Note: Descriptions are shown in the official language in which they were submitted.
2~2~8~2
HOECHST AKTI~NGESELLSCHAFT HOE 89/F 245R Dr.WI/rh
Description
Renin-inhibiting amino oligohydroxy derivatives
Amino diol derivatives having a renin-inhibiting action
are known from European Patent Applications EP-A 184,855,
189,203, 202,577, 229,667, 230,266 and 237,202 and
International Patent Application WO 87/05302.
Renin-inhibiting amino diol derivatives are furthermore
described in Biochem. Biophys. Res. Commun. 132, 155 -
161 (1985), in Biochem. Biophys. Res. Commun. 146, 959 -
963 (1987), in FEBS Lett. 230, 38 - 42 (1988) and in J.
Ned. Chem. 30, 976 - 982 (1987).
Surprisingly, it has now been found that those compounds
which differ from the compounds described in the docu-
ments mentioned in that they contain additional hydroxyl
functions on the C terminus are highly active renin
inhibitors in vitro and in vivo and have advantageous
properties compared with the known compounds.
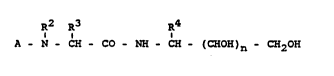
The invention relates to compounds of the formula I
R2 R3 R4
A - N - CH - CO - NH - CH - (CHOH)n - CH2OH (I)
in which
A denotes a radical of the formula II, III or IV
R6 R5 O
Rl _ N - CH - C - (II)
R7 R5 O
Rl _ CH - CH - C - (III)
R5 O
Rl - O - CH - C - (IV)
2 ~ 2 ~
R1 al) denotes (C3-C8)-cycloalkyl, (C4-C1O)-bicycloalkyl,
(C8-Clz)-tricycloalkyl, (c3-c8)-cycloalkyl-(cl-cB)-
alkyl, (C4-C10)-bicycloalkyl-(Cl-C6)-alkYl, ( CB_
C12)-tricycloalkyl-(C1-C6)-alkyl, (C3-C8)-cyclo-
alkylcarbonyl or (C3-C8)-cycloalkylsulfonyl, in
which the cycloalkyl, bicycloalkyl and tricyclo-
alkyl substituent in each case can be substituted
by one or two identical or different radicals
from the series comprising F, Cl, Br, I,
hydroxyl, (Cl-C6)-alkoxy, (Cl-C6)-alkyl, carboxyl,
(Cl-C6)-alkoxycarbonyl,carbamoyl,carboxymethoxy,
amino, (Cl-C6)-monoalkylamino, (Cl~c6)~
dialkylamino, amino-(Cl-C6)-alkyl, (C1-C6)-alkyl-
amino-(Cl-C6)-alkyl,di-(Cl-C6)-alkYlamin-(C1-C6)-
alkyl, amidino, hydroxyamino, hydroximino,
hydrazono, imino, guanidino, (Cl-C6)-alkoxy-
sulfonyl,(C1-C6)-alkoxysulfenyl,trifluoromethyl,
(C1-C4)-alkoxycarbonylamino and (C6-C12)-aryl_
( C1_C4 )-alkoxycarbonyl-amino; hydroxyl,
hydrogen, (Cl-ClB)-alkyl, ( C1_CB ) -alkoxy, (C1-C13)-
alkanoyl, (Cl-Cl8)-alkox~carbonyl, (C1-C1B)-alkyl-
sulfonyl or (Cl-Cl8)-alkylsulfinyl, in which the
alkyl radicals in each case can be substituted by
optionally protected amino, trimethylsilyl,
hydroxyl, mercapto, (Cl-C4)-alkylthio, halogen,
(C1-C4)-alkoxy, mono- or di-(Cl-C8)-alkylamino,
carboxyl,carbamoyl, guanidino,(Cl-C4)-alkoxycar-
bonyl, ( C1_CB ) -a1kanOY10XY~ phenyl-(Cl-C4)-alkoxy
or a radical CONR8R~;
(C6-Cl4)-aryl, (C8-Cl~)-aryl-(Cl-C4)-alkyl or (C6-
Cl4)-aryl-(C1-C4)-alkoxy, in which the aryl radical
in each case can be substituted by one, two or
three identical or different radicals from the
series comprising (Cl-C6)-alkyl, amino, mono- or
di-(Cl-C4)-alkylamino, amino-(Cl-C4)-alkyl,
hydroxy-(Cl-C4)-alkyl, mono- or di-(Cl-C4)-alkyl-
amino-(Cl-C4)-alkyl, hydroxyl, (Cl-C4)-alkoxy,
haloqen, formyl, ~Cl-C4)-alkoxycarbonyl,
2 3 2 1 ~ 2 ~
-- 3 --
carboxamido, mono- or di-(C1-C4)-alkylamino-
carbonyl and nitro;
Het or Het-~Cl-C4)-alkyl, in which Het repre~ents
a 5-, 6- or 7-membered heterocyclic ring which
can be additionally benzo-fu~ed an~ either
aromatic, partly hydrogenated or completely
hydrogenated and contains one or two identical or
different radicals from the series compri~ing N,
O, S, NO, SO and SO2 as the hetero element and can
be substituted by one or two identical or dif-
ferent radicals from the series comprising
(Cl-C4)-alkyl, (Cl-C4)-alkoxy, (Cl-C4)-alkoxy-
carbonyl, hydroxyl, halogen, amino, amino-(Cl-C4)-
alkyl and mono- or di-(C1-C4)-alkylamino, or a
radical NRaR9, in which
R8 and R~ are identical or different and independ-
ently of one another denote hydrogen; (C1-C8)-
alkyl, which can be substituted by amino,
~Cl-C4)-alkylamino, di-(C1-C4)-alkylamino,
hydroxyl or (Cl-C4)-alkoxy; or (C3-C~-cyclo-
alkyl; mercapto; (C1-C4)-alkylthio;phenylthio;
~C1-C4)-alkoxycarbonyl; carboxyl or ( C6-C14 ) -
aryl, which can be substituted in the aryl
radical as described above; or Het or Het-
~C1-C4)-alkyl, in which Het is defined as
described above, or in which
R8 and R~, together with the nitrogen atom carry-
ing them, form a S- to 12-membered ring which
can be mono- or bicyclic, can al80 contain 1
or 2 nitrogen atoms, 1 sulfur atom or 1 oxygen
atom as further ring members and can be
substituted by (Cl-C4)-alkyl,
or
R1 a2) denotes a radical of the formula V
R1 - W (V)
in which R1 is defined as Rl under al) and W
-- 4 --
xepresents -CO-/ ~CS , -O-CO-, SO2-, -SO , -NH
-SO2-, -NH-CO-, -CH(OH)- or -N(OH)-;
R' and R5, together with ~he nitrogen atom carrying
them, form a 5- to 12-membered ring which can be
S mono- or bicyclic, aromatic, partly hydrogenated or
completely hydrogenated, and can also contain, as
further ring members, 1 ox 2 nitrogen atoms,
sulfur atom or 1 oxygen atom and can be substituted
by (Cl C4)-alkyl,
R2 and R6 independently of one another denote hydrogen
or (Cl C4)-alkyl,
R3 and R5 independently of one another are defined as
under al);
R4 denotes (C3-Cl2)-alkyl; mono-, bi- or tricyclic (C3-
C18 ) -CyC loalkyl, ( C3-c 18 ) -cyc loalkylmethyl or ( C3-Cl8 ) -
cycloalkylethyl, in which the cycloalkyl part is
optionally substituted by (C1-C6)-alkyl; dithiolanyl;
( C6-C14 ) -arylmethyl; dithiolanylmethyl; dithiolanyl-
ethyl; dithianyl; dithianylmethyl or dithianylethyl;
R7 is hydrogen or (Cl-C8)-alkyl, or together with Rl or R5
and the atoms carrying these, forms a mono~ or bi-
cyclic, saturated or partly unsaturated ring system
having 5 - 12 ring members, which, in addition to
carbon, can also contain 1 ~ulfur atom, which can
optionally be oxidized to ~ulfoxide or sulfone;
and
n denotes 2 - 10,
and physiologically tolerated salts thereof.
The chirality centers in the compounds of the formula I
can have the R, S or R-S configuration.
Alkyl can be straight~chain or branched. The same applies
to radicals derived ther0from.
h J "; j_ ( ! ¦,, v
-- 5 --
(C~-C8)-Cycloalkyl is under~tood as meaning cyclopropyl,
cyclobu~yl, cyclopentyl, cyclohexyl, cycloheptyl or
cyclooctyl.
(C4-C10)-Bicycloalkyl or (C8-Cl2)-tricycloalkyl are under-
s~ood as meaning an isocyclic aliphatic non-aromatic
radical which can optionally contain unsymmetrically
distribu~ed double bonds and can optionally also be
substituted by open-chain aliphatic side chains~ The two
or three rings as components of such a radical are
condensed or spiro-linked and linked via a ring carbon
atom or a side chain carbon atom. Examples of these
radicals are bornyl, norbornyl, pinanyl, norpinanyl,
caranyl, norcaranyl, ~hujanyl, adamantyl, bicyclo(3.3.0)-
octyl, bicyclo(l.l.O~butyl and spiro(3.3)heptyl sub-
stituents.
If the rings mentioned carry more than one substituent,these can be either cis or trans to one another.
(C6-C14)-Aryl is,forexample, phenyl, naphthyl, biphenylyl
or fluorenyl; phenyl is preferred. The ~ame applies to
2~ radical~ deriv~d therefram, such a6, for example, ary--
oxy, aroyl, aralkyl and aralkyloxy. Axalkyl is underætood
as meaning an unsubstituted or substituted aryl radical
linked to alkyl such as, for example, benzyl, ~- and ~-
naphthylmethyl, halobenzyl and alkoxybenzyl, but aralkyl
would not be limited to the radical6 mentioned.
A radical Het in the contex~ of the above definition is,
for example, pyrrolyl, furyl, thienyl, imidazolyl,
pyrazolyl, oxazolyl, thiazolyl, pyridyl, pyrazinyl,
pyrimidinyl, indolyl, quinolyl, isoquinolyl, quinoxal-
inyl, ~-carbolinyl or a benzo-fused ox cyclopenta ,
cyclohexa- or cyclohepta-fused derivative of these
radicals. This heterocyclic radical can be substituted on
a nitrogen atom by oxido, (Cl-C6)-alkyl, for example
methyl or ethyl, phenyl or phenyl-(Cl-C4)-alkyl, for
~2 ~ $2~
-- 6 --
example benzyl, and/or on one or more carbon atoms by
( C~-C4 )-alkyl, for example methyl, phenyl, phenyl-(Cl-C4~-
alkyl, for example benzyl, halogen, for example chlorine,
hydroxyl, (Cl-C4)-alkoxy, for example methoxy, phenyl-
(C1-C4)-alkoxy, for example benzyloxy, or oxo and partly
saturated and is, for example, 2- or 3-pyrrolyl, phenyl-
pyrrolyl, for example 4- or 5-phenyl-2-pyrroly~, 2-furyl,
2-thienyl, 4-imidazolyl, methyl-imidazolyl, for example
l-methyl-2-, -4- or -5-imidazolyl, 1,3-thiazol-2-yl, 2-,
3- or 4-pyridyl, 1-oxido-2-, -3- or -4-pyridyl, 2-pyraz-
inyl, 2-, 4- or 5-pyrimidinyl, 2-, 3- or 5-indolyl,
substituted 2-indolyl, for example 1-methyl-, 5-methyl-,
5-methoxy-, 5-benzyloxy-, 5-chloro- or 4,5-dimethyl-2-
indolyl, l-benzyl-2- or -3-indolyl, 4,5,6,7-tetrahydro-
2-indolyl, cyclohepta[b]-5-pyrrolyl, 2-, 3- or 4-quino-
lyl, 4-hydroxy-2-quinolyl, 1-, 3- or 4-isoquinolyl, 1-
oxo-1,2-dihydro-3-isoquinolyl, 2-quinoxalinyl, 2-benzo-
furanyl, 2-benzoxazolyl, 2-benzothiazolyl, benz~e]indol-
2-yl or ~-carbolin-3-yl.
Partly hydrogenated or completely hydrogenated hetero-
cyclic rings are, for example, dihydropyridinyl, pyrro-
lidinyl, for example 2-, 3- or 4-N-methylpyrrolidinyl,
piperidinyl, piperazinyl, morpholino or thiomorpholino.
Halogen repre~ents fluorine, chlorine, bromine or iodine,
fluorine, chlorine and bromine being preferred.
Salts of compounds of the formula I are to be understood
as meaning, in particular, pharmaceutically usable or
non-toxic salts.
Such salts are formed, for example, from compounds of the
formula I which contain acid groups, for example
carboxyl, with alkali metals or alkaline earth metals,
such as Na, K, Ng and Ca, and with physiologically
tolerated organic amines, such as, for example, triethyl-
amine and tri-(2-hydroxyethyl)-amine.
~?~2~.
-- 7 --
Compounds of the formula I which contain basic group~,
for example an amino group or a guanidino group, form
salts with inorganic acids, such as, for example, hydro-
chloric acid, sulfuric acid or phosphoric acid, and with
organic carboxylic or ~ulfonic acids, such as, for
example, acetic acid, citric acid, benzoic acid, maleic
acid, fumaric acid, tartaric acid and p-toluenesulfonic
acid.
Preferred compounds of the formula I are those in which
A is as defined on page 1;
Rl denotes hydrogen or represents tC,-C10)-alkyl; cyclo-
pentyl; cyclohexyl; cyclopentyl-(C1-C6)-alkyl; cyclo-
hexyl-( Cl-c6 ) -alkyl; phenyl-( Cl-c4 ) -alkyl, in which the
phenyl radical is optionally substituted as described
on page 2; thienyl or thienyl-( Cl-c4 ) -alkyl, in which
the thiophene radical can in each case be substituted
by one or two identical or different radicals from the
series comprising tCl-C4)-alkyl, ( Cl-c4)-alkoxy and
halogen, or 2-, 3- or 4-pyridyl or 2-, 3- or 4-pyri-
dyl-(Cl-C4)-alkyl, in which the pyridine radical can be
substituted by one or two identical or different
radicals from the series compri~ing (C,-C4)-alkyl,
( C,-C4 )-alkoxy and halogen; amino-(C~-C10)-alkyl;
hydroxy-(C~-C10)-alkyl; ( Cl-C4 ) -alkoxy-(C~-C~0)-alkyl;
( Cl-C4 ) -alkoxycarbonyl-(C~-C~0)-alkyl; ( Cl-C8 ) -alkylsul-
fonyl; (C1-C8)-alkylsulfinyl; (C~-C8)-hydroxyalkylsul-
fonyl; (Cl-C8)-hydroxy-alkylsulfinyl; hydroxy-(Cl-C1O)-
alkanoyl; ( Cl-C8 ) -alkanoyloxy-(C,_C,0)-alkyl; (C~-C11)-
alkanoyl; optionally protected amino-(C1-C11)-alkanoyl,
such as (3-amino-3,3-dLmethyl)-propionyl, 4-aminobuty-
ryl, 5-aminopentanoyl, 6-aminohexanoyl, 4-N-tert.-
butoxycarbonylaminobutyryl, 5-N-tert.-butoxycarbonyl-
aminopentanoyl or 6-N-tert.-butoxycarbonylaminohexa-
noyl; di-( Cl-C7 ) -alkylamino-( C2-Cll ) -alkanoyl; ( C3-C8 ) -
cycloalkylcarbonyl; amino-substituted (C3-C8)-cyclo-
alkyl-carbonyl; amino-~ubstituted (C3-C8)-cycloalkyl-
2~2:~ ~2;~
-- 8 --
sulfonyl or ( C6-Clo ) -aryl-~ C2-cl~ )-alkanoyl; 2-pyridyl-
(C1-C~)-alkanoyl; 3-pyridyl-( Cl-c8 ) -alkanoyl; 4-pyridyl-
(Cl-C~)-alkanoyl; benzoyl which i6 optionally
substituted by halogen, (Cl-C6)-alkyl, (Cl-C4)-alkoxy or
( Cl-C4 ) -alkoxycarbonyl; benzenesulfonyl; (Cl-Cl~)-
alkoxycarbonyl, optionally ~ubstituted by trimethyl-
silyl, halogen, (Cl-C6)-alkyl or halo-(Cl-C6~-alkyl;
( C6-C14 ) -aryl-( Cl-C6 ) -alkoxy-carbonyl; 4-amino-piperi-
dino-l-carbonyl; 4-aminomethyl-piperidino-1-carbonyl;
N-(4-piperidino)-carbamoyl;orN-methyl-t2-<N-(morpho-
linocarbonyl)N-methylamino>-ethyl]aminocarbonyl;
Rl and R5 together with the nitrogen atom carrying them,
form a 5- to 12- membered ring, which can be mono- or
bicyclic, aromatic, partly hydrogenated or completely
hydrogenated;
R2 and R6 independently of one another denote hydrogen or
(C1-C4)-alkyl,
R3 and R5 independently of one another are defined as
al) on page 2;
R4 denotes (C3-Cl2)-alkyl; mono-, bi- or tricyclic (C3-
Cl~)-cycloalkyl or (C3-C1~)-cycloalkylmethyl, in which
the cycloalkyl part i8 optionally substituted by
( Cl-C4 ) -alkyl; ( C~-C14 ) -arylmethyl; dithiolanyl; di-
thiolanylmethyl; dithianyl or dithianylmethyl;
R7 denotes hydrogen or methyl, or together with Rl or R5
and the atoms carrying these forms a mono- or bicyclic
saturated or partly unsaturated ring system having 5 -
12 ring members which, in addition to carbon, can also
contain 1 sulfur atom, which is optionally oxidized to
sulfoxide or sulfone;
and
n denotes 2 - 8.
Particularly preferred compounds of the formula I are
9 ,_ ~i , ,J ,"_ f _ ~ J ~ ~
those in which
R' denotes hydrogen, (Cl-C~) alkylsulfonyl; (cl-c8)-alkyl-
sulfinyl; 2-hydroxye~hyl6ulfonyl; 2-hydroxypropyl-
sulfonyl; ~-hydroxypropionyl; 3-hydroxypropionyl;-3-
hydroxybutyryl; 2-hydroxy 3-methylbutyryl; ( C~-C8 ) -
alkanoyloxy-(C1-C10)-alkyl; n-decanoyl, formyl; acetyl;
propionyl; pivaloyl; isovaleryl; iæobutyryl; (3-amino-
3,3-dimethyl)-propionyl;4-aminobutyryl;5-aminopenta-
noyl î 6-aminohexanoyl; dimethylaminoacetyl; piperi-
dino-4-carbonyl; morpholino-4-carbonyl; cyclopropyl-
carbonyl; cyclobutylcarbonyl; cyclopentylcarbonyl;
cyclohexylcarbonyl; 3-aminocyclobutylcarbonyl; 4-
aminocyclohexylcarbonyl;3-aminocyclobutylsulfonyl;4-
aminocyclohexylsulfonyl; phenylacetyl, phenylpropa-
noyl; phenylbutanoyl; 2 pyridyl-(C1-C~)-alkanoyl; 3-
pyridyl-( Cl-Ca ) -alkanoyl; 4-pyridyl~( Cl-C8 ) -alkanoyl; 4-
chlorobenzoyl; 4-methylbenzoyl; 2-methoxycarbonyl-
benzoyl; 4-methoxybenzoyl; pyrrolyl-2-carbonyl;
pyridyl-3-carbonyl; benæenesulfonyl; methoxycarbonyl;
ethoxycarbonyl; isobutoxycarbonyl; tert.-butoxycar~
bonyl; 2-(trLmethylsilyl)-ethoxycarbonyl; 2,2,2-
trichloroethoxycarbollyl; l,1-dLmethyl-2,2,2-trichloro-
ethoxycarbo:nyl; benzyloxy-carbonyl; 1- or 2-naphthyl-
methoxycarbonyl; ~-fluorenylmethoxycaxbonyl; 4-amino-
piperidino-l-carbonyl; 4-aminomethyl-piperidino 1-
carbonyl; N-methyl-[2-<N-(morpholinocarbonyl)-N-
methylamino~-ethyl]-aminocarbonyl, or N-(4~piperi-
dino)-carbamoyl;
Rl and R5, together with the nitro~en atom carrying them,
form an 8- to 12-membered bicyclic ring, which can be
aromatic, partly hydrogenated or completely hydrogena-
ted;
R2 and R6 independently of one anothex denote hydrogen or
methyl;
R3 and R5 independently of one another denote hydrogenr
methyl r ethyl, isopropyl, n-propyl, n ~utyl, isobutyl,
J
-- 10 --
sec.-butyl, 3-guanidinopropyl, carbamoylmethyl, 2-
carbamoylethyl, carboxymethyl, 2-carboxyethyl, mercap-
tomethyl,2-(methylthio)-e~hyl,(l-mercapto-1-methyl)-
ethyl, hydroxymethyl, 1-hydroxyethyl, aminomethyl, 2-
aminoethyl, 3-aminopropyl, 4-aminobutyl~ N,N-dimethyl-
amino, cyclohexylmethyl, imidazol-4-yl-methyl, benzyl,
2-methyl-benzyl, 3-methylbenzyl, indol-3-yl-methyl, 4-
hydroxybenzyl, 4-methoxybenzyl, 3,4-dihydroxybenzyl,
3,4-dimethoxybenzyl, (benzodioxolan-5-yl)-methyl, 2-
thienyl, 2-thienylmethyl, 2-(2-thienyl)-ethyl, 3-
thienyl, 3-thienylmethyl, 2-(3-thienyl)-ethyl, 4-
chlorobenzyl, 2-(methylsulfinyl)-ethyl, 2-(methyl-
sulfonyl)-ethyl, 2-pyridylmethyl, 3-pyridylmethyl, 4-
pyridylmethyl, cyclohexyl, (l-methyl-imidazol-4-yl)-
methyl, (3-methyl-imidazol-4-yl)-methyl, phenyl, 1-
naphthylmethyl, 2-naphthylmethyl, 2-phenylethyl, 2-
thiazolylmethyl,4-thiazolylmethyl,3-pyrazolylmethyl,
4-pyrimidinylmethyl, indol-2-yl-methyl, 2-benzo[b]-
thienylmethyl,3-benzo~b]thienylmethyl~2-furylmethyl~
cyclohexyl or cyclopentyl;
R4 denotes (C3-C12)-alkyl; mono- or bicyclic (C3-C12)-
cycloalkyl or (C3-C12)-cycloalkylmethyl, in which the
cycloalkyl part is optionally substituted by (C1-C4)-
alkyl; ( C6-Clo ) -aryl-methyl; dithiolanyl; dithiolanyl-
methyl; dithianyl or dithianylmethyl;
R7 denotes hydrogen or, together with R1 and the atoms
carrying these, forms a mono- or bicyclic saturated or
partly unsaturated ring system having 5 - 12 ring
members, which, in addition to carbon, also contains
1 sulfur atom, which is particularly preferably
oxidized to sulfone, or together with R5 and the atoms
carrying these forms a thiochromane system, the sulfur
atom of which is particularly preferably oxidized to
sulfone, and
n denotes 3 - 6.
~ ~ 2 ~ " 2 ~ J
Compounds of the formula I which may furthermore be
mentioned as particularly preferred are those in which
R1 denotes hydrogen; (Cl-C6)-alkylcarbonyl; (Cl-C6)-alkyl-
sulfonyl; amino-(C5-~8)-cycloalkylcarbonyl; 4-amino-
piperidino-l-carbonyl; 4-aminomethyl-piperidino-1-
carbonyl or N-methyl-[2-<N-(morpholinocarbonyl)-N-
methylamino>-ethyl]-aminocarbonyl;
R1 and R5, together wi~h the nitrogen atom carrying them,
denote indolyl;
R2,R6 and R7 denote hydrogen;
R3 denotes (Cl-C6)-alkyl or imidazolyl-(Cl-C4)-alkyl;
R4 denotes ( C5-C~ ) -CyC loalkyl-(C1-C4)-alkyl;
R5 denotes phenyl-(C1-C4)-alkyl or thienyl-( Cl-c4 )-alkyl;
and
n denotes 2-4.
The invention furthermore relates to a process for the
preparation of compounds of the formula I, which com-
prises coupling a fragment having a terminal carboxyl
group or a reactive derivative thereof with a correspond-
ing fragment having a free amino group, if appropriate
splitting off (a) protective group(s) temporarily intro-
duced to protect further functional groups and if approp-
riate converting the compound thus obtained into its
physiologically tolerated salt.
Fragments of a compound of the formula I having a ter-
minal carboxyl group have tbe following formulae VI and
VII
A - OH (VI)
R2 R3
A - N - CH - C - OH (VII)
Fragments of a compound of the formula I having a ter-
minal amino group have the following formulae VIII to X
2 ~ 2 ~
- 12 -
R6 R5 o R2 R3 R4
u l I ~ I
HN - CH - C - N - CH - C - HN - CH - (CHOH)n - CH20H (VIII)
R2 R3 R4 (IX)
HN - CH - C - HN - CH - (CHOH)n - CH20H
R4 (X)
H2N - CH - (CHOH)n - CH2OH
Methods which are suitable for the production of an amide
S bond are described, for example, in Houben-Weyl, Methoden
der organischen Chemie (Methods of Organic Chemistry),
Volume 15/2; ~odanszky et al., Peptide Synthesis, 2nd ed.
(Wiley & Sons, New York 1976) or Gross, Meienhofer, The
Peptides: Analysis, synthesis, biology (Academic Press,
New York 1979). The following methods are preferably
used:
Active ester method with N-hydroxy-succinimide, l-hydrox-
ybenzotriazole or 3-hydroxy-4-oxo-3,4-dihydro-1,2,3-
benzotriazine as the alcohol component, coupling with a
carbodiimide, such as dicyclohexylcarbodiimide, with
propanephosphonic anhydride or methylethylphosphinic
anhydride and the mixed anhydride method with pivaloyl
chloride or ethyl or isobutyl chloroformate, or coupling
with phosphonium reagents, such as benzotriazol-l-yl-oxy-
~ris-(dimethylamino)-phosphonium-hexafluorophosphate
(BOP), or uronium reagents, such as 2-(lH-benzotriazol-
l-yl)-1,1,3,3-tetramethyluroniumtetrafluoroborate(TBTU)
or 2-(lH-benzotriazol-l-yl)-1,1,3,3-tetramethyluronium
hexafluorophosphate (for example Chem. Ber. 103 (1970)
788 and 2034, Z. Naturforsch. 21b (1966) 426; Angew.
Chem. Int. Ed. 19 (1980) 133 and US Patent 4,426,325).
Fragments of the formula VI which fall under
a) formula II are synthesized by the generally known
methods for the preparation of amino acids;
b) formula III are synthesized either from the corres-
ponding ~-amino acids, the chirality center thereof
$ 2 ~
- 13 -
being retained. Diazotization at -20C to 50C in
dilute mineral acid~ leads to ~-bromo carboxylic acids
or, via lactic acid, to u-trifluoromethanesulfonyloxy
carboxylic acids, which can be reacted with a nucleo-
phile carrying Rl and R7; or
c) formula IV are synthesized from the corresponding
~-amino acids, the chirality center thereof being
retained. Diazotization at -20C to 50C in dilute
mineral acids leads to lactic acids, which can be
reacted with an electrophile carrying Rl.
Fragments of the formula VII are synthesized by generally
known methods for the preparation of amino acids and
peptides.
Fragments of the formula X are prepared from suitable
carbohydrates by the corresponding protected ~-lactones.
Reaction with a C-nucleophile, such as a Grignard com-
pound or an alkyllithium compound, first takes place,
followed by aminoglycosidation and reductive ring opening
with complex hydrides, such as LiAlH4 or catalytic hydro-
genation.
Alternatively, fragments of the formula X can be prepared
from the suitable protected aralkylaminoglycosides. In
this case the aralky~ radical is advantageously chosen 80
that hydrogenolytic removal i8 possible before the amide
coupling.
Reaction with a C-nucleophile, such as an alkyllithium
compound, in a solvent which is inert towards these
nucleophiles, such as diethyl ether, tetrahydrofuran,
tetrahydropyran, formaldehyde dimethyl acetal or 1,2-
dimethoxyethane, at a temperature between -30-C and the
boiling point of the solvent, preferably between 0C and
the boiling point of the solvent, gives the aralkyl
derivative of the protected fragment of the formula X.
This can be liberated, for amide coupling, by catalytic
hydrogenation with hydrogen or catalytic transfer hydro-
2~2:~ ~2~
- 14 -
genation with ammonium formate.
The preliminary and subsequent operations required for
preparation of the compounds of the formula I, such as
introduction and splitting off of protective groups, are
known from the literature and are described, for example,
in T.W. Greene ~Protective Groups in Organic Synthesis"
(John Wiley & Sons, New York, 1981). Salts of compounds
of the formula I having salt-forming groups are prepared
in a manner which i8 known per 6e, for example by react-
ing a compound of the formula I having a basic group witha stoichiometric amount of a suitable acid, or reacting
compounds of the formula I having an acid group with a
stoichiometric amount of a suitable base. Stereoisomer
mixtures, in particular diastereomer mixtures, which are
obtained, if appropriate, during the synthesis of
compounds of the formula I, can be resolved in a manner
which is known per se by fractional crystallization or by
chromatography.
The compounds of the formula I according to the invention
have enzyme-inhibiting properties, and in particular they
inhibit aspartyl proteases, such as renin.
Renin is secreted into the blood circulation from the
~uxtaglomerular cells of the kidney as a consequence of
various stimuli ~volume depletion, sodium deficiency, ~-
receptor sti~ulation). In the blood circulation it ~plitsoff the decapeptide angiotensin I from the angio-
tensinogen discharged by the liver. This decapeptide is
converted by angiotensin converting enzyme (ACE) into
angiotensin II. Angiotensin II plays an essential role in
blood pressure regulation, ~ince it directly increases
the blood pressure by vasoconstriction. It additionally
stimulates the secretion of aldosterone from the adrenals
and in this manner increases the extracellular fluid
volume via inhibition of sodium excretion, which in turn
contributes towards increasing the blood pressure.
Inhibitors of the enzymatic activity of renin have the
~i ` J r .
~ 15 ~
effect of a reduced formation of angtiotensin I, the
consequence of which is a reduced formation of angioten-
sin II. The reduction in the concentration of this active
peptide hormone is the direct cause of ~he antihyperten-
sive action of renin inhibitors.
The activity of renin inhibitors can be investigated by
in vitro tests. In these, the reduction in the formation
of angiotensin I is measured in various systems (human
plasma and purified human renin3.
l. Test prihciple
For example, human plasma which contains both renin and
angiotensinogen is incubated at 37C with the compound to
be tested. During this incubation, angiotensin I is
liberated from angiotensinogen by the action of renin and
can then be measured using a commercially available
radioimmunoassay. This angiotensin liberation is in-
hibited by renin inhibitors.
2. Isolation of the pl88ma
The blood is obtained from volunteer6 (about 0.5 1 per
person; Bluko sampler from ASID Bonz und Sohn, Unter-
schleiBheim) and collected in partly evacuated bottles,
while cooling with ice. Coagulation is prevented by
addition of EDTA (final concentration 10 mM). After
centrifugation (Rotor HS 4 (Sorvall), 3,500 revolutions
per minute, 0 - 4DC, 15 minutes; repeated if necessary),
the plasma is carefully pipetted off and frozen at -30C
in suitable portions. Only plasmas having a sufficiently
high renin activity are used for the test. Plasmas having
a low renin activity are activated tprorenin . renin) by
a low temperature treatment (-4C, 3 days).
2 f~ 2
3. Test procedure
Angiotensin I i8 determined with a Renin-Maia- kit (Serono
Diagnostics S.A., Coinsins, Switzerland). The plasma is
incubated in accordance with the instructions given with
the kit:
Incubation batch: 1000 ~1 of plasma (thawed at 0-4C)
100 ~1 of phosphate buffer (pH 7.4,
addition of 10-4 M ramiprilat)
10 ~1 PMSF solution
10 ~1 of 0.1% of Genapol PFIC
12 ~1 of dimethyl sulfoxide or test
preparation
The test preparations are in general dissolved as a 10-2
M solution in 100~ pure dimethyl sulfoxide (DMS0) and the
solutions are diluted accordingly with water; the incuba-
tion mixture contains not more than 1% of DNS0.
The mixtures are mixed in ice and placed in a waterbath
(37C) for 1 hour for incubation. A total of 6 samples
(in each case 100 ~1) are removed from an additional
mixture without an inhibitor and without further
incubation in order to determine the starting angiotensin
I content of the plasma used.
The concentrations of the test preparstions are chosen 80
that approximately the range of 10 - 90% enzyme inhibi-
tion is covered (at }east five concentrations). At theend of the incubation period, three 100 ~1 samples from
each mixture are frozen on dry ice in pre-cooled
Eppendorf vessels and kept at about -25-C for the
angiotensin I determination (mean value of three
individual samples).
4. Angiotensin I radioi mNnoassay (RIA)
The instructions for using the RIA kit (Renin-Maia kit,
- 17 ~ , 3
Serono Diagnostics S.A./ Coinsins, Swi~zerland) are
followed exactly.
The calibration plot includes the range from 0.2 to
25.0 ng of angiotensin I per ml. The bas 1 angiotensin I
content of the plasma is subtracted from all the measured
values. The plasma renin activity (PRA) is stated as ng
of Ang I/ml x hour. PR~ values in ~he pres0nce of the
test substances are related to a mixture without
inhibitor t= 100~) and stated as ~ residual activity. The
IC50 value is read off from the plot of ~ residual
activity against the concentration (M) of the test
preparation (logarithmic scale3.
The compounds of the general formula I described in the
present invention exhibit inhibitory actions at concen
trations of abou~ 10-5 to 10-1 mol/l in the in vitro test.
In detail, the following values were determined:
Table 1:
Example IC50 [M~ IC50 [M]
No. human plasma renin purified human renin
1 ~4.2 x 1~-7 1.2 x 10-7
2 4O0 x 109 ~.0 x 10
3 2.2 x 10 1.2 x 10
4 .1.6 x 10 a 2.4 x 10
1 . 1 x 10 1 . 4 x 10
7 2 . 2 x 10-7 3 . 0 x 10-7
g 1.8 x 10-8 1.2 x 10-8
1 . 1 x 10-7 5 . 0 x 10-8
11 1.9 x 10-6 3.6 x 10-6
1;~ > 10 1.O x 10
Renin inhibitors cause a reduction in blood pressure in
animals with salt depletion. Since human renin differs
from the renin of other species, primates, such as, for
example, Rhesus monkeys, are used for in vivo testing of
- 18 - 2~2~
renin inhibitors.
1. Test principle
Primate renin and human renin are largely homologous in
their sequence. An endogenous secretion of renin is
stimulated by intravenous injection of furosemide. The
test compounds are then administered and their effect on
blood pressure and heart rate is measured.
2. Test procedure
6 Rhesus monkeys were pretreated orally with
10 mg/kg x day of furosemide on 6 successive days. On the
7th day, a further 10 mg/kg of furosemide were adminis-
tered intravenously about 30 minutes before the start of
the experiment. Anasthesia was then induced with 20 mg/kg
of ketamine intramuscularly and continued with 40 mg/kg
of pentobarbitone intravenously. A side arm of the
femoral artery was exposed and a cannula inserted for
blood pressure measurement by means of a blood pressure
transducer (P 23 ID). Blood samples for determination of
the piasma renin activity were removed via a Braunule in
the saphena vein.
The title compound of Example 2 gives the following
result: 2 mg/kg intraduodenally (in 0.1 N citric acid of
2 mg/ml)
t [min.] Change in blood change in Change in plasma
pressure [%] pulse [%] renin activity
1~
1 - 0.72 -1.16
3 - 8.21 -1.61
-11.53 -2.42
-11.82 -2.51
-12.68 -3.49 -20
-13.48 -1.97
-16.43 -1.61 -62
~ ~ 2 ~ ~ ~ rJ
-- 19 --
-12.82 +0.09 -53
-13.98 -0.09 -58
- 8.79 +1.16
- 6.77 +3.04 -51
120 ~47
The compounds of the present invention are effective here
in a dose range of about 0.1-5 mg/kg intravenously, and
in the dose range from about 0.5-50 mg.kg on intraduo-
denal administration by a gastroscope.
The compounds of the general formula I described in the
present invention can be used as anti-hypertensives and
for the treatment of cardiac insufficiency.
The invention furthermore relates to the use of compounds
of the formula I for the preparation of pharmaceutical6
for antihypertensive therapy and treatment of congestive
cardiac insufficiency.
Pharmaceutical preparations contain an active amount of
the active compound of the formula I together with an
inorganic or organic pharmaceutically usable excipient.
They can be used intranasally, intravenously, subcutane-
ously, perorally or intraduodenally. The dosage of the
active compound depend~ on the warm-blooded species, the
body weight, the age and the mode of admini~tration.
The pharmaceutical preparations of the present invention
are prepared in dissolving, mixing, granulating or
tablet-coating proce~ses which are known per 8e.
For the oral use forms, the active compounds are mixed
with the additives customary for these, such as excipi-
ents, stabilizers or inert diluents, and the mixture is
brought by customary methods into suitable pre3entation
forms, such as tablets, coated tablets, two-piece cap-
sules~ aqueous, alcoholic or oily su~pensions or aqueous,
2 ~
- 20 -
alcoholic or oily solutions. Inert excipients which can
be used are, for example, gum arabic, magnesia, magnesium
carbonate, potassium phosphate, lactose, glucose, mag-
nesium stearyl fumarate or starch, in particular maize
starch. The formulation here can be in the form either of
dry granules or of moist granules. Examples of possible
oily excipients or solvents are vegetable or animal oils,
such as sunflower oil and cod-liver oil.
For subcutaneou6 or intravenous administration, the
active compounds or physiologically tolerated salts
thereof are dissolved, suspended or emulsified if desired
with the substances customary for this purpose, such as
solubilizing agents, emulsifiers or other auxiliaries.
Possible solvents are, for example: water, physiological
saline solutions or alcohols, for example ethanol,
propanediol or glycerol, and in addition also sugar
solutions, such as glucose solutions or mannitol
solutions, or a mixture of the various solvents
mentioned.
List of abbreviations used:
Ac acetyl
Boc tert.-butoxycarbonyl
BOP benzotriazol-l-yl-oxy-tris-(dimethyl-
amino)-phosphonium
BuLi n-butyllithium
TLC thin layer chromatography
DCC dicyclohexylcarbodiimide
DCI desorption chemical ionization
DIP diisopropyl ether
DNP 2,4-dinitrophenyl
DNE 1, 2-dimethoxyethane
DMF dimethylformamide
DMSO dimethyl sulfoxide
EA ethyl acetate
EI electron impact
Etoc ethoxycarbonyl
2~2~2~
- 21 -
FAB fast atom bombardment
H hexane
Hep n-heptane
HOBt l-hydroxybenzotriazole
Iva isovaleryl
M molecular peak
MeOH methanol
NS mass spectrum
MTB methyl tert.-butyl ether
NEM N-ethylmorpholine
Nva norvaline
Nle norleucine
PPA N-propanephosphonic anhydride
R . T . room temperature
m.p. melting point
b.p.~ boiling point at xx mm Hg
TBTU 2-(lH-benzotriazol-1-yl)-1,1,3,3-tetra-
methyluronium
Thi 2-thienylalanine
THF tetrahydrofuran
Z benzyloxycarbonyl.
List of abbreviation~ of selected C termini
(D)-manno-pentol (XX) (D)-manno-pentol
(L)-gulo-pentol (XX) (L)-gulo-pentol
-- 2 2 -- i ~ ",, ~, j;
~ o 7L
X ~ ~
(D)-talo-pentol (~X~ tD)-talo-pentol
~S ~ C~ C~
0~0 C~ ~
(L)-allo-pentol (XX) (L)~allo-pentol
The other abbreviations used for amino acids correspond
to the three~letter code customary in peptide chemistry,
such as is described, for example, in Eur. J. Biochem.
138, 9 - 37 (1984). Unless expressly stated otherwise,
the amino acids are always in the L configuration.
The following examples serve to illustrate the present
invention without limiting it to these.
Example 1
Iva-Phe-Nva [(L)-gulo-pentol]
200 mg of Iva-Phe-Nva-[(L)-gulo-pentol(XX)] are dissolved
in 10 ml of 859i 6trength aqueous trifluoroacetic acid and
the mixture is stirred at R.~. for 2.5 hours. The sol-
vents are remo~ed in vacuo and chromatographed on silicagel using CH2Cl2JMeOH 10:1. 140 mg of the title compound
are obtained as a colorless amorphous powder.
Rf (CH2Cl2/MeOH 10:1) = U.06 MS (FAB + LiI~o
614 (M+Li)
a) Iva-Phe-Nva-[(L)-gulo-pentol(XX)~
204 ~1 of pivaloyl chloride are added to 578 mg of
Iva-Phe-Nva-OHI 229 ~1 of N-ethylpipsridine and 230 ~1
2 ~ 2 ~
- 23 -
of triethylamine, dissolved in 25 ml of anhydrous
CH2Cl2, at -15C. The mixture is stirred at R.T. for 10
minutes and cooled to -10C and a solution of 599 mg
of H-(L)-gulo-pentol(XX) in 10 ml of anhydrous CH2Cl2
is added. The mixture is stirred at R.T. for 20 hours,
the solvent is removed in vacuo and the residue is
taken up in 150 ml of EA. The mixture is washed three
times with 50 ml of KH2PO; solution each time and three
times with 50 ml of NaHCO3 solution each time, the EA
phase is dried over R2C03 and the solvent is removed in
vacuo. Chromatography on silica gel using DIP/MT~
gives 200 mg of the title compound as a colorless
amorphous powder.
Rs (MTB/DIP 1:1) = 0.10 MS(FAB): 688 (M+l)
b) tH-(L)-gulo-pentol(XX)~
740 mg of -~N-benzyl-(L)-gulopQntol(XX)] are dissolved
in 20 ml of anhydrous NeOH, and 150 ml of Pd/C (10%)
and 1.1 g of HC02NH4 are added under argon. The mixture
is heated under reflux for 1.5 hours, the catalyst is
filtered off and the solvent is removed in vacuo. 600
mg of the title compound are obtained as a pale yellow
oil, which is employed further without purification.
R (MTB) - 0.05
c) ~N-Benzyl-(L)-gulo-pentol(XX)]
880 mg of 2-benzylamino-2-cyclohexylmethyl-(3,4-
isopropylidene)-3(S),4(S)-dihydroxy-5-(R)-t(l, 2-
isopropylidene)-l(S)-2-dihydroxyethyl]-tetrahydrofuran
are dissolved in 25 ml of anhydrous THF and 188 mg of
LiAlH4 are added. The mixture is ~tirred at R.T. for
20 hour~, 50 ml of NaHCO3 solution are added and the
mixture is extracted three times with 100 ml of EA
each time. The extract is dried over Na2SO4 and the
solvent is removed in vacuo. 690 mg of the title
- 2~ -
compound are obtained as a colorless oil.
Rf (MTB/Hep 1:5~ = 0.31 MS (DCI): 448 (M+1)
d) 2-~enzylamino-2-cyclohe~ylmethyl-(3,4 isopropylidene)-
3(S),4(S)-dihydroxy-5-(R)-[(1,2-isopropylidene)-l(S)-
2-dihydroxyethyl]-tetrahydrofuran
4.65 g of 2-cyclohexylmethyl-(3,4-isopropylidene)-
2,3(S),4(S)-trihydroxy-5-(R)-[(1,2-isopropylidene)~
l(S),2-dihydroxyethyl]-tetrahydrofuran and 5.7 ml of
benzylamine are dis~olved in 150 ml of anhydrous
toluene, and 910 ~1 of TiC14 are added at -20C~ The
mixture is stirred at R.T. for 3 hours, 100 ml of
saturated aqueous NazCO3 solution are added and the
mixture is diluted with 300 ml of EA and washed with
XH2P04 solution until pH 5 is reached. The organic
phase is dried with NazSO4 and the solvent is removed
in vacuo. Chromatography on silica gel using MTB/Hep
1:5 gives 1.0 g of the title compound as a colorless
oil.
Rf (MTB/Hep 1:5) = 0.24 MS (DCI): 446 (M+l)
e) 2-Cyclohexylmethyl-(3,4-isopropylidene)-2,3(S),4(S)-
trihydroxy--5-(R)-[(1,2-isopropylidene)-l(S)-2-di-
hydroxyethy]L]-tetrahydrofuran
5.16 g of (2,3-5,6-diisopropylidene)-L-gulonic acid ~-
lactone are suspended in 300 ml of diethyl ether and
1.1 equivalents of cyclohe~ylmethyl-magnesium bromide
in 50 ml of diethyl ether are added dropwise at the
reflu~ temperature under argon in the course of 2
hours. 100 ml of saturated aqueous NaHCO3 solution are
then added, the mixture is extracted twice with 100 ml
of E~ each time, the or~anic phase is dried over Na2S04
and the solvent is removed in vacuo. 4.9 g of the
title compound are obtained as a colorless oil
slightly contaminated with the bis-adduct.
2U~l2 ~
-- 2S --
Rf (DIP) = 0.40 MS (DCI): 357 (M+l)
f) (2,3-5,6-Diisopropylidene)-L-gulonic acid ~-lactone
42 g of L-gulonic acid ~-lactone and 100 mg of p-
toluenesulfonic acid are heated under reflux in 300 ml
of 2,2-dimethoxypropane for S hours. After 1 hour, a
clear solution forms. The volatile constituents are
removed in vacuo and the residue i8 recrystallized
from DIP. 45 g of the title compound are obtained as
colorless crystals, m.p.: 144C.
Rf (DIP) = 0-12 MS (DCI) t 259 (M+l)
Fxample 2
(2(S)-Benzyl-3-t-butylsulfonyl)propionyl-Hi~-t(D)-manno-
pentol]
960 mg of (2(S)-benzyl-3-t-butylsulfonyl)propionyl-Hi~-
t(D)-manno-pentol(XX)] are dissolved witn 722 mg of p
toluenesulfonic acid in 100 ml of methanol and 2 drops of
water are added. The mixture i8 stirred at RT for 17
hours, the pH i~ then brought to 7 with NaHC03 ~olution,
the methanol is removed in vacuo, the residue iB extrac-
ted three times with 100 ml of MTB and the extract i8dried over NazSO4. The solvent is removed in vacuo and the
residue is chromatographed on silica gel using acetone/
water 10:1. 120 mg of the title compound are obtained as
white crystals.
Rf (acetone/water 10:1) - 0.46 MS (FAB): 681 (M+l)
m.p. 100 - 110C (decomposition)
a) (2-(S)-Benzyl-3-t-butylsulfonyl)propionyl-Hi~-t(D)-
manno-pentol(XX)]
850 mg of (2(S)-benzyl-3-t-butyl~ulfonyl~propionyl-
2~2
-- 26 --
His(DNP)-[(D)-manno-pentol(XX)] and 0.94 ml of thio-
phenol are dissolved in 15 ml of acetonitrile and the
solution is stirred at RT for 2 hours. The solvent is
removed in vacuo, the residue is digested with DIP,
and the residue is chromatographed on silica gel using
toluene/MeOH 5:1. 400 mg of the title compound are
obtained as pale yellow crystals.
m.p. ~ 160C (decompo6ition)
Rf (toluene/MeOH 5:1) = 0.39 MS (FAB): 761 (M+l)
b) (2(S)-Benzyl-3-t-butylsulfonyl)propionyl-His(DNP)-
[(D)-manno-pentol(XX)l
1.3 g of (2(S)-benzyl-3-t-butylsulfonyl)propionyl-
His(DNP)-OH, 0.31 ml of N-ethylpiperidine and 0.31 ml
of triethylamine are dissolved in 30 ml of CH2Cl2 and
0.28 ml of pivaloyl chloride are added dropwise at
- 15C under argon. The mixture is stirred at RT for
10 minutes and cooled to -10C and a solution of 400
mg of H-(D)-manno-pentol(XX) in 5 ml of CH2Cl2 is added
dropwise. The mixture is stirred at RT for 18 hours,
diluted with 100 ml of NTB and extracted once each
with 100 ml of 0.66 N KH2PO~ and 100 ml of snturated
NaHCO3 solution. The extract is dried over Na2SO~ and
the solvent is removed ln vacuo. 850 mq of the title
compound are obtained a8 an amorphous powder which is
further reacted without purification.
Ff (EA/MeOH lOsl) = 0.53 NS (FAB): 927 (N+l)
c) (2(S)-Benzyl-3-t-butylsulfonyl)propionyl)-His(DNP)-OH
10.7 g of (2(S)-benzyl-3-t-benzylsulfonyl)propionic
acid N-hydroxy-succinimide ester and 11.3 g of H-
His(DNP)-OH hydrochloride are dissolved in 500 ml of
THF/ethanol 1:1, and 470 ml of saturated aqueous
NaHCO3 solution are added. The mixture is stirred at
2~2~2~
- 27 -
RT for 5 hours, the organic solvents are removed in
vacuo, the pH i6 brought to 1-2 with NaHS04 ~olution,
the mixture is extracted three times with 500 ml of EA
each time, the extract is dried over Na2S04 and the
solvent is removed in vacuo. The residue is taken up
in 350 ml of acetone/E~ 1:1 and the product is pre-
cipitated with diethyl ether in an ultrasonic bath.
12.5 g of the title compound are obtained a~ a yellow
powder.
Rr (MeOH/CH2Cl2 1:5) = 0.11 MS (FAB): 588 (M+l)
d) (2(S)-Benzyl-3-t-butylsulfonyl)propionic acid N-
hydroxysuccinimide ester
8.0 g of (2(S)-benzyl-3-t-butylsulfonyl)propionic acid
(J. Med. Chem. 31, 1839 (1988)) and 3.3 g of N-
hydroxysuccinimide are dissolved in 200 ml of 1,2-
dimethoxyethane, and a solution of 5.8 g of DCC in
50 ml of 1,2-dimethoxyethane is added dropwise at 0C.
The mixture is left to stand at 6-C for 18 hours, the
solvent is removed in vacuo at 20C and the residue is
taken up in 100 ml of acetonitrile. The urea is
filtered off and the solvent is removed at 20C in
vacuo. 10.7 g of the title compound are obtained as a
colorless oil which i8 reacted further without purifi-
cation.
e) H-(D)-Manno-pentol(XX)
The compound was prepared analogously to Example lb
from N-benzyl-(D)-manno-pentol(XX).
f) tN-Benzyl-(D)-manno-pentol(XX)]
8.8 g of 2-benzylamino-(3,4-isopropylidene)-3(S),4(S)-
dihydroxy-5(R)-[(1,2-isopropylidene)-l(R)-2-dihydroxy-
ethyl]-tetrahydrofuran and 7.1 ml of cyclohexylmethyl
bromide are dissolved in 250 ml of THF and reacted
2 ~ 2 ~
- 28 -
with 0.7 g of lithium wire (3.2 mm diameter, about 1%
of Na) in an ultrasonic bath at RT under argon. After
6 hours, the solvent is removed in vacuo, the residue
is taken up in 500 ml of 0.67 M KH2PO4 solution and the
mixture i8 extracted three times with 300 ml of MTB.
The extract is dried over Na2S04, the solvent i8
removed in vacuo and the residue is chromatographed on
silica gel using MTB/Hep 1:1. 3.3 g of the title
compound are obtained a~ a colorless oil.
Rf (MTB/Hep 1:1) = 0.39 MS(DCI): 448 (N+l)
g) 2-Benzylamino-(3,4-isopropylidene)-3(S), 4 ( s ) -di-
hydroxy-5(R)-[(1,2-isopropylidene)-l(R)-2-dihydroxy-
ethyl]-tetrahydrofuran
31.5 g of 2,3-5,6-diisopropylidene-D-mannofuranoside
and 20.0 ml of benzylamine are heated in 250 ml of
toluene using a water separator. After 7 hours, the
water separator is replaced by a Soxhlet extractor
filled with molecular sieve 4A and the mixture is
heated under reflux for a further 16 hours. The
solvent is then removed in vacuo, the residue i~ tsken
up in 500 ml of EA and the mixture is washed three
times with 250 ml of 0.67 M RH2PO4 ~olution. The
mixture i~ dried over Na2S04 and the ~olvent is removed
in vacuo. 41.5 g of the white crystalline title
compound are obtained.
F~ (toluene/EA 2:1) = 0.29 MS(DCI): 350 (M+l)
Alternative syntheses
h) (2(S)-Benzyl-3-t-butylsulfonyl)-propionyl-~is-[(D)-
manno-pentol(XX)~ (2a))
785 mg of (2(S)-benzyl-3-t-butylsulfonyl)-propionic
acid and 382 ~1 of triethylamine are dissolved in
20 ml of DMF, 1.1 g of 0-benzotriazol-1-yl-1,1,3,
~ ~;Vi ~ 2 ~
- 29 -
3-tetramethyluronium hexafluorophosphate are added at
RT and the mixture is stirred at RT for 5 minuteg. A
solution of 1.6 g of H-Hi~-[(D)-manno-pentol(XX)] in
15 ml of DMF is then added dropwise and the mixture is
stirred at RT for 20 hours. The solvent i8 removed in
vacuo, the residue is taken up in 500 ml of EA and the
mixture is washed three times with 100 ml of NaaCO3
solution. It is dried over Na2SO4, the solvent is
removed in vacuo and the re~idue i~ chromatographed on
silica gel using EA/MeOH 5:1. 1.1 g of the title
compound of Example 2a) are obtained.
i) H-His-[(D)-manno-pentol(xx)]
3 g of Z-His-t(D)-manno-pentol(XX)] and 3 g of 2mmo-
nium formate are dissolved in 50 ml of methanol, 2 g
of Pd/C (10% strength) are added and the mixture is
stirred under argon at RT for 5 hours. The catalyst is
then filtered off, the solvent is removed in vacuo,
the residue is taken up in 500 ml of EA and the
mixture is washed three times with 50 ml of Na2CO3
solution. It i8 dried over Na2SO4 and the solvent is
removed in vacuo. 1.8 g of the title compound are
obtained as a foam, which is kept under argon and is
further reacted as soon as possible.
k) Z-Hi~ (D)-manno-pentol(XX)]
500 mg of H-(D)-manno-pentol(XX) and 405 mg of
Z-His-OH are dissolved in 20 ml of DNF under argon,
and first 303 ~1 of diphenylphosphoryl azide and then
208 ~1 of 1-diethylamino-2-propanol are subsequently
added at 0C. The mixture is stirred at 0C for 2
hours and then at RT for 4 days. The reaction mixture
is diluted with 200 ml of EA and washed in each case
once with 100 ml of 0.7 M RH2PO~ solution and 100 ml of
saturated NaHCO3 solution. It is dried over Na2SO~ and
chromatographed on silica gel using EA/MeOH 10:1.
530 mg of the title compound, colorless foam, are
- 30 -
obtained.
R (EA/MeOH 10:1) = 0.17 MS (FAB): 629 (M+l)
1) H-(D)-manno-pentol(XX)
410 mg of N-benzhydryl-(D)-manno-pentol(XX) and 490 mg
of ammonium formate are dissolved in 15 ml of anhy-
drous methanol, 82 mg of Pd/C (10% strength) are added
and the mixture is stirred under argon at RT for
6 hours. The solvent i8 removed in vacuo, the residue
is taken up in 100 ml of EA and the mixture is washed
three times with 50 ml of Na2CO3 solution. The organic
pha8e i8 dried with Na2SO4 and the solvent is removed
in vacuo. After drying under a fine vacuum to remove
the diphenylmethane, 275 mg of the title compound of
Example 2e) are obtained.
m) N-Benzhydryl-(D)-manno-pentol(XX)
1.8 g of 2-benzhydrylamino-(3,4-isopropylidene)-
3(S),4(S)-dihydroxy-5(R)-tl,2-isopropylidene)-l(R)-2-
dihydroxyethyll-tetrahydrofuran and 1.2 ml of cyclo-
hexylmethyl bromide are dissolved in 40 ml of formal-
dehyde dimethyl acetal (distilled from ~/Na alloy) and
are reacted with 115 mg of lithium wire (3.2 mm
diameter, about 1% of Na) under argon in an ultrasonic
bath at 20-40-C for 3.5 hours. 115 mg of lithium wire
and 1.2 ml of cyclohexylmethylbromide are then added
again and the mixture is reacted at 40-60C for
another hour. The reaction mixture i8 poured into
200 ml of NaHCO3 solution and extracted three times
with 100 ml of MTB. It i8 dried over Na2SO~, the
solvent is removed in vacuo snd the residue i8 chroma-
tographed on Bilica gel using DIP/toluene 1:3. 1.1 g
of the title compound are obtained as a colorless oil.
Rf (DIP/toluene 1:3) = 0.28 MS(DCI): 524 (M+l)
2~c2~
n) 2-Benzhydrylamino-(3,4-isopropylidene)-3~S),4(S)-
dihydroxy-5(R)-tl,2-isopropylidene-l(R)-2-dihydroxy-
ethyl]-tetrahydrofuran
10 g of D(+)-mannose and about 10 mg of p-toluenesul-
S fonic acid are suspended in 40 ml of 2,2-dimethoxypro-
pane and the suspension i8 stirred at 40C for 1 hour.
A clear solution i8 formed by this procedure. 10 ml of
benzhydrylamine are added and the mixture is boiled
under reflux for 24 hours. A further 10 ml of 2,2-
dimethoxypropane and 2 ml of benzhydrylamine are then
added and the mixture is boiled under reflux for a
further 18 hours. Volatile constituents are removed in
vacuo, the residue is taken up in 100 ml of EA and the
mixture i8 washed three times with 100 ml of NaHC03
solution. It is dried over Na2S04, the solvent is
removed in vacuo and the residue i8 chromatographed on
silica gel using DIP/toluene 1:5. 17 g of the title
compound are obtained as pale yellow crystals, melting
; points 82-84C.
R~ (DIP/toluene 1:3) = 0.41 NS(DCI): 426 (M+l)
The title compound of Example 2g) can also be prepared
analogously from D(+)-mannose in a one-pot process.
Esample 3
cis-4-Aminocyclohexylcarbonyl-Phe-His-~(D)-manno-pentol]
180 mg of (N-tert.-butoxycarbonyl-cis-4-amino-cyclohexyl-
carbonyl)-Phe-His-t(D)-manno-pentol(XX)] are dissolved in
S ml of CH2C12, and S ml of trifluoroacetic acid are added
at 0C. The mixture is stirred at RT for 24 hours, 100 ml
of ~aturated Na2C03 solution are added and the mixture iB
extracted 3 time~ with 100 ml of EA. The extract is dried
over Na2S0~, the solvent is removed in vacuo and the
residue is chromatographed on silica gel using acetone/
H20/concentrated NH3 100:10:5. 24 mg of the title compound
are obtained as a colorless powder.
Rf (acetone/H2Otconcentrated N~3 100:10:5) = 0.12
MS(FAB): 687 (M~l)
a) (N-tert.-Butoxycarbonyl-cis-4-aminocyclohexylcarb-
onyl)--Phe-His-[(D)-manno-pentol(XX)]
The title compound is prepared analogously to Example
2a), 2b~ from (N-tert.-butoxycarbonyl-cis-4-amino-
cyclohexylcarbonyl)-Phe-His(DNP)-OH and H-(D)-manno-
pentol(XX).
0 Rf (EA/MeOH 5:1) = 0.43 MS~FAB): 868 (M+l)
b) (N-tert.-Butoxycarbonyl~cis-4-aminocyclohexylcarb-
onyl)-Phe~His(DNP)-OH
The title compound is prepared analogously to Example
2c), 2d) from (N-tert.-butoxycarbonyl-cis-4-amino-
cyclohexylcarbollyl) Phe-OH and H-His(DNP)-OH.
R~ (EA/MeOH 3:1) = 0.20 MS(FA~): 694 (M+l)
c) N-tN-tert.-Butoxycarbonyl-c i8 -4~aminocyclohexylcarb-
onyl)-L-phenylalanine
5.5 g of N--(N-tert.-butoxycarbonyl-ci~-4 aminocyclo-
hexylcarbonyl)-L phenylalanine ~enzyl ester are
hydrogenated in 230 ml of ethanol over 1 g of Pd/char-
coal under normal pressure. When the reaction had
ended, the catalyst was filtered off and the solvent
was distilled off. Recrystallization from n-heptane/
ethyl acetate gave 4.1 g of colorless product.
Melting point 160 - 161C (decomposition)
MS(DCI): 391 (M+l)
f~ J .~
~ 33 -
d) N-(N-ter~.-Butoxycarbonyl-cis-4-aminocyclohexylcarb-
onyl)-L-phenylalanine benzyl ester
6.0 g of N-tert.~butoxycarbonyl cis-1,4-aminocyclo-
hexanecarboxylic acid and 6.3 g of L-phenylalanine
benzyl ester are di~solved in 75 ml of DMF, the
solution was mixed with 24 ml of n-PPA and 15.7 ml of
NEM at 0~C and the mixture is reacted overnight at RT.
The solution is dilu~ed with CH2Cl2, washed in each
~ase with semi~saturated NaHCO3 solution, 10% ~trength
citric acid and water, dried oYer MgSO4 and concentra-
ted in vacuo. Flash chromatography ga~e 5.7 g of pure
product.
[~]20 -14.6 (c = 1.1 in CH30H)
Example 4
2(S)-{N-Methyl-N-<2-[N-(morpholinocarbonyl)-N-methyl-
amino]-ethyl>-aminocarbonyloxy}-3-phenylpropionyl~His-
[(D)]mannopentol]
The title compound is prepared analogously to Example
2a), 2b), 2c:~ and 2d) from 2(S) {N-methyl-N-<2-[N-
(morpholinocarbonyl)-N-methylamino]ethyl>-aminocarbonyl-
oxy]-3-phenylpropionic acid.
R~ (EA/MeOH 1:1) = 0.22 MS(FAB): 790 (M+1)
a) 2(S)-{N-methyl-N-<2-[N-(morpholinocarbonyl)~N-methyl-
amino]ethyl>-aminocarbonyloxy]-3-phenylpropioni~ acid
840 g of ethyl 2(S)-{N-methyl-N-<2-~N-(morpholinocarb-
onyl)-N-methylamino]ethyl~-aminocarbonyloxy]-3-phenyl-
propionate are dissolved in 100 ml of methanol, and
20 ml of 0.1 N sodium hydroxide solution are added.
After 16 hours at RT, the methanol is distilled off
and the residue is brought to pH 1 - 2 with hydro-
chloric acid. Extraction with EA 3 times gives, after
2~2~
- 34 -
drying with Na2S04 and concentration, the title com-
pound (24%), which i8 used for the next reaction
without further purification.
MS tDCI): 394 (M+l)
b) Ethyl 2(S)-{N-methyl-N-<2-[N-(morpholinocarbonyl)-N-
methylamino]ethyl~-aminocarbonyloxy}-3-phenylpropio-
nate
0.9 q of ethyl phenyllactate in CH2C12 (10 ml) are
added dropwise to 2 g of di(4-benzotriazolyl) car-
bonate and 0.23 ml of pyridine in CH2C12 (20 ml) at RT.
After 6 hours, 900 mg of N-methyl-N-2-tN-(morpholino-
carbonyl)-N-methylamino]-ethylamine in 10 ml of CH2Cl2
are added dropwise. After 16 hours, 100 ml of ethyl
acetate are added, and the mixture is then washed
twice with saturated Na2C03 solution, twice with
saturated NaHS04 and once with saturated NaCl solution.
After drying with Na2S04 and concentration, a yellow
oil is obtained which, after chromatography on SiO2
(eluent EA), gives the title compound.
Rf (EA) = 0.3 MS (DCI): 422 (M+l)
c) N-Methyl-N-2-[N-(morpholinocarbonyl)-N-methylamino]-
ethylamine
2.1 g of Boc-N-methyl-N-2-[N-(morpholinocarbonyl)-N-
methylamino]ethylamine are stirred in 75 ml of a ~6N
solution of HCl in DME at RT for 3 hours. After
concentration, 50 ml of ~aturated Na2C03 solution are
added and the mixture is extracted three times with
EA. Drying and concentration give the title compound,
which is employed for the further reaction~ without
additional purification.
d) Boc-N-methyl-N-2-[N-(morpholinocarbonyl)-N-methyl-
amino]ethylamine
~f : i ~ ; j
.J '_i ! J , _ . .- ..t~;
~ 3~ -
The title compound is obtained analogously to Example
4b~ from 2.1 g of morpholine, 10 g of di~ benzotri-
azolyl)carbonate, 2 ml of pyridine and 4.7 g of Boc-
N-methyl-2~(methylamino)ethylamine.
Rf (EA) = 0.25 MS (DCI): 302 (M+l)
e) Boc-N-methyl-2-~methylaminn)ethylamine
12.5 g of di-t-butyl dicarbonate in 25 ml of CH2C12 are
added dropwise to 100 g of N,N'-dimethylethylenedi-
amine at 5C. After 4 hour~ at RT, the exces~ diamine
is distilled off. The residue i~ taken up in EA
(100 ml) and the mixture is washed with saturated
NazCO3 ~olution and sa~urated NaCl ~olution. Drying
with Na2SO4 and concentration gives the title compound,
which is used in the crude form for further reactions.
MS (DCI): 189 ~M+l)
Example 5
3-(4-Amino-l-piperidinyl-carbonyl)-2(R)-benzylpropionyl~
His-[(D)-manno-pentol]
The title comE~ound is prepared analogously to Example 3
from 3-[4-(tert.-butoxycarbonyl)amino-1-piperidinyl-
carbonyl]-2(R)-benzylpropionic acid.
R (acetone/H2O/concentrated NH3 100:10:5) = 0.15
MS (FAB): 687 ~M+1)
a) 3-[4-(tert.-Butoxycarbonyl)amino-1-piperidinyl-carb-
onyl]-2(R)-benzylpropionic acid
1.3 g of benzyl 3-[4~(tert.-~utoxycarbonyl~amino-1-
piperidinyl-carbonyl]-2(R)~benzylpropionate are
hydrogenated in 60 ml of EtOH with 200 mg of PdtC at
RT for 1 hour. After filtration and removal of the
2~23L~22
- 36 -
solvent in vacuo, the title compound crystallizes out
of cold diethyl ether.
m.p.: 135 - 136C
Rf (CH2Cl2/MeOH 9:1) = 0.30 MS (DCI): 391 (M+l)
b) Benzyl 3-~4-(tert.-butoxycarbonyl)amino-l-piperidinyl-
carbonyl]-2(R)-benzylpropionate
1.0 g of benzyl 2(R)-(carboxymethyl)-3-phenyl-propion-
ate (J. Med. Chem. 31 2277 (1988)) are stirred in
50 ml of CH2Cl2 with 0.31 ml of oxalyl chloride and
1 ml of DNF at 0C for 1 hour. The ~olvent is removed
in vacuo, the residue is taken up in 25 ml of CH2C12,
the pH is brought to 7 with 2 drops of triethylamine,
0.71 g of 4-(tert.-butoxycarbonyl)-amino-piperidine
and 0.47 ml of triethylamine in 50 ml of CH2C12 are
added dropwise to this solution, the mixture is
stirred at 0C for 3 hours, the solvent is removed in
vacuo, the residue is taken up in 50 ml of EA and the
mixture is washed once each with 50 ml of 2N HCl and
saturated NaHC03 solution. The mixture is dried over
MgS04 and the solvent is removed in vacuo to give 1.3 g
of the title compound as a colorless oil.
Rf (CH2Cl2/NeOH 9sl) = 0.50 NS (DCI)s 479 (M+H)
c) 4-(tert.-Butoxycarbonyl)amino-piperidine
10.0 g of l-benzyl-4-[(tert.-butoxycarbonyl)amino]
piperidine are hydrogenated in 60 ml of ethanol/glac-
ial acetic acid 9:1 with 1 g of Pd/C for 1 hour at RT
under a pressure of 1 bar. The catalyst is filtered
off, the solvent is removed in vacuo (residues of
glacial scetic acid are distilled off azeotropically
with toluene) and the residue is taken up in 100 ml of
EA. The mixture i8 then washed once each with 100 ml
of saturated NaHC03 solution and saturated NaCl solu-
tion and dried over NgS04 and the solvent is removed in
?.', ~'~ ', ' i , ,)
- 37 -
vacuo. The residue is recrystallized from EA to give
5.5 g of the title compound as white crystals~
m.p.: 159 - 161C
R~ (CH2Cl2/MeOH 9:1) = 0.11 MS (DCI)o 201 (M+l)
d~ l~Benzyl-4-[(tert.-butoxycarbonyl)amino]-piperidine
10.O g of 4-amino-N-benzylpiperidine are dissolved in
100 ml of CH2Cl2, 11.5 g of di-tert.-butyldicarbonate
are added and the solution is stirred at RT for 2
hours and left to stand overnight. The solvent is
removed in vacuo and the residue is recrys~allized
from EA. 12.9 g of the title compound are obtained as
white crystals.
m.p.: 123C
Rf (CH2Clz/MeOH 8:1) = 0.23 MS (DCI): 291 (M+l)
~xample 6
2(S)-(4-Amino-l-piperidinocarbonyloxy)-3-phenylpropionyl-
His-[(D)-manno-pentol]
The title compound i5 prepared analogously to Example 3
from 2(S)-[4-(t:ert.~utoxycarbonyl)amino-l-piperidinocar-
bonyloxy]-3-ph~nylpxopionic acid.
R~ (acetone/H2O/concentrated NH3 100:10:5) = 0.15
MS (FAB): 689 (M+l)
a) 2(S)-[4-(tert.-Butoxycarbonyl)amino-l-piperidinocarb-
onyloxy]-3-phenylpropionic acid
1.8 g of ethyl (2(S)-[4-(tert.-buto~ycarbonyl)amino-
l-piperidinocarbonyloxy]-3-phenylpropionate and 5.1 ml
of lN NaOH are dissolved in 30 ml of ethanol and the
mixture is stirred at RT for 18 hours. It is then
diluted with 50 ml of H2ol the ethanol is removed in
~2~
- 38 -
vacuo and the pH is brought to 1 - 2 with NaHS04
solution. The mixture is extracted three times with
100 ml of EA, the extract is dried over Na2S04 and the
solvent is removed in vacuo. The title compound, which
crystallizes from ether~n-heptane, is obtained, 1.0 g
of white crystals.
m.p.: 100 - 101C
Rf (CH2Cl2/MeOH) = 0.29 MS (DCI): 393 (M+l)
b) Ethyl 2(S)-t4-(tert.-butoxycarbonyl)amino-1-piperi-
dinocarbonyloxy]-3-phenylpropionate
825 mg of ethyl phenyllactate and 1.8 g of di-(l-
benzotriazolyl)carbonate (70~) are dissolved in 40 ml
of CH2Cl2, 386 ~1 of ethyl diisopropylamine are added
and the mixture is stirred at RT for 18 hours. 850 mg
of 4-(tert.-butoxycarbonyl)aminopiperidine (Example
5c)), dissolved in 10 ml of CH2Cl2, are then added
dropwise and a further 386 ~1 of ethydiisopropylamine
are added. The mixture is stirred at RT for a further
2 hours, the solvent is removed in vacuo and the
residue is taken up in 100 ml of MTB. The mixture is
washed with in each case 100 ml of Na2C03 solution and
100 ml of NaHS04 solution and dried over Na2S0~ and the
solvent is removed in vacuo. 1.8 g of the title
compound are obtained a~ a colorless oil which is
employed further without purification.
R~ (Hep/EA 2:1) = 0.2 MS (DCI): 421 (M+l)
~ample 7
2(S)-(4-Aminomethyl-l-piperidinocarbonyloxy)-3-phenylpro-
pionyl-His-[( D)-manno-pentol]
The title compound is synthesized analogously to Example
6.
~2~
-- 39 --
Rf ( acetone/H20/concentrated NH3 lOO:lOs5) = 0.15
MS (FAB)s 703 (N+l)
E~cample 8
(3-t-Butoxysulfonyl-2-thienylmethyl)propionyl-His-t(D)-
manno-pentol]
The title compound i8 synthesized analogously to Example
2.
R (acetone/water lOsl) = 0.50 MS (FAB)s 687 (M+l)
E~cample 9
(2(S)-Benzyl-3-t-butylsulfonyl)propionyl-His-4(S)-(5-
cyclohexyl-l,2,3-trihydroxy)-pentylamide
240 mg of (2(S)-benzyl-3-t-butylsulfonyl)propionyl-His-
(DNP)-OH are dissolved with 69 mg of HOBt, 93 mg of DCC
and 0.3 ml of NEN in 8 ml of DNF (absolute) and the 801u-
tion is cooled to 0C. The solution, described under 9a)
of 4(S)-(5-cyclohexyl-1~2~3-trihydroxy)pentylamine is
then added. After warming up, the solution remains at RT
for 48 hours. 0.5 ml of H20 are added to the reaction
solution, the dicyclohexylurea formed is then filtered
off and the filtrate i8 taken up in 25 ml of EA. This
solution is washed with 10% strength NaHCO3 solution,
water and saturated NaCl solution and filtered over
cotton absorbent and the filtrate is concentrated in
vacuo. The oily crude product thus obtained is
chromatographed over silica gel using CH2C12/CH30H 20sl.
The product of Rf 0.5 (CH2Cl2/CH30H 9:1 on silica gel) is
obtained as a vitreous solid in ~I yield of 60 mg.
MS (FAB) 787 (N+l)
The product is dissolved in 5 ml of CH2C12 (absolute) and
the solution was stirred at RT with 0.2 ml of thiophenol
~ i~ 2 ~ ~ t~
- 40 -
for 4 hours. After the solvent has been filtered off in
vacuo, the crude mixture is chromatographed on silica gel
using CH2Cl2/CH30H 20:1. 16 mg of the title compound are
obtained.
5 MS (FAB): 621 (M+l)
Rf (CH2C12/MeOH 9:1) = 0.25
a) 4(S)-(5-Cyclohexyl-1,2,3-trihydroxy)-pentylamine
130 mg of (2RS,3RS,4S)-4-tert.-butoxycarbonylamino-5-
cyclohexyl-1,2,3-trihydroxypentane (Example ~b) are
dissolved in 2 ml of DNE, 4 ml of saturated HCl~DNE
solution are added and the mixture is stirred at 0C
for 30 minutes. After w~rming up to RT, the solution
is concentrated in vacuo and the residue is concentra-
ted three times, in each case after addition of
toluene (absolute). This oil thus obtained is immedi-
ately employed as a solution in DNF in the subsequent
reaction step.
b) (2RS,3RS,4S)-4-tert.-Butoxycarbonylamino-5-cyclohexyl-
1,2,3-trihydroxypentane
293 mg of the pentenol obtained in Example 9c) are
dissolved in 10 ml of THF (ab~olute) together with 240
mg of trimethylamine N-oxide, and 11 mg of osmium
tetroxide are added. After a few minutes, the solution
changes color to green-brown and i8 stirred overnight
at RT. 10~ strength NaHS03 solution i8 added to the
solution, the mixture is stirred for 30 minutes and
then concentrsted in vacuo, the residue i8 taken up
in 50 ml of ethyl acetate and the aqueous phase is
separated off. The organic phase is washed with 1N HCl,
10% strength Na2S0~, dried and concentrated in vacuo.
An amorphous solid of 140 mg remains.
MS (DCI): 318 (M+l)
~2~822
- 41 -
c) 4S-tert~Butoxycarbonylamino-5-cyclohexyl-cis-2-
pentenol
5.5 ml of a 1.2 M solution of diisobutylaluminum
hydride in toluene were added to 0.98 g of 4S-tert.-
butoxycarbonylamino-5-cyclohexyl-cis-2-pentenoic acid
(prepared in accordance with the method of W.C. Still
et al. Tetrahedron Lett. 1983, 4405) in 20 ml of
absolute methylene chloride at -78C. After 1 hour,
the mixture was allowed to warm to RT. Addition of
1 ml of methanol ends the reaction, and after dilution
with 80 ml of CH2Cl2 the solution i8 shaken in each
case once with 10% ~trength K Na tartrate solution,
10~ strength citric acid solution and 6aturated NaCl
solution. The solution is dried over MgSO4 and con-
centrated in vacuo and the resulting oily crude
product is chromatographed over silica gel (cyclo-
hexane/ethyl acetate). 480 mg of a slightly yellow oil
are obtained.
E~ample 10
Iva-Phe-Nva-t(D)-manno-pentol]
The compound is prepared snalogously to Example 1) and
la) from Iva-Phe-Nva-OH and H-(D)-manno-pentol(XX).
Rf (CH2Cl2/MeOH) = 0.06 NS (FAB + LiI)s 614 (N+Li)
The title compounds of Examples 11 and 12 are prepared
analogously to Example 2:
E~ Iple 11
Indolyl-2-carbonyl-His-[(D)-manno-pentol]
R~ (CH2CH2:MeOH:concentrated aqueous NH3 = 50:10:1) = 0.10
MS (FAB): 558 (M+l)
b:, ' : ~ ,. ., `j
- 42 -
Example 12
2(S)-~ydroxy-3-phenylpropionyl-His-[~D)-manno-pentol]
R~ (acetone/H20 10:1) = 0~25 MS (FAB): 563 (M+l)