Note: Descriptions are shown in the official language in which they were submitted.
2~1222
Novel benzimidazole and azab~nzimi~azole ~riYa~iY~e~
their methods of preparation! ~n~bc~i~.l U~ e:~ ~d
~}~r~ aL QQmPo~i~ ir ~9h t~y~r~
which are useful e~pecially for the treatment of cardioL-
05 vascular diseases and duodenal ulcers
The present invention relates, by way of novelproducts, to the benzimidazole and azabenzimidazole deri-
- vatives of general formula (I) below and their tautomeric forms, and if appropriate to their acid addition salts,
in particular the addition salts with non-toxic or
pharmacologically acceptable acids. The compounds in
~uestion have a very valuable pharmacological profile
~`~ insofar as they possess cardiotonic, vasodilative, anti-
hyperten~ive and platelet aggregation inhibiting proper-
~; ties. They are therefore indicated especially for the
treatment of cardiovascular diseases and in particular
for the treatment of cardiac insufficiency.
Furthermore, some derivatives of the invention
pos~ess ulcer inhibiting properties. They are thereforealso claimed for this activity and their use in the
I treatment of ga~troduodenal ulcers.
¦ The present invention further relate~ to the
method of preparing ~aid product~ and to their appli-
~¦ 25 cations in therapy. It further relates to the novel
;~ intermediates which enable said products to be ~ynthe-
sized.
US patent document 3 336 192 (British patent
~;d 1,085,634) has disclo~ed benzazole derivatives with
~0 anthelmintic activity which are substituted in the 5(6)-
position and possibly in the 2-position. The sub-
stituents in the 5-position or 6-position are generally
heteroaromatic radicals, in particular imidazolyl,
thiazolyl, isothiazolyl, 1,2,5-thiadiazolyl, pyrryl,
. . .~
1 35 furyl and thienyl The substituent in the 2-position i~
.`.~ :-
.. ~ - :.
. 0,.~,.. ~.,". .... , . : ., :
2~ ~222
; 2 -
a five-membered or six-membered heteroaromatic ring con-
taining from 1 to 3 heteroatoms selected from oxygen,
sulfur and/or nitrogen. The pyrryl or pyridyl radical is
mentioned among the nitrogen-containing radicals, the
05 furyl radical i~ mentioned among the oxygen-containing
radical~ and the thienyl radical i3 mentioned among the
sulfur-containing radicals. The preferred ~ubstituents
-- are the thiazolyl, isothiazolyl and thiadiazolyl groups.
~ The combination~ of Rubstituents which are
;- 10 actually described are mainly thiazole~i~othiazole com-
binations.
'- The compounds which are most similar to the
invention are 2-(thiazol-4'-yl)-5(6)-(imidazol-1'-yl)-
benzimidazole and 1-acetyl-2-(pyrid-3'-yl)-5-(1,2,5-
thiadiazol-4'-yl)benzimidazole.
The most similar compounds of the invention
differ radicallY from those described in said document in
that they necessarily have a 2-phenyl or 2-pyridyl sub-
; stituent combined with an imidazolyl in the 5-position or
6-position, whereas the abovementioned document does not
specify thi~ particular combination; on the contrary, it
suggest~ thiazolyl, isothiazolyl or thiadiazolyl as pre-
~ ferred combinations.
: Now, it ha~ been discovered by the inventors that
the precise combination 2-phenyl- or 2-pyridyl-5(6)-
imidazole, inter alia, produces an unexpected pharma-
` ceutical activity, especially for the treatment of car-
diovascular diseases and duodenal ulcer~.
Furthermore, it has also been possible to observe
that substitution of the benzimidazole by a lower alkyl
group, in particular a methyl, in the 6-position or 7-
- po~ition leads to derivatives which are particularly
`` active when admini~tered orally or parenterally.
; Thus the invention provide~ novel benzimidazole
35 and azabenzimidazole derivatives of general formula (I~: -
' -'. ~:
~. ~
2~1 1222
: - 3 -
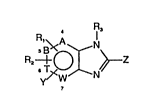
06 ~ X N
,
FORMULA (I)
. :
in which:
- Y is located in the 4-, 5-, 6- or 7-position of the
: benzimidazole or azabenzimidazole ring and is an imid-
azole, benzimidazole, triazole or imidazothiazole deri-
vative which is unsubstituted or substituted by groups
such as halogen, COR6, OR~, SR6, COOR6 (R6 being a
; 15 hydrogen atom or a lower alkyl radical) or a lower alkyl
~: radical which is unsubstituted or substituted by halogen,
: OR6, SR6, COOR6, NHR6, NHCOR6 or COR6 groups, R6 being as
.; defined above;
. - Z can be a phenyl or pyridyl ring directly bonded to
' 20 the benzimidazole or azabenzimidazole or indirectly
. :
bonded via a nitrogen atom which i~ unsub~ti.tuted or sub-
stituted by a lower alkyl radical, in particular aniline
~ or aminopyridine, the phenyl or pyridyl ring being unsub-
stituted or substituted in particular by one or more
. 25 lower alkyl radicals, one or more halogen atoms, one or
more OR~, SR6, SOR6, NHCOR6 or NHR6 groups (R6 bein~ as
` defined above) or a 5-membered to 10-membered heterocycle
containing 1 to 3 heteroelements selected from nitrogen,
oxygen and sulfur, or Z can also be an OH, SH, SR6 or :~
SOR6 group, R6 being a lower alkyl, a Cz-C8 alkenyl, in
particular an allyl, or a C2-C8 alkynyl, in particular a
propargyl;
- Rl and R~ independently of one anot.her are the hydrogen
.~ atom, a halogen atom, a CF3, NOz, NHR~, NHCOR4, OR4 or
~i 35 SR4 group (R4 being a hydrogen atom or a lower alkyl
. . ~ - - .
: .
.,-.i .
.. ;. . .
-~ ~
2~1~ 22~
radical) or a lower alkyl and can be located in the 4-,
5-, 6- or 7-po~ition of the benzimidazole or azabenz-
imidazole;
- Rs i~ the hydrogen atom and can be a lower alkyl
05 radical or a benzyl group if Z is an OH, SH, SR6 or SOR6
group; and
- A, B, T and W can be a carbon atom or a heteroelement
such as nitrogen.
In the description and the claims, the term
"lower alkyl radical" will be understood as meaning a
linear or branched chain containing 1 to 6 carbon atoms
or a Cs-C6 ring.
In the description and the claims, the term
"halo~en" will be understood as meaning a chlorine,
bromine, iodine or fluorine atom, preferably a chlorine
atom.
The total number of carbon atoms in the group Z
comprising the phenyl or pyridyl ring can be between 5
and 20.
In one particular variant, Y is imidazole.
In another variant, Z is pyridine, in particular
the pyrid-4-yl radical.
' In yet another variant, Z is aminopyridine and in
particular 4-aminopyridine.
In yet another variant of the invention, Z i3 the
OH group.
In yet another variant, Z i~ the SH group.
'' In one particular embodiment, Rl is the hydrogen
atom.
In another particular embodiment, R1 is a lower ~-~
alkyl radical, in particular a methyl, preferably in the
6-position or 7-position.
¦ In yet another variant, R1 is the chlorine atom.
In one variant, R1 is the trifluoromethyl group.
In one particular embodiment, R2 is the hydrogen
,~i ;~ ;
2~3L12"2
- 5 -
atom
In another particular embodiment, R2 is a lower
alkyl radical and in particular a methyl, preferably in
the 7-position or 6-position.
05 In yet another variant, R3 is the i~opropyl
radical if Z is the OH or SH group.
In one variant, W is the nitrogen atom.
In another variant, B and W are nitrogen atom~.
~ In another variant, Rl i~ a lower alkyl radical,
; 10 in particular methyl, in the 7-po~ition, Rz i~ the hydro-
gen atom, Y is an imidazolyl radical and Z is a pyrid-4-
yl radical.
;Particularly preferred compounds of the invention
j are those selected from the products of the formulae
N
: H H3C H
~,
N~; . .:
6_ ~N~ ~N~H~
CH3 H
::
~N :~:
~N N/~N/~H
H ~ :
~.
2~1~ 22~
-- 6 --
1~ ~N 6N~N~
~ ~N
: 15 According to the invention, the derivatives of
formula (I) in which Y i~ an imidazole, triazole or ben-
zimidazole derivative can be prepared according to the
~ following ~chemes:
:, The derivatives of formula (II): :
. RJ
~W)~ NH
11 FORMULA (II)
.1 ', ','~
in which A, B, T, W, Rl, Rz, R3 and Y are a~ defined
above, are reacted with urea, thiourea or carbon diqul-
3Q fide in the ca~e of the derivatives in which Z is the OH
l or SH group, in an organic solvent or without a ~olvent,
:3 at a temperature in the range from 40 to 200C.
i~ To obtain the compound~ in which Z is the S-R~
`' group, R~ being a~ defined above, the derivative in which
Z i~ the SH group will be reacted with a halogen compound
,
.'. . ~:
, ~ '
2Q1 ~?,22
-- 7 --
of the formula X-R6, X being a halogen and R~ being a~
defined above, in an organic ~olvent ~uch a~ an alcohol
or dimethylformamide, in a ba~ic medium, at a temperature
in the range from 20 to 150C.
05 The derivative~ in which Z i~ ~SORc, R~ being as
defined above, will be obtained by oxidizing the deriva-
tive~ in which 2 iB SR6 with a peracid, for example
; metachloroperbenzoic acid, in an organic solvent ~uch a~chloroform or methylene chloride, at temperature~ in the
range from -5 to 2~C.
In the case where Z i~ a phenyl or pyridyl rin8,
it will be possible:
either to heat the compounds of formula (II) in which
R3 i~ the hydrogen atom, in an organic solvent auch a~ an
alcohol, with an aldehyde of the formula Z-CHO, Z being a
phenyl or pyridyl ring, and subject the re~ulting deri-
vative to oxidation by a heat treatment in the tempera-
ture range from 150 to 200~C in nitrobenzene,
or to form the benzimidazole by the cla~ical methods
with the acid chloride~ Z-COCl, the acid~ Z-COOH or the
nitriles Z-CN, in which Z i~ a phenyl or pyridyl ring,
~ according to the following reference: J.B. WRIGHT, Chem.
i Rev. 1951 J ~ 397.
In the case where Z i~ an aniline or an amino-
~ 25 pyridine, the derivative~ of formula (II) in which R3 i~
;j the hydrogen atom will be reacted with compound~ of the ;
formula
S ~ SCH
Z -NH-C-SCH3, Z -N=C \ or Z'-N=C=S,
SCH3
in which Z i~ a phenyl or pyridyl ring, according to the
method de~cribed in the following reference~: A. MOHSEN -~
et al., Synthe~i~ 1977, 864j F. MERCHAN et al., Synthe~
'.1 ...
.~ -
},
~:.. . . . . .
2~ 222
1982, 482.
To have an -SOCH3 group on the phenyl or pyridyl
ring, the derivative substituted by an -SCH3 group will
be oxidized, as before, with a peracid, for example meta-
05 chloroperbenzoic acid, in a ~olvent such a~ chloroform or
methylene chloride, at a temperature in the range from
~ -50C to 50C.
~ The compounds of formula (II) may be obtained by
hydrogenating nitroamine~ of formula (III):
R,
. R,E~,<A NH
R2 T~
y W 2 NO
FORMULA (III)
in which A, B, T, W, R1, R2, R3 and Y are a3 defined -
s above, in the presence of a catalyst such a~ Raney : -
., 20 nickel, or by reducing them with Fe/HCl in known manner. :
1 The compounds of formula (III) can be obtained by simply
j deacetylating compound3 of formula (IV):
'' ~'
0 ~ CH~
R2~ RJ ;
W N2
": :
, 30 FORMULA (IV)
,1
The compound~ of formulae (III) and (IV) in which
Y i~ an imidazole, triazole or benzimidazole derivative
~- can be obtained by reacting a derivative of imidazole (or
its sodium salt), a derivative of triazole (or its ~odium
. .1
,
: ~7 . . .. : ' . . ' '
2~ ~222
- salt), a derivative of benzimidazole (or it~ sodium salt)
~; or the sodium ~alt of imidazolone, in an organic Qolvent
such as dimethYlformamide, or direct without a ~olvent,
: by heating at a temperature in the range from 80 to
05 200C, with derivatives of formula (V) or (VI):
R, ~
', FORMULA (V) ~ -
. O ~ CHa
:, y , :
T~
FORMULA (VI) ~ :
. 20 ~:
,..
in which A~ B, T, W, Rl, R2 and R3 are as defined above
and X i~ a halogen atom.
j The compounds of formula (V) may be obtained:
- either by deacetylating compounds of formula (VI),
~ or by reacting amine~ of the formula R3NH2, R3 being as
. defined above, with derivative~ of formula (VII):
~ :30 ~NO,
.. ~ . , :
. FORMULA (VII)
,. ' ~
in which A, B, T, W, Rl and R2 are as defined above and X
.~
2i~222
-- 10 --
and X~ are a halogen atom, without a solvent, in an
organic solvent such as alcohol or in water, at atmos-
pheric pressure or under positive pressure, at a tempera-
ture in the range from 25 to 200C.
05 In the case where R3 is a hydrogen, the compounds
of formula (V) can also be obtained either by a Hofmann
: degradation of the amides [E.S. Wallis and J.F. Lane,
Organic Reactions 3, 267 (1949)] or by partial reduction
of the corresponding dinitro compounds (B.M. Wepster and
P.E. Verkade; Recueil des travaux chimiques des Pays Bas
' 1949 vol. 68 p. 105).
The compounds of formula (VI) may be obtained by : :
the nitration, by methods known per se, of corresponding
acetamide derivatives which are commercially available or ~:
which can themselve~ be prepared from commercially avail~
able derivatives of amino compounds by reaction with
acetic anhydride or acetyl chloride by methods known per : ::
se.
The compounds of formula (VII) may be obtained~
- by the simple nitration of commercially available deri~
., vatives by methods known per se, or ~::
j - from the amines of formula (VIII): : -:
,
~ 25 R,--~ O X
``. R
.~ . :
~ FORMULA (VIII) -~
: 30
in which A, B, T, W, R1 and R2 are as defined above, X :
and X being a halogen atom,
. either by oxidation with an oxidizing agent such
,~ as hydrogen peroxide,
! 35 or via diazonium fluoroborates by methods des-
.,. ~ :.
-.~
. .: : . . - . . .. . .. : . : . : , . .
2 ~ 2 ~
"
11 --
cribed per ~e (Vogel A.I., Third Edition p. 5~5).
The compounds (VIII) can be obtained by halo-
genating the aromatic amines by methods known per se,
starting from commercially available compounds or com-
05 pounds described in the literature. ::
The compound~ of formula (I) in which Y is an
~ imidazothiazole derivative can be obtained~
-, a) by the method~ described above, from derivatives of
formula (II) in which A, T, R1, Ra and R3 are as defined
above and Y is an imidazothiazole derivative.
~t, In this case the compounds of formula (II) can be :-
. obtained from derivatives of formula (IX): :~
"' ~-
Y ~ A ~ N02
~X ~:
R, 3 R2
. ~ .
FORMULA (IX) :
'l~ 20
:, in which Y is an imidazothiazole derivative, R1 and R~
.l are a hydrogen atom or a lower alkyl radical, X is a
halogen and A and T are carbon atom~.
l These compounds of formula (IX) can be obtained
j 25 by the intramolecular cyclization of products of formula
; ~ ( X):
~`I O
` 30 SR, ~ N02
.l R, R2
. .
~ FORMULA (X)
., . ~
, 35 in which R1, Rz and Rs are hydrogen atoms or lower alkyl
i
.':
2~ 3 22~
- 12 -
radicals, X is a halogen atom and R7 i~ an imidazole
derivative, in an organic solvent, for example toluene,
xylene, DMF or N-methylpyrrolidone, in the presence or
~ absence of paratoluenesulfonic acid, or in PPA, at tem-
:l, 05 peratures in the range from 25 to 180C.
ç The compound~ of formula (X) are obtained from
halogenoalkyl halogenonitrophenyl ketones which are un-
substituted or substituted on the phenyl and which are
~l prepared by method~ described in the literature (Beil. 7J
`~ 10 285), by reaction with mercaptoimidazole compounds by
., known methods (Beil. 23, 353). ~:
b) by the intramolecular cyclization, under conditions
described above (for example in PPA), of compounds of
formula (XI)~
:
.
R,~N~
:~ FORMULA (XI)
in which R1, Rz, R3, R7 and Rs are as defined above.
These compound~ of formula (XI) are prepared by
reacting 2-mercaptoimidazole derivative~ with compounds
of formula (XII):
R,~N~
y 35 FORMULA (XII)
;'. ~
~, .
. :
., .
2 2 2
.
.
- 13 -
. in which R1, Rz, Rs and Rs are as defined above and X is a halogen atom, by known methods mentioned above.
The compounds of formula (XII) are obtained by a
- classical Friedel-Crafts reaction between unsubstituted ~-
05 or variou~ly substituted benzimidazoles and halogenated
~` acid chlorides:
O
' R"--I~
X Ci
Rs and X being as defined above, in the presence of Lewis
acids, in solvents such as carbon disulfide or methylene
; chloride.
Acid addition salts of some of the compounds of
' formula (I) can be obtained by reacting these compounds
with a mineral or organic acid by a method known per se.
The addition salts with non-toxic, pharmacologically
' acceptable acids will advantageously be used. -
`~ 20 Hydrochloric, hydrobromic, sulfuric, phosphoric,
,i toluene-4-sulfonic, methanesulfonic, cyclohexylsulfamic,
1 oxalic, succinic, formic, fumaric, maleic, citric, a~par-
'~! tic, cinnamic, lactic, glutamic, N-acetylaspartic, N-
`~ acetylglutamic, ascorbic, malic, benzoic, nicotinic and
acetic acids may be mentioned among the acids which can
be used for this purpo~e.
.~ ~
The novel compounds according to the invention
possess remarkable pharmacological properties and can be
used in therapy in the treatment of cardiovascular
; 30 diseases and in particular in the treatment of cardiac
insufficiency, since they possess cardiotonic, va~odi-
lative, antihypertensive and platelet aggregation in-
hibiting properties.
Furthermore, as pointed out earlier, the com-
pounds of the invention can have ulcer and secretion
i`l
.
..,
, .
: :
2~ ~ 222
- 14 -
.
inhibiting properties and may therefore be u~eful in the
treatment of gastroduodenal ulcers.
Thus the invention also covers a pharmaceutical
'' compo~ition which comprises a pharmaceutically effective
'!, 05 amount of at least one compound of formula (I) as defined
above, as well as its addition salts with pharmacologi-
cally acceptable acids, which may or may not be incor-
~ porated in a pharmaceutically acceptable excipient,
`3 vehicle or carrier
~! 10 In one variant, this pharmaceutical composition
,~
has cardiovascular, cardiotonic, vasodilative, antihyper-
tensive and platelet aggregation inhibiting activity
In another variant, this pharmaceutical compo-
sition has secretion and ulcer inhibiting activity.
In human and animal therapy, the compounds of
formula (I) and their addition salts with non-toxic acids
can be administered by themselves or in association with
a physiologically acceptable excipient, in any form, in
particular orally in the form of gelatin capsules and
~ 20 tablets or parenterally in the form of an injectable
;~l solution.
~i As will be clearly evident from the pharmaco-
-l logical tests gi~en at the end of the description, the
compounds according to the invention can be administered
25 in human therapy, for the indications mentioned above,
~ orally in the form of tablets or gelatin capsule~ con-
`~ taining from 150 to 500 mg of active ingredient, or
parenterally in the form of injectable preparations con-
j taining from 30 to 200 mg of active ingredient, in one or
i 30 two dosage units per day for an adult with an average
`~ weight of 60 to 70 kg.
I In animal therapy, the daily dose which càn be
-~ used should normally be between 3 and 30 mg per kg~
~ The invention further relates to a method of pre-
J 35 paring a pharmaceutical composition, which comprises in-
.. ~
`.c
~i
- 2011 ~22
- 15 -
corporating a therapeutically effective amount of at
least one compound of formula (I) a~ defined above, a~
well a~ it~ addition ~alt~ with pharmacologically accep-
table acid~ if appropriate, into a pharmaceutically
05 acceptable excipient, vehicle or carrier.
In one variant, thi~ method of preparation
involves preparing a pharmaceutical composition with
~' cardiovascular, cardiotonic, va~odilative, antihyper-
tensive ard platelet aggregation inhibiting activity or
secretion or ulcer inhibiting activity.
In another variant of this method, the pharma-
ceutical compo~ition is prepared in the form of gelatin
capsules or tablets which can be administered orally, or
in the form of ~olutions which can be injected paren-
terally.
These tabletæ or gelatin capsules can advan-
tageously contain from 150 to 500 mg of active ingredient
and the injectable preparations can contain from 30 to
200 mg of active ingredient.
According to yet another feature, the invention
provides a method of therapeutic treatment, which com-
j prises administering to a mamm~l, includin~ an animal or
a human, a therapeutically effective amount of at least
one compound of formula (I) as defined above, or one of
its addition ~alts with pharmacologically acceptable
acid~ if appropriate, which may or may not be incor-
porated in a pharmaceutically acceptable excipient,
, vehicle or carrier.
In one variant of this method of treatment, in
;~ 30 human therapy, a daily dose of between 150 and 1000 m8 is
administered in the form of tablets or gelatin cap~ules,
or a daily dose of 30 to 400 mg is adminiqtered paren-
terally, for a human weighing between 60 and 70 kg. ~ -
In another variant, in animal therapy, a daily
35 dose of between 3 and 30 mg per kg is administered. ~-~
. ! ' :
'
:: ~
.':
2 ~ 2
- 16 -
:; :
-~ In yet another variant of the invention, cardio-
vascular disease, cardiac in~ufficiency or hyperten~ion
i~ treated. or a platelet a~gregation inhibitin~ treat-
- ment, secretion inhibi,ting treatment or ulcer inhibiting
05 treatment, especially for duodenal ulcers, is effected.
Further characteristics and advantage~ of the
invention will be understood more clearly from the
following description of some Preparatory Examples, which
in no way imply a limitation but are given by way of
illustration
~, Ex~mple 1 2-Nitro-3-chloroaniline
-~ Formula (V) A = B = T = W = C, R1 = Rz = Rs = H, X =
i 15 3-Cl
e~ 11.5 cm3 of bromine are added to a solution of
i 350 cm3 of water and 33.6 g of KOH. 40.1 g of 2-nitro-3-
,i chlorobenzamide, obtained by condensing ammonia with the
corresponding acid chloride, are then added all at once.
The mixture is stirred for 45 min. The insoluble
"r` material is filtered off to give a clear solution.t The filtrate is added all at once to a solution
;i: of 44.8 g of KOH in 100 cm3 of water. The mixture i~
heated at 70-75~C for 45 min and then brought back to
: room temperature. The brown precipitate is filtered off
and washed with water and then with pentane. It is dried
and purified by chromatography on silica gel to give 10.5
g of 2-nitro-3-chloroaniline
Melting point: llO~C
.
,~.
~ x~mPle 2 2,4-Dichloro-6-ethylaniline
.~.
. Formula (VIII) A = B = T = W = C, R1 = H, R2 = 6-Et, X =
4 Cl, X = 2 Cl
,~,..~
..~
,~... ... . . ~ :
2 ~ 2 2
17
A solution of 483 ml of 95% ethanol, 483 ml of
concentrated hydrochloric acid and 81 g of 2,4-dichloro-
6-ethylacetanilide (melting point: 146-148C), obtained
from 2-ethylaniline by the method described in J. Am.
05 Chem. Soc. (1950) p. 2454-7, is refluxed for 14 h. It is
concentrated and then neutralized with a solution of
sodium hydroxide. The mixture is extracted with ethyl
acetate and the organic phase~ are washed with water.
After evaporation of the solvent, the brown oil obtained
is purified by distillation to give 60 g of 2,4-dichloro-
6-ethylaniline.
Boiling point: 135-145C under 20 mm of mercury.
; Example 3 3,5-Dichloro-2-nitrotoluene
Formula (VII) A = B = T = W = C, R1 = H, R2 = 6-CH3, X =
j 4-Cl, X' = 2-Cl
21.6 g of 2,4-dichloro-6-methylaniline ~J. Am.
Chem. Soc. (1950) p. 2454-7] are placed in 32 ml of con-
centrated HCl and 32 ml of water. The mixture is stirred
at 50-60C for 30 min and then cooled to 5C and a
solution of 9 g of NaNOz in 20 cm3 of water is added
dropwise in such a way that the temperature is between 5
and 7C. The mixture i8 then stirred for 1 h at 5C
before a solution of 20 of NaBF4 in 40 cm3 of water i3
added dropwise at 5C. The white precipitate formed i~
filtered off and washed with cold water and then with
isopropanol to give 28.5 g of the corresponding diazonium
fluoroborate.
Melting point ~ 200C.
The salt prepared in this way is then added
slowly to 300 cm3 of water containing 100 g of NaN02 and
20 g of copper powder freshly prepared from CuS04/Zn by -
, 35 the classical method de~cribed in the literature. The
,.
.,
.. , . . ::
2 ~ 2 2
~i - 18 -
.~ .
copper is filtered off when the evolution of nitrogen
cea3es. The filtrate i~ extracted with ether and the
extract is dried and concentrated under vacuum to give a
red oil, which is purified by di~tillation under nitrogen
05 to give 14 g of 3,5-dichloro-2-nitrotoluene.
Boiling point: 142-150C under 24 mm Hg.
` Melting point: 68C.
i
~` ~mPle 4 2,4-Dichloro-6-ethylnitrobenzene
Formula (VII) A = B = T = W = C, R1 = H, R2 = 6-ethyl,
X = 4-Cl, X = 2-Cl
112 g of 2,4-dichloro-6-ethylaniline, prepared
according to Example 2, are treated a~ in Example 3 to
' give 35 g of 2,4-dichloro-6-ethylnitrobenzene.
J. Boiling point: 135-140C under 20 mm of mercury.
rJ ~ ~
Y Examp]c .~ 2,4-Dichloro-5,6-dimethylnitrobenzene
'? 20
Formula (VII) A = B = T = W = C, R1 = 5-CH3, Ra = 6-CH3,
X = 4-Cl, X = 2-Cl
~i
113.2 g of 2,4-dichloro-5,6-dimethylaniline, pre-
25 pared according to J. Chem. Soc. (1934) p. 283-7, are
treated a~ in Example 3 to give 46 g of 2,4-dichloro-5,6-
dimethylnitrobenzene after di~tillation.
Boiling point: 138-152C under 6 mm of mercury.
`~ Melting point: 72C. -
Ex~ple 6 2,4,5-Trichloro-6-methylnitrobenzene
Formula (VII) A = B = T = W = C, Rl - 5-Cl, R2 = 6-CH3,
X = 4-C1, X = 2-Cl
~ 35
:'
,.,.. ~ . : :
.: ~: - : -:
: ~ ~ , : . . . , :
~ 2~ ~222
- 19 -
; 149.8 g of 2,4,5-trichloro-6-methylaniline, pre-
pared according to J. Org. Chem. (1951) p. 328-33, are
treated according to Example 3 to give 81.2 g of 2,4,5-
trichloro-6-methylnitrobenzene after distillation under
05 nitrogen.
- Boiling point: 124-132C under 6 mm of mercury.
Melting point: 66C.
Example 7 2,4-Dichloro-5-methylnitrobenzene
` Formula (VII) A = B = T = W = C, R1 = 5-CH3, X = 4-Cl,
X' = 2-C1
.;,
8.2 g of sodium nitrite are dissolved in 90 ml of
,`~ 15 sulfuric acid. A ~olution of 20 g of 5-chloro-4-methyl-
2-nitroaniline in 220 ml of acetic acid is introduced
into the above mixture without the temperature exceeding
40C. The resulting mixture is heated for a further 30
min at 40C. It is then added ~lowly to a cold solution
(5C) of 23.6 g of cuprous chloride in 220 ml of hydro-
!i'~'~i chloric acid. The resulting mixture is heated in a water
bath until there is no further evolution of gas. 600 ml
of water are then added and the mixture is left cold
overnight. The solid formed is filtered off, wa~hed with
~i ~5 water and then taken up with ether. The organic phase
obtained is washed with a basic aqueou~ ~olution, dried
and concentrated to give 17.9 g of 2,4-dichloro-5-methyl-
nitrobenzene in the form of an oil, which crystallizes. ~ d
Melting point: 50C.
~xa~ple ~ N-Isopropyl-5-chloro-4-methyl-2-nitroaniline
`i Formula (V) A = B = T = W = C, Rl = 4-CH3, Rz = H, R~
~1 iPr, X = 5-Cl
"`,1 ' .:
i~ 35
,, : .
:,
~., - - :~
2~ 22~
- 20 -
17.9 g of 2,4-dichloro-5-methylnitrobenzene, 100
ml of ethanol and 40 ml of isopropylamine are placed in
an autoclave. The mixture i~ heated at 100C for 24 h
under pres~ure. The ethanol is evaporated off After
05 purification on silica gel, 11 g of N-isopropyl-5-chloro-
4-methyl-2-nitroaniline are obtained in the form of an
orange oil, which is used in the crude state for the next
step.
.
Example 9 N-Methyl-5-chloro-2-nitroaniline
.
Formula (V) A = B = T = W = C, R1 = R2 = H, R3 = CH3,
X = 5-Cl
300 ml of an 8 M solution of monomethylamine in
ethanol are added to a cooled solution of 115.2 g of 2,4-
,,.
~l dichloronitrobenzene in 400 ml of ethanol. The mixture
,i1 i~ heated at 50C for 10 h. It is cooled and the solid
obtained i5 filtered off, washed with water and then with
20 isopropanol and dried to give 106.7 g of N-methyl-5-
. chloro-2-nitroaniline.
j Melting point: 106-107C.
E~m~le 10 N-Isopropyl-~-fluoro-2-nitroaniline
' Formula (V) A = B = T = W = C, Rl = R2 = H, R3 = iPr,
:, X = 5-F
.~
25 g of 2,4-difluoronitrobenzene are dissolved in
30 100 ml of ethanol. 67 ml of isopropylamine are then
added slowly because the reaction is very exothermic,
after which the solution is refluxed for 2 h. The medium
is concentrated and the residue is taken up with water
and extracted with chloroform. The organic pha~e is
dried and concentrated and the residue is chromatographed
.:
~1
:i :
20 ~ .L~22
- 21 -
'
on silica gel to give 15 g of N-isopropyl-5-fluoro-2-
~; nitroaniline.
Melting point: 72C.
~, 05 E~gmDlQ_11 N-Benzyl-5-fluoro-2-nitroaniline
(
' Formula (V) A = B = T = W = C, R1 = R2 = H? R3 = CHz0,
X = 5-F
! . j .
N-Benzyl-5-fluoro-2-nitroaniline is obtained by
r~l following the procedure described in Example 10, using
benzylamine and 2,4-difluoronitrobenzene as reactants.
Melting point: 100C.
. ~ .
`~ 15 E~a=}l=_12 ~-Chloro-4-methyl-2-nitroacetanilide
..,
Formula (VI) A = B = T = W = C, Rl = 4-CHe, R~ = R3 = H,
X = 5-Cl
A mixture of 128 ml of nitric acid and 175 ml of
acetic acid is added dropwise to a ,olution, cooled to
-5C, of 357 g of 3-chloro-4-methylacetanilide in 700 ml
of acetic anhydride and 350 ml of acetic acid. The tem-
perature is kept between -5 and 0C. The resulting mix-
~, 25 ture is stirred for a further 2 h at 0C and the solution
;i~; is then poured into water. The precipitate is tritura-
ted, filtered off and washed with water and then with
isopropanol. It is recrystallized from ethanol to give
254.~ g of 5-chloro-4-methyl-2-nitroacetanilide.
Melting point: 114C.
`~ E~mPle 13 N-Methyl-5-chloro-4-methyl-2-nitroacetanilide
... ; ~ ~:
~' Formula (VI) A = B = T = W = C, Rl = 4-CH3, Rz = H, R3 =
CH3, X = 5-Cl
, . . .
,,,
.^'~, .
~.' :~:-' . ~ : ~ . ' :. . , : : . :
~ 2~11222
.
- 22 -
A solution of 37.2 g of 5-chloro-4-methyl-2-
; nitroacetanilide, prepared according to Example 12, in
200 ml of methyl ethyl ketone i~ placed in a three-necked
flask equipped with a stirrer, a condenser and a dropping
05 funnel. 27 g of KzC03 and 30.6 ml of methyl iodide are
then added. The suspension is refluxed for 24 h and then
`~ for a further 20 h after the addition of another 30.6 ml
of methyl iodide. After cooling, the insoluble material
is filtered off and the filtrate is concentrated The
residue is chromatographed on silica gel to give 27 7 g
of N-methyl-5-chloro-4-methyl-2-nitroacetanilide in the
form of a yellow oil, which is used in the crude ~tate
for the next step? and 10 g of starting material
.
~j 15 Example 14 5-Chloro-4-methyl-2-nitroaniline
Formula (V) A = B = T = W = C, R1 = 4-CH~, R2 = R3 = H,
X = 5-Cl
26 g of sodium are dissolved in 970 ml of
methanol 254 5 g of 5-chloro-4-methyl-2-nitroacetani-
lide, prepared according to Example 12, are introduced
" and the mixture is stirred for 3 h at room temperature.
`~' It is poured into water. The solid obtained is tritu-
rated, filtered off and washed with water and then with
isopropanol to give 200.5 g of 5-chloro-4-methyl-2-nitro-
' aniline.
Melting point: 166C.
Examel~ 15 ~-Chloro-6-methyl-2-nitroaniline
Formula (V) A = B = T = W - C, R1 = 6-CH3, Rz = R3 = H,
'~ X = 5-Cl
~ 35 A mixture containing 400 cm3 of concentrated
.... .
,
2~ 222
hydrochloric acid, 400 cm3 of 95% ethanol and 72.4 g of
5-chloro-6-methyl-2-nitroacetanilide, prepared according
to Beilstein 12 II 461, i~ refluxed for 23 h. After
coolin~, the precipitate i~ filtered off. The filtrate
05 i~ concentrated to give a ~econd precipitate. The pre-
cipitates obtained are combined and wa~hed with an alka-
, line aqueou~ ~olution to give 57.6 g of 5-chloro-6-
methyl-2-nitroaniline.
' Melting point: 153C.
1, 10
j Exa~ple 1~ 5-Chloro-3-methyl-2-nitroaniline
. ~
i Formula (V) A = B = T = W = C, R1 = 3-CH3, Rz = R3 = H,
X = 5-Cl
~ -
116.7 g of 3,5-dichloro-2-nitrotoluene, prepared ;-
g in Example 3, and 500 cm3 of 2-methoxyethanol are placed
in an autoclave. The mixture i~ cooled to -35C and 145
cm3 of li~uid ammonia are added.
The autoclave i~ heated at 150C for 48 h and
cooled. The crude compound obtained i~ purified by
chromatoEraphy on ~ilica gel to give 57.6 g of 5-chloro-
~ 3-methyl-2-nitroaniline.
:;~ Melting point: 76C.
~, The compound~ of Example~ 17 to 19 below were
;~ prepared according to Example 16: ;~
,~ ' '.
~ E~sm}l~ 1~ 5-Chloro-3-ethyl-2-nitroaniline
-j 30
Formula (V) A = B = T = W = C, Rl = 3-C2H6, R2 = R3 = H,
X = 5-Cl
: . ~.,
Oil u~ed without further purification for the
next ~tep.
`'`.~i '
,~
- 20~ ~222
- 24 -
.
Example 18 4,5-Dichloro-3-methyl-2-nitroaniline
Formula (V) A = B = T = W = C, Rl = 4-Cl, R~ = 3-CH3,
, R3 = H, X = ~-Cl
05
Melting point: 122C.
... .
~;
Example 19 ~-Chloro-2-nitro-4-trifluoromethylaniline
~10 Formula (V) A = B = T = W = C, R1 = 4-CF3, Rz = R3 = H,
n. /X = 5 Cl
1. .,
Meltin~ point: 114C.
":~
15 E~m~ls~2Q 5-Chloro-3,4-dimethyl-2-nitroaniline
'~
::
5-Chloro-3,4-dimethyl-2-nitroaniline is obtained
in the ~ame manner a~ in Example 16~ except that the
~ autoclave is heated at 200C for 48 h.
;~ 20 Melting point: 90C.
1 ~amP-le 21 5-(Imidazol-1-yl)-4-methyl-2-nitroacetanilide
¦ Formula (IV) A = B = T = W = C, R1 = 4-CH3, R2 = R3 = H,
.~ 25 Y = 5-(imidazol-1-yl)
`'';~
:-
20.6 g of 5-chloro-4-methyl-2-nitroacetanilide,
prepared in Example 12, and 9.2 g of imidazole are inti-
;~ mately mixed and heated for 2 h at 180C. After cooling,
'~ 30 the medium i~ poured into a mixture of 100 ml of chloro-
form and 100 ml of water, with ~tirring. The organic
pha~e i~ decanted and extracted with a dilute solution of
~-~hydrochloric acid. The acid pha~e i~ rendered basic with
~ ~:
1 N sodium hydroxide ~olution and extracted with chloro-
` ~'`3 35 form. The chloroform i~ dried over magne~ium ~ulfate and
" ,~ .
. ~ ,,.
. ~ .
2~11222
- 25 -
then concentrated.
This gives 10.8 g of 5-(imidazol-1-yl)-4-methyl-
2-nitroacetanilide.
Melting point: 146C.
05
3 Example 22 N-Methyl-5-~imidazol-1-yl)-4-methyl-2-nitro-
$ acetanilide
Formula (IV) A = B = T = W = C, R1 = 4-CH3, R~ = H, R3 = :-~
CH3, Y = 5-(imidazol-1-yl)
14.2 g of N-methyl-5-chloro-4-methyl-2-nitroacet-
anilide, synthesized in Example 13, are treated with 8 g
d of imidazole according to the procedure of Example 21. ~-
¦ 15 This gives 14.7 g of N-methyl-5-(imidazol-1-yl)-4-methyl-
2-nitroacetanilide in the form of an oil, which is used
in the crude state for the next step.
,.~
Exa~p~Q 23 N-Methyl-5-(2-methylimi~azol-1-yl)-4-methyl-
2-nitroacetanilide
.,~ , .
`; Formula (IV) A = B = T = W = C, Rl = 4-CH3, Ra = H, R3 =
CH3, Y = 5-(2-methylimidazol-1-yl)
By following the same procedure as in Example 21,
but replacing the imidazole with 2-methylimidazole, N-
methyl-5-(2-methylimidazol-1-yl)-4-methyl-2-nitroacet-
anilide is obtained in the form of an oil, which is used
in the crude ~tate for the next ~tep.
E~mP~e 24 N-Methyl-5-(imidazol-1-yl)-4-methyl-2-nitro-
aniline
~,
s Formula (III) A = B = T = W = C, Rl = 4-CH3, R2 = H,
~ 35 R3 = CH3, Y = 5-(imid~zol-1-yl)
~ ';
''` '
~: : .
:
~122
::;
- 26 -
., .
N-Methyl-5-(imidazol-1-yl)-4-methyl-2-nitroacet-
anilide, synthesized in Example 22, is treated according
to Example 14 to give N-methyl-5-(imidazol-1-yl)-4-
~l methyl-2-nitroaniline.
~I 05 Melting point: 219C.
.i E~mElç 25 5-(Imidazol-1-yl)-4-methyl-2-nitroaniline
~'`3 Formula (III) A = B = T = W = C, Rl = 4-CH3, R2 = H,
R3 = H, Y = 5-(imidazol-1-yl)
A mixture containing 18.7 g of 5-chloro-4-methyl-
2-nitroaniline and 40.8 g of imidazole is heated at
180C. The reaction is followed by thin layer chromato-
; 15 graphy. When no more starting material remains, the
mixture is cooled, water is added and the solid obtained
~ is filtered off. After washing with water, the compound
-~ is taken up with ether and pentane and then dried to give
20.6 g of 5-(imidazol-1-yl)-4-methyl-2-nitroaniline.
Melting point: 220C.
~ This same product can be obtained by deacetyla-
`~ ting the compound of Example 21 by the method de~cribed
in Example 14.
The compounds of Example~ 26 to 34 below were
prepared by following the procedure of Example 2~:
.~
Example 26 5-(Imidazol-l-yl)-3-methyl-2-nitroaniline
Formula (III) A = B = T = W = C, R1 = 3-CH3, Rz = R3 =
H, Y = 5-(imidazol-1-yl)
.
Melting point: 182C. -~
.. ,~ .,~,,
2~2~2
~: -
- 27 -
' Ex~m~le 27 3-(Imidazol-l-yl)-2-nitroaniline
., ,
1 ,
` Formula (III) A = B _ T = W = C, R1 = Rz = R~ = H, Y = ~ :
3-(imidazol-1-yl) : :
05
Melting point: 1~0C
~"-
EX~m~ R 4-Chloro-5-(imidazol-1-yl)-2-nitroaniline
~,
l 10 Formula (III) A = B = T = W = C, R1 = 4-Cl, R~ = R3 = H,
i Y = 5-(imidazol-1-yl)
.~
i Melting point: 204C. ~:
;l 15 Ex~mpl ~ 29 5-(Imidazol-l-yl)-6-methyl-2-nitroaniline ~:
: ~ :
Formula (III) A = B = T = W = C, R1 = 6-CH3, Rz = R3 =
H, Y = 5-(imidazol-1-yl)
~' 20 Melting point: 157C. :
:
i.
: Ex~mple 30 3-Ethyl-5-(imidazol-1-yl)-2-nitroaniline
.
1 Formula (III) A = B = T = W = C, R~ = 3-CH2CH3, R~ =
25 R3 = H, Y = 5-(imidazol-1-yl)
'.` Melting point: 142C~
.
.~ Ex~mple 31 5-(Imidazol-l-yl)-2-nitro-4-trifluorome$h~Yl~~ 30 aniline
,~,,~
.. ~ Formula (III) A = B = T = W = C, R1 = 4-C~3~ ~æ ~ R~
i~i H, Y = 5-(imidazol-1-yl) ~ ~
~ 35 Melting point: 221C. ~.
201~22?,
- 28 -
~mPle 32 4-Chloro-S-(imidazol-1-yl)-3-methyl-2-nitro-
aniline
Formula (III) A = B = T = W = C, Rl = 4-Cl, Rz = 3-CH3,
05 R3 = H, Y = 5-(imidazol-1-yl) ~:
Melting point: 208~C.
Ex~a~ple 33 3~4-Dimethyl-5-(imidazol-1-yl)-2-nitroaniline
Formula (III) A = B = T = W = C, Rl = 4-CH3~ R2 = 3-CH3,
R3 = H, Y = 5-(imidazol-1-yl)
., '
Meltin~ point: 188C.
Example 34 N-I~opropyl-5-timidazol-1-yl)-4-methyl-2-
nitroaniline
Formula (III) A = B = T = W = C, Rl = 4-CH3, R2 = H,
R3 = iPr, Y = 5-(imidazol-1-yl)
Melting point: 159C.
The derivative~ of Example~ 35 to 41 below were
prepared by following the procedure of Example 25, except
~ that the appropriately substituted imidazole wa~ u~ed~
3 .
.
Example 35 N-Methyl-5-(2-methylimidazol-1-yl)-2-nitro~
-~ aniline
- :~
Formula (III) A = B = T = W = C, R1 = R2 = H, R3 = CH3,
Y = 5-(2-methylimidazol-1-yl) -~
. Melting point: 186C.
.~ .
.~
2~1~2~
- 29 -
ExamPle 36 5-(4-Methylimidazol-l-yl)-3-methyl-2-nitro-
aniline
Formula (III) A = B = T = W = C, R1 = 3-CH3, R~ = R3 =
05H, Y = 5-(4-methylimidazol-1-yl)
Melting point: 240~C.
E~m~l e 37 5-(2,4-Dimethylimidazol-l-yl)-3-methyl-2-
10nitroaniline
l Formula (III) A = B = T = W = C, R1 = 3-CH3, Rz = R3 =
', H, Y = 5-(2,4-dimethylimidazol-1-yl) :
.,
~}
i 15Meltin~ point: 196C.
~! Example 3~ 5-(4-Methylimidazol-1-yl)~4-methyl-2-nitro-
: aniline
r 20 Formula (III) A = B = T = W = C, Rl = 4-CH3, Rz = R3 =
,i H, Y = 5-(4-methylimidazol-1-yl)
:~ Melting point: 205C.
:,`. 25Ex.amEl~ 3~ 5-(2-Methylimidazol-l-yl)-4-methyl-2-nitro-
aniline
ii `'' :~
Formula (III) A = B = T = W = C, R1 = 4-CH3, R2 = R3 = :~
. H, Y = 5-(2-methylimidazol-1-yl)
.
Melting point: 222C.
,
,' ..
2Q1~22~
- 30 -
E~m~lQ_~Q 5-(2~4-Dimethylimidazol-1-yl)-4-methyl-2-
nitroaniline
Formula (III) A = B = T = W = C, P.1 -- 4-CH3, Rz = R3 =
05 H, Y = 5-(2,4-dimethylimidazol-1-yl)
Melting point: 248C.
:'~
Exa~ple 41 4-Chloro-5-(2-methylimidazol-1-yl)-2-nitro-
. 10 aniline
. ,
Formula (III) A = B = T = W = C, Rl = 4-Cl, R2 = R3 = H,
. Y = 5-(2-methylimidazol-1-yl)
~A
~ 15 Melting point: 170C.
:~ E~mDl~_~2 4-Chloro-5-(4-methylimidazol-l-yl)-2-nitro-
'~ aniline
20 Formula (III) A = B = T = W = C, Rl = 4-Cl, Rz = R3 = H, ;:
~ Y = ~-(4-methylimidazol-1-yl) ~:
'.'
Melting point: 220C. :
,. ,
, 25 The compounds of Examples 43 to 45 below were : ~
~` prepared by following the procedure of Example 25, except~:
;; . .
..that 5-fluoro-2-nitroaniline and substituted or unsub- ::
stituted imidazoles were used as starting material~
~30 E~smnle_g~ 5-(2-Methylimidazol-1-yl)-2-nitroaniline
.~
. Formula (III) A = B = T = W = C, Rl = Rz = R3 = H, Y = ::
i5-(2-methylimidazol-1-yl)
'. '
Melting point: 229C. ~
. ~
.,
~$
2~22~ :
- 31 -
E~m}l=~9~ 2-Nitro-5-(2,4,5-trimethylimidazol-1-yl)-
aniline
, - ' .
Formula (III) A = B = T = W = C, R1 ~ R~ = R3 = H, Y =
` 05 5-(2,4,5-trimethylimidazol-1-yl)
Melting point ~ 250C.
~x~mE1~45 5-(4-Methylimidazol-1-yl)-2-nitroaniline
For~ula (III) A = B = T = W = C, Rl = Rz = R3 = H, Y =
i 5-(4-methylimidazol-1-yl)
. .
Melting point: 188C.
E~amPl-e 46 5-(Imidazol-1-yl)-2-nitroaniline
Formula (III) A = B = T = W = C, R1 = Rz = R3 = H, Y =
5-(imidazol-1-yl)
~, A solution of 13.7 g of imidazole in 300 ml of
dimethylformamide is placed in a three-necked flask
equipped with a condenser and a dropping funnel. 10 g of 1
sodium hydride, a~ a 50% suspen~ion in oil, are then
added in portions. The medium i9 heated for 1 h at 60
' and then cooled to room temperature and a solution of
-¦ 33.3 g of 5-fluoro-2-nitroaniline in 100 ml of dimethyl-
formamide is added dropwi~e
1 The reaction medium is then heated for 2 h at
70C, with stirring, cooled and poured into 1 1 of cold
j water. The solution is then extracted with ether and the
`~3 organic phase is dried over magnesium sulfate and con~
centrated to give 26 3 g of 5-(imidazol-1-yl)-2-nitro-
aniline.
Melting point: 185-7C.
.
, .
., .~ -: .- . , ,.................. , :
-d,`J~
2 ~ 2 2
- 32 -
:'~
~ Example 47 5-(Benzimidazol-1-yl)-2-nitroaniline
:
' Formula (III) A = B = T = W = C, R1 = R~ = R3 = H, Y =
;~ 5-(benzimidazol-1-yl)
05
5-(Benzimidazol-1-yl)-2-nitroaniline is obtained
by following the procedure of Example 46, except that 5-
chloro-2-nitroaniline i~ reacted with benzimidazole.
Melting point: 204-206C.
~, 10
Example 48 N-Methyl-5-(imidazol-1-yl)-2-nitroaniline
Formula (III) A = B = T = W = C, R1 = Rz = H, R3 = CH3,
; Y = 5-(imidazol-1-yl)
J A solution of 9.3 g of N-methyl-5-chloro-2-nitro-
aniline, prepared according to Example 9, 3 4 g of
,~ imidazole, 5.3 g of sodium carbonate and 50 ml of DMF i~
'l~ refluxed for 16 h. It is left to cool and concentrated
20 to dryness and the residue is then taken up with water, -
filtered off, wash~d with acetonitrile and dried to ~ive
5.9 g of N-methyl-5-(imidazol-1-yl)-2-nitroaniline.
Melting point: 254C.
~; 25 E~smPle 49 5-(2,4-Dimethylimidazol-l-yl)-2-nitroaniline
.
Formula (III) A = B = T = W = C, R1 = Rz = R3 = H, Y =
5-(2,4-dimethylimidazol-1-yl)
, 30 According to Example 48, 4-fluoro-2-nitroaniline
reacts with 2,4-dimethylimidazole to give 5-(2,4-di-
methylimidazol-1-yl)-2-nitroaniline.
`~ Meltin~ point: 194C.
"l 35
:.
'~.'~ :
2~1222
- 33 -
Example 50 2~ (3-Amino-4-nitrophenyl)-2-methyl-
imidazo1-4-yl]-2-methyl-1,3-dioxolan
Formula (III) A = B = T = W = C, R1 = Rz = R3 = H, Y =
055-[4-(2-methyl-1,3-dioxolan-2-yl)-2-
methylimidazol-1-yl]
~.Z
According to Example 48, 4-fluoro-2-nitroaniline
react~ with 2-(2-methylimidazol-4-yl)-2-methyl-1,3- -
Z 10 dioxolan, prepared from 4-acetyl-2-methylimidazole and
ethylene glycol in the pre~ence of paratoluene~ulfonic
acid~ to give 2-~1-(3-amino-4-nitrophenyl)-2-methyl-
imidazol-4-yl]-2-methyl-1,3-dioxolan.
. ~ :
Melting point: 196C.
Example 51 2-Amino-6-(imidazol-1-yl)-3-nitropyridine
ZZ Formula (III) B = T = W = C, A = N, R1 = R2 = R3 = H,
~ Y = 5-(imidazol-1-yl)
:~ 20 -~
Imidazole and 2-amino-6-chloro-3-nitropyridine
react under the conditions of Example 48 to give 2-amino-
`~ 6-(imidazol-1-yl)-3-nitropyridine.
Melting point: 224C. ;;
E~mple 52 4-Amino-2-(imidazol-1-yl)-5-nitropyrimidine
Formula (III) A = T = N, B = W = C, R1 = Rz = R3 = H, ~
Y = 5-(imidazol-1-yl) ~-
The reaction of imidazole with 4-amino-2-chloro-
ii 5-nitropyrimidine according to Example 48 yield~ 4-amin~-
2-(imidazol-1-yl)-5-nitropyrimidine.
Melting point: 248C.
`'Z
~ 2~222
- 34 -
Esam~lQ_~ N-I~opropyl-5-(imidazol-1-yl)-2-nitroaniline
'
Formula (III) A = B = T = W = C, Rl = R2 = H, R3 = iPr,
Y = 5-(imidazol-1-yl)
05
4.2 g of the sodium salt of imidazole are added
, in portion3 to a solution of 9.4 g of N-isopropyl-5-
` fluoro-2-nitroaniline, prepared in Example 10, in 75 ml ~
, of dimethylformamide. The reaction medium is then re- ~-
1 10 fluxed for 5 h 30 min, cooled and poured into 200 ml of
water. The precipitate obtained i~ filtered off, washed ~-
with water and then dissolved in chloroform.
The organic phase is washed with water, dried and
concentrated to give 8.2 g of N-isopropyl-5-(imidazol-1-
yl)-2-nitroaniline.
. Melting point: 152C.
.
..
; Example 54 N-Benzyl-5-(imidazol-1-yl)-2-nitroaniline
.,.,~
.~
-¦20 Formula (III) A = B = T = W = C, Rl = Rz = H, R3 = CH20,
Y = 5-(imidazol-1-yl) -~
.~ : ::
~,
Under the same condition~ a~ in Example 53, N-
benzyl-5-fluoro-2-nitroaniline, obtained in Example 11, -~
react~ with the sodium salt of imidazole to give N-
benzyl-5-(imidazol-1-yl)-2-nitroaniline.
Melting point: 171C.
, .-i;~m~ 5 N-Methyl-5-(2-methylimidazol-1-yl)-4-methyl-
2-nitroaniline
~r'`~ Formula (III) A = B = T = W = C, Rl = 4-CH3, R2 = H,
R3 = CH3, Y = 5-(2-methylimidazol-1-yl)
N-Methyl-5-(2-methylimidazol-1-yl)-4-methyl-2-
''~
~, ,.
," ~
,. ~i .: .
2 2 2
- 35 -
.
nitroacetanilide, prepared in Example 23, is deacetylated
by following the procedure described in Example 14 to -
give N-methyl-5-(2-methylimidazol-1-yl)-4-methyl-2-nitro- -
, aniline.
05 Melting point: 155C.
I Example 56 N-I~opropyl-5-(2-methylimidazol-1-yl)-2-
.` nitroaniline
`3 10 Formula (III) A = B = T = W = C, R1 = R~ = H, R3 = iPr,
3 Y = 5-(2-methylimidazol-1-yl)
i N-Isopropyl-5-fluoro-2-nitroaniline, prepared
according to Example 10, and 2-methylimidazole are re-
15 acted as in the ca~e of Example 25 to give N-isopropyl-5-
(2-methylimidazol-1-yl)-2-nitroaniline.
, Melting point: 166C. ~
~ ':
E~am~l~ fi~ 2-Nitro-~-(1,2,4-triazol-1-yl)aniline
Formula (III) A = B = T = W = C, R1 = Rz = R3 = H, Y =
5-(1,2,4-triazol-1-yl)
., '
2-Nitro-5-(1,2,4-triazol-1-yl)aniline is obtained
25 by following the procedure of Example 53, but using the
sodium salt of 1,2,4-triazole and 2-nitro-5-fluoroani-
line.
Melting point: 260-265C.
,
.
Example 58 N-Methyl-2-nitro-5-(1,2,4-triazol-1-yl)-
aniline
.
Formula (III~ A = B = T = W = C, R1 = Rz = H, R3 = CH3,
Y = 5-(1,2,4-triazol-1-yl)
3~
,
' ,
2~ ~22~
- 36 -
8.1 g of the ~odium salt of 1,2,4-triazole are
refluxed for 60 h in 250 ml of DMF with 16.7 g of N-
methyl-5-chloro-2-nitroaniline (Example 9). After
cooling, the insoluble material is filtered off and ~ -
05 washed with methanol. The DMF is concentrated and the
residue i~ taken up with hot methanol and filtered off. ~-
The 2 solids are identical. 12.8 g of N-methyl-
2-nitro-5-(1,2,4-triazol-1-yl)aniline are obtained in
this way.
Melting point: 234C.
s
i Example 59 N-Isopropyl-2-nitro-5-(1~2,4-triazol-1-yl)-
aniline
~15 Formula (III) A = B = T = W = C, R1 = R~ = H, R3 = iPr, ;-~
iY = 5-(1,2,4-triazol-1-yl)
N-Isopropyl-2-nitro-5-(1,2,4-triazol-1-yl)aniline
,is obtained a~ described in Example 53, but u ing N-iso-
:!20 propyl-5-fluoro-2-nitroaniline and the sodium qalt of
1,2,4-triazole as reactants.
lMelting point: 144C.
¦Example 60 2-Amino-4-(imidazol-1-yl)aniline
Formula (II) A = B = T = W = C, R~ = Rz = R3 = H, Y = 4-
(imidazol-1-yl)
26.3 g of 5-(i~idazol-1-yl)-2-nitroaniline, pre-
pared according to Example 46, are dissolved in 150 ml of
¦ ethanol. 35 ml of water and 120 g of powdered iron are
¦ then added~ With stirring, 2 ml of concentrated hydro-
l chloric acid are added and the medium iq refluxed for 4
-l~ h. The ~uspension is then filtered hot on Célite. The
filtrate is cooled and extracted with methylene chloride.
!
~`
2 2
~ - 37 -
., ,
- The organic pha~e i~ dried over magnesium ~ulfate and
s then concentrated to give 17 g of 2-amino-4-(imidazol-1-
yl)aniline.
Melting point: 168-170C.
:~ 05 ~:
-, The compounds of Example~ 61 to 69 below were ~
prepared by following the procedure of Example 60: :.
Example 61 2-A~ino-4-(imidazol-1-yl)-5-methylaniline
,: i
1 0
;~ Formula (II) A = B = T = W = C, Rl = 5-CH3, Ra = R3 = H7
Y = 4-(imidazol-1-yl)
Melting point: 104C. ~
~.
Ex~mp~e 62 2-Amino-4-(2-methylimidazol-1-yl)-5-methyl-
aniline
~ .
7 Formula (II) A = B = T = W = C, Rl = 5-CH3, R2 = R3 = H,
Y = 4-(2-methylimidazol-1-yl)
Melting point: 177C.
;; .
Example 63 2-Amino-4-(2-methylimidazol-1-yl)aniline
,~ Formula (II) A = B = T = W = C, Rl = R2 = R3 = H, Y = 4-
~ (2-methylimidazol-1-yl)
~ ~ 3
. Melting point: 192C.
~. Example 64 2-N-Methylamino-4-(2-methylimidazol-1-yl~-
;;. aniline
Formula (II) A = B = T = W = C, Rl = R2 = H, R3 = CH3,
Y = 4-(2-methylimidazol-1-yl)
~.-
i~'
.:
~:: ~:~hi~ .. . ., "
2~ ~222
.. .. .
~ 38 - ~
', ,' ,
Melting point: 204C.
-~ Example 65 2-N-Methylamino-4-~imidazol-1-yl)-5-methyl-
~ aniline
'.! 05
Formula (II) A = B = T = W = C, R1 = 5-CH3, R2 = H, R3 =
~ CH3, Y = 4-(imidazol-1-yl)
`:`t Melting point: 135C.
~ Example 66 2-N-Methylamino-4-(2-methylimidazol-1-yl)-
;j 5-methylaniline
.. . .
Formula (II) A = B = T = W = C, R1 = 5-CH3, Ra _ H, R3 =
CH3, Y = 4-(2-methylimidazol-1-yl)
'I ~
Oil used in the crude state for the next ~tep.
~ Exa~ple 67 2-N-Methylamino-4-(1,2,4-triazol-1-yl)aniline
`~ 20
Formula (II) A = B = T = W = C, R1 = Rz = H, R3 = CH3,
Y = 4-(1,2,4-triazol-1-yl)
.,
~: Melting point: 108C.
!i 25
I E~Em~ 8 2-N-Isopropylamino-4-(imidazol-1-yl)aniline
`,~
Formula (II) A = B = T = W = C, R1 = Rz = H, R3 ~ iPr,
Y = 4-(imidazol-1-yl)
Melting point: 118C.
~ "~
2~1~ 2~2
., ,
i Examp~e 69 2-N-Benzylamino-4-(imidazol-1-yl)aniline
,~
~ Formula (II) A = B = T = W = C, Rl = Rz = H, R3 = CH20,
t Y = 4-(imidazol-1-yl)
05
Melting point: 149C.
¦ Example 70 2-N-Methylamino-4-(imidazol-1-yl)aniline
'
t iO Formula (II) A = B = T = W = C, R1 = Rz = H, R3 = CH3,
i Y = 4-(imidazol-1-yl)
:3
! A solution of 16-3 g of N-methyl-5-(imidazol-1-
yl)-2-nitroaniline, prepared in Example 48, in 900 ml of
15 methanol i~i hydrogenated at normal preisisure in the
presence of Raney nickel. When the necessary amount of -~
hydrogen has been absorbed, the catalyi3t is filtered off
and the filtrate is concentrated under vacuum. The con-
centrate isi taken up with isopropyl ether to give 12.9 g
`~ 20 of 2-N-methylamino-4-(imidazol-1-yl)aniline.
~ Melting point: 160C.
::
The compoundæ of Examplei~i 71 to 96 below were
prepared by following the procedure of Example 70:
~m;i~_~1 2-Amino-4-(1,2,4-triazol-1-yl)aniline
Formula (II) A = B = T = W = C, R1 = R2 = R3 = H, Y = 4-
(1,2,4-triazol-1-yl)
Melting point: 182C.
, .
: 2~222
- 40 -
Example 7~2 2-N-Isopropylamino-4-(1,2,4-triazol-1-yl)-
aniline ;~
I Formula (II) A = B = T = W = C, R1 - R2 = H, R3 = iPr,
;, 05 Y = 4-(1,2,4-triazol-1-yl)
Melting point: 94C.
ExamPle 73 2-N-Isopropylamino-4-(2-methylimidazol-1-yl)-
aniline
~ Formula (II) A = B = T = W = C, R1 = R2 = H, R3 = iPr,
-~ Y = 4-(2-methylimidazol-1-yl)
1 15 Melting point: 160C.
~I
~ Example 74 2-N-I~opropylamino-4-(imidazol-1-yl~-5-
~:
; methylaniline ;~
Formula (II) A = B = T = W = C, R1 = 5-CH3, R2 = H, R3 =
iPr, Y = 4-(imidazol-1-yl)
,.~
: Melting point: 139C.
;~ 25 Ex~m~le 75 2-Amino-3-(i~idazol-1-yl)aniline
,~ Formula (II) A = B = T = W = C, Rl = R2 = R3 = H, Y = 3- :
, (imidazol-1-yl)
~,~ 30 Melting point: 128C.
~. .~':
.E~am~le.76 2-Amino-4-(imidazol-1-yl)-~-methylaniline ;~
: Formula (II) A = B = T = W = C, R1 = 6-CH3, R~ = R3 = H, :::
Y = 4-(imidazol-1-yl) ~.
r
:~`
2~ 2~2
- 41 -
Meltin~ point: 152~C.
E~am~l~_~ 2-Amino-4-(imidazol-1-yl)-6-ethylaniline
05 Formula (II) A = B = T = W = C, R1 = 6-CzH6, R~ = R3 =
H, Y = 4-(imidazol-1-yl)
Melting point: 139C.
10 E~xample 78 2-Amino-5-chloro-4-(imidazol-1-yl~aniline
Formula (II) A = B = T = W = C, R1 = 5-Cl, R2 = R3 = H,
Y = 4-(imidazol-1-yl)
Melting point: 164C.
ExamplQj'9 2-Amino-4-(imidazol-1-yl)-3-methylaniline
Formula (II) A = B = T = W = C, R1 = 3-CH3, Rz = R3 = H,
Y = 4-(imidazol-1-yl) ~:
Melting point: 152C.
E~a~ le 80 2-Amino-4-(imidazol-1-yl)-5-trifluoromethyl-
aniline
Formula (II) A = B = T = W = C, R1 = 5-CF3, R2 = R~ = H,
Y = 4-(imidazol-1-yl)
Melting point: 186C.
.
. : ~:
:`
: .. ; ~ - . . . : .
201~22~
- 42 -
; ExamplQ_~1 2-Amino-5-chloro-4-(imidazol-1-yl)-6-methyl-
aniline
:
Formula (II) A = B = T = W = C, R1 = 5-Cl, Rz = 6-CH3,
05R3 = H, Y = 4-(imidazol-1-yl)
. .
--. Melting point: 131C.
;:,
E~=Glsm~2 2-Amino-5,6-dimethyl-4-(imidazol-1-yl)aniline
.,, 10
`~ Formula (II) A = B = T = W = C, R1 = 5-CH3, Rz = 6-CH3,
~-~ R3 = H, Y = 4-(imidazol-1-yl) -.
, . . .
Melting point: 132C.
~, Ex.amPl~ ~3 2-Amino-4-(4-methylimidazol-1-yl)-6-methyl-
aniline
Formula (II) A = B = T = W = C, R1 = 6-CH3, R2 = R3 = H,
20Y = 4-(4-methylimidazol-1-yl)
.~
:`
Melting point: 177~C.
.j
~,s,~3 ~am~l~ 84 2-Amino-4-(2,4-dimethylimidazol-1-yl)-6-
25methylaniline
''I
Formula (II) A = B = T = W = CJ Rl = 6-CH3, R~ = R3 = H,
Y = 4-(2,4-dimethylimidazol-1-yl)
30Melting point: 178C.
" " ~
i;3 35:~::
'3 ~
20~1222 -
.
:: - 43 -
. Example 85 2-Amino-4-(4-methylimidazol-1-yl)-5-methyl-
aniline
. ~ ,
Formula (II) A = B = T = W = C7 R1 = 5-CH3, Rz = R3 = H,
05 Y = 4-(4-methylimidazol-1-yl)
. Melting point: 146C.
.. :
E~mPle 86 2-Amino-4-(2-methylimidazol-1-yl)-5-methyl-
aniline
Formula (II) A = B = T = W = C, Rl = 5-CHs, Rz = R3 = H,
Y = 4-(2-methylimidazol-1-yl)
., .
~ 15 Melting point: 172C.
b
. Ex~mEle 87 2-Amino-4-(2,4-dimethylimidazol-1-yl)-5-
methylaniline
Formula (II) A = B = T = W = C, Rl = 5-CH3, R2 = R3 = H,
Y = 4-(2,4-dimethylimidazol-1-yl)
Oil u~ed in the crude ~tate for the next ~tep.
.
ExamPle 88 2-Amino-5-chloro-4-(2-methylimidazol-1-yl)-
aniline
~;,
Formula (II) A ~ B = T = W = C, Rl = 5-Cl, R2 = R3 = H,
~ Y = 4-(2-methylimidazol-1-yl) :
!i~ 30
Melting point: 191C.
, :
~
:i ....
'l
F'. ' - : :: . ~ ': ; : . . - , .
. ~ , . . ... . .. . . . . . .
20~ 222
- 44 -
Exa~ple 89 2-Amino-5-chloro-4-(4-methylimidazol-1-yl)-
aniline
I Formula (II) A = B = T = W = C, R1 = 5-Cl, R2 = R3 = H, ~:
05Y = 4-(4-methylimidazol-1-yl)
~ Melting point: 168C.
,~ ExamPle 90 2-Amino-4-(2,4,5-trimethylimidazol-1-yl)-
10aniline
~ Formula (II) A = B = T = W = C, R1 = R2 = R3 = H, Y = 4-
.3 ( 2,4,5-trimethylimidazol-1-yl)
15Melting point: 194C.
.1 ,E,~ample 91 2-Amino-4-(4-methylimidazol-1-yl)aniline
' ~
Formula (II~ A = B = T = W = C, Rl = R2 = R3 = H, Y = 4-
20(4-methylimidazol-1-yl) ~-
Melting point: 153C.
.:
Ex~mPle 92 2-Amino-4-(benzimidazol-1-yl)aniline ~,
. 25 ',~
`1 Formula (II) A = B = T = W = C, R1 = R2 = R~ = H, Y = 4- :
(benzimidazol-1-yl)
!` Meltin~ point: 175C.
Example 93 2-Amino-4-(2,4-dimethylimidazol-1-yl)aniline
Formula (II) A = B = T = W = C, R1 = Rz = R3 = H, Y = 4
'~ (2,4-dimethylimidazol-1-yl)
.
f~f ~222
- 45 -
i
, Melting point: 133C
. .,
f
~mEle S'~ 2-[1-(3,4-Diaminophenyl)-2-methylimidazol-4-
yl]-2-methyl-1,3-dioxolan
f 05
Formula (II) A = B = T = W = C, R1 = R2 = R3 = H, Y = 4-
'f [4-(2-methyl-1,3-dioxolan-2-yl)-2-methyl-
imidazol-1-yl]
f
1 10 Melting point: 206~C.
.,1
;, Exampl_ 95 2,3-Diamino-6-(imidazol-1-yl)pyridine
, ., ~
ff Formula (II) A = N, B = T = W = C, Rl = Rz = R3 = H, Y =
4-(imidazol-1-yl)
'~ Melting point: 186C.
E~ le 9~ 4,5-DiaDin~-2~ idaz~l-1-yl)pyrimidiDe
Formula (II) A = T = N, B = W = C, R1 = R2 = R3 = H, Y =
4-(imidazol-1-yl)
~'f Melting point: 240C (decompo~ition).
` 25
` Example 97 5-[(Imidazol-2-yl)thioacetyl~-2-chloronitro-
benzene
, :` Formula (X) R1 = R2 = Rs = H, R7 = imidazol-2-yl, X = Cl
'i 30
A solution of 14.4 g of 5-(a-bromoacetyl)-2-
f chloronitrobenzene and 5.2 g of 2-mercaptoimidazole in
~, 310 ml of methanol is ~tirred for one hour at room tem-
~- perature. It i~ concentrated under vacuum and the con-
centrate i~ taken up with ether and filtered off to give
.
."';'
. ~1 :
-~f
. :L f~i : . .. : ~ . . : . .: : . : . : ~ . : ,
2~222
?
- 46 -
~;
19 g of 5-[(imidazol-2-yl)thioacetyl]-2-chloranitro-
~' benzene hydrobromide.
',J Melting point: 240-242C.
The 19 ~ of solid obtclined above are added to a
05 solution of 5.5 g of sodium carbonate in 100 ml of water.
The mixture i~ ~tirred for 1 hour and the product is
filtered off and washed with water and then with
; isopropyl alcohol and isopropyl ether to give 13.9 ~ of5-[(imidazol-2-yl)thioacetyl]-2-chloronitrobenzene.
Melting point: 160C.
Exam~lQ 98 3-(4-Chloro-3-nitrophenyl)imidazoC2,1-b]-
~ ç
thiazole
..
Formula (IX) A = T = C, R1 = Rz = H, X = Cl, Y =
imidazo[2,1-b]thiazol-3-yl ~ -
~ ~:
38.6 g of 5-[(imidazol-2-yl)thioacetyl]-2-chloro-
nitrobenzene, prepared above, are added to 1400 g of PPA
20 kept at 110C. The mixture is heated at 120C for 3 h ~--
and then left overnight at room temperature. It i8
poured into a mixture of water and ice.
~; The pH is brought to 7 by the addition of a solu-
tion of ammonia. The precipitate obtained is filtered
off and wa~hed with water and then with isopropyl ether
and acetone to give 41.1 g of 3-(4-chloro-3-nitrophenyl)-
-$ imidazo~2,1-b]thiazole.
Melting point: 206C.
30 Ex~mple 99 ~,6-Dihydro-3-(4-chloro-3-nitrophenyl)-
1 imidazo[2,1-b]thiazole ~
.,~ .ç Formula (IX) A = T = C, R1 = R2 = H~ X = Cl, Y = 5,6-
dihydroimidazo[2,1-b]thiazol-3-yl
~ .
' ~ :
.,~
20~ ~22
- 47 -
10.4 ~ of 5-(a-bromoacetyl)-2-chloronitrobenzene
and 3.8 g of imidazolidine-2-thione in 200 ml of methanol
are reacted for one hour at room temperature. The inter-
mediate hydrobromide (melting point: 1~0C) is neutrali-
05 zed as in Example 97 to give 10.5 g of 2,3 7 5,6-tetra-
, hydro-3-hydroxy-3-(4-chloro-3-nitrophenyl)imidazo r 2,1-b]-
i~ thiazole (melting point: 187C), which is treated in
200 g of PPA according to Example 98 to give 8.7 g of
5,6-dihydro-3-(4-chloro-3-nitrophenyl)imidazo[2,1-b]-
thiazole.
Melting point: 174C.
~1 Fx~mPle 100 2-Amino-4-(imidazo[2,1-b]thiazol-3-yl)-
, aniline
~~ Formula (II) A = B = T = W = C, Rl = Rz = R3 = H, Y = 4-
,~ (imidazot2,1-b]thiazo1-3-yl)
.~
3 55 g of ammonia are dissolved in a 1 l autoclave
containing 500 ml of cold 2-methoxyethanol. 41.1 g of 3-
`~ (4-chloro-3-nitrophenyl)imidazo~2,1-b~thiazole, prepared
in Example 98, are then added and the mixture is heated
~¦ at 150C for 24 h under pressure. It i~ left to cool.
i The solvent is evaporated off and the reqidue is taken up
¦25 with water and filtered off to give 30 g of 2-nitro-4-
,(imidazo~2,1-b]thiazol-3-yl)aniline.
;lMelting point: 266C.
Without further purification, 15.6 g of this com-
,pound are hydrogenated in the presence of Raney nickel,
according to Example 70, to give 6.5 g of 2-amino-4-
(imidazor2,1-b~thiazol-3-yl)aniline.
Melting point: 210C.
. '~
.'"'~ .
'.'-
:,~
20~ 1222
- 48 -
ExamPle 101 5-(a-Chloroacetyl)benzimidazol-2-one
! .
- Formula (XII) R1 = Rz = R3 = Rs = H, X = Cl
.-;~
i, 05 26.8 g of benzimidazolone are added in portions,
,~J at a temperature below 5C, to a mixture of 80 g of
aluminum chloride, 34 ml of chloroacetyl chloride and 100
ml of anhydrous methylene chloride. When the addition is
complete, the reaction mixture i~ brought ~lowly to the
reflux point and kept at this temperature for 3 h. It is
then cooled and poured into an ice/hydrochloric acid mix-
3 ture. The precipitate obtained is filtered off and ~-~
, washed with water until the washings are neutral, then
,~ with ethyl alcohol and finally with ether.
35.1 g of 5-(a-chloroacetyl)benzimidazol-2-one ~;
~; are obtained after drying.
Melting point: 281-286C (decompo~ition).
.
The compounds of Examples 102 and 103 below were
prepared by following the procedure of Example 101:
-!
`~I E~ample 102 ~-(2-Chloropropionyl)benzimidazol-2-one
., ~ - .
Formula (XII) R1 = Rz = R3 = H, X = Cl, Rs = CH3
~ 25 `
; Melting point: 270-280C (decomposition).
', ..
Example lQ~ S-(a-Chloroacetyl)-6-methylbenzimidazol-2-
one
. 30
Formula (XII) R1 = 6-CH3, R2 = R3 = Rs = H, X = Cl
~. ~:
Melting point: 263-266C (decomposition).
-,~
!, . 35
-
.,~
2 2 ~C~
- 49 -
.
Example 104 ~-[(Imidazol-2-yl)thioacetyl]benzimidazol-2-
one hydrochloride
,~ ,
Formula (XI) R1 = R2 = Rs = R~ = H, R7 = imidazol-2-yl
05
13.8 g of 5-(a-chloroacetyl)benzimidazol-2-one,
I prepared in Example 101, and 6.6 g of 2-mercaptoimidazole
;~ in 75 ml of N~methylpyrrolidone are heated at 100 for
3 h. The crystals obtained are filtered off cold, washed
~ 10 with acetone and ether and then dried.
;j This ~ives 19.9 g of 5-[(imidazol-2-yl)thio-
acetyl]benzimidazol-2-one hydrochloride.
Melting point: 194C.
15 ~Am~le 105 5-(~6-Dihydroimidazo[2,1-b]thiazol-3-Yl)-
, benzimidazol-2-one fumarate
Formula (I) A = B = T = W -- C, R1 = R2 = R~ = H, Y = 5-
(5,6-dihydroimidazo[2,1-b]thiazol-3-yl), Z =
! 20 OH
. ~ .
~ A solution of 9 g of imidazolidine-2-thione and
1 22.5 g of 5-t-chloroacetyl)benzimidazol-2-one, prepared
in Example 101, in 450 ml of N-methylpyrrolidone is
heated at 150 for 3 h. The precipitate obtained is
filtered off, w~shed with acetone and ether and then
dissolved in 500 ml of water.
The resulting solution is filtered, with passage
over charcoal, and then rendered alkaline in the cold
i 30 with concentrated ammonia, and the precipitate formed is
filtered off, washed with water until the washings are
`~ neutral, and then with acetone and dried. This give~
¦ 16.8 g of 5-(5,6-dihydroimidazo[2,1-b]thiazol-3-yl)benz-
imidazol-2-one in the form of the base, which is dis-
', 35 solved in 750 ml of hot methanol. 11.3 g (1.5 eq) of
,~
.. "r~ !., . .... . . ~ . . . . . . .
2~ ~ 22~
- 50 -
fumaric acid in 100 ml of methanol are added to this hot
solution and the mixture is then filtered hot, with
passage over charcoal. The solution is then concentrated
to half its volume. The product crystallizes in the cold
05 as the 3/2 fumarate to give 7.5 g of 5-(5,6-dihydro- -
imidazo[2,1-b]thiazol-3-yl)benzimidazol-2-one 3/2
fumarate.
~`~j Melting point: 211-213C.
The compounds of Examples 106 and 107 below were
prepared by following the procedure of Example 105:
.,
,1
ExamElQ_106 5-(5,6-Dihydro-2-methylimidazo[2,1-b]thia- ~ -
zol-3-yl)benzimidazol-2-one hydrochloride
. Formula (I) A = B = T = W = C, Rl = R2 = R3 = H, Y = 5-
(5,6-dihydro-2-methylimidazo[2,1-b]thiazol-
3-yl), Z = OH
" ' .
Melting point: 329-330C (decomposition).
Ex~mple 107 5-(5,6-Dihydroimidazo~2,1-b]thiazol-3-yl)-
6-methylbenzimidazol-2-one hydrochloride
Formula (I) A = B = T = W = C, Rl = 6-CH3, R2 = R3 = H,
Y = 5-(5,6-dihydroimidazo[2,1-b]thiazol-3-
yl), Z = OH
. . .
` Melting point: 340-345C (decomposition).
~; ExBmple 108 5-(Imidazo~2,1-b]thiazol-3-yl)benzimidazol-
2-one
.!
,~ Formula (I) A = B = T = W = C, Rl = Rz = R3 = H, Y = 5-
~, 35 (imidazo[2,1-b]thiazol-3-yl), Z = OH
,:
2~ 2~
.
- 51 -
,j 19.9 g of 5-[(imidazol-2-yl)thioacetyl]benz-
imidazol-2-one hydrochloride, prepared in Example 104,
and 600 g of PPA are heated at 90 for 8 h. While still
. hot, the mixture is poured slowly into 5 l of water/ice.
05 The solution obtained is rendered alkaline in the cold
with sodium hydroxide ~qolution. A copious precipitate
forms, which is filtered off and washed with water.
This precipitate iq taken up with 250 ml of DMF under
reflux. The insoluble material is removed and the DMF
solution is concentrated, after passing over charcoal,
until crystallization starts. The crystals obtained are
filtered off, washed with water, acetone and ether and
then dried
This gives 6 2 g of 5-(imidazo[2,1-b]thiazol-3-
yl)benzimidazol-2-one
Melting point > 330C (decomposition)
Exam~le 109 5-(Imidazol-1-yl)-6-methylbenzimidazol-2-one
Formula (I) A = B = T = W = C, R1 = 6-CH3, R2 = R3 = H,
Y = 5-(imidazol-1-yl), Z = OH
-:!
4 8 g of 2-amino-4-(imidazol-1-yl)-5-methylani-
! line, prepared in Example 61, are intimately mixed with
~ 25 2.3 g of finely ground urea. The mixture is heated at
¦ 180C for 2 h 30 min. The medium is seen to melt, with
`~ evolution of the ammonia formed, and then solidify.
After cooling, the solid is dissolved in 50 ml of 1 N
/ sodium hydroxide solution The solution i~ extracted
~l 30 with ether and then paqsed over charcoal and filtered. 1
The filtrate is acidified to pH 7 with dilute hydro-
chloric acid. A precipitate forms, which is filtered off
~? and washed with water and then with acetone.
.¦ This gives 3.7 g of 5-(imidazol-1-yl)-6-methyl-
~l 35 benzimidazol-2-one.
.. ~ :
.; . .
2~1 ~ 2~
- 52 -
Melting point > 340C.
The compound~ of Example~ 110 to 122 below were
obtained by following the procedure of Example 109:
05
Exa~Ple 110 5-(Imidazol-1-yl)benzimidazol-2-one
hydrochloride
Formula (I) A = B = T = W = C, Rl = R~ = R3 = H, Y = 5-
(imidazol-1-yl), Z = OH
:,
i Melting point > 330C.
~' .,
Ex~mPle 111 5-(Imidazol-1-yl)-3-methylbenzimidazol-2-one
l 15
i~, Formula (I) A = B = T = W = C, R1 = Rz = H, R3 = CH3,
~ Y = 5-(imidazol-1-yl~, Z = OH
i
I Melting point: 254C.
i;i~ 20
j ~xa~p~ 5-(2-Methylimidazol--1-yl)-6-methylbenz-
`1 imidazol-2-one hydrochloride
i,'i
Formula (I) A = B = T = W = C, R1 = 6-CH3, R2 = R3 = H,
~ 25 Y = 5-(2-methylimidazol-1-yl), Z = OH
,~ .
Melting point ~ 340C.
~, ~
Exa~ple 113 5-(2-Methylimidazol-1-yl)benzimidazol-2-one
~3 30 hydrochloride
Formula (I) A = B = T = W - C, R1 = R~ = R3 = H, Y = 5-
(2-methylimidazol-1-yl~, Z = OH
Melting point: 286-288C.
''. ...
...
:
2~ 1 2 2 2
. .
- 53 -
ExamPle 114 5-(2-Methylimidazol-1-yl)-3-methylbenz-
imidazol-2-one hydrochloride
Formula (I) A = B = T = W = C, R1 = R~ = H, R3 = CH3,
05 Y = 5-(2-methylimidazol-1-yl~, Z = OH
Melting point of the hydrochloride monohydrate:
: 315C.
::~
~, 10 E~amPle 115 3,6-Dimethyl-5-(imidazol-1-yl)benzimidazol-
2-one hydrochloride
,
Formula (I~ A = B = T = W = C, Rl = 6-CH3, Rz = H, R3 =
, CH3, Y _ 5-(imidazol-1-yl), Z = OH
::, 15
Melting point of the hydrochloride monohydrate:
335-340C.
.,
:~ Exa~ple 116 3,6-Dimethyl-5-(2-methylimidazol-1-yl)benz-
`~ 20 imidazol-2-one hydrochloride
.'''
Formula (I) A = B = T = W = C, Rl = 6-CH3, Rz = H, R3 =
;i CH3, Y = 5-(2-methylimidazol-1-yl~, Z = OH
',i, '
. 25 Melting point of the hydrochloride monohydrate >
: 340C. ~:
.~.
Ex~m~ L7 3-Methyl-5-(1,2,4-triazol-1-yl)benzimidazol- ~ -
. 2-one hydrochloride :
-~
` Formula (I) A = B = T = W = C, R1 = Rz = H, R3 = CH3,
:~l, Y = 5-(1,2,4-triazol-1-yl), Z = OH ::~
~'3i ~ '
`' Melting point: 238-241C.
~.
.';`.~, ~ ~
',i, :~'~ '
,
20~ 222
.
- 54 -
,1
ExamPle 118 3-I~opropyl-5-(imidazol-1-yl)benzimidazol-2-
~ one hydrochloride
', :
Formula (I) A = B = T = W = C, R1 = Rz = H, R3 = iPr,
05 Y = 5-(imidazol-1-yl), Z = OH
, :
Melting point: 260-265C.
Example 119 3-Benzyl-5-(imidazol-1-yl)benzimidazol-2-one
i'. 10
..
~: Formula (I) A = B = T = W = C, R1 = Rz = H, R3 = CHz-0,
Y = 5~(imidazol-1-yl), Z = OH
Melting point: 289-293C.
~.1 15
,; ~
~l ExamPle 120 5-(1,2,4-Triazol-1-yl)benzimidazol-~-one
! .,
Formula ~I) A = B = T = W = C, R1 = Rz = R3 = H, Y = 5-
(1,2,4-triazol-1-yl), Z = OH
Melting point: 360~C.
Example 121 3-I~opropyl-5-(imidazol-1-yl)-6-methylbenz-
imidazol-2-one ~:
Formula (I) A = B = T = W = C, Rl = 6-CH3, Rz = H, R3 =
iPr, Y = 5-(imidazol-1-yl), Z = OH -:
Melting point: 268C. -~
" r ~ 3 0
Exa~ple 122 5-(Imidazol-1-yl)imidazo~4,5-b]pyridin-2-one ~ -
Formula (I) A = N, B = T = W = C, R1 = Rz = Rs = H, Y =
5-(i~idazol-1-yl), æ = OH
,~ 35 :
~: J
.,
2~2~
- 55 -
Melting point: 331C.
Example 123 5-(Imidazol-1-yl)benzimidazole-2-thione
05 Formula (I) R1 = Rz = R3 = H, Y = 5-(imidazol-1-yl), Z =
SH
,~
20.4 g of 2-amino-4-(imidazol-1-yl)aniline, pre-
pared in Example 60, are di~solved in 800 ml of ethanol.
9 ml of carbon di~ulfide are added dropwise. The mixture
is refluxed for 6 h and cooled. The precipitate is fil-
tered off and then washed with ethanol. Recry~talliza-
tion from dimethylformamide gives 14.5 g of 5-(imidazol-
1-yl)benzimidazole-2-thione.
-, 15 Melting point: 347C.
,
The compound~ of Example~ 124 to 127 below were
prepared by following the procedure of Example 123
E~mPle 124 5-(Imidazol-1-yl)-3-methylbenzimidazole-
2-thione
:::
Formula (I) A = B = T = W = C, R1 = Rz = H, R3 = CH3,
Y = 5-(imid~zol-1-yl), Z = SH
Melting point: 295C.
, .
~' ~
~ Example 125 5-(l,Z,4-Triazol-1-yl)benzimidazole-2-thione ~ ~
., ~:
`:! 30 Formula (I) A = B = T = W = C, R1 = Rz = R3 = H, Y = 5-
`~ (1,2,4-triazol-1-yl), Z = SH
Melting point: 335C.
., .
` 35 ~
': '
.. ..
.- ~
~ 2~ ~22~
.~ .
.~ .
- 56 -
Exam~le 126 5-(Imidazol-1-yl)-3-isopropyl-6-~ethylbenz-
I imidazole-2-thione
:,
Formula (I) A = B = T = W = C, R1 = 6-CHs, Rz = H, Rs =
05 iPr, Y = 5-(imidazol-1-yl~, Z = SH
Melting point: 277C.
Example 127 5-(Imidazol-1-yl)-3-isopropylbenzimidazole-
' 10 2-thione
~,.. , .
Formula (I) A = B = T = W = C, Rl = R2 = H, Rs = iPr,
Y = 5-(imidazol-1-yl), Z = SH
Melting point: 281C. ~;~
E~mPle 128 5-(Imidazol-1-yl)-2-methylthiobenzimidazole -
Formula (I) A = B = T = W = C, Rl = Rz = R3 = H, Y = 6- -
(imidazol-1-yl), Z = SCH3
:,
$
~;~ 10 ml of 5 N NaOH are added to a suspension of
11 g of 5-(imidazol-1-yl)benzimidazole-2-thione, obtained
~3 in Example 123, in 150 ml of ethanol. The mixture is
25 stirred for 30 min to give a total solution. 8.5 g of -~
methyl iodide in 30 ml of ethanol are added dropwise.~ -~
The reaction is allowed to proceed overnight at room tem~
perature The mixture is concentrated under vacuum, the
concentrate is taken up with chloroform and the chloro-
`~ 30 form is decanted. The resulting organic phase i~ washed
~ with water, dried and then concentrated under vacuum to
: . .. .
`, give an oil, which is treated with ethyl acetate/pentane
to give crystals. Recrystallization from acetonitrile ;~
gives 7.3 g of ~-(imidazol-1-yl)-2-methylthiobenzimida-
~, 35 zole
. ~
. ,
~, ,
~ r:`,
2~ ~2~
- 57 -
:.,
~ Melting point: 153C.
. .
~' The compound~ of Examplei 129 to 132 below were
` obtained by following the procedure of Example 12B:
: 05
ample 129 5-(Imidazol-1-yl)-2-allylthiobenzimidazole
~, Formula (I) A = B = T = W = C, R1 = R2 = R3 = H, Y = 5-
--' (imidazol-l-yl), Z = allylthio ::
' 10 : :~
`i~ Melting point: 114C.
: .
;: Exam~le 130 5-(Imidazol-l-yl)-2-propargylthiobenz- -~
imidazole ~ .
:l 15
I Formula (I) A = B = T = W = C, Rl = Rz = R3 = H, Y = 5-
-l (imidazol-1-yl), Z = propargylthio ~
~ Melting point: 246C. : ::
.l 20
ii~ Ex~mE~e 131 3-Isopropyl-2-propargylthio-~-(1,2,4-
triazol-l-yl)benzimidazole
I Formula (I) A = B = T = W = C, R1 = R2 = H, Ra = iPr,
Y = 5-(1,2,4-triazol-1-yl), Z = propargyl- : :
;. thio
~ Melting point: 100C. ~:
;~ 30 Example 132 ~-(2-Methylimidazol-1-yl)-3-isopropyl-2-
~, propargylthiobenzimidazole
Formula (I) A = B = T = W = C, Rl = R2 = H, R3 = iPr,
Y = 5-(2-methylimidazol-1-yl), Z = ~:
propargylthio
:
, ( :
,,.~, :
2~ 22~
.
- 58 -
Melting point: 159C.
., ,
Example 133 5-(Imidazol-1-yl)-2-methylsulfinylbenz-
imidazole
05
Formula (I) A = B = T = W = C, Rl = Rz = R3 = H 7 Y = 5-
(imidazol-1-yl), Z = SOCH3
,~ ,
' 4.2 g of metachloroperbenzoic acid in 40 ml of
CHC13 are added dropwise to a solution, cooled to O~C? f
, 4.3 g of 5-(imidazol-1-yl)-2-methylthiobenzimidazole,
;~ obtained in Example 128, in 200 ml of a methanol/chloro-
form solution (50~50). After a reaction time of 20 min,
the temperature is allowed to rise to room temperature.
¦ 15 The mixture is concentrated and the concentrate is taken
up with a ~aturated solution of sodium bicarbonate and
then extracted with methylene chloride. The organic
phase is dried, concentrated and treated with aceto- ~-~
nitrile to give 3.5 g of 5-(imidazol-1-yl)-2-methyl-
sulfinylbenzimidazole.
Melting point: 214C.
Example 134 2-(Pyrid-4-ylamino)-5-(imidazol-1-yl)-
benzimidazole :
; Formula (I) A = B = T = W = C, R1 = R2 = R3 = H, Y = 5-
~- (imidazol-l-yl), Z = pyrid-4-ylamino
: . .:
A solution of 5.5 g of S-methyl pyrid-4-yl di-
thiocarbamate in 40 ml of dimethylformamide is added
~1 dropwise to a solution of 5.2 ~ of 2-amino-4-(imidazol-1-
`! yl)aniline, prepared in Example 60, and 6.6 g of red
mercury(II) oxide in 60 ml of dimethylformamide. The
mixture i~ stirred for 3 h at room temperature. The
solution is poured into 1800 ml of 3% hydrochloric acid
, .
.`'-1 .
~ . .
`;
.''
2~1122~
- 59 -
, .
and the mixture iisi refluxed for 1/4 h. It is filtered
~, hot and cooled. The pH is brought to 8 with a solution
of ammonia. The re~ulting precipitate is taken up in hot
~, ethanol and treated with active charcoal. An acid-base
!, 05 conver~iion makes it pos~ible to obtain 1.8 g of 2-(pyrid-
~ 4-ylamino)-5-(imidazol-1-yl)benzimidazole.
- Melting point: lôOC.
:; :
g Ex~mple 135 5-(Imidazol-1-yl)-2-(2,4-dimethoxyphenyl)-
benzimidazole
Formula (I) A = B = T = W = C, Rl = Rz = R3 = H, Y = 5-
(imidazol-1-yl), ~ = 2,4-dimethoxyphenyl
9.1 g of 2-amino-4-(imidazol-1-yl)aniline, pre-
pared in Example 60, are dis~olved in 150 ml of methanol.
8.ô g of 2,4-dimethoxybenzaldehyde are added. The mix-
ture is refluxed for 13 h. It ii concentrated under
vacuum and the concentrate is taken up with methylene -~
chloride~ethyl acetate to give a precipitate, which is
purified on silica gel to give 2 g of 5-(imidazol-1-yl)-
l 2-(2~4-dimethoxyphenyl)benzimidazole.
g Melting point: 188C.
~`.
25 E~mple 136 ~-(I~idazol-1-yl)-2-(2-methoxy-4-methylthio- -
phenyl)benzimidazole
Formula (I) A = B = T = W = C, R1 = R2 = R3 = H, Y = 5-
(imidazol-l-yl), Z = 2-methoxy-4-methylthio-
phenyl
. :
A mixture of 8.7 g of 2-amino-4-(imidazol-1-yl)-
aniline (Example 60) and 9.9 g of 2-methoxy-4-methylthio-
benzoic acid is added in portions to 200 ml of POCl3.
The mixture is refluxed for 4 h. The black solid ob-
''` ,
:~ :
, ~
2~ 2~
- 60 -
-
tained is filtered off and washed with ether. The
crystals are placed in 200 ml of 1 N hydrochloric acid
The mixture is filtered and the mother li~uors are ren- -
dered alkaline with 30% NaOH and extracted with chloro-
05 form. The extract is dried and concentrated. After par- ~ -
tial purification on silica gel, recrystallization from
! acetonitrile gives 2.1 ~ of 5-(imidazol-1-yl)-2-(2-
¦ methoxy-4-methylthiophenyl)benzimidazole.
Melting point: 182C.
'~ Example 137 5-(Imidazol-1-yl)-2-(2-methoxy-4-methyl-
~ulfinylphenyl)benzimidazole
Formula (I) A = B = T = W = C, Rl = Rz = R3 = H, Y = 5-
(imidazol-1-yl), Z = 2-methoxy-4-methyl-
sulfinylphenyl
~ ' '
3.6 g of metachloroperbenzoic acid are added in
small portions to a solution of 5.8 g of 5-(imidazol-1-
yl)-2-(2-methoxy-4-methylthiophenyl)benzimidazole, pre-
pared in Example 136, in 60 ml of CHCl3, the temperature
being kept below -30C. The mixture is stirred for 1 h
at -30C and the temperature is then allowed to ri~e to
room temperature. A saturated solution of NaHCO3 is
added. The mixture i~ extracted with chloroform, the
extract is dried and concentrated and the product is
taken up in acetonitrile to give 2.ô g of 5-(imidazol-1- ;~
` yl)-2-(2-methoxy-4-methylsulfinylphenyl)benzimidazole.
Meltin~ point: 208C. ~-
E~m~le 13R 5-(Imidazol-1-yl)-2-(pyrid-4-yl)benzimi-
dazole
Formula (I) A = B = T = W = C, R1 = Rz = R3 = H, Y = 5-
(imidazol-1-yl), Z = pyrid-4-yl
; ~.,; - : - ,
:~5:
2~ 222
- 61 -
9.4 g of 4-formylpyridine are added to a ~olution
of 15.3 g of 2-amino-4-(imidazol-1-yl)aniline (Example :
, 60) in 2~0 ml of methanol. The mixture is refluxed for
-~ 8 h. It i~ concentrated under vacuum and the crude pro-
. 05 duct is then taken up in 70 ml of nitrobenzene and heated
at 180C for 4 h. The ~olid obtained after distillation
of the solvent i~ partially purified on ~ilica gel and
~ then recry~tallized from methanol to give 4.5 g of 5-
- (imidazol-1-yl)-2-(pyrid-4-yl)benzimidazole.
` 10Melting point: 265C. : :
: :~
The compounds of Example~ 139 to 145 b~low could
be prepared by following the procedure of Example 138:
"
~:: 15ExamPle 139 5-(Imidazol-1-yl)-6-methyl-2-(pyrid-4-yl)-
benzimidazole ~:
Formula (I) A = B = T = W = C, Rl = 6-CH3, R2 = R3 = H, -~
Y = 5-(imidazol-1-yl), Z = pyrid-4-yl
' 20
~ Melting point: 288C.
Exampl~_140 5-(Imidazol-1-yl)-2-(pyrid-2-yl)benzimida-
zole
Formula (I) A = B = T = W = C, R1 = R2 = R3 = H, Y = 5-
:~ (imidazol-1-yl), Z = pyrid-2-yl
..
. Melting point: 219C.
;` 30
`- E~am~ 1 5-(Imidazol-1-yl)-2-(pyrid-3-yl)benzimida- zole :
x Formula (I) A = B = T = W = C, Rl = Rz = R3 = H 9 Y = 5-
^. 35(imidazol-1-yl), Z = pyrid-3-yl
. :
,.,
" .. ~
: l :
2 ~ 2
.1 - 62 -
"
:~ Melting point: 250C.
3 Example 142 2-(Pyr;d-4-yl)-5-(1,2,4-triazol-1-yl)benz- ~ -
imidazole :
l 05
-1~ Formula (I) A = B = T = W = C, R1 = Rz = R3 = H, Y = 5- ~ :
, ~1,2,4-triazol-1-yl), Z = pyrid-4-yl
.i~
Melting point: 248~C. -:
i 10 , -
I E~amGlQ-l9~ ~-(Imidazol-1-yl)-2-(2-methoxypyrid-3-yl)-
~, benzimidazole
~ ..
Formula (I) A = B = T = W = C, R1 = Rz = R3 = H, Y = 5- :
, 15 (imidazol-1-yl), Z = 2-methoxypyrid-3-yl
¦ Melting point: 242C.
~; :
Example 1~4 5-(2-Methylimidazol-l-yl)-2-(pyrid-4-yl)-
benzimidazole
. Formula (I) A = B = T = W = C, R1 = Rz = R3 = H, Y = 5- ~.
'j (2-methylimidazol-1-yl), Z = pyrid-4-yl
i~".l
Melting point: 295C.
Ex~mEle 145 5-(Imidazo~2,1-b]thiazol-3-yl)-2-(pyrid-4- :~
~: yl)benzimidazole
Formula (I) A = B = T = W = C, R1 = Rz = R3 = H, Y = 5-
(imidazo~2,1-b]thiazol-3-yl), Z = pyrid-4-yl
Meltin~ point: 332~C.
.,
. ,
,, :
;, j
",~: ."~.. " . " "
2011222
.
- 63 -
Ex~mple 146 2-(Pyrid-4-yl)-5-(2,4,5-trimethylimidazol-
1-yl)benzimidazole
Formula (I) A = B = T = W = C, Rl = Rz = R3 = H, Y = 5-
05 (2,4,5-trimethylimidazol-1-yl), Z = pyrid-4
yl
. . .
Melting point: 278C.
10 Exam~l~147 5-(4-Methylimidazol-1-yl)-2-(pyrid-4-yl)-
benzimidazole
Formula (I) A = B = T = W = C, R1 = R2 = R3 = H, Y = 5-
(4-methylimidazol-1-yl), Z = pyrid-4-yl
1 15
i Meltin~ point: 264C.
~`
Exa~ple 148 5-(2,4-Dimethylimidazol-l-yl)-2-(pYrid-4
yl)benzimidazole
Formula (I) A = B = T = W = C, R1 = Rz = R3 = H, Y = 5-
(2,4-dimethylimidazol-1-yl), Z = pyrid-4-yl
i
1 Melting point: 291~C.
~i 25
~ Ex~mple l49 5-(Benzimidazol-1-yl)-2-(pyrid-4-yl)benzimi-
i dazole
E'ormula (I) A = B = T = W = C, R1 = R2 = R3 = H, Y = 5-
i 30 (benzimidazol-1-yl), Z = pyrid-4-yl
`t Melting point: 280-282~C.
',' : ~
`' 35 ~
201~222
::~
64 -
:`
E~mPlQ_l~Q 6-Chloro-5-(imidazol-1-yl)-2-(pyrid-4-yl)-
, benzimidazole
1 .
Formula (I) A = B = T = W = C 7 R1 = 6-Cl, R2 = R3 = H,
Oi~ Y = 5-(imidazol-1-yl), Z = pyrid-4-yl
~j
. Melting point: 326C.
.,
EX~mElQ 151 4-[5-(Imidazol-1-yl)benzimidazol-2-yllacet-
anilide
~. Formula (I) A = B = T = W = C, R1 = R2 = R3 = H, Y = 5-
- (imidazol-1-yl), Z = 4-N-acetyla~inophenyl
~ 15 Melting point: 362C.
. ~
E~mPle 152 5-(Imidazol-l-yl)-7-methyl-2-(pyrid-4-yl)-
benzimidazole
~,
.`:,2
Formula (I) A = B = T = W = C, R1 = 7-CH~i, R2 = R3 = H,
Y = 6-(imidazol-1-yl), Z = pyrid-4-yl
,. ~ .
.. i : :
~ Melting point: 289C.
' !
;- 25 E~ample 163 7-Methyl-~-(4-methylimidazol-1-yl)-2-(pYrid-
~ 4-yl)benzimidazole
`. Formula (I) A = B = T = W = C, R1 = 7-CH3, Rz = Rs = H,
~ Y = i6-(4-methylimidazol-1-yl), Z = pyrid-4-
i~ 30 yl
. .
Melting point: 286C.
.~
.~
: '.
. .
' ~ " ' ~. . ' . ' ' :,
~ 2011222
- 65 -
Examp1~ 154 5-(2,4-Dimethylimidazol-1-yl)-7-methyl-2-
(pyrid-4-yl)benzimidazole
Formula (I) A = B = T = W = C, R1 = 7-CH3, R2 = R3 = H,
Z 05 Y = 5-(274-dimethylimidazol-1-yl), Z =
, pyrid-4-yl
:,
Z Melting point: 286C.
~'.`' ,
: 10 ~x2n~ 155 4-(Imidazol-1-yl3-2-(pyrid-4-yl)benzimida-
zole
Formula (I) A = B = T = W = C, R1 = Rz = R3 = H, Y = 4-
(imidazol-1-yl), Z = pyrid-4-yl
:l 15
¦ Melting point: 313C.
Melting point of the dihydrochloride: 320-325C.
~Z -
Ex~mple 156 8-Methyl-5-(4-methylimidazol-~-yl)-2-(pyrid-
4-yl)benzimidazole
Formula (I) A = B = T = W = C, Rl = 6-CHs. R2 = R3 = H,
Y = 5-(4-methylimidazol-1-yl), Z = pyrid-4-
-1 yl
~Z Melting point: 332C.
j~, E~amPle 157 6-Methyl-5-(2-methylimidazol-1-yl~-2-~pyrid~
~. 4-yl)benzimidazole
Z~
:~
,`3~ Formula (I) A = B = T = W = C, R1 = 6-CH3, Rz = R3 = H,
Y = 5-(2-methylimidazol-1-yl), Z = pyrid-4-
yl :'~
~':Z ' ~ :
,~Z 35 Melting point: 308C.
~.
, , . '~ ~
:
~ : .:
201~ 222
- 66 -
E~am~e~ , 5-(2,4-Dimethylimidazol-1-yl)-6-methyl-2-
(pyrid-4-yl)benzimidazole
Formula (I) A = B = T = W = C, Rl = 6-CH3, Rz = R3 = H,
05 Y = 5-(2,4-dimethylimidazol-1-yl), Z =
pyrid-4-yl
.' :
~ Melting point: 295C. ~ ~
`' 10 E~m~le 1~9 ~-(Imidazol-l-yl)-6-methyl-2-C4-(imida
' yl)phenyl]benzimidazole
Formula (I) A = B = T = W = C, R1 = 6-CHa, R2 = R3 = H,
Y - 5-(imidazol-1-yl), Z = 4-(imidazol-1-
yl)phenyl
Melting point: 300C
Ex~mE~ Q 6-Chloro-5-(2-methylimidazol-1-yl)-2-(pyrid-
~, 20 4-yl)benzimidazole
~,
Formula ~I~ A = B = T = W = C, R1 = 6-Cl, R2 = R3 = H,
il Y = 5-(2-methylimidazol-1-yl), 2 = pyrid-4-
Melting point: 324C.
~,`.
;~' Example 1~1 6-Chloro-5-(4-methylimidazol-1-yl)-2-(pyrid-
~, 4-yl)benzimidazole
Formula (I) A = B = T = W = C, Rl = 6-Cl, R2 = R3 = H,
~',, Y = 5-(4-methylimidazol-1-yl), Z = pyrid-4-
yl .'~.
~ 35 Melting point: 285C. ~ ~
.,,, ' '.
.~
: 20~222
:
- 67 -
` ~xample 1~2 5-(Imidazol-l-yl)-4-methyl-2-(pyrid-4-yl)-
benzimidazole
Formula (I) A = B = T = W = C? R1 = 4-CH3, R2 = R3 ~ H,
. 05 Y = 5-(imidazol-1-yl), Z = pyrid-4-yl
. ~ ,
I Melting point: ~83C.
'
Example 163 5-(Imidazol-1-yl)-2-(pyrid-4-yl)imidazo[4,6-
b]pyridine
, .
~, Formula (I) A = N, B = T = W = C, R1 = Rz = R3 = H, Y =
~` 2-(imidazol-1-yl), Z = pyrid-4-yl
I ,.
,,i
`~ 15 Melting point: 358-359C
. .
Ex~m~le 164 5-(Imidazol-1-yl)-2-(2-methylthiopyrid-3-
yl)benzimidazole ::
,,i ,
~ 20 Formula (I) A = B = T = W = C, R1 = Rz = R3 = H, Y = 5-
p, ~
(imidazol-1-yl), Z = 2-methylthiopYrid-3-yl
Melting point: 217-218C.
25 E~mElQ 16~ 2-(Imidazol-l-yl)-8-(pyrid-4-yl)purine ~:-
ù~l Formula (I) A = T = N, B = W = C, R1 = R2 = R3 = H~ Y = :
5-(imidazol-1-yl), Z = pyrid-4-yl
~i 30 Melting point: 340C (decompo~ition).
i''`'`'l :
; ' ~
~ 35
..
2 a ~ 2
- 68 -
Exqmple 166 5-(Imidazol-1-yl)-2-(pyrid-4-yl)-6-tri-
i fluoromethylbenzimidazole
Formula (I) A = B = T = W = C, Rl = 6-CF3, Rz = R3 = H,
05 Y = 5-(imidazol-1-yl), Z = pyrid-4-yl
~j
Melting point: 340C.
,
Example 167 6-Chloro-5-(imidazol-1-yl)-7-methyl-2-
~ 10 (pyrid-4-yl)benzimidazole
; Formula (I) A = B = T = W = C, R1 = 6-Cl, R2 = 7-CHs,
. R3 = H, Y = 5-(imidazol-1-yl), Z = pyrid-4-
' yl
Melting point: 316C.
:,
~, ExamPle 1~R ô~7-Dimethyl-5-(imidazol-1-yl)-2-(pyrid-4-
yl)benzimidazole
Formula (I) A = B = T = W = C, Rl = 6-CH3, Ra = 7-CH3,
R3 = H, Y = 5-(imidazol-1-yl~, 2 = pyrid-4-
~i yl
Melting point: 318C.
., ,Exa~mple 169 2-~1-(2-(Pyrid-4-yl)benzimidazol-5-yl)-2-
methylimidazol-4-yl~-2-methyl-1,3-dioxolan :~:
Formula (I) A = B = T = W = C, Rl = R~ = R3 = H, Y = 4-
(2-methyl-1,3-dioxolan-2-yl)-2-methylimi-
: dazolyl, Z = pyrid-4-yl
Meltin~ point: 255C.
.~ :
-' ~
2~1~222
~ 69 -
~,
Ex~ple 17~ 7-Ethyl-6-(imidazol-1-yl)-2-(pyrid-4-yl)-
-~ benzimidazole
Formula (I~ A = B = T = W = C~ Rl = 7-CHzCH~, R~ = R3 =
05 H, Y - 5-(imidazol-1-yl), Z = pyrid-4-yl
.,,~
~ Melting point: 280C.
. .
;,3
xample 171 5-(Imidazol-1-yl)-2-(6-chloropyrid-3-yl)-
benzimidazole
~; ,
f`.~' ~
~;! Formula (I) A = B = T = W = C, R1 = R2 = R3 = H, Y = 5-
(imidazol-1-yl), Z = 6-chloropyrid-3-yl
8.8 g of 6-chloronicotinoyl chloride in 50 ml of
toluene are added dropwi e to a solution of 10.2 g of 5-
(imidazol-1-yl)-2-nitroaniline, prepared in Example 46,
in 100 ml of pyridine. The reaction i~ exothermic. The
mixture is ~tirred for 3 h at room temperature and then ~ -
for 3 h at 50C. It i9 concentrated under vacuum, the
concentrate i~ taken up with water and the æolid obtained ~-~
filtered off and washed with water and then with iso-
propanol to give 13.7 g of 6-chloro-N-~5-(imidazol-1-yl)-
2-nitrophenyl~nicotinamide.
;~ 25 Melting point: 180C.
Catalytic hydrogenation of this compound in
~ methoxyethanol in the pre~ence of Raney nickel, according
`- to Example 70, is followed by treatment in 100 ml of
` acetic acid under reflux for 3 h. The compound obtained
i9 partially purified on silica gel and then recry~tal-
lized from 2-methoxyethanol to give 4.8 g of 5-(imidazol-
1-yl)-2-t6-chloropyrid-3-yl)benzimidazole.
- Melting point > 330C.
, '
.:
. ' . . !
': ~ ~ ' '' ' ' , `
20~222
- 7 0 -
TABIE
Code Formula Example
05
8057~12~ ~N
H , 3~2FUMAR~ATE
;3
1 5 S~CH,
66'1 ~N ~N 106
H . HCL ::
'
`~1 s
66-9 ~ ~C >cO 107
. HCL
. , .
S -
N9( ~1~ H
. 66-28 ~ ~N>CO 108
.` H
,
2~ ~222
- 7 1
CodeFormula ExamPle
1 05 ~j H
49-6 N~N>=
49 2 ~N~N~ 110
`, H , HCL
49'3 6N~
`' H :
~' 25 `
,':
N~CH3
49-4 ~_N NH)= 112
H H~
1 35
., .
, .Y~
~ 2~ ~222
;j - 72 -
j Code Formula Example
! ~
'~ 05 N~CH,
49-5 6 N~N~
, ~i H HCL
'1 10
.,
~CH,
49-8 6~ 114
H HCL 1
.'~ ,,.j"~
,
~, 20
49 9 H,C
., ~ .
49 10 6~ C )=0
". ,
~ ::
~,` , .: .
2Q1~222
-- 73 --
Code Formula Example
~j ~ 117
, N_N~N HCL ~'
49-18 ,~ y 1 18
~ ~
. HCL
~ ,:
49~19 ~ ~ 119
`Z 25 ~ ~cO
H :
~, .
` 30 N~
.j 49-20 ~N_l N 120
;'i~ ~ >CO : .'
H :
'''`~ , . ~
.: ,: ::
` 2~222
-- 7 4
Code Formula Example
:,i
'3 05 ~j y
1 49-24 N~ N 121
i ~--N
H,C H
' 10
49-27 ~N~cN 122
H HCL :
~,
. ' .
; 20 N~
49-7 6_N~NH~S 123
:, ,
49-13 ~N~N 124 ~
`. H :
. . .
, :
! .' ::
2~ 222
. . ,
- 75
,i~
.
j Code Formula Ex~mple -:
49-16 ~N 125
H
: 10
,~ .
i": '
~N~/CS 126
,,
1,
~1 ~
... .
N9 Y
49 29 ~ ~S
P H
~3
,. .
N~ -,
~I~HN~ 128
~; 35
r j ~ ~
2 ~ 2 ~
. - 76 -
Code Formula ExamPle
.1 .
49-15 ~ ~SCH,--CH=CH, 129
N
eNi N
49-17 ~C ~S--CH2--C=CH 130
H
49-21 N~N~ .~S--CH,--C= ICH 131
N~CH~ y ~ ~:
49-22 ~ ~S--CH,--Cil--CU 132
~` 35
', ,~' '
2~1222
~ .
-- 77 --
:. :
- Code Formula ExF~mple
05 ~N~j o :~
49-12 ~_N~cN~ 133
. H
,, 10
....
~`
S 15 100-6 N~N~ ~N 134
~'`''~ .
6,~CN~ ~;
~5 ~:
~ ':.'"'',~
D 30 100 2 ~N~SOH~ 136
H
~'~:3 . ~: ~
. ~ 35
-::
` - 2~ ~222
,
- 78 -
Code Formula Ex~mplo
; . ,
"
100~4 ~N~SCH, 137
H
,, 10
15100 3 ~ 9--~N 133
:i H :
: ~,'.;
i 20 N~
H,C~CH
~ .
~3 2~ ~:
.-..-..
s, 30100-7 6--N~ ~ 140
.`,'t H ~ ::
" '
. ~:
.. i~C ~ , ~ , . . .. . ~ . " . :. ,
2~ ~222
-- 79 --
Code Formula Example
,
~ ~
100-8 ~ ~ 141
H
J 10
100~10 ~ ~N 142 ~ ~
~ -:
N~
H
.1 25
CH~
100-9 ~ ~N 144
: H ;~:
~
: :
, ~ ~ . : .: .:: -. , : - `, , -
.
20~222
-- 80 --
.` Codo Formula Example
'.
~`
05 No(S--~
100-14 ~N~N~N 145
H
, .,
N~C~
" 15 100-16 ~N~
H
i~'l "~' ''
.. ~ . . .
CH~
100-17 ~CN~N 147
:~
100-18 CH~ CHJ 148 ;
\[~N~N
r I ~ . . :
' l
2~11222
-- 8 1
Code Formula Example
~ .
05
100-20 ~-- ~ ~N 149 ~ . ~
H .
'~ 10
.j :
' ': - '
100-Zl ~-~9~N 15
N~
100~22~}CN~ )~
~ 30 100-23 ~ 152
2 H ~
::
~]
~0~ ~22~
-- 82 --
Code Formula ExamPle
. .
o~ 9
100-24 ~C ~N lSl
CHJ
,,
" '';'';
~ .
N~CH3
100 25 ~C ~N 154
H ~-
CH3
¢N~ ~
100-26 ~N~N ~ :
: 30
N~ :
CH-~6 N~
H3C H
'. ' ~ '
:` 2~222
~ ~
-- 83 --
CodeFormula Example
.~
'i
05 N~CH, ~ :
100-2C ~N~
i, 10 -~:,
N~ H3
15100 29--~HN~N 15a
ZO
00-30~ 9~N~ 159
H,C H
: 1
, ` .
~,l, N~CH3
`i 100~31 )~ ~N 160
ji a H
:~ .
~, 35
~,,
.~,
.. ,~.. j... ;. . ,. ~ .,, . . -~ , . . .
2~1~ 222
~ B4 - ;
-I Code Formula Example ~
~ ~'
~,, 05 CHJ~
100-32 a~N~N 161
.~1 10 :
~ ~N 162
., . .
~ 20
;, 100-34 N//~N~ ~N
1 ~ H 163
:i 25
100-35 ~N~NN~ 164
i H
;~ 35
..
:' 1
~,;
20~1222
,;
- 85 - :
.. ,
-i Code Formula Exampl~ ~
, :
1 :
`: N~ 166
,~1 10
100-37 F,C H 166
100-38 6--1 N~ 167
CH3
r. I :
)I 100-39 ~ 168
~1 35
^~,",~
201 ~ 222
,
- 86 -
Code Formula Example ::
. s
. ~
~( j[ ~N 169
' 10
.j -, .
, ' .:
100 41 ~C ~N 170
:i H
CzH5
.
~0
100-12 ~N~ ~ 171
`~ :
:.
i,3 30 ~:
h
3~
:2`!
i.,~ .
':
.i .
,~
-~ ~
,........................................................................... ~:
i . .: ~ ~ :: -
` 20~222
, - 87 -
., ,~'~
P~sM~oroGY
'
~, The products of the Examples claimed possess
positive inotropic propertie~ and ulcer inhibiting pro-
l 05 perties. The following methods were u~ed to demonstrate
-~ and ~uantify these activities.
:i, - ,
Positive inotropic activity
, 10 Principl~:
The positive inotropic activity was assessed on
~i the isolated left guinea-pig atrium preparation stimu-
lated at constant fre~uency
The recording of the contractile force of the
atrium is used to define the po~itive inotropic effect.
Procedure
Tricolored male guinea-pigs are stunned and bled.
The heart is quickly removed and rinsed in a carbonated
l 20 Krebs-Henseleit solution. The atria are separated from
~ the ventricles and isolated from one another. The basal
;`j part of the left atrium is fixed to the organ support
!~4~ between two electrodes and the free part is wired to an
isometric tension sensor. The organ i9 immersed in a 50
ml cell thermoqtated at 32C and containing a Krebs-
;~ Henseleit solution. The preparation is subjected to a ~-~
i tension of 1 g and then stimulated by s~uare electrical
pulses with a fre~uency of 3 Hz, a duration of 1 ms and a
voltage 20 to 30% higher than the threshold voltage ~1 to
4 V). The contractile force is measured by means of a
tension sensor connected to a recorder. The preparation
is then left to stand for a 60-minute period, during
which the physiological medium is renewed every 15
minutes and the tension readjusted if necessary.
~ i` : -
1~ `
`"~
.
- 2 ~ 2
- 88 -
Expression of the re~ult.q:
The contractile force i~ measured after 5 minutes
of contact with the preparation
Wherever pos~ible, a 50% active concentration (M)
05 and it~ confidence limitis at p < 0.05 are calculated.
The ACso expresises the concentration of product which
cau~es an increase in inotropism equal to 50% of the
maximum inotropic effect obiserved, expressed by the
i maximum tenision recorded.
;, 10
Product Code AC~o - (M) and ¦ Maximum teni3ion
of no confidencedifference
Example intervaldeveloped in mg
(m + ~em)
81 100-3 8.8 E-5 558 + 68
(7.2 E-5; 1.1 E-4)
54 49-2 9.4 E-5 442
' 55 49-3 3.5 E-5 494
57 49-5 9.6 E-5 488 + 54
(7.4 E-5; 1.3 E-4)
53 49-6 4.5 E-5 682 + 121
(3.6 E-5; 5.5 E-5)
66 49-7 6.0 E-5 425 ~ 110
(4.8 E-5; 7.5 E-5)
58 49-8 4.9 E-5 283 + 30
59 49-9 5.4 E-5 558 + 61
(4.6 E-5; 6.2 E-S)
i` 71 49-11 6 0 E-5 604 + 84
(5.5 E-5; 7.3 E-5)
76 49-12 4.0 E-4 463 + 155
(3.3 E-4; 4.8 E-4)
67 49-13 2.7 E-5 275 + 63
61 49-14 2.0 E-5 529 + 90
__ _ _
,.::: :
'-
- 89 -
~i '
:' . .
Product Code A~60 - (M) and Maximum tension
of no. confidence difference
Example interval developed in mg
(m + G,em)
',
3 05 72 49-15 1.8 E-5 496 + 62
i (1.6 E-5; 2.2 E-5)
.J 68 49-16 1.4 E-4 513 + 61
(1.2 E-4; 1 8 E-4)
'~ 73 49-17 9.7 E-6 563 + 55
-j 10 (7.7 E-6; 1.2 E-5)
62 49-18 1.1 E-5 608 + 52
(9.0 E-6; 1 4 E-5)
63 49-19 1.4 E-5 338 + 38
, (1.0 E-5; 1.8 E-5)
'¦ 15 64 49-20 3.7 E-4 364 + 90
`,3 (2.7 E-4; 5.1 E-4)
69 4g-23 1.9 E-5 391 + 46
~ (1.6 E-5; 2.2 E-5)
,~ 49 8057-12 1.1 E-5 746
(6.0 E-5; 1.9 E-5)
66-1 2.0 E-4 325
51 66-9 1.4 E-5 669
~3 52 66-28 2.0 E-5 258 + 36
(1.2 E-5; 3.6 E-5)
134 100-6 6.5 E-5 533 + 89
(4.9 E-5; 8.4 E-5)
139 100-5 2.4 E-5 670 + 98
I,3 (1.7 E-5; 3.5 E-5)
?!, 30 141 100-8 8.2 E-5 625 + 69
(6.7 E-5; 1.01 E-4)
142 100-10 1.5 E-4 455 + 102
~, (8 E-5; 3 E-4)
~, 143 100-11 1.4 E-4 496 + 82
_ (9 E-5; 2.10 E-4) -
. .-!
"i
. ' ~
2Q.~:~2~
- 90 -
:,
Product Code AC60 - (M) and Maximum tension
s of no. confidence difference
Example interval developed in mg
; (m + sem)
i 05 144 100-9 4.3 E-5 708 + 89
(3 5 E-5; 5.3 E-5)
; 146 100-16 15 E-5 518 + 180
(8.10 E-6; 2.19 E-5)
148 100-18 2.6 E-5 337 + 77
` 10 (2.20 E-5; 2.80 E-5)
149 100-20 1.4 E-5 378 + 68
(1.1 E-5; 1.9 E-5)
152 100-23 1 3 E-5 443 + 76
(9 E-7; 1 8 E-5)
~ 15 156 100-27 5.6 E-5 490 + 87
; (3.27 E-5; 7.9 E-5)
,l~ 157 100-28 5.8 E-5 585 + 81
.~ ~3.97 E-5; 7.63 E-5)
158 100-29 3.7 E-5 453 ~ 52
~-, 20 (2.8 E-5; 4.5 E-5)
160 100-31 3.9 E-5 620 + 73
^ _ (3.56 E-5; 4.24 E-5)
. ~
i~ Ulcer inhiibiting activity
;~ 25
t~ i~hQ~
Groups of 10 to 30 male rats of the OFA ~train
(Iffa Credo), weighing 160-180 g, are placed on a water
diet 24 h before oral administration of the product
studied. Thirty minutes later, an ulcerogenic drug or a
necrosing agent (for example absolute ethanol) is admini~
stered orally at do~es which cause maximum ga~tric ulcer-
'~ ation in the control animal~. The stomachs are removed
either 6 or 1 h after the last treatment, depending on
the test. The gastric le~ions are then evaluated macro~
2 ~
, - 91 -
,
scopically (grading and measurement of the extent of the
~¦ ulcers).
~, ExpressiQn_~f_~h~ res~lt_:
~, 05 The result~ are expressed in the form of the 50%
active dose, determined graphically. This dose expres~es
the dose which is necessary to cause a 50% reduction in
¦ the ulcerous lesions observed in the control animals.
.. 1
~ 10 Resul~:
,,,~
,' _
Product Code Ulcer inhibiting Ulcer inhibiting
; of no.activity towards activity towards
i Example a necrosin~ agent a non-~teroidal
AD60 mg/kg p.o. antiinflammatory
; ADso mg~kg p.o.
,.,
, 123 49-711.11
s 128 49-114.~ 18.29
'`~ 20 124 49-134.5 _
;~; 129 49-150.33 _
130 49-170.48 9.24
i;''. ..
~ 25
`, Preliminary toxicity studies were able to show
.;
that the 50% lethal doses determined after oral adminis-
; tration to rats were greater than 300 mg.kg-1J represen-
. ting an advantageous therapeutic index.
CONCI.~SION
,` . -
In conclusion, the molecule~ de~cribed in the
present patent application possess particularly valuable -
positive inotropic, platelet aggregation inhibiting and
2 ~ 2 2
- 92 -
ulcer inhibiting properties.
The stimulation of the myocardial function
induced by these derivatives, and the protection induced
against ulcerogenic lesions, can be advantageously and
05 beneficially utilized in the treatment of cardiac in-
sufficiency and gastroduodenal ulcers by oral administra-
tion in the form of tablets or gelatin capsules con-
taining from 150 to 500 mg of active ingredient, or by
parenteral administration in the form of injectable
preparations containing from 30 to 200 ~g of active
ingredient
The invention also covers a method of preparing a
pharmaceutical composition, which comprises incorporating
a pharmaceutically effective amount of at least one
¦ 15 compound of formula (I) as defined above, or one of its
addition salts with a pharmacologically acceptable acid,
into a pharmaceutically acceptable excipient, vehicle or
carrier. -
The invention also covers a method for the thera-
peutic treatment of a mammal, including a human, whichcomprises administering to thi~ mammal a therapeutically
effective amount of at least one compound of formula (I)
as defined above, or one of it~ addition ~alts with a
pharmacologically acceptable acid, which may or may not
¦ 25 be incorporated in a pharmaceutically acceptable excipi-
ent, vehicle or carrier~
In one variant, this method of therapeutic treat-
ment consists in treating cardiovascular diseases.
In another particular embodiment, thiq method of
treatment involves the therapeutic treatment of gastro-
duodenal ulcers.
.
b
i: . ~ , - . . . . . . . . . .