Note: Descriptions are shown in the official language in which they were submitted.
CA 02020142 2000-02-24
- 1 -
DERIVATIZED DTPA COMPLEXES
Background of the Invention
The invention relates to novel complexes and complex
salts, agents containing these compounds, their use in
diagnostics and therapy, as well as processes for
preparing these compounds and agents.
Metallic complexes have been scrutinized as early as
at the beginning of the fifties as contrast media for
radiology. The compounds then employed were, however, of
such toxicity that utilization on human patients could
not be considered. It was, therefore, entirely
surprising to find that certain complex salts exhibit
adequate compatibility for considering__routine
administration to human patients for diagnostic purposes.
The first representative of this class of compounds was
the dimeglumine salt of Gd DTPA [gadolinium(III) complex
of diethylenetriaminepentaacetic acid] described in the
European Patent Application, Publication No. 71564, which
proved itself very well in the form of a contrast medium
for nuclear spin tomography. This compound has been
registered, under the name of "Magnevist'", worldwide as
the first NMR diagnostic agent.
- 2 -
Contrast media exhibiting an at least only partial
extrarenal excretion would be desirable, in particular
for patients with limited kidney function.
Consequently, there is a need for NMR contrast media
exhibiting various pharmacokinetic behaviors.
Summary of the Invention
The invention makes such compounds and media
available, and also provides a process for their
production. .-
The compounds according to this invention display
renal elimination as well as excretion with feces.
Surprisingly, elimination via the gallbladder,
however, is not the only extrarenal path of elimination:
in NMR studies on rats, upon intravenous administration
of the compounds of this invention, a contrast
enhancement of the gastrointestinal tract has also been
unexpectedly observed. The kidneys, as well as implanted
tumors, are likewise visualized with improved contrast.
The elimination (secretion) by way of the stomach
has the advantage that demarcation of abdominal
structures (e. g., the pancreas) from the gastrointestinal
tract is made possible, with a simultaneous contrast
enhancement of pathological processes (tumors,
inflammations). Imaging of the renal system, of the
liver and gallbladder, and the bile ducts can moreover
likewise be achieved. Besides the improved visualization
of ulcers and stomach carcinomas, it is also possible
~0~~1~'~
_ 3
to perform studies regarding gastric acid secretion with
the aid of imaging procedures.
Accordingly, by making the compounds of this
invention available, help can be extended to patients
with renal insufficiency as well as patients suffering
from gastrointestinal disorders (at least 10% of the
population in the Western industrial countries).
Most of these patients, as well as a large number of
patients suspected of harboring such disease, must submit
to diagnostic tests. At present, two methods suitable
for this purpose are utilized above all: Endoscopy and
X-ray diagnostics with the aid of barium contrast media.
These tests exhibit various drawbacks: they
carry the risk of radiation stress, cause trauma, are
:L5 connected with inconvenience, occasionally even with risk
to the patient, and thus can evoke psychological stress.
In most instances, these tests must be repeated; their
performance is relatively complicated, require the
patient's active cooperation (e.g. assumption of a
specific bodily attitude) and frequently cannot be
employed in case of frail and high-risk patients.
Provision of novel diagnostic
methods for the identification and localization of
gastrointestinal diseases, which methods do not exhibit
these drawbacks, has thus likewise been attained by tile
complex compounds and agents according to this invention.
Their. pharmacokinetics permits, even without
specific measures, an improvement in the diagnosis of
numerous diseases. The cornplexes for the most: part
are excreted again in unchanged form and rapidly so
that, especial.l.y also in case of using relatively toxic
metallic ions, no damaging effects are observed even at
high dosage.
The practical use of the novel complexes is
also facilitated by their favorable chemical stability.
CA 02020142 2000-02-24
- 4 -
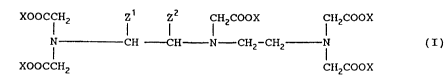
The compounds of this invention are characterized by
general Formula I
XOOCCHZ Z~ ZZ CHZCOOX CIiZCOOX
i CH -CH N-CH2-CHZ i ( I ) ,
XOOCCHZ CIiZCOOX
wherein:
one of Z1 and ZZ is H, and the other is
-(CHZ)m-(C6H9)q-(0)x-(CH2)n-(CsHa)m(O)r-R.
wherein
m and n, independently, are each 0-20,
k, l, q and r are each, independently, 0 or 1,
R is H, C1-C6-alkyl, OR1-substituted C1-C6-alkyl
or CH2COOR1, where R1 is H, C1-C6-alkyl or benzyl;
and
X is, in each case, a hydrogen atom or a metal ion
equivalent of an element of atomic number 21-29, 42, 44 or
57-83;
with the provisos that:
at least two of the substituents X represent a metal
ion equivalent;
when n and 1 each are 0, then k and r are not
simultaneously 1;
at least one of q and 1 is 1;
-(O)r-R is not OH; and
Z1 and ZZ are not -CHZ-C6Hq-O-CHz-COOCH2C6H5 or -CHZ-C6H4-
0- (CHZ) 5-COOCHzC6H5;
or a physiologically-acceptable salt thereof with an
inorganic and/or organic base, an amino acid or amino acid
amide.
CA 02020142 2000-02-24
_ 5 _
If the agent of this invention is intended
for use in NMR diagnostics, then the central ion
of the complex salt must be paramagnetic. These are,
in particular, the divalent and trivalent ions of the
elements of atomic numbers 21-29, 42, 44 and 58-70.
Suitable ions are, for example, the chxomium(III),
manganese(II), iron(II), cobalt(II), nickel(II),
copper(II), praseodymium(III).,neodymium(III),
samarium(III) and ytterbium(III) ions. On account of
p their very strong magnetic moment, the gadolinium(III),
terbium(III), dysprosium(III), holmium(III), erbium(III)
and iron(III)ions are especially preferred.
If the agent of this invention is meant for
X-ray diagnostics, then the central ion must be derived
~5 from an element of a higher atomic number in order to
obtain adequate absorption of the X-rays. It has been
found that suitable diagnostic media for this purpose
are those containing a physiologically compatible
complex salt with central ions of elements of atomic
20 numbers between 21-29, 42, 44, 57-83; these are, for
example, the lanthanum(III) ion and the above-cited
ions of the lanthanide series.
The numbers standing for m and n are prefer-
ably 0 to 5.
25 Suitable as the alkyl substituents R and Rl
are straight-chain or branched hydrocarbons of up to
6, preferably up to 4 carbon atoms which, in case of R,
are optionally substituted by one or several, preferably
1-3, hydroxy or C1-C6-, preferably Cl-C4-alkoxy groups.
~~~~~.4?
- 6 -
Examples that can be cited for optionally
substituted alkyl groups are the methyl, hydroxymethyl,
ethyl, 2-hydroxyethyl, 2-hydroxy-1-(hydroxymethyl)ethyl,
1-(hydroxymethyl)ethyl, propyl, isopropyl, 2- and 3-
hydroxypropyl, 2,3-dihydroxypropyl, n-, sec- and tert-
butyl, 2-, 3- and 4-hydroxybutyl, 2- and 3-hydroxyiso-
butyl, pentyl, 2-, 3- and 4-hydroxy-2-methylbutyl,
2,3,4-trihydroxybutyl, 1,2,4-trihydroxybutyl, cyclo-
pentyl, cyclohexyl, 2,3,4,5,6-pentahydroxyhexyl groups
as well as -- in case of the hydroxyalkyl groups ---
their C~-C6-, preferably C~-C4-alkyl derivatives, i.e., the
corresponding C~_6-alkoxy groups.
Preferred substituents Z~ and ZZ are the
CHZ-C6H4-OCH3, -CHZ-CaHs, -CFiz-C6H4-O-CHZ-C6H4-OCH3.
-CHZ-O-CHZ-CbHs, -CHz-C6H4-O-CHZ-COOH, -CHZ-C:6H4-OCZI-15,
-CHZ-C6H4-OC4H9, -CHz-C6H4-O-CHZ-C6H5 residue:>. Thus, m
preferably is l, and/or q preferably is 1, k and/or r
preferably is 1, etc., and two phenyl rings are
preferably separated by -O-CHz, etc.
In case not all of the acidic hydrogen atoms are
substituted by the central ion, it is possible to replace
one, several, or all remaining hydrogen atoms) by
rations of inorganic and/or organic bases or amino acids.
Suitable inorganic rations are, for example, the lithium
ion, the potassium ion, the calcium ion, the magnesium
ion and, in particular, the sodium ion. Suitable rations
of organic bases are, inter alia, those of primary,
secondary or tertiary amines, such as, for example,
ethanolamine, diethanolamine, morpholine, glucamine, N,N-
dimethyglucamine and, in particular, N-methylglucamine.
Suitable rations of amino acids are, for example, those
of lysine, of arginine, and or ornithine. Suitable
rations of amino acid amides axe lysine methyl amide,
glycine ethyl amide and serine methylamide.
~~2~~.4?
The production of the complex compounds of
this invention in accordance with general Formula I
takes place by converting, in a manner known per se,
compounds of general Formula II
Rz00C iFiz i3 i~ iH2C00R2 'HZCOORz
N C H--C H-N-C H z--C ti Z N ( I I ) ,
RZOOCCIIZ CHZCOORZ
wherein
R2 means an acid blocking group,
Z3 and Z4 each means a hydrogen atom or the residue
- (CH2) m (C6H4) q-OH, with the proviso that
one of the substituents Z3 and Z4 is a hydrogen
atom and the other is the indicated residue,
and m and q are as in formula I
into a compound with the residue indicated for Z1 and Z2,
splitting off the acid blocking groups R2, reacting
I5 the thus-obtained complex-forming acids of general
Formula I where X' is a hydrogen atom (I: ormul.a I' ) with at
least one metal oxide or metal salt of an element of
atomic numbers 21-29, 92, 49 ar 57-83, and subsequently
-- if desired -- substituting any present acidic
?0 hydrogen atoms by canons of inorganic and/or organic
bases, amino acids or amino acid amides.
CA 02020142 2000-02-24
Suitable acid blocking groups R2 are lower
alkyl, aryl and aralkyl groups, e.g. the methyl, ethyl,
propyl, n-butyl, tert-butyl, phenyl, benzyl, diphenyl-
methyl, triphenylmethyl, bis(p-nitrophenyl)methyl
groups, as well as trialkylsilyl groups.
Splitting off of the blocking groups R2 takes
place according to methods known to one skilled in the
art [for example, E. Wtinsch, "Methoden der Org. Chemie"
[Methods of Organic Chemistry] (Houben-Weyl), vol. XV/1,
4th ed., 1974, pp. 315 et seq.), for instance by
hydrolysis, hydrogenolysis or alkaline saponification
of the esters with an alkali in aqueous-alcoholic
solution at temperatures of 0-50° C. Organic or
inorganic acids are used for splitting off the tert-
butyl esters which are especially advantageous for the
present reactions: The ester compound dissolved in a
suitable anhydrous organic solvent, but preferably the
pulverized dry material, is combined either with a
hydrogen halide solution in glacial acetic acid, with
trifluoroacetic acid, or also with boron trifluoride
diethyl etherate in glacial acetic acid and split off
at temperatures of -10° C to 60° C, preferably at room
temperature.
The compounds of general Formula II, serving as
educts for the production of the complex compounds of this
invention, are known or can be synthesized.
A series of literature methods known to a
person skilled in the art is available for reacting
the known aliphatic or aromatic hydroxy compounds to
the corresponding arylalkyl or dialkyl ethers (for
example, J. March, Advanced Organic Chemistry, 3rd ed.,
1985, pp. 342 et seq.).
~o~o.~~~
- 9 -
For this purpose, the compounds of Formula II
wherein R2 stands for an alkali-stable acid blocking
group are dissolved in a polar aprotic solvent, such
as, for example, tetrahydrofuran, dimethoxyethane or
dimethyl su~.foxide, and combined with a base, such as,
for example, sodium hydride, sodium hydroxide or alkali
or alkaline earth carbonates, at temperatures of between
-30° C and the boiling point of the respective solvent,
but preferably between 0° C and 60° C.
A compound of general Formula III is added
to this mixture
Y-(CHL)~-(C6H~)~-(011,-R (III)
wherein Y means a nucleofugal entity, such as, for ex-
ample, C1, I3r, I, CH3-C6II4S03 or CF3S03, and the
remaining indices have the same meanings as in
general Formula I.
The reaction periods are 30 minutes to 8 hours,
depending on the steric hindrance of the residues
participating in the reaction.
As an alternative to the aforedescribed re-
action conditions, it is possible to produce aryl-
alkyl as well as dialkyl ethers in a very advantageous
way by phase transfer catalysis (Stacks and Liotta,
Phase Transfer Catalysis, Academic Press, N.Y. 1978,
pp. 128-138).
For this purpose, the reaction is performed
in a two-phase mixture of an aqueous base, preferably
30~ sodium hydroxide solution, and a water-immiscible
organic aprotic solvent. Suitable phase transfer
catalysts are the compounds known to a person skilled
in the art, but preferably tetraalkylamrrlonium or
tetraalkylphosphonium salts.
20~~~.4~
- ,o -
If it is desired to synthesize compounds of
general Formula t wherein k, n, 1 and r = 0 and R means
a hydrogen atom, then it is possible to conduct the
synthesis in analogy to the methods known from the
literature, starting with the corresponding unsubstituted
amino acid (e. g. phenylalanine).
However, if a series of analogous compounds
is to be synthesized, then it is recommended to prepare
the phenol derivatives described in DOS 3,710,730 and
to reductively remove the phenol function in accordance
with literature methods known to those skilled in the
art. Above all, the reduction of aryl diethyl phosphates
with titanium can be cited which can be performed in a
very advantageous way also in the presence of ester
groups [S.C. Welch et al., J. Org. Chem. 43 . 4797-99
(1978) and literature cited therein]. In this procedure,
the corresponding aryl diethyl phosphate is first
formed from the phenolic educt by reaction with phos-
phoric acid diethyl ester chloride in a 70-100 yield,
preferably by the use of sodium hydride as the base in
a polar aprotic solvent. Subsequently, the reduction
is 'performed with freshly prepared titanium metal.
Preferably, anhydrous titanium(III) chloride is reduced
by magnesium or potassium in anhydrous tetrahydrofuran
under an inert gas for preparing highly active titanium.
The above-described diethyl phosphate is added
to such a mixture and heated under reflux for 2-24 hours,
preferably 6-16 hours. llfte.r the reaction is ter-
rninated, the mixture is optionally worked up by
chromatography. It is also possible to employ the
palladium-catalyzed reduction of the corresponding aryl
triflates according to S. Cacchi et al., Tetr. Lett.
27 . 5541-44 (1986).
2~~fl~4
_ 11 _
The thus-obtained compounds of general
Formula I' wherein X means a hydrogen atom represent
complexing agents. They can be isolated and purified
or can be converted, without isolation, into metal
complexes of general Formula I with at least two of
the substituents X meaning a metal ion equivalent.
The metal complexes of this invention can be
produced in a way disclosed in Patent DE 3,401,052,
by dissolving or suspending tine metal oxide or a metal
salt (e. g. the nitrate, acetate, carbonate, chloride
or sulfate) of the element of atomic numbers 21-~9, 42,
44 or 58-70 in water and/or a lower alcohol (such as
methanol, ethanol or isopropanol) and reacting with a
solution or suspension of the equivalent amount of the
complex-forming acid of general Formula I' wherein X
means a hydrogen atom, preferably at temperatures of
between 40° and 100° C, and subsequently -- if desired --
substituting any present acidic hydrogen atoms of acid
groups by cations of inorganic and/or organic bases,
amino acids or amino acid amides.
Neutralization is herein effected with the
aid of inorganic bases (for example, hydroxides,
carbonates or bicarbonates) of, for example, sodium,
potassium, lithium, magnesium or calcium and/or with
the aid of organic bases, such as, inter alia, primary,
secondary and tertiary amines, e.g. ethanolamine,
morpholine, glucamine, N-methyl- and N,N-di.methyl-
glucamine, as well as basic amino acids, such as, for
example, lysine, arginine and ornithine.
In order to prepare the neutral. complex com-
pounds, it is possible, for example, to add to the
acidic complex salts in an aqueous solution or
suspension such an amount of the desired bases that
~~~~~4~
_ ,Z _
the neutral point is reached. The resultant solution
can subsequently be evaporated to dryness under vacuum.
It is frequently advantageous to precipitate the thus-
formed neutral salts by adding water-miscible solvents,
such as, for example, lower alcohols (methanol, ethanol,
isopropanol, and others), lower ketones (acetone and
others), polar ethers (tetrahydrofuran, dioxane, 1,2-
dimethoxyethane, and others), and to obtain in this way
crystallized products which can be readily isolated
and easily purified. It proved to be especially advan-
tageous to add the desired base as early as during the
complexing to the reaction mixture, thereby saving a
process step.
If the acidic complex compounds contain
several free acidic groups, then it is frequently ex-
pedient to prepare neutral mixed salts containing
inorganic as well as organic cations as the counterions.
This can be done, for example, by reacting
the complexing acid in an aqueous suspension or solu-
tion with the oxide or salt of the element yielding the
central ion and with half the amount of an organic
base needed for neutralization, isolating the thus-
formed complex salt, purifying same if desired, and then
combining same for complete neutralization with the
required amount of inorganic base. The sequence of
adding the bases can also be reversed.
~L'he pharmaceuticals of i~his invention can be
prepared in a likewise conventional way by suspending
or dissolving the complex compounds according to the
invention -- optionally adding the additives customary
in galenic pharmacy -- in an aqueous medium and 'then
optionally sterilizing the suspension or solution.
Suitable additives are, for example, physiologically
acceptable bu:Efers (e. g. tromethamine), small additions
CA 02020142 2000-02-24
- ~3 -
of complexing agents (such as, for example, diethylene-
triaminepentaacetic acid) or, if necessary, electro-
lytes (such as, for example, sodium chloride) or,
if necessary, antioxidants, e.g. ascorbic acid.
If, for enteral administration or other
purposes, suspensions or solutions of the agents of
this invention in water or a physiological saline solu-
tion are desirable, they are mixed with one or several
auxiliary agents) customary in galenic pharmacy
(for example methylcellulose, lactose, mannitol)
and/or tenside(s), e.g. lecithins, "Tween~', "Myrj"
and/or flavoring substances) for taste improvement
(e. g. ethereal oils).
In principle, it is also possible to prepare
the pharmaceuticals of this invention even without
isolation of the complex salts. In any event, special
care must be directed toward effecting the chelate
formation so that the salts and salt solutions accord-
ing to this invention are practically devoid of toxic-
ally active metal ions that are not complexed.
This can be ensured, for example, with the
aid of color indicators, such as xylenol orange, by
control titrations during the manufacturing process.
Consequently, the invention also relates to processes
for preparing the complex compounds and their salts.
The final safety feature resides in purification of
the isolated complex salt.
The pharmaceutical agents of this invention can
be administered to mammals, including humans, in a dose of
1 ~mol/kg to 5 mmol/kg, preferably 10 /~mol to 0.5 mmol/kg
of the complex salt according to the invention. For intra-
venous injection, aqueous formulations are utilized with a
concentration of 50 ~mol/1 to 2 mol/1, preferably 100 mmol/1
to 1 mol/1. Rectal as well as oral administration is
preferably performed with solutions of a concentration
of 0.1 mmol/1 to 100 mmol/1. The volumes administered
are between 5 ml and 2 l, depending on the diagnostic
problem.
The agents according to this invention meet
the variegated prerequisites for suitability as contrast
media. Thus, they are excellently suited, upon enteral
or parenteral administration, to improve the information
content of the image obtained with the aid of the NMR
tomograph, by increasing the signal intensity. They show
furthermore -the high efficacy necessary for burdening the
body with minimum amounts of foreign substances, and
the good compatibility required for maintaining the non-
invasive character of the tests.
The high water solubility and low osmolality
of the agents according to this invention permits the
production of highly concentrated solutions so that the
volume load on the circulation is maintained within
tolerable limits and dilution by body fluids is com-
pensated. Furthermore, the agents of this invention
exhibit not only a high stability in vitro but also a
surprisingly high stability in vivo so that release or
exchange of the --actually toxic -- ions not covalently
bound in the complexes takes place only extremely
gradually within 'the time wherein 'the novel contrast
media are aclain entirely eliminated.
The agents of this invention can also be
utilized for radiation therapy. Thus, complexes of
gadolinium are excellently suited due 'to the large
capture cross section for neutron capture therapy.
If the agent of this invention is intended for use in
the version of radiation therapy proposed by R.h. Mills
et al. [Nature, 336 . 787 (1988)l, then the central
~~~~~.4
- 55 -
.ion must be derived from a MBssbauer isotope, such as,
for example, S~Fe or 151.Eu.
When administered, the agents of this inven-
tion can also be given together with a suitable carrier,
such as, for example, serum or physiological saline
solution andlor together with a protein, such as, for
example, human serum albumin. The dosage herein is
dependent on the type of cellular disorder and on the
properties of the metal complex utilized.
CA 02020142 2000-02-24
- 16 -
Without further elaboration, it is believed that one
skilled in the art can, using the preceding description,
utilize the present invention to its fullest extent. The
following preferred specific embodiments are, therefore,
to be construed as merely illustrative, and not
limitative of the remainder of the disclosure in any way
whatsoever.
In the foregoing and.in the following examples, all
temperatures are set forth uncorrected in degrees Celsius
and unless otherwise indicated, all parts and percentages
are by weight.
2~~~~ 4~
- 17 -
Example 7.
(a) 3,6,9-Triaza-3,6,9-tris(tert-butoxycarbonylmethyl)-
4-(4-methoxybenzyl)undecanedioic Acid
Di-tert-butyl Diester
At 0° C, 1.56 g (2 miLlimoles) of 3,6,9-
triaza-3,6,9-tris(tert-butoxycarbonylmethyl)-4-(4-
hydroxybenzyl)undecanedioic acid di-tert-butyl diester
(Example 9f of DOS 3,710,73U) is combined in tetra-
hydrofuran with 66 mg (2.2 mmol) of 80o strength-
sodium hydride. This mixtrure is combined with
0.31 g (2.2 mmol) of iodomethane and stirred for
30 minutes. Then the solution is combined with water,
tetrahydrofuran is removed by distillation, and the
aqueous emulsion is extracted with diethyl ether.
The organic phase is washed with water, dried over
Na2S04, arid concentrated.
Yield: 1.55 g (97.60)
Calculated: C 63.53 H 9.01 N 5.29
Found: C 63.37 H 8.96 N 5.32
(b) 3,6,9-Triaza-3,6,9-tris(carboxymethyl)-4-
(4-methoxybenzyl)undecanedioic Acid
1.27 g (1.6 mmol.) of the tert-butyl ester
described in Example 1(a) is dissolved in 25 ml of
trifluoroacetic acid and stirred for one hour at room
temperature. 'fhe solution is the~n combined with
diethyl ether, the precipitate is suctioned off,
washed with ether and dried at 40° C under vacuum
over phosphorus pentoxide. 'flue crude product is dis-
solved in water and combined under agitation with
active carbon. The mixture is filtered off from the
carbon and lyophilized three times to remove residual
trifluoroacetic acid.
~~~~~.4~
- 18 -
Yield: 0.62 g (75.40)
Calculated: C 51.46 H 6.09 N 8.18
Found: C 51,27 H 6.02 N 8.11
(c) Gadolinium Complex of 3,6,9-Triaza-3,6,9-tris-
(carboxymethyl)-4-(4-methoxybenzyl)undecanedioic
Acid
513 mg (1 mmol) of the complexing acid
described in Example 1(b) is dissolved in about 30 ml
of water and combined at 80° C with 181 mg (0.5 mmol)
of Gd203. After 30 minutes, the almost clear solution
is filtered and the filtrate freeze-dried.
Yield: 649 mg (97.20 based on the anhydrous
material
Calculated: C 39.57 I-1 4.23 N 6.29 Gd 23.55
Found: C 39.47 Ii 4.29 N 6.21 Gd 23.19
Disodium Salt of the Gadolinium Complex
The complex (500 mg, 0.75 mmol) obtained as
described above is dissolved in 10 times the amount o.f
water and combined by means of a microburette with
1.5 ml of a 1N sodium hydroxide solution.
After freeze-drying, 533 mg of white crystals
is obtained.
'f1 relaxation (1/mmol~sec) is
in water 4.54 ~ U.13
in plasma 6.89 v 0.17
- 19 --
Di-N-methyl-D-glucamine Salt of
the Gadolinium Complex
3.34 g (5 mmo1) of the gadolinium complex is
combined in 40 ml of water in portions with 1.96 g
(10 mmol) of N-methyl-D-glucamine under agitation.
After the base has been completely dissolved, the
product is freeze-dried. There remains 5.55 g of a
colorless crystalline compound.
H20 content (Karl Fischer determination):
4.73%
(d) Europium Complex of 3,6,9-Triaza-3,6,9-tris-
(carboxymethyl)-4-(4-methoxybenzyl)undecanedioic
Acid
5.13 g (10 mmol) of the complexing acid
described in Example 1(b) is dissolved in about 30 ml
of water and combined at 80° C with 1.76 g (5 mmol) of
Eu203. After 30 minutes,the almost clear solution is
filtered and the filtrate freeze-dried.
Yield: 6.62 g
Analysis (based on anhydrous substance)
Calculated: C 39.89 I-I 4.26 N 6.34 Eu 22.94
Found: C 39.71 II 4.38 N 6.17 Eu 22.58
D:isodirzm Salt of the Europium Complex
The complex described above (497 mg, 0.75 mmol)
is dissolved i.n lU times the quantity of water and
combined by means of a microburette with 1.5 ml of a 1N
sodium hydroxide solution. After freeze-drying, 540 mg
of white crystals is obtained.
~fl~~~.4?
- 20 -
Di-N-methyl-D-glucamine Salt of the
Europium Complex
3.31 g (5 mmol) of the europium complex
is mixed in 40 ml of water in portions with 1.96 g
(10 mmol) of N-methyl-D-glucamine under agitation.
After the base has been completely dissolved, the
mixture is freeze-dried. There remains 5.63 g of a
colorless, crystalline compound.
(e) Iron(III) Complex of 3,6,9-Triaza-3,6,9-tris-
1C (carboxymethyl)-4-(4-methoxybenzyl)undecanedioic
Acid
5.13 g (10 mmol) of the complexing acid dis-
closed in Example 1(b) is dissolved in about 30 ml of
water and combined at 80° C with 798 mg (5 mmol) of
Fe203. After 30 minutes, the almost clear solution is
filtered and the filtrate freeze-dried.
Yield: 5.66 g
Analysis (based on anhydrous substance):
Calculated: C 46.66 II 4.98 N 7.42 Fe 9.86
Found: C 46.71 II 5.03 N 7.38 Fe 9.81
Disodium Salt of the Irorr(III) Complex
'i'he complex obtained as described above
(425 mg, 0.15 mmol) is dissolved in 10 times the amount
of water and combined by means of a microburette with
1.5 ml of a 1N sodium hydroxide solution. After freeze-
drying, 460 mg of white crystals is obtained.
- 21 -
Di-N-methyl-D-glucamine Salt of the
Iron(III) Complex
2.83 g (5 mmol) of the iron(III) complex is
combined in 40 ml of water in portions with 1.96 g
(10 mmol) of N-methyl-D-glucamine under agitation.
After the base has been completely dissolved, the
solution is freeze-dried. There remains 4.83 g of a
colorless, crystalline compound.
Analogously, with bismuth oxide, Bi203'
the bismuth complex is obtained as the disodium salt
and, respectively, as the di-N-methyl-D-glucamine salt.
Example 2
(a) 3,6,9-Triaza-3,6,9-tris(tert-butoxycarbonyl
methyl)-5-(4-methoxybenzyl)undecanedioic
Acid Di-tert-butyl Ester
In accordance with the directions given in
Example 1(a), 3.9 g (5 mmol) of 3,6,9-triaza-3,6,9-
tris(tert-butoxycarbonylmethyl)-5-(4-hydroxybenzyl)-
undecanedioic acid di-tert-butyl ester (Example 17d in
DOS 3,710,73U) is reacted to 3.61 g (91~ of theory) of
the title compound.
Calculated: C 63.53 I-I 9.01 N 5.29
Found: C 63.59 II 9.07 N 5.27
(b) 3,6,9-'1'riaza-3,6,9-tris(carboxymethyl)-5
(4-methoxybenzyl)undecanedioic Acid
3.18 g (4 mmol) of the tert-butyl ester
described in Example 2(a) is treated in accordance
with the directions set forth in Example 1(b) with
trifluoroacetic acid and worked up, thus obtaining
1.62 g (79% of theory) of a colorless lyophilized
product.
Calculated: C 51.46 HI 6.09 N 8.18
Found: C 51.34 H 6.14 N 8.11
(c) Gadolinium Complex of 3,6,9-Triaza-3,6,9-tris-
(carboxymethyl)-5-(4-methoxybenzyl)undecanedioic
Acid
According to the directions in Example 1(c),
1.03 g (2 mmol) of the complex-forming acid described
in Example 2(b) is complexed with Gd203, yielding
1.32 g (99~ of theory) of a colorless lyophilized
product.
Calculated: C 39.5'7 H 4.23 N 6.29 Gd 23.55
Found: C 39.51 H 4.19 N 6.25 Gd 23.61
The T~ relaxation ( 1/rmnol ~ sec ) is
in water 4.17 r 0.14
in plasma 6.61 -1~ 0.18
~~~'0~ 4~
- 23 -
Example 3
(a) 3,6,9-Triaza-3,6,9-tris(tart-butoxycarbonylmethyl)-
4-[4-(4-methoxybenzyloxy)benzyl)undecanedioic
Acid Di-tart-butyl Ester
At 0° C, 1.56 g (2 mmol) of 3,6,9-triaza-3,6,9-
tris(tart-butoxycarbonylmethyl)-4-(4-hydroxybenzyl)-
undecanedioic acid di-tart-butyl ester (Example 9f of
DOS 3,710,730) is combined in tetrahydrofuran with
66 mg (2.2 mmol) of 80~ strength sodium hydride.-
To this mixture is added 0.3 ml (2.2 mmol) of 4-
methoxybenzyl chloride and the mixture is stirred
overnight. 'fhe solution is then combined with water,
tetrahydrofuran is removed by distillation, and the
aqueous emulsion is extracted with diethyl ether.
The organic phase is washed with water, dried over
Na2S0~, and concentrated. The resultant colorless oil
is chromatographed on silica gel (ether/hexane 1:1).
Yield: 1.17 g (65'~ of theory) of a
colorless oil.
Calculated: C 65.38 H 8.62 N 4.67
Found: C 65.29 H 8.65 N 4.59
(b) 3,6,9-Triaza-3,6,9-tris(carboxymethyl)-4-[4-
(4-methoxybenzyloxy)benzyl]undecanedioic Acid
1.80 g (2 mmol) of the tart-butyl ester set
forth in Example 3(a) is treated analogously to the
directions given in Example 1(b) with trifluoroacetic
acid and reacted to 905 mg (73$ of theory) of colorless,
flaky lyophilized product.
Calculated: C 56.21 II 6.02 N 6.78
Found: C 56.10 Ii 5.98 N 6.82
~~~~1~~
- 24 -
(c) Gadolinium Complex of 3,6,9-Triaza-3,6,9-tris-
(carboxymethyl)-4-[4-(4-methoxybenzyloxy)benzyl]-
undecanedioic Acid
Analogously to the directions given for
Example 1(c), 620 mg (1 mmolj of the complexing acid
described in Example 3(b) is complexed and worked
up, yielding 758 mg (980 of theory).
Calculated: C 45.01 H 4.43 N 5.43 Gd 20.32
Found: C 44.93 H 4.49 N 5.37 Gd --20.18
The T1 relaxation (1/mmol~sec) amounts to
in water 4.23 ~ 0.16
in plasma 6.99 t 0.13
Example 4
(a) Diethyl Phosphate of 3,6,9-Triaza-3,6,9-tris-
(tert-butoxycarbonylmethyl)-4-(4-hydroxybenzyl)
undecanedioic Acid Di-tent-butyl Ester
11.2 g (14.36 mmol) of the phenol disclosed
in DOS 3,710,730 (Example 9f) is dissolved in 100 ml
of absolute tetrahydrofuran (THF). To this mixture is
added 380 mg (15.8 mmol) of sodium hydride (prepared
from 50'~ NaI-I in paraffin oil by washing three times
with 10 ml of 'fI-IF) . After 30 minutes at room tempera-
ture, 2.60 g (15.0 mmol) of phosphoric acid diethyl
ester chloride is added and the mixture stirred for
24 hours at room temperature.
The solution is diluted with 500 ml of
ether and washed three times with 300 ml of 10~ sodium
hydroxide solution. After drying the: organic phase over.
magnesium sulfate, the product is concentrated under
vacuum and the residue purified by flash chromatography
(eluent: ether/hexane = 1:1).
~~~4?
- 25 -
Yield: 11.97 g (91$ of theory) of a
pale-yellow oil.
Calculated: C 59.00 H 8.58 N 4.59 P 3.38
Found: C 58.88 H 8.63 N 4.63 P 3.30
(b) 3,6,9-Triaza-3,6,9-tris(tert-butoxycarbonyl-
methyl)-4-benzylundeeanedioic Acid Di-tert-butyl Ester
A mixture of 1.33 g (8.62 mmol) of anhydrous
titanium(III) chloride and 1.02 g (26.09 mmol) of
finely chopped potassium in 20 ml of tetrahydrofuran
is heated under reflux in an argon atmosphere for
one hour.
Within 15 minutes, a solution of 11.5 g (12.55
mmol) of the compound described in Example 4(a) in
50 ml of tetrahydrofuran is added dropwise to this
mixture. Then the mixture is heated under reflux for
8 hours, cooled in an ice bath, 20 ml of methanol is
gently added, then 100 ml of water is added, and the
mixture is extracted three times with 200 ml of ether.
The organic phases are dried over magnesium sulfate
and concentrated under vacuum. The residue is
chromatographed on silica gel (eluent: hexane/ether
- 2:1), thus obtaining 8.9 g (93~ of theory) of the
title compound as a colorless oil which crystallizes
upon standing.
Calculated: C 64.46 II 9.10 N 5.50
hound: C 64.54 II 9.15 N 5.41
~fl~~~~~
- 2.6 -
{c) 3,6,9-Triaza-3,6,9-tris(carboxymethyl)-4-
benzylundecanedioic Acid
Analogously to the directions set forth in
Example 1(b), 7.64 g (10 mmo1) of the tert-butyl ester
described in Example 4(b) is reacted to 4.01 g (83% of
theory) of the title compound.
Calculated: C 52.17 II 6.05 N 8.69
Found: C 52.23 II 5.99 N 8.73
(d) Gadolinium Complex of 3,6,9-Triaza-3,6,9-tris-
(carboxymethyl)-4-benzylundecanedioic Acid
2.42 g (5 mmol) of the complex-forming acid
described in Example 4(c) is reacted analogously to
the directions given in Example 1(c) to 3.14 g
(98.5 of theory) of the title compound, obtaining
the gadolinium complex as a colorless, flaky
lyophilized product.
Calculated: C 39.55 II 4.11 N 6.59 Gd 24.66
Found: C 39.47 Ii 4.19 N 6.52 Gd 24.88
The T1 relaxation (1/rnmol.~sec) is
in water. 4.54 ~ 0.13
in plasma 6.89 ~1~ 0.17
Ytterbium Complex of 3,6,9-Triaza-3,6,9-
tr:is(carboxymethyl)-4-benzylundecanedioic
Acid
Analogously to the directions for preparing
the gadolinium complex, the corresponding ytterbium
complex is obtained by using Yb203 in place of
Cd203.
~~~~~ 4)
- 27 __
Example 5
(a) 3,6,9-Triaza-3,6,9-tris(tert-butoxycarbonyl-
methyl)-4-benzyloxymethylundecanedioic Acid
Di-tert-butyl Ester
Within 30 minutes, 7.2 ml (60 mmol) of benzyl
bromide is added dropwise at room temperature to a
thoroughly stirred suspension of 14.1 g (20 mmol) of
4-hydroxymethy 1- 3,6,9-triaza-3,6,9-tris(tort-butoxycarbonylmethyl)-
undecanedioic di-tert-butyl diester described in i)OS
3,710,730 (Example 37d) and 0.3 g of tetrabutylammonium
hydrogen sulfate in 200 ml of dichloromethane/200 ml of
30$ strength sodium hydroxide solution, and the mix-
ture is then agitated for 8 hours.
400 ml of water is added to this suspension;
the organic phase is separated and the aqueous phase
extracted twice with respectively 150 ml of dichloro-
methane. After drying the combined organic phases
over magnesium sulfate, the product is chromatographed
on silica gel (ether/hexane = 1:1), thus obtaining
13.0 g (82~ of. theory) of the title compound as a
colorless oil.
Calculated: C 63.53 fI 9.01 N 5.29
Found: C 63.42 I-I 9.07 N 5.21
(b) 3,6,9-~'riaza-3,6,9-tris(carboxymethyl)-4-
benzyloxymethylundecanedioic Acid
Analogously to the directions given for Ex-
ample 1.(b), 7.94 g (10 mmol) of the tort-butyl ester
set forth in Example 5(a) is reacted with trifluoro-
acetic acid to 4.06 g (79'L of theory) oL 'the title
compound.
~~~~~ 4?
_ 28 _
Calculated: C 51.46 H 6.09 N 8.18
Found: C 51.51 H 6.06 N 8.12
(c) Gadolinium Complex of 3,6,9-Triaza-3,6,9-tris
(carboxymethyl.)-4-benzyloxymethylundecanedioic
Acid
In analogy to the directions in Example 1(c),
2.57 g (5 mmol) of the complexing acid described in
Example 5(b) is reacted to 3.30 g (98.9 of theory)
of the title compound, yielding a colorless, flaky
solid.
Calculated: C 39.57 H 4.23 N 6.29 Gd 23.55
Found: C 39.51 Ii 4.26 N 6.35 Gd 23.27
The 'f1 relaxation (1/mmol~sec) is
in water 4.39 ~ 0.12
in plasma 6.31 ~ 0.15
Example 6
(a) 3,6,9-Triaza-3,6,9-tris(tert-butoxycarbonyl-
methyl)-4-(4-carboxymethoxybenzyl)undecanedioic
Acid Bis(tert-butyl) Ester
At 0° C, 2:3.40 g (30 mtnol) of 3,6,9-triaza-
3,6,9-tris(tort-butoxycarbonylmethyl)-4-(4-hydroxy-
benzyl)undecanedioic acid di-tert-butyl ester (Ex-
ample 9f of UOS 3,710,730) i.s combined in tetrahydro-
furan with 2.7 cJ (90 mmol) of 8U'k strength sodium
hydride. 'fo this mixture is dropped 6.25 g (45 mmol) of
bromoacetic acid in tetrahydrofuran, and the mixture
is stirred for one hour at 0° C and overnight at room
temperature.
The solution is then combined with water,
tetrahydrofuran is removed by distillation, and the
2~~~~.4?
_. 2 g _
aqueous phase is extracted with ethyl acetate. The
organic phase is dried over sodium sulfate and con-
centrated.
The residue is chromatographed on silica gel in
an eluent mixture of dioxane/methanol/tri.ethylamine
(15:4:1); the combined fractions are concentrated and
divided between ethyl acetate and 1N citric acid.
The organic phase is then dried over sodium sulfate
and concentrated, thus obtaining 21.8 g (87~ of theory)
as a colorless oil.
Calculated: C 61.63 H 8.54 N 5.01
Found: C 61.62 H 8.62 N 4.95
(b) 3,6,9-Triaza-3,6,9-tris(carboxymethyl)-4-
(4-carboxymethoxybenzyl)undecanedioic Acid
Analogously to the directions given for Ex-
ample 1(b), 21.0 g (25 mmol) of the tert-butyl ester
described in Example 6(a) is reacted to 11.0 g (78.9
of theory) of the title compound.
Calculated: C 49.55 H 5.60 N 7.54
Found: C 49.31 II 5.51 N 7.47
(c) Gadolinium Complex of 3,6,9-'Priaza-3,6,9-tris-
(carboxymethyl)-4-(4-carboxymethoxybenzyl)-
undecanedioic Acid
5.57 g (10 mmo:L) of the complex-forming acid
described in Example 6(b) is .reacted analogously to
the dire ctions set forth in Example 1(c) to yield
7.01 g (98.5' of theory) o.f the title compound.
Calculated: C 38.87. Ii 3.96 N 5.90 Gd 22.09
Found: C 38.75 II 3.89 N 5.97 Gd 21.93
- 30 --
The T1 relaxation (1/mmol~sec) is
in water 5.00 ~ 0.01
in plasma 7.10 ~ 0.08
Example 7
Preparation of a Solution of the Sodium Salt of the
Gadolinium(III) Complex of 3,6,9-Triaza-3,6,9-tris-
(carboxymethyl)-4-benzyloxymethylundecanedioic Acid
6.68 g (10 mmol) of the gadolinium complex
obtained according to Example 5(c) is dissolved in
70 ml of water pro injectione (p.i.) and combined
dropwise with 1N sodium hydroxide solution until a
pH of 7.2 has been reached. After adding 0.02 g of
tromethamine, the mixture is filled up to 100 ml with
water p.i.; the solution is dispensed into bottles and
heat-sterilized.
Example 8
(a) 3,6,9-T.riaza-3,6,9-tris(tert-butoxycarbonyl-
methyl)-4-(4-ethoxybenzyl)undecanedioic Acid
Di-tert-butyl Diester
At U° C, 5.85 g (7.5 mmol) of 3,6,9-triaza-
3,6,9-tris(tart-butoxycarbonylmethyl)-4-(4-hydroxy-
benzyl)undecanedioic acid di-tart-butyl diester
(Example 9f of DOS 3,710,730) is combined in 100 ml
of tetrahydrofuran with 0.30 g (10 mmol) of 80~
strength sodium hydride. 'fo this mixture is added
1.56 g (10 mmol) of iodoethane and the mixture is
stirred for 3 hours. 'Then the solution is combined
with water, tetrahydrofuran is distilled off, and the
aqueous emulsion is extracted with diethyl ether.
The crude product obtained after drying over sodium
~~%~~~?
- 31 -
sulfate and concentration of the solvent is chromato-
graphed on silica gel (system: hexane/ether/triethyl-
amine 70:30:5).
Yield: 4.0 g (660)
Analysis (based on anhydrous material):
Calculated: C 63.91 H 9.11 N 5.20
Found: C 63.67 H 9.05 N 5.28
(b) 3,6,9-Triaza-3,6,9-tris(carboxymethyl)-4-
(4-ethoxybenzyl)undecanedioic Acid
3.64 g (4.5 mmol) of the tert-butyl ester
disclosed in Example 8(a) is dissolved in 25 ml of
trifluoroacetic acid, stirred for one hour at room
temperature, and worked up analogously to Example 1(b).
Yield: 1.2 g (50.60
Analysis (based on anhydrous substance)-
Calculated: C 52.36 H 6.13 N 7.97
Found: C 52.21 H 6.39 N 7.84
(c) Disodium Salt of the Gadolinium Complex of
3,6,9-Triaza-3,6,9-tris(carboxymethyl)-4-
(4-ethoxybenzyl)undecanedioic Acid
528 mg (1 mmol) of the complex-farming acid
described in t1e preceding example is dissolved in
40 ml of water and complexed at 80° C with 181 mg
(0.5 mmol) of Gd203. 'then the mixture is neutralized
with 2 ml of 1N Na0Il, stirred with activated carbon,
filtered, and the filtrate is freeze-dried.
Yield: 700 mg (96.5$)
~0~~~.~?
- 32 -
Analysis (based on anhydrous mater_ial):
Calculated: C 38.06 II 3.89 Gd 21.67 N 5.79 Na 6.34
Found: C 37.91 H 3.99 Gd 21.30 N 5.69 Na 6.57
The Tl relaxation (1/mmol~sec) is
in water 5.33 ~ 0.13
in plasma 8.69 ~ 0.53
Analogously, the corresponding europium
complex is obtained with europium oxide, Eu203.
Calculated: C 38.34 H 3.92 Eu 21.09 N 5.83 Na 6.38
Found: C 38.20 H 4.01 Eu 20.87 N 5.79 Na 6.49
With iron oxide, Fe203, 'the corresponding iron
complex is obtained analogously:
Calculated: C 44.25 H 4.52 Fe 8.95 N 6.73 Na 7.37
Found: C 44.17 H 4.59 Fe 8.52 N 6.81 Na 7.49
Example 9
(a) 3,6,9-Triaza-3,6,9-tris(tert-butoxycarbonyl-
methyl)-4-(4-butoxybenzyl)undecanedioic Acid
Di-tert-butyl Diester
Analog ousl.y to Example 8(a), 5.85 g
(7.5 mmol) of 3,6,9-triaza-3,6,9-tris(tert-butoxy-
carbonylmethyl)-4-(4-hydroxyberxzyl)undecanedioic acid
di-tert-butyl diester (Example 9f of DOS 3,710,730) is
reacted with :1.84 g (:LO nut~ol) of 1-iodobutane and
worked up as described therein.
Yield: 4.1 g (65.40
- 33 -
Analysis (based on anhydrous compound):
Calculated: C 64.64 H 9.28 N 5.03
Found: C 64.82 H 9.37 N 4.96
(b) 3,6,9-Triaza-3,6,9-tris(carboxymethyl)-4-
(4-butoxybenzyl)undecanedioic Acid
3.34 g (4 mmol) of the teat-butyl ester
described in Example 9(a) is dissolved in 20 ml of
trifluoroacetic acid, stirred for one hour at room
temperature, and worked up analogously to Example 1(b).
Yield: 1.36 g (6l.Oo)
Analysis (based on anhydrous material):
Calculated: C 54.04 I-I 6.71 N 7.57
Found: C 53.88 I-I 6.63 N 7.41
(c) Disodium Salt of the Gadolinium Complex of
3,6,9-Triaza-3,6,9-tris(carboxymethyl)-4-
(4-butoxybenzyl)undecanedioic Acid
556 mg (1 mmol) of the complexing acid
described in the preceding example is combined with
40 ml of water and complexed at 80° C with 181 mg
(0.5 mmol) of Gd203. The mixture is then neutralized
with 2 ml of 1N NaOH, stirred with activated carbon,
filtered, and 'the filtrate freeze-dried.
Yield: 711. mg (94.30
Analysis (based on anhydrous material):
Calculated: C 39.83 II 4.28 Gd 20.86 N 5.58 Na 6.10
Found: C 39.61 I-I 4.35 Gd 20.51 N 5.49 Na 6.17
~~.~r~
- 34 -
The T1 relaxation (1./mmol~sec) is
in water 5.80 ~ 0.26
in plasma 14.20 ~ 0.98
Analogously, with the use of europium oxide,
Eu203, the corresponding europium complex is obtained:
Calculated: C 40.11 H 4.31 Eu 20.30 N 5.61 Na 6.14
Found: C 39.97 H 4.39 Eu 20.02 N 5.72 Na 6.25
With iron oxide, Fe203, the corresponding
iron complex is analogously obtained:
Calculated: C 46.03 H 4.94 Fe 8.56 N 6.44 Na 7.05
Found: C 45.88 H 5.03 Fe 8.30 N 6.50 Na 7.11
Example 10
(a) 3,6,9-Triaza-3,6,9-tris(tert-butoxycarbonyl-
methyl)-4-(4-benzyloxybenzyl)undecanedioic
Acid Di-tert-butyl Diester
Analogously to Example 8(a), 5.85 g
(7.5 mmol) of 3,6,9-triaza-3,6,9-tris(tert-butoxy-
carbonylmethyl)-4-(4-hydroxybenzyl)undecaned.ioic
acid di-tert-butyl diester (Example 9f of DOS
3,710,730) is reacted with 1.71 g (10 mmol) of
benzyl bromide and worked up as described therein.
Yield: 4.9 g (75.10
Analysis (based on anhydrous substance):
Calculated: C 66.25 HI 8.69 N 4.83
E'ound: C 66.14 II 8.77 N 4.83
- 35 -
(b) 3,6,9-Triaza-3,G,9-tris(carboxymethyl)-4
(4-benzyloxybenzyl)undecanedioic Acid
3.48 g (4 mmol) of the tert-butyl ester
disclosed in Example 10(a) is dissolved in 20 ml of
trifluoroacetic acid, stirred for one hour at room
temperature, and worked up analogously to Example 1(b).
Yield: 1.33 g (56.50)
nnalysis (based on anhydrous material):
Calculated: C 57.04 H 5.98 N 7.13
Found: C 56.89 H 6.03 N 7.21
(c) Disodium Salt of the Gadolinium Complex of
3,6,9-Triaza-3,6,9-tris(carboxymethyl)-4-
(4-benzyloxybenzyl)undecanedioic Acid
590 mg (1 mmol) of the complexing acid
described in the preceding example is combined with
40 ml of water and 1 ml of 1N NaOH and complexed at
80° C with 181 mg 40.5 mmol) of Gd203. Then the
mixture is neutralized furthermore with 1 ml of 1N
NaOH, stirred with active carbon, filtered, and the
filtrate freeze-dried.
Yield: 703 mg (89.20
Analysis (based on anhydrous material):
Calculated: C 42.69 I-I 3.84 Gd 19.96 N 5.33 Na 5.84
Found: C 42.63 Ii 3.91 Gd 19.57 N 5.26 Na 5.99
The T1 .relaxation (1/mmol~sec) is
in water 5.81 ~ 0.11
in plasma 16.35 ~ 1.01
~~~~3~.4~
- 36 -
the corresponding europium complex is
obtained analogously with europium oxide, Eu203:
Calculated: C 42.98 H 3.86 Eu 19.42 N 5.37 Na 5.88
Found: C 43.10 H 3.91 Eu 19.13 N 5.27 Na 5.99
With iron oxide, Fe203, the corresponding
iron complex is obtained analogously:
Calculated: C 48.99 H 4.41 Fe 8.14 N 6.12 Na 6.70
Found: C 48.73 II 4.57 i'e 8.29 N 6.03 Na 6.85
CA 02020142 2000-02-24
- 37 -
Examples for in vivo NMR Diagnostics
Example 1
Images were obtained at various times after
administration of the disodium salt of the gadolinium
complex of Example 1(c) to rats with the aid of an
NMR tomograph by General Electric, specifically
developed for animal experimental research.
Spin echo scans were made with the NMR
tomograph (CSI 2 T) at 2 tesla (TR time of 400 ms and
TE time of 20 ms). The layer thickness of this
Tl-weighted imaging sequence was 3 mm; the image
matrix was 128 x 128.
The contrast medium was administered intra-
venously into a caudal vein of a male hairless rat
(Lew/Mol) weighing 190 g, in a dose of 0.06 mmol/kg.
The animal had a Brown Pearce tumor in the thigh and
was anesthetized for the study by means of an intra-
muscular administration of "Ketavet"/"Rompun".
Various dark structures are visible in the
abdomen in the coronary blank scan (baseline, No. 1).
No differentiation was possible between.intestinal
lumen and stomach.
One minute after administration (No. 2), the
first enhancement is already apparent in the urinary
bladder. A strong increase in contrast is visible in
the stomach 45 minutes after injection (No. 3). A
good visualization of the tumor (at the level of the
reference tube), of the urinary bladder, and of the
stomach can be observed 60 minutes after injection
(No. 4). Moreover, contrasting of the intestine can
likewise be observed. This makes it possible to
distinguish among intestinal loops, fat, as well as
lymphatic nodes (lymphomas). Contrasting of the
CA 02020142 2000-02-24
- 38 -
renal pelvis is also striking; this image can be
even more improved 65 minutes after injection in a
somewhat different layer (No. 5). In Figure 6,
180 minutes after injection, the contrast enhancement
is likewise clearly recognizable in an axial scan in
the zone of the liver. This makes it possible to
differentiate among the stomach, the liver, the
duodenum, and the pancreas.
Example 2
The test animals were female rats of the
strain Lew/Mol weighing 160-180 g. Prior to imaging,
the animals were anesthetized ("Rompun' + "Ketavet~')
and provided with a catheter in the caudal vein to
administer the contrast medium. Imaging took place
in an MRI experimental device by General Electric
(field strength 2 tesla). First of all, the images
(7, 9, 11) were made without contrast medium with a
Tl-weighted spin echo sequence (TR = 400 msec, TE =
msec, axial section plane, layer thickness 3 mm).
20 The liver appears in each case with the normal signal
intensity; the stomach is darker in tendency than the
liver. In case of animal 1, the stomach exhibits, in
part, a rather high signal intensity. This is due to
feed residues, the feed containing manganese in
relatively high concentrations (at the time of
the test, the animals had been fasting for 6 hours). Ani-
mal 3 had been implanted with an osteogenic sarcoma
three weeks previously; this sarcoma was of equal
contrast in the blank image and could not be defined.
The administration of contrast medium took place via
the venous catheter with a dose of 0.1 mmol Gd/kg
(concentration of the solutions 0.05 mmol Gd/ml in
0.9o NaCl) for all 3 compounds.
2~~~~ 4j
- 39 -
A marked enhancement of the liver can be found
for all 3 compounds after 90 minutes [Figure 8,
Example 8(c)] and, respectively, after 60 minutes have
elapsed upon administration [Figure 10, Example 9(c);
Figure 12, Example 10(c)]; this is due to uptake by
the hepatocytes and cannot be observed at this point in
time after administration with the contrast medium
for NMR tomography, "Magnevist", heretofore the sole
contrast medium available on the market. In case of
animal 3 [Figure 12, Example 10(c)], the tumor is now
additionally clearly visible, which has not absorbed
the contrast medium at all, or only to a lesser propor-
tion.
Furthermore, all compounds -- most strongly in
case of Example 10(c), least in case of Example 8(c) --
show great enhancement of the stomch. This offers
additional diagnostic possibilities in view of an improved
distinction of liver and stomach.