Note: Descriptions are shown in the official language in which they were submitted.
201212~
D E S C R I P T I O N
The present invention relates to a transdermal system with
graduated drug release and to its use for the local or
systemic dermal drug administration in human and veterinary
medicine, or in cosmetics.
Transdermal therapeutic systems have become well-estab-
lished in the treatment of various diseases. Their major
advantage is the fact that the active substance after per-
meation through the skin is immediately systemically effec-
tive, thereby avoiding the primary liver passage which
always occurs in the case of orally administered active
substances, and that very constant plasma levels can be
achieved, if the system is adequately prepared. This is of
special importance for active substances which exhibit
short half-lives and therefore make necessary a constant
fresh delivery of the drug.
Since the system is applied externally, the intended func-
tion can thus be performed for a very long period of time -
some of the commercially available systems may remain on
the site of application for up to one week. This effect can
virtually not be achieved with oral systems, since they
leave the organism after one day at the latest due to diges-
2~2123
tion.
Such transdermal systems usually consist of a backing layerwhich is impermeable to the active substance, an active
substance reservoir, a fixing device for anchoring the
system to the skin, and a removable protective foil for the
skin side of the system. In a preferred embodiment of the
fixing device the skin side is rendered at least partially
self-adhesive.
In those systems the reservoir may have the form of a bag
containing the liquid or dissolved active substance, or it
may be a foil-like article comprising the active substance
as a polymer containing preparation.
The latter systems are also called matrix systems, and the
following statements relate to those kinds of systems.
If the reservoir of such a matrix system consists of only
one homogeneous layer and no further layers controlling the
active substance release are present between the side
facing the skin after application and the skin itself, the
system controls the active substance release; if the matrix
is supersaturated this is performed according to equation
1, if this is not the case according to equation 2.
2012~23
1/2
Q = ( DDR [CDR-cSDR~ * CSDR * t ) equation 1
1/2
Q = 2 * CDR * ( DDR t / 3.14 ) equation 2
Q : amount of active substance released at time t
t : time
DDR : diffusion coefficient of active substance in the
matrix
CDR : active substance concentration in the reservoir
CSDR : saturation solubility of the active substance in
the reservoir
Since Q is directly proportional the square root of time,
these equations are also called the root-t-law.
The active substance release is not constant in these
systems and rapidly decreases in the course of time.
If a more constant active substance release is desired,
this can be achieved by the use of so-called controlling
membranes.
Such a system consists of a backing layer, an active sub-
stance reservoir, a controlling membrane, a pressure-sensi-
2012123
tive adhesive layer for securing the system to the skinand a removable protective foil.
-D /1 * t
Q = Co * e equation 3
Co = initial concentration of active substance
D = diffusion coefficient of active substance in the mem-
brane
1 = thickness of membrane
For some groups of active substances constant release
rates and constant plasma levels may be undesired. For
example, in the case of blood pressure and circulation
influencing agents, calmatives and soporifics, psycho-
pharmacological agents, anodynes, angina pectoris agents,
antiasthmatics, and agents facilitating the curing of drug
addiction, e.g., nicotine.
In case of these substances it is advantageous to achieve
plasma levels adapted to the indiviual need.
In addition, some drugs are not administered constantly,
but only when need arises at probably very large inter-
vals. Such a substance which is already on the market
as transdermal system, e.g., is scopolamine against motion
sickness.
2012123
Other active substances suitable for such a temporary
administration, e.g., are anodynes, psychopharmacological
agents, calmatives, soporifics, or appetite-suppressing
agents.
In the case of a transdermal application of such sub-
stances, the transdermal system has to effect a variable
active substance flux through the skin during the period
of application, whereby an initial dosage provides for a
rapid commencement of effect, and a maintenance dosage
provides for a sufficiently long constancy or a prepro-
grammed decrease of plasma levels.
A transdermal therapeutic system to solve this problem has
been described in EP-A O 227 252. In this case, the active
substance in a reservoir is brought into contact with an
amount of penetration accelerator merely sufficient to
maintain the accelerated penetration only during a defined
initial phase of application. It is of disadvantage in
this case that each active substance has to assigned a
suitable penetration accelerator.
Another solution to this problem has been proposed in
DE-OS 36 42 931. In this case, at least two plaster cham-
bers lying side by side and being separate from each other
201 2123
are provided with different active substance concentra-
tions so that in the first application phase the release
of active substance from all chambers effects a high initi-
al dosage, while after evacuation of the chambers with low
active substance concentration only those chambers with
higher active substance concentration contribute to the
release and thus effect a lower maintenance dosage. This
system is expensive merely because of this chamber con-
struction, and requires special measures with respect to
the different adjustments of concentration in the cham-
bers.
It is accordingly the object of the present invention to
provide a plaster used as therapeutic system with graduat-
ed drug release for the administration of active sub-
stances to the skin, which avoids the compelling presence
of a penetration accelerator and - in excess of the prior
art - offers additional possiblities to control the active
substance release, and which furthermore can be manufac-
tured in a simple manner.
According to the present invention, this object is surpris-
ingly achieved in that the active substance containing
reservoir comprises at least one membrane located parallel
to the releasing surface, the surface of said membrane
being smaller in its dimension than the releasing area.
2012123
According to one aspect of the present invention
there is provided a transdermal system for the controlled,
graduated admlnlstratlon of an actlve substance to the skln,
for the release of a high initial dosage and a lower
maintenance dosage which consists of
a backing layer averted from the skin and
impermeable to the active substance,
an active substance reservoir adiacent to said
backlng layer the reservoir having a releasing surface, the
reservolr integrating at least one membrane located parallel
to the releasing surface, said membrane being less permeable
to the active substance than the reservolr and having a
surface area smaller than said releasing surface, ad~acent to
the skin,
a pressure-sensitive adhesive for securing the
system to the skin, and
a removable protective layer optionally covering
the surface of the system facing the skin.
In this connection, a membrane means an areal
flexible article whose permeability to components of the
active substance reservoir can also equal nil. Thus, for
example a thin metal foil is also comprised by the term
membrane. Usually the thickness of such a membrane rarely
exceeds 50 ~, however, thicker membranes are not excluded for
' 1
specla cases.
Normal membrane thlcknesses are from 20 to 100 ~m.
The membrane may elther be incorporated or embedded
in the
B 28483-11
2012123
membrane, or adjoin the reservoir on the skin side.
According to an embodiment of the present invention, the
membrane is impermeable to the active substance or sub-
stances to be released. According to a further embodiment,
the membrane exhibits a limited permeability to the active
substance or active substances. According to the present
invention it is also possible to combine two membranes
having different permeabilities to the substances to be
released. In this connection, at least one of these membra-
nes has a smaller surface than that of the releasing area
of the system. In this case it is of advantage that said
smaller membrane is impermeable to the substance or sub-
stances to be released, and incorporated in the reservoir.
The active substance or substances may be present in the
reservoir at a concentration below the saturation concen-
tration or at a concentration exceeding the saturation
concentration.
The reservoir itself may consist of several layers of
different composition.
In principle, the same materials as described for common
systems can be used for all components of such a system.
These materials are known to the man skilled in the art.
2012123
The backing layer may consist of flexible or inflexible
material, and may be constructed single or multi-layered.
Substances suitable for its production are polymeric sub-
stances, such as, e.g., polyethylene, polypropylene, poly-
ethylene terephthalate, polyurethane, or polyamide. As
further materials metal foils, e.g., an aluminum foil
alone or coated with a polymeric substrate may be used.
Textile fabrics may be used, too, if the components of the
reservoir cannot leave the reservoir via the gas phase due
to their physical properties.
In principle, the same materials may be used for the remov-
able protective foil, however, they must additionally be
rendered dehesive.
This dehesive preparation can be achieved by a special
siliconization.
The reservoir or the layers of the reservoir, respective-
ly, consist of a polymeric matrix and the active substance
or substances, whereby the polymeric matrix exhibits such
a self-adhesiveness that the coherence in case of a multi-
-layer construction is guaranteed. The polymeric material
of the matrix may, e.g., be built up of polymers, such as
rubber, rubber-like synthetic homo-, co-, or blockpoly-
2012123
mers, polyacrylic acid esters and their copolymers, poly-
urethanes, copolymers of ethylene and polysiloxanes. In
principle, all polymers are suitable which are used in the
manufacture of pressure-sensitive adhesives and are physio-
logically acceptable. Additives may also be used, their
nature depends on the polymer used and the active sub-
stance or substances. Depending on their function they can
be divided into softeners, tackifiers, resorption agents,
stabilizers, or fillers. Substances suitable for this
purpose and physiologically acceptable are known to the
man skilled in the art.
All physiologically acceptable foil-like materials having
the adequate permeability to the active substance or sub-
stances or auxiliary agents, respectively, are suitable
for the manufacture of the membranes. Membranes on the
basis of polyethylene, polyamide, ethylene-vinyl ace-
tate-copolymers, and polysiloxanes are particularly suit-
able.
In the following, the invention is further illustrated but
not limited by the drawings:
igure 1 shows a section through a system provided with a
controlling membrane according to the
prior art,
2012123
igure 2 shows an embodiment of a system according to the
present invention,
igure 3 shows another view of the matrix of figure 2
with a membrane incorporated into the
matrix,
igure 4 shows different embodiments of the membrane,
igure 5a shows a section through an embodiment of the
present invention exhibiting a combination
of an impermeable membrane with a membrane
of higher permeability,
igure 5b shows a top view on the embodiment of Figure 5a,
igure 6 shows diagrammatically the release behaviour of
an embodiment with a membrane incorporated
in the reservoir and impermeable to the
active substance in the form as shown in
Figure 4, whereby the cumulated released
amount of active substance is shown as
function of time,
igure 7 shows the release behaviour of the same system
2012123
- 12
as shown in Figure 6, however, diagram-
matically the active substance release
rate per system and hour as function of
time,
Figure 8 shows in a diagram the release behaviour of a
system according to the present invention
with a membrane of limited permeability to
the active substance, whereby the cumula-
tive active substance release is plotted
versus time,
Figure 9 shows diagrammatically the release rate of the
same system as shown in Figure 8 per sys-
tem and hour as function of time.
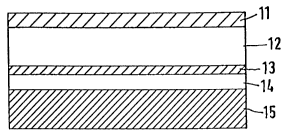
The system described in Figure 1 consists of a backing
layer (11), the active substance containing reservoir
(12), the controlling membrane (13), a pressure-sensitive
adhesive layer for securing the system to the skin (14),
and a removable protective foil (15).
In some cases the pressure-sensitive adhesive layer (14)
has the same formulation as the reservoir (12) so that the
membrane is actually incorporated in the reservoir, and
thus one can imagine the reservoir being built up of two
2012123
parts.
If the active substance is present in the reservoir at an
oversaturated concentration, a release according to a
kinetic O is achieved by the membrane. Order, i.e., a
constant release within the application period; and if the
active substance is present below this concentration, a
release according to a kinetic 1 is achieved.
Order according to equation 3.
Figure 2 shows the general construction of a system accord-
ing to the present invention. It consists of a backing
layer (21), a reservoir (22), a membrane (23), a self-ad-
hesive skin coat (24), and a removable protective foil
(25).
Membrane (23) is smaller than the reservoir surface, since
the membrane has a central, circular recess.
In Figure 3 a membrane (31) is incorporated in the reser-
voir (32) which for this reason is divided into two halves
(33 and 34). If the reservoir formulation is self-adhe-
sive, the self-adhesive skin coat (24) of Figure 2 can
naturally be omitted. Due to the fact that the surface of
the membrane is always smaller than the total area of the
reservoir, reservoir and skin, or reservoir and pressure-
2012~23
- 14
-sensitive adhesive layer, or both parts of the reservoir,
respectively, are in direct contact with each other on
that surface which is not covered by the membrane.
Figure 4 shows some examples of geometric forms of such
membranes according to the present invention, in which
either the hatched areas or the areas without hatches are
membranes.
The embodiments according to the present invention as
shown in Figures 3 and 4 are particularly suitable for
systems with only one membrane being impermeable to the
active substance, e.g., such as is shown in Figure 4.1 and
integrated into the reservoir according to Figure 3.
At first, this system behaves like a common matrix system,
i.e., the active substance is released over the whole
releasing surface according to the so-called root-t-law.
However, as soon as the reservoir member, which is posi-
tioned below the membrane area, is emptied so far that the
depletion zone has reached the membrane, the behaviour of
the reservoir compared to a common matrix system changes
drastically. The active substance release decreases rapid-
ly on the surface having the same dimension as the membra-
ne, while on the partial surface which is not covered by
the membrane the release continues undiminishedly accord-
~012123
ing to the root-t-law until the depletion zone reaches the
backing layer. Thus, the additional initial dosage origi-
nates from the area lying below the membrane. By way of
changing the absolute area sizes and the relation between
membrane surface and total surface of the reservoir, the
amount of initial dosage and maintenance dosage can be
influenced within wide ranges.
As a matter of fact, such a release behaviour can also be
achieved in that the reservoir is given a steplike geo-
metry. However, this bears the disadvantage that such a
system is more difficult to produce, and that due to the
plastic deformability of usual reservoir formulations such
a system does not maintain its steplike shape.
Embodiment 4.5 is particularly advantageous, since there
are no problems concerning positioning due to the variety
of holes within the membrane.
By way of changing the ratio of membrane surface to the
total surface, and the choice of membranes of different
permeabilities to the active substance, the release be-
haviour of the system can be influenced in wide ranges, as
is stated below. It is particularly possible administer
very high initial dosages.
201~23
16
Figures 5a and 5b show in sectional view and in top view
an embodiment of the present invention which is provided
with a combination of tow different membranes.
The combination of a membrane having permeability "O" with
a membrane of higher permeability is particularly suit-
able. Such a system is shown in Figure 5a. It consists of
the impermeable backing layer (51), the reservoir (52), a
membrane of permeability O to the active substance or
substances (53), a membrane of a higher permeability than
O to the active substance or substances (54), and a remov-
able protective layer (55).
Both membranes are once more shown in Figure 5b in top
view. As a matter of fact, the membrane having permeabil-
ity O must be smaller than the total releasing surface of
the system, thus limiting the maximum active substance
release on that partial area of the total releasing sur-
face corresponding to the membrane size, since that por-
tion of active substance lying above the membrane cannot
pass it.
The membrane having the permeability higher than O effects
an active substance release according to a kinetic of
order O or 1 on that partial area of the total releasing
surface corresponding to its size.
2012:123
_ 17
Both membranes need not necessarily lie in the same plane
within the system. Their exact position depends on the
individual requirements, and it is an additional means to
achieve the desired release behaviour.
If the membrane having permeability O lies closer to the
releasing surface, the other membrane may be as large as
the total releasing surface without changing the release
behaviour, since said membrane is of no effect, if it lies
above the impermeable membrane.
Figures 6 and 7 show the release behaviour of those
systems having a membrane which is impermeable to the
active substance, as example a scopolamine plaster is
chosen. The following indications apply to all samples
described in the following: the active substance content
amounts to 450 ,ug/cm2 and the weight per unit area of the
self-adhesive reservoir amounts to 12.5 mg/cm2.
Figure 6 shows the cumulated released amount of scopol-
amine as function of time.
Curve I corresponds to a normal system of 2 cm2 size with-
out membrane, and serves as comparison.
Curve II corresponds to a system of a total size of 3 cm2;
~012~23
_ 18
an impermeable membrane is incorporated into the reser-
voir. The membrane has an area of 1 cm2 and divides the
reservoir into one layer having an area weight of 10.4
mg/cm2 and one having an area weight of 2.1 mg/cm2.
Curve III corresponds to a system of a total area of 4 cm2
and a membrane surface of 2 cm2.
It can clearly be recognized that on the whole the active
substance release of the systems provided with membrane is
higher. However, in Figure 7 it can be recognized more
clearly that this increased active substance release only
applies to the initial phase of release. Figure 7 shows
the release rate per system and hour as function of time,
i.e., the flux is indicated.
Thus, this system is particularly useful, if relatively
high initial dosages shall be combined with a maintenance
dosage which is not necessarily constant.
Figures 8 and 9 show an embodiment according to the pres-
ent invention having a membrane of limited permeability to
the active substance; as example a scopolamine plaster was
used under the same conditions as described for Figures 6
and 7.
2012123
_. 19
The cumulative release is shown in Figure 8, the flux is
shown in Figure 9. Curve I or flux I, respectively, corres-
ponds to a system of 2 cm2 having a membrane of the same
size (comparison), curve II or flux II, respectively,
correspond to a system of 2.5 cm2 having a membrane of 2
cm2, and curve III or flux III, respectively, correspond
to a system of 3 cm2 size having a membrane of 2 cm2.
Even the system according to curve I and flux I is able to
release a certain initial dosage. This initial dosage
corresponds to a release according to the root-t-law ac-
cording to equation 1 and 2, which takes place until the
depletion zone of the active substance has reached the
membrane.
The other two systems provided with membrane, which are of
smaller dimension than the releasing surface of the system
the initial dosage can be increased very easily. In this
case, the intitial dosage is followed by a constant main-
tenance dosage the amount of which depends on the
permeability and the surface of the membrane.
Production of the systems according to the present
invention used in Figures 6 to 9 (samples)
2012123
_ 20
230 g polyacrylate resin adhesive (50 % in acetic ester)
6 g scopolamine base
10 g Cetiol S
50 g methanol
were mixed and the mixture homogenized.
A siliconized polyester foil of 100 ~ thickness was coated
with this mixture as films of 400 ~ (film I) and 100 ~
(film II), the films were dried at 50C for 15 minutes.
After drying, film I had a weight per unit area of 103
g/mZ and film II one of 21 g/m2.
The membrane having circular recesses of adequate size was
laminated on film II, and film I in turn was laminated
thereon. The siliconized polyester foil of film I was
removed and substituted for an unsiliconized foil of 15
thickness.
The individual samples were punched in such a way that the
adequate total area resulted and the recess became posi-
tioned centrally.
Performance of the in vitro-release
- 2012~3
The release was carried out at 32C according to the
paddle-over-disk-method using 50 ml physiological saline.
In order to determine the samples the total release medium
was changed completely and the content determined accord-
ing to a HPLC-method.