Note: Descriptions are shown in the official language in which they were submitted.
Background of the Invention
Field of the Invention
This invention relates to a process for the
preparation of nitrilo- mono-, di- or tri- amides in high
purity, in aqueous solution and under moderate reaction
conditions.
Description of the Prior Art
It is known in the prior art that nitrilo-
monoaceticamide and nitrilotriaceticamide can be prepared
by chemical hydrolysis. For example USP 3,560,551 teaches
a method of synthesizing a nitrilomonoamide having the
formula:
N - C - R - N - R - C - NH
3 , 1 " 2
R2 O
CN
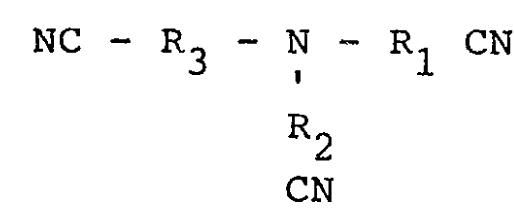
by reacting a compound having the formula:
NC - R3 - N - R1 CN
R2
CN
wherein R1, R2, and R3 are independently selected
from alkylene radical having 1-20 carbon atoms, with
an aqueous hydrogen peroxide solution at about
15°-95°C, and separating and recovering the product.
However, this process involves expensive solvents,
intermediate separations, high temperatures, high pressure
and suffers from low-yield and low selectivity.
Nitrile hydratase enzymes have been known for their
ability to hydrate mono- and di- nitriles to form
- 3 -
corresponding amides. However, these enzymes are
substrate specific and are only capable of converting
nitrites which are completely soluble in aqueous
solutions.
It now has been discovered that trinitriles which are
practically insoluble in aqueous solution, having the
formula:
NC - R3 - N - R1 CN
R2
CN
wherein R1, R2, and R3 are independently selected from
alkylene radical having 1-20 carbon atoms, under the
process of this invention, can be selectively hydrolyzed
into the nitrilo mono-, di-, or tri- amides with the aid
of enzymes or microorganisms having nitrite hydratase
activity.
Detailed Description
The subject process is directed to a means of
hydrolyzing nitrilotrinitriles having the formula:
NC - R3 - N - R1 CN
R2
CN
wherein R1, R2, and R3 are independently selected from
alkylene radical having 1-20 carbon atoms, by employing
whole cells of microorganisms or enzymes having high
nitrite hydratase activity to selectively transform only
one or two or all three of the cyano groups into the
corresponding mono- or di- or tri- amides.
Specifically, under the process of this invention a
- 4 -
substrate comprising an aqueous saturated suspension of
nitrilotrinitrile having the formula:
NC - R3 - N - R1 CN
R2
CN
wherein R1, R2, and R3 are independently selected from
alkylene radical having 1-20 carbon atoms, preferably in a
pH 7.0 phosphate buffer is prepared in a slurry reactor.
The substrate is pumped continuously into contact with the
whole cells of microorganisms or enzymes having high
nitrite hydratase activity, under reactive conditions to
produce the corresponding nitrite mono-, di-, or tri-
amides.
Suitable microbial species having high nitrite
hydratase activity are Pseudomonas, Gluconobacter,
Agrobacterium, Acetobacter. Achromobacter, Acinetobacter,
Alcaligenes, Citrobacter, Enterobacter, Erwinia,
Escherichia, Klebsiella, Proteus, Serratia, Yersinia,
Chromobacterium, Aeromonas, Vibrio, Flavobacterium,
Micrococcus, Staphylococcus, Streptococcus, Bacillus,
Clostridium, Lactobacillus, Leuconostoc, Brevibacterium,
Cellulomonas, Corynebacterium, Microbacterium,
Propionibacterium , Mycobacterium, Streptomyces,
Bacteridium, Chaetomella, Septoria, Diplodia,
Arthrobacter, Nocardia, Stenphylium, Torylopsis, Phoma,
Conothyrium, Myrothecium, Pestalotia, Melanconium,
Epicoccum, Penicillium, Aspergillus, Sepedonium, Fusidium,
Oidiodendron, Ce~ohalosporium, Sco~ulariopsis,
Paecilomyces, Verticillium, Tricothecium, Pullularia,
Monotospora, Cladosporium, Helminthosporium,
Ch~sosporium, Rhodotorula,, Kloeckera, Geotrichum and
Fusarium, strains of Agrobacterium radiobacter,
- 5 - 2~3.~1~~
Pseudomonas aeroqinosa, Pseudomonas fluorescens,
Pseudomonas utida, Corynebacterium n.itrilophilus,
Corynebacterium pseudodiphteriticum, Nocardia rhodochrous,
Escherichia coli, Neurospora crassa, Lathyrus sylvestris,
Lathyrus odoratus, Vicia villosa, and Brevibacterium,
strains.
In a first embodiment, the substrate from the slurry
reactor is pumped first through a filter to separate the
i
solubilized nitrile from the slurry suspension and then
through a bioreactor column containing immobilized enzymes
or cells having high nitrile hydratase activity. The
immobilized cells are prepared by blending a selected
biomass with filtered sterile water and sodium alginate.
This mixture is placed in a grilling column and dispersed
in a solution of calcium chloride where it forms grills
which are then screened to between about 10 to 20 mesh.
The grills are then washed and packed into the bioreactor
column. The filtered solubilized nitriles from the slurry
reactor are circulated through the bioreactor, brought
into reactive contact with the immobilized enzymes and
selectively hydrolyzed into the corresponding mono-, di-,
or tri- amide product. The product is continuously
recirculated back to the slurry reactor where it further
solubilizes the nitrile substrate. Both the bioreactor
and the slurry reactor should be maintained at
temperatures in the range 5° to 25°C.
In a second embodiment, the substrate from the slurry
reactor is pumped first through a filter to separate the
solubilized nitrile from the remaining slurry suspension,
and then into reactive contact with free cells of the
microorganisms or enzymes having high nitrile hydratase
activity in a controlled temperature bioreactor. The
free cells hydrolyze the nitrile to form the corresponding
nitrile mono-, di- or tri- amide product. The mixture of
free cells and product within the bioreactor is then
pumped through an ultrifilter wherein the cells are
separated from the liquid product, the free cells are
returned to the bioreactor, and the liquid product is
returned to the slurry reactor where it aids in
solubilizing the nitrile substrate. The reactor systems
should be maintained at a temperature in the range 5° to
25°C.
Without further elaboration, it is believed that one
skilled in the art, using the preceding detailed
description can utilize the present invention to its
fullest extent. The principles, preferred embodiments and
modes of operation of the present invention have been
described in the foregoing specification. The following
examples are provided to illustrate the invention in
accordance with the principles of this invention, but are
not to be construed as limiting the invention in any way
except as indicated in the appended claims. Variations
and changes may be made by those skilled in the art
without departing from the spirit of the invention. All
parts and percentages are by weight unless otherwise
indicated.
Example 1
A substrate slurry solution was prepared by
suspending and saturating 40 grams of
nitrilotriacetonitrile in 2 liters of pH 7 phosphate
buffer. The substrate slurry solution was mixed in a
slurry reactor and filtered through a fine glass frit
filter and continuously circulated to a bioreactor
containing 63 g, cell pasta of Brevibacterium A4 in 280
ml. of pH 7 phosphate buffer. The liquid (product) and
7 _
cells were simultaneously separated using an ultrifilter
(100,000 M.W. cutoff); the product effluent returned to
the slurry reactor, arid the cells returned to the
bioreactor. The temperature of the reactors was
maintained at 10°C. After 24 hours of continuous
operation the nitrile was converted to pure NTA- monoamide
dinitrile, aftex 96 hours there was 100$ conversion to NTA
diamide mononitrile and NTA triamide in aqueous ssolution.
The products were recovered from the aqueous solution by
freeze drying and were identified by HPLC, HPLC/MS, and
NMR as 90$ NTA-diamide and 10$ NTA-triamide.
Example 2
100 grams of nitrilotriacetonitrile were suspended
and saturated in 3 liters of pH 7 phosphate buffer. This
substrate solution was filtered and pumped continuously to
a reactor containing 70 grams of cell pasta of
Corynebacterium N771. The reactor was maintained at 10°C
at 1 atm. for about 72 hours. The cells were separated
from the aqueous solution using an ultrifilter as in
Example 1. The products were identified by HPLC and mass
spectrometer as 90~ nitrilodiacetamide and 10~
nitrilotriacetamide.
Example 3
A substrate slurry solution was prepared by
suspending and saturating 25 grams of
nitrilotriacetonitrile in 2 liters of pH 7 phosphate
buffer. This substrate slurry solution was circulated in
a slurry reactor, filtered and pumped through a column
containing immobilized cells having nitrile hydratase
following method: 60 gr of cells of Brevibacterium R-312
were mixed with 240 ml of deionized water. 20 gr of
g -
sodium Alginate (I6 400, Sinoport International) were
mixed with 1200 ml of deionized water until the alginate
was fully dissolved. The mixture containing the cells was
mixed with the alginate under icy conditions. This
mixture was then placed in a grilling column which was
equipped at the bottom with syringe EFD tips 5114B (2 mm),
and the mixture was dispersed into 1 liter of 14 g/1 of
CaCl solution. 625 gr of immobilized grills were.,
0
recovered. The grills were placed in a column and the
solution from the nitrilotriacetonitrile slurry reactor
was circulated through the column under the same
conditions as described in Example 1. After 72 hours of
operation we recovered 85~ nitrilodiacetamide and 150
nitrilotriacetamide in an aqueous solution.
Example 4
This example illustrates that nitrilotrinitriles can
be hydrolyzed to corresponding nitrile mono-, di- or tri-
amides in a continuous process. Three batches of
substrate were prepared by suspending and saturating 50,
50 and 75 grams of nitrilotriacetonitrile in 2 liters of
pH 7 phosphate buffer, respectively. A column containing
immobilized cells was prepared as described in Example 3.
The first substrate suspension was continuously circulated
through the column at 10°C until 100 conversion was
obtained. The second batch of 50 grams was added to the
slurry reactor with continuous circulation through the
column until conversion was complete. The third batch of
75 grams was added and circulated as before. The reactors
were in continuous operation for 14 days, The three
batches of nitrilotriacetonitrile were hydrolyzed to form
a product mixture comprising 85~ NTA-diamide and 150
NTA-triamide (as identified by HPLC). The product mixture
_ 5 _
of NT~-diamide and NTA-triamide was recovered by
recrystallization and yielded a total of 168 grams of
dried crystals.
Example 5
10 grams of the mixed product obtained from Example 4
were suspended in one liter of water and loaded on an ion
exchange column containing a weak anion exchange xesin
s
such as Dowex AD-8 using 1M H2S04 with a flow rate of 15
ml/min. The column was then eluted using 10o ammonium
hydroxide. The eluate was collected and evaporated to
produce 8 grams of purl nitrilodiacetamide crystals.