Note: Descriptions are shown in the official language in which they were submitted.
~t~~.~9'~8
18-586-0
25/
TITLE OF THE INVENTION
METHOD FOR TREATING HEPATIC DISEASES USING FK 506
AND RELATED COMPOUNDS
BACKGROUND OF THE INVENTION
Field of the Invent=ion:
The invention relates to the use of macrolide
compounds, such as FK 506, for treatment of liver
diseases and for r~=_generation of liver tissue. More
specifically, the :invention relates to the use of
macrolide compounds to promote liver hypertrophy and
hyperplasia and thereby facilitate regeneration of
liver tissue.
Discussion of the Background:
Immunosuppressive agents such as azathioprine,
adrenocortical steroids and cyclosporin are used to
suppress the immune system during organ transplant
operations. Azath,ioprine and the adrenocortical
steroids are also known to depress the regeneration of
liver tissue after partial hepatectomy (Gonzalez et al,
Surgery, 1970, 68:254-59 and Guzek, Nature, 1964,
201:930-31). In contrast, cyclosporin facilitates
hepatic regeneration in both rats and dogs. (Makowka
et al, Surg Forum,. 1986, 37:352-54: Kahn et al,
Transplant Proc, x_988. 20 (Suppl 3):850-52; Kim et al,
-2-
Surg. Gynecol. Obstet., 1988, 166:317-22; Mazzaferro et
al., Surgery, May 1990, Vol. 107, No. 5, pp. 533-9.
Macrolide compounds, in particular the macrolide
FK 506, are known immunosuppressive compounds which
prevent acute and chronic liver allograft rejection in
humans more reliably and completely than has been
possible with previous compounds (Starzl et al., Lancet,
1989, 2:1000-1009:). Several compounds belonging to this
class of immunosuppressive macrolides are obtained from
cultures of species belonging to the genus Streptomyces.
Compounds within this class are described in U.S. Patent
Nos. 4,894,366 and 4,929,611.
The ability of immunosuppressive drugs to
facilitate liver regeneration is important to patient
survival following transplant operations. Liver
restoration is also important in the recovery of liver
tissue following severe liver disease. Accordingly, a
need continues to exist for improved methods of
facilitating liver regeneration.
SUMMARY OF THE INVENTION
Accordingly, one object of the present invention is
to provide a method for enhancing the regeneration of
liver tissue in mammals by administering to a mammal
jv
~"~.r i
_3_
212978
in need thereof a regeneration effective amount of a
liver regenerating macrolide compound.
A further object is to provide a method for
regenerating mammal liver tissue by facilitating or
stimulating hypertrophy and hyperplasia of liver cells,
i.e. hepatocytes.
A further object is to provide a method for -
treating hepatic disease, in particular hepatic disease
having an autoimmune component. °
These and other objects have been achieved by the
present method in which a liver regenerating effective
amount of a macrolide compound is administered to a
mammal to facilitate hepatocyte, hypertrophy and
hyperplasia.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
It has now been discovered that by administration
of the immunosuppressive macrolide compounds of the
present invention to a mammal, liver regeneration is
facilitated by means of hypertrophy and hyperplasia of
hepatocytes. The macrolide compounds of the present
invention are therefore useful to treat liver diseases,
particularly if there is an autoimmune component to the
diver disease. Further, the macrolide compounds of the
present invention are also effective in promoting
hepatic healing even in the absence of immune system
deficiencies, for example after hepatectomy.
201297
-4-
The ability of the present compounds to regenerate
liver tissue is surprising since many compounds which
exhibit metabolic actions on the liver have no
hepatotrophic actions. For example, glucagon which has
a powerful metabolic effect on the liver does not have
the hepatotrophic properties of the present macrolide
compounds (Starzl et al, Lancet, 1976, 1:821-25). _
Naturally occurring peptides, such as insulin and
°cytosolic hepatic stimulatory substance (HSS) exhibit
hepatotrophic effects similar to those of the present
compounds (Starzl et al, Lancet, 1976, 1:821-25; Starzl
et al, Lancet, 1979, 1:127-130). However, the effect
of the present mac:rolide compounds is somewhat greater
than these natura~~ly occurring peptides and furthermore
they can be given orally instead of by infusion into
the main vein (portal vein) supplying the liver with
blood.
The present rnacrolide compounds are believed to .
modulate hepatocyi~e cellular growth either by means of
their immunosuppressive action, by means of growth
control effectors such as cytokines or through other
mediators which a:re not necessarily directly linked to
immunosupression. The hepatotrophic mechanism of the
present compound .is not known in detail. However, it
is believed that 'the regeneration mechanisms take place
substantially within the liver itself rather than
-5- 2012978
systemically throughout the mammal. It is known, for
example, that the macrolide FK 506 has no direct effect
on hepatocytes in culture (Francavilla et al,
Transplant Proc, i.n press). Therefore, intermediary
substances or cells such as intrahepatic macrophages,
endothelial cells or lymphoid aggregates may be
involved.
The macrolider compounds useful in the present
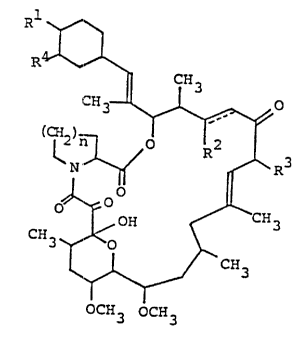
invention are compounds having structure I shown below.
R1
R.
CH3
i~H3 _ O
,.-
(CH2)n O R2
R3
N'
OO (I)
O
OH ~CH3
CH3 ~/ n
CH3
OCH3 OCH3
In structure I, R1 is hydroxy or protected
hydroxy, R2 is hydrogen, hydroxy or protected hydroxy,
R3 is methyl, ethyl, propyl or allyl, R4 is hydroxy,
methoxy or oxo (=O), n is an integer of 1 or 2 and the
symbol of a line and a dotted line is a single bond or
a double bond, provided that R2 is not protected
hydroxy when R4 i:a hydroxy or oxo, and salts thereof.
-6-
20~2~~
Such macrolide compounds may be prepared by both
fermentation processes and synthetic organic processes as
disclosed in U.S. Patent No. 4,894,366; U.S. Patent No.
4,929,611 and EP-A-353,678.
The term "lower" used in the specification is intended
to mean 1 to 6 carbon atoms, unless otherwise indicated.
Suitable hydrox.y-protective groups in the "protected
hydroxy" may include . 1-(lower alkylthio) (lower) alkyl such
as lower alkylthiomethyl (e. g. methylthiomethyl,
ethylthiomethyl, propylthiomethyl, isopropylthiomethyl,
butylthiomethyl, isobL~tylthiomethyl, hexylthiomethyl, etc.),
and the like, in which the preferred one is C1-C4
alkylthiomethyl and the most preferred one is
methylthiomethyl; tris;ubstituted silyl such as tri(lower)
alkylsilyl (e. g. trimethylsilyl, triethylsilyl, tributylsilyl,
tert-butyl-dimethylsilyl, tri-tert-butylsilyl, etc.), lower
alkyl-diarylsilyl (e. g. methyl-diphenylsilyl, ethyl-
diphenylsilyl, propyl-diphenylsilyl, tert-butyl-diphenylsilyl,
etc.),
..': .~ ,
~.,, x.
201.2~~'~~
and the like, in which the preferred one is tri(C1-C4)
alkylsilyl and C1-C4 alkyl-diphenylsilyl, and the most
preferred one is t~ert-butyl-dimethylsilyl and tert-
butyl-diphenylsily:l; acyl such as aliphatic acyl,
aromatic acyl and .aliphatic acyl substituted with
aromatic groups, which are derived from carboxylic,
sulfonic and carbamic acids; and the like. _
The aliphatic acyl may include lower alkanoyl
which may have one of more suitable substituent(s) such
as carboxy (e. g. formyl, acetyl, propionyl, butyryl,
isobutyryl, valeryl, isovaleryl, pivaloyl, hexanoyl,
carboxyacetyl, carboxypropionyl, carboxybutyryl,
carboxyhexanoyl, etc.),
cyclo(lower)alkyloxy(lower)alkanoyl which may have one
or more suitable substituent(s) such as lower alkyl
(e. g. cyclopropyloxyacetyl, cyclobutyloxypropionyl,
cycloheptyloxybutyryl, menthyloxyacetyl,
menthyloxypropionyl, menthyloxybutyryl,
menthyloxypentanoyl, menthyloxyhexanoyl, etc.),
camphorsulfonyl, lower alkylcarbamoyl having one or
more suitable substituent(s) such as carboxy and a
protected carboxy, for example, carboxy(lower)
alkylcarbamoyl (e. g. carboxymethylcarbamoyl,
carboxyethylcarbamoyl, carboxypropylcarbamoyl,
carboxybutylcarbamoyl, carboxypentylcarbamoyl,
carboxyhexylcarbamoyl, etc.). protected
2(~ 12978
-8-
carboxy(lower)alkylcarbamoyl such as
tri(lower)alkylsil:yl(lower)alkoxycarbonyl(lower)alkyl-
carbamoyl (e.g trimethylsilylmethoxycar-
bonylethylcarbamoy:l, trimethylsilyl-
ethoxycarbonylprop;ylcarbamoyl, triethylsilyl-
ethoxycarbonylprop:ylcarbamoyl, tertbutyl-
dimethylsilylethoxycarbonylpropylcarbamoyl, -
trimethylsilylpropoxycarbonylbutylcarbamoyl, etc.), and
the like:
The aromatic acyl may include aroyl which may have
one or more suitable substituent(s) such as nitro (e. g.
benzoyl, toluoyl, xyloyl, naphthoyl, nitrobenzoyl,
dinitrobenzoyl, nitronaphthoyl, etc.), arenesulfonyl
which may have one or more suitable subsitutent(s) such
as halogen (e. g. benzenesulfonyl, toluenesulfonyl,
xylenesulfonyl, naphthalenesulfonyl,
fluorobenzenesulfonyl, chlorobenzenesulfonyl,
bromobenzenesulfonyl, iodobenzenesulfonyl, etc.), and
the like.
The aliphatic' acyl substituted with aromatic group
may include ar(lower) alkanoyl which may have one or
more suitable substituent(s) such as lower alkoxy and
trihalo(lower)alkyl (e. g. phenylacetyl,
phenylpropionyl, phenylbutyryl, 2-trifluoromethyl-2-
methoxy-2-phenylacetyl., 2-ethyl-2-trifluoromethyl-2-
phenylacetyl, 2-trifluoromethyl-2-propoxy-2-
phenylacetyl, etc.), and the like.
The more preferred acyl group thus defined is Cl-
C4.alkanoyi which may have carboxy, cyclo(C5-C6)- . -
alkyloxy(C1-C4)alkanoyl having two (Cl-C4) alkyl groups
on the cycloalkyl moiety, camphorsulfonyl, carboxy {Cl-
C4)-alkylcarbamoyl, tri(Cl-C4)alkylsilyl(Cl-
C4)alkoxycarbonyl-(Cl-C~)alkylcarbamoyl, benzoyl which
may have one or two nitro groups, benzenesulfonyl -
having halogens, phenyl{Cl-C4)alkanoyl having Cl-C4
alkoxy and trihalo (Cl-C4) alkyl, and the most
preferred are acetyl, carboxypropionyl,
menthyloxyacetyl, camphorsulfonyl, benzoyl,
nitrobenzoyl, dinitrobenzoyl, iodobenzenesulfonyl and
2-trifluoromethyl-2-methoxy-2-phenyiacetyl.
Particularly preferred macrolide compounds
include:
(1) the macrolide compound in which R1 and R2 are
each hydroxy, R3 is. allyl, R4 is methoxy, n = 2 ; and the
symbol of a line and a dozzed 1W a is a single bond. This c~ound
is known as FR-900506 or FK 506;
( 2 ) the compound in which R1 and R2 are each hydroxy,
Pthvl. R4 is methoxv, n = 2 ; and zhe symbol of a line
and a dotted line :Ls a single bond. This compound is
also known as FR-900520 or WS 7238A;
( 3 ) the compound in which Rl and R2 are each hydr'oxy.
R3 is methyl, R4 is rcu~thoxy, n = 2, and the symbol of a line
and a dotted line :is a single bond. This compound is
known as.FR-900523 or WS 7238B; and
-10-
201298
(4) the compound in which R1 and R2 are each hydroxy,
R3 is allyl, R4 is methoxy, n = 1 and the symbol of a line
and a dotted line is a single bond. This compound is
known as FR-900525.
With respect to the macrolide compounds (I) of
this invention, it is to be understood that there may
be one or more conformers or stereoisomeric pairs such
as optical and geometrical isomers due to asymmetric
carbon atoms and double bonds, and such-isomers are
also included within the scope of the present
invention.
Salts of the macrolide compounds of the present
invention include all pharmaceutically acceptable salts
without limitation. ~ _
The macrolide compounds of the present invention
may be administered as pure compounds or msxtures of
compounds or preferably, in a pharmaceutical vehicle or
carrier. '-
The pharmaceutical compositions of this invention
can be used in the form of a pharmaceutical
preparation, for eaample, in solid, semisolid or liquid
form, which contains the macrolide compounds of the
present invention, as an active ingredient, in
admixture with an organic or inorganic carrier or
excipient suitable for external, enteral, intravenous,
intramuscular, or parenteral applications. The active
-11- zo1 z97
ingredient may be compounded, for example, with the
usual non-toxic, pharmaceutically acceptable carriers
for tablets, pellets, capsules, suppositories,
solutions (saline, for example), emulsions, suspensions
(olive oil, for ex;~mple), and any other form suitable
for use. The carriers which can be used are water,
glucose, lactose, gum acacia, gelatin, mannitol, starch -
paste, magnesium trisilicate, talc, corn starch,
keratin, colloidal silica, potato starch, urea and
other carriers suitable for use in manufacturing
preparations, in solid, semisolid, or liquid form, and
in addition auxiliary, stabilizing, thickening and
coloring agents and perfumes may be used. The active
object compound is included in the pharmaceutical
composition in an effective amount sufficient to
produce the desired effect upon the process or
condition of the disease.
Mammals which. may be treated using the method of
the present invention include livestock mammals such as
cows, horses, etc., domestic animals such as dogs,
cats, rats, etc. a~.nd humans.
For applying this composition to a human, it is
preferable to apply it by oral, parenteral, enteral,
intravenous, or intramuscular administration. For
promoting liver rE~generation, the oral route is
preferable. The compound is absorbed from the
-12-
intestine and brought to the liver on first pass in
high concentration. These macrolide compounds are the
only liver regeneration promoting substances known
which can be given by mouth and presented to the liver
in this way and this is why they possess unique
advantages for therapy. While the dosage of
therapeutically effective amount of the macrolide
compounds varies from and also depends upon the age and
condition of each individual patient to be treated, a
daily dose of about 0.01-1000 mg, preferably 0.1-500 mg
and more preferably 0.5-100 mg, of the active
ingredient is generally given for treating diseases and
for regeneration o~f liver tissue, and an average single
dose of about 0.2-0.5 mg, 1 mg, 5 mg, l0 mg, 50 mg, 100
mg, 250 mg and 500 mg is generally administered. Daily
doses for chronic administration in humans will be in
the range of about: 0.3 mg/kg/day.
Other features of the invention will become
apparent in the course of the following descriptions of
exemplary embodiments which are given for illustration
of the invention and are not intended to be limiting
thereof .
13
G'Y~MDT.FC
Example 1 - Comparative Effect of Cyclosporin and FK
- 506 on Rat Liver Regeneration
Adult male inbred Fisher 344 rats weighing i80-200
g were purchased from Hilltop Lab Animals Inc
(Scottdale, Pennsylvania). The animals were given
standard rat laboratory diet and water ad libitum in a
_ r
temperature and Iigvht controlled room (light 0730-
1930). The rats were assigned to groups and treated
for 4 days as controls or with cyclosporin or FK 506
(Table I). On the fourth day, between 0900 and 1030,
the rats in groups 5-10 had a standard 40$ or 70$
hepatectomy under light ether aneasthesia. Animals in
groups 3 and 4 had sham operations in which the liver
was manipulated at laparotomy. Food and. drink were
allowed immediately. Parenteral fluid and electrolyte
support were not required.
24 h after the hepatectomies, 185 x104 Bq 3H-
thymidine was administered to all rats by intraperi-
toneal injection. The rats, including groups 1 and 2,
were killed 2 h later by guillotine. Extraction and
purification of hepatic DNA were done with the method
of Ove et al (Liver and hormones, New York: Raven
Press, 1987:265-76) and DNA content was measured with
calf thymus DNA (Cigma) as standard. Specimens from
each liver were prepared for histological examination
with hematoxylin and eosin and the proportion of
-14-
hepatocytes in mitosis was counted. All results are
means and SE.
As expected, DNA synthesis and the proportion of
hepatocytes in mitosis were increased in rats with a
40~ or 70~ hepatectomy that were not given cyclosporin
or FK 506 (groups 5, 7 and 8; Table II). After pre-
treatment for 4 days before hepatectomy with intramus-
cular FK 506 (groups 2; 4 and 10) or oral cyclosporin
(group 9), regeneration was significantly augmented
compared with controls. The effect was greater with FK
506 than with cyclosporin (group 10 compared with group
9). FK 506 did not increase resting hepatocyte mitosis
or DNA synthesis. These indices were slightly in-
creased in rats submitted to sham operation. When FK
506 was added to the sham operation group, hepatocyte
mitosis and DNA synthesis were further and signi-
ficantly increased..
TABLE I - REGIMENS
Cyclosporin FK 506
Group Route ~(mg/kg) (mg/kg) Yehicle~ Hepatectomy
1 (n=5) IM .. .. Saline
2 (n=5) IM .. 1 Saline ..
3 (n=10)IM .. .. Saline Sham
4 (n=10)IM .. 1 Saline Sham
(n=8) IM .. .. Saline 40%
6 (n=8) IM .. 1 Saline 40%
7 (n=20)PO .. Olive oil 70%
8 (n=20)IM .. .. Saline 70%
9 (n=15)PO 10 .. Olive oil 70%
10(n=15)IM .. 1 Saline 70%
X250 ul saline or 200 ul olive oil. IM = intramuscular, PO = oral.
-15- ~'~~2~~s
TABLE II - EFFECTS OF CYCLOSPORIN ON
AND FK 506
RAT L:IYER REGENERATION (MEAN, SE)
3H-thymi~ir.~e incorporationProportion
of hepatocytes
Group (g 10 c:pm/mg DNA) in mitosis
3.3 (0.4) 1.6 (0.1)
2 3.2 (0.3) 1.7 (0.1)
3 ~.9 (0.5) 6.8 (0.6)+
10.5 (0.8)~ 9.5 (0.5)++
12.5 (1.3) . . _
6 32:4 (8.2)~~ . .
7 138.1 (13.1) 31.0 (2.0)
8 130.0 (9:2) 29.0 (2.8)
g 179.0 ( 14.0)~'~'~ 44.0 (2. 1 )~~~
242.0 (2$.0)* 59.0 (3.0)*
Student's t test: *p<0.005 vs groups 1, 2 and 3.
**p<0.001 vs group 5. ***p<0.05 vs group 7. *p<0.01
vs group 8. +p<0.01 vs groups 1 and 2. ++p<0.05 vs
groups 1 and 2.
Example 2 - Effect.s of FK 506 in Dogs
Twenty adult female beagle dogs underwent Eck
fistula (Starzl et: al, Lancet, 1976, 1:821-25). After
performing a large side to side portacaval shunt, the
left and right portal vein branches were ligated. A
small infusion catheter was tied into the left branch
and led through the abdominal wall and subcutaneously
to a battery charged infusion pump that was
incorporated into a non-restraining body cast. A
constant infusion was started of the control or test
fluids at the volume of 20 to 30 ml/day. Oral fluids
and diet were allowed ad lib. Four days later, the
animals were administered 0.2 mCi/kg (CH3 - 3H)-
-16- ~l~L~''~~
thymidine with specific activity of 80-90 Ci/mMol (New
England Nuclear, Hoston). Two hours later, the dogs
were anesthetized and killed.
Specimens were taken from 2 of the right hepatic
Lobes and 2 of the left lobes, fixed in 10$ buffered
formalin, and stained using standard hematoxylin-eosin
staining techniques. -
Autoradiography was carried out using Ilford KS
nuclear track liquid emulsion and an exposure time of
at least 30 days. The number. of mitoses, as an index
of hepatocyte regeneration, was determined by counting
the number of 3H-thymidine labelled nuclei per 1000
hepatocytes. The size of individual hepatocytes (index
of hypertrophy) wa.s determined by tracing out a large
number of midzonal. liver cells projected on standard-
thickness paper, cutting out the individual silhouettes
and weighing each (Starzl et al, Surg Gynecol Obstet,
1973, 137:179-199). -This method has been shown to be
accurate for determining hepatocyte cell size and has
been validated by planimetry and by studies of
unicellular organi.srns, the size of which have been
determined directly. In normal, unaltered dogs, about
1.6 ~ 0.4 mitosis per 1000 hepatocytes are present in
the liver, and midzonal hepatocytes are about 0.17 ~
0.01 size-units (Starzl et al, Lancet, 1979, 1:127-
130): The exceptional reproducibility of these values
-17- 2p1 ~9
and the small standard deviations make it easy to
identify changes caused by operations such as Eck
fistula, drugs, or other experimental variables.
For studies of: the organelles, small cubes of each
hepatic sample were taken for electron microscopy. The
tissue was post-fried in glutaraldehyde followed by
osmic acid. After embedding in Polarbed 812 resin, -
ultrathin sections were cut, stained with lead citrate
and examined in a Philip's 300 electron microscope.
Measurements of the organelles were made on electron
micrographs by Loud's method (Loud. J Cell Biol, 1968,
37:27-46).
Results are expressed as mean ~ standard deviation
(SD-). The Student's t-test was employed in individual
experimental groups to compare differences between
right and left lobes or between groups. A p value less
than 0.05 was considered to be significant.
Infusion of the drug vehicle did not effect the
hepatocyte atrophy typical of the Eck fistula liver or
change the low grade hyperplasia (Table l, vehicle
controls). However, when FK 506 was infused into the
left portal vein, atrophy of the left lobar hepatocytes
was prevented in proportion to the dose, and the rate
of mitoses was increased. These changes were
significantly greater in the directly infused Lobes at
all doses, but even the non-infused lobes were
-18-
significantly effected compared to the vehicle controls
at the high FK 506 doses (Table 1).
Comparison of the ultrastructure of the left and
right lobar hepatocytes showed that the hepatocytes
exposed to infused. FR 506 were almost normal even at
the smallest doses (Tables 2 and 3). The amount of
rough endoplasmic reticulum was restored relative to
controls; in addition, dilatation and disruption of the
cisternae were minimal. The number of microbodies,
lysosomes and small lipid containing vacuoles were near
i
normal levels in t:he FK 506-infused lobes. The
i
mitochondria in these lobes were neither enlarged
(Table 3) nor abnormal.
The changes i.n the hepatocytes in the right lobes
did not differ greatly from those seen in the controls
at low doses of FK 506. However, at the l mg/kg/day
dose, there was better preservation of the RER in the
right lobes compared to right lobar hepatocytes in the
vehicle controls (Table 2), and reduced-lipid
accumulation (p<.001). At this high dose, the right
lobar hepatocytes also had reduced microbodies (p=.07)
and lysosomes (p<"05).
19
Example 3
FK 506 lg
Hydroxypropyl methy1ce11ulose 2910 (TC-5R} Ig
Lactose 2g
Croscarmellose sodium (Ac-Di-Sol) lg
The FK 506 (lg) was dissolved in ethanol (lOmi},
and thereto was adc3ed hydroxypropyl methylcellulose -
2910 (TC-5R) (lg) ato prepare a suspension. To this
suspension was added dichloromethane (5m1) to prepare a
homogeneous solution. Lactose (2g) and croscarmellose
sodium (Trade Mark: Ac-Di-Sol, maker: Asahi Chemical
Industry) were homogeneously suspended to this
solution, and then the organic solvent was removed by
evaporation. The residual product was dried under
reduced pressure for 10 hours by vacuum dryer, milled
for 2 minutes by coffee mill and then passed through a
sieve (32 mesh) to give the solid dispersion
composition of FK 506 (5g). This composition was
ecapsulated by a conventional manner to provide capsules
containing lnig or 5mg of FK 506 per each capsule.
There was no evidence of drug toxicity in the
lobes infused with FK 506:
Human diseases for which the present macrolide
compounds may be useful for their regeneration
promoting properties include but are not limited to:
........ . ~.. _.
- 2Q ) ~9~7
1. Postoperative patients after partial liver
resection.
2. Acute liver necrosis caused by: (a) toxins,
(b) viral hepatitis. (c) shock, (d) anoxia and (e)
unknown causes.
3. Autoimmune liver diseases which are chronic
including: (a) autoimmune hepatitis, (b) primary _
biliary cirrhosis, and (c) sclerosing cholangitis.
4. Chronic liver diseases without an autoimmune
hepatitis including: (a) B-virus hepatitis. (b) non
Anon B hepatitis, (c) alcoholic cirrhosis, and (d)
cirrhosis of unknown etiology.
.r. o o C 3
-n
* OI ~ O
~ ~ ,
w a
1 m
...
m ,
_ _
C ~ ~ I N . N. ~ I i Z
I I
I 1 *
cT 3 - ! ~ : :. ; j
;
p N V ~ ~ i. m j i
i i
~
- + + 1 + ..
+ ~ .
.
w ~ ~ ~ ~
1'~ ~ Gf7
N i f9
I
N~
O O O ~ ~ ~ CD
m
I
va o p $ C
O
!
t G1 ~1 ~ ~ _ j
~
(
I ~ O 1 r p 1 s.~
! -~ ~! 8 ~ N ~ . wp '
,~I ~
1
j ;! , =
+' f
I
O O i I o i I
d ~ ~ ~ ,
?
C :
O
'
~ Y I
~
t i
o 1 i
j o i a ~ sj .
.
Q I g I V I ~I I ,
~
~
O N I
1 t
s
.
=. ; I
~
o i
0 0 0 ~ i
I
~ 1 ~. ~
~ ~ 0 ~
i
w , I
a . m j y~ , c~ ~ :~ ~ ;
A ~
N W ~ I r~. I c, ~ '
_ r, r .
I +
y y y, i ' ~ ';
,~ so
m 1 I
m- ~ W ' l ~ t Op
N
t c 1 ~ 0
C n ~
j
I ~ _ i
~ O.
.
O O ~ < ~ I
o . ~
'L7
j o p I ~ ~ o ~
~ p
_
CT _ ~ 4w I O V Jo. I 6ND I C O
(J~ i'' o.r
.
V i tt~ I V I N I ..r. O .
O
+ + ! + +
_
I
~
~ ~ . w , ~ ~ ~ ~ ~ ,
m a ;
; _ ~
I ~ I
I
1
. O O O
x
i
N
~ ~
_ C
w ~
.
N ~' O O
N
p ~
C / ~ ~
22
o i O < Wrt
iD ; ~. p ~
i
m
~ !m
O ' t ~
i
I i
Z
~
:; ~~ ~k ~ ~ ~
o
N ~ N W' I
'G N : m : m ~ I
O ; ~ ~
d ~ . i ~i 1
_ ~ i c~ ~ N a .,.
I c
t
O .r 1 T i i f
~ 1 v ~ t ~ j
~~. ,
~
i r i r N i
~ : i ~ C
-, N ~ o N I
~O O I ! o i ' :~ I r
0 ; ~ m
I ,
~
$ ~ ~
:~
O N N N ! m N ~ ~, j _
! _' ~ ~ 'I
O i N ~ ~ ~ ~ p
I
O 4. ; cn j ~ N
i
~i _ i t t = t t
~
_ ' ~ t J CD ~7 I
O ~ '
~
Qy : V . ~ ~ ~
~ N N ~ I . sv s~'
; ~ '
~ 1
= . ~ i 1 ;
'
v . I f ~
.
C I : ;
I ~ ~ ~
I
C7 $ ~ O ~ I
~
< ,
I i
o o i ; ~ :'
m
~
~ 1 I ....
m ~ ~ o
i
-
.
m O O t o ~ ~; ~ ;
,
5
S i g N ~
o ~ ~ i corn~ .
' ' ~
. , i
C1 J ; .r. ~, t N 1~' I
I ! i,
I
C ; O ~
m '
G ~ ~ ~ ~ ~ ~ .
V7
i
CI: ~
.~
o g y ~ ~ a ; ~
a
;
C N i cr ' r 3
I
~ O ~ O ! 0 .
O ~ ~ . ~ p
O ..
~ ~
p W N N ~ O
O ( ~ ~ n' r
~
tJ a V V O
;
f ,~ -
1
~ j
J
~ N ~
0
t
7r'
i
0 0 0 .~ -
O
gr N -' .
-23-
~L
~1 ? ,
;
(~
* . ~ ,~
X1.1
p I ~ ~ i
t i
m 1
! ~ ~ ~ !
t
*
1 . .
_ ~ r !
m i I ! N ! I
N N
I
m : , ~ j
I 07 ~ CJ r I !
> : cJ ~ !
~
=
O ~ tm V ~,,~ ~ ; t
i ;
f T ~ y -f. '
C1 t 1 I 1 1 . f ~
! ' I
S
~ .Na r 'y~~ ~ ! ~ ~
' O r 1 O
~ m ~ N ~ O t c7
io i f 1
~ o ...
~
_ n, a. ~, n p
! :
~ ~ o ~ : !
~ N ~
a' : r : r
r _ ~
~ I ~ ~ ,~ a ~
~ ' ~
'
(L7
o ~ s~ ~ ...
I ~ ..
!
1
~
. _ :,7 r ~
' '
_ ~ _ N ~
'
r
a: ~: ~. N
~ ~
N ;~ V . p , s
~_ . : , r~ o
'
+ s. y p .
y
1 _ r r N m ~S
' O .
1
,
, a ~.I '~ N ..v
s ' ' O
O O t O I O a - O 1
3
~ ~ ~ p w ' ~
'
i N r~ i ~ C
_ r CJs trr O V m o ~ Q t O
~ ; ~
C
01 ; N (J ~ 3 _
: t , rJ t
.
+ I m O fIf
O A + . ~ t ; -
i f
;
~ ?
. ~ L1 N ~ ~ ~ ~ t . r"~,
~ i
'
_ t I t C7 ~ 1 /
. i 1
? m 1 t~ N O
O ~
' ~
! O
O 1 + 1 i t
C I ~- 1 I ~
y ;
:
3 t _ r .
' ~ r
~f' ~ O (.1 (Jt N y Zp 1
. ~ .
~a ~ r " O ~/
~
O r '. O 0 'd <.
~
, ~ ~
p O . O y ' O
O
.
~
1 N O i Q !
! ~ m Of O O tD ~ I
0 ~ ~ j t5 ~ t
r N
; 1
O Of Qf O : : ~
i t. i I
I I
i rj ~ T ~ ~; ~
tn ~ '' ' ' ~ eW 'C
, ; j a
w ~. W ~ y ~ ~ ~
t t ~
o ~ ~a -~ t 1 "'!
j , ~
r.
N N a ~ >- i I
t t
N ; ( ~ 1
I "~
1
m
~ !
! 1 ~ 1 w '
n ~ :
. !
!
i ~'' ~ d r
' ;
v '~ 'd
' f
o i o 1
~
$ m, ~
1
. I r ,
~l ~ m ~ m ~ ' ~ i
p ~
Go to N O O o~
t s y + I
1 \ 1
y
,
m t
!
iJ " ~ "' i 3 ~ t ' _
~ ~
24
Obviously, numerous modifications and variations
of the present invention are possible in light of the
above teachings. It is therefore to be understood that
within the scope of the appended claims, the invention
may be practiced otherwise than as specifically
described therein.