Note: Descriptions are shown in the official language in which they were submitted.
1 1622 CIP 1
DEVICE AND METHOD OF
IONTOPHORETIC DRUG DELIVERY
TECHNICAL FIELD
This invention relates to a device and method for delivering an
agent transdermally or transmucosally by iontophoresis. More
particularly, this invention relates to an electrically powered
iontophoretic delivery device having a selectively permeable
separator membrane positioned between an agent reservoir and an
electrode in the device.
BACKGROUND ART
Iontophoresis, according to Dorland°s Illustrated Medical
Dictionary, is defined to be "the introduction, by means of
electric current, of ions of soluble salts into the tissues of the
body for therapeutic purposes." Iontophoretic devices have been
known since the early 1900's. British patent specification No.
410,009 (1934) describes an iontophoretic device which overcame one
of the disadvantages of such early devices known to the art at that
time, namely the requirement of a special low tension (low voltage)
source of current which meant that the patient needed to be
immobilized near such source. The device of that British
specification was made by forming a galvanic cell from the electrodes
and the material containing the medicament or drug to be delivered
transdermally. The galvanic cell produced the current necessary for
iontophoretically delivering 'the medicament. This ambulatory device
thus permitted iontophoretic drug delivery with substantially less
interference with the patient's daily activities.
More recently, a number of United States patents have issued in
the iontophoresis field, indicating a renewed interest in this mode
of drug delivery. For example, U.S. Patent No. 3,991,755 issued to
Vernon et al; U.S. Patent No. 4,141,359 issued to Jacobsen et al;
U.S. Patent No. 4,398,545 issued to Wilson; and U.S. Patent No.
r;;r. w .r ~
2 1622 CIP 1
4,250,878 issued to Jacobsen disclose examples of iontophoretic
devices and some applications thereof. The iontophoresis process has
been found to be useful in the transdermal administration of
medicaments or drugs including lidocaine hydrochloride,
hydrocortisone, fluoride, penicillin, dexamethasone sodium phosphate,
insulin and many other drugs. Perhaps the most common use of
iontophoresis is in diagnosing cystic fibrosis by delivering
pilocarpine salts iontophoretically. The pilocarpine stimulates
sweat production; the sweat is collected and analyzed for its
chloride content to detect the presence of the disease.
In presently known iontophoretic devices, at least two
electrodes are used. Both of these electrodes are disposed so as to
be in intimate electrical contact with some portion of the body. One
electrode, called the active or donor electrode, is the electrode
from which the ionic substance, medicament, drug precursor or drug is
delivered into the body by electrodiffusion. The other electrode,
called the counter or return electrode, serves to close the
electrical circuit through the body. In conjunction with the
patient's skin contacted by the electrodes, the circuit is completed
by connection of the electrodes to a source of electrical energy,
e.g., a battery. For example, if the ionic substance to be delivered
into the body is positively charged (i.e., a cation), then the anode
will be the active electrode and the cathode will serve to complete
the circuit. If the ionic substance to be delivered is negatively
charged (i.e., an anion), then the cathode will be the active
electrode and the anode will be the counter electrode.
Alternatively, both the anode and cathode may be used to
deliver drugs of opposite charge into the body. In such a case, both
electrodes are considered to be active or donor electrodes. For
example, the anode can deliver a positively charged ionic substance
into the body while the cathode can deliver a negatively charged
ionic substance into the body.
rt
3 1622 CIP 1
It is also known that iontophoretic delivery devices can be
used to deliver an uncharged drug or agent into the body. This is
accomplished by a process called electroosmosis. Electroosmosis is
the transdermal flux of a liquid solvent (e. g., the liquid solvent
containing the drug or agent) which is induced by the presence of the
electric field imposed across the skin by the donor electrode. In
theory, all iontophoretic delivery devices exhib it an electroosmotic
flux component. However, when delivering a charged drug ion from a
donor electrode having the opposite charge (i.e., drug delivery by
electrodiffusion), the electroosmotic flux component is quite small
in relation to the electrodiffusion flux component. On the other
hand, when delivering uncharged drug from an iontophoretic delivery
device, the electroosmotic transdermal flux component becomes the
dominant flux component in the transdermal flux of the uncharged
drug.
Furthermore, existing iontophoresis devices generally require a
reservoir or source of the beneficial agent (which is preferably an
ionized or ionizable agent or a precursor of such agent) to be
iontophoretically delivered or introduced into the body. Examples of
such reservoirs or sources of ionized or ionizable agents include a
pouch as described in the previously mentioned Jacobsen U.S. Patent
No. 4,250,878, or a pre-formed gel body as described in ~Jebster U.S.
Patent No. 4,382,529. Such drug reservoirs are electrically
connected to the anode or the cathode of an iontophoresis device to
provide a fixed or renewable source of one or more desired agents.
Typical electrotransport systems combine the agent or drug to
be delivered with other electrolyte components such as buffers, salts
and electrochemical reactants. These electrolyte components can in
some cases react directly with the drug or change the composition of
the drug reservoir such that the performance of the delivery system
is adversely affected. For example, a reaction product which
precipitates the drug and subsequently blocks and insulates the
electrode surface would adversely affect the operation of the device.
~~3 ~~y
4 1622 CIP 1
Damage to the skin can also occur due to transport of metal ions
produced during discharge of the electrodes.
There may also be a problem controlling pH in an iontophoretic
drug delivery device. Protons may be produced at the anode and
hydroxide ions may be produced at the cathode by water electrolysis
under conditions that may exist during iontophoretic drug delivery.
If the ions produced have the same charge as the drug ions, they will
compete with the drug for transport into the body tissue. In the
case of a positively charged drug ion which is delivered from the
anode electrode assembly, protons tend to be produced at the anode by
the electrolysis of water: H20 --> 2H+ + 1/2 02 + 2e-. The protons
are more mobile than the positively charged drug ions, and therefore
are delivered into the skin more easily than the drug ions. The
increase in proton concentration in the subcutaneous tissue is due to
increasing proton transport from the donor electrode assembly caused
by the continuous production of protons at the anodic donor
electrode. The delivery of protons into the skin can cause severe
irritation (e.g., acid burns). The pH of the drug reservoir is
likewise affected by the increasing proton concentration. In many
cases, a pH change can adversely affect the stability of the drug.
Changes in the pH of the drug reservoir can yield drastic changes in
drug transport characteristics as well as cause irritation and damage
to the skin. Similar problems can occur with the production of
hydroxyl ions at a cathodic donor electrode when iontophoretically
delivering a negatively charged drug ion.
Selectively permeable membranes have been employed in both the
donor and counter electrode assemblies of iontophoretic delivery
devices. For example, Sibalis U.S. Patent 4,640,689 discloses an
iontophoretic delivery device having a donor electrode assembly with
a two-compartment drug reservoir. The cower compartment contains a
low concentration of drug while the upper compartment contains a high
concentration of drug. The two compartments are separated by a
"semipermeable" membrane which is permeable to the passage of drug
ions. Parsi U.S. Patent 4,731,049 discloses an iontophoretic
:~. ~..
1622 CIP 1
delivery device wherein the ionized drug is bound within the drug
reservoir using an ion-exchange resin or a ligand affinity medium as
the drug reservoir matrix. Parsi also positions a selectively
permeable membrane (e.g. either an ion exchange membrane or a
5 conventional semipermeable ultrafiltration-type membrane) between the
drug reservoir and the electrolyte reservoir in the donor electrode
assembly of the device. Unfortunately, many conventional
semipermeable ultrafiltration-type membranes of the type disclosed by
Sibalis and Parsi have a high electrical resistivity (i.e., a high
resistance to ionic transport) making them unsuitable for use with
small portable iontophoretic delivery devices which are powered by
low voltage batteries (e. g., batteries having a voltage of less than
about 20 volts). Therefore, there is a need for an improved means
for separating the agent reservoir and the electrode, and optionally
for separating the agent and electrolyte reservoirs, of a donor
electrode assembly in an electrically-powered iontophoretic agent
delivery device.
The transdermal delivery of peptides and proteins, including
genetically engineered proteins, by iontophoresis has received
increasing attention. Generally speaking, peptides and proteins
being considered for transdermal or transmucosal delivery have a
molecular weight ranging between about 500 to 40,000 daltons. These
high molecular weight substances are too large to passively diffuse
through skin at therapeutically effective levels. Since many
peptides and proteins carry either a net positive or net negative
charge and because of their inability to passively diffuse through
skin, they are considered likely candidates for iontophoretic
delivery. Unfortunately, peptides and proteins may react at the
donor electrode surface and undergo inactivation and/or metal
catalyzed degradation. In addition, peptides and proteins may adsorb
on the electrode surface and thereby increase the resistivity of the
delivery system. This is a particular problem in conventional
iontophoresis devices which do not provide any means for separating
the drug reservoir from the electrode.
~.' .~,~'Q Y~. y
~c~...~t.a_i5 s..ei o.~.sA
s 1x22 cIP 1
Another problem with conventional iontophoretic delivery
devices is the tendency for charged materials in the patient's skin
or bloodstream to be driven into the donor and counter electrode
assemblies of the delivery device. Certain materials, such as fats
and lipids, may foul the electrodes and lower the transdermal flux of
the agent being delivered. Other materials, such as the drug counter
ion or other components) 'in the drug reservoir, may also undesirably
interact with, or corrode, the electrode material itself, thereby
compromising the performance of the device.
DISCLOSURE OF THE INVENTION
Therefore, it is an object of this invention to provide a
method of improving the delivery efficiency of an electrically
powered iontophoretic agent delivery device.
It is another object of this invention to provide such a method
which minimizes the electrical power requirements of the
iontophoretic delivery device.
It is a further object of this invention to provide an
iontophoretic agent delivery device, which device has a donor
electrode assembly including a donor electrode, an agent (e. g., drug)
reservoir, and optionally an electrolyte reservoir, which device
inhibits agent ions from interacting with the donor electrode, and
optionally inhibits passage of agent ions from the agent reservoir
into the electrolyte reservoir and inhibits the passage of
electrolyte ions of similar charge to the agent ions from the
electrolyte reservoir into the agent reservoir.
These and other objects are met by a device and method for
increasing the agent delivery efficiency of an electrically powered
iontophoretic agent delivery donor electrode assembly. The donor
electrode assembly is adapted to be placed on a body surface, such as
intact skin or a mucosal membrane, for iontophoretic delivery of a
beneficial agent therethrough. The donor electrode assembly includes
p~,°~°.
a
,~~t.,..~;.
7 1622 CIP 1
an agent reservoir adapted to be placed in agent transmitting
relation with the body surface, a donor electrode adapted to be
electrically connected to a source of electrical power and a
selectively permeable membrane intermediate the agent reservoir and
the electrode. The membrane is more permeable to the passage of
species of less than a predetermined molecular weight than to the
passage of species of greater than the predetermined molecular
weight. In other words, the membrane is selectively permeable based
upon the size or molecular weight of the diffusing species. An agent
is selected for delivery from the agent reservoir. The agent is
capable of dissociating into agent ions and counter ions of opposite
charge.
In a first embodiment, the selectively permeable membrane is
positioned between the agent reservoir and the donor electrode and is
in direct contact with the donor electrode, preferably forming a
laminate therewith. The agent and the membrane are selected so that
upon dissociation of the agent into agent ions and counter ions of
opposite charge, the agent ions have a molecular weight greater than
the predetermined molecular weight, and therefore are substantially
prevented from passing through the membrane. Preferably, the counter
ions have a molecular weight less than the predetermined molecular
weight, and therefore can easily pass through the membrane.
In a second embodiment, the selectively permeable membrane is
likewise positioned between the agent reservoir and the donor
electrode and is in direct contact with the donor electrode,
preferably forming a laminate therewith. The agent ions which are to
be delivered from the device have a low molecular weight and
accordingly are difficult to contain using known size exclusion type
selectively permeable membranes. In such a case, the agent and the
membrane are selected so that upon dissociation of the agent into
agent ions and counter ions of opposite charge, the counter ions have
a molecular weight greater than the predetermined molecular weight,
and therefore are substantially prevented from passing through the
membrane. In this embodiment, at least the membrane and preferably
~'"~P3,:~ t".~t." ~~,~
r~ :~.~.;~.a.
8 1622 CIP 1
both the membrane and 'the agent reservoir are maintained in a
substantially dry condition before operation of the device. By
maintaining the membrane in a dry condition, the low molecular weight
agent ions are unable to permeate from the agent reservoir into and
through the selectively permeable membrane, where they might
undesirably interact with 'the electrode. In operation, the agent
reservoir and the membrane are hydrated and placed in agent
transmitting relation with a body surface and thereafter the device
begins to pass current. As current flows through the device, agent
ions are displaced from the agent reservoir into the body. The
counter ions are substantially unable to pass through the selectively
permeable membrane because of their size.
In a third embodiment, an electrolyte reservoir is positioned
intermediate the electrode and the selectively permeable membrane.
An electrolyte is selected for the electrolyte reservoir which is
capable of dissociating into positively charged and negatively
charged electrolyte ions. In this embodiment, the agent, the
electrolyte and the membrane are selected so that upon dissociation
of the agent into agent ions and counter ions of opposite charge, the
agent ions have a molecular weight greater than the predetermined
molecular weight, and therefore are substantially prevented from
passing through the membrane. The counter ions have a molecular
weight less than the predetermined molecular weight, and therefore
can easily pass through the membrane. The electrolyte is selected so
that upon dissociation of the electrolyte into positively and
negatively charged ions, the electrolyte ions of similar charge to
the agent ions have a molecular weight greater than the predetermined
molecular weight, and therefore are substantially prevented from
passing through the membrane.
In a fourth embodiment, an electrolyte reservoir is likewise
positioned intermediate the electrode and the selectively permeable
membrane. The agent ions which are to be delivered from the device
have a low molecular weight and accordingly are difficult to contain
using known size exclusion type selectively permeable membranes. The
~yrF~ f"C~',v..,,,~,'~'
'~48jt. ~ ie7 ai' 2
9 1622 CIP 1
agent, the electrolyte and the membrane are selected so that upon
dissociating, the agent counter ions have a molecular weight greater .
than the predetermined molecular weight and therefore are
substantially prevented from passing through the membrane. The
electrolyte is selected so that upon dissociating, the electrolyte
ions of similar charge to the agent ions have a molecular weight less
than the predetermined molecular weight, and therefore can easily
pass through the membrane. The electrolyte ions of similar charge to
the counter ions have a molecular weight greater than the
predetermined molecular weight and therefore are substantially
prevented from passing through the membrane. In this embodiment, at .
least the membrane and preferably all of the membrane, the agent
reservoir and the electrolyte reservoir are maintained in a
substantially dry condition before operation of the device. By
maintaining the membrane in a dry condition, the low molecular weight
agent ions are unable to diffuse from the agent reservoir through the
membrane and into the electrolyte reservoir where they might
undesirably interact with the electrode. The device is placed into
operation by hydrating the membrane, the agent reservoir and
electrolyte reservoir and placing the agent reservoir in agent
transmitting relation with a body surface. Thereafter, the device
begins passing current. As current flows, electrolyte ions having a
similar charge as the agent ions are transported from the electrolyte
reservoir through the selectively permeable membrane and into the
agent reservoir. Simultaneously, agent ions are discharged from the
agent reservoir into the body. Neither the counterions or the
electrolyte ions having a similar charge as the counterions are able
to pass through the semipermeable membrane due to their high
molecular weight.
Preferably, in all four embodiments the membrane is also
substantially impermeable to a material in the body which has a
tendency to be driven into the electrode assembly by reverse
iontophoresis during operation of the device. Since the membrane is
positioned between the electrode and the body, the membrane inhibits
T
~y f
Rs6.IZ: s.~7~ %.C
1622 CIP 1
passage of the body material which might otherwise cause fouling of
the electrode.
In a fifth embodiment, the electrically powered iontophoretic
5 agent delivery device includes a donor electrode assembly, a counter
electrode assembly and a source of electrical power adapted to be
electrically connected to the donor electrode assembly and the
counter electrode assembly. The donor electrode assembly comprises
an agent reservoir for containing an agent and which is adapted to be
10 placed in agent transmitting relation with a body surface such as
intact skin or a mucosal membrane. The agent is capable of
dissociating into agent ions and counter ions of opposite charge.
The donor electrode assembly also includes a donor electrode adapted
to be electrically connected to the source of electrical power. A
selectively permeable membrane containing a chelating agent is
positioned intermediate the electrode and the agent reservoir. The
chelating agent in the membrane is capable of trapping ions having a
charge similar to the charge of the agent ions, but does not trap or
impede the permeation of ions having the opposite charge. This
causes the membrane to be permeable to species having a charge
similar to the charge of the counter ions and substantially less
permeable to species having a charge similar to the charge of the
agent ions.
BRIEF DESCRIPTION OF THE DRAWINGS
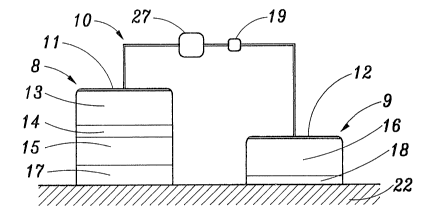
Figure 1 is a schematic view of one embodiment of a device for
iontophoretic delivery of a beneficial agent according to the present
invention;
Figure 2 is a schematic view of another embodiment of an
iontophoretic delivery device according to the present invention;
Figure 3 is a schematic view of another embodiment of an
iontophoretic delivery device according to the present invention; and
~id~, ~ ~.,~'. !~'1fn
~u~,fd.~raa:~
11 1622 CIP 1
Figure 4 is a schematic of another embodiment of an
iontophoretic delivery device according to the present invention.
MODES FOR CARRYING OUT THE INVENTION
Figure 1 is a schematic view of an iontophoretic delivery
device 10 for delivering a beneficial agent through a body surface
22. Body surface 22 is typically intact skin or a mucosal membrane.
Iontophoretic delivery device 10 includes a donor electrode assembly
8 and a counter electrode assembly 9. Electrode assemblies 8 and 9
are connected in series with an electrical power source 27, which is
typically one or more low voltage batteries, and an optional control
circuit 19 which is described in more detail hereinafter. When the
device 10 is in storage, no current flows because the device does not
form a closed circuit.
The donor and counter electrode assemblies 8 and 9 normally
inc'tude a strippable release liner, not shown, which is removed prior
to application of electrode assemblies 8 and 9 to body surface 22.
The combined skin-contacting areas of electrode assemblies 8 and 9
can vary from less than 1 cm2 to greater than 200 cm2. The average
device 10 however, will have electrode assemblies with a combined
skin-contacting area within the range of about 5-50 cm2.
The donor electrode assembly 8 includes a donor electrode 11,
an electrolyte reservoir 13, a selectively permeable separator
membrane 14 and an agent reservoir 15. The agent reservoir 15
contains a beneficial agent which is capable of dissociating into
agent ions (which ions are to be iontophoretically delivered by
device 10) and counter ions of opposite charge. The donor electrode
assembly 8 is adhered to the body surface 22 by means of an ion-
conducting adhesive layer 17.
Device 10 includes a counter electrode assembly 9 which is
placed on the body surface 22 at a location spaced apart from donor
electrode assembly 8. Counter electrode assembly 9 includes a
CA 02015597 2000-09-OS
67696-157
12
counter electrode 12 and an electrolyte reservoir 16. Counter
electrode assembly 9 is adhered to the body surface ZZ by means of an
ion-conducting adhesive layer 18.
When the device 10 is placed on the skin of a patient the
circuit between the electrodes is closed and the power source begins °
to deliver current through the device and through the body of the
patient. At least a portion of the current, and preferably a high
proportion of the current, is carried by agent ions delivered from
the donor electrode assembly 8 into the skin 22. The fraction of
current carried by a particular ionic species j is called the
transference number of species j and may be expressed mathematically
as:
t~ = i j/i
where:
i = the total current density; and
i~ - the current density carried by ionic species j.
The devices of the present invention increase the transference number
of the ionized agent, thereby increasing the agent delivery
efficiency of the device.
Electrodes 11 and 12 can be metal foils, e.g., silver, aluminum
or zinc foils or a polymer matrix loaded with metal powder, powdered
graphite, carbon fibers or other suitable electrically conductive
material. Numerous other electrode materials are well known in the
art and disclosed, for example, in U.S. Patent Nos. 4,474,570 and
4,557,723.
As an alternative to a battery as the power source Z7, device
10 can be powered by a galvanic couple formed by the donor electrode
11 and counter electrode 12 being composed of dissimilar
electrochemical couples and being placed in electrical contact with
r °f~~s t'~t".n~
=Cb ~:'i-A'lAsj
13 1622 CIP 1
one other. Typical materials include a zinc donor electrode 11 and a
silver/silver chloride counter electrode 12. A Zn-Ag/AgCI galvanic
couple provides an electrical potential of about 1.0 volt.
The electrolyte reservoir 16 contains a suitable
pharmacologically acceptable electrolyte species which is capable of
dissociating, once reservoir 16 becomes hydrated, into cations and
anions of appropriate molecular weight. In the device illustrated in
Figure 1, the molecular weight of the electrolyte species in
reservoir 16 is not critical. Accordingly, the electrolyte species
may be selected from any pharmacologically acceptable salt. Suitable
salts include sodium chloride, alkali metal salts and alkaline earth
metal salts such as chlorides, sulfates, nitrates, carbonates,
phosphates, and organic salts such as ascorbates, citrates, acetates
and mixtures thereof. Reservoir 16 may also contain a buffering
agent. Sodium chloride is a suitable electrolyte when the counter
electrode 12 is the cathode and is composed of silver/silver
chloride, optionally with a sodium phosphate buffer.
Selectively permeable membrane 14 is more permeable to ions of
less than a predetermined molecular weight than to ions of greater
than the predetermined molecular weight under the conditions of
operation of the iontophoretic delivery device. Thus, membrane 14 is
permeable to ions having a molecular weight less than the
predetermined molecular weight and allows such ions to freely pass
through the membrane, but is substantially less permeable to ions
having a molecular weight greater than the predetermined molecular
weight and substantially prevents the passage of such high molecular
weight ions. It should be kept in mind that no membrane has perfect
selectivity and the passage of a small amount of ions having a
molecular weight greater than the predetermined molecular weight is
unavoidable. The size exclusion characteristics of membrane 14 may
be expressed in terms of the mass ratio, Rmass, which is defined as
follows:
:.~'~
14 1622 CIP 1
p Mperm
'mass =
Mres
where:
Mperm is the mass of agent species j which permeates through the
membrane during the operational life of the device; and
to
Mres is the total mass of agent species j initially contained in
the agent reservoir.
In general, the selectively permeable membrane should exhibit a mass
ratio, "ass, of less than about 0.3 and preferably less than about
0.1.
In addition to inhibiting the passage of selected drug ions,
counter ions and/or electrolyte ions, the separator membrane 14
2O should have a sufficiently low voltage drop across the membrane to
enable a portable low voltage power source, such as one or more low
voltage batteries, to deliver therapeutically effective amounts of
beneficial agent through the skin or mucosa of a patient. Membrane
14 should exhibit an area resistance of less than 50 kohm~cm2,
preferably less than about 5 kohm~cm2 and most preferably less than
about 1 kohm~cm2. The area resistance of a membrane is determined by
measuring the voltage drop across the membrane while 100 ~A/cm2 of
direct current density is flowing. The resistance is then calculated
using Ohm's Law, i.e., R = V/i. For example, if a current density of
100 ~CA/cm2 produces a potential drop of 100 mV, the membrane area
resistance is 1 kohm~cm2.
Any known selectively permeable membrane which is selectively
permeable based upon the size or molecular weight of the permeating
species can potentially be used as the membrane 14 or 14a. The
membrane may be either a homogeneous material or non-homogeneous,
incorporating for example water soluble pore forming agents such as
polyethylene glycol. Suitable materials for making membranes 14 and
CA 02015597 2000-09-OS
67696-157
14a include, without limitation, polycarbonates, i.e., linear
polyesters of carbonic acids in which carbonate groups recur in the
polymer chain by phosgenation of a dihydroxy aromatic such as
bisphenol A, polyvinylchlorides, polyamides such as polyhexamethylene
5 adipamide and other such polyamides commonly known as "nylon",
modacrylic copolymers such as those formed of polyvinylchloride and °
acrylonitrile, and styrene-acrylic acid copolymers, polysulfones such
as those characterized by diphenylene sulfone groups in the linear
chain thereof, halogenated polymers such as polyvinylidene fluoride
10 and polyvinylfluoride, polychloroethers and thermoplastic polyethers,
acetal polymers such as polyformaldehyde, acrylic resins such as
polyacrylonitrile, polymethyl methacrylate and poly n-butyl
methacrylate, polyurethanes, polyimides, polybenzimidazoles,
polyvinyl acetate, aromatic and aliphatic polyethers, cellulose
15 esters such as cellulose triacetate, cellulose, collodion, epoxy
resins, polyolefins such as polyethylene and polypropylene, porous
rubber, cross-linked polyethylene oxide), cross-linked
polyvinylpyrrolidone, cross-linked polyvinyl alcohol); derivatives
of polystyrene such as poly (sodium styrenesulfonate) and
polyvinylbenzyltrimethyl-ananonium chloride, poly(hydroxyethyl
methacrylate), poly(isobutyl vinyl ether), poiyisoprenes,
polyalkenes, ethylene vinyl acetate copolymers such as those
described in U.S. Patent No. 4,144,317,
polyethylene oxides such as Polyox~ manufactured by Union
Carbide of New York, NY or Polyox~ blended with polyacrylic acid or
Carbopol~, cellulose derivatives such as hydroxypropyl methyl
cellulose, hydroxyethyl cellulose, hydroxypropyl cellulose, pectin,
starch, guar gum, locust bean gum, and the like, along with blends
thereof. This list is merely exemplary of the materials suited for
use in this invention. A more extensive list can be found in J. R.
Scott & W. J. Roff, Handbook of Common Polymers (CRC Press, 1971) and
in patents disclosing suitable materials for use in manufacturing
microporous membranes such as U.S. Patent No. 3,797,494.
Preferably, the separator membranes 14 and 14a
have an equilibrium water content of about 0.1 to 30 wty., preferably
about 1 to 20 wt~.. A preferred membrane material is cellulose
~~w''v~.''. F.., ;,.,
x-outJ ,~
16 1622 CIP 1
acetate loaded with up to 20 wt% of a pore forming agent such as
polyethylene glycol having a molecular weight in the range of 400 to
8000. Specific examples of preferred membranes are listed below.
TABLE I
Molecular ThicknessResistance
Weight
Membrane/CompositionCutoff (daltons)mils (kohmcm )
1
O
CA 398-10* with < 300 2.8 8.4
5% PEG 400+
CA 398-10 with < 300 3.0 2.3
10% PEG 400
1 CA 398-10 with < 300 3.9 1.2
5 15% PEG 400
CA 398-10 with < 300 5.9 1.8
10% PEG 3350
CA 398-10 with < 300 5.9 3.9
15% PEG 335D
20
CA 398-10 with < 300 3.8 3.6
12.5% PEG 8000
CA 398-10 with < 300 4.0 2.4
15% PEG 8000
2 CA 398-10 wish < 300 4.3 4.5
5 17.5% PEG 8000
Spectrapor~** natural s 1 --
cellulose 500
to 106
* cellulose acetate sold by FMC Corp.
resin having triacetate of Philadelphia,
substitution of
39.8% and
30 PA.
** sold by MedicalInc. of Los
Industries, Angeles,
CA.
+ polyethylene Union Carbide,
glycol sold by of Long Beach,
CA.
35
Figure 2 illustrates another iontophoretic delivery device
designated by the numeral 20. Like device 10, device 20 also
contains an electrical power source 27 (e.g., a battery) and an
40 optional control circuit 19. However, in device 20 the donor
electrode assembly 8 and the counter electrode assembly 29 are
attached to an insulator 26 and form a single self-contained unit.
Insulator 26 performs the function of preventing shorting of the
device by preventing direct ion transport from the donor electrode
45 assembly 8 to the counter electrode assembly 29 without ion transport
through body surface 22. Insulator 26 is preferably formed of a
hydrophobic non-conducting polymeric material which is impermeable to
both the passage of ions and water. Preferred insulating materials
are nonporous ethylene vinyl acetate and closed cell foamed plastics.
50 The donor electrode assembly 8 has the same structure as assembly 8
in device 10 (Figure 1). The counter electrode assembly 29 (Figure
2) includes a counter electrode 12, an electrolyte reservoir 16, an
L"' >-~,f"i~y
~~_,.rt.si. '.6'~~
17 1622 CIP 1
agent reservoir 25 and a selectively permeable separator membrane ,
14a. In this embodiment therefore, both the donor electrode assembly
8 and the counter electrode assembly 29 may be used to
iontophoretically deliver different beneficial agents through body
surface 22. For example, positive agent ions can be delivered
through body surface 22 from 'the anode electrode assembly, while
negative agent ions can be delivered from the cathode electrode
assembly. Alternatively, neutral drugs can be introduced from either
electrode assembly by electroosmosis.
As an alternative to the side-by-side alignment of the donor
electrode assembly 8, the insulator 26 and the counter electrode
assembly 29 shown in Figure 2, the electrode assemblies can be
concentrically aligned with the counter electrode assembly positioned
centrally and surrounded by the insulator 26 and the donor electrode
assembly. The electrode assemblies can, if desired, be reversed with
the counter electrode assembly surrounding the centrally positioned
donor electrode assembly. The concentric alignment of the electrode
assemblies can be circular, elliptical, rectangular or any of a
variety of geometric configurations.
Figure 3 illustrates another iontophoretic delivery device
designated by the numeral 30. Device 30 is substantially the same as
device 10 illustrated in Figure 1 with the following exception. In
device 30, the donor electrode assembly 8 contains no electrolyte
reservoir 13. Thus, selectively permeable membrane 14 is in direct
contact with the donor electrode 11.
Figure 4 illustrates yet another iontophoretic delivery device
designated by the numeral 40. Device 40 is substantially the same as
device 20 illustrated in Figure 2 with the following exceptions. The
donor electrode 8 of device 40 contains no electrolyte reservoir 13
and the counter electrode assembly 29 contains no electrolyte
reservoir 16. Thus, in device 40 the selectively permeable separator
membrane 14 is in direct contact with the electrode 11 vahile the
r:d. ~,:a '
18 1622 CIp 1
selectively permeable separator membrane 14a is in direct contact
with electrode 12.
A control circuit 19 is optionally provided. Control circuit
19 may take the form of an on-off switch for "on-demand" drug
delivery (e. g., patient-controlled of an analgesic for pain control),
a timer, a fixed or variable electrical resistor, a controller which
automatically 'turns the device on and off at some desired periodicity
to match the natural or circadian patterns of the body, or other more
ld sophisticated electronic control devices known in the art. For
example, it may be desirable to deliver a predetermined constant
level of current from device 10 since a constant current level
ensures that the drug or agent is delivered through the skin at a
constant rate. The current level can be controlled by a variety of
known means, for example, a resistor or a simple circuit that employs
a resistor and a field effect transistor. Control circuit 19 may
also include an integrated circuit which could be designed to control
the dosage of beneficial agent, or even to respond to sensor signals
in order to regulate the dosage to maintain a predetermined dosage
regimen. A relatively simple circuit can control the current as a
function of time, and if desired, generate complex current waveforms
such as pulses or sinusoidal waves. In addition, the control circuit
19 may employ a bio-feedback system which monitors a biosignal,
provides an assessment of the therapy, and adjusts the drug delivery
accordingly. A typical example is the monitoring of the blood sugar
level for controlled administration of insulin to a diabetic patient.
According to the first embodiment of the present invention, the
donor electrode assembly 8 includes a donor electrode 11 and an agent
reservoir 15, the electrode 11 and reservoir 15 being separated by a
selectively permeable 14 (i.e., the donor electrode assembly 8 has
the configuration shown in Figures 3 and 4). The agent and the
membrane 14 are selected so that the agent ions have greater than the
predetermined molecular weight and the counter ions have less than
the predetermined molecular weight. Thus, membrane 14 inhibits agent
ions from permeating from agent reservoir 15 into and through
r°, :~a'.~
ryy, r.r~ ,.s..~x-~'~-~
19 1622 CIP 1
membrane 14, where they might otherwise adversely react with
electrode 11 and/or other ionic species present in the electrolyte
reservoir 13.
According to the second embodiment of the present invention,
the donor electrode assembly 8 includes a donor electrode 11 and an
agent reservoir 15, the electrode 11 and the reservoir 15 being
separated by a selectively permeable membrane (i.e., the donor
electrode assembly 8 has the configuration shown in Figures 3 and 4).
The agent and the membrane 14 are selected so that the agent counter
ions have greater than the predetermined molecular weight while the
agent ions have less than the predetermined molecular weight. This
embodiment is used to deliver agent ions having a molecular weight
which is too low to be contained using known size exclusion type
selectively permeable membranes. Size exclusion type membranes
generally are unable to inhibit the passage of ionic species having a
molecular weight of less than about 100 daltons without increasing
the area resistance of the membrane to an unacceptable 7eve1 (i.e.,
to a level significantly above 50 kohm~cm2). Thus, when delivering
small mobile agent ions (i.e., agent ions having a molecular weight
of less than about 100 daltons) according to the present invention,
the agent is selected so that the counter ions have a molecular
weight greater than the predetermined molecular weight. In the
second embodiment, the selectively permeable membrane, and preferably
both the agent reservoir and the selectively permeable membrane, are
maintained in a substantially non-hydrated condition until use. 8y
maintaining the membrane in a non-hydrated condition, ionic species
are unable to permeate through the membrane prior to placement on the
body. Once the agent reservoir is placed in agent transmitting
relation with the body surface, and the agent reservoir and the
membrane become sufficiently hydrated, the device begins 'to pass
current. The non-hydrated initial condition of the membrane, in
combination with the electrical field imposed across the membrane
after hydration, inhibits the passage of the low molecular weight
agent ions from the agent reservoir into and through the membrane
where they might undesirably interact with the electrode 11.
~:~.~~'~'~
20 1622 CIP 1
According to the third embodiment of the present invention, the donor
electrode assembly 8 includes a donor electrode 11, an agent
reservoir 15 and an electrolyte reservoir 13, the reservoirs 13 and
15 being separated by a selectively permeable membrane 14 (i.e., the
donor electrode assembly 8 has the configuration shown in Figures 1
and 2). The agent and the membrane 14 are selected so that the agent
ions have greater than the predetermined molecular weight and the
counter ions have less than the predetermined molecular weight. The
electrolyte is selected so that the electrolyte ions having a similar
charge to the agent ions have greater than the predetermined
molecular weight. In this manner, both the agent ions and the
electrolyte ions having the same charge as the agent ions are
inhibited from permeating through membrane 14. Thus, membrane 14
inhibits agent ions from permeating from agent reservoir 15 into
electrolyte reservoir 13, where they might otherwise adversely react
with electrode 11 and/or other ionic species present in the
electrolyte reservoir 13. Similarly, electrolyte ions in electrolyte
reservoir 13 having a similar charge to the agent ions are inhibited
from permeating through membrane 14 into agent reservoir 15, where
they would undesirably compete with the agent ions for delivery
through body surface 22 and lower the transference number of the
agent ions, thereby lowering the agent delivery efficiency of the
device.
According to the fourth embodiment of the present invention,
the donor electrode assembly 8 includes a donor electrode 11, an
agent reservoir 15 and an electrolyte reservoir 13, the reservoirs 13
and 15 being separated by a selectively permeable membrane 14 (i.e.,
the donor electrode assembly 8 has the configuration shown in Figures
1 and 2). The agent and the membrane 14 are selected so that the
agent ions have less than the predetermined molecular weight and the
counter ions have greater than the predetermined molecular weight.
This fourth embodiment of the invention is useful for delivering
agent ions having a molecular weight which is too low to be contained
using known size exclusion type selectively permeable membranes.
Size exclusion type membranes generally are unable to inhibit the
t' i!/'A .-'.,'~ fi~,' 1""~ i
~Li.~2:ltrid tl ~~
21 1622 CIP 1
passage of ionic species having a molecular weight of less than about
100 daltons without increasing the area resistance of the membrane to
an unacceptable level (i.e., to a level significantly above 50
kohm~cm2). When delivering small mobile agent ions (i.e., agent ions
having a molecular weight of less than about 100 daltons) according
to the present invention, the agent is selected so that the counter
ions have a molecular weight greater than the predetermined molecular
weight. In addition, the electrolyte is selected so that the
electrolyte ions of similar charge to the agent ions have a molecular
weight less than the predetermined molecular weight, while the
electrolyte ions of similar charge to the counter ions have greater
than the predetermined molecular weight. In the fourth embodiment of
the invention, at least the membrane, and preferably both the
membrane and the agent reservoir, are maintained in a substantially
non-hydrated condition until placement on the body surface. The non-
hydrated initial condition of the membrane, in combination with the
electrical field imposed across the membrane after hydration,
inhibits the passage of the low molecular weight agent ions from the
agent reservoir into and through the membrane where they might
undesirably interact with the electrode 11.
In those embodiments of the present invention wherein the
selectively permeable membrane 14 must be maintained in a
substantially non-hydrated condition until placement on the body, the
term "non-hydrated" means that there is insufficient solvent
contained in the membrane to allow ionic species to become dissolved
in the solvent and transported across the membrane 14. In most
cases, the solvent will be water. However, the terms "hydrated" and
"non-hydrated" are broad enough to encompass the use of solvents
other than water (i.e., non-aqueous solvents).
Suitable high molecular weight ions for use with the present
invention should have a molecular weight of at least about 100
daltons, preferably greater than about 300 daltons, and good
solubility in the solvent (2.g., water) used in the device. Specific
examples of high molecular weight ions include the following:
r ~ ~'r_,-~r s~
~.3Lz,.at~0,,~ .r
22 1622 CIP 1
TABLE II
High Molecular Molecular Weight
Weiqh_t Ions (daltons) Tyoe
tetraethylammonium 13U cation
tetrabutylammonium 242 cation
cholestyramine >100,000 cation
dextran carbonates 5,000 to 500,000cation
aminated styrenes 500 to 100,000 cation
polyvinylimine 500 to 100,000 cation
polyethyleneimine 500 to 100,000 cation
polyvinyl 4-alkylpyri-
dinium) 500 to 100,000 cation
poly(methylene-N,N-
dimethyl-piperidinium)500 to 100,000 cation
poly(vinylbenzyltri-
methyl ammonium) 500 to 100,000 cation
polyacrylates 500 to 100,000 anion
polymethacrylates 500 to 100,000 anion
polystyrene sulfonates500 to 100,000 anion
gluconate 195 anion
hyaluronate >100,000 anion
alginate 240,000 anion
lauryl sulfate 265 anion
tartrate 171 anion
tetradecyi sulfate 293 anion
dextran sulfates 5,000 to 500,000anion
Suitable low molecular weight ions for use with the present
invention include alkali metal ions such as sodium, potassium and
lithium ions; alkaline earth metal ions such as magnesium, calcium
and barium ions; halogen ions such as fluoride, chloride, bromide and
iodide ions; as well as ammonium, phosphate, sulphate, perchlorate,
carbonate, citrate, acetate, benzoate, oxalate, and borate ions. Of
these, sodium, potassium and chloride ions are preferred.
According to the fifth embodiment of the present invention, the
electrically powered iontophoretic agent delivery device may have a
~~.~.~ a~%'~
23 1622 CIP 1
structure as shown in either of Figures 1, 2, 3 or 4. In the fifth
embodiment, the selectively permeable membrane 14 intermediate the
electrode 11 and the agent reservoir 15 (or the selectively permeable
membrane 14a intermediate the electrode 12 and the reservoir 25) is
selectively permeable based upon the charge of the permeating
species. In this embodiment, the membrane 14 contains a chelating
agent which is capable of trapping ions having a charge similar to
the charge of the agent ions but does not trap or impede the
permeation of ions having the opposite charge. 'Thus, the membrane is
permeable to species having a charge similar to the counter ions but
is substantially less permeable to species having a charge similar to
the charge of the agent ions. This embodiment is particularly useful
when the donor electrode is at least in part composed of an
oxidizable metal which is oxidized during operation of the device to
form metal ions. Thus, the chelating agent acts to trap the metal
ions produced during the discharge of the electrode. This is
particularly desirable when the metal ions may damage the skin or
body surface. The chelating agent containing membrane is preferably
comprised of a hydrogel. The hydrogel can be any state of the art
material including, without limitation, polyvinylalcohol,
polyacryiamide, hydroxypropylmethylcellulose, hydroxyethylcellulose,
hydroxymethylcellulose, polyacrylic acid, polyvinylpyrrolidone,
hydroxyethylmethacrylate, albumin, gelatin and ceilulosic polymers.
Suitable cheiating agents include, without limitation,
ethyienediamine-tetraacetic acid (EDTA) and chelating resins such as
Chelex 100, sold by Bio-Rad Laboratories. Other suitable chelating
agents are discussed at length in Martin, Swarbrick and Cammarata,
Physical Pharmacy, 3rd edition (1983).
This invention has utility in connection with the delivery of
drugs within the broad class normally delivered through body
surfaces, including intact skin, mucosal membranes and nails. As
used herein, the expressions "agent" and "drug" are used
interchangeably and are intended to have their broadest
interpretation as any therapeutically active substance which is
delivered to a living organism to produce a desired, usually
24 1622 CIP 1
beneficial, effect. In general, this includes therapeutic agents in
all of the major therapeutic areas including, but not limited to,
anti-infectives such as antibiotics and antiviral agents, analgesics
and analgesic combinations, anesthetics, anorexics, antiarthritics,
antiasthmatic agents, anticonvulsants, anti depressants, antidiabetic
agents, antidiarrheals, antihistamines, anti-inflammatory agents,
antimigraine preparations, antimotion sickness preparations, anti-
emetics such as metoclopramide, antinauseants, antineoplastics,
antiparkinsonism drugs, antipruritics, antipsychotics, antipyretics,
antispasmodics, including gastrointestinal and urinary,
anticholinergics, sympathomimetrics, xanthine derivatives,
cardiovascular preparations including calcium channel blockers,
beta-blockers, antiarrythmics, antihypertensives, diuretics,
vasodilators, including general, coronary, peripheral and cerebral,
central nervous system stimulants, cough and cold preparations,
decongestants, diagnostics, hormones, hypnotics, immunosuppressives,
muscle relaxants; parasympatholytics, parasympathomimetrics,
proteins, peptides, polypeptides and other macromolecules including
genetically engineered peptides and proteins, psychostimulants,
sedatives and tranquilizers.
It is most preferable to use a water soluble salt of the drug
or agent to be delivered.
The embodiments of the present invention which utilize agent
ions having a high molecular weight (and which agent ions are
therefore inhibited from passing through the selectively permeable
membranes 14 and 14a) are particularly useful in the controlled
delivery of peptides, polypeptides, proteins and other macromolecules
which typically have a molecular weight of at least about 300
daltons, and most typically a molecular weight in the range of about
300 to 40,000 daltons. Specific examples of peptides and proteins in
this size range include, without limitation, LHRH, LHRH analogs such
as buserelin, gonadorelin, naferelin and leuprolide, insulin,
heparin, calcitonin, endorphin, TRH, NT-36 (chemical name: N-[[(s)-
4-oxo-2-azetidinyl]carbonyl]-L-histidyl-L-prolinamide), liprecin,
CA 02015597 2000-09-OS
67696-157
pituitary hormones (e. g., HGH, HMG, HCG, desmopressin acetate, etc.),
follicle luteoids, aANF, growth factor releasing factor (GFRF), ~MSH,
somatostatin, bradykinin, somatotropin, platelet-derived growth
factor, asparaginase, bleomycin sulfate, chymopapain,
5 cholecystokinin, chorionic gonadotropin, corticotropin (ACTH)
erythropoietin, epoprostenol (platelet aggregation inhibitor),
glucagon, hyaluronidase, interferon, interleukin-2, menotropins
(e. g., urofollitropin (FSH) and LH), oxytocin, streptokinase, tissue
plasminogen activator, urokinase, vasopressin, ACTH analogs, ANP, ANP
10 clearance inhibitors, angiotensin II antagonists, antidiuretic
hormone agonists, antidiuretic hormone antagonists, bradykinin
antagonists, CD4, ceredase, CSF's, enkephalins, FAB fragments, GHRH,
IgE peptide suppressors, IGF-1, neurotrophic factors, parathyroid
hormone and agonists, parathyroid hormone antagonists, prostaglandin
15 antagonists, pentigetide, protein C, protein S, renin inhibitors,
thymosin alpha-1, thrombolytics, TNF, vaccines, vasopressin
antagonist analogs, alpha-1 anti-trypsin (recombinant) and
interferon.
ZO As an alternative to the ion-conducting adhesive layers 17 and
18 shown in Figures 1-4, the agent reservoirs 15 and 25 and the
electrolyte reservoir 16 may comprise self-adhering matrices.
Suitable self-adhering matrix materials include, without limitation,
polystyrene-butadiene) and polystyrene-isoprene-styrene) block
25 copolymers, and high and low molecular weight polyisobutylene
copolymers. Other suitable self-adhering matrix materials are
described in U.S. Patent Nos. 4,391,278, 4,474,570 , 4,593,053,
4,702,732 and 4,820,263.
When using a self-adhering agent or electrolyte reservoir, the
adhesive properties of the polymer matrix may be enhanced by adding a
resinous tackifier. Examples of suitable tackifiers include those
sold by Hercules, Inc. of Wilmington, DE under the tradenames
Staybelite Ester ~5 and X10, Regal-Rez and Piccotac. Additionally,
the matrix may contain a rheological agent, such as mineral oil or
silica.
v~.~-~~'
26 1622 CIP 1
As a further alternative to the ion-conducting adhesive layers
17 and 18 shown in Figures 1-4, the iontophoretic delivery devices
10, 20, 30 and 40 may be adhered to the skin using an adhesive
overlay. Any of the conventional adhesive overlays used to secure
passive transdermal delivery devices to the skin may be used.
The agent reservoirs 15 and 25 and the electrolyte reservoirs
13 and 16 can be a polymer matrix structure formed by blending the
desired agent, drug, electrolyte or other component(s), with the
polymer and forming the matrix (e. g., as a film) by melt pressing,
solvent casting or extrusion, for example. The drug and/or
electrolyte loading in the polymer matrix is generally about 20 to
95% by weight, preferably about 30 to 80% by weight and most
preferably about 30 to 60% by weight.
Suitable polymers for use as the matrix of reservoirs 13, 15,
16 and 25 include, without limitation, polyethylene, polypropylene,
polyisoprenes and polyalkenes, rubbers, copolymers such as Kraton~,
polyvinylacetate, ethylene vinyl acetate copolymers, polyamides,
polyurethanes, polyvinylchloride, cellulose acetate, cellulose
acetate butyrate, ethylcellulose, cellulose acetate, ethylene vinyl
acetate, polyurethane, nylons, and blends thereof. The matrix can be
crosslinked with the components in place such as a silastic matrix,
or the polymers can be prefabricated and sorbed with the components
from solutions as is the case with cellulose, woven fiber pads and
sponges.
The matrix of reservoirs 13, 15, 16 and 25 can alternately be
formed of a hydrophilic polymer which is swellable or soluble in
water, e.g., hydrogels. Examples of suitable hydrophilic polymers
include polyvinyl alcohols, polyacrylates, polyethylene oxides,
Polyox~, Polyox~ blended with polyacrylic acid or Carbopol~,
cellulose derivatives such as hydroxypropyl methyl cellulose,
hydroxyethyl cellulose, hydroxypropyl cellulose, pectin, starch, guar
gum, locust bean gum, and the like, along with blends thereof.
~~~ ~~"°T~i~
fvJ "'.~ ~ :'-4lAa d
27 1622 CIP 1
In addition to the agent, drug or electrolyte, the reservoirs
13, 15 and 16 may also contain other conventional materials such as
dyes, pigments, inert fillers, plasticizers and other excipients.
Having thus generally described our invention, the following
examples will illustrate certain preferred embodiments of the
iontophoretic delivery device of the present invention.
EXAMPLE 1
A device for iontophoretically delivering metoclopramide is
constructed as follows. The donor electrode assembly has a
multilaminate construction including a zinc foil donor electrode, a
polyethylene oxide based electrolyte reservoir, a cellulose acetate
selectively permeable membrane and a polyvinylpyrrolidone based drug
reservoir.
The drug reservoir is made by dry blending 65 parts by weight
of powdered polyvinyl pyrrolidone having a weight average molecular
weight of 360,000 (PUP-K90 manufactured by GAF Corporation) and 35
parts by weight of metoclopramide HCl at 65°C using a Brabender
mixer. The mixture is extruded into a sheet having a thickness of 6
mils and a square section having an area of 5 cm2 is cut.
An electrolyte reservoir is made by dry blending 70 parts by
weight of polyethylene oxide (Polyox~ manufactured by Union Carbide
of New York, NY) and 30 parts by weight of cholestyramine chloride
salt. The cholestyramine cation has a molecular weight of more than
100,000 daltons. The mixture is extruded into a sheet having a
thickness of 6 mils and a square section having an area of 5 cm2 is
cut.
The selectively permeable membrane is made by mixing 90 parts
by weight cellulose acetate (CA 398-10 manufactured by FMC Corp. of
Philadelphia, PA); and 10 parts by weight of polyethylene glycol (PEG
400 manufactured by Union Carbide of Long Beach, CA) in a Hobart
CA 02015597 2000-09-OS
67696-157
28
mixer with methylene chloride solvent. The mixture is solvent cast
into a sheet having a thickness of 3 mils. A 5 cm2 square section of
the cast sheet is cut. The area resistance of the membrane is about
2 kohm~cmz. The membrane is freely permeable to ionic species having
a molecular weight of less than about 100 daltons. For the high
molecular weight metoclopramide ions, the membrane exhibits an R,~ss
of about 0.02. The transference number, t, for cholestyramine
through the hydrated cellulose acetate membrane is less than about
0.01.
The drug reservoir and the electrolyte reservoir are laminated
onto opposite sides of the selectively permeable membrane using heat
and pressure. Thereafter, the zinc foil electrode is laminated onto
the free surface of the electrolyte reservoir using heat and
pressure.
The counter electrode assembly is made by dry blending 70 parts
by weight of sodium polyacrylate (Acoflock*A-130 manufactured by
Mitsui Cyanamide Co.) and 30 parts by weight of sodium chloride at
65'C using a Brabender mixer. The mixture is extruded as a film
having a thickness of 6 mils. A square section of the film having an
area of 5 cm2 is cut. A sintered Ag/AgCI disk having an area of 5
cm2 is laminated onto one side of the polyacryiate film.
The zinc foil donor electrode and the Ag/AgCI counter electrode
are electrically connected to an electrical power source which
supplies a constant level of direct current of 500 uA or 100 ~A/cm2.
The zinc electrode is connected to the positive terminal of the power
supply and the Ag/AgCI electrode is connected to the negative
terminal. The entire device is adhered to a body surface using a
conventional transdermal type adhesive overlay comprising a flexible
polyethylene sheet having a peripheral silicone based adhesive.
During operation of the device, both the drug cation
(metoclopramide, molecular weight - 300 daltons) and the electrolyte
cation (cholestyramine, molecular weight >100,000 daltons) are
*Trade-mark
..
%~~a.~_;,~~a~,y'~
29 1622 CIP 1
substantially unable to penetrate through the cellulose acetate
membrane. As the metoclopramide ions are driven into the body, the
drug counter ions (i.e., chloride ions) pass through the
semipermeable membrane and into the electrolyte reservoir. Because
the electrolyte cations (i.e., the cholestyramine ions) are unable to
penetrate the selectively permeable cellulose acetate membrane, they
do not pass into the drug reservoir where they would otherwise
undesirably compete with the metoclopramide ions for delivery into
the body. Therefore a higher percentage of the applied current is
carried by metoclopramide ions being transported into the body,
thereby increasing the transference number for metoclopramide ions
and the metoclopramide delivery efficiency of the device.
EXAMPLE 2
A device for iontophoretically delivering lithium has a similar
structure as the device of Example 1. The agent in the
polyvinylpyrroiidone agent reservoir is comprised of lithium
gluconate. The agent ions (i.e., lithium ions) have a molecular
weight of only 7 daltons which is too small to be effectively
contained using a size exclusion type membrane. The gluconate
counter ion has a molecular weight of 195 daltons and is therefore
substantially unable to pass through the membrane (once the membrane
is hydrated). The electrolyte in the polyethylene oxide electrolyte
reservoir is sodium alginate. The sodium ions have a molecular
weight of only 23 daltons and therefore can easily pass through the
membrane once hydrated. The alginate anion has a molecular weight of
about 240,000 daltons which is too large to pass through the
membrane. The donor electrode is a silver foil. Because the lithium
ions have a low molecular weight (and therefore can easily permeate
through the selectively permeable membrane) the membrane, and
preferably all three of the membrane, the drug reservoir and the
electrolyte reservoir, are maintained in a non-hydrated condition
prior to placement on the patient. By maintaining the membrane in a
non-hydrated condition, the lithium ions are unable to permeate from
... y~~~'(~.'J. i.
30 1622 CIP 1
the drug reservoir through the membrane prior to placement on the
body.
In use, the membrane, the drug reservoir the and electrolyte
reservoir are hydrated at the time the device is placed on the body.
Hydration may be accomplished by absorption of water from the body
(e. g., absorption of sweat, absorption of transepidermal water loss
or absorption of saliva in the case of a buccal mucosal membrane) or
by using water from an exterior source. Once the agent reservoir,
the electrolyte reservoir and the membrane are hydrated and the agent
reservoir is placed in agent transmitting relation with a body
surface, the device begins to pass current. As current flows through
the device, the lithium ions are displaced from the drug reservoir
into the body. The gluconate counter ions are substantially unable
to pass through the selectively permeable membrane because of their
size. For the low molecular weight lithium ions, the hydrated
membrane exhibits an f~,ass of less than 0.3. For the high molecular
weight alginate and gluconate ions, and the hydrated membrane
exhibits an f~,ass of less than about 0.1. The area resistance of the
membrane is about 2 kohm~cm2.
In this device, both the drug counter ion (gluconate ion,
molecular weight = 195 daltons) and the electrolyte anion (alginate
ion, molecular weight = 240,000 daltons) are substantially unable to
pass through the cellulose acetate membrane. Prior to operation, the
system is not hydrated, thus inhibiting diffusion of all ionic
species. In use, the system is hydrated and placed upon the body and
immediately thereafter begins to pass current. As current flows,
sodium ions are transported from the electrolyte reservoir through
the semipermeable membrane and into the agent reservoir.
Simultaneously, lithium ions are discharged from the agent reservoir
into the skin. PJeither the alginate or the gluconate ions are able
to cross the semipermeable membrane. The non-hydrated initial
condition of the membrane, the drug reservoir and the electrolyte
reservoir, in combination with the electrical field imposed across
31 1622 CIP 1
the semipermeable membrane after hydration, inhibits the passage of
lithium ions from the agent reservoir into the electrolyte reservoir.
EXAMPLE 3
A device for iontophoretically delivering insulin (as the
chloride salt) has a similar structure and composition as the device
described in Example 1 with the following exceptions. First, no
electrolyte reservoir is utilized. Secondly, the electrode is a
silver foil. 'thus, the cellulose acetate membrane is laminated
directly onto the silver foil using heat and pressure. The ,ass for
insulin through the hydrated is less than 0.05. The area resistance
of the hydrated membrane is about 10 kohm~cm2.
Because the insulin ions (molecular weight of about 6000
daltons) are unable to penetrate the selectively permeable cellulose
acetate membrane, no undesirable reactions between the insulin and
the metal electrode can take place. On the other hand, the drug
counter ion (chloride ions) are able to penetrate the semipermeable
membrane and react with silver ions produced at the electrode to form
an insoluble AgCI precipitate within the membrane itself.
EXAMPLE 4
A device for iontophoretically delivering lithium has a similar
structure and composition as the device of Example 3 except the drug
reservoir contains lithium gluconate instead of insulin and the donor
electrode is comprised of zinc foil rather than silver foil. Thus,
the cellulose acetate membrane is laminated directly onto the zinc
foil using heat and pressure. The gluconate counter ion (molecular
weight of 195 daltons) is substantially unable to permeate through
the cellulose acetate membrane because of its size.
Prior to operation, the drug reservoir and the membrane are
maintained in a non-hydrated condition. Accordingly, the ionic
species within the drug reservoir are unable to permeate through the
~~ t-_~ r_i~ fi~
~.».~ma~ ,e
32 1622 CIP 1
non-hydrated membrane. Accordingly, the low molecular weight lithium
ions are retained in the drug reservoir.
In use, the drug reservoir and the membrane are hydrated after
(or immediately before) placement upon the body, and the device
begins to pass current. As current flows, lithium ions are
discharged from the drug reservoir into the skin. The gluconate ions
are substantially unable to cross the semipermeable membrane. The
Pn,ass for lithium through the hydrated membrane is less than 0.3. The
lb area resistance of the hydrated membrane is about 30 kohm~cm2. The
transference number, t, for the gluconate ions through the hydrated
membrane is less than 0.1. The non-hydrated initial condition of the
drug reservoir and the membrane, in combination with the electrical
field imposed across the membrane after hydration, inhibits the
passage of lithium ions from the drug reservoir into and through the
membrane where they might undesirably interact with the zinc
electrode.
Having thus generally described our invention and described in
detail certain preferred embodiments thereof, it will be readily
apparent that various mod ifications to the invention may be made by
workers skilled in the art without departing from the scope of the
invention as defined by the following claims.