Note: Descriptions are shown in the official language in which they were submitted.
TYRPHOSTINS FOR TREATMENT OF ALLERGIC, 012634
INFLAMNATORY AND CARDIOVASCULAR DISEASES
FIELD OF THE INVENTION
This invention pertains to the novel use of composi-
tions containingbenzylidenemalononitrileand hydroxycinnamamide
derivatives in the treatment of asthma, allergic diseases, hay
fever, skin rashes, inflammatory bowel diseases, arthritis, adult
respiratory distress syndrome (ARDS), migraine, cardiac shock,
septic shock, thrombosis, hypotension, hypertension and ischemia. -
BACKGROUND OF THE INVENTION
15Tyrphostins are a group of low molecular weight
compounds, having the nucleus of benzvlidenemalononitrile and/or
hydroxycinnamamide. These compounds are reported to be inhibi-
tors of protein tyrosine kinases and their use in the treatment
of cancer has been recommended. I have found that these agents
also inhibit the action of the mediators of asthma, inflammation
and cardiovascular diseases.
Compounds having the nucleus of benzylidene malono-
nitrile such as N-~2-(2, 5-dihydroxyphenyl~ ethenyl) formamide,
identified with the trade-mark Erbstatin, have been known for
several years. Such compounds have the following formula:
I~D / ~CHo
H
OH
20~2634
These compounds were initially described as inhibitors
of protein tyrosine kinase and their use in cancer chemotherapy
was recommended due to their effects on blocking the epidermal
growth factor receptor (EGF) kinase (Umezawa, H. et al., J.
5Antibiotics, vol. 39, 170-173, 1986 and Yaish, P. et al.,
Science, vol. 242, 933-935, 1988).
u.s. Patent No. 4,686,308, granted August 11, 1987,
protects a novel compound 2,2-formamidoethenyl-1,4-hydroxy-
10quinone. Preparation comprises cultivation of a Streptomyces
strain or by its mutant treated by ultraviolet irradiation, or
by recombinant DNA techniques of gene coding. This compound
purportedly has antitumour and antimicrobial activities, with
inhibitory activity against tyrosine specific protein kinase.
Sseveral articles disclose tyrosine kinase inhibitors.
One is entitled "Blocking of EGF-Dependent Cell Proliferation by
EGF Receptor Kinase Inhibitors", Pnina Yaish, Aviv Gazit, Chaim
Gilon, Alexander Levitzki, Science, vol.242, Nov. 11, 1988, pp.
20933-935. This article describes the synthesis of a systematic
series of low mole¢ular weight protein tyrosine kinase inhibi-
tors. They had progressively increasing affinity over a 2500-
fold range toward the substrate site of epidermal growth factor
(EGF) receptor kinase domain. These compounds inhibited EGF
25receptor kinase activity up to three orders of magnitude more
than they inhibited insulin receptor kinase, and they also
effectively inhibited the EGF-dependent autophosphorylation of
the receptor. The most potent compounds effectively inhibited
the EGF-dependent proliferation of A431/clone 15 cells with
30little or no effect on the EGF-independent proliferation of these
cells. The potential use of tyrosine protein kinase inhibitors
as antiproliferative agents is demonstrated.
Another is entitled "Tyrphostins Inhibit Epidermal
35Growth Factor (EGF)-Receptor Tyrosine Kinase Activity in Living
Cells and EGF-stimulated Cell Proliferation", R. M. Lyall, A.
Zilberstein, A. Gazit, C. Gilon, A. Levitzki and J. Schlessinger,
;~012634
e Journal of Biological Chemistry, Vol. 264, No. 24, Aug. 25,
1989, pp. 14503-14509. Synthetic compounds ca]led tyrphostins
were examined for their effects on cells which are mitogenically
responsive to epidermal growth factor (EGF). The writers studied
in detail the effects of two tyrphostins on EGF binding, tyrosine
phosphorylation in intact cells, EGF-receptor internalization,
and mitogenesis. These compounds inhibited EGF-stimulated [3H]
thymidine incorporation in a specific manner and the degree of
selectivity varied. Both compounds inhibited EGF-stimulated
receptor autophosphyorylation and tyrosine phosphorylation of
endogenous substrates in intact cells at doses that correlated
with the ICso for [3H] thymidine incorporation. These results are
consistent with the notion that tyrosine phosphorylation is a
crucial signal in transduction of the mitogenic message delivered
by EGF. The compoun~ RGs0864 demonstrated specificity at
inhibiting EGF-stimulated cell growth compared with stimulation
with either platelet-derived growth factor or serum. These novel
synthetic inhibitors, specific for EGF-receptor kinase, allegedly
offer a new method to inhibit EGF-stimulated cell proliferation
which may be useful in treating specific pathological conditions
involving cellular proliferation, including different types of
cancers.
A third article is entitled "Specific Inhibitors of
Tyrosine-Specific Protein Kinases: Properties of 4-Hydroxycin-
namamide Derivatives in Vitro", T. Shiraishi, M. K. Owada, M.
Tatsuka, T. Yamashita, K. Watanabe and T. Kakunaga, Cancer
Research 49, 2374-2378, May 1, 1989. Inhibition by seven
synthetic 4-hydroxycinnamamide derivatives, ST 271, ST 280, ST
458, ST 494, ST 633, ST 638 and ST 642, of tyrosine-specific
protein kinases (tyrosine kinase) of oncogene or proto-oncogene
products (pl30gag-v-fps, p70gag-actin-v-fgr, pp60v-src, pp60c-
src) and epidermal growth factor (EGF) receptor kinase were
investigated. ST 638 (~-cyano-3-ethoxy-4-hydroxy-5-phenylthio-
methylcinnamamide) strongly inhibited more of the tyrosine
kinases than any of the other compounds. The susceptibilities
of these tyrosine kinases to ST 638 increased in the following
20~Z634
rder: EGF receptor > p70gag-actin-v-fgr > pp60c-src > pl30gag-
v-fps, pp60v-src, with 50% inhibitory concentration values of
1.1, 4.2, 18, 70, and 87 ~M, respectively. The phosphorylation
of the tyrosine residues in particulate fractions from RR1022
5cells expressing pp60v-src was inhibited by ST 638 in a dose-
dependent way, while it had a negligible effect on the phosphory-
lations of threonine and serine residues. Kinetic analysis
showed that ST 638 competitively inhibited the phosphorylation
of an exogenous substrate by the EGF receptor kinase with a K; of
102.1 ~M. ST 638 noncompetitively inhibited autophosphorylation
by EGF receptor kinase. These results indicate that ST 638 is
a potent and specific inhibitor of tyrosine kinases in vitro, and
that its inhibitory activity is caused by competing with the
substrate protein for the tyrosine kinase binding site.
~ --
A fourth article relates specifically to Erbstatin:
"Effective Synthesis of Erbstatin and its Analogs~', E. L. Dulaney
and C.A. Jacobsen, The Journal of Antibiotics, Vol. XL, No. 8,
Aug. 1987, pp. 1207-1212. Erbstatin, purported to be a new
20potent inhibitor for tyrosine protein kinase (TPK), was isolated
from the broth of Stre~tomyces sp. (MH435-hF3) and the structure
was determined by X-ray crystallographic analysis. Erbstatin -
(3a) and its analogs were expected to be useful for the studies
of the functions of oncogenes (tumour inducing genes), and may
25have therapeutic activity for the treatment of cancer. ~
', ':
SUMMARY OF THE INVENTION
The invention is directed to a composition useful in
30the treatment of inflammation induced diseases comprising~
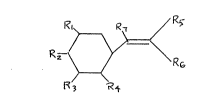
(a) a compound of the formula
Rz-
R
` 201Z634
wherein Rl is H, OH, OCH3, ETO;
R2 is EtO, CHC(CH3)2, iso-Proline, CH3SCH2, H, OH, NO2, OCH3, OCH,
R6Cl;
R3 is H, OH, OCH3, phenyl SCH2, CH. (CH3)2, iso-Proline, CH3SCH2;
R4 is H, OH;
R5 is H, CN, COOH, NHCHO;
R6 is H, CN, COOH, NHCHO, O, S;
R7 is H, OH; and wherein
R5 and R6 can form the following cyclic structures:
~ ' ''
C/
J
when R1 and R3 are CH3SCH2, R2 is OH, and R4 and R7 are H;
O
1~
<C~_~,3 '' '
C~
O ~:
when R1 is ETO, R2 is OH, R3 is PHSCH2, R4 and R7 are H;
~
~, . i ' '-'~
;'
-- 5
len R1 is ETO, R2 is OH, R3 is PhenylSCH2 and R4 and R7 are ~ a;~
/ \ ~: .
O
when R1 and R3 are iso-Proline, R2 is OH, and R4 and R7 are H,
and pharmaceutically acceptable acid addition salts thereof; and -:
"'"-'-.'~'.
(b) a pharmaceutically acceptable carrier.
' ~' '
The invention includes a composition for treating
inflammatory diseases comprising:
(a) a benzylidene malononitrile of the formula: ~:
"'~
, . -.
/ ~3
~ ~
wherein: : ::
( 1 ) R1 =OH, Rz=H, R3=H, R4=OH, Pc5=NHCHO, R6=H
( 2 ) R1=H, R2=OH, R3=H, R4=H, R5=C02H, R6 H
( 3 ) R1 H ~ R2=OH, R3=H, R4=H, R5=C02H, R6=COzH
( 4 ) Rl H , R2=OH , R3=H , R4=H , R5=CN , R6=CN ::
(5) Rl=OH, R2=OH, R3=H, R4=H ~ Rs=C2H, R6 N
( 6 ) Rl OH, Rz H, R3=H, R4=OH, R5=H, R6=NHCHO
( 7 ) Rl=H, R2=H, R3=OH, R4=H, R5=CN, R6=CN
( 8 ) Rl OH, R2=H, R3=H, R4=OH, R5=CN, R6=CO2H
( 9 ) Rl H, Rz=OH, R3=OH, R4=H, R5=CO2H, R6=CN
( 10 ) Rl=H, R2=OH, R3=OH, R4=H, R5=CN, R6=CN
( 11 ) Rl=OCH3, R2=OH, R3=OH, R4=H, R5=CN, R6=CN ~ ~:
-- 6 -- ~
:
:
- ~2) Rl=OH, R2=OH, R3=OH, R4=H, Rs=CN, R6=CN Z012634
(13) R1=OH, ~R2=OH, R3=OH, R4=OH, Rs=CN, R6=CN
( l4 ) R1=OH, R2=OH, R3=OH, R4=H, Rs=NHCHO, R6=H
(l5) R1=H, R2=OH, R3=H, R4=H, Rs=CN, R6=CN
( l 6 ) R1=H, R2=OH, R3=H, R4=H ~ Rs=CN ~ R6=H
(17) R1=OH, R2=02N, R3=H, R4=H, Rs=CN, R6=CN
( l8 ) R1=H, R2=OH, R3=H, R4=H, Rs=CN, R6=CN, R7=OH
(l9) R1=CH30, R2=OH, R3=H, R4=H, R5=CN, R6=CN
( 2 0 ) R1=OH, R2=H, R3=OH, R4=H, Rs=CN, R6=CN
( ) R1 OH, Rz OH, R3=OH, R4=H, R5=CN, R6=CN, R7=OH
(22) R1=H, R2=CH30, R3=H, R4=H, Rs=C02H, R6=CN
(23) R1=H, R2=FlCl, R3=H, R4=H, Rs=C02H, R6=CN
(24) R1=CH30, R2=OH, R3=CH30, R4=H, R5=CO2H, R6 CN
( 2 5 ) R1 H, R2=OH, R3=H, R4=H, R5=C02H, R6=CNlS (26) R1=H, R2=oCH, R3=H, R4=H, R5=C02H, R6 CN
(27) R1=OH, R2=H, R3=H, R4=H, Rs=CN, R6=CO2H
and pharmaceutically acceptable acid addition salts thereof; and
(b) a pharmaceutically acceptable carrier.
The invention also includes a composition for treating
inflammatory diseases comprising: -
(a) a cinnamamide of the formula:
R6
R J~
R~ Rb
wherein:
( l ) R1=ETO, R2=OH, R3=PhenylSCH2, R4=H, Rs=CN, R6=O . - -
( 2 ) Rl=CH . CMe2, R2=OH, R3=CH . Me2, R4 H, Rs CN, 6
(3) Rl=ETO, R2=OH, R3=PhenylSCH2, R4=H, Rs=CN, R6=S
( 4 ) R1=OH, R2=OH, R3=H, R4=H, R5=CN, R6=S : ~ -
(5) R1=iso-Proline, R2=OH, R3=iso-Proline, R4=H, R5=CN, R6=O
- 7 -
'' ~' ;'.
~,) R1=H, R2=OH, R3=H, R4=H, R5=CN, R6=o 201Z634
(7) R1=OH, R2=OH, R3=OH, R4=H, R5=CN, R6=o; or -- -
(8) R~=OH, R2=OH, R3=OH, R4=I, R5=CN, R6=S; or wherein R5 and R6 -
can combine to one of the following structures; and having
R1=OCH3, iso-proline or OH, R2=OCH3 or OH, R3=iso-proline or OCH
~ ~ =Q IH ~ ~ ~
o O
oc~3 C~
~ 3 3 =C~H~ C~C~z
2 0
and acid addition salts thereof; and -
(b) a pharmaceutically acceptable carrier. ~
. ~ . . :-
In the composition as illustated above, R1 can be OH
2 5 and R2 and R3 can be H, R4 can be OH, Rs can be NHCHO and R6 and
R7 can be H. In the composition, the carrier is distilled water.
In the composition as described, compound (a) can be present in
compound (b) at a concentration ranging from 0.5 mg/l to l00
mg/l. The composition can include an effective amount of an
iron chelation agent, andjor oxygen radicals scavenger.
. . . ~ ,
I The composition is administered orally, as an aerosol, ;-
subcutaneously or intravenously. The composition can be in the
form of a tablet. The composition can be used in the treatment
of asthma, allergic diseases, hay fever, skin rashes, inflamma-
tory bowel diseases, arthritis, adult respiratory distress
- 8 -
ZOlZ634
~yndrome (ARDS), migraine, cardiac shock, septic shock, thrombo-
sis, hypotension, hypertension and ischemia.
DETAILED DESCRIPTION OF SPECIFIC
5EMBODIMENTS OF THE INVENTION
Platelet activating factor (PAF) and Leukotriene D4
(LTD4) are important components of bronchial hyperresponsiveness
and inflammation. Elucidation of their mode of cellular action
is a crucial step towards the understanding of pathophysiological
states caused by PAF and LTD4 and subsequently the management of
diseases associated with those molecules.
Initially, the role ofprotein-tyrosinephosphorylation
in the signal transduction of PAF was investigated in rabbit
platelets. Two tyrosine kinase inhibitors, N-(2-(2,5-dihydroxy-
phenyl)ethenyl)formamide-methanolate (coded; TR-lA) and ~-cyano-
3,4-dihydroxythiocinnamamide (TR-18) were found to inhibit PAF
responses at the ICso = 15 and 50 ~g/ml, respectively. Inhibi-
tion of protein-tyrosine phosphorylation blocked PAF-induced
phosphoinositide breakdown, membranous protein kinase C activity,
platelet aggregation and serotonin release. These data imply
that protein-tyrosine phosporylation plays a critical role in
PAF-signal transduction systems. This suggests that the PAF
receptor may be a tyrosine kinase or a tyrosine phosphorylatable
protein coupled to a phospholipase C, or alternatively coupled
to a phospholipase C amenable to phosphorylation by tyrosine
kinases.
30This work provides firm evidence that specific
inhibitors of protein-tyrosine kinase should prove to be valuable -
tool$ in the management of bronchial hyperresponsiveness and
inflammation.
::,--:-: ~ :,
35The drug TR-lA was initially believed to be useful for
the study of the functions of oncogenes (tumour inducing genes) ~ ~ ;
due to its action on the inhibition of protein tyrosine kinase. ;~
:, ''`
:,'..~ "-- - .,~':
2012634 ~ ~
~ e to the fact that the receptors for a number of mediators of
inflammatory and allergic reactions might have protein tyrosine
kinase activity, I have discovered that the drug TR-lA can
inhibit the activation of these mediators' receptors and affect
the normal interaction of these receptors with their effector
system.
Existing drugs used in the treatment of asthma,
inflammatory diseases, or allergic diseases, are designed either
to inhibit certain enzymes responsible for the syntheses of these
mediators, or to block their mode of signal transduction. TR-
lA, by inhibiting protein tyrosine kinase, a common enzyme for
the transduction of signals for many mediators, should be more
effective in preventing the above diseases, since it will work
against many mediators at the same time.
I have found the drug TR-lA inhibits platelet activat-
ing factor (PAF) (an inflammatory mediator) responses. These
included aggregation of platelets and release of serotonin. TR-
lA also inhibited constriction o guinea pig's trachea inresponse to leukotiene D4 (LTD4) (a major mediator of allergic
and asthmatic reactions). From this, it is evident that TR-lA
has important use in the treatment of asthma, allergy cardiovas-
cular and inflammatory diseases.
From the foregoing demonstrations, it follows that the
compound TR-lA is useful for the treatment of virtually all kinds
of inflammatory diseases (eg. arthritis, inflammatory bowel
disease, etc.), all kinds of allergic diseases (eg. asthma,
rhenitis, skin allergy, hay fever, systemic anaphylaxis), and all
kinds of heart and vascular diseases (eg. septic shock, ARDS,
cardiogenic shock, arrythmias, hypotension, hypertension,
thrombosis and blood clot).
I have discovered that the compounds of the invention
block the action of PAF and LTD4. Although the receptors for
these molecules are not characterized, nevertheless, it is
-- 10 --
~012634
lieved that these agents inhibit tyrosine phosphorylation of
the receptor. Alternatively, the compounds of the invention are
interfering with biochemical parameters involved in the trans-
duction of signals for PAF or LTD4. Such biochemical parameters
could be the component of guanyl nucleotide regulatory proteins
(G-proteins), phospholipases, protein kinases or phosphatases.
The compounds of the invention, with their novel uses,
can be broadly divided into two main specific groups:
Group A Compounds (Benzvlidene Malononitrile Com~ounds)
A composition for treating asthma, allergic diseases, ~:
hay fever, skin rashes, arthritis, inflammatory diseases (bowel,
colon, etc.), adult respiratory distress syndrome (ARDS), septic
shock, cardiac shock, thrombosis, hypertension, hypotension,
tissue ischemia and migraine, comprising a benzylidene malono- .
nitrile of the formula: :
:~
R~ R_
~3 R~
wherein~
(l) R~=OH~ R2=H, R3=H, R4=OH, Rs=NHCHO, R6=H (TR-lA) ;
(2) R1=H, R2=OH, R3=H~ R4=H~ R5=C2H~ R6 H
( 3 ) R~ H, R2=OH, R3=H, R4=H, Rs=C02H, R6=CO2H :: -
( 4 ) ., R~=H , R2=OH ~ R3=H ~ R4=H ~ Rs=CN ~ R6=CN
( 5 ) R1=OH, R2=OH, R3=H ~ R4=~ ~ Rs=C2H ~ R6 N
(6) R1=OH, R2=H, R3=H~ R4=H~ Rs=H~ R6=NHCH ~ ~ -
(7) R~=H, R2=H~ R3=OH~ R4=H~ Rs=CN, R6=CN ;
( 8 ) R~=OH, R2=H, R3=H, R4=OH, Rs=CN, R6=CO2H
( 9 ) R~ H, R2=OH, R3=OH, R4=H, Rs=C02H, R6=CN
0) R1=H, R2=OH, R3=OH, R4=H, ~=CN, R6=CN
(ll) R1=0CH3, R2=OH, R3=OH, R4=H, ~=CN, R6=CN 2ol2
(12) R1=OH, R2=OH, R3=OH, R4=H, ~=CN, R6=CN 634 ~ :
(l3) R1=OH, R2=OH, R3=OH, R4=OH, ~=CN, R6=CN
(l4) R1=OH, R2=OH, R3=OH, R4=H, Rs=NHcHo, R6=H
(l5) R1=H, R2=OH, R3=H, R4=H, ~=CN, R6=CN
(l6) R1=H, Rz=OH, R3=H, R4=H, Rs=CN, R6=H
(l7) R1=OH, R2=02N, R3=H, R4=H, ~=CN, R6=CN
(l8) R1=H, R2=OH, R3=H, R4=H, Rs=CN, R6=CN, R7=OH
(l9) R~=CH30, R2=OH, R3=H, R4=H, Rs=CN, R6=CN
(20) R1=OH, R2=H, R3=OH, R4=H, ~=CN, R6=CN
(2l) R1=OH~ R2=OH, R3=OH, R4=H, Rs=CN, R6=CN, R7=OH
(22) R1=H, R2=CH30, R3=H, R4=H, Rs=C02H~ R6 CN
(23) R1=H, R2=FICl, R3=H, R4=H, Rs=C02H~ R6=CN
(24) R1=CH30, R2=OH, R3=CH30, R4=H, ~=C02H, R6 CN
(25) R1 H, R2=OH, R3=H, R4=H, Rs=C02H, R6=CN
( 6) R1 H, R2=OCH, R3=H, R4=H, Rs=C02H~ R6=CN
( ) R1 OH, R2 H, R3=H, R4=H, R5=CN, R6=C02H
Results of Ex~eriments Conducted with Group A Compounds
Initial Experiments
.:, .
A number of initial experiments were performed with N-
25 (2-(2-,5-dihydroxyphenyl)ethenyl) formamide methanolate (TR-
lA), to inhibit biological activity of PAF on rabbit platelets.
Rabbit platelets were prepared according to the well-
known method of Pinckard et al. J. Immuno~. 123, 1847-1853, 1979.
Platelets at 2 x lO8~ml were challenged with PAF (0.2 nN) at 37
in Tyrode's buffer, pH 7.2. Platelets (0.5 ml) were tested in
a Bio-Data aggregometer for aggregation in response to PAF. TR-
lA or PAF was added in 50 ul of 0.25% BSA in Tyrode's buffer.
Activities were measured as percent increase in light trans-
mission.
- 12 -
ZOlZ634
As can be seen from Table 1, TR-lA, at 10 ~g/ml
inhibited PAF-induced platelets aggregation by approximately 30%.
At 25 ~g/ml, it is seen that TR-lA blocked the action of PAF by
100%.
Table 1
Effect of Various Concentrations of TR-lA on
PAF Induced Rabbit Platelets Aaareaation
.,
PAF (200 PM) Induced Aggregation
TR-lA conc. (uq/ml) (% of Control
mean of five experiments ~
100 ~ ~ "
155 100 ;~
-~
o
Second Set of Ex~eriments
' ~''~'
In another set of studies, at similar concentrations, -~-~
it was found that TR-lA inhibited PAF induced serotonin release ;~
from rabbit platelets. Platelets were labelled with t3H] ; ;~
serotonin for 1 hour. After this period of time, the unincorpor-
ated [3H] serotonin was washed off and the platelets were used ~; ;
for challenge with PAF in the presence and absence of TR-lA. ~;~
TR-lA, from 10 ~g/ml, was found to inhibit PAF (200 PM) induced
serotonin release. The IC50 was determined to be about 10 ~g/ml.
At 25 ~g/ml, it was noted that TR-lA entirely blocked PAF induced
serotonin release.
. ~. i. . ;,
TR-lA, at concentrations below 10 ~g/ml, did not
appreciably prevent PAF induced serotonin release from rabbit
platelets. At 20 ~g/ml, TR-lA inhibited PAF action by about
50%. At 25 ~g/ml, TR-lA almost completely blocked the action
of PAF. These determinations are tabulated in Table 2.
- 13 -
."'~
~ '~
2012634
Table 2
Inhibitory Effect of Various Concentrations of TR-lA
on PAF Induced Serotonin Release from Rabbit Platelets
TR-lA Conc. (~q/ml)Serotonin Release (% of Control)
mean of five experiments
0 100
0.5 97
1 102
2 98
92
42
o
0
o
Example;
In order to evaluate the specific site of action of
TR-lA on platelets, a series of experiments were performed to
investigate the cellular second messenger system following PAF
activation. Platelets were labelled with [3H] inositol which
became incorporated into phosphatidylinositol. The metabolism
of phosphatidylinositol in response to PAF was then investigated.
the results are tabulated in Table 3.
As Table 3 shows, PAF (200 PM), without the presence
of TR-lA, caused rapid hydrolysis of phosphatidylinositide.
However, when the platelets were first pretreated with various
concentrations of TR-lA for 5 minutes prior to the addition of
PAF, the formation of metabolites of phosphoinositide (inositol
monophosphate, inositol bisphosphate and inositol trisphosphate)
were inhibited. The IC50 for TR-lA was found to be between 20
and 25 ~g/ml. At 50 ~g/ml, it was noted that TR-lA entirely
blocked the action of PAF. Similarly, TR-lA blocked the
- 14 -
~'
Z01263~
~ctivation of protein kinase C induced by PAF. Protein kinase
tPKC) is also a major component of a cell signalling system.
In this assay system, platelets were preincubated for
5 minutes with TR-lA (0-50 ~g/ml) and subsequently treated with
PAF (200 PM) for 1 minute. The sample material was chromato-
graphed on a mono Q column. 0.5 ml of NP 40-solubilized
particulate protein from the platelet extracts was chromato-
graphed, and the column fractions assayed for phosphorylating
activity. It was found that TR-lA from 10 ~g/ml inhibited (>40%)
the activation of PKC by PAF. At 25 ~g/ml, TR-lA blocked about ~
80% of the action of PAF. These results suggest that TR-lA ; -
blocks PAF induced activation of rabbit platelets. Thus it ~ ~ -
follows that TR-lA can be used in the treatment of diseases where
PAF plays an important role (such as asthma, cardiovascular and
inflammatory diseases).
Table 3
Inhibitory Effects of TR-lA on PAf
Induced Polyphosphoinositide Metabolism
in Rabbit Platelets
Phosphoinosidide Metabolites Formation
(% of control) - -~
TR-lA (~g/ml) IPl IP2 IP3
O 100 100 100 ':
0.5 100 100 100
1 100 100 100 ~;
100 -~ -
- 15 -
ZOlZ634
roup B Compounds (Hydroxy Cinnamamide Compounds)
Composition containing hydroxy cinnamamide compounds
according to the following formula are useful for treating
asthma, allergic diseases, hay fever, skin rashes, arthritis,
inflammatory diseases (bowel, colon, etc.), ARDS, septic shock,
cardiac shock, thrombosis, hypertension, hypotension, tissue
ischemia and migraine. The hydroxycinnamamides have the
following formula:
R6
~L~ ~ N~z
wherein:
(1) R1 ETO, R2=OH, R3=PhenylSCH2, R4=H, R5=CN, R6=O
(2) R1=CH.CMe2, R2=OH, R3=CH.CMe2, R4=H, R5=CN, R6=O
(3) R1 ETO, R2=OH, R3=PhenylscH2, R4=H, R5=CN, R6=S
(4) Rl=OH, R2=OH, R3=H, R4=H, R5-CN, R6=S
(5) R1=iso-Proline, R2=OH, R3=iso-Proline, R4=H, R5=CN, R6=O
(6) Rl=H, R2=OH, R3=H, R4=H, R5=CN, R6=O
(7) Rl=OH, R2=OH, R3=OH, R4=H, R5=CN, R6=O
(8) Rl=OH, R2=OH, R3=OH, R4=I,F,Cl, Rs=CN, R6=S :
'~ '
The hydroxycinnamamide can also have the following
structures~
~ ~N~
O ~;~-Pv
C~3 5 c~
- 16 -
Z012634
C
E I--O ~ ~ Ph L= 1~0
7N~ H~//~
Ph Sa~ V ~hSC~17
Experiments Conducted with Group B Compounds
Results of the studies conducted with four compounds
lo of the groups B, ~-cyano-4-hydroxy-3, s-diisopropylcinnamamide
and ~-cyano-3, 4-dihydroxythiocinnamamide are shown in Table 4
with LTD4 induced smooth muscle contraction. Leukotriene D4 is
the major component of bronchial asthma, contracts bronchial and
tracheal smooth muscle cells at the concentrations of 210l0M.
The effects of four different protein tyrosine kinase inhibitors
were evaluated against contraction induced by LTD4 in isolated
guinea pig tracheal preparation. Trachea from guinea pigs were
suspended in a jacketed organ bath containing oxygenated kreb's-
Henseleit colution. The tissues were allowed to equilibrate for
60-~0 minutes under 1. 5 g tension and then optimal tension
obtained using electrical field stimulation at 0.25 g tension
increments. Isometric force generation was measured with Grass
FT 0.3 force transducer, and recorded on a polygraph. Responses
of each tissue to Acetylcholine (103M) was first evaluated. The
tissues were then washed and stimulated with various concentra~
tions of LTD4 (ranging from 101M to 3 x 107M).
As can be seen from Table 4, pretreatment of tissue
with tyrosine kinase inhibitors (10 ~g/ml) for 60 minutes, caused
significant inhibition of LTD4 (3 x 10-7) induced smooth muscle
contraction. The most potent of all the four tested inhibitors
was found to be ~-cyano-4-hydroxy-3, 5-diisopropylcinnamamide.
This was greater than ~-cyano-3, 4-dihydroxythiocinnamamide which
was greater than 2,5-bis-(3,4-dimethoxybenzylidene) cyclo-
pentanone which was more effective than N-(2-(2,5-dihydroxy~
phenyl)ethenyl) formamide methanolate.
:''''''';~';
-- 17 --
, 2012634
~'
H o Ul
~0
~ ~ ~ r~
E~ ~ a)
_l
O .,
C~
~ O ~ ~ ~ ~
O ~ ~ . .
_I X ~o ~r o
H I H O 111
C I C
~ 1 . I ~ ~ U ~ ,
U ~ ~,
0~ 0 ~ ~ :
o ~
U~ t) N
~1 0
R f~
~ In ~ ~
,~ ` X X 0-
,~ ~ ~ ~ X
~ ~ ~ ~ O
Y O ~ ~ ~ :
o ~ ,~ ,1 :~ .
~ ~ ~ ~ . .
In ~ ` ` I
~r ~q
o , , _
~ o o ~n ~ .,
.,~
Q
~1 ~ ~ I
H i I `~
:. :
- 18- ; -.
201263~
- These results clearly indicate that tyrosine kinase
inhibitors with the common structure of hydroxycinnamamide are
effective in preventing leukotriene D4 and presumably other
arachidonic acid metabolites which induce smooth muscle contrac-
tion. Therefore, these substances can be used in the treatmentof asthma and other inflammatory and allergic diseases, in which
arachidonic acid metabolites play a major role.
Conclusion -~
Leukotrienes are polyunsaturated conjugated trienes
derived from arachidonic acid. They consist of leukotriene A4
(LTA4), LTB4~ LTC4, LTD4, and LTE4. Other arachidonic acid
metabolites are called prostaglandins and thromboxane A2. Their
role in inflammatory diseases such as arthritis and in airway
asthma has been recognized for several years. For example, LTC4,
LTD4, LTE4, PGD2, and TXAz are potent smooth muscle constrictors.
They also contract vascular smooth muscles. LTB4, PAF and PGE2
are major components of inflammatory diseases such as arthritis.
Recruiting leukocyte to the site of inflammation causes these
cells to degranulate and release their dysosomal enzyme. The
combination of intracellular enzymes and leukocytes accumula- ;
tion causes acute inflammation. Other arachidonic acid metabo-
lites are shown to be involved in the diseases associated with ;
cardiovascular system. For example, TXA2 which is a potent
constrictor of vasculature, can induce hypertension. To the
contrary, PGI2, a potent vasodilator, induces hypotension.
Cardiac shock and ischemia are also associated with PGI2. In
regard to septic shock and ARDS, PAF may play a major role.
Several available reports indicate that PAF, to a large extent,
and TXA2, to a lesser extent, play a critical role in the
initiation of cardiac shocks associated with endotoxin (septic
shock). In regard to migraine, several prostaglandins such as
PGE2, El, etc., have been shown to have an important role in the -~
development of migraine. It follows, therefore, that the
tyrosine kinase inhibitors of the invention could be valuable
drugs for inhibition of the cellular activation by a number of
;;., ~ .,: .
. ,
-19
201Z634
.i, .,
'"~rostaglandins, l~ukotrienes, and PAF and thus can be valuable
tools for the treatment of the above diseases.
Mode of Application
The compounds of Groups A or B may be present in the
composition of the invention at a concentration in the range of
0.5-100 mg/l or kg of body weight of a pharmaceutically accept-
able carrier.
Suitable doses are 1-1,000 mg/kg, especially 1-10 mg,
preferably taken 2 to 3 times daily, orally, subcutaneously,
intravenously or by aerosol. The pharmaceutically acceptable
carrier may be distilled water, a mixture of saline, glucose,
lactose or ethylcellulose N100 and water or starch talc. The
composition of the invention may be administered orally, by
aerosol, subcutaneously or intravenously. Tablets for oral
ingestion may be made via compression of approximately 100 mg of
a compound of Group A or B, 100 mg of an iron chelator, 200 mg
of lactose and 100 mg Avicel.
Capsules may be prepared by makinq micelles of
liposomal drugs with lecithin. Micelle injections can be made
either in water and propylene glycol with an upwardly adjusted
pH in phosphate buffer. The product is typically sterilized
through a filter. The micelle can be made in 20 percent
propylene glycol and a preservative such as ascorbic acid. The
aerosol composition can be made by making liposomes of the
compounds of Group A or B in a pharmaceutically acceptable
buffer/lecithin, with preservative, and solubilizing agent such
as 0.1% ethanol.
.
As will be apparent to those skilled in the art in the
light of the foregoing disclosure, many alterations and modifica-
tions are possible in the practice of this invention without -
departing from the spirit or scope thereof. Accordingly, the
scope of the invention is to be construed in accordance with the
- 20 -
_~bstance defined by the following claims. Z012634
,`,,`,'' ",`,
.' `,..'"'-':
, `. ~`.
`.,,; ~
,; . -. .
- 21 -