
limited.
Returning again to Fig. 1, unit dose package 10
includes a generally cylindrical body 14, a metal container
bottom 16 secured to the bottom of the cylindrical body 14,
a plastic filament 18 secured to an upper portion of
cylindrical body I4, a foil seal membrane 20 which is
removably sealed to plastic fitment 18, and a protective
overcap 22 that overlies and protects metal foil membrane
20. This unit dose package 10 is ideally structured for
fillage on a high speed packaging line with a dry infant or
adult nutritional formula and when filled and closed, forms
a hermetically seated package in which the powdered formula
- 9 -
. .
201(:OU
may be sealed under a nitrogen atmosphere to give the
product a long shelf life.
Cylindrical body 14 of unit dose package 10, as
may be seen in Figs. 1 and 4, is a composite which
preferably includes an inner liner 24 of a foil and
polypropylene coated kraft paper, and one or more outer body
plies 26 which may be of a suitable kraft paper. Outer
surface 28 of cylindrical body 14 may be a suitable print
receiving paper or aluminum foil which may be treated, after
printing, with a generally well known lacquer. Metal
package bottom 16 is provided with an outer peripheral
flange 30 which may define an appropriately dimensioned
channel (not shown) into which a bottom edge 32 of
cylindrical body 14 may be sealingly secured. As will be
understood by those in the art, this bottom 16 is typically
attached to cylindrical body 14 after the contents have been
placed inside unit dose package 10. The bottom end 16 is
scarred onto bottom edge 32 of cylindrical body 14 with
automatic can end seaming equipment.
As may be seen most clearly in Fig. 3, the upper
portion of inner liner 26 of cylindrical body 14 terminates
in a radially outwardly and downwardly extending curl 34.
This curl rolls over an upper edge 36 of the outer body
plies ?6. Curl 34 provides a surface to which the foil seal
membrane 20 may be bonded. Membrane 20, as may be seen most
clearly in Fig.l, overlies plastic fitment 18 and curl 34.
The foi.'_ membrane 20 is preferably formed of a foil with a
hot melt adhesive that can be bonded by suitable R.F.
- 10 -
201000
sealing means or other heat means to the top of fitment 18
and to the foil and polypropylene on curl 34. When foil
membrane seal 20 is removed, such as by grasping the
integral pull tab 38, a portion of the foil and poly-
propylene on curl 34 will also be removed. This will expose
a portion of the paper portion of liner 24. Thus, if an
attempt is made to wash unit dose package 10 after it has
been opened, it will start to decompose. This will
discourage reuse of package 10.
Turning now to Figs. 3 and 4, plastic fitment 18
will be seen as being situated within cylindrical body 14 of
unit dose package 10 generally adjacent an upper portion 40
of body 14 when the unit dose package 10 is in the upright
position depicted in Fig. 1. Plastic fitment 18 includes a
central open mouth 42 which is defined by an annular mouth
ring 44. A transverse web 46 extends radially outwardly
from a bottom portion 48 of mouth ring 44. An attachment
ring 50, which is generally concentric With mouth ring 44,
is formed integrally with, and extends generally perpen-
2p dicular to transverse web 46 of plastic fitment 18.
Attachment ring 50 has an upper rim 52 which is situated
adjacent curl 34 when plastic fitment 18 is slid into the
upper portion 40 of cylindrical body 14 of unit dose package
10. An outer peripheral surface 54 of attachment ring 50 is
coextensive with an upper inner surface 56 of inner linear
24 of cylindrical body 14. Surface 54 of attachment ring 50
of plastic fitment 18 and inner surface 56 of inner liner 24
are bonded together by suitable R.F. heating or other
- 11 -
01000
similar heating. This bonding is strong enough to resist
any rotational torque which might be applied to plastic
fitment 18 when it is secured to, or removed from bottle 12,
as will be discussed subsequently. The bonding also
5 provides a water tight seal that does not leak when the
powder and liquid are mixed together.
A threaded ring 60 is also formed as a segment of
plastic fitment 18. As may best be seen in Fig. 3, threaded
ring 60 is concentric with, and spaced between inner mouth
10 ring 17 and outer attachment ring 50. Threaded ring 60 is
joined at a lower portion 62 to transverse web 46 and
terminates in an upper rim 64 which is generally co-planar
with upper rim 52 of attachment ring 50 and an upper rim 66
of mouth ring 44. A helical screw thread 68 is molded on
15 the radially inner surface 70 of threaded ring 60. This
thread 68 is sized to cooperate with the generally conven-
tional helical screw threat 72 that is found on the outer
neck surface 74 of a neck portion 76 of bottle 12. An
inner, bottle neck receiving channel 80 is defined by mouth
20 ring 44, threaded ring 60 and their connecting portion of
transverse web 46. A spacing channel 82 is defined between
threaded ring 60, outer attachment ring 50 and their
connection portion of transverse web 46.
An optional feature of the invention not shown by
25 the drawings comprises a water soluble membrane covering
open mouth 42 of plastic fitment 18 or spaced within the
annular mouth ring 44 to contain the contents of the unit
dose package. Preferably, the water soluble membrane is
- 12 -
2~1(~OQ i
formed of rice paper but other water soluble membranes of
carbohydrate based material such as corn starch, potato
starch, wheat starch, tapioca starch, etc. can be used. The
membrane is attached to lower ring 65 or secured within the
annular mouth ring 44 by suitable R.F. sealing means. The
membrane insures that no spillage of the nutritional powder
formula will occur when unit dose package 10 is turned into
the inverted position shown in Fig. 3. Upon shaking the
assembly, the water soluble membrane dissolves and mixing of
the contents of unit dose package 10 and bottle 12 can be
accomplished.
As previously alluded to, bottle 12 is preferably
a graduated nursing bottle that is molded from a suitable
plastic in a generally conventional configuration. Bottle
12 has a bottom 90, a generally cylindrical sidewall 92
which may have a reduced diameter central region 94 to
facilitate grasping, and a plurality of graduation marks 96
which may include an upper maximum fill line. Bottle 12 has
an open mouth 98 which is defined by bottle neck 76. This
neck 76 terminates in an upper neck rim 100, all in a
generally conventional manner. As may be seen in Fig. 3,
the surface of bottle neck rim 100 will abut the upper
surface of transverse web 46 of plastic fitment 46 when unit
dose package 10 is crewed onto the neck 76 of bottle 12.
Empty unit dose packages 10 which have been
provided by the fabricator with metal bottoms 16 not
attached, are given a highly accurate filling of a powdered
reconstitutable infant or adult nutritional formula on a
- 13 -
201n00 i
high speed packaging line. Once the formula has been placed
in the package, the metal bottom is attached by normal can
end seaming equipoment. The unit dose package 10 can now be
shipped and stored until usage. When the user is ready to
reconstitute and mix the formula, he first adds the appro-
priate volume of reconstituting liquid to bottle 12 using
graduations 96 as a guide. He then may remove protective
overcap 22 by grasping a rim portion 102 thereof. This
overcap 22 is preferably fabricated of a thin polystyrene
material and snap fits over the upper end of a unit dose
package 10. Once overcap 22 has been removed, the user can
visually inspect foil membrane 20 to insure that it has not
been tampered with. Having done this, the user may then
remove foil membrane 20 by grasping the pull tab 38 and
pulling upwardly on it. This force will separate the foil
membrane seal 20 from the upper rims 52, 64 and 66 of the
plastic fitment, and from the curl 34 of the inner liner 24.
Grasping bottle 12 in one hand and now open unit dose
package 10 in the other, the user will insert the neck 76 of
bottle 12 into the neck receiving channel 80 of plastic
fitment 18 while turning unit dose package 10 into the
inverted position shown in Fig. 3. Any slight discrepancy
between the diameter of bottle neck 76 and the inner
circumference of threaded ring 60 can be accommodated by
flexure of ring 60 into spacing channel 82. Unit dose
package 10 is typically not completely filled with formula
so this inversion of package 10 is accomplished without
spillage of the package's contents. With the unit dose
- 14 -
~:U1UUU~
package 10 securely affixed atop bottle 12 through the
cooperation of the screw threads 68 on threaded ring 60 of
plastic fitment 18, and the screw threads 72 on bottle neck
76, thorough mixing of the contents of the unit dose package
~ 5 10 and the bottle 12 can be accomplished by shaking the
assembly. The added headspace created through the attach-
ment of unit dose package 10 to the bottle 12 provides
adequate room for efficient formula reconstitution by
shaking. Once such reconstitution has been accomplished,
the now empty unit dose package 10 may be removed from the
neck 76 of bottle 12 and discarded, as shown in Fig. 4. A
suitable closure device, such as a well known resilient
nipple and seal ring assembly (not shown) may now be applied
to bottle neck 76 and the bottle 12 can now be used for its
intended function.
The unit dose package 10 in accordance with the
present invention has many beneficial attributes. Since
each unit dose package was accurately filled, uniformity of
the reconstituted product is assured. There is no mess,
spillage or wastage associated with the unit dose packages,
and essentially sterile condition may be maintained as the
unit dose package iu kept closed and sealed until immed-
iately prior to usage. The unit dose package 10 of the
present invention is intended primarily for use with an
8 oz. plastic purser bottle 12. However, unit dose packages
and bottles in other sizes could also be provided. Also, as
was discussed above, the unit dose package of the present
invention is equally suited for use with liquid products to
- 15 -
201()00
be mixed with other liquids. It will thus be apparent that
the unit dose package in accordance with the present
invention provides an accurate, reproducible device for
reconstituting or mixing two constituents in a manner which
' S eliminates mess and saves time in measuring and mixing.
While a preferred embodiment of a unit dose
package in accordance with the present invention has been
set forth fully and completely hereinabove, it will be
apparent to one of skill in the art that a number of changes
in, for example, the type of plastics used for the plastic
' fitment and bottle, the types of heat activated adhesives
used, the overall size of the package, and the contents of
the package and the reconstituting liquid may be made
without departing from the true spirit and scope of the
subject invention which is accordingly to be limited only by
the following claims.
- 16 -