Note: Descriptions are shown in the official language in which they were submitted.
' 2~21~5
,~ .?`
: ~ocket No. 72-21G
PHOTOCHEMICAL TREATMENT OF BLOOD VESSELS
:
Back~round of the Invention
1. Field of the Invention
The present invention relates generally to the use
of photoreactive dyes to treat vascular tissue. More
particularly, the present invention involves using
photoreactive dyes to selectively destroy unwanted blood
vessels in normal tissue.
2. Description of Related Art
In the mid-1960's, researchers began using
photosensitive agents to treat malignant tumors. One of
the most popular photosensitive agents used in such
treatments is a dye known as hematoporphyrin derivative
(HPD). In purified form, HPD is known as Photofrin II.
It was found that the HPD preferentially congregates in
cancer celis or in vessels feeding the malignant tumor.
The HPD has no effect on the tumor until it is energized
by radiation. The energized HPD creates toxic molecules
that selectively kill the tissue where the ~PD is
located. The use of such photoreactive dyes to destroy
abnormal tissue is commonly referred to as photodynamic
therapy.
HPD has been used to treat patients for many kinds
of solid tumors, including those of the skin, lung,
bladder, eye, neck, and esophagus. Usually, the patient
who is being treated will receive about two milligrams
of Photofrin II per kilogram of body weight. The
Photofrin is typically injected into the bloodstream of
the patient over a period of about five minutes. The
patient must be immediately protected from bright light
to prevent undesirable non-selective photoactivation of
the Photofrin II. The physician then waits two to three
days for the Photofrin II to congregate in the malignant
tumor while the remainder of the Photofrin II is washed
;' '~
, ~: .: '~
! ~ .
2 0 ~ 2 ~
-2-
from normal tissues by the patient's system. The
physician generally then uses an argon-pumped dye laser
to shine liyht on the patient's tumor and surrounding
area. The laser is focused directly on exterior tumors
or those located within 3 to 10 mm of the skin surface.
When tumors are located inside the body, the doctor
utilizes an optical fiber to deliver the radiation to
the tumor.
The use of photodynamic therapy has been limited to
the treatment of malignant tissues. The limitation is
due, for the most part, because of the unique ability of
malignant tissues to retain HPD long after it has been
:, :
washed from normal tissues. Even so, there is a
continuing need to use this valuable form of therapy to
treat other disorders which are not malignant.
There is presently a need to provide improved
therapeutics for treating hypervascular dermal lesions,
such as port-wine stains. Hypervascular dermal lesions
and other non-malignant tissue disorders are presently
treated with lasers. The treatment utilizes
photothermal ablation whereby absorbed energies are
converted to heat. The lesion is destroyed either by
direct thermal denaturization or by propagated shock
waves due to near instantaneous heating provided by the
laser. A problem with this type of treatment is
confining and localizing the thermal injury to a
specific tissue location.
In order to reduce injury to surrounding tissues,
shorter laser pulse durations have been used which
produce a more confined shock wave effect and less
thermal conductivity. Although some success has been
achieved in reducing injury to the surrounding tissues,
there continues to be a "photothermal overflow" which
causes injury to surrounding tissues. The thermal
injury to surrounding tissues is especially undesirable
- :-~ . .-,
,.,. ~. . . ~:
,',: ~:' ' ':. ,
. ' ~'
~21 7.~
. . .
when dealing with skin lesions where cosmetic appearance
is an important consideration.
The use of photosensitive dyes in combination with
laser treatment would appear to be desirable in reducing
the thermal damage to normal tissues surrounding the
hypervascular dermal lesions. ~lowever, photosensitive
dyes have not been shown to selectively congregate at
such non-malignant dermal lesions. Accordingly,
photodynamic therapy has not been suggested for use in
treating such non-malignant dermal lesions. It would be
desirable, however, to provide a process based on -
photodynamic therapy for treating such blood vessel
disorders, even though the photosensitive dyes do not
selectively migrate to such tissues. ~ ~-
` -
Summarv of the Invention
In accordance with the present invention, it was
discovered that blood vessels can be photochemically ~ `-
treated to provide selective destruction while, at the
20 same time, limiting damage to surrounding tissues. -
The present invention is based on a method wherein
a therapeutic amount of a photoreactive compound is
introduced into the blood flowing through the blood
vessel. It was discovered that photoreactive compounds ~ ;
associate with the blood vessel wall. The photoreactive
compound is allowed to accumulate in the blood vessel
wall for a sufficient time to provide a sufficient
amount of photoreactive compound within the blood vessel
wall to destroy the blood vessel when the photoreactive
compound is activated by radiation from a laser or other
source.
As a feature of the present invention, the -~
photoreactive compound associated with the blood vessel
wall is activated prior to the time at which the
35 photoreactive compound leaves the vessel and either -~
enters the surrounding tissue or is washed away. It was
2~217~
discovered that sufficient photoreactive compound is
present within blood vessel walls to allow photodynamic
destruction of the blood vessel tissue withou-t affectiny
surrounding tissue. As a result, the method of the
present invention allows the destruction of undesirable
blood vessels while limiting the amount of harm to
surrounding tissues.
The method of the present invention is particularly
well suited for treating hypervascular dermal lesions,
such as port-wine stains. The method eliminates many of
the cosmetic problems associated with thermal
destruction of surrounding tissue. Further, the method
takes advantage of the selectivity of tissue destruction
provided when using photoreactive compounds.
The above discussed and many other features and
attendant advantages of the present invention will
become better understood by reference to the following
detailed description when considered in conjunction with
the accompanying drawings.
Brief Description of the Drawings
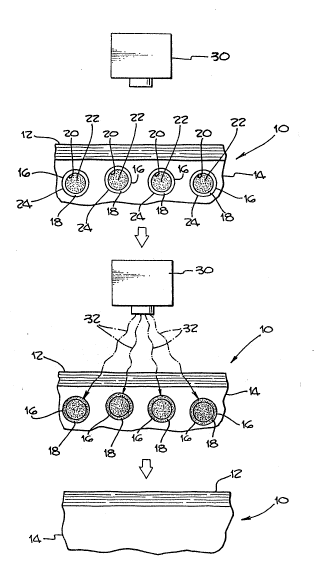
The drawing is a schematic representation of a
preferred exemplary method in accordance with the
present invention.
Detailed Descri~tion of the Preferred Embodiments
The present invention involves photochemical
therapy which may be used to treat a wide variety of
abnormal blood vessel conditions. The method may be
used for a wide variety of situations wherein it is
desired to selectively destroy or-e or more blood
vessels. The invention is particularly well suited for
treating hypervascular dermal lesions. Accordingly, the
following description of a preferred embodiment will be
limited to describing the treatment of hypervascular
dermal lesions with it being understood that the method
~11 217~
, . .. .
-5-
may be used for photochemically treating other blood
vessel abnormalities.
Hypervascular dermal lesions, such as port-wine-
stains or spider veins are abnormal assemblies of blood
vessels located within the dermis. The drawiny depicts
schematically the process in accordance with the present
invention for treating such abnormal blood vessel
assemblies. A portion of the skin to be treated is
shown at 10. The skin is shown as an extremely
simplified cross section showing the epidermis 12 and
dermis or corium 14.
The dermis 14 includes blood vessels 16 which are
shown in the simplified cross section. 'l`~le various
structures present in the dermis, such as nerve endings,
sweat glands, hair follicles and sebaceous glands are
not depicted in order to simplify the pictorial
description of the method. The blood vessels 16 include
a blood vessel wall 18 which is made up of blood vessel
tissue cells. The blood vessels 16 have an interior
surface 20 which defines a blood flow zone 22 through
which blood flows. The blood vessels 16 also include an
exterior surface 24 which defines the outer perimeter of
the blood vessel 16.
The first step in the method involves introducing a
therapeutic amount of a photoreactive compound into the
blood flowing through the blood flow zone 22. The
photoreactive compound is shown schematically as dots
within the blood vessel 16. The photoreactive compound
can be any of the known photosensitive dyes which are
suitable for use in photodynamic therapy. These
compounds include hematoporphyrin derivative (llPD) and
the purified form of HPD known as Photofrin II. These
compounds are commercially available. These compounds
and other porphyrin derivatives for use in photodynamic
therapy are described in United States Patents Nos.
4,649,151; 4,699,903; 4,692,439; and 4,753,958. The
~2~7~
-6-
contents of these United States Patents are ~ e~y
incorporated by reference. Although a variety of
porphyrin based compounds are available, Photofrin II is -
a preferred photoreactive compound. Other suitable -~
5 photoreactive compounds include chlorins, -~
.:. : .
phthalocyanines and purpurin.
The photoreactive compound is injected
intravenously into the patient. The dosage level should
be between about 1 milligram per kilogram of body weight
to about 3 milligrams per kilogram of body weight. The
dosage level may vary depending upon the compound being
administered and the lesion being treated. The
particular dosage levels which will be most effective
can be established by routine experimentation. Such
dosage levels may be as low as 0.1 milligram per
kilogram of body weight or as high as 5 milligra]ns per
kilogram of body weight. The photoreactive compound is
injected as a solution in which the photoreactive
compound is dissolved in a suitable pharmaceutical
carrier. Any of the pharmaceutical carriers which have
been used in the past for injecting porphyrin dyes for
photodynamic therapy may be used. These pharmaceutical
carriers include physiological saline which is a
preferred pharmaceutical carrier.
. : -.. ,, ,. ,:,
The top portion of the drawing at (l) depicts a -~
portion of skin immediately after intravenous injection
of the photoreactive compound. As can be seen, the dots ~ -
representing the photoreactive compound are limited to ~ s~
the interior of the blood vessels 16. The photoreactive
30 compound is then allowed to accumulate in the blood ;`~
vessel wall 18 for a sufficient time to provide a
sufficient amount of photoreactive compound within the
blood vessel wall 18 to destroy the blood vessel when
the photoreactive compound is activated by a laser in ; ;,
35 accordance with known techniques. This condition is i
shown in the middle of the drawing at (2) where the
: ~ ~: ~ : ,: .
: ` - ', ' "'
~ 217~
.
.
--7--
photoreactive compound, as represented by the dots, has
migrated from the interior of the blood vessel 22 out
into the blood vessel walls 18. At this point, the
laser 30 is directed onto the blood vessel 16 to
activate the photoreactive compound and destroy the
blood vessel. The irradiation of the vessels by laser 30
is represented by arrows 32. The laser does not have to
focus on individual blood vessels. In fact, any light
source of correct color absorbed by the dye is suitable.
It was discovered in accordance with the present
invention that sufficient quantities of photoreactive
compound are present in the blood vessel wall to cause
destruction of the blood vessel even when accumulation
of the photoreactive compound is limited to the blood
vessel. Limitation of accumulation is achieved by
irradiating the skin with laser 30 after only a
relatively short period of time. As was previously
discussed, conventional photodynamic therapy of tumors
requires a waiting period of at least twenty-four hours
prior to irradiation. In the present method, the skin
is irradiated within one to four hours after intravenous
injection of the photoreactive compound. Shorter or
longer waiting periods may be used depending upon the
type of patient being treated and the particular
compound being used. A waiting period of about two
hours is preferred. The two-hour time period was found
to optimize blood vessel destruction while limiting
damage to surrounding tissues.
The wavelength and intensity of laser radiation
directed at the blood vessels 16 can be varied within
the range of intensities and wavelengths commonly used
in photodynamic therapy. Wavelengths of between about
400 to 700 nanometers are acceptable. The preferred
wavelength range for HPD is between 00 to 650
nanometers. Wavelengths in this range are preferred
since they have sufficient energy to activate hemato
23121~
..:
porphyrin compounds to destroy the blood vessels while
at the same time being able to penetrate throuyh the
skin to depths sufficient to reach the blood vessels.
630 nanometer laser light has been found to be
particularly effective in treating blood vessels located
in the skin. For blood vessels located at locations
other than in the dermis, suitable means must be used
for focusing laser light onto blood vessels. Various
optical fiber devices commonly used to direct laser
light onto tumors can be used for this purpose. The
controlling consideration is to match the wavelength
with the absorption by the dye and the location of the
lesions. For example, shorter wavelengths which
penetrate less deeply into the tissue, are more suitable
for superficial lesions.
Destruction of the blood vessels as represented at
(2) in the drawing, results in hemorrhaging and
coloration of the skin 10. However, after normal
healing, the skin 10 remains devoid of the abnormal
blood vessels as represented at (3) at the bottom of the
drawing.
Examples of a practice are as follows:
Example 1
The effectiveness of the present invention was
demonstrated by treating chicken combs. It has been
shown that chicken combs provide an accurate model which
represents the hypervascular lesions which are found in
abnormal human skin. Accordingly, demonstration that
the method is effective in destroying chicken comb blood
vessels is indicative of the effectiveness of the method
in connection with treating human hypervascular lesions.
Twenty chickens weighing 2-1/2 to 3-1/2 kg
underwent anesthesia with methohexitone sodium 1%
solution at 10 mg/kg/ IV 2 cc. Some of the chickens
were not anesthetized because the procedure is painless
due to the lower power densities which are used.
; ~ : ,.:
, ~ ' . ~, ': -
2~2~7~
,~ ~
g
Photofrin II (DHE) was obtained and prepared by the
method of Nelson et al. and injected without dilution
intravenou~ly at lo mg/kg. Tl-e Photofrill II was
injected as a solution of 2.45 mg Photof'rin per 1 ml of
solu. The laser used was a Krypton laser coherent
Innova so of 405 nm wavelength. Using a 1 sq cm
circular hole stencilled in a cardboard template, 3
chicken combs were irradiated immediately post-
injection. 3 additional combs were each rradiated at
post-injection times of 1 hr., 2 hr., 3 hr., and 4 hr.
Irradiation parameters were 90 mW for 3 min. 42 sec. for
a total energy of 30 j. Animals were examined and
..; . ,
photographed for a period of 2 weeks.
Post-injection intervals prior to irradiation were
compared by observing the chronological extent of
persistent comb blanching. Blanching of the chicken
comb is recognized as an effective measure of blood ;~
vessel destruction. The 2 hr. areas showed the most
prominent blanching effects which persisted for 7, 9 and
20 14 days. All other post-injection intervals areas had ~ -
less pronounced blanching and returned to baseline
within 2 to 4 days. ;~
After determining the optimal post-injection
interval of 2 hours, two different portions of 10
chicken combs underwent 2 irradiations, each after a 2-
hour injection interval. The radiation levels were:
-9OmW for 554 seconds for a total irradiation of -~
50 joules
-90mW for 828 seconds for a total irradiation of
75 joules
Animals were photographed prior to being - ;~
sacrificed. Two animals were then sacrificed at post-
irradiation times of 1 hr., 24 hr., 72 hr., and 1 week. ~ ~ -
Samples of the 50 joule regions were sectioned for light
microscopy (LM), scanning electron microscopy (SEM) and
transmission (TEM) electron microscopy (TEM). , -
` 2~2~7~ ~:
.~. .
-10-
,. .
Comparative histology was achleved by examininy
combs in which
Baseline - no Photofrin II or irrad.ation
administered
5Control - (LM) and transmission (EM) were compared
to a control group that underwent the same irradiation
protocol but without prior sensitization with DHE.
Evaluation of the epidermal effect was performed by
SEM on each area irradiated with 50 joules. In addition
lo to comparison with the baseline micrographs, each area
was compared to a neighboring un-irradiated zone.
All of the combs showed immediate post-irradiation
darkening which persisted for 15 to go minutes before
gradual conversion to blanching. Initial blanching was
more prominent in the 75 joule group. For the ensuing
several days that blanching persisted; zones of
intermittent darkening and blanching were observed.
Return to baseline color was more rapid in the 50 joule
group which had nearly returned to baseline by 4 to 5
days. The 75 joule group had scattered areas of
persistent blanching at 1 week.
It was noted that the changes occurred specifically
in the region of the comb vasculature. Dramatic
swelling of erythrocytes and apparent ballooning of the
vessels was noted on both LM and EM corresponding to the
period of darkening. There was subsequent progression
to prominent vessel wall swelling with absence of
intraluminal erythrocytes and presence of enlarged
endothelial cells which corresponded to the development
of gross blanching. From 24 hours to 1 week, histologic
sections revealed zones of patent vessels alternating
with zones of persistent occlusion. At one week, there
was both areas of patent vasculature and areas of
occlusion. ~owever, open vessels re~ealed persistent
stasis and crowding of normal RBC's. The epidermal
- 2~217~
-11- , .
:
layer remained unchanged compared to the baseline
epidermal layer.
The blanching of the combs remained for up to 12
days followed by a gradual return to normal coloration.
Example 2
The same method as Example 1 was used to treat a
number of chicken combs except that 630 mm red light
from a dye laser was used instead of 405 nm light and
all times between injections and irradiation were 2
hours. The results were similar except that permanent
blanching of the chicken combs was achieved. As in
Example 1, there was no damage found to the epidermis or
other tissue of the comb surrounding the destroyed blood
vessels.
15Having thus described exemplary embodiments of the ~
present invention, it should be noted by those skilled -~ -
in the art that the within disclosures are exemplary -
only and that various other alternatives, adaptations,
and modifications may be made within the scope of the
present invention. Accordingly, the present invention
is not limited to the specific embodiments as
illustrated herein, but is only limited by the following
claims.
,.,',.....
''"' ~'-~"~""'.'"'