Note: Descriptions are shown in the official language in which they were submitted.
2015671
BACKGROUND OF THE INVENTION
Field of the Invention
The present invention concerns nucleotide analogs and
their compositions and use. In particular, the disclosure
concerns antiviral (including antiretroviral) and antitumor
phosphonomethoxymethyloxymethyl purine/pyrimidine
derivatives and the invention concerns 4'-phosphonomethoxy-
tetrahydrofuranyl-1'- purine/pyrimidine derivatives.
Backqround Information
Infectious viral diseases are recognized as an
important medical problem. Progress against infectious
viral disease requires the development of drugs with
selective antiviral activity while remaining benign to
normal cell lines. A number of antiviral agents currently
under study which seem to possess some selectivity are
nucleoside analogs. In general, these compounds are
structural analogs of the naturally occurring nucleosides.
Structural modification in either the purine or pyrimidine
base nucleus and/or the saccharide component results in a
synthetically modified nucleoside derivative which, when
incorporated into a viral nucleic acid forming process, acts
to disrupt further synthesis of viral nucleic acid.
-2-
c
2~1567i
Effectiveness of these antiviral agents depends on selective
conversion by viral erizymes, but not by host enzymes, to the
corresponding nucleotide analog which is then converted to
the triphosphate and incorporation into viral nucleic acid
occurs. A problem with this antiviral strategy has been the
emergence of certain viral strains whose enzymes poorly
promote phosphorylation of the nucleoside analogs. To
circumvent this problem, intact nucleotide analogs appear to
be potentially quite useful as antivirals for incorporation
into viral nucleic acid.
Reist and Sturm in WO 84/04748, published December
6, 1984, disclosed new phosphonic acid analogs of nucleoside
phosphates which are useful as antivirals for incorporation
into viral DNA. The structural formula for these compounds
is shown below as 1'
R3
Z20 0 X
jP-C- < CH2 ) n-CH 0 B
Z10 Y \
CH \CH
R2 R1
1'
-3-
~~~ 5b71
In the Reist and Sturm compounds, B is a purine or
pyrimidine base: R1 and R2 together complete a
beta-pentofuranose sugar or R1 is H and R2 is H or
hydroxmethyl; R3 is H or OH: X is H, OH or together with Y
is carbonyl oxygen and Y can also be H; Z1 and Z2 are H or
alkyl.
Similarly, synthesis and anti-Herpes-Virus activity of
phosphate and phosphonate derivatives of
9-[(1,3-dihydroxy-2-propoxy)methyl]guanine (Formula 2' was
disclosed by Prisbe, et al., in J. Med. Chem., 1986, 29,
671.
OH
N-
H H
z
0
(HO)Z~P-X
OH (X=0, CH2>
2'
Other phosphonate nucleotide analogs of the Formula 2'
type wherein X=CH2 have been described by R. M. Riggs et
al., Nucleosides and Nucleotides, 8(5&6, 1119-1120 (1989);
D.H.R. Bouton, et al., Tetrahedron Letters, Vol. 30, No. 37,
pp 4969-4972 (1972); and H. Tanaka, et al., Tetrahedron
Letters, 30, 2567-2570 (1989).
-4-
C
2015671
Adenine phosphonic analogs (Formula 3') and their
syntheses are disclosed in the UK Patent Application of
Holy, et al., GB 2,134,907A published 8/22/84.
NH2
N
~~N~~
CH2
R? ~OR~
R3 ~HOR~
3'
In formula 3', R2 and R3 are H or together complete a
ribonucleoside ring; and both R4 are alternately a hydrogen
and -CH2P(O) (OH)2 group.
A preferred example of one of these compounds, known as
(S)-HPMPA (Formula 4') was disclosed by DeClercq, et al., in
Nature, 1986, 323, pp. 464-467 and earlier by Holy, et al.,
Nucleic Acids Research, Symposium Series No. 14, 1984, pp.
277-278. Phosphonate compounds which are HPMPA analogs are
described in South African Patent 1987/8607. In applicant's
hands, (S)-HPMPA is only slightly active in mice inoculated
with Herpes simplex virus - 2. In a 21 day protocol 30% of
a group of animals treated i.p. with 50 mg/kg/day of
(S)-HPMPA survived.
-5-
2015671
NHz
N
O~P<OH>2
OH
4'
There is no teaching contained in these references, or
a suggested combination thereof, which would make obvious
the compounds, compositions, and uses involved in the
present invention.
SUMMARY OF THE INVENTION
Phosphonomethoxymethoxymethyl purine and pyrimidine
derivatives, 4'-phosphonomethoxytetrahydro-2-fur-yl-9-purine
and 1-pyrimidine derivatives, phosphonomethoxymethoxymethyl
9-purine and 1-pyrimidine derivatives having a cyclic
phosphonate group and 4'-phosphonomethoxytetrahydro-2-fur-
yl-9-purine and 1-pyrimidine derivatives having a cyclic
phosphonate group have been synthesized and found to possess
useful antiviral and antitumor activity.
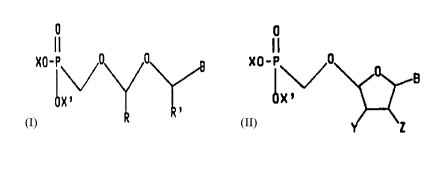
The present disclosure describes a phosphonomethoxy-
methoxymethyl purine/pyrimidine derivative of the formula
-6-
2015671
0
ii
XO-P ~ 0 0 g
<I)
X'
wherein X and X' independently are hydrogen or alkyl
having 1 to 6 carbon atoms, or the cation of a salt-forming
base,
R and R' independently are hydrogen,
alkyl having 1 to 6 carbon atoms, hydroxyalkyl with 1 to 6
carbon atoms, alkanoyl having 2 to 7 carbon atoms, and B is
a (9-substituted) purine or (1-substituted) pyrimidine base
selected from the group consisting of xanthine, substituted
xanthine, for example, hypoxanthine, guanine, substituted
guanine, for example, 8-bromoguanine, 8-chloroguanine,
8-aminoguanine, 8-hydrazinoguanine, 8-hydroxyguanine,
8-methylguanine, 8-thioguanine and 3-deazaguanine, purine,
substituted purine, for example, 2-aminopurine,
2,6-diaminopurine, cytosine, substituted cytosine, for
example, 5-ethylcytosine and 5-methylcytosine, thymine,
uracil, 5-substituted uracil, for example, 5-chlorouracil,
5-bromouracil, 5-ethyluracil, 5-iodouracil, 5-propyluracil
and 5-vinyluracil, adenine and substituted adenine, for
example, 3-deazaadenine, and pharmaceutically acceptable
salts thereof.
2015671
The present invention concerns a
4'-phosphonomethoxytetrahydro(or dihydro)fur-2-yl-purine or
pyrimidine derivative of the formula
0
I I
xo-P~~ o
OX' CII)
Y
wherein X and X' independently are hydrogen or alkyl
having 1 to 6 carbon atoms, or the cation of a salt-forming
base the broken line represents an optional bond,
Y and Z are the same or different and are unsubstituted
or substituted alkyl with 1 to 6 carbon atoms or together
they constitute an oxygen atom or methylene group in which
event the broken line is absent, and
B is a 9-substituted purine or a 1-substituted
pyrimidine base selected from the group consisting of
xanthine, substituted xanthine, for example, hypoxanthanine,
guanine, substituted guanine, for example, 8-bromoguanine,
8-chloroguanine, 8-methylguanine and 8-thioguanine and
3-deazaguanine, purine, substituted purine, for example,
2-aminopurine, 2,6-diaminopurine, cytosine, substituted
cytosine, for example, 5-ethylcytosine and 5-methylcytosine,
thymine, uracil, 5-substituted uracil, for example,
-8-
x
C
2015671
5-chlorouracil, 5-bromouracil, 5-ethyluracil, 5-iodouracil,
5-propyluracil and 5-vinyluracil, adenine and substituted
adenine, for example, 3-deazaadenine, and pharmaceutically
acceptable salts thereof.
The invention also concerns oligonucleotides derived
from a 4'-phosphonomethoxytetrahydrofuranyl-1'-
purine/pyrimidine derivative according to formula II.
The invention also concerns to a 4'-phosphonomethoxy-
tetrahydrofur-2-yl-purine or pyrimidine derivative having a
cyclic phosphonate group of the formula
0
X 0 - PI~O
CIII>
b.
wherein X is hydrogen or alkyl with 1 to 5 carbon
atoms,
Rb is H or OH
and B is a (9-substituted) purine or a (1-substituted)
pyrimidine base selected from the group consisting of
xanthine, substituted xanthine, for example, hypoxanthine,
guanine, substituted guanine, for example, 8-bromoguanine,
8-chloroguanine, 8-aminoguanine, 8-hydrazinoguanine,
8-hydroxyguanine, 8-methylguanine, 8-thioguanine and
3-deazaguanine, purine, substituted purine, for example,
2-aminopurine, 2,6-diaminopurine, cytosine, substituted
cytosine, for example, 5-ethylcytosine and 5-methylcytosine,
thymine, uracil, 5-substituted uracil, for example,
5-chlorouracil, 5-bromouracil, 5-ethyluracil, 5-iodouracil,
_g_
y
__
2015671
5-propyluracil and 5-vinyluracil, adenine and substituted
adenine, for example,~3-deazaadenine,
and pharmaceutically acceptable salts thereof.
The disclosure further discusses a
phosphonomethoxymethoxymethyl-9-substituted purine or
1-substituted pyrimidine derivatives having a cyclic
phosphonate group of the formula
0
xo- P~ o ~ a
CIV)
c
wherein X is hydrogen, alkyl with 1 to 6 carbon atoms,
Rcis hydrogen, alkyl with 1 to 6 carbon atoms or
alkanoyl having 2 to 7 carbon atoms, and B is a purine or
pyrimidine base selected from the group consisting of
xanthine, substituted xanthine, for example, hypoxanthine,
guanine, substituted guanine, for example, 8-bromoguanine,
8-chlorogranine, 8-aminoguanine, 8-hydrazinoguanine,
8-hydroxyguanine, 8-methylguanine, 8-thioguanine and
3-deazaguanine, purine, substituted purine, for example,
2-aminopurine, 2,6-diaminopurine, cytosine, substituted
cytosine, for example, 5-ethylcytosine and 5-methylcytosine,
thymine, uracil, 5-substituted uracil, for example,
5-chlorouracil, 5-bromouracil, 5-ethyluracil, 5-iodouracil,
-10-
C
2015671
5-propyluracil and 5-vinyluracil, adenine and substituted
adenine, for example,'3-deazaadenine, and pharmaceutically
acceptable salts thereof.
The disclosure also concerns the following intermediates:
(V)
wherein B is a 9-substituted purine or a 1-substituted
pyrimidine base selected from the group consisting of
xanthine, substituted xanthine, for example, hypoxanthine,
guanine, substituted guanine, for example, 8-bromoguanine,
8-chloroguanine, 8-aminoguanine, 8-hydrazinoguanine,
8-hydroxyguanine, 8-methylguanine, 8-thioguanine and
3-deazaguanine, purine, substituted purine, for example,
2-aminopurine, 2,6-diaminopurine, cytosine, substituted
cytosine, for example, 5-ethylcytosine and 5-methylcytosine,
thymine, uracil, 5-substituted uracil, for example,
5-chlorouracil, 5-bromouracil, 5-ethyluracil, 5-iodouracil,
5-propyluracil and 5-vinyluracil, adenine and substituted
adenine, for example, 3-deazaadenine.
The present invention also concerns the following
derivatives:
X g
(VI)
Y
-11-
C
2015671
wherein X is a halogen, for example, chlorine, bromine,
iodine or fluorine,
Y is phenylthio, phenylseleno or a halogen atom, for
example, chlorine, bromine, iodine or fluorine, and B is a
9-substituted purine or 1-substituted pyrimidine base
selected from the group consisting of xanthine, substituted
xanthine, for example, hypoxanthine, guanine, substituted
guanine, for example, 8-bromoguanine, 8-chloroguanine,
8-aminoguanine, 8-hydrazinoguanine, 8-hydroxyguanine,
8-methylguanine, 8-thioguanine and 3-deazaguanine, purine,
substituted purine, for example, 2-aminopurine,
2,6-diaminopurine, cytosine, substituted cytosine, for
example, 5-ethylcytosine and 5-methylcytosine, thymine,
uracil, 5-substituted uracil, for example, 5-chlorouracil,
5-bromouracil, 5-ethyluracil, 5-iodouracil, 5-propyluracil
and 5-vinyluracil, adenine and substituted adenine, for
example, 3-deazaadenine;
8
VII
. wherein B is guanine, substituted guanine, for example,
8-bromoguanine, 8-chloroguanine, 8-aminoguanine,
8-hydrazinoguanine, 8-hydroxyguanine, 8-methylguanine,
8-thioguanine and 3-deazaguanine, cystosine or substituted
-12-
2015671
cytosine, for example, 5-ethylcytosine and 5-methylcytosine;
5-substituted uracil,' for example, 5-chlorouracil, 5-
bromouracil, 5-ethyluracil, 5-iodouracil, 5-propyluracil
and 5-vinyluracil except 5-flourouracil and substituted
adenine, for examle, 3-deazaadenine,
and
(RO)2P~~ 2
(VIII)
wherein Y is a halogen, for example chlorine, fluorine,
bromine or iodine, phenylthio, or phenylselano, R is
hydrogen or alkyl with 1 to 6 carbon atoms and B is a purine
or pyrimidine base selected from the group consisting of
xanthine, substituted xanthine, for example, hypoxanthine,
guanine, substituted guanine, for example, 8-bromoguanine,
8-chloroguanine, 8-aminoguanine, 8-hydrazinoguanine,
8-hydroxyguanine, 8-methylguanine, 8-thioguanine and
3-deazaguanine, purine, substituted purine, for example,
2-aminopurine, 2,6-diaminopurine, cytosine, substituted
cytosine, for example, 5-ethylcytosine and 5-methylcytosine,
thymine, uracil, 5-substituted uracil, for example,
5-chlorouracil, 5-bromouracil, 5-ethyluracil, 5-iodouracil,
5-propyluracil and 5-vinyluracil, adenine and substituted
adenine, for example, 3-deazaadenine.
-13-
C
2015671
The present disclosure further discusses the following
processes:
<ai) II
0 0 0 P(ox~~)= 0
HO J
CI V V OCR ~ R CI0~0~ ~~R~~p)=P V
Leis acid Leis acid
<IX) 0°C to 80°C
wherein R"is an alkyl having 1 to 6 carbon atoms or an
unsubstituted aryl or an aryl substituted by a substituent
such as a halogen, e.g., bromine or chlorine, vitro, alkyl
having 1 to 6 carbon atoms or an alkoxy having 1 to 6 carbon
atoms, B is a silylated purine or pyrimidine base, and R"' is
hydrogen or alkyl with 1 to 6 carbon atoms; preferably the
molar ratio of compound (IX) to B is 1:1;
(R2)
<XI>
C I p 0 C 1 (R°o) 2 PNa a P 0 0 C I
-20°C to 25°CR ~) 2 ~ ~ ~
B <XII>
<X> 0 B
II
(R~~) zP~~~~J
wherein R°is an alkyl having 1 to 6 carbon atoms and B is a
purine or pyrimidine base; preferably compound (X) is 5 to
-14-
2015671
times in excess of compounds (XI) and the preferred molar
ratio of compound (XII) to B is 1.1:
(R3)
8 4 <Xill) 0
II 'CV) HOVP(OR")z (R"'O)z ~O OJ (Ia) ~
Pera- cid
0°C l0 80°C H
wherein compound (XIII) is preferably in a S to 10 times
excess of compound (V).
The present invention further relates to the following
processes: (R4)
a
a x
X-Y
-70°C to 25°C (VI)
(VII)
wherein X-Y is a halogen,e.g., Br2, C12, phenyl-Se-Z,
phenyl-S-Z (wherein Z= halogen) and B is a purine or
pyrimidine base; preferably the molar ratio of compound
(VII) to X-Y is 1:1;
(R5)
8 0
/~ 0 8
/(VII) (R"O)zP OH (XIV) ~~ 0
''~~!! (R~'o)= P ~
Perac l d
-70°C to 25°C H
wherein R"'is hydrogen or alkyl with 1 to 6 carbon atoms;
preferably compound (XIV) is 5 to 10 times in excess of
compound (VII).
-15-
C
2015671
DETAILED DESCRIPTION OF THE INVENTION
The compounds of~the present invention can exist as
optical isomers and both racemic and diasteromeric mixtures
of these isomers which may exist for certain compounds, as
well as the individual optical isomers are all within the
scope of the present invention. While the racemic mixtures
can be separated into their individual isomers through
well-known techniques such as, for example, the separation
of diastereomeric salts formed with optically active
adjuncts, e.g., acids or bases followed by conversion back
to the optically active substrates; in most instances, for
the compounds of the present invention, the preferred
optical isomer can be synthesized by means of stereospecific
reactions, beginning with the appropriate stereoisomer of
the desired starting material.
As indicated above, the present invention also pertains
to pharmaceutically acceptable non-toxic salts of these
compounds, containing, for example, Na+, Li+, K+, Ca++ and
Mg++. Such salts may include those derived by combination
of appropriate cations such as alkali and alkaline earth
metal ions or ammonium and quaternary amino ions with the
acid anion moiety of the phosphonic acid group. Metal salts
can be prepared by reacting the metal hydroxide with a
compound of this invention. Examples of metal salts which
can be prepared in this way are salts containing Li+, Na+,
-16-
2015671
and K+. A less soluble metal salt can be precipatated from
the solution of a more soluble salt by addition of the
suitable metal compound. In addition, salts may be formed
from acid addition of certain organic and inorganic acids,
e.g., HC1, HBr, H2S04 or organic sulfonic acids, with basic
centers of the purine, specifically guanine, or pyrimidine
base. Finally, it is to be understood that compounds of the
present invention in their un-ionized, as well as
zwitterionic form, and/or in the form, of solvates are also
considered part of the present invention.
Compounds of the present invention also exist in
subclasses, with two broad subclasses being those wherein B
is either a purine or a pyrimidine base. Of these broad
subclasses there are preferred classes wherein the purine
base is a guanine or a substituted guanine moiety and where
the pyrimidine bases are either thymine or cytosine. The
most preferred class of compounds are those wherein B is
guanine or substituted guanine.
Compounds of the present invention may also be
subclassed according to the structure of the phosphonate
moiety. These classes are comprised of the diester, the
monoester, and the diacid. Preferred subclasses of the
phosphonate moiety are the monoester and the diacid.
The compounds of this invention, including the
physiologically acceptable salts thereof, have desirable
-17-
2015671
antiviral and antitumor activity. They exhibit activity
against viruses, for example, Herpes Simplex virus I, Herpes
Simplex virus II, cytomegalovirus, Varicella Zoster virus,
influenza virus, vaccinia, polio, rubella, small pox,
cowpox, Epstein-Barr virus, measles virus, human respiratory
virus, papillomavirus and sinbis virus, just to mention a
few and also against retroviruses, for example, human
immunodeficiency virus (HIV). The inventive compounds also
have an antitumor effect. They are active against murine
leukemia P388 and other experimental tumors.
As mentioned above, the compounds of the present
invention are useful active ingredients in human and
veterinary medicine for the treatment and prophylaxis of
diseases caused by retroviruses. Examples of fields of
indication in human medicine regarding retroviruses are as
follows:
(1) the treatment or prophylaxis of human retrovirus
infections;
(2) the treatment or prophylaxis of diseases caused by
HIV (virus of human immune deficiency; previously called
HTLV III/LAV or AIDS) and the stages associated therewith
such as ARC (AIDS related complex) and LAS (lymph adenopathy
syndrome) and the immune weakness and encephalopathy caused
by this retrovirus;
-18-
C
2015671
(3) the treatment or prophylaxis of HTLV I infection
or HTLV II infection;
(4) the treatment or prophylaxis of the AIDS carrier
state (AIDS transmitter state); and
(5) the treatment or prophylaxis of diseases caused by
hepatitis B virus.
Examples of indications in veterinary medicine are as
follows:
(1) Maedivisna (in sheep and goats),
(2) progressive pneumonia virus (PPV) (in sheep and
goats),
(3) caprine arthritis encephalitis virus (in sheep and
goats),
(4) Zwoegerziekte virus (in sheep),
(5) infectious virus of anemia (of the horse), and
(6) infections caused by cat leukemia virus.
For use against viral infections and against tumors,
the compounds of this invention can be formulated into
pharmaceutical preparations. Such preparations are composed
of one or more of the inventive compounds in association
with a pharmaceutically acceptable carrier. The reference
Remington's Pharmaceutical Sciences, 17th Edition by A.R.
Gennaro (Mack Publishing Company, 1985) discloses typical
carriers and methods of preparation.
-19-
C
2015671
For antiviral purposes, the compounds may be
administered topically or systemically to warm blooded
animals, e.g., humans. For antitumor use, systemic, and
preferably, parenteral administration is employed. By
systemic administration is intended, oral, rectal, and
parenteral (i.e., intramuscular, intravenous, subcutaneous
and nasal) routes. Generally, it will be found that when a
compound of the present invention is administered orally, a
larger quantity of the reactive agent is required to produce
the same effect as the smaller quantity given parenterally.
In accordance with good clinical practice, it is preferred
to administer the instant compounds at a concentration level
that will produce effective antiviral or antitumor effect
without causing any harmful or untoward side effects.
Therapeutically and prophylactically the instant
compounds are given as pharmaceutical compositions comprised
of an effective antiviral or antitumor amount of a compound
according to the invention or a pharmaceutically acceptable
salt thereof and a pharmaceutically acceptable carrier, as
stated hereinabove. Pharmaceutical compositions for
effecting such treatment will contain a major or minor
amount, e.g., from 95 to 0.5% of at least one compound of
the present invention in combination with a pharmaceutical
carrier, the carrier comprising one or more solid,
semi-solid, or liquid diluents, fillers and formulation
adjuvants which are non-toxic, inert and pharmaceutically
-20-
2015671
acceptable. Such pharmaceutical compositions are preferable
in dosage unit form; i.e., physically discrete units
containing a predetermined amount of the drug corresponding
to a fraction or multiple of the dose which is calculated to
produce the desired therapeutic response. Other therapeutic
agents can also be present. Pharmaceutical compositions
providing from about 1 to 50 mg of the active ingredient per
unit dose are preferred and are conventionally prepared as
tablets, lozenges, capsules, powders, aqueous or oily
suspensions, syrups, elixirs, and aqueous solutions.
Preferred oral compositions are in the form of tablets or
capsules and may contain conventional excipients such as
binding agents, (e. g., syrup, acacia, gelatin, sorbitol,
tragacanth or polyvinylpyrrolidone), fillers (e. g., lactose,
sugar, corn starch, calcium phosphate, sorbitol, or
glycine), lubricants (e. g., magnesium stearate, talc,
polyethylene glycol or silica), disintegrants (e. g., starch)
and wetting agents (e. g., sodium lauryl sulfate). Solutions
or suspensions of an inventive compound with conventional
pharmaceutical vehicles are employed for parenteral
compositions, such as an aqueous solution for intravenous
injection or an oily suspension for intramuscular injection.
Such compositions having the desired clarity, stability and
adaptability for parenteral use are obtained by dissolving
from 0.1% to 10% by weight of an active inventive compound
-21-
2015671
in water or a vehicle comprising a polyhydric aliphatic
alcohol such as glycerine, propylene glycol, and
polyethylene glycol or mixtures thereof. The polyethylene
glycols comprise a mixture of non-volatile, usually liquid,
polyethylene glycols which are soluble in both water and
organic liquids and have molecular weights from about 200 to
1500.
Considering the biological activities possessed by the
compounds of the instant invention, it can be seen that
these compounds have antitumor and antiviral properties,
particularly suited to their use in combating viral
infections or tumors: Thus, another aspect of the instant
invention concerns a process for treating viral (including
retroviral) infections or tumors in a mammal in need of such
treatment which comprises systemic or topical administration
to such mammal of an effective dose of an inventive compound
or a pharmaceutically acceptable salt thereof. On the basis
of testing, an effective dose could be expected to be from
about 0.01 to about 30 mg/kg body weight with about 1 to
about 20 mg/kg body weight a preferred dosage range. It is
envisioned that for clinical antiviral application compounds
of the instant invention will be administered in the same
manner as for the reference drug acyclovir. For clinical
applications, however, the dosage and dosage regimen must in
each case be carefully adjusted, utilizing sound
-22-
2015671
professional judgment and consideration of the age, weight
and condition of the recipient, the route of administration
and the nature and gravity of the illness. Generally a
daily oral dose will comprise from about 150 to about 750
mg, preferable 250-500 mg of an inventive compound
administered from one to three times a day. In some
instances, a sufficient therapeutic effect can be obtained
at lower doses, while in others, larger doses will be
required.
In the reaction (process) (R1) described above,
non-limiting examples of Lewis acids include BF3ether, TiCl4
and BC13.
In the reactions (processes) (R3) and (R5) described
above, non-limiting examples of peracids include the
following: m-chloroperbenzoic acid, trifluoroperacetic acid
and perbenzoic acid.
The reactions (processes) (R1) to (R5) described above
are preferably conducted at atmospheric pressure and
preferably conducted in the presence of a solvent, e.g.,
CH3CN, CH2C12, C1CH2CH2C1, CHC13, THF, dioxane,
diethylether, benzene or toluene.
Description of the Specific Embodiments
The compounds which constitute this invention and their
methods of preparation will appear more fully from a
consideration of the~following examples which are given for
the purpose of illustration only and are not to be construed
-23-
2015671
as limiting the invention in sphere or scope. In addition
to the compounds described in the following examples,
further compounds encompassed by the present invention are
as follows:
9-[(2-Hydroxy-1-phosphonomethoxyethoxy)methyl]adenine
disodium salt
NHz
N
0 ~ I ~N
~i 0 0 N
(Na0>2P ~/
HO
9-[(2-Hydroxy-1-phosphonomethoxyethoxy)methyl]cytosine
disodium salt
NH2
i
0 OJ
<Na0>2P ~
H
9-[(2-Hydroxy-1-phosphonomethoxyethoxymethyl]thymine
disodium salt
0
CH3
H
0 0
~ NaO )=P ~
H
-24-
,~,./
... 2015671
In the following examples, all temperatures are
understood to be in degrees C when not specified. The
nuclear magnetic resonance (NMR) spectral characteristics
refer to chemical shifts (b) expressed in parts per milion
(ppm) versus tetramethylsilane (TMS) as a reference
standard. The relative area reported for the various shifts
in the proton NMR spectral data corresponds to the number of
hydrogen atoms of a particular functional type in the
molecule. The nature of the shifts as to multiplicity is
reported as broad singlet (bs), singlet (s), multiplet (m),
doublet (d), doublet of doublets (dd), triplet (t) or
quartet (q). Abbreviations employed are:
ACV (acyclovir)
BID (twice a day)
CDC13 (deuterochloroform)
DMf (dimethylformamide
DMSO-d6
(perdeuterodimethylsulfoxide)
EMEM (Earle's Minimum Essential medium)
Et (ethyl)
HIV (human
immune
deficiency
HSV (Herpes simplex virus)
MuLV (murine leukemia virus)
NOE (Nuclear Overhouser/Effect)
PffU (plaque forming units)
-25-
C
._ 2015671
TMS (trimethylsilyl)
Me (methyl) '
Ac (acetyl)
Pv (pivaloyl)
Ph (phenyl)
All compounds gave satisfactory elemental analyses.
The invention will now be described with reference to
the following non-limiting examples.
Examples:
Example 1: Bisbenzoyloxymethyl ether (1)
0 0
II II
Ph C p ~0~0 C Ph < 1 )
N
To a suspension of sodium benzoate (5.0 g, 34.7 mmol)
in DMF (70 ml) was added bischloromethyl ether (20 g, 17.3
mmol) and the mixture was heated at 70°C for 16 hours. The
insoluble material was removed by filtration. The filtrate
was concentrated in vacuo to give a white crystal which was
recrystallized from ether-pentane: yield 4.5 g (91%); mp
39°C.
Analysis: Calc. for C16H14~5' C~ 67.12; H, 4.92.
Found: C, 66.87; H, 4.94.
1H-NMR (200 MHz, CDC13)' b 5.66 (s, 4H), 7.75-8.05
(m,lOH).
-2 6-
2015671
Example 2: 1-[(Benzoyloxymethyoxy)methyl]thymine (2)
0
H
o d~ / cHa
Pn c o ~oV < z ~
(1) A suspension of thymine (12.6 g, 0.1 mole), ammonium
sulfate (300 mg) and trimethylsilyl chloride (2.5 ml) in
hexamethyldisilazane (150 ml) was heated at 140°C for 16
hours under nitrogen. The volatiles were removed in yacuo
at 50°C and the residual oil was dissolved in xylene (30 ml)
and concentrated to dryness.
(2) To a solution of the silylated thymine in CH2C12 (200
ml) was added bisbenzoyloxymethyl ether (30 g, 0.1 mol) and
trimethylsilyl trifluoromethanesulfonate (50 ml). The
solution was stirred for 8 hours at 25°C under nitrogen.
The reaction was diluted with ethyl acetate (400 ml) and
washed with aqueous sodium carbonate, brine, dried (MgS04),
filtered and concentrated in vacuo. The crude oily material
was purified by silica gel column chromatography using
CH2C12-5% MeOH as an eluent to give the title compound as
white crystals: yield 14.5 g (50%); mp 141-143°C.
Analysis: Calc. for C14H14N205' C~ 57.93; H, 4.82; N,
9.65.
Found: C, 57.59; H, 4.90; N, 9.52.
-27-
2015671
13C-~R (50.3 MHz, d6DMS0): d 70.779, 72.477, 72.915,
73.349, 82.985, 105.563, 123.376, 124.076, 124.368, 128.383,
134.397, 146.260, 159.451, 160.211.
1H-NMR (CDC13): d 1.82 (s,3H), 5.38 (s, 2H), 5.62 (s,
2H), 7.10 (s,lH), 7.4-8.0 (m,SH).
Example 3:
1-[(Diethylphosphonomethoxy)methoxymethyl]thymine (3)
H3
0
II
<Et0)2P
<3>
To a solution of 1-[3(benzoyloxy)methoxymethyl]thymine
(2.9 g, 10 mmol) and diethylphosphonomethanol (1.85 g, 11
mmol) in benzene (180 ml) was added trimethylsilyl
trifluoromethanesulfonate (0.05 ml) via a syringe under
nitrogen. The solution was heated at 85°C for 20 minutes.
After cooling to room temperature, ethyl acetate (50 ml) was
added and washed with aqueous bicarbonate, brine dried
(MgS04), filtered and concentrated in yacuo. The resultant
yellow oil was purified by silica gel column chromatography
using CH2C12-5% MeOH as an eluent to give the title compound
as a white oil: yield 980 mg (30%).
-28-
C
2015671
H1-NMR (200 MHz, CDC13): d 1.39 (t, J = 6.6 Hz, 6H),
1.98 (s, 3H), 3.85 (d', J = 9.9 Hz, 2H), 4.1-4.3 (m, 4H),
4.82 (s, 2H), 5.20 (s, 2H), 7.20 (s, 1H), 9.0 (broad s, 1H).
Example 4:
1-[3-(Ethylphosphonomethoxy)methyloxymethyl)]thymine sodium
salt (4)
0
~i%~CH3
0 ''
<Et0)2P ~0~~~
<4)
ONa
A solution of 1-[(diethylphosphonomethoxy)methoxy-
methyl]thymine (400 mg, 1.2 mmol) in 1N NaOH (8 ml) was
stirred for 3 hours at 25°C. The solution was concentrated
in vacuo and the resultant solid was purified by C-18
reverse phase column chromatography using water as an eluent
under 0.56 bar (8 psi) pressure. The fractions having
ultraviolet absorption were checked with HPLC, combined and
lyophilized to give the title compound as a white amorphous
powder:
yield 220 mg (55%).
Analysis: Calc. for C10H16N207PNa H20: C, 34.48; H,
5.17; N, 8.05. Found: C, 34.92; H, 5.37; N, 8.35.
W (H20): a max 226 nm (E =6966).
-29-
2015671
13C-~R (50.3 MHz, D20): d 11.451, 15.843, 61.067,
61.826, 63.585, 75.247, 94.631, 111.049, 141.277, 155.20,
170.907.
1H-NMR (200 MHz, D20): d 1.19 (t, J = 6.8 Hz, 3H), 1.81
(s, 3H), 3.62 (d, J = 8.9 Hz, 2H), 3.8-4.1 (m, 2H), 3.62 (s,
2H), 4.78 (s, 2H), 5.20 (s, 2H), 7.45 (s, 1H).
Example 5: 1-((Phosphonomethoxy)methoxymethyl]thymine
disodium salt (5)
0
H ~CH3
0
C
II
<Na0)2 P ~~OJ C5)
To a solution of 1-[3'-(ethylphosphonomethoxy)
methoxymethyl]thymine sodium salt (300 mg, 0.9 mmol) in dry
DMF (5 ml) was added bromotrimethylsilane (1.5 m 1) under
nitrogen. After stirring 3 hours at 25°C, volatiles were
removed in vacuo and the residue was dissolved in aqueous
saturated bicarbonate and re-evaporated in vacuo to a solid
foam. Purification of this material by a C-18 reverse phase
column chromatography using water as eluent under 0.56 bar
(8 psi) pressure and lyophilization of combined fractions
gave the title compound as a white amorphous foam: Yield
140 mg (48%).
-30-
C
2015671
Analysis: Calcd. for C8N11N207PNa2: C, 29.64; H,
3.42; N, 8.64. Found: C, 29.91; H, 3.61; N, 9.16.
W (H20): a max 266 nm (e = 8100).
13H-~R (200 MHz, D20): d 1.75 (s, 3H), 3.27 (d, J =
8.5 Hz, 2H), 4.71 (s, 2H), 5.11 (s, 2H), 7.74 (s, 1H).
Example 6:
2-Amino-6-chloro-9-[(diethylphosphonomethoxy)methoxymeth-
yl]purine (6)
C1
~/ I w.N
N
p~ ~ ~N H
(Et0)2 F ~~CJ
<6>
To a suspension of 60% sodium hydride in mineral oil
(1.4 g, 34.5 mmol) in n-pentane (100 m ) at 0°C was added
dropwise diethyl phosphate (4.4 ml, 34.5 mmol) under
nitrogen. After stirring for 1 hour at 0°C, a solution of
bis(chloromethoxy)methane (25 g, 172 mmol) (prepared
according to the literature procedure: P.R. Strapp, J. Org.
Chem., 34, 1143 (1969) in n-pentane (50 ml) was added at
-70°C. The mixture was stirred for 90 minutes at 0°C, and
then the solvent was evaporated under reduced pressure. The
-31-
C
2015671
residual oil was dissolved in xylene and volatiles were
removed in vacuo to give crude chloromethoxy-(diethoxyphos-
phonomethoxy)methane. Without further purification, this
material was used for the next reaction.
To a suspension of 60% sodium hydride in mineral oil
(1.4 g, 34.5 mmol) in DMF (100 ml) was added
2-amino-6-chloropurine (5.78 g, 34.2 mmol) and the mixture
was stirred for 1 hour at 25°C. To the resulting yellow
solution was added dropwise a solution of above
chloromethoxy(diethylphosphonomethoxy)methane in DMF (20 ml)
under nitrogen. After stirring 15 hours at 25°C, volatiles
were removed in vacuo. The residual oil was suspended in
ethyl acetate (100 ml), washed with water (30 m1), brine and
dried (MgS04). The solvent was removed under reduced
pressure, and the residual oil was chromatographed on silica
gel using CH2C12-3% MeOH as an eluent to give the title
compound as a colorless oil: yield 3.0 g (23%).
1H-NMR (300 MHz, CDC13): d 1.395 (t, J = 6.9 Hz, 6H),
3.850 (d, J = 9.0 Hz, 2H), 4.05-4.20 (m, 4H), 4.697 (s, 2H),
5.323 (broad s, 2H) 5.578 (s, 2H, 7.889 (s, 1H).
-32-
C
2015671
Example 7:
9-[(Methylphosphonomethoxv)methoxymethyl]auanine sodium salt
0
<iN i
0
fle0-~P ~0~0 N NH2
I ~ <7)
ONa
To a solution of 2-amino-6-chloro-9-[3-(diethyl-
phosphonomethoxy)methoxy-methyl] purine (325 mg, 0.84 mmol)
in methanol (5 ml) was added 1N sodium methoxide in methanol
(10 m1). The solution was heated at 80°C for 1 hour under
nitrogen. Volatiles were removed under reduced pressure.
The residual oil was then dissolved in water (10 ml) and the
solution was heated at 100°C for 1 hour. The pH of the
solution was carefully adjusted to 8.0 at 0°C by dropwise
addition of 1N-HC1. Water was then evaporated in yacuo and
the residual oil was purified by a C18 reverse phase column
using water as an eluent to give the title compound as a
white solid: yield 185 mg(60%).
Analysis: Calcd. for C9H1N506PNa 4H20: C, 26.13; H,
5.12; N, 16.95. Found: C, 26.05; H, 4.99; N, 16.64.
UV (H20): a max 254 nm (e = 14,372), 274 nm (e =
9.788).
-33-
C
2015671
13C-~R (75.47 MHz, D20): d 51.875, 60.464, 63.627,
70.005, 94.711, 94.952, 116.171, 139.925, 151.665, 154.428,
159.278.
1H-NMR (300 MHz, D20): 3.677 (d, J = 10.3Hz, 3H),
3.620 (d, J = 9.0 Hz, 2H), 4.817 (s, 2H), 5.539 (s, 2H),
7.882 (s, 2H).
Example 8: 9-(3-(Phosphonomethoxy)methoxymethyl]guanine
disodium salt (8)
0
~NH
0 ~
C N a0 ) ~P 0 0 N"N H 2
2 ~ ~ ~ <8>
To a solution of 9-[(methylphosphonomethoxy)methoxy-
methyl]guanine (1.5 g, 4.4 mmol) in DMF (5 ml) was added
bromotrimethylsilane ( 5 ml) under nitrogen. After stirring
3 hours at 25°C, the volatiles were removed in vacuo and the
residue was neutralized to pH 8.0 by addition of aqueous
saturated sodium bicarbonate. Water was then evaporated in
vacuo, and the residue was purified by a C18 reverse phase
column using water as eluent under 0.56 bar (8 psi)
pressure to give the title compound as a white powder:
yield 900 mg (59%).
Analysis: Calcd. for CBHlON506PNa 3H20: C, 23.84; H,
4.01; N, 17.37. Found: C,23.99; H, 3.92; N, 17.21
W (H20): a max 252 nm (e - 12,113), 274 nm (e =
8,201).
-34-
2015671
13C-~R (75.47 MHz, D20): d 67.016, 69.018, 70,746,
95.680, 95.831, 118.192, 141.812, 153.576, 157.386, 162.493.
1H-NMR (300 MHz, D20): d 3.525 (d, J = 8.9 Hz, 2H), 4.766
(s, 2H), 5.539 (s, 2H), 7.892 (s, 1H).
Example 9:
9-[(Diethylphosphonomethoxy)methoxymethyl]adenine (9)
NH2
~~N
' J
< E t 0 ) 2~P ~0~ ~ N
(9)
To a suspension of 60% sodium hydride in mineral oil
(1.4 g, 34.5 mmol) in DMF (100 ml) was added adenine (4.7 g,
34.5 mmol) and the mixture was stirred at 80°C for 1 hour.
To the resulting yellow solution was added dropwise a
solution of chloromethoxy-(diethoxyphosphinolmethoxy)methane
((prepared from diethylphosphate (4.4 ml, 34.5 mmol) and
bis-(chloromethoxy)methane (25 g, 172 mmol)] in DME (20 ml)
under nitrogen. After stirring at 25°C for 15 hours,
volatiles were removed in vacuo, and the resulting oily
residue was purified by silica gel column chromatography
using CH2C12-10% MeOH as an eluent to obtain the title
compound as a colorless oil: yield 6.0 g (50%).
-35-
C
2015671
1H-NMR (300 MHz, CDC13): d 1.390, (t, J = 6.7 Hz, 6H),
3.821 (d, J = 9.2 Hz,' 2H), 4.05-4.18 (m, 4H), 4.785 (s, 2H),
5.690 (s, 2H), 6.20 (broad s, 2H), 7.921 (s, 1H), 8.295 (s,
1H).
Example 10: 9-[3-(Phosphonomethoxy)methoxymethyl]adenine
disodium salt (10)
NH2
~~N
0
' J
< N a0 ) 2~P ~0~0~ N
<10)
To a solution of 9-[(diethylphosphonomethoxy)-
methoxymethyl]adenine (600 mg, 1.7 mmol) in DMF (4 ml) was
added bromotrimethylsilane (5 ml) under nitrogen. After
stirring 3 hours at 25°C, the volatiles were removed in
vacuo and the residue was neutralized to pH 8.0 by addition
of aqueous saturated sodium bicarbonate. Water was then
evaporated in vacuo, and the residue was purified by a C18
reverse phase column using water as an eluent under 0.56
bar (8 psi) pressure to give the title compound as a white
powder: yield 280 mg (50%)
Analysis: Calcd for C8N10N505PNa2 (3H20 + 0.2 mol
NaCl): C, 22.08; H, 4.72; N, 16.10. Found: C, 22.15; H,
4.64; N, 16.26.
W (H20): a max 260 nm (E = 12,016)
-36-
2015671
13C-~R (75.47 MHz, D20): d 66.913, 68.917, 71.033,
95.729, 95.940, 120.228, 144.611, 150.754, 154.766, 157.370.
1H-NMR (300 MHz, D20): 3.486 (d, J = 8.9 Hz, 2H),
4.779 (s, 2H), 5.710 (s, 2H), 8.177 (s, 1H), 8.226 (s, 1H).
Example 11: .
1-[(Diethylphosphonomethoxy)methoxymethyl]cytosine (11)
NH2
0
II N
<Et0)z P ~~0
<11)
To a suspension of 60% sodium hydride in mineral oil
(700 mg, 17 mmol) in DMF (50 ml) was added cytosine (1.9g,
17 mmol) and the mixture was heated at 80°C for 2 hours
under nitrogen. To the resulting yellow solution was added
dropwise a solution of chloromethoxy(diethylphosphono-
methoxy)methane [prepared from diethylphosphate (2.4 g, 17
mmol) and bis(chloromethoxy)methane (12.5 g, 86 mmol)] in
DMF (10 ml) under nitrogen. After stirring 15 hours at
25°C, the volatiles were removed in vacuo. The residue was
dissolved in ethyl acetate (120 mL) and water (30 ml). The
organic phase was washed with brine and dried (MgS04).
After removal of the solvent in vacuo, the residual oil was
chromatographed on silica gel using CH2C12-10% MeOH as an
eluent to give the title compound as a white oil: yield 1.2
g (22%).
-37-
C
2015671
1H-NMR (300 MHz, CDC13): d 1.390 (t, J = 6.9 Hz, 6H),
1.90 (broad s, 2H), 3'.815 (d, J = 9.0 Hz, 2H), 4.05-4.20 (m,
4H), 4.752 (s, 2H), 5.20 (s, 1H), 5.853 (d, J = 7.4 Hz, 1H),
7.312 (d, J = 7.4 Hz, 1H).
Example 12- 1-(Phosphonomethoxy)methoxymethylcytosine
disodium salt (12)
NH2
0
il N
< N a0 ) 2 P ~0~0~
<12)
To a solution of 1-[diethylphosphonomethoxy)-
methoxymethyl]cytosine (1.2 g, 3.7 mmol) in DMF (5 ml) was
added bromotrimethylsilane (5 ml) under nitrogen. After
stirring 3 hours at 25°C, the volatiles were removed in
vacuo and the residue was neutralized to pH of 8.0 by the
addition of aqueous saturated sodium bicarbonate. Water was
then evaporated in vacuo and the residue was purified by a
C18 reverse phase column using water as eluent under 0.56
bar (8 psi) pressure to give the title compound as a white
solid: yield 460 mg (47~)
Analysis: Calcd. for C7H11N306PNa2 (3 H20 + 5% NaCl);
C, 21.99; H, 4.22; N, 10.99. Found: C, 21.72; H, 4.65; N,
10.78.
-38-
_ 2015671
W (H20): a max 268 nm (E = 8,245)
13C-~R (75.47 MHz, D20): b 66.876, 68.873, 77.710,
96.141, 96.284, 98.178, 148.355, 160.409, 162.543. 1H-NMR
(300MHz, D20): d 3.560 (d, J = 9.0 Hz, 2H), 4.849 (s, 2H),
5.313 (s, 2H), 6.03 (d, J= 7.3 Hz, 1H), 7.714 (d, J = 7.3
Hz, 1H).
Example 13~ [2-(Phenylselenyl)ethoxy]methyl chloride (13)
0~~1
Ph S e~~ ( 13 )
N
To a solution of 2-(phenylselenyl)ethanol (4.0 g, 20
mmol) [prepared according to the literature procedure: P.
Rollin, V.V. Bencomo, P. Sinay, Synthesis, 13 (1984] in
CH2C12 15 ml) was added paraformaldehyde (620 mg, 20 mmol).
HC1 gas was then bubbled into the solution at 5°C for 2
hours. The solution was dried (MgS04), and the solvent was
removed under reduced pressure to give the title compound as
a colorless oil in a quantitative yield.
1H-NMR (300 MHZ, CDC13: d 3.059 (t, J = 7.0 Hz, 2H),
3.882 (t, J = 7.0 Hz, 2H), 5.449 (s, 2H), 7.2-7.5 (m, 5H).
-39-
C
2015671
Example 14:
2-Amino-6-chloro-9-((2-(phenylselenyl)ethoxy)methyl]purine
(14)
CI
~~N
i
Ph Se ~~/ ~ NHe
C14)
A mixture of 2-amino-6-chloropurine (20 g, 118 mmol)
and ammonium sulfate (400 mg) in hexamethyldisilazane (400
ml) and chlorotrimethylsilane (6m1) was heated at 145°C for
hours under nitrogen. Volatiles were removed in yacuo and
the residue was evaporated with xylene twice, and further
dried in vacuo for 3 hours. The crude silylated 2-amino-6-
chloro-purine (15 g, 72 mmol) and mercuric cyanide (15 g, 59
mmol) in benzene (900 ml) was heated at reflux for 30
minutes, then a solution of 2-(phenylselenyl)ethoxymethyl
chloride (17 g, 68 mmol) in benzene (100 ml) was added. The
mixture was refluxed for 3 hours, and then allowed to stir
for 15 hours at 25°C. The reaction was diluted with CH2C12
(300 ml.), and then quenched with aqueous saturated
bicarbonate (2 1). The organic phase was washed with 2N
-40-
C
2015671
potassium iodide (200 ml), dried (MgS04) and the solvents
were removed in vacuo'. The residual oil was chromatographed
on silica gel using CH2C12-5% MeOH as an eluent to provide
the title compound as a slightly yellow foam: yield 15.0 g
(63%).
Analysis: Calcd. for C14H14N50C1Se 1/2 H20 C, 42.93;
H, 3.86; N, 17.88. Found: C, 42.92: H, 3.80: N, 17.59
H1-NMR (300 MHz, CDC13): d 2.961 (t, J = 6.9 Hz, 2H),
3.704 (t, J - 6.9 Hz, 2H), 5.196 (broad s, 2H), 5.420 (s,
2H), 7.1-7.4 (m,SH), 7.806 (s, 1H).
13C-~R (75.47 MHz, CDC13): d 26.041, 69.144, 72.710,
127.206, 128.653, 128.846, 131.232, 136.062, 144.946,
151.190, 151.833,. 152.298.
Example 15: 2-Acetamino-6-chloro-9-[(2-(phenylselenyl)-
ethoxy)methyl]purine (15)
ct
WN
Ph Se ~/~/
0
(15)
A solution of 2-amino-6-chloro-9-[(2-phenylselenyl)-
ethoxy)methyl]purine (8 g, 21 mmol) in acetic anhydride (80
ml) was heated at 55°C for 40 hours. Volatiles were removed
in vacuo and the residual oil was purified by silica gel
column chromatography using CH2C12-40% EtOAc as an eluent to
give the title compound as a yellow powder: yield 5.8 g
(65%).
-41-
C
2015671
Analysis: Calcd. for C16H16N202C1Se: C, 45.25: H,
3.80; N, 16.49. Found: C, 45.12; H, 3.90; N, 16.47.
1H-NMR (300 MHz, CDC13): d 2.49 (s, 3H), 2.961 (t, J =
6.9 Hz, 2H), 3.756 (t, J = 6.9 Hz, 2H), 5.541 (s, 2H),
7.2-7.4 (m, 5H), 8.063 (s, 1H).
Example 16:
2-Acetamino-6-chloro-9-fvinyloxymethyl)purine (16)
C1
WN
i~
N"NH
0~ ~ 0
C16)
To a solution of 2-acetamino-6-chloro-9-[(2-(phenyl-
selenyl)ethoxy)methyljpurine (424 mg, 1 mmol) in methanol
(20 ml) was added sodium bicarbonate (92 mg, 1.1 mmol) and
sodium periodate (320 mg, 1.5 mmol). After stirring at 25°C
for 30 minutes the mixture was filtered and evaporated to
dryness. The residue was dissolved in dioxane (20 ml) and
the solution was heated at 80°C for 20 minutes under
nitrogen. The solution was evaporated in vacuo and the
residual oil was chromatographed on silica gel using
CH2C12-20% MeOH as an eluent to give the title compound as a
slightly yellow foam: yield 220 mg (60%).
Analysis: Calcd. for ClOH10N502C1: C, 44.88; H, 3.77;
N, 26.17.
Found: C, 44.57; H, 3.83; N, 25.82.
-42-
c
2015671
Example 17:
2-Acetamino-6-chloro-9-((1-(dimethylphosphonomethoxy)-
ethoxy)methyl]purine (17)
C1
~N
0 i
I I 0 ~N H
<CH30)2 p ~ ~0
CH3 <17)
To a solution of 2-acetamino-6-chloro-9-(vinyloxy)-
purine (2.2 g, 6.0 mmol) and dimethylphosphonomethanol (1.67
g, 12.0 mmol) in chloroform (100 ml) was added 120 mg of
methanesulfonic acid. After heating at 60°C for 2 hours,
the solvent was removed in vacuo and the residual oil was
chromatographed on silica gel using CH2C12-10% MeOH as an
eluent to give the title compound as a colorless oil: yield
1.2 g (50%).
13C ~R (75.47 MHz, CDC13): 18.794, 25.054, 53.064,
53.218, 55.743, 59.056, 68.071, 99.254, 99.502, 127.921,
144.576, 151.598, 152.645, 170.299.
1H-NMR (300 MHz, CDC13): d 1.347 (d, J = 8.1 Hz, 3H),
2.519 (s, 3H), 3.852 (d, J = 16.2 Hz, 6H), 5.029 (q, J = 8.1
Hz, 1H), 5.648 (d, J = 15.6 Hz, 1H), 5.791 (d, J = 15.6 Hz,
1H), 7.275 (s, 1H), 8.201 (s, 1H).
-43-
C
2015671
Example 18:
9-[(1-(Phosphonomethoxyethoxy)methyl]ctuanine disodium salt
0
~NH
0
II ~NH2
(Na0)2 P ~/ 0
CH3 <18>
To a solution of 2-acetamino-6-chloro-9-[(1-dimethyl-
phosphonomethoxy)ethoxy)methyl]purine (1.2 g, 2.95 mmol) in
methanol (5 ml) was added 1N sodium methoxide in methanol
(10 ml). After stirring at 25°C for 1 hour, water (10 m1)
was added and the solution was heated at 90°C for 1 hour
under nitrogen. Volatiles were removed in vacuo and the
residual oil was purified by C18 reverse phase column
chromatography using water as an eluent under 0.56 bar (8
psi) pressure. Each 10 ml fraction was assayed by high
pressure liquid chromatography. The combined fractions
were lyophilized to give a white solid. This material was
dissolved in DMF (20 ml) followed by bromotrimethylsilane (5
ml). After stirring 2 hours at 25°C, volatiles were removed
in vacuo and the residue was purified by a C18 reverse phase
column using water as an eluent under 0.56 bar (8 psi)
pressure to give the title compound as white amorphous
powder after lyophilization: yield 245 mg (24%)
-44-
C
2015671
Analysis: Calcd. for C9H12N506PNa2 4H20: C, 25.78, H,
4.80; N, 16.70.
Found: C, 25.93; H, 4.44; N, 16.91.
W (H20): a max 252 nm (e=9751).
13C-~ (75.47 MHz, D20): d 20.859, 64.079, 66.088,
70.423, 102.054, 102.241, 119.11, 140.287, 153.110, 162.211,
168.712.
1H-NMR (300 MHz, D20): d 1.195 (d, J = 6.3 Hz, 3H),
3.305 (dd, J = 8.9, 8.4 Hz, 1H), 3.496 (dd, J = 8.9, 8.4 Hz,
1H), 4.874, (q, J = 6.3 Hz, 1H), 5.475 (dd, J = 14.0, 11.1
Hz, 1H), 5.523 (dd, J = 14.0, 11.1 Hz, 1H), 7.790 (s, 1H).
Example 19:
1-(5-Methoxytetrahydro-2-furyl)thymine (19)
0
H
CH3
CH30
<19>
To a suspension of thymine (2.5 g, 20 mmol) in
hexamethyldisilazane (30 ml) was added ammonium sulfate (50
mg) and chlorotrimethylsilane (0.5 ml) and the mixture was
heated at 145°C for 4 hours under nitrogen. The excess
hexamethyldisilazane was removed at reduced pressure, and
the residual oil was dissolved in xylene and evaporated in
vacuo to give a colorless viscous oil. To this silylated
thymine in dichloroethane (40 ml) was added 2,5-dimethoxy-
-45-
2015671
tetrahydrofuran (7 ml). After cooling the solution to
-30°C, tin tetrachloride (2.3 ml) was added dropwise via a
syringe under nitrogen. The mixture was allowed to warm to
-10°C and was then poured into ice cold aqueous sodium
bicarbonate (100 ml) and ethyl acetate (150 ml). The
mixture was filtered and the organic phase was separated and
dried (MgS04). The solvent was removed under reduced
pressure and the residual oil was chromatographed on silica
gel using CH2C12-5% MeOH as an eluent to give the title
compound as a cis/trans mixture in a ratio of 1:1 as shown
by analytical HPLC and 1H-NMR: yield 3.4 g (75%).
Analysis: Calcd. for C10H14N204' C~ 53.08; H, 6.24; N,
12.39.
Found: C, 52.81; H, 6.22; N, 12.38.
W (EtOH): Amax 266 nm (e = 9076).
1H-NMR (300 MHz, CDC13): d 1.4-2.1 (m, 7H), 3.40 and
3.425 (two s, 3H), 5.20 and 5.328 (two broad s, lh), 6.208
and 6.417 (two dd, J = 3.5, 7.0 HZ and 7.2, 7.2 Hz, 1H),
7.021 and 7.40 (broad s, 1H).
The cis/trans (19A/19B) mixture was separated by a
careful silica gel column chromatography. Thus, the cis
isomer 19A was eluted first with CH2C12-3% MeOH and obtained
as a white needle. The X-ray crystallography and the NOE
(Nuclear Overhauser Effect) nmr confirmed the cis stereo-
chemical arrangement of 19A. mp 153-154°C.
-46-
2015671
Analysis: Calcd. for C10H14N204' C~ 53.09; H, 6.24; N,
12.38.
Found: C, 52.92; H, 6.20; N, 12.10.
13C-~R (75.47 MHz, CDC13): d 12.634, 29.417, 32.157,
55.202, 105.883, 111.411, 135.952, 139.553, 150.938,
163.906.
1H-NMR (300 MHz, CDC13): d 1.950 (s, 3H), 1.9-2.3 (m,
4H), 3.40 (s, 3H), 5.20 (t, J = 2.9 Hz, 1H), 6.417 (dd, J =
4.0, 7.2 Hz, 1H), 7.40 (s, 1H), 8.781 (s, 1H).
Continuing the column with CH2C12-3% MeOH, the trans
isomer 19B was eluted after the cis isomer 19A and was
obtained as white needles. The NOE observation of 19A was
consistent with the assigned structure: mp 124-125°C.
Analysis: Calcd. for C10H14N204' C~ 53.09; H, 6.24;
N, 12.38.
Found: C 53.10; H, 6.10; N, 12.00.
1H-NMR (75.47 MHz, CDC13): d 1.920 (s, 3H), 2.0-2.5 (m,
4H), 3.425 (s, 3H), 5.328 (dd, J = 2.5, 6.0 Hz, 1H), 6.208
(dd, J = 3.5, 7.0 Hz, 1H), 7.021 (s, 1H), 8.885 (s, 1H).
Example 20:
1-(4-Dimethylphosphonomethoxytetrahydro-2-furyl)thymine (20)
3
0
II
<he0)~ P
ccm
-47-
C
2015671
To a solution of 1-(5-methoxytetrahydro-2-furyl)thymine
(5.2 g, 23 mmol) and dimethylphosphonomethanol (6.5 g, 44
mmol) in toluene was added acetic acid (5 ml) and p-toluene-
sulfonic acid monohydrate (500 mg, 2.6 mmol). The solution
was heated at 100°C for 2 hours and the resulting insoluble
solid was removed by suction filtration. After removal of
the solvent under reduced pressure, the residual oil was
chromotographed on silica gel using CH2C12-5% MeOH as an
eluent to give the title compound as a cis/trans mixture
(6:4): yield 5.0 g (60%).
1H-NMR (300 MHz, CDC13): d 1.9-2.2 (m, lOH), 3.8-4.1
(m, 6H), 5.198, 5.445 (broad s, 0.6 and 0.4H), 6.250 (dd, J
- 2.8, 7.5 Hz, 0.4H), 6.437 (t, J = 7.4 Hz, 0.6H), 7.052 (s,
0.4H), 7.405 (s, 0.6H), 9.60 (broad s, 0.6H), 7.628 (broad
s, 0.4H).
Example 21:
1 (4 Methylphosphonomethoxytetrahydro-2-furyl)thymine sodium
salt (21)
0
H3
0
II
tle0-P
b~
-48-
C
2015671
To a solution of 1-(4-dimethylphosphonomethoxytetra-
hydro-2-furyl)thymine'(5 g, 14.6 mmol) in methanol (10 ml)
was added 2N sodium hydroxide (20 ml). After stirring 2
hours at 25°C, the reaction was neutralized to pH 8.0 by
addition of 3N-HC1 with stirring. Water was then evaporated
in vacuo and the residual oil was purified by a C18 reverse
phase column using water as an eluent to give the title
compound as a white powder. This material was shown to be a
1:1 cis/trans (21A/21B) mixture by analytical HPLC and
1H-NMR: yield 3.2 g (65%).
Analysis: Calcd. for C11H16N207NaP 2H20: C, 34.92; H,
5.29; N, 7.40.
Found: C, 34.93; H, 4.99; N, 7.43.
UV (H20): a max 268 nm (E = 8668).
1H-NMR (300 MHz, D20): d 1.842 (s, 1.5H), 1.894 (s,
1.5H), 1.9-2.5 (m,4H), 3.571 (d, J = 9.8 Hz, 1H), 3.589 (d,
J = 9.2 Hz, 1H), 3.59-3.85 (m, 2H), 5.239 (d, J = 3.5. Hz,
0.5H), 5.483 (d, J = 4.7 Hz, 0.5H), 6.198 (q, J = 2.9 Hz,
0.5H), 6.331 (t, J = 6.0 Hz, 0.5H), 7.371 (s, 0.5H), 7.561
(s, 0.5H).
The cis/trans mixture was separated by a C18 reverse
phase column (100 time weight) using water -3% acetonitrile
as an eluent under 0.42 bar (6 psig) pressure. Each 15 ml
fraction was assayed by HPLC. The cis isomer 21A was
eluted first and obtained as a white powder.
-49-
2015671
1H-NMR(300 MHz, D20): d 1.894 (s, 3H), 2.0-2.45 (m,4H),
3.571 (d, J = 9.8 Hz,~2H), 3.595 (dd, J = 7.4, 10.0 Hz, lH),
3.781 (dd, J = 7.4, 10.8 Hz, 1H), 5.239 (d, J = 3.5 Hz, 1H),
6.331 (t, J = 6.0 Hz, 1H), 7.561 (s, 1H).
After cis/trans mixture fractions, the pure trans
isomer 21B was also obtianed as a white powder.
1H-NMR (300 MHz, D20): d 1.842 (s, 3H), 2.0-2.5 (m,
4H), 3.589 (d, J = 9.2 Hz, 2H), 3.611 (dd, J - 9.2, 10.0
Hz, 1H), 3.840 (dd, J = 9.2, 10.0 Hz, 1H), 5.483 (d, J = 4.3
Hz, 1H), 6.198 (q, J = 2.9 Hz, 1H), 7.371 (s, 1H).
The steroechemical assignment of the cis and trans
isomers was confirmed by the NOE (Nuclear Overhauser Effect)
nmr.
Example 22:
1-(4-Phosphonomethoxytetrahydro-2-furyl)thymine disodium
salt (22)
0
i~
<Na0)~P
W n
To a solution of 1-(4-methylphosphonotetrahydro-2-
furyl)thymine sodium salt (1.2 g, 3.5 mmol) in DMf (15 ml)
was added bromotrimethylsilane (10 ml) under nitrogen.
After stirring 4 hours at 25°C, the volatiles were removed
-50-
c
2015671
in vacuo and the residual oil was neutralized to pH 8.0 by
the addition of aqueous sodium bicarbonate. Water was then
evaporated in vacuo, and the residue was purified by a C18
reverse phase column using water as an eluent under 0.56
bar (8 psi) pressure to give the title compound as a white
powder: yield 857 mg (70%)
Analysis: Calcd. for C10H13N207PNa2 5H20: C, 27.26;
H, 5.20; N, 6.36.
Found: C, 27.14; H, 5.26; N, 6.03.
W (H20): a max 268 nm (e = 7.350).
1H-NMR (300 MHz, D20): d 1.864 (s, 1.5H), 1.928 (s,
1.5H), 1.95-2.90 (m, 4H), 3.4-3.6 (m, 2H), 5.363 (t, J = 3.0
Hz, 0.5H), 5.56 (d, J = 4.2 Hz, 0.5H), 6.212 (dd, J = 2.7,
5.8 Hz, 0.5H), 6.321 (t, J = 3.9 Hz, 0.5H), 7.435 (s, 0.5H),
7.679 (s, 0.5H).
Example 23:
1-(2,3-Dideoxy-4-beta-chloro-3-(phenylselenyl)-beta-D-
erythrofuranosyl]thymine (23)
H3
C
Ph 5 a
To a solution of 1-(2,3-dideoxy-3,-4-didehydro-beta-D-
ethyro-furanosyl)thymine (1.94 g, 10 mmol) prepared
-51-
2015671
according to the literature procedure: J. Zemlicka, R.
Gasser, J. V. Freisler, J. P. Horwitz, J. Amer. Chem. Soc.,
94, 3213 (1972)] in CH.2C12 (30 mL) was added at - 70°C
dropwise a solution of phenylselenyl chloride (1.92 g, 10
mmol) in CH2C12 (5 ml) under nitrogen. After stirring at
-70°C for 1 hour, the solvent was removed in vacuo to give
the title compound as a yellowish oil. This material was
used for the next reaction without further purification.
1H-NMR (300 MHz, CDC13): d 1.95 (s, 3H), 2.5-2.8 (m,
2H), 4.18 (d, J =- 6.5 Hz, 1H), 6.20 (s, 1H), 6.55 (q, J -
6.0, 7.5 Hz, 1H), 7.1-7.7 (m, 6H), 9.30 (broad, 1H).
Example 24:
1-[2,3-Dideoxy-4-beta-(dimethylphosphono)methoxy-3-(pheny-
selenyl)-beta-D-erythrofuranosyl]thymine (24)
3
0I
(CF13U)2 'P
To a solution of 1-[2,3-dideoxy-4-chloro-3-(phenyl-
selenyl)-beta-D-erythro-furanosyl]thymine (3.85 g, 10 mmol)
and dimethoxyphosphinylmethanol (1.5 g, 11 mmol) in CH2C12
(20 ml) was added dropwise at -70°C a solution of silver
perchlorate (2.3 g, 11 mmol) in CH3CN (3 ml) over 3 minutes
under nitrogen. The~mixture was allowed to warm to O°C and
-52-
c
2015671
was then poured into aqueous saturated bicarbonate (10
ml)-brine (15 ml). The organic phase was separated after
filtration and dried (MgS04). The solvents were removed
under reduced pressure, and the residual oil was purified by
silica gel column chromatography using CH2C12-5% MeOH as an
eluent to give the title compound as a colorless oil: yield
1.3 g (31%).
1H-NMR (300 MHz, CDC13): d 1.93 (s, 3H), 2.45 (m, 2H),
3.70 (dd, J = 8.4, 8.0 Hz, 1H), 3.75 (d, J = 12.0 Hz, 6H),
3.85 (d, J = 6.9 Hz, 1H), 3.90 (dd, J = 8.4, 8.0 Hz, 1H),
5.10 (s, 1H), 6.52 (s, 1H), 7.35 (s, 1H), 8.86 (broad s,
1H).
Example 25:
1 [2,3 Dideoxy-2,3-didehydro-4-phosphonomethoxy-beta-D-ery-
throfuranosyl]thymine disodium salt (25)
0 0~ NH 0
(Na0>2 ~~ ~0 0
CH3
<25)
To a solution of 1-[2,3-dideoxy-4-(dimethylphosphono)-
methoxy-3-(phenylselenyl-beta-D-erythrofuranosyl]thymine
(1.15 g, 2.76 mmol) in DME (4 ml) was added at 5°C
bromotrimethylsilane (3 ml) under nitrogen. After stirring
-53-
C
2015671
for 4 hours at 5°C, volatiles were removed in vacuo and the
residue was dissolved~in aqueous saturated bicarbonate (3
ml) and evaporated again in vacuo to give a slightly yellow
solid.
1H-NMR (200 N~iz, D20): d 1.79 (s, 3H), 2.3-2.5 (m, 2H),
3.27 (1, J = 7.6, 8.4 Hz, 1H), 3.50 (1, J = 7.6, 8.4 Hz,
1H), 3.93 (d, J = 6.9 Hz, 1H), 5.23 (s, 1H), 6.0 (t, J = 6.9
Hz, 1H), 7.53 (s, 1H).
The reaction product from the above example was
dissolved in water (5 ml) followed by sodium periodate (1.7
g, 8.0 mmol). After stirring for 30 minutes, the reaction
mixture was heated at 80°C for 8 minutes and then filtered.
The filtrate was evaporated to dryness and the residual
solid was purified by a C18 reverse phase column
chromatography using water as an eluent under 0.56 bar (8
psi) pressure. Each 15 ml fraction was assayed by high
pressure liquid chromatography. Lyophilization of combined
fractions gave the title compound as a white amorphous
solid: yield 538 mg (50%): mp 233-237oC.
Analysis: Calcd. for C10H11N207Na2P H20: C, 32.61; H,
3.51; N, 7.61.
Found: C, 32.31; H, 3.63; N, 7.35.
13C-~R (50.3 N~iz, D20): d 13.921, 67.870, 69.848,
89.868, 111.242, 111.364, 113.908, 131.177, 134.655,
139.350.
-54-
c
2015671
1H-NMR (300 MHz, D20): d 1.848 (s, 3H), 3.565 (dd, J =
8.4, 8.7 Hz, 1H), 3.738 (dd, J - 8.4, 8.7 Hz, 1H), 5.987 (s,
1H), 6.180 (d, J = 6.0 Hz, 1H), 6.432 (d, J = 6.0 Hz, 1H),
6.817 (s, 1H), 7.377 (s, 1H).
W (H20): a max 266 nm (e - 10.134).
Example 26:
1-[2,3-Dideoxy-2,3-didehydro-4-beta-(dimethylphosphono)-
methoxy-beta-D-erythrofuranosyl)thymine (26)
NH
<Me0)2 ~P ~0
CH3
(26>
To a solution of 1-(2,3-dideoxy-4-beta-(dimethyl-
phosphono)methoxy-3-(phenylselenyl)-beta-D-erythrofuranosyl]
thymine (6.0 g, 12.2 mmol) in methanol (20 ml) was added
dropwise a suspended solution of sodium bicarbonate (1.8 g,
21 mmol) and sodium periodate (3.2 g, 15 mmol) in water (20
ml). After stirring at 25°C for 1 hour, the mixture was
heated at 80°C for 60 minutes. Volatiles were removed in
vacuo and the residue was suspended in CH2C12 (120 ml).
After removal of insoluble material, the organic phase was
dried (MgS04) and evaporated in vacuo. The residue was
chromatographed on silica gel using CH2C12-5% MeOH as eluent
to give the title compound as a white amorphous powder:
yield 3.4 g (85%).
-55-
C
2015671
Analysis: Calcd. for C12H17N207P: C, 43.38; H, 6.16;
N, 8.43.
Found: C, 43.53; H, 5.20; N, 8.26.
13C-~R (75.47 MHz, CDC13): d 12.339, 52.889, 52.976,
53.080, 60.491, 62.738, 87.837, 108.402, 108.569, 111.653,
130.613, 131.675, 135.435, 150.480, 163.503.
1H-NMR (300 MHz, CDC13): d 1.862 (s, 3H), 3.748 (d, J =
12.8 Hz, 3H), 3.814 (d, J = 12.8 Hz, 1H), 3.826 (dd, J =
8.9, 8.4 Hz, 1H), 3.902 (dd, J = 8.9, 8.4 Hz, 1H), 5.711 (s,
1H), 6.075 (d, J = 5.7 Hz, 1H), 6.233 (d, J = 5.7 Hz, 1H),
6.915 (s, 1H), 7.129 (s, 1H), 8.95 (broad s, 1H).
Example 27:
1-[2,3-Dideoxy-2,3-didehydro-4-beta-(methylphosphono)
methoxy-beta-D-erthyrofuranosyl]thymine sodium salt (27)
0 ~~ NH
(Me0>-IP ~0 0
i
Na0 ~H3
<27)
A solution of 1-[2,3-dideoxy-2,3-dihydro-4-beta-
(dimethylphosphono)methoxy-beta-D-2-erthyrofuranosyl]thymine
(180 mg, 0.54 mmol) in 1N-NaOH (2 ml) was stirred at 25°C
for 2 hours. The reaction was carefully neutralized to pH
8.0 by dropwise addition of 1N-HC1 with good stirring.
Water was then evaporated in vacuo and the residue was
-56-
C
2015671
purified by a C18 reverse phase column using water -3%
acetonitrile as an eluent to give the title compound as a
white solid: yield 125 mg (68%).
Analysis: Calcd. for C11H14N207pNa 1.5 H20: C, 34.28;
H, 4.93; N, 7.27.
Found: C, 34.02; H, 49.4; N, 7.19.
UV (H20): ~ max 269 nm (e=8160)
1H-NMR (300 MHz, D20): d 1.847 (s, 3H), 3.525 (d, J =
11.0 Hz, 3H), 3.917 (dd, J = 13.6, 17.0 Hz, 1H), 3.765 (dd,
J = 13.6, 17.0 Hz, 1H), 5.849 (s, 1H), 6.198 (d, J = 4.6 Hz,
1H), 6.415 (d, J = 4.6 Hz, 1H), 6.847 (s, 1H), 7.374 (s,
1H).
Example 28:
1-[2,3-Dideoxy-4-beta-(methylphosphono)methoxy-beta-D-
erythrofuranosyl]thymine sodium salt (28)
0
0 NH 0
CH30 -IP ~0
i
Na0 CH3
<28)
To a solution of 1-[2,3-dideoxy-2,3-didehydro-4-beta-
(methylphosphono)methoxy-beta-D-erythrofuranosyl]thymine
sodium salt (300 mg, 0.9 mmol) in water (20 ml) was added
10% palladium on active carbon (200 mg) and hydrogenated for
-57-
C
2015671
30 minutes under 2.49 bar (35 psi) HZ pressure. The
catalyst was filtered'and washed with methanol (30m1). The
combined filtrate and wash was evaporated in vacuo and the
residue was purified by a C18 reverse phase column using
water -2% acetonitrile under 0.56 bar (8 psi) pressure to
give the title compound as a white solid: yield 250 mg
(s4%) .
This material showed an identical nmr with compound 21A
which was prepared from 2,5-dimethoxytetrahydrofuran and the
stereochemical assignment of compound 21A was confirmed by
the NOE.
1H-NMR (300 MHz, D20): d 1.943 (s, 3H), 2.168 (m, 2H),
2.418 (m, 2H, 3.608 (d, J = 6.9 Hz, 3H), 3.6B (dd, J = 13.5,
18.0 Hz, 1H), 3.85 (dd, J = 13.5, 18.0 Hz), 5.303 (s, 1H),
6.390 (d, J = 6.3 Hz, 1H), 7.627 (s, 1H).
In a manner similar to the above Exampl$ 28, the
thymine-containing reactant can be replaced with a
corresponding adenine, guanine or cytosine reactant to
produce 1-[2,3-dideoxy-2,3-didehydro-4-beta-(methylphos-
phono)methoxy-beta-D-erythrofuranosyl] adenine sodium salt,
or 1-[2,3-dideoxy-2,3-didehydro-4-beta-(methylphosphono)-
methoxy-beta-D-erythrofuranosyl] guanine sodium salt, or
1-[2,3-dideoxy-2,3-didehydro-4-beta-(methylphosphono)-
methoxy-beta-D-erythrofuranosyl cytosine sodium salt.
-58-
C
2015671
Example 29:
1-(4-beta-(Dimethylphosphono)methoxy-beta-D-erythrofurano-
syl]thymine (29)
0
C ~NH C
<tle0)2~P ~C
CH3
H~ ~H ~2g)
To a solution of 1-[2,3-dideoxy-2,3-didehydro-4-beta-
(dimethylphosphono)methoxy-beta-D-erythrofuranosyl]thymine
(3.31 g, 10 mmol) in pyridine (20 ml) was added osmium
tetroxide (2.548, lOmmol at O°C and stirred for 2 hours.
hours. The solvent was removed in yacuo. The residue was
dissolved in ethyl acetate (100 ml), washed with 10%
phosphoric acid (30 ml), water (20 ml), aqueous sodium
bicarbonate (20 ml), brine and dried MgS04). The solvent
was removed in yacuo, and the residual oil was
chromatographed on silica gel using CH2C12-5% MeOH as an
eluent to give the title compound as a white powder: yield
2.56 g (70%).
Analysis: Calcd. for CH12H19N20gP: C, 39.35; H, 5.23;
N, 7.65.
Found: C, 38.98; H, 5.09; N, 7.42.
-59-
C
2015671
13C-~R (75.47 MHZ, d6-DMSO): b 11.861, 52.672, 52.797,
59.114, 61.411, 72.608, 73.316, 73.399, 87.419, 107.607,
107.863, 110.810, 135.217, 150.973, 163.581.
1H-NMR (300 MHz, d6-DMSO): b 1.675 (s, 3H), 3.,540 (d,
J = 10.5 Hz, 6H), 3.736 (1, J = 4.0 Hz, 1H), 3.817 (d, J =
9.6 Hz, 2H), 4.03 (dd, J = 6.0, 11.2 Hz, 1H), 4.763 (s, 1H),
5.35 (d, J = 4.0 Hz, 1H), 5.45 (d, J = 7.0 Hz, 1H), 5.919
(d, J = 6.9 Hz, 1H), 7.128 (s, 1H), 11.228 (broad s, 1H).
In a manner similar to the above Example 29, the
thymine-containing reactant can be replaced with a
corresponding adenine, guanine or cytosine reactant to
produce 1-[4-beta-(dimethylphosphono)methoxy-beta-D-
erythrofuranosyl]adenine, or 1-[4-beta-(dimethylphosphono)
methoxy-beta-D-erythrofuranosyl]guanine, or
1-[4-beta-(dimethylphosphono)methoxy-beta-D-erythro-
furanosyl]cytosine.
Example 30:
1-[4-(Methylphosphono)methoxy-beta-D-erythrofuranosyl]-
thymine Sodium Salt (30)
0 NH 0
II
CH30 - P
Nal CH3
<30)
-60-
C
2015671
To a solution of 1-[4-beta-(dimethylphosphono)methoxy-
beta-D-erythrofuranosyl]thymine (200 mg, 0.5 mmol) in
1N-NaOH (2 ml) was stirred at 25°C for 2 hours. The
reaction was carefully neutralized to pH 9.0 by dropwise
addition of 1N-HC1 with stirring. Water was then evaporated
in vacuo and the residual oil was purified by a C18 reverse
phase column using water-2% acetonitrile as an eluent under
8 psi pressure to give the title compound as a white
amorphous powder: yield 114 mg (56%).
Analysis Calcd. for C11H15N209pNa 2H20: C, 32.27; H,
4.64; N, 6.84.
Found: C, 31.94 ; H, 4.32; N, 6.86.
W (H20): a max 268 nm (c = 8153).
13C-~R (75.47 MHz, D2): d 35.644, 35.719, 45.366,
47.479, 57.032, 57.411, 71.915, 92.426, 92.599, 96.463,
120.503, 137.007, 144.422, 151.377.
1H-NMR (300 MHz, D20): b 1.922 (s, 3H), 3.615 (D, J =
10.1 Hz, 3H), 3.687 (dd,J = 11.3, 11.1 Hz, lh), 3.885 (dd, J
- 11.3, 11.1 Hz, 1H), 4.220 (d, J = 4.3 Hz, 1H), 4.574 (dd,
J = 6.7, 4.3 Hz, 1H), 5.09 (s, 1H), 6.175 (d, J = 6.7, Hz,
1H), 7.485 (s, 1H).
In a manner similar to the above Example 30, the
thymine-containing reactant can be replaced with a
corresponding adenine, guanine or cytosine reactant to
produce 1-[4-beta-(methylphosphono)methoxy-beta-D-
-61-
C
2015671
erythrofuranosyl]adenine, or 1-[4-beta-(methylphosphono)-
methoxy-beta-D-erythrofuranosyl]guanine, or
1-[4-beta-(methylphosphono)methoxy-beta-D-erythrofuran-
osyl]cytosine.
Example 31:
1-f4-beta-Phosphonomethoxy-beta-D-erythrofuranosyl)-
thymine disodium salt (31)
0
0 NH 0
<Na0)2 ~P ~0
CH3
H~ ~H (31)
To a solution of 1-[4-beta-(diemthylphosphono)methoxy-
beta-D-erythrofuranosyl]thymine (320 mg, 0.87 mmol) in DMF
(2 ml) was added at 0°C bromotrimethylsilane (1.4 ml) under
nitrogen. After stirring 90 minutes at O°C, volatiles were
removed in vacuo and the residue was neutralized to pH 8.0
by addition of aqueous saturated sodium bicarbonate. Water
was then evaporated in yacuo and the residual solid was
purified by a C18 reverse phase column using water-2%
acetonitrile as an eluent to give the title compound as a
white solid: yield 153 mg (46%). mp >250°C.
(decomposition).
Analysis: Calcd. for C10H13N209pNa 2H20: C, 27.52; H,
4.35; N, 6.42.
Found: 27.05; 3.99; N, 6.12.
C, H,
W (H20): a max,268 nm (e = 7,568).
-62-
C
2015671
130-~R (75.47 Ngiz, D20): b 49.590, 51.582, 57.347,
57.541, 71.861, 93.034, 93.169, 95.541, 121.178, 136.393,
144.222, 150.601.
1H-NMR (300 Ngiz, D20): d 1.936 (s, 3H), 3.469 (dd, J =
12.3, 12.6 Hz, 1H), 3.756 (dd, J = 12.3, 12.6 Hz, 1H), 4.258
(d, J = 4.5 Hz, 1H), 4.578 (dd, J = 4.5, 6.2 Hz, 1H), 5.175
(s, lh), 6.182 (d, J = 6.2 Hz, 1H), 7.591 (s, 1H).
In a manner similar to the above Example 31, the
thymine-containing reactant can be replaced with a
corresponding adenine, guanine or cytosine reactant to
produce 1-[4-beta-phosphonomethoxy-beta-D-erythrofuranosyl]-
adenine disodium salt, or 1-[4-beta-phosphonomethoxy-beta-D-
erythrofuranosyl]guanine disodium salt, or
1-[4-beta-phosphonomethoxy-beta-D-erythrofuranosyl]
cytosine disodium salt.
Example 32:
1-[2-Deoxy-4-beta-(diethylphosphono)methoxy-beta-D-
erythrofuranosyl]thymine (32A) and the trans isomer (32B).
0 0
0 NH O 0 ~NH 0
<Et0) ~~ 0 ~ <Et0)2 ~~
i ~/ ., i
CH3 CH3
H (32A) H (328>
-63-
C
2015671
To a solution of 1-(2,3-dideoxy-3,4-didehydro-beta-D-
erythrofuranosyl)thymine (2.4 g, 12.4 mmol) and diethyl-
phosphonomethanol (17.4 g, 103 mmol) in CH2C12 ( 2 ml) was
added 80-85% 3-chloroperoxybenzoic acid (17.4 g, 13.14 mmol)
at 5°C. After stirring for 60 minutes at 25°C, the reaction
mixture was purified by column chromatography on silica gel
using CH2C12-3% MeOH to obtain the crude product, which was
carefully rechromatographed on silica gel to separate the
two isomers. Using CH2C12-1% MeOH, the minor isomer B was
first eluted and obtained as a colorless oil: yield 75 mg
(1.7%).
1H-NMR (300 MHz, CDC13) of 32B: d 1.287 (t, J = 6.9 Hz,
6H), 1.900 (s, 3H), 1.97-2.58 (m, 2H), 3.807 (dd, J = 9.0,
13.8 Hz), 4.016 (dd, J = 9.0, 13.8 Hz, 1H), 4.138 (m, 4H),
4.30 (m, 1H), 4.934 (d, J = 4.2 Hz, 1H), 6.321 (t, J = 6.6
Hz, 1H), 7.417 (s, 1H), 9.80 (broad s, 1H).
The silica gel column was continuously eluted with
CH2C12-3% MeOH to obtain the major isomer 32A as a colorless
oil: yield 735 mg (17%).
1H-NMR (300 MHz, CDC13) of 32A: d 1.310 (t, J = 7.5 Hz,
6H), 1.872 (s, 3H), 1.95 (m, 1H), 2.70 (m, 1H), 3.780 (dd, J
- 9.3, 13.8 Hz, 1H), 3.928 (dd, J = 9.3, 13.8 Hz, 1H), 4.139
(m, 4H), 4.330 (d, J = 5.7 Hz, 1H), 5.268 (s, 1H), 6.238
(dd, J = 2.7, 8.4 Hz, 1H), 7.624 (s, 1H), 9.176 (broad s,
1H).
-64-
C
2015671
The stereochemical assignment of 32A and 32B was
consistent with nmr NOE observation.
In a manner similar to the above Example 32,
1-(2,3-deoxy-3,4-didehydro-beta-D-erythrofuranosyl)thymine
can be replaced with a corresponding adenine, guanine or
cytosine reactant to produce 1-[2-deoxy-4-beta-(diethyl-
phosphono)methoxy-beta-D-erythrofuranosyl]adenine,
1-[2-deoxy-4-beta-(methylphosphono)methoxy-beta-D-erythro-
furanosyl]adenine sodium salt, or 1-[2-deoxy-4-beta-
(diethylphosphono)methoxy-beta-D-erythrofuranosyl]guanine,
or 1-[2-deoxy-4-beta-(diethylphosphono)methoxy-beta-D-
-erythrofuranosyl]cytosine.
Example 33:
1-(2-Deoxy-4-beta-phosphonomethoxy-beta-D-erythrofuranosyl)-
thymine disodium salt (33)
n
0 N 0
II
(Na0)2 P
CH3
(33)
To a solution of 1-[2-deoxy-4-(diethylphosphono)-
methoxy-beta-D-erythrofuranosyl]thymine (480 mg, 1.3 mmol)
in DMF (2 ml) was added bromotrimethylsilane (2 ml) under
nitrogen. After stirring 3 hours at 25°C, volatiles were
-65-
C
2015671
removed in vacuo and the residue was carefully neutralized
to pH 8.5 by addition~of aqueous saturated sodium
bicarbonate. Water was evaporated to dryness and the
residual solid was purified by a C18 reverse phase column
using water-3% acetonitrile as a eluent to give the title
compound as a white powder: yield 165 mg (40%).
Analysis: Calcd. for C10H13N2~8pNa2 3H20: C, 28.57;
H, 4.52; N, 6.67.
Found: C, 28,48; H, 4.49; N, 6.52.
UV (H20): Amax 268 nm (e = 7,602).
13C-~R (75.47 MHz, D20): d 20.803, 48.289, 50.291,
56.714, 69.570, 94.015, 94.157, 94.994, 122.321, 135.750,
150.715.
1H-NMR (300 MHZ, D20): d 1.853 (s, 3H), 1.924 (dd, J =
2.6, 13.4 Hz, 1H), 2.8=785 (dd, J = 2.6, 5.3, 8.0 Hz, 1H),
3.415 (dd, J = 8.9, 12.4 Hz, 1H), 3.669 (dd, J = 8.9, 12.4
Hz, 1H), 4.372 (d, J = 5.3 Hz, 1H), 4.372 (d, J = 5.3 Hz,
1H), 4.372 (d, J = 5.3 Hz, 1H), 5.310 (s, 1H), 6.285 (dd, J
- 2.6, 8.0 Hz, 1H), 7.776 (s, 1H).
In a manner similar to the above Example 33, the
thymine-containing reactant can be replaced with a
corresponding adenine, guanine or cytosine reactant to
produce 1-(2-deoxy-4-beta-phosphonomethoxy-beta-D-erythro-
furanosyl)adenine disodium salt, or 1-(2-deoxy-4-beta-
phosponomethoxy-beta-D-erythrofuranosyl)-guanine disodium
salt, or 1-(2-deoxy-4-beta-phosphonomethoxy-beta-D-erythro-
furanosyl)cytosine disodium salt.
-66-
C'
2015671
Example 34:
2-Acetamino-6-diphenylcarbamoyloxy-9-[2-(phenylselenyl)
ethoxymethyl]purine (34):
0
OCNPh,
WN
H
Ph S e'~~~ NAc
(34)
A mixture of 2-acetamino-6-diphenylcarbamoylpurine
(36.78, 94.6 mmol) [prepared according to the following
literature procedure: R. Zou and M.J. Ropbins, Can. J.
Chem., 65, No. 6, 1436 (1987)) and N,O-bis(trimethylsilyl)-
acetamide (47.6 ml, 193 mmol) in dry dichloroethane (700 ml)
was heated at 80°C for 60 minutes. Volatiles were removed
in vacuo and the residue was evaporated with toluene twice.
The silylated purine and mercuric cyanide (29.6g, 117 mmol)
in benzene (800 ml) was heated at reflux for 60 minutes,
then a solution of 2-(phenylselenyl)ethoxymethyl chloride
(24g, 94.5 mmol) in benzene (100 ml) was added dropwise.
The mixture was refluxed for 4 hours and then allowed to
stir for 15 hours at 25°C. The reaction was diluted with
CH2C12 (500m1) and quenched with aqueous saturated
bicarbonate (11.). The organic phase was washed with 2N
potassium iodide (200 ml), dried (MgS04) and the solvents
-67-
a
2015671
were removed in vacuo. The residual oil was chromatographed
on silica gel using CH2C12-5% MeOH as an eluent to provide
the title compound as a slightly yellow powder: yield 22g
(39%).
Analysis: Calcd. for C29H25N604Se: C, 57.91, H, 4.36;
N, 13.98. Found: C, 57.76; H, 4.46; N, 13.48.
1H-NMR (300 MHz, CDC13): d 2.459 (s, 3H), 2.951 (t,J
6.9 Hz, 2H), 3.714 (t, J = 6.9 Hz, 2H), 5.477 (s, 2H),
7.07-7.7 (m, 15H), 8.001 (s, 1H), 8.171 (s, 1H).
13C-~R (75.45 MHz; CDC13): d 25.133, 26.196, 69.192,
72.602, 120.363, 126.970, 127.014, 129.174, 141.686,
143.734, 150.247, 152.487, 155.232, 156.247, 156.297,
170.793.
Example 35~ 2-Acetamino-6-diphenylcarbamoyl-9-(yinyloxy-
methyl) purine (35):
0
ii
OCN~a
NAc
t35)
-68-
C
2015611
To a solution of 2-acetamino-6-diphenylcarbamoyl-9-
[2-(phenylselenyl)-ethoxymethyl]purine (4.92g, 8.16 mmol) in
dioxane (80 ml) was added to 30% H202 (4 ml, 35 mmol) and
sodium bicarbonate (2.1g, 24.5 mmol). The mixture was
heated at 60°C for 20 minutes. The reaction was then
concentrated to about lOmL, diluted with ethyl acetate (100
ml), dried (MgS04) and the solvents were removed in vacuo.
The residue was dissolved in dioxane (40 ml),
diisopropylethylamine (1.278, 10 mmol) was added and the
solution was heated at 80°C for 30 minutes under nitrogen.
The solvent was evaporated in vacuo and the residual oil was
chromatographed on silica gel using CH2C12-ethyl acetate
(1:1) as an eluent to give the title compound as a yellowish
powder: yield 2.3g (65%).
Analysis: Calcd. for C23H20N604 O~SH20: C, 60.98; H,
4.67; N, 18.55. Found: C, 61.20; H, 4.76; N, 18.84.
1H-NMR (300 MHz, CDC13): d 2.485 (s, 3H), 4.170 (dd, J
- 2.7, 6.6 Hz, 1H), 44.473 (dd, J = 2.7, 14.1 Hz, 1H), 6.395
(dd, J = 6.6, 14.1 Hz, 1H). 7.0-7.5 (m, lOH), 7.961 (s,
1H), 7,996 (s, 1H).
13C-~R (75.47 MHz, CDC13): d 25.121, 70.572, 92.427,
126.272, 126.344, 126.443, 126.496 " 126.566, 126.644,
141.628, 143.226, 148.925, 152.552, 170.505.
-69-
2015671
Example 36:
2-Acetamino-6-diphenylcarbamoyloxy-9-[(2-hydroxy-
1-(dimethylphonomethoxy)ethoxymethyl]purine (36):
o
OCNPh=
~,~ -N
~NAc
<tle0)2 P ~
H
<36)
To a suspension of 2-acetamino-6-diphenylcarbamoyloxy-
9-(vinyloxymethyl)purine (l.Og, 2.25 mmol) and
dimethylphosphonomethanol (6 ml) in CH2C12 (6 ml) was added
80-85% m-chloroperbenzoic acid (611 mg, 3 mmol). After
stirring for 18 hours at 25°C, the clear solution was
diluted with CH2C12 (100 ml) and washed with ice cold
1N-NaOH (4 ml) and brine (20 ml). The organic phase was
washed again with brine (20 ml), dried (MgS04) and the
solvent was removed in vacuo. The residual oil was
chromatographed on silica gel using using CH2C12-5% MeOH as
an eluent to give the title compound as a colorless oil:
yield 360 mg (27%).
Analysis: Calcd. for C26H29N609P 0.5CH2C12: C, 49.22;
H, 4.64; N, 13.00. Found: C, 49.89; H, 4.47; N, 12.76.
-70-
2015671
1H-NMR (300 MHz, CDC13): b 2.384 (s, 3H), 3.582 (dd, J
- 4.5, 12.5 Hz, 1H), 3.722 (dd, J =4.5, 12.5 Hz, 1H), 3.768
(dd, J = 2.9, 10.7 Hz, 6H), 3.798 (dd, J = 8.9, 14.0 Hz,
1H), 3.994 (dd, J = 8.9, 14.0 Hz, 1H), 4.910 (t, J =4.9 Hz,
1H), 5.649 (d, J = 10.8 Hz, 1H), 5.723 (d, J =10.8 Hz, 1H),
7.0-7.4 (m, lOH), 8.032 (s, 1H), 8.662 (s, 1H).
Example 37: 9[(2-Hydroxy-1-(methylphosphonomethoxy)ethoxy-
methyl]cruanine ammonium salt (37):
0
~N
,i~
fle0 ~ ~NH
2
OJ
+b_
H4N H
C37)
A solution of 2-acetamino-6-diphenylcarbamoyloxy-9[(2-
hydroxy-1-(dimethylphosphonomethoxy)ethoxy)methyl]guanine
(2.9g, 4.8 mmol) in methanol (300 ml) and 28% NH40H (300 ml)
was heated at 60°C for 90 minutes. The solution was
concentrated in vacuo and the residual oil was purified by
C-18 reverse phase column chromatography using water as
eluent under 0.56 bar (8 psi) pressure. The fractions
having ultraviolet fractions were checked with HPLC,
combined and lyophilized to give the title compound as a
white powder: yield 1.15g (65%).
-71-
C
2015671
Analysis: Calcd. for C10H1gN607P H20: C, 31.26; H,
5.51; N, 21.87. Found: C, 31.63; H, 5.43; N, 21.72.
1H-NMR (300 MHz, D20): d 3.549 (d, J = 10.5 Hz, 3H),
3.571 (dd, J = 4.8, 13.2 Hz, 1H), 3.675 (dd, J = 9.3, 13.2
Hz, 1H), 3.4-3.6 (m, 2H), 4.844 (t, J = 3.9 Hz, 1H), 5.573
(d, J = 11.4 Hz, 1H), 5.638 (d, J = 11.4 Hz, 1H), 7.934 (s,
1H).
W (H20): a max 252 nm (e = 13, 871).
Example 38: 9[(2-Hydroxy-1-(phosphonomethoxy)ethoxy)-
methyl]guanine disodium salt (38):
0
NH
NH2
<Na0>z P ~ 0
H
<38)
To a solution of 9-[2-hydroxy-1-(methyl-
phosphonomethoxy)ethoxy)methyl]guanine ammonium salt (780
mg, 2.0 mmol) in dry DMF (20 ml) was added at 5°C
bromotrimethysilllyl (8 ml. 60 mmol) under nitrogen. After
stirring for 3 hours at 5°C, volatiles were removed in vacuo
and the residue was dissolved in aqueous saturated
bicarbonate and re-evaporated in vacuo to a solid.
Purification of this material by C-18 reverse phase column
chromatography using water as eluent under 0.56 bar (8 psi)
pressure and lyophilization of combined fractions gave the
title compound as a white powder: yield 520 mg (62%).
-72-
C
2015671
Analysis: Calcd. for CgH12N507PNa2 2H20: C, 26.04;
H, 3.89; N, 16.87; Found: C, 26.25; H, 4.05; N, 16.89.
W (H20): a max 252nm ( a = 15,150).
1H-NMR (300MHz, D20): d 3.40-3.50 (m, 2H), 3.605 (dd,
J = 5.4, 11.6 Hz, 1H), 3.698 (dd, J =9.0, 11.6 Hz, 1H),
4.877 (t, J = 4.5 Hz, 1H), 5.665 (d, J = 11.3 Hz, 1H), 5.725
(d, J = 11.3 Hz, 1H), 7.999 (s, 1H).
13C-~R (75.47 MHz, D20): d 63.268, 65.832, 67.834,
71.636, 104.561, 104.712, 117.980, 141.823, 153.521,
156.320, 161.129.
Example 39: 1-(4-Methoxytetrahydro-2-furyl)thymine (3A)
0
TfiSO
CH 0 OCH3 ~ SnCl4 ~N
3
+ ~w ~ CH3
TtlSO"NJ
lA 2A 3R
To a suspension of 2.5 g (20 mmol) of dry, powdered
thymine in 30 ml of hexamethyldisilazane was added 50 mg of
ammonium sulfate and 0.5 ml of trimethylsilyl chloride. The
mixture was heated at 140-145° (for 4 hours to obtain a
clear solution). The excess hexamethyldisilazane was
removed at reduced pressure, then the residual white oil was
dissolved in xylene and evaporated in high vacuum to give a
-73-
C
2015671
colorles viscous oil. The crude silyated thimine was
dissolved in 40 ml of dichloroethane and cooled to -30°;
followed by 7.8g (60 mmol) of 2.5 dimethoxytetrahydrofuran.
To this solution was added 2.3 mL of tin tetrachoride via a
syringe over 2 minutes, then stirred for 10 minutes under
nitrogen. The mixture reaction was poured into ice-cold
aqueous NaHC03 (100 ml)-ethylacetate (100 ml). The milky
solution was filtered through celite and the organic layer
was separated and dried over MgS04. Evaporation of the
dried solvents gave a yellow oil which was chromatographed
on Si02 (CH2C12-MeOH) to give 4.3 g (95%) of 3A as a
colorless oil. This oil was a mixture of the two isomers
(cis/trans) in a ratio of 1:1 as seen by analytical HPLC and
1H-NMR.
1H-NMR (CDC13) d 1.4-2.2 (m, 7H), 3.40 and 3.42 (two s, 3H),
5.2 and 5.25 (two broad s, 1H), 6.20 and 6.41 (two q, 1H, J
- 3.5, 7.0 Hz, and 4.0, 7.2 Hz), 7.02 and 7.40 (two s, 1H)
Example 40:
1-(5-Diethylphosphonomethoxytetrahydro-2-furyl)thymine (5A)
0 0
TIISBr ~~ 0
il
3R --~ Br ~CEtO)zP~
<Et0)2PI. OH
4A 5A
-74-
c
2015671
To a solution of 600 mg (2.65 mmol) of 3A (Example 39)
in 15 ml of methylene chloride was added 0.6 ml of
trimethylsilyl bromide and heated at 40-45°C for 10 minutes
under nitrogen. The reaction was evaporated in yacuo to
give 4AW as a yellow oil which was dissolved in 20 ml of
methylne chloride followed by 440 mg (2.60 mmol) of
diethylphosphonomethyl alcohol. This solution was cooled to
-10°C followed by 0.6 ml of triethylamine and stirred for 15
minutes without the cooling bath. After dilution with 40 ml
of ethylacetate, the reaction was washed with water and
brine. Evaporation of the dried (MgS04) solvent gave a
yellow oil which was chromtographed over Si02(CH2CH2-MeOH)
to give 180mg (18.5%) of 5A as a white oil. This oil was a
mixture of the two isomers cis/trans in a ratio of 1:1, as
seen by analytical HPLC and 1H NMR; 1H-NMR(CDC13) d 1.25 (t,
6H, J = 7.0 Hz), 1.95 and 2.0 (two S, 3H), 3.8-4.0 (m,
2H),4.0-4.2 (m, 4H), 5.2 and 5.3 (two broad s, 1H), 6.2 and
6.42 (q,q, 1H, J =3.5, 7.0 Hz and 4.0, 7.2 Hz), 7.0 and 7.4
(two s, 1H).
Example 41: Testing and evaluation of compounds against
herpes virus
A. Plactue Reduction Assa
Herpes simplex virus (HSV) strains were grown and
titered at 37°C in vero cells (African Green Monkey Kidney
cells) and used for~virus work before the tenth passage.
-75-
C
2015671
Cells were grown and maintained in Earle's Minimum
Essential Medium (EMEM), Gibco Laboratories, supplemented
with 0.75% sodium bicarbonate, 2mM 1-glutamine, Pen-strep.
and 5-10% fetal calf serum.
The titer of HSV strains is determined by a plaque
titration method (Roizman and Roane, Virology, 115:75-79,
1961). Tissue culture 24-well petri dishes are seeded with
cells and used for assays when approximately 75% monolayer.
Volumes (O.lml) of logarithmic dilutions of the virus strain
are inoculated onto each of triplicate wells, and absorbed
for one hour with intermittent shaking. The inoculum
thereafter is removed, and 1 ml of 5-10% EMEM containing
0.3% human immune serum globulin is added. After a 48 hour
incubation period at 37°C in a 5% C02 atmosphere, the
overlay medium is removed and the cell sheets stained with
Giemsa stain. The number of plaques is counted, the
triplicate is averaged, and the number of plaque-forming
units per ml is calculated.
The compounds are tested for activity against the
herpes simplex stains using a stock solution of each
compound freshly prepared. Appropriate dilution of each
compound are made in 10% EMEM before usage. The antiviral
efficacy of each compound is determined using the plaque
reduction assay described above. Briefly, tissue culture
24-well plates, with approximately 50 plaque forming units
of HSV per 0.1 ml, and the virus absorbed for 1 hour, with
-76-
n
2015671
intermittent shaking. After removal of the inoculum, 1 ml
of 10% EMEM containing two-fold dilutions of the appropriate
drug are added in triplicates. Triplicate wells/plate
receives no drug and are used as a virus control. After a
48-hour incubation period, at 37°C in a 5% C02 atmosphere,
the overlay medium is removed, the cells are stained as
described above, and plaques are counted. The counts of
triplicate wells are averaged, and the number of plaques in
the presence of each drug dilution are calculated.
The antiviral potency of the drug is determined by
ID50, the drug concentration necessary to reduce the number
of plaques by 50% of those in the virus control cultures.
The results are shown in Table 1 herein below.
Table 1
Antiviral Test REsults'of Compound 8 (see Example 8) against
HSV-1 and HSV-2
ID~"(u
Compound HVS-1 HVS-2
ACV (Acyclovir) 0.5 0.5
Compound 8 2.6 11
Example 42: Comparison of Compound 8 and Acycloyir (ACV) In
Vivo
Groups of ten mice were inoculated intraperitoneally
with from 200 to 600 PFU/0.2m1 of Herpes simplex virus-1
(HL-34 strain). Different doses of test compound were
_77_
C
2015671
administered to separate groups of animals on a BID basis
for five consecutive days commencing three hours after
inoculation. Treatment was by an intraperitoneal route.
The experiment was teminated 21 days post inoculation and
the number of survivals in each group was counted. The mean
survival time (MST, days) was calculated.
The results are shown in Table 2. Compound 8 is
equally as active as ACV.
Table 2
Antiviral Effect of Compound 8 Against HSV-1 Systemic
Infection in Mice
Compound Dose (mg/kg/d) Route Survival
Compound 8 300 i.p. 10/10
100 i.p. 9/10
i.p. 3/10
ACV 200 i.p. 6/7
100 i.p 7/10
Treatment was initiated 3 hours post-infection and was given
BID for 5 consecutive days.
_~8_
C
2015671
Example 43~ Testing andevaluating of compounds against
Murine Retroviruses:
The compounds were evaluated for antiviral activity
against murine leukemia virus (MuLV) strains using the W-XC
plaque assay (Rowe, et al., Virology, 42:1136, 1970).
The MuLV strains were grown in feral mouse cells (SC-1)
and used for antiviral tests using the UV-XC plaque assay.
Briefly, SC-1 cells are grown as monolayers in 4-well tissue
culture plates and inoculated with approximately 50-100
plaque forming units of MuLV in 0.5 ml of 5% EMEM containing
20 ug/ml DEAE/Dextran. After 1 hour adsorbtion, the
inoculum is removed and 5 ml of 5% EMEM containing
three-fold dilutions of the appropriate drug are added.
Five days later, the cultures are UV-irradiated with an
ultraviolet lamp and rat XC sarcoma cells are added to the
cultures. Three-four days after UV-irradiation, the cell
cultures are stained with Giemsa stain and the plaques are
enumerated. Antiviral activity is expressed in terms of the
reduction in the mean number of UX-XC plaques counted in the
drug treated, virus-infected cultures compared with mean
number of plaques counted in untreated, virus-infected
control cultures.
Example 44~ In Vitro Eyaluation Against HIV.
The HIV in vitro assay described as follows was used:
The anti-HIV/LAV activity is measured in cultures of CEM-F
cells. The CEM cells are infected with approximately 30
-79_
C
2015671
TCID50 (50% tissue culture infectious dose of HIV (LAV
strain). The cells are then incubated for 45 minutes at
37°C. The test compounds in culture medium are added at
various concentrations to the infected cells and then
incubated for a further 8 days. After 8 days the antiviral
activity was evaluated in the culture media supernatant for
p-24 gag protein by an enzyme capture assay (ELISA). The
antiviral activity was expressed as the dose that inhibits
50% of the virus expression (ID50 in ug/mL) as detected by
the assay described.
Table 3
Antiviral Test Results of Compound 25 (see Example 25)
against Retroviruses
IDS mL
Compound R-MuLV HIV
AZT 0.05 0.1
Compound 25 0.3 1.8
It will be appreciated that the instant specification
and claims are set forth by way of illustration and not
limitation and that various modifications and changes may be
made without departing form the spirit and scope of the
present invention.
-80-
c
2015671
Example 45
6-N-Pivaloyl-9-(2,3-dideoxy-3.4-didehvdro-Q-D-ervthrofuran-
osyl)-adenine
HNPv
~~ N
J
N N
To a solution of 9-(2,3-dideoxy3,4-didehydro-[i-D-ery-
throfuranosyl)adenine (2.0 g, 10 mmol) [prepared according
to the literature procedure: J. Zemlicka, R. Gasser, J. V.
Freisler, J. P. Horwitz, J. Amer. Chem. Soc., 94, 3213
(1972)] in 1,2-dichloroethane (10 ml) was added pyridine (1
ml), dimethylaminopyridine (170 mg) and pivaloyl chloride
(1.5 g, 12 mmol). The resulting solution was heated at
55-60°C for 6 h under nitrogen. The mixture was then
concentrated in vacuo, taken up in CH2C12 and washed with
water, 20% H3P04 and brine, dried over MgS04, and evaporated
in vacuo. The residual oil was chromatographed on silica
gel using CH2C12-3% MeOH as eluent to give 2 (2.45 g, 85%)
as a white powder.
1H NMR (CDC13) d 1.24 (s, 9H), 2.29 (ddd, J=3.5, 5.0,
17.1 Hz, 1H), 3.33 (dddd, J=2.4, 5.0, 9.4, 17.1 Hz, 1H),
5.24 (dd, J=2.4, 5.O Hz, 1H), 6.41 (dd, J=3.5, 9.4 Hz, 1H),
6.48 (dd, J=2.4, 5.0 Hz, 1H), 8.18 (s, 1H), 8.73 (s, 1H).
-81-
c
2015671
Example 46
6-N-Piyaloyl-9-[2,3-dideoxy-4-S-chloro-3-a-(phenylselenyl)-
S-D-erythrofuranosyl]adenine
HNPv
<',
J
PhSe
To a solution of the product of Example 45 (3.5 g, 12.2
mmol) in CH2C12 (40 ml) was added at -25°C a solution of
phenylselenyl chloride (2.7 g, 14.0 mmol) in CH2C12 (7 ml)
over 5 min under nitrogen. After stirring for 30 min at
-25°C, the solvent was removed in vacuo to give 3 as a
yellow oil. This material was used promptly for the next
reaction:
1H NMR (CDC13) d 1.41 (s, 9H), 2.88 (m, 1H), 3.23 (m,
1H), 4.36 (d, J=6.3 Hz, 1H), 6.29 (s, 1H), 6.80 (dd, J=6.6,
8.4 Hz, 1H), 8.53 (s, 1H), 8.77 (s, 1H).
-82-
C
2015671
Example 47
6-N-Pivaloyl-9-(2,3-dideoxy-4-~-dimethylphosphono)methoxy-
-3-a-(phenylselenyl)-~-D-erythrofuranosyl]adenine
HNPv
0
II
tNeO)p P
To a solution of the product of Example 46
(approximately 12 mmol) and dimethyl hydroxymethylphospho-
nate (16.8 g, 120 mmol) [prepared according to the
literature procedure: D.P. Philion and S.S. Andres,
Tetrahedron Lett. 27, 1477 (1986)] in CH2C12 (15 ml) was
added at -25oC a suspended solution of silver perchlorate
(4.0 g, 20 mmol) in CH2C12 (10 ml) and dimethyl hydroxy-
methylphosphonate (5 ml) over 5 min under nitrogen. The
mixture was allowed to warm to 0°C, stirred for 60 min and
was then poured into CH2C12 (100 ml)-aqueous bicarbonate
(100 ml)-brine (50 ml). The organic phase was separated
after filtration, fried over MgS04 and evaporated. The
residual oil was chromatographed on silica gel using
CH2C12-5% MeOH as eluent to give 4 (3.2 g, 45%) as a
colorless oil:
1H NMR (CDC13) d 1.25 (s, 9H), 2.61 (ddd, J=2.7, 6.6,
14.4 Hz, 1H), 2.96 (ddd, J=6.6, 7.5, 14.4 Hz, 1H), 3.68 (d,
J=11 Hz, 6H), 3.7-4.0 (m, 3H), 5.24 (s, 1H), 8.27 (s, 1H),
8.67 (s, 1H). ,
-83-
C
2015611
Example 48
6-N-Pivaloyl-9-j2,3-dideoxy-2,3-didehydro-4-Q-(dimethylphos-
phono)-methoxy-S-D-erythrofuranosyl]adenine
HNPv
0
II
CneO)2P~0
To a solution of the product of Example 47 (3.3 g, 5.6
mmol) in dioxane (30 ml) was added a solution of sodium
periodate (6.3 g, 30 mmol) in water (30 mll and the
resulting solution was stirred at 23°C for 4 h. CH2C12 (200
ml)was added and the mixture was filtered through celite.
The organic phase was washed with water, brine, dried over
MgS04 and evaporated in vacuo. The residual oil was
chromatographed on silica gel using CH2C12-5% MeOH as eluent
to give 5 (1.4 g, 57%) as a colorless oil:
1H NMR (CDC13) d 1.31 (s, 6H), 3.64 (d, J=10.8 Hz, 3H),
3.71 (d, J=10.8 Hz, 3H), 3.84 (dd, J=8.7, 9.9 Hz, 1H), 5.87
(s, 1H), 6.27 (d, J=6.0 Hz, 1H), 6.34 (dd, J=1.5, 6.0 Hz,
1H), 7.00 (d, J=1.5 Hz, 1H), 8.04 (s, 1H), 8.66 (s, 1H);
13C ~R (CDC13) d 27.524, 40.607, 53.318 (d, J=6.2 Hz),
61.598 (d, J-165 Hz), 86.247, 109.202, 123.406, 130.657,
132.679, 141.966, 150.205, 152.056, 176,547.
-84-
C
2015611
Example 49
9-[2,3-Dideoxy-2,3-didehydro-4-S-(methylphosphono)methoxy-S-
-D-erythrofuranosyl]adenine ammonium salt.
NHZ
~~N
+ _ 0 0
NH40 - P ~
tle0
A solution of the product of Example 48 (330 mg, 0.78
mmol) and sodium methoxide (250 mg, 4.6 mmol) in methanol
(10 ml) was stirred at 23°C for 18 h. The reaction was
carefully neutralized to pH 5.0 by dropwise addition of
2N-HC1 in an ice bath. The pH of the solution was
readjusted to 8.0 by concentrated NH40H and volatiles were
removed in vacuo. The solid residue was purified by C18
reverse-phase column using water as eluent under 0.56 bar
(8 psi) pressure to give 6 (141mg, 49%) as a white
amorphous powder:
1H NMR (D20) d 3.34 (d, J=10.5 Hz, 3H), 3.65 (dd,
J=9.2, 13.2 Hz, 1H), 3.80 (dd, J=9.2, 13.2 Hz, 1H), 5.99 (s,
1H), 6.57 (s, 2H), 6.83 (s, 1H), 8.11 (s, 1H), 8.14 (s, 1H);
13C ~R (D20) 58.542 (d, J=3.0 Hz), 68.483 (d, J-150
Hz), 92.797, 116.319, 125.121, 136.147, 139.719, 147.131,
155.275, 159.781, 162.359;
W max (H20) 260 nm (~ 12,964).
-85-
C
2015671
Anal. Calcd for CH11H13N505 PNa H20: C, 35.97; H, 3.90;
N, 1907.
Found: C, 35.53;.H, 3.71; N, 18.78.
Example 50
9-j2 3-Dideoxy-2 3-didehydro-4-Q-phosphonomethoxv-a-D-
erythrofuranosyl]adenine ammonium salt
NH2
0
~/N~ W N
+ - II
NH40- P ~0
HO
A solution of the product of Example 49 (310 mg, 0.9
mmol) and trimethylsilylbromide (1.0 ml) in DMF (4 ml) was
stirred at 0°C for 3 h under nitrogen. Volatiles were
removed in vacuo and the residue was dissolved in
concentrated NH40H (2 ml). Water was evaporated in vacuo
and the residual solid was purified by C18 reverse-phase
column using water as eluent under 0.56 bar (8 psi) pres-
sure to give 7 (128 mg, 43%) as a white amorphous powder:
UV max (H20) 260 nm (~ 14,982);
1H NMR (D20) b 3.59 (dd, J=9.3, 22.2 Hz, 1H), 3.69 (dd,
J=9.3, 22.2 Hz, 1H), 5.99 (s, 1H), 6.46 (d, J=6.0 Hz, 1H),
6.50 (d, J=6.0 Hz, 1H), 6.83 (s, 1H), 7.87 (s, 1H), 8.13 (s,
1H);
-86-
2015671
13C ~R (D20) d 70.756 (d, J-150 Hz), 93.065, 116.510,
122.434, 136.579, 13 9.835, 147.763, 155.424, 158.990,
161.809.
Anal. Calcd for C10H15N605P 3H20: C, 31.25; H, 5.46; N,
21.87.
Found: C, 31.32; H, 5.85; N, 22.15.
This compound was evaluated for anti-retroviral
activity by the methods described in Examples 43 and 44.
The following results were obtained. AZT controls were run
simultaneously to confirm the validity of the test.
Virus Cell-line Cell-tox. ID50
HIV CEM >100um 45um
MuLV SC-1 >100um <O,lum
HIV MT-4 >500um l.5um
MuLV-R SC-1 >100um O.Olum
Example 51
6-N-Piyaloyl-9-(2,3-dideoxy-4-~-(dimethylphosphono)methoxy
-3-a-iodo-S-D-erythrofuranosyl]adenine
HNPv
WN
0
<tle0>2 P ~0
I
-87-
C
2015611
To a solution of the product of Example 45 (3.5 g, 12.2
mmol) and dimethyl hydroxymethylphosphonate (16.8 g, 120
mmol) in CH2C12 (40 ml) was added portionwise at O°C
N-iodosuccinimide (2.75 g, 12.2 mmol) and the mixture was
stirred for 2 h at O°C. The reaction was diluted with
CH2C12 (50 ml), washed with water, brine, and dried over
MgS04. The residual oil was chromatographed on silica gel
using CH2C12-3% MeOH as eluent to give 8 (4.9 g, 72%) as a
slightly yellow oil.
1H NMR (CDC13) d 1.36 (s, 9H), 2.89 (dd, J=6.3, 9.7 Hz,
1H), 3.20 (dd, J-6.3, 10.3 Hz, 1H), 3.7-3.9 (m, 8H), 4.49
(d, J-5.4 Hz, 1H), 5.50 (s, 1H), 6.86 (t, J=7.2 Hz, 1H),
8.29 (s, 1H), 8.50 (broad s, 1H), 8.74 (s, 1H).
Example 52
6-N-Pivaloyl-9-~2 3-dideoxy-2-3-didehydro-4-Q-(dimethyl-
phosphono)methoxy-a-D-erythrofuranosylladenine
HNPv
~~N
U
II
<He0)2 P ~0
A solution of the product of Example 51 (1.7 g, 3.0
mmol) and 1,8-diazabicyclo [5,4,0] under-7-ene (912 mg, 6.0
mmol) in CHC13 (20 mL) was heated at 65°C for 2 h. The
-88-
C
2015611
reaction was washed with ice-cold 20% H3P04, brine, dried
over MgS04 and evaporated in vacuo. The residual oil was
purified on silica gel using CH2C12-3% MeOH to give 5 (1.2
g, 90%) as a colorless oil. This material was identical
with the product of Example 48.
Example 53
1-f2,3-Dideoxy-4-S-(phosphonomethoxy)-~-D-erythrofuranosyll-
thymine disodium salt
0
CH3
H I
0
ll
<Na0)2P ~0
A mixture of the product of Example 25 (200 mg, 0.57
mmol) and 10% palladium on active carbon (180 mg) in water
(20 ml) was hydrogenated for 30 min. under 2.13 bar (30
psi) HZpressure in the Parr hydrogenator. The catalyst was
filtered through celite with the aid of a water wash.
Water was removed by lyophilization to give the desired
product (205 mg, 100%) as a white amorphous powder:
W max (H20) 268 nm (~ 8844);
1H NMR (D20) 6 1.86 (s, 3H), 2.0-2.4 (m, 4H), 3.41 (dd,
J=8.1, 12.9 Hz, 1H), 3.62 (dd, J=8.1, 12.9Hz, 1H), 5.32 (t,
J-2.7Hz, 1H), 6.24 (t, J=7.0 Hz, 1H), 7.60 (s, 1H);
-89-
2015671
13C ~R (D20) b 18.496, 35.384, 38.041, 72.628, (d,
J=150 H3), 93.249, 113.423, 118.884, 159.494, 174.169,
Anal. Calcd. for C10H13N207 PNa2 1 1/2 H20: C, 31.83;
H, 4.24; N, 7.42.
Found: C, 31.62; H, 3.95; N, 7.28
Example 54
6-N-Pivaloyl-9-[4-S-(dimethylphosphono)methoxy-~-D-erythro-
furanosyl]adenine
NHPv
/ I ~N
0
(I
CtleO)tP~O
HO OH
To a solution of phenylboric acid (860 mg, 7.0 mmol)
and 4-methylmorpholine N-oxide (900 mg, 7.5 mmol)in CH2C12
(20 ml) was added at 23°C osmium tetroxide (25 mg) followed
by the product of Example 52 (2.75 g, 6.4 mmol) in CH2C12
(10 ml). The mixture was stirred for 2h and 10% aqueous
sodium bisulfite (4 ml) was added. After stirring for lh,
the CH2C12 was separated, washed with brine and dried over
MgS04. Evaporation of solvent gave a white oil which was
dissolved in acetone (15 ml) and 1,3-dipropanol (760 mg, 10
mmol). All volatiles were removed in vacuo and the
-90-
C
2015671
resulting oil was chromatographed over silica gel using the
above named compound CH2C12-7% MeOH as eluent to give (2.3
g, 76%) as a white foam:
1H NMR (CDC13) d 1.28 (s, 9H), 3.72 (d, J=10.5Hz, 6H),
3.73 (dd, J=10.6, 13.5Hz, 1H), 3.95 (d, J=10.6, 13.5 Hz, 1H)
4.91 (dd,J=4.5, 6.3 Hz, 1H), 4.33 (d, J=4.5 Hz, 1H), 5.09
(s, 1H), 6.38 (d, J=6.3Hz, 1H), 8.35 (s, 1H), 8.49 (s, 1H),
8.83 (broad s, 1H).
Example 55
9 [4-S-(Methoxyhydroxyphosphinyl)methoxy-~-D-erythrofurano-
syl]adenine ammonium salt
NH2
0
(I
ne0 -P ~0
t
NH40
OH OH
A solution of the product of Example 54 (2.2g, 4.8
mmol) and sodium methoxide (1.49, 26 mmol) in methanol (50
ml) was stirred at 23oC for 7 h under nitrogen. Volatiles
were removed in vacuo and the residual oil was dissolved in
water (20 ml). The aqueous solution was heated at :~5~oc
for 10 h. The reaction was carefully neutralized to pH 5.0
by dropwise addition of 2N-HC1 in an ice bath. The solution
-91-
2015671
was then readjusted to 8.0 with concentrated NH40H and
volatiles were removed in vacuo. The solid residue was
purified by C18 reverse-phase column using water -5% CH3C1
as eluent under 0.56 bar (8 psi) pressure to give 11 (1.3g,
75%)
t1V max (H20) 260 nm (>; 10,019);
1H NMR (D20) 3.56 (d, J-10.5 Hz, 3H), 3.61 (dd, J=10,
12.9 Hz, 1H), 3.83 (dd, J=10, 12.9 Hz, 1H), 4.38 (d, J-11.0
Hz, 1H), 5.0 (dd, J-6.2, 11.0 Hz, 1H), 5.19 (s, 1H);
13C ~R (D20) S 53.897 (d, J-6.0 Hz), 64.192 (d, J-150
Hz), 75.589, 75,909, 111.096, 141,824, 151.079, 154,785,
157.354.
Anal. Calc. for CH11H14N507 p' 1 3/4 NaCl: C, 28.46; H,
3.47; N, 15.08.
Found: C, 28.59; H, 3.43; N, 14.72.
Example 56
9 [4 S (phosphonomethoxy)methoxy-~-D-erythrofuranosvl]-
adenine ammonium salt
NH2
WN
+ 0
NH40-I I
p ~0
HO
OH OH
A solution of the product of Example 55 (1.5 g, 4.1
mmol) and trimethylsilyl bromide (7 ml) in DMF (30 ml) was
stirred at 23~~C fob 6 h under nitrogen. The volatiles
-92-
C
2015671
were removed in vacuo and the residue was dissolved in
concentrated NH40H (3~m1). Water was evaporated in vacuo
and the residual solid was purified by C18 reverse-phase
column using water as eluent to give 12 (600 mg, 40%) as a
white amorphous powder:
W max (H20) 262 nm ( ~ 12,640);
1H NMR (D20) d 3.56 (dd, J=10.0, 12.9 Hz, 1H), 3.79
(dd, J=10.0, 12.9Hz, 1H), 4.31 (d, J=11.0 Hz, 1H), 4.92 (dd,
J=6.0, 11.0 Hz, 1H), 5.17 (s, 1H), 6.08 (d, J=OHz, 1H), 8.24
(s, 1H), 8.25 (s, 1H).
13C ~R (D20) b 66.296 (d, J=157 Hz), 75.881, 76.967,
88.982, 111.148, 119:927, 142.208, 150.689, 153,560,
156.330.
Anal. Calcd. for C10H17N607 P H20: C, 30.05; H, 5.26,
N. 21.03.
Found: C, 30.09; H, 5.06; N, 20.38.
-93-
_. _.~.. _ _. . __. _._.__ _ _ _ ..