Note: Descriptions are shown in the official language in which they were submitted.
20~37~3
The invention relates to new substituted 1,8-
naphthyridines, to intermediate~ or their preparation,
and to their preparation and their use in medicaments
It is known that lactone derivatives isolated
from fungal cultures ~re inhibitors of 3-hydroxy-3-
methyl-glutaryl-coenzyme A reductase (HMG-CoA reductase)
~mevinolin, EP-A 22,478; US 4,231,9381 Moreover, certain
indole derivatives or pyrazole derivatives ~re inhibitors
of HMG-CoA reductase tEP-A 1,114,027; US Patent
4,613,610]
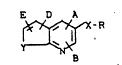
New substituted 1,8-naphthyridines o$ the general
formula (I)
E D A
~- R
B
in which
A - repre~ents a 3- to 7-membered heterocycle which
lS m~y contain up to 4 sulphur, oxyqen or nitroqen
heteroatoms and which is optionallY
monosubstituted to trisubstituted
by identic~l or different ~ubstituents from the
erie- comprl~ing halogen, trlfluoromethyl,
trlfluorometho~y, tralght-chaln or br~nched
alkyl, alkoxy or ~lko ycarbonyl ln ach ca-e
havlng up to 8 carbon atoms or by ~ryl h~vlng 6
to 10 carbon atoms,
or
~e A 26 759 - 1 -
20~3783
A - repre~ents aryl having 6 to 10 carbon atom~ which
is optionally monosubstituted to pentasubstituted
by identical or different substituents from the
series comprising straight-chain or branched
S alkyl, alkylthio, alkylsulphonyl, alkoxy or
alkoxycarbonyl in each ca~e having up to 10
carbon atoms, which may in turn be substltuted by
hydroxyl, ~lkoxy having up to 6 c~rbon atoms,
phenyl or by a group of the formula -NRlR2,
in which
Rl and R2 ar~ identical or different and
- denote hydrogen, aryl or arylsu1phonyl
having 6 to 10 carbon atoms, straight-
chain or branched alkyl or alkyl~ulphonyl
having up to 8 carbon atom~, where the
last mentioned radicals are optionally
sub~tituted by aryI having 6 to 10 carbon
~toms,
- or denote a group of the formula -CoR3
in which
R3 - denotes str~ight-ch~in or br~nched
aIkyl or ~lkoxy having up to 8 carbon
~tomR, or phenyl,
or the ~ryl i9 substituted by aryl, aryloxy, arylthlo or
Aryl-ulphonyl h~vlng 6 to 10 c~rbon ~toms, or by
halogen, nltro, cyano, trlfluoromethyl,
6 7$~ - 2 -
2~ 3 ~g3
trifluoromethoxy, trifluoromethylthio, benzyloxy
or a group of the formul~ -NRlR2,
in which
Rl and R2 have the ~bovementioned meaning,
5 B - represents cyclo~lkyl having S to 8
carbon atoms, straight-chain or
br~nched alkyl having up to 12 carbon atoms,
which i~ optionally substituted by halogen,
trifluoromethyl or alkylthio havlng up to 8
carbon atoms,
- represents aryl having 6 to 10 carbon atoms,
- which is optionally substituted by halogen,
cyano, nitro, trifluoromethyl, straight-chain or
branched ~lkyl, alkoxy or alkoxycarbonyl in each
case having up to 8 carbon atoms, or amino,
D and E are identical or different and
- represent hydrogen, halogen, mercapto, hydroxyl,
a}koxy having up to 8 carbon atoms, str~ight-
chain or branched alkyl having up to 10 carbon
~toms, which is option~lly substituted by
:~ hydroxyI, phenoxy, halogon, trifluoromethyl or
~lkylthio h~ving up to 8 carbon ~toms, or repre-
: sent a group of the fonmula -NRlR2,
ln whlch
Rl ~nd R2 h~v the ~bovqmentloned mean$ng,
or D and E each independently
Le A 26 ?S9 3
20~37~3
- repre6ent aryl, aryloxy or arylthio having 6 to
lO carbon atom6, which is optionally sub~tituted
by halogen, cyano, nitro, trifluoromethyl,
straight-chain or branched alkyl, alkoxy or
S alkoxycarbonyl in each case having up to 8 carbon
atoms, or amino,
Y - represent~ a group of the formula
-frN- or
J Z G
in which
lO J - denotes hydrogen, hydroxyl, mercapto or halogen,
or denote~ straight-chain or branched alkyl,
alkoxy or alkylthio having up tolO carbon atoms,
which are optionally ~ubstituted by phenyl, or
denotes aryloxy, benzyloxy or arylthio having 6
to 10 carbon atom~ or a group of the formula
R2
- in which
Rl and R2 have the ~bov~mentioned meaning,
Z - donote~ oxyg n or ulphur,
G - d not-~ hydrog n, tr~iqht-chain or branched
alkyl or alkenyl in -ach ca-e ha~ing up to lO
carbon atoms, which 1~ optionally ~ubst$tutQd by
~e A 26 759 _ 4 _
20~ 3~3
halogen, cyano, alkoxy having up to 8 carbon
atoms, benzyloxy, aryl or aryloxy having.6 to 10
carbon atoms, by a 5- to 7-membered heterocycle
having up to 4 nitrogen, oxygen or sulpur
heteroatoms or by a
group of the formula -NRlR2, -COR~ or -COOR~,
in which
R1, R2 and R3 have the abovementioned meaning,
R~ - denotes hydrogen or straight-chain or
branched alkyl having up to 10 carbon
atoms, which is optionally substituted by
hydroxyl, phenyl, halogen or cyano,
_ or R~ denotes aryl having 6 to 10 ~rbon atoms,
which may in turn be substituted by
halogen, amino, hydroxyl, nitro or cyano,
X - represents a group of the formula -CH2-CH2- or
-CH=CH-,
and
R - represents a group of the formula
I R5
7H CH2 7-CH2-COoR6 or HO
OH OH
$n wh$ch
e A 26 759 - 5 -
2 ~ 1 3 r~l ~ 3
Rs _ denotes hydro~en or ~traight-chain or
branched alkyl having up to lQ carbon
atoms
and
R5 _ denstes hydrogen or straight-chain or
branched alkyl having up to 10 carbon
atoms, which may be substituted by phenyl,
or
- denote~ aryl having 6 to lO carbon atoms
or a cation,
and their salts have now been found.
If R~ forms an ester radical with the carboxyl
group, then a physiologically tolerable e~ter radical,
which i8 ea~ily hydrolyzed in vivo to give a free car-
boxyl group and a corresponding physiologically tolerablealcohol, io preferably meant by this. These include, for
example, alkyl e~ter~ (C1 to CE) and aralkyl ester6 (C~ to
C10), preferably (C~-C~)-alkyl esters and benzyl esters.
Moreover, the following e~ter radical~ may be mentioneds
methyl e~ter~, ethyl e~ter~, propyl esters and banzyl
e6ters.
If RB represents a cation, then ~ physiologically
tolerable metal cation or a~monium cation ~8 psefer~bly
meant. Preferred cations in this connection ~re alkali
metal or al~aline earth aetal oatlon~ uch as, for
ex~mple, ~odium, pota~ium, aagne~lum or calcium cations,
and al~o aluminum or ammonium cation~, and non-toxic
.
Le ~ 2 ~ 7~2 - 6 -
2~3 3r~3
sub6tituted ammonium cations of a~ines such a~ (Cl-C~)-
dialkylamines, Cl-C~)-trialkylamines, procaine,.diben-
zylamine, N,N~-dibenzylethylenedi~mine, N-benzyl-~-
phenylethylamine, N-methylmorpholine or N-ethylmorpho-
line, l-ephenamine, dihydro~bietylamine, N,N'-bi~-
dihydroabietylethylenediamine, N-lower alkyl piperidine
and other amines which may be used for the formation of
salts.
Surprisingly, the substituted 1,8-naphthyridines
according to the invention show a ~uperior inhibitory
action on HNG-CoA reductase (3-hydroxy-3-methyl-glutaryl-
coenzyme A reducta~e).
In the context of the general formula (I),
compounds of the general formula (Ia) and (Ib)
~ and ~-R
(I~) (Ib)
in which
A, B, D, E, X, Y and R have the ~bovementioned meaning,
are preferred.
Preferred compounds are those of the general
formula (Ia) and (Ib),
in which
A - represents pyridyl or pyrimidyl, which i~ option-
ally monosubst$tuted or disubstituted by
e A 26 759 - 7 -
2~3783
identical or different subst$tuents from the
series comprising fluorine, chlorine, bromine,
trifluoromethyl, straight-chain or branched alkyl
having up to 6 carbon atoms or phenyl,
S ~ or A represents phenyl or naphthyl which is oPtion-
ally monosubstituted to tetrasubstituted by
identical or different ~ubstituents from the
series comprising straight-chain or branched
alkyl, alkylthio, alkylsulphonyl, alko~y or
alkoxycarbonyl in e~ch case having up to 8 carbon
atoms, which may in turn be sub~tituted by
hydroxyl, alkoxy having up to 4 carbon atoms,
phenyl or by ~ group of the formula -NR~R2,
in which
Rl and R2 are identic~l or different and
- denote hydrogen, phenyl, phenylsulphonyl,
~traight-chain or branched alkyl or
alkylsulphonyl h~ving up to 6 carbon
atom~, benzyl or benzylsulphonyl, or
- denote ~ group of the formula -CoR3,
in which
R3 - denote~ straight-chain or branched
alkyl or alkoxy having up to 6 carbon
atoms or phenyl,
or the phenyl or naphthyl is optionally mono- to tetrasubstituted
by i- ub-tituted by phenyl, phenyloxy, fluorine,
chlorine, bromine, nitro, ¢yano, trifluoromethyl,
trlfluoromethoxy, ben~yloxy or by a group of the
e A 26 759 - 8 -
~0137~3
formula -NRlR2,
in which
R~ and R2 have the abov~mentioned meaning,
B - repre~ents cyclopropyl, cyclobutyl, cyclopentyl
or cyclohexyl,
- repre~ents straight-chain or branched alkyl
having up to 10 carbon atoms, which may option-
ally be substituted by fluorine, chlorine,
bromine, trifluoromethyl or methylthio,
D and E are identical or different and
- represent hydrogen, hydroxyl, alkoxy having up to
6 carbon atom~, straight-chain or branched alkyl
having up to 8 carbon atoms, phenyl or a group of
the formula
-NRlR2,
in which
R~ and R~ have the abovementioned meaning,
Y - repre-ents a group of the formula
-C~N- -C-N-
I or ll l
J z G
ln wh$ch
J - denotes hydrogen, hydro yl, mercapto,
Le A 26 759 - 9 -
2~ 37~3
fluorine, chlorine or bromine, or denotes
~traight-chain or branched alkyl, alkoxy
or alkylthio having up to 8 carbon atoms,
which are optionally substituted by
phenyl, or denotes phenoxy, benzyloxy or
a group of the formula -NRlR2,
in which
Rl and R2 have the abovementioned meaning,
Z - denote~ o~ygen or sulphur,
10 G - denote~ hydrogen, straight-chain or branched
alkyl or alkenyl in each case having up to 8
carbon atoms, which iB optionally sub~tituted by
fluorine, chlorine, bromine, cyano, alkoxy having
up to 6 carbon atoms, phenyl, phenoxy, benzyloxy,
lS pyrryl, furyl or by a group of the formula -NRlRZ,
-CoR3 or -COOR~,
in which
Rl, R2 ~nd R3 h~ve the ~bovementioned meaning,
R~ - denotes hydrogen, str~ight-ch~$n or
branched alkyl having up to 8 carbon
~toms, which 1~ opt$on~11y ~ubstituted by
hydroxyl, phenyl, fluor~ne, chlor$ne or
bromine,
- denote- phenyl whl¢h aay ln turn be
2S ub-tltuted by fluor$n , ohlor$ne, bromlne
Le A 26 759 - 10 -
2~3~v3
or hydroxyl,
x - represents a group of the formula -CH2-C~2- or
-CH=CH-
and
S R - represents a group of the formula
R5
-ICH-cH2-l_c~2_cOOR or HO
OH OH
in which
R5 - denotes hydrogen or traight-chain or
branched alkyl having up to 8 carbon atoms
and
R6 _ denotes hydrogen or straight-chain or
branched alkyl having up to 8 carbon
atoms, or benzyl, or
- denotes phenyl or a cation
and their ~alts.
Part$cularly preferred compounds of the general
formulae ~Ia) and ~Ib) are tho~e
in wh$ch
A - represents phenyl wh$ch 1- opt~onally
Le A 26 ~2 - 11 -
2~ 37v3
monosubstituted to trisubstituted by identical or
different substituent~ from the ~eries comprising
~traight-chain or branched alkyl having up to 6
carbon atoms, which may in turn be substituted by
hydroxyl, methoxy, ethoxy, propoxy or phenyl,
or the phenyl is substituted by~phenyl, phenoxy, fluorfne,
chlorine, bromine or benzyloxy,
B - represents cyclopropyl, cyclobutyl, cyclopentyl
or cyclohexyl,
- represents methyl, ethyl, propyl, isopropyl,
butyl, isobutyl, tert butyl or trifluoromethyl,
D and E are identical or different and
- represent hydrogen, hydroxyl, methyl, ethyl,
propyl, isopropyl, methoxy or ethoxy,
15 Y - represent~ a group of the formula
_fsN_ or -Il- 1-
J Z G
in which
J - denotes hydrogen, hydroxyl, fluorine or
chlorine, or denotes ~traight-chain or
branched alkyl, alkoxy or ~lkylthio having
up to ~ c~rbon atom~, wh$ch ~re optionally
ub~t$tuted by phenyl, or denote- benzyl-
oxy or a group of the formula -NRlR2,
Le A 26 759 - 12 -
2~13~3
in which
R1 and R2 are identical or different and
- denote hydrogen or straight-chain
or branched alkyl having up to 4
carbon atoms, or benzyl,
Z - denotes oxygen or ~ulphur,
G - denote~ hydrogen, straight-chain or
branched alkyl having up to 6 carbon
atom~, wh~ch is optionally substituted by
fluorine, chlorine, cyano, alkoxy having
up to 4 carbon atoms, phenyl, benzyloxy or
by a group of the formula -CoR3 or -COOR~,
in which
R3 - denotes straight-chain or branched
alkyl or phenyl,
R~ - denote~ hydrogen, straight-chain
: or branched alkyl ha~ing up to 6
carbon atoms or phenyl,
X - repre~ents a group -CH~CH- or -CH2-CH2-
and
R - repre~ents a group of the for~ula
e A 26 759 - 13 -
2~137~3
R5
I R5`_~A~G~o
-IH-cH2-l-cH2-cooR6 or Ho ~ T
OH OH
in which
R~ - denotes hydrogen, methyl, ethyl, propyl,
$sopropyl, butyl, isobutyl or tert.butyl
S and
R6 _ denotes hydrogen, methyl, ethyl, propyl,
isopropyl, butyl, i~obutyl, tert.butyl or
benzyl, or
- denotes a sodlum, pota~sium, calcium,
magnesium or ammonlum ion
and their ~alts.
The substituted 1,8-naphthyridines of the general
formula (I) according to the invention have several
asymmetric carbon atoms and can therefore exlst in
various stereochemical form~. The invention relates both
to the individual ~omers ~nd to thelr mixtures.
Depending on the meaning of the group X or the
radical R, different stereoisomars result, which are
intended to be lllu-trated in more detail ~n the
following~
a) if the group -S- repr-sent~ a group of the formula
-CH-CH-, the ¢ompound~ ~ccordlnq to the lnvention
Le A 26 759 - 14 -
20~37~3
can exi8t in two stereoisomeric forms which can have
the E configuration (II) or Z configuration ~III) on
the double bond:
E~ D A (II) E form
E D A
~ R
~ (III) Z form
(A, B, D, E and R have the abovementioned meanin~).
Preferred compounds of the genersl formula (I)
are those which have the E configur~tion (II).
b) If the radical -R- represents a group.of the formula
IR5
-IH-cH2-cl-cH2-cooR6
OH OH
the compounds of the general formuls ~I) have at
least two a~ymmetric carbon ~tom~, n~mely the two
carbon atom~ to whlch the hydroxyl group~ are
bonded. Dopendin9 on the r l~ti~e po~ltlon of the~e
hydroxyl groups to one another, the compounds
Le A 26 759 - 15 -
20~37~3
according to the invention msy be present in the
erythro configuration ~IV) or in the threo con-
figuration (v).
E D A
~ ~H CH2-~-cH2-cooR6 erythro form (IV)
R5
E D A
~X- CH- CH2 - C - CH2 - COOR6
S ~ OH OH threo form (V)
In turn, two enantiomers each exist both of the
compounds in the erythro and in the threo configu-
ration, namely the 3R,SS-isomer or the 3S,SR-isomer
(erythro form) ~nd the 3R,SR-isomer and 3S,5S-isomer
(threo form).
In this connection, the isomers in the erythro
configuration are preferred, particularly preferably
the 3R,5S-isomer ~nd the 3R,SS-3S,SR-racemate.
c) If the radical -R- reprooents a group of the formula
HO ~
,?<
15 the ub~tituted 1,8-naphthyridino~ have at loaot two
~e A 26 759 - 16 -
2013~3
a~ymmetric carbon atoms, namely the carbon atom to
which the hydroxyl group i8 bonded, and thç carbon
atom to which the radical of the formula
E ~ A
i8 bonded Depending on the po~ition of the hydroxyl
group to the free v~lency on the l~ctone ring, the
substituted 1,8-naphthyridines may be present as
cis-lactones (VI) or ~ trans-lactones (VII)
, HO~ \R
cis-lactone (VI)
H0",~ R5
E~ tr~n~-l~cton (VII~
In turn, two isQmers each exi~t of the cis-lactone
and the tr~ns-lactone, namely the 4R,6R-isomer or
the 4S,6S-i-omer ~ci~ ctone), ~nd tho 4R,6S-i-omer
or 4S,6R-l~omer (tr~n~ ctone) Pref-rred l~omers
~re the tr~n~ ctono~ ~he 4R,6S-lsQmer ~tran-) ~nd
the 4R,6S-4S,6R-r~com~te ~re p~rtlcul~rly preferred
Le A 26 759 - 17 -
2~13783
in this connection.
For example, the following isomeric forms of the
substituted 1,8-naphthyridines may be mentioned:
A
E D ~ I
~\\\`~0'' ~o
A R ~ OH
E D ¦ ¦ ¦
~0~0
Le A 26 759 - 18 -
20~37~3
A ~
~ o~o
A ~
~lo~o
A OH OH
~H C ~ 5 6
A OH OH
E D I C ~
~CH - CH2 - CR5 - CH2 - COOR6
B
A OH OH
~-CH2-CR5-CH2-CooR6
A OH OH
E D I . ~
~CH-CH2- CR5 -CH2-COOR6
~B
~ ~/H-CH2-~R5-CH2-COOR6
I.e A 26 759 - 19 -
2~137~3
B OH OH
E D I - ~
~CH - CH2 - CRS - CH2 - COOR6
~A
B OH OH
~CH-CH2-CR5-CH2-CooR~
B OH OH
~ ~U-CH2-CR5-CH2-CooR6
In addition, a process for the preparation of the
substituted 1,8-naphthyridines of the general formula ~I)
~ -R (I)
in which
A, B, D, E, ~, Y and R have the abovementioned meaning,
has been found, which i~ characterized in that
ketone~ of the general formula tVIII)
O
E CHSCH-CH-CH2-C-CH2-CooR7 (VIII j
in which
Le A 26 759 - 20 -
20137~3
A, B, D, E and Y have the abovementioned meaning,
and
R7 - represents alkyl,
are reduced,
in the case of the preparation of the acid~ the esters
are hydrolyzed,
in the case of the preparation of the lactones the
carboxylic acids are cyclized,
in the case of the preparation of the 8alt~ elther the
esters or the lactones are hydrolyzed,
in the case of the preparation of the ethylene compounds
(x = -CH2-CH2-) the ethene compounds (X - -CH~CH-) are
hydrogenated according to customary method~,
and, if appropriate, isomers are separated.
The proces~ according to the invention can be
illustrated by the following equations
Le A 26 759 - 21 -
20137~3
CH 2C OOCH :3
~OH
reductl on
CH2COOCH3
0~
¦ hydrolysis
COOeN~
~ ~ OH
hCOOH
cycl l ~ atl on ~ ~ ~OH
~ .
~e A 26 759 - 22 -
20~37~3
~'
H
The reductlon can be carried out u~ing the
customary reducing agentE, preferably those which are
suitable for the reduction of ketone~ to hydroxy com-
pounds. Reduction u~ng metal hydrides or complex met~l
hydrides in inert solvents is particularly suitable in
thi6 connection, if appropriate in the pre3ence of a
trialkylborane. Preferably, the reduction is carried out
using complex metal hydride~ such as, for example,
lithium borohydride, sodium borohydride, poeassium
borohydride, zinc borohydride, lithium trialkylboro-
hydrides, sodium trialkylborohydrides, sodium cyanoboro-
hydride or lithium aluminum hydride. Very particularly
preferably, the reduction is carried out using ~odium
borohydride in the presence of triethylborane.
Suitable solventQ in this connection are the
customary organic solvents which do not change under the
reaction conditions. These preferably include ethers such
as, for example, diethyl ether, d~oxane, tetrahydrofuran
or dimethoxyethane, or halogenated hydrocarbons uch as,
for example, dichloromethane, tr~chloromethane, tetra-
chlorometh~ne, 1,2-dichloroethane, or hydrocarbons ~uch
as, for example, benz-ne, toluene or xylene. It is
lik~wi-e pos~ble to employ mixture- of the olvents
Le A 26 759 - 23 -
~0~3~$3
mentioned.
Particularly pre$erably, the reduction of the
ketone group to the hydroxyl group i8 carried out under
conditions in which the customary functional groups such
S as, for example, the ~lkoxycarbonyl group do not change.
The use of ~odium borohydride as ~ reducing agent in the
presence of triethylborane in inert solvents such ~,
preferably, ethers is particularly suitable for this
purpose.
The reduction is in general carried out in a
temperature range from -80-C ~o +30-C, preferably from
-78C to O-C.
The proce~s according to the invention is in
general carried out at ~tmospheric pressure. However, it
is also possible to carry out the proces~ at reduced
pressure or at elevated pressure (for ex~mple in a range
from 0.5 to 5 bar).
In general, the reducing agent i~ employed in an
~mount from 1 to 2 moles, preferably from 1 to 1.5 moles,
relative to 1 mole of the keto compound.
Under the abovementioned reaction conditions, the
carbonyl group i8 in general reducad to the hydroxyl
group without reduction of the double bond to a ~ingle
bond taking plAce.
In order to prepare compound~ of the general
formula (I), in which ~ repre~ent~ an ethylene grouping,
the reduction of the ketone~ (III) c~n be c~rried out
under those condition- under ~hich both the carbonyl
group ~nd the double bond are r duced.
Noreover, $t 1~ o po-~lble to o~rry out the
Le A 26 7~9 - 24 -
201 37~3
reduction of the carbonyl group and the reduction of the
double bond in two separate steps.
The carboxylic acids in the context of the
general formula (I) correspond to the formula (Ic)
E D A l5
~ -ICH-CH2-Cl-CH2-COOH ~Ic)
~ OH OH
in which B
A, B, D, ~, and R5 have the abovementioned meaning.
The carbo~ylic ~cid ester~ in the context of the
general formula (I) correspond to the formula (Id)
R5
~ -ICH-cH2-l-cH2-cooR7 (Id)
in which
A, B, D, E, Y and R5 have the abovementioned meaning,
and
R7 - represents alkyl.
The s~lts of the compounds in the context of the
general formula (I) according to the in~ention correspond
to the formul~ (Ie)
~ -CIH-CH2-cl-cH2-coo ~ Mn- (I.)
I,e A ~b 7~2 - 25 -
2013733
in which
A, B, D, E, Y and R5 have the abovementioned meanLng,
and
Mnt represents a cation, where n indicates the valency.
The lactones in the context of the general
formula (I) corre6pond to the formula (If)
HO~y~R5
E D A ~ ~
~0~
in which
A, B, D, E, Y ~nd Rs h~ve the ~bovementioned mean$ng.
In order to prepare the carboxylic acids of the
general formul~ (Ic) according to the invention, the
carboxylic acid e~ters of the general formula (Id) or the
lactones of the general formula (If) are in general
hydrolyzed according to cu~tomary method~. Hydrolysi~ i~
in general carried out by treating the e~ters or the
lactones in inert ~olvents with cu-tomary bases, in
qeneral the salts of the general formula (Ie) initially
resulting, which c~n aub~equently be converted in a
second step by treating wlth acid into the free acids of
the general formula ~Ic).
Suitable base~ for hydroly-is are the cu~tomary
inorg~nic baae-. Theoe pref-rably lnclude alkali metal
hydroxidea or alkaline earth etal hydroxlde- uch aa,
for example, ~odium hydroxide, pota~aium hydroxlde or
; 25 barium hydroxide, or alkali metal c~rbonate~ ~uch a8
.
Le A 26 759 - 26 -
20137~3
sodium carbonate or potassium carbonate or sodium hydro-
gen carbonate, or alkali metal alkoxides such as sodium
ethoxide, sodium methoxide, potassium methoxide,
potassium ethoxide or potassium tert. butoxide. Sodi~m
S hydroxide or potassium hydroxide are particularly prefer-
ably employed.
Suitable solvents for hydrolysis are water or the
organic solvents customary for hydrolysi~. These pre-
ferably include water, alcohols ~uch as ~ethanol,
ethanol, propanol, i-opropanol or butanol, or ethers such
as tetrahydrofuran or dio~ane, or dimethylformamide or
dimethyl sulphoxide. Particularly preferably, methanol,
tetrahydrofuran or water are used. It is al~o possible to
employ mixtures of the solvents mentioned.
The hydrolysis is in general carried out in a
temperature range from O-C to +lOO~C, preferably from
+20C to +80-C.
In general, the hydrolysis is carried out at
atmospheric pressure. Nowever, it is also possible to
work at reduced pressure or elevated pre~sure (for
example from 0.5 to 5 bar).
When carrying out the hydrolysis, the ba~e i~ in
general employed in an amount from 1 to 3 moles, pre-
ferably from 1 to 1.5 moles, relative to 1 mole of the
ester or the lactone. Molar amounts of the reactants are
particularly preferably u~ed.
When carry$ng out the reactlon, the alt- of the
compounds (Ie) accordlng to the lnv ntlon ~ro fonmed ln
the fir~t ~tep ~ lntermedlato- ~hlch c~n bo l~olat-d.
The acids ~Ic) according to the inventlon are obtained by
Le A 26 759 - 27 -
2~:137~3
treating the salts (Ie) with customary inorganic acids.
These preferably include mineral acids such as, for
example, hydrochloric acid, hydrobromic acid, sulphuric
acid or phosphoric acid. It has proved advantageous in
S this connection in the preparation of the carboxylic
acid~ (Ic) to acidify the basic reaction mixture from the
hydroly~i~ in a second step without isolation of the
salts. The acids can then be i~olated in a customary
manner.
In order to prepare the lactone~ of the formula
( If ) according to the invention, the carboxylic acids
(Ic3 according to the invention are in general cyclized
according to customary methods, for example by heating
the corresponding acid in inert organic solvents, if
appropriate in the presence of mo}ecular sieve.
Suitable solvents in this connection are hydro-
carbons such as benzene, toluene, ~ylene, mineral oil
fractions, or tetralin or diglyme or triglyme. Benzene,
toluene or xylene are preferably employed. It i~ also
possible to employ mixture~ of the sol~ent~ mentioned.
Hydrocarbons, in particular toluene, in the presence of
molecular sieve are particularly preferably u~ed.
The cyclizatinn 18 in general carried out in a
temperature range from -40-C to +200-C, proferably from
-25-C to +50-C.
The cycllzation i8 in general carried out at
atmospheric pre-sure, but lt i~ al~o po88ib1e to carry
out the proce~ at reduced pre~ure or at elevated
pres~ure ~for example in a range from 0.5 to 5 bar).
Moreo~er, the cy~lization i~ aIso carriod out in
Le A 26 759 - 28 -
20 ~ 37~3
inert organic solvents, with the _id of cyclizing or
dehydrating _gents Carbodiimides _re preferably used as
dehydrating agents in this connection N,N~-dicyclohexyl-
carbodiimide paratoluenesulphonate, N-cyclohexyl-N'-~2-
(N'-methylmorpholinium)ethyl]carbodiimide or N-(3-di-
methylaminopropyl~-N~-ethylc_rbodiimidehydrochlorideare
preferably employed _8 C_rbodiimideB.
Suit_ble solvents in thi~ connection _re the
customary org_nic ~olvents The~e preferably include
ethers such a8 diethyl ether, tetrahydrofur_n or dioxane,
or chlorinated hydroc_rbons such _8 methylene chloride,
chloroform or carbon tetrachloride, or hydrocarbons such
as benzene, toluene, xylene or miner_l oil fractions
Chlorinated hydrocarbons such a8, for example, methylene
chloride, chloroform or c_rbon tetr_chloride, or hydro-
carbons such as benzene, toluene, xylene or mineral oil
fractions are particularly preferred Chlorinated hydro-
carbons such as, for ex_mple, methylene chloride,
chloroform or carbon tetr_chloride _re particularly
preferably employed
The re~ction i8 in gener_l carried out in _
temper_ture r_nge from O C to +80 C, preferably from
+lO C to +50 C
When carrying out the cycliz_tion, it h_s proved
advant_geous to employ the cycli~ation method with the
aid of carbodiimides _8 dehydratinq _g nts
~ he re-olution of the i-o~or- lnto the
~tereoi~omerlcally uniform con-titu-nt- i- in gen r_l
c_rried out by cu-tomary method- uch a- ar- de~cribed,
for e~_mple, by E L ~liel, Ster~ochemi-try of C_rbon
Le A 26 759 - 29 -
2~37~3
Compounds, McGraw Hill, 1962. Resolution of the isomers
in the racemic ester Btep iB preferred in this con-
nection. The racemic mixture of the trans-lactones (VII)
is particularly preferably converted in this case by
treating either with D-(+)- or L-(-)-~-methylbenzylamine
by customary methods into the diastereomeric dihydroxy-
amides (Ig)
1~ IH3
E D A ~ -cH2-coNH-cH-c6H5
~ ~ H (Ig)
which can then be resolved into the individual dia~tere-
omers as i~ customary by chromatography or crystal-
lization. Subsequent hydrolysis of the pure dia~tere-
omeric amides by customary methods, for ex~mple by
treating the dia~tereomeric amides with inorganic base~
such as sodium hydroxide or potassium hydro~ide in water
and/or organic solvents such a8 alcohols, for example
methanol, ethanol, propanol or isopropanol, gives the
corresponding enantiomerically pure dihydroxy acids tIc)
which can be converted lnto the enantiomerically pure
lactones by cyclization as descrlbed above. In general,
it is true for the prep~ration of the compounds of the
general formula (I) according to the invention in enan-
t~omerically pure form that the configur~tlon of the
final product- accordlng to the method descrlbed ~bove
is dependent on the configuratlon of the t~rtlnq ub-
stances.
Le A 26 7S9 - 30 -
20137~3
The resolution of isomers is intended to be
illustrated by way of example in the following ~cheme:
'~
~ A.~6 ~59 - 31 -
20~37~3
~ COOCH3
erythro racQmate
H3
~I ~ H2N-CH-C6HS
F OH IH
H2-CO-NH-CH-c6~5
"`~OH
H~ mixture of diastereomers
1) separation of diastereomers
2) hydroly~is
3) l~ctoniz~tion
F ~H F
Le A 26 75~ - 32 -
2~ 37~3
The ketones (VIII) employed as starting sub-
stances are new.
A process for the preparation of the ketones of
the general formula (VIII) according to the invention
o
E D A ¦¦
~ CH=CH-IH_CH2_C_CH2_COOR7 (VIII)
in which
A, B, D, E, Y ~nd R7 have the abovement~oned meaning,
has been found, which i~ characterized in that
aldehydes of the general formula (IX)
R
E D A ~
~ (IX)
in which
A, B, D, ~ ~nd Y h~ve the abovementioned meaning,
sre reacted in inert solvents with ~cetoacetic e~ters of
the general formula (X)
7 ~X)
15 in which H3c-c-cH2-cooR
R7 has the abovementioned meaning,
in the pre~ence of base~.
~ he process according to the invention c~n be
illu~trated, for e~mple, by the followlng equatlont
Le A 2~ 75~_ 33 _
2al137~3
~ 1lH
o ~ ~ H3C-C-CH2-COOCH3
~ ¦ B~
CH2COOCH3
Suit~ble b~ses in this connection ~re the CU8-
tomary 6trong b~sic compounds. These preferably include
organolithium compounds such as, for example, N-butyl
lithium, sec. butyllithium, tert. butyllithium or phenyl
lithium, or amides uch a~, for ex~mple, lithium diiso-
propylamide, sodium ~mide or pot~ssium ~mide, or lithium
hexamethyldisilyl~mide, or ~lkali met~l hydr~des such a~
sodium hydride or pot~ssium hydride. It is likewise
possible to ~mploy mixture~ of the b~ses mentioned.
N-butyllithlum or ~odium hydride or ~ mlxture thereof i8
p~rtlcul~rly pref-r~bly employed.
Addition- of met~l h~lide~ uch ~8, for ex~mple,
Le A 26 752 - 34 -
20137~3
magnesium chloride, zinc chloride or zinc bromide may be
advantageous. The addition of zinc halides iB particu-
larly preferred.
Suitable solvents in this connection nre the
customary organic solvents which do not change under the
reaction conditions. These preferably include ether~ such
as diethyl ether, tetrahydrofuran, dioxane or dimethoxy-
ethane, or hydrocarbons ~uch as benzene, toluene, ~ylene,
cyclohexane, hexane or mineral oil fractions. It i8
likewise possible to employ mixture~ of the solvents
mentioned. Ethers such às d$ethyl ether or tetrahydro-
furan are part$cularly preferably used.
The reaction is in general carried out in a
temperature range from -80-C to +50-C, preferably from
-20-C to room temperature.
The process is in general carried out at
atmospheric pressure, but it is al~o possible to carry
out the process at reduced pressure or elevated pressure,
for example in a range from 0.5 to 5 bar.
When carrying out the process, the acetoacetic
ester is in general employed in an amount from 1 to 5,
preferably from 1 to 3, moles, relative to 1 mole of the
aldehyde.
The acetoacetic e-ters of the formula (~)
employed as ~tarting ubstances are known or can be
prepared by known method~ ~eilstein's Handbuch der
organi~chen Chemie ~eil-t-in~ Handbook of Organic
Chemistry) ~I~, 632t ~381-
Example- of ao-toac-tlc -t-r- for th proce~s
according to the invention which ~ay be mention d aret
Le A 26 759 - 35 -
201378~
methyl acetoacetate, ethyl acetoacetate, propyl aceto-
acetate and i~opropyl acetoacetste
The preparation of the aldehydes of the general
formula (IX) employed a~ ~tarting substances is intended
to be illustrated below by way of ex~mple for the 1,8-
naphthyridines of the type (Ia)
[A]
~COOR8 ~ CHzC)H
(XI~/X~b) ~XII)
A A H 'CHO
r 2 ] ~CHO ~ 3 ~ ~H
~X2II ) ~ IX)
~ .
In this connection, ~ccording to ~ch~me A, 1,8-
naphthyridines of the formula (XI), ln which R~ represent~
an alkyl radical having up to 4 carbon atoms, are reduced
to the hydroxymethyl compound~ (XII) in the first step
[1] in inert olvent- uch a- eth r-, for x~mple di-
ethyl-ther, tetrahydrofur~n or dloxane or hydroc~rbon-
~uch a- toluene, pr-f-r~bly toluene, u-lng ~et~l hydrlde~
15 ~B r duclng ~gent-, for x~mpl- lithlu~ aluminum
hydrlde, odium cyanoborohydride, odlum aluminum
~e A 26 759 - 36 -
2~137~3
hydride, dii~obutylaluminum hydride or sodium bi~(2-
methoxyethoxy~-dihydro~luminate, in temperature ranges
from -75-C to +lOO-C, preferably from -80-C to room
temperature, or from room temperature to -78-C depending
on the reducing agent u~ed. Preferably, the reduction i8
carried out using diisobutylaluminum hydride in tetra-
hydrofuran or toluene in a temperature range from -78-C
to room temperature. The hydroxymethyl compounds (XII)
are oxidized by cu~tomary methods to the aldehydes ~III)
in the second ~tep [21. The o~id~t$on c~n be c~rried out,
for example, with pyridinium chlorochromate, if appropri-
ate in the presence of ~lumina, in inert solvents such as
chlorinated hydrocarbon~, preferably methylene chloride,
in a temperature range from O-C to 60-C, preferably at
room temperature, or else are carried out usinq di~ethyl
sulphoxide by the customary method~ of Swern oxidation.
The aldehyde~ (XIII) are reacted to give the aldehydes
~IX) in the third step [3] using diethyl-2-(cyclohexyl-
amino)-vinylphosphonate in the presence of sodium hydride
in inert solvents ~uch a~ ether~, for example diethyl-
ether, tetrahydrofuran or dio~ane, preferably in tetra-
hydrofuran, in a temperature range from -20-C to +40-C,
prefer~bly from -5-C to room temper~ture.
The 1,8-naphthyridines of the formula (XI)
employed as ~tarting subst~nces in this connection are
new.
Compound~ of th formula (XIa), ln which Y
repre~ent~ the group of th for~ul~
-C-N-
11 1
Z G
Le A 26 759 _ 37 _
2~37~3
in which
G and Z have the abovementioned meaning, can be obtained
by oxidizing 3,4-dihydro-1,8-naphthyridines of the
formula (XIV), in which A, B, D, E, G, Z and R~ have the
abovementioned meaning, according to scheme lBl The
oxidation can carried out, for example, with chromic
oxide or sodium nitrite $n glacial acetic acid, with
nitric acid in a aqueous suspension, with cerium salts
such as, for example, ~mmonium cerium nitrate in a
solvent mixture of acetonitrile and water, or with
dichlorodicyano-p-benzoguinone in the abovementioned
inert solvents, in a temperature range from -20 C to
+150 C A
ooR8 G COOR8
~XIV) (%~)
lS The 3,4-dihydro-1,8-naphthyridines of the general
formula (XIV) employed as starting substances in this
connection are new
They ~re obt~ined according to ~cheme ~C] by
reaction of suitably sub~tituted ~ un~aturated
carboxylic acid e~ter~ of the general formula (XV), $n
which A, B and R have the abovementioned meaning, with
substituted 6-~mino-2-pyridone~ of the general formula
(XVI), in whlch D, E, G and ~ hav the abo~ementioned
meaning,
Th r action can be illu-trated by th following
e A 26 759 - 38 -
2~37~3
equation
[C] A D A
oOR8
B z ~ NI~l~NH2z ~ N~l~N~'~B
G G
~XV) ~XVI) (XIV~)
The process can be carried out in ~ubst~ncQ or in
a high-boiling solvent such a~, for example, ethylene
S glycol or dimethylform~mide, if appropriate in the
presence of acetic acid, at room temperature to ~200 C
Reaction in sub~tance or dimethylformamide at +120 C to
+160 C is preferred
In addition, compounds of the general formula (I)
in which Y represents the group of the formula
-C-N-
11 1 .
Z G
and G denote~ a C1-C6-~lkyl radical, can be prepared by
alXylating compounds of the general formulae (XIII),
(XIV) or tI), in which G repre~ents hydrogen, with Cl-C6-
~lkyl halides, uch as, for example, methyl iodide in the
presence of bases, ~uch as, for example, pot~ssium
tert butoxide, in the ~bovementioned olvents, preferably
in dimethylformamide at room temperature
Compound- of the formula ~XIb), in which Y
represent- the group of th formul~
-I-N-
L~_A_~ 152 - 39 -
2~ 37~3
in which J has the abovementioned me_ning, can be pre-
pared by reacting compounds of the formula (XIa) by
methods known from the literature [cf. L.F. Pieser,
M. Fieser, Reagent6 for Organic Synthesis, Vol. l, p.1232
5(1967)l either with trialkyloxonium salts, preferably
with trimethyloxonium tetrafluoroborohydride at room
temperature, or with bases such as, for example, potas-
sium tert.butoxide or sodium hydride and C1-C~-alkyl
halides such as, for ex_mple, methyl iodide, ethyl iodide
lOor isopropyl iodide, preferably with isopropyl iodide, in
the abovementioned solvents at roo~ temperature. It i8
also possible first to carry out a chlorination reaction
with phosphorus chlorides, such as, for example, phos-
phorus oxychloride, $n the presence of C1-C~-dialkyl-
15anilines at O-C to +lOO-C and then to $ntroduce the group
F by nucleophilic substitution.
The compounds of the general formula (I) accord-
ing to the invention possess useful pharmacological
properties and can be employed in medic_ments. In par-
20ticul_r, they _re inhibitors of 3-hydroxy-3-methyl-
glutaryl-coenzyme A (HMG-CoA) reduct_se and, aB a result
of this, inhibitors of cholesterol biosynthesis. They can
therefore be employed for the treatment of hyperlipo-
proteinaemia, lipoproteinaemia or atherosclerosis. The
25active substances according to the invention additionally
cause a lowering of the cholesterol content in the blood.
The enzyme aetivity dete-m~n~tlon wa- earried out
as modified by G.C. Ne~- et al., Arehiv ~ of Bioehemi-try
_nd Blophy~ics 197, ~93 ~ ~99 E 1979~ le Rleo rats
30(body weight 300 - ~00 g) were treated for ll d_ys with
L4 A 26 759 - 40 -
29~37~3
sltromin powdered feed to which 40 g of cholestyramine/kg
of feed had been added. After decapitation, the liver was
removed from the animals and placed on ice. The liver6
were comminuted and homogenized 3 times in 3 ~olumes of
S 0.1 M ~ucrose, 0.05 M ~Cl, 0.04 M R~ pho~phate, 0.03 M
ethylenediaminetetraacetic acid, 0.002 m dithiothreitol
tSPEI buffer pH 7.2 $n a Potter-Elve~em homogenizer. The
mixture was then centrifuged at 15,000 g for 15 minutes
and the sediment was discarded. The supernatant was
sedimented at 100,000 g for 75 minute~. The pellet i~
taken up in 1/4 volume~ of SPE buffer, homogenized once
more and then centrifuged again at 100,000 g for 60
minutes. The pellet iB taken up using a 5-fold amount of
its volume of SPE buffer, homogenized and frozen and
~tored at -78-C (- enzyme ~olution).
For testing, the test compound~ (or mevinolin a~
a reference substance) were di~olved in dimethylfor-
mamide with the addition of 5 vol.-% of 1 N NaOH and,
u~ing 10 ~1, employed in the enzyme test in various
concentrations. The test wa~ started after preincubation
of the compound~ with the enzyme at 37-C for 20 minutes.
The test batch was 0.380 ml and contalned 4 ~col of
gluco~e-6-phosphate, 1.1 mg of bovlne ~erum albumin,
2.1 ~mol of dithiothreitol, 0.35 ~mol of NADP, 1 unit of
glucose-6-phosphate dehydrogenase, 35 ~mol of R~ phos-
phate pH 7.2, 20 ~1 of enzyme preparation ~nd 56 nmol of
3-hydro~y-3-methyl-glutaryl-coenzyme A (glutaryl-3-1~C~
100,000 dpm.
After lncubating for 60 minutes ~t 37-C, the
batch wa~ centrlfuged and 600 ~1 of the ~upernatant was
Le A 26 759 - 41 -
2013733
applied to a 0.7 x 4 cm column packed with a 5-chloride
100-200 mesh (anion exchanger). The column W48 sub-
sequently wa~hed with 2 ml of di~td. water and 3 ml of
Aquasol were added to runnings plus washing water and
c~unted in an LRB scintillation counter. IC50 value~ were
determined by intrapolation by plotting the percentage
inhibition against the concentration of the compound in
the test. In order to determine the relative inhibitory
potency, the IC~ value of the reference ~ubstance mevino-
lin was set at 1 and compared with the s~multaneouslydetermined IC50 value of the test compound.
The new active substances can be converted in a
known manner into the customary formulations, such as
tablets, coated tablets, pills, granules, aerosols,
syrups, emulsions, suspensions and solutions, using
inert, non-toxic, pharmaceutically suit~ble excipient~ or
solvents. In this connection, the therapeutically active
compound ~hould in each ca~e be present in a concen-
tration of about 0.5 to 98% by weight, preferably 1 to
90~ by weight, of the total mixture, i.e. in amounts
which are sufficient in order to achieve the dosage range
indicated.
The formulations are prepared, for example, by
extending the act$ve compounds with solvent~ and/or
excipients, if appropriate using emulsifiers and/or
dispersants, where, for example, in the case of the use
of water as a diluent, lf appropriate organic olvents
can be u~ed a- auxiliary olv nt-.
Example~ of auxillarie- which may be mentloned
aret
Le A 26 759 - 42 -
2~37~3
water, non-toxic organic solvents, such as paraffins (for
example mineral oil fractions), vegetable oils (for
example groundnut~sesame oil), alcohol~ (for example:
ethyl alcohol, glycerol), excipients, such as, for
example, ground natural minerals (for example kaolins,
clays, talc, chalk), ground synthetic minerals (for
example highly disperse silica, silicates), sugars (for
example sucrose, lacto~e and dextrose), emulsifier~ (for
example polyoxyethylene fatty acid esters, polyoxy-
ethylene fatty alcohol ethers, alkylsulphonate~ andarylsulphonates), dispersing agents (for example lignin-
sulphite waste liguors, methylcellulo~e, st~rch and poly-
vinylpyrrolidone) and lubricants (for example magnesium
stearate, talc, stearic acid and sodium lauryl sulphate).
lS Admini~trstion is carried out in a customary
manner, preferably ornlly, parenterally, perlLngually or
intravenously. In the case of oral administration,
t~blet~ may of course also contain additions, such as
sodium citrate, calcium carbonate and dicalcium phosphate
together with vsrious additives, such as starch, prefer-
ably potato starch, gelatin and the like in addition to
the excipients mentioned. Furthermore, lubricants, such
as magnesium stearate, ~odium lauryl sulphate and talc
can addition~lly be u~ed for t~bleting. In the case of
agueous ~uspensions, variou8 flavor enhancers or
colorants may be added to the active compounds in addi-
tlon to the abovementionod auxiliaries~
In tho ca~e of parenteral administration, olu-
tion~ of the active compounds u~lng ~ultable liquid
excipient~ may be employed.
Le A 26 75~ - 43 -
2~ 37~3
In general, it ha~ proved advantageou~ on in-
travenous administrat$on to admin$ster amounts of about
0 001 to 1 mg~kg, preferably about 0 01 to O S mg/kg of
body weight to attain effective results, and on oral
S administration the dosage is about 0 01 to 20 mg/kq,
preferably 0 1 to 10 mg/kg of body we~ght
In ~pfte of thi~ it may be nece~sary to deviate
from the amounts mentioned, depending on the body weight
or the type of adm$ni~tration route, on individual
behavior towards the medicament, the manner of its
formulation ~nd the point $n time or interval ~t which
administration takes place
Thus in some cases it may be ~ufficient to manage
with less than the minimum amount previously mentioned,
lS whereas in other cases the upper limit mentioned must be
exceeded In the case of the admini~tration of larger
amounts, it may be advisable to divide these $nto a
number of individual doses over the day
Preparation examples
Example 1
E,Z-2-Ethoxycarbonyl-1-(4-fluorophenyl)-4-methyl-pent-1-
en-3-one
F ~ ~COOCH2CH3
lCI~
O
A olution of 20 ml ~0 2 1) of piperidine and
12 ml (0 21 ~mol) of acetic ac~d ln 200 ~1 of i-opropanol
is addod to 554 g (3 5 mol) of thyl l-obutyryl aoet~te
and 434 g (3 5 mol) of 4-fluoroben~ldehyde ln 1 8 1 of
Le A 26 759 - ~4 -
2~L3~3
isopropanol. The mixture i8 ~tirred at room temperature
for 1 day and concentrated in vacuo, and the residue is
distilled in a high vacuum.
Yield: 796 g (86~ of theory) of yellowish oil
S b.p.: 135 - 140-C (0.2 mbar)
Example 2
6-Ethoxycarbonyl-5-(4-fluorophenyl)-1,2-dihydro-7-i~o-
propyl-2-oxo-1,8-naphthyridine
F
~COO~:H2CH~
~ N ~
66 g (0.6 mol) of 6-~mino-pyridin-2-one
tO.A. Seide, A.I. Titow, 8er. dtsch. Chem. Ges. 69, 1884
(1936)] and 159 g (0.6 mol) of the compound from Example
1 are stirred at lOO-C for 3 h in 50 ml of dimethyl-
formamide. All volatile constituents are then distilled
off up to a b~th temperature of 200-C and a pressure of
0.3 mbar. The dark re~idue is filtered through 1 kg of
silica gel u~ing chloroform and chloroform/methanol
(10:1). After stripping off the olvent, a brown foum
(60 g) rem~ins.
This crude product i- di-~olved in 0.7 1 of
dichloromethane, 39.7 g (0.175 mol) of d~chloro-dicyano-
p-benzoquinone ~r- ~dd d and th ml~ture 1- tlrred at
room temperatur- for 1 h. After fllt-rlng off the pre-
cipitate with uction from the reactlon mirture ~nd
.
.
Le A 26 759 _ 45 -
2 ~ 3
concentrating the filtrate, a residue r~main~ which i8
filtered through 1 kg of silica gel u~ing petroleum
ether/ethyl acetate (S 1) to (2 1) The residue obtained
from the filtrate is recrystallized from ethyl acetate/-
S ether
Yield: 21 7 g (10~ of theory) of colorless crystals
m p 182 C
Example 3
5-(4-Fluorophenyl)-1,2-dihydro-6-hydroxy-methyl-7-iso-
propyl-2-oxo-1,8-naphthyridine
F
2H
H ~
200 ml of a 1 5 molar ~olution of diisobutyl
alumlnum hydride in toluene are added dropwi~e at -78 C
during the course of 2 h to a ~olution of 21 7 g
(61 mmol) of the compound from ~xample 2 in 700 ml of
anhydrous toluene and the mixture 18 stirred for a
further hour at thi~ temperature It i8 then allowed to
warm slowly to room temperature, during which 200 ml of
water are cautiously added dropwise The mixture is
filtered off with ~uction through ~ieselguhr and wa~hed
with ethyl ac-tate, and the ~ie-elguhr 18 boilod ag~in
with ethyl acetate Tho organic pha-e 1~ ~a~hed with
~odium chloride olutlon, drled over odium ulph~te and
concentrated to dryne~ ~nd the re-idue i~ cry-tallized
Le A 26 759 - ~6 -
2~733
from ethyl acetate/ether.
Yield: 18.8 g (97~ of theory) of colorless crystals
m.p.: 230-C (from ethyl acetate)
Example 4
5-(4-Fluorophenyl)-6-formyl-1,2-dihydro-7-isopropyl-2-
oxo-1,8-naphthyridine
¢~ ' '
~
H
12.3 g of alumina and 25.9 g (120 mmol) of
pyridinium chlorochromate are added to a ~olution of
18.8 g ~60 mmol) of the compound from Example 3 in 300 ml
of tetrahydrofuran and the mixture iB ~tirred at room
temperature for 1 h. It is filtered through 500 g of
silica gel and washed with 1 1 each of dichloromethane,
petroleum ether~ethyl acetate (lsl) and ethyl acetate.
The filtrate is concentrated on a rotary evaporator and
the residue i~ cry~tallized from methanol.
Yields 13.3 g (71% of theory) of colorless cry~tals
m.p.s 223-C
~ A 26 759 - 47 -
20~37g3
Example S
(E)-3-[5-(4-Fluorophenyl)-1,2-dihydro-7-isopropyl-2-oxo-
1,8-naphthyridin-6-yl]-prop-2-enal
~q
~ ~CHO
A ~olution of 13.1 g (S0 mmol) of diethyl 2-
S (cyclohexylamino)-vinyl-phosphonate in 80 ml of tetra-
hydrofuran is added dropwise at O-C under argon to a
suspension of 3.0 g (100 mmol) of 80% strength ~odium
hydride in 80 ml of anhydrous tetrahydrofuran and the
mixture is stirred at this temperature for 30 min. A
solution of 13.0 g ~42 mmol) of the compound from Example
4 in 80 ml of tetrahydrofuran is then added dropwise at
O-C-5-C and the mixture is then heated under reflux for
1 h. 200 ml of water are added cautiously and the mixture
i8 extracted three times with ethyl acetate. After con-
centrating the organic phases, the residue i8 heated
under reflux for 2 h with a mixture of 500 ml of toluene,
500 ml of water and 27.5 g (218 mmol) of oxalic acid
dihydrate. The toluene phase is concentrated and the
residue i- treated with ethyl acetate. 11.35 g (80~ of
theory) of colorless ory-tal~ of m.p. 261-C are
obta~ned.
Le A 26 759 - 48 -
20~L3r~33
Example 6
Methyl erythro-(E)-7-~5-~4-fluorophenyl)-1,2-dihydro-7-
isopropyl-2-oxo-1,8-naphthyridin-6-yl]-3,s-dihydroxy-
hept-6-enoate F
~ ~ COOCH3
~'
5.47 g (50.7 mmol) of methyl acetoacetate in 5 ml
of tetrahydrofuran are added dropwi~e at -5-C to O-C
under argon to a ~uspension of 1.69 g (56.4 mmol) of 804
strength sodium hydride in SO ml of anhydrous tetrahydro-
furan. After 15 min, 41 ml (67.6 mmol) of 15% ~trength
butyl lithium in hexane are added dropwise at the same
temperature and, after a further 15 m$n, a ~olution of
5.68 g (16.9 mmol) of the compound from Example S in 150
ml of tetrahydrofuran. The mixture is stirred at room
temperature for one hour, then 11.2 g of acet$c acid in
120 ml of water are caut$ou~1y added dropwise and the
mixture i~ extracted three t~s with ethyl acetate. The
organic pha~e~ are wa~hed with ~aturated ~odium hydrogen
carbonate and sodium chlor$de solution, dr~ed over ~od~um
~ulphate and concentrated. 7.8 g of crude methyl-(~)-7-
t5-(4-fluorophenyl)1,2-dihydro-7-i-opropyl-2-oxo-1,8-
n~phthyridin-C-yll-5-hydroxy-3-oxo-h pt-6-enoate are
obtained a- orange oll.
This crude product i~ dl~olved under argon in
Le A 26 759 - 49 -
2~137~3
100 ml of anhydrous tetrahydrofuran, 20.3 ml of a 1 M
solution of triethylborane in tetrahydrofuran are added
and air iB passed through the solution for 5 min. The
mixture i8 cooled to -78-C, 767 mg (20.3 mmol) of sodium
borohydride are added, then 11 ml of meth~nol are 810wly
added dropwise and the mixture is stirred for a further
hour at -78-C to -75-C. The m~xture i~ then allowed to
warm to room temperature, 53 ml of 30~ ~trength hydrogen
peroxide and 50 ml of water being added dropwi~e from
about -30-C. The mixture i~ extracted three time~ with
ethyl acetate, and the organic phase~ are dried over
sodium sulphate and concentrated. The residue is chro-
matographed on 120 g of silica gel (230 - 400 mesh) using
ethyl acetate/petroleum ether (2:1) to ~4:1). A color-
less foam (4.09 g) i~ obtained, which is recrystallized
from methanol/water.
Yield: 2.32 g (30% of theory) of colourless crystals
m.p.: 148-C
Example 7
Sodium erythro-(E)-7-tS-(4-fluorophenyl)-1,2-dihydro-7-
isopropyl-2-oxo-1,8-naphthyridin-6-yl]-3,5-dihydroxy-
hept-6-ene-c~rboxylate
F
~00~
100 mg (0.22 mol) of the oompound from E~ample 6
L5LJL;~i~Za~ - 50
2~13~3
are fitirred at room temperature for 1 h in 2.2 ml of
tetrahydrofuran and 2.2 ml of 0.1 N sodium hydroxide
solution. The solvent is ~tripped off and the residue is
dried over phosphoru~ pentoxide in a high vacuum.
Yield: 85 mg of colorless crystals
m.p.: from 170-C (dec.)
Example 8
~ethyl erythro-(E)-7-15-(4-fluorophenyl)-1,2-dihydro-7-
isopropyl-l-methyl-2-oxo-1,8-naphthyridin-6-yl]3,5-
dihydroxy-hept-6-enoate F
~ ~ COOCH3
CH3
A solution of 275 mg (0.6 mol) of the compound
from Example 6 in 5 ml of d$methylformamide and 86 mg
(0.6 mol) of methyl iodide ~re added to ~ ~olution of
69 mg (0.6 mmol) of pot~ssium tert.-butoxide in 5 ml of
dimethylformamide ~nd the mixture iB stirred ~t room
temperature for 4 h. A further 138 mq (1.2 rmol) of
potas~ium tert.-butoxide ~nd 344 mg (2.4 mmol) of methyl
iodide ~re then ~dded ~nd the mixture is ~tirred over-
night. 2he mixture i~ poured into water ~nd extrscted
three times wlth ethyl ~cet~te, ~nd the organlc ph~e is
dried ~nd concentr~ted ~0.28 g of yellow oil). Column
chrom~togr~phy on 20 g of llic~ gel ~230-~00 ~e-h) u-ing
petroleum ether~ethyl ~cet~te (1~ nd ethyl ~cet~te
~e A 26 759 - Sl -
20137~3
gives 52 mg (18~ of theory) of a colorless oil.
H-NMR (CDCl3)s ~ - 1.25 - 1.45 (m, 8H, CH(Ç~b)2 + 4-H);
2.45 (m, 2H, 2-X); 3.07 (d, lH, OH);
3.45 (sept~ lH, ~(CH3)2); 3.53 (d,
S lH, OH); 3.72 (8, 3H; O-CH3); 3.9 (8,
3H, N-CH3); 4.1 (m, lH, C-H); 4.35
(m, lH, C-H); 5.3 (dd, lH, 6-H); 6.43
(d, lH, 7-H); 6.6 (d, lH, 3'-H); 7.13
(m, 4H, ~rom~tic-H); 7.28 (d, lH,
4'-H) ppm.
~xample 9
(E)-3-~1-Ethyl-5-(4-fluorophenyl)-1,2-dihydro-7-i~o-
propyl-2-oxo-1,8-n~phthyridin-6-yl~-prop-2-enal
¢~ HO
~.,
lH2
1.0 g (3 mmol) of the compound from Ex~mple 5 ~re
suspended in 20 ml of dimethylform~mide ~nd ~ ~olution of
366 mg (3.3 m~ol) of pot~ssium tert.-butoxide in 5 ml of
dimethylform~mide ~nd 510 mg (3.3 mmol) of ethyl iodide
in 1 ml of dimethylform~mide ~re ~uccessively ~dded
dropwi~e. ~he mixture i- ~tirrod ~t 60-C for 90 min,
poured $nto 150 ml of lc- w~t-r ~nd xtracted three tlmes
with ethyl ~cet~te. The org~nlc ph~-es ~re drlod ~nd
concentr~ted, ~nd the re~idue i- chrom~togr~phed on 30 g
Le A 26 759 - 52 -
2013 ~83
of ~ilica gel (230-400 mesh, column di~meter 2.5 cm)
using petroleum ether/ethyl acetste (5sl) ~nd (3:1).
Yield: 850 mg (78%), colorless cryst~ls of melting
point: 123-C.
S In analooy to example 9 are prepared starting from
the compound of example 5:
F
~ HO
No. R Rea~ent m.p. Yield
ExamDle
C~2~ Esr-CH2--~3 164 8 X
11 --~ `CH
Example 12
Methyl erythro-(~)-7-[1-ethyl-5-(4-fluorophenyl)-1,2-
dihydro-7-ieopropyl-2-oxo-1,8-n~phthyr~dln-6-yl]-3.5-
dihydroxy-hept-6-enoate . F
¢1 ~OOCH3
J~,
f~2
C}~3
Le A 26 759 - 53 -
2~37~33
830 mg (2.3 mmol) of the compound from E~ample 9
are reacted analogously to the procedure of E~ample 6,
103 mg (3.4 mmol) of sodium hydride, 0.27 ml (2.5 mmol)
of ethyl acetate ~nd 2.79 ml (4.6 mmol) of 15% butyl
lithium being used.
Yield: 155 mg (14%)
colorless crystal8, melting point llO-C
(crystallized from ether ~nd petroleum ether).
In analogy to example 12 are prepared:
F
OH OH
COOCH3
~N ~
No. R Starting m. p . lH-NMR
r~Example ¦ ¦ (Exarnple) l _I(COC13)
2,45 tm,2H)
1~ CH2 ~ 10 3 7 ~s 3H)
4,1 (m,lH)
4,35 (n,lH)
5,3 tdd,lH)
5,8 (5,2H)
6,4 (d,lH)
14 ~ 11 - 6,6 td,4H)
Le A 26 759 - 54 -
2~137~3
Example =I5
Sodium erythro-(E)-7-[l-ethyl-5-(4-fluorophenyl)-l~2-
dihydro-7-isopropyl-2-oxo-1,8-naphthyridin-6-yl]3,5-
dihydroxy-hept-6-ene-carboxylate
¢l ~oo N.''
CH2
100 mg (0.2 mmol) of the compound from Example 12
are reacted analogously to the procedure of Example 7.
Yield: 82 mg (81%), colorless foam
FAB-MA: 513 (100%, M + Na + H, 491/20~, M + H)
,Example 1~
trans-6-~2-[5-(4-(Fluorophenyl)-7-isopropyl-2-oxo-1,2-
dihydro-1,8-naphthyridin-6-yl]-ethenyl}-4-hydroxy-
3,4,5,6-tetrahydro-2H-pyran-2-one
F OH
92S mg ~2 mmol) of tho compound from ~xample 7
~re dissolved in 20 ml c THF and 20 81 of wat-r, the
solution is ot at pH S u-ing 1 N HCl, 384 mg ~2 ~mol) of
N-(3-dimethyl d nopropyl)-N'-ethyloarbodi~mide are added
,e,, A 26 759 - 55 -
2~137~3
and the mixture i~ stirred at room temperature for 1 day.
A further 192 g (1 mmol) of the carbodiimide are added
and the mixture is stirred for 2 more days. Extraction is
carried out with ethyl acetate; drying of the organic
phase and chromatography are carried out on 30 q of
silica gel (230 to 400 me6h) usinq chloroform and
chloroform/methanol ~Ssl), and crystallization from
chloroform/ether/petroleum ether gi~es 179 mg (2~%) of
colorless cry~tal~ of m.p.s 233-C.
Example 17
tran~-6-{2-~ enzyl-5-~4-fluorophenyl)-7-isopropyl-2-
oxo-1,2-dihydro-1,8-n~phthyridin-6-yl]-ethenyl}-4-
hydroxy-3,4,5,6-tetrahydro-2H-pyran-2-one
OH
f~2
A solution of 73 mg ~0.65 mmol) of potassium
- 15 tert.-butoxide in 1 ml of DMF and a ~olution of 110 mg
~0.65 mmol) of benzyl br~mide in 1 ml of DMF are added to
a su6pension of 250 mg ~0.59 mmol) of the compound from
~x~mple 16 in 5 ml of DMF and the mixture i- tirred at
60-C for 2.5 h. Working up a- ln Fxamplo 8 ~ive- llS mg
(38~) of colorless foam.
e A 26 759 - 56 -
20137.~3
H-NMR (CDC13)s 6 - 1.25 ~d, 6H); 1.3 - 1.7 (m, 2H); 2.6
(m~ 2H); 3.4 (sept~ lH); 4.2 (m, lH);
5.1 (m, lH); 5.3 (dd, 3H); 5.8 (5,
2H); 6.5 (d, lH); 6.6 (d, lH); 7.0 -
7.3 (m, 8H); 7.5 (m, 2H).
Example 18
trans-6-{2-tl-cy~nomethyl-5-(4-fluorophenyl)-7-isoprop~l-
2-oxo-1,2-d~hydro-1,8-nnphthyridln-6-yl]-ethenyl}-4-
hydroxy-3,4,5,6-tetrahydro-2H-pyr~n-2-one
OH
~,
l H2
CN
The title compound i8 prep~red analogously to the
procedure of Ex~mple 8 from 120 mg (0.28 mmol) of the
compound from Ex~mple 7, 57 mg (0.5 mmol) of potas~ium
tert.-butox~de and 38 mg (0.5 mmol) of chloroaceto-
nitrile.
Yield: 68 mg (53~) of beige oil.
H-NMR (CDC13) 6 - 1.37 (d, 6H); 1.4 - 1.8 ~m, 2H~; 2.25
~b, lH); 2.65 ~m, 2H); 3.45 ~sept,
lH)t 4.25 ~m, lH); S.lS ~m, lH)~ 5.38
(dd, lH)~ 5.47 (-, 2H)~ 6.03 (d, lH)~
6.12 (d, lH)~ 7.13 (m, 4H); 7~38 (d,
- 57 -
2~137~3
Example 19
(E)-3-~2-Chloro-5-(4-fluorophenyl)-7-i~opropyl-1,8-
naphthyridin-6-yl]-prop-2-enal
F
~3
Cl ~ CH0
7.8 g (23 mmol) of the compound from Example S
are heated to 75-C in 20 ml of phosphorus oxychloride.
After a clear solution has formed, the mixture i~ con-
centrated in vacuo, the residue iB di~solved in ethyl
acetate, and the solution i8 poured into ice water and
stirred vigorously. The ~queous phase i~ extracted twice
with ethyl acetate, the combined organic phase~ are dried
and the solvent i~ ~tripped off. 5.78 g (71S) of color-
less crystal~ of m.p.s 142-C cry~tallize from ether.
The compounds shown in ~able 1 are prepared by
the following procedure or by ~nalogous methodss
A solution of 167 mg (1.55 mmol) of benzyl
alcohol in 1 ml of IHF is added dropwi~e at O-C to a
suspen~ion of 46 mg (1.55 mmol) of 80S strength sodium
hydride in 5 ml of THF and the mixture i~ stirred for 15
min~
A olutlon of 0.5 g (1.4 mmol) of the compound
from ~xample 19 in 5 ml of THF 1- added dropwl-e at the
~me temperature ~nd the mixture 1- then tlrred at room
temperature for 2.5 h. 1.6 ml of acetic acld in 20 ml of
e A 26 759 - 58 -
2~ 1 37~3
water are added, the mixture is extracted twice with
20 ml of ethyl acetate, the combined organic pha~es are
dried and concentrated, and the residue is chromato-
graphed on 20 g of silica gel 230-400 mesh using
S petroleum ether/ethyl acetate 6~ 3:1.
286 mg (49%) of colorless crystals of m.p.:
182C crystallize from ether/petroleum ether.
Lç_~ 26 759 - 5S - .
20137~3
_ 65L9Z ~r
~_ O~
!~ _ ~ N N 'O ~ .
~ 0
N ~ 2 ~, à~ 2' ~ -
O ~ 8. 0
`C~
8 u ~ , U ~ , u x;
U ` ~ U ` ~
o ~ ~ ~ ~ ~ Z--U X
8 ~ z ~ Z N ~ ~ s _
u ~ u u
0 O _I N ~ ~t
E-1~; z ~ N (~
2~137~3
The compounds shown in Table 2 were prep~red from
the compounds of Examples 19, 20, 21, 22, 23 and 24 in
analogy to the procedure of Example 12.
Table 2: 1
OH OH
~ ~ COOCH3
Example R m.p. (-C) lH-NNR
No. solvent (CDCl3)
_ 25 -O-CHz~C0Hs 170 ether
26 -OCH3 155 ether/
petroleum ether
27 -O-CH2-C(CH3)3 amorphous 1.08 (~, 9H);
2.45 (m, 2H);
3.73 (8~ 3H);
4.1 (m, lH);
4.3 (8, 2H);
4.36 (m, lH);
6.57 (d, lH);
7.15 (m, 4H);
7.58 (d, lH)
28 -S-CH2-C~Hs 165
29 -N-CH2-C~H5 134 ether
CH~
Cl 207 ether
Le A 26 759
- 20137~3
The compounds shown in Table 3 were prepared in
analogy to the procedure of Example 7.
Table 3: F
~ ~ COO Na
~ . -
Example Y Starting Yield m.p. FAB-MS
No. Compound (%~ C
_ _ _ _ _ _ _ _ _
31 Cl-C=N- 30 92 decomposi-
tion from
150-
32 ~ CH2-O-C=N- 25 100 amorphous 575 ~M+Na`
553 (M+Na)
33 o l N~ 10 92
CH2
Tt.~ e a~preci~t.e~ that the instant. snecifica~ion
: anc claims are set forth by way of illustration and not li-
mitation, and that various modifications and changes may be
made without departing from the spirit and scope of the pre-
sent invention.
Ie A 26 759 - 62 -