Note: Descriptions are shown in the official language in which they were submitted.
CA 02022018 2000-OS-08
HEMODYNAMICALLY RESPONSIVE SYSTEM
FOR TREATING A MALFUNCTIONING HEART
BACKGROUND OF THE INVENTION
1. Field of the Invention
This invention relates to a system for
treating a malfunctioning heart and, more particularly,
to such a system which effects
cardioversion/defibrillation in response to sensing a
heart malfunction. The term "hemodynamic parameter",
as used herein, means any parameter which may be sensed
or determined and either directly or indirectly affects
the motion or constituents of blood or performance of
the heart within the circulatory system. The invention
provides for the cardioverting/defibrillation of a
malfunctioning heart as well as the possibility of
overcoming a tachycardia manifestation without
resorting to either cardioverting or defibrillating the
heart.
2. Description of the Prior Art
In recent years, substantial progress has been
made in pacemakers and in the development of
cardioverting/defibrillating techniques for effectively
treating various heart disorders and arrhythmias. Past
efforts have resulted in the development of implantable
electronic pacemakers and standby cardioverters-
defibrillators which, in response to the detection of
an abnormal cardiac rhythm, discharge sufficient energy
via electrodes connected to the heart to depolarize and
restore it to normal cardiac rhythm. An early example
f? ,A 7
~~~~,~~,r~.~~
of this cardioverting/defibrillating technique is
disclosed in U. S. Pat. No. 3,942,536 of Mirowski et
al., the technique involving responses to a sensed
peak right ventricular systolic pressure dropping to a
fixed predetermined threshold level. This known
technique did not involve mean pressure changes in
either direction from a baseline. Nor did it involve
sensing of pressure within any vessels which extends
between the heart and lung(s).
Efforts have also been directed toward developing
techniques for reliably monitoring heart activity in
order to determine whether cardioversion/defibrillation
are desirable or necessary. Such techniques include
monitoring ventricular rate or determining the presence
of fibrillation on the basis of a probability density
function (PDF). A system using the PDF technique
statistically compares the location of points of a
cardiac waveform with the expected locations of points
of the normal waveform. When the waveform becomes
irregular, as measured by its probability density
function, an abnormal cardiac function is suggested.
The latter technique is described in U. S. Pat. Nos.
4,184,493 and 4,202,340 both of Langer et al.
A more recent system, as disclosed in U. S. Pat.
No. 4,475,551 of Langer et al. utilizes both the PDF
technique to determine the presence of an abnormal
cardiac rhythm and a heart rate sensing circuit for
distinguishing between ventricular fibrillation and
high rate tachycardia (the latter being indicated by a
heart rate above a predetermined minimum threshold), on
the one hand, and normal sinus rhythm or a low rate
tachycardia (indicated by a heart rate falling below a
pre-determined minimum threshold), on the other hand.
Still further, research in this area has resulted
in the development of a heart rate detector system
which accurately measures heart rate from a variety of
-2-
~~,. ;~~
P.~~%:~.~0
different electrocardiogram (ECG) signal shapes. One
such system is disclosed in U. S. Pat. No. 4,393,877 of
Imran et al.
Despite these past efforts and the level of
achievement prevalent among prior art systems, there
are potential difficulties and drawbacks which may be
experienced with such devices.
Currently antitachycardia systems detect
arrhythmias primarily by sensing rate and perform
inadequately in the differentiation of hemodynamically
stable from unstable rhythms. These devices, for
example, may fire during a stable supraventricular
tachycardia (SVT) inflicting pain and wasting energy;
damage to the heart may resu~a.
A commonly used implantable antitachycardia device
is the automatic implantable cardioverter-
defibrillators (A~CD) which is commercially available
under~the model designations 1500, 1510 and 1520 from
Cardiac Pacemakers, Inc. whose address is: 4100 North
Hamlin Avenue, St. Paul, Minnesota 55164. These
devices continuously monitor myocardial electrical
activity, detecting ventricular tachycardia (VT) and
ventricular fibrillation (VF), and delivering a shock
to the myocardium to terminate the arrhythmia. The
AICD has been shawn to reduce the mortality rate in
patients with malignant arrhythmias with initial
studies at Johns Hopkins Hospital and Stanford Medical
Center demonstrating a 50 percent decrease in the
anticipated total incidence of death, as reported by
Mirowski et al., "Recent Clinical Experience with the
Automatic Implantable Cardioverter Defibrillator",
Medical Instrumentation, Vol. 20, pages 285-291 (1986).
Arrhythmias are detected by (1) a rate (R wave) sensor
and (2) a probability density function (PDF) which
defines the fraction of time spent by the
differentiated electrocardiogram between two amplitude
-3-
'da~~iJ.'..~.~
~~ ~~'~'~ '
limits located near zero potential. Presently, the
functional window of the PDF is wide to permit the
detection of both VT and VF, and therefore, this device
functions essentially as a rate-only sensing system.
As reported by Mirowski, "The Automatic Implantable
Cardioverter-Defibrillator: An Overview°', JACC, Vol. 6,
No. 2, pages 461-466, (August, 1985), when an
arrhythmia fulfills either the rate or PDF criteria,
the device delivers Schuder's truncated exponential
pulse of 25 Joules same 17 seconds after the onset of
the arrhythmia. The device can recycle as many as
three times if the previous discharge is ineffective
with the strength of the second, third and fourth
pulses being increased to 30 Joules. After the fourth
discharge, approximately 35 seconds of nonfibrillating
rhythm are required to reset the device. The Mirowski
et al., su ra, and the Mirowski, su ra publications set
out, in summary form, background material relating to
the defibrillating/cardioverting arts against which the
present invention was made.
In addition to the standard automatic implantable
cardioverter-defibrillator characterized by the
above-noted, dual detection algorithm, a variant of the
device which features a sensing system that relies only
on the analysis of heart rate is also available. This
"rate-only" version of the known cardioverter-
defibrillator preferred by some investigators, is more
sensitive than the dual detection version unit and
theoretically less likely to miss ventricular
tachycardias with narrow QRS complexes. It is believed
that the "rate-only" system, on the other hand, may be
too sensitive, delivering cardioverting/defibrillating
pulses too often or too soon, no hemodynamic parameter
having been taken into consideration.
One problem with current systems is that they
function primarily as a rate-only sensing systems and
-4-
CA 02022018 2000-OS-08
may fire for nonmalignant as well as malignant
tachycardias. These firings are not benign;
potentially endangering myocardium, wasting energy and
inflicting pain on the conscious patient, all distinct
shortcomings and disadvantages.
Prior proposals of the present invention involving
mean pressure determinations at some points in a
circulatory system are disclosed in EPO application of
Todd J. Cohen published May 24, 1989 under publication
No. 0317065A2.
The principal object of the present invention is
to provide a system for cardioverting/defibrillating
which avoids unnecessary firings, thereby reducing the
danger to the myocardium, saving energy and avoiding
pain.
Another object of the present invention is to
provide an implantable system for cardioverting/-
defibrillating which avoids unnecessary firings,
thereby reducing the danger to the myocardium, saving
energy and avoiding pain.
A further object of the present invention is to
provide a system for cardioverting/defibrillating which
is hemodynamically responsive to change in a
hemodynamic parameter, such as pressure, at a site in
the circulatory system of a patient.
An additional object of the present invention is
to provide a system for cardioverting/defibrillating
which is hemodynamically responsive to change in a
selected parameter from a baseline (either fixed or
varying) and to rate criteria.
-5-
CA 02022018 2000-OS-08
In accordance with preferred embodiments of the
present invention, new sensing algorithms are proposed
using hemodynamic or both hemodynamic and rate
criteria, the latter being taken in series or parallel.
The series configuration algorithm could be effected by
detecting rate with an intracardiac, extracardiac, or
body-surface R-wave sensor. When rate exceeds the
programmed cut-off value, at least one hemodynamic
parameter, such as mean pulmonary artery pressure
(MPAP), mean pulmonary vein pressure (MPVp), right
ventricular systolic pressure (RVSP), right ventricular
end diastolic pressure (RVEDP) or right ventricular
pulse pressure (RVPP) departures from a fixed or
variable baseline would be monitored. If the
hemodynamic parameter departs from the fixed or
variable baseline level within a time period of
predetermined duration, indicating hemodynamic
compromise, the system would fire. If the respective
pressure changes were less than the respective
predetermined magnitudes, pressures would be monitored
to determine if respective changes from the respective
baseline levels take place, as long as the rate
criteria is satisfied. The system and method of the
invention as disclosed herein may involve mean
pulmonary artery pressure (MPAP), mean pulmonary vein
pressure (MPVP) or mean pulmonary capillary wedge
pressure (MPCWP).
A parallel configuration algorithm in which rate
and hemodynamic criteria function simultaneously is
also proposed; however, continuous pressure change
determination would probably be less energy efficient.
Either configuration of algorithm could be adapted, in
some cases, to a single catheter consisting of a
-6-
~~ _~ t3
pressure transducer in either the right atrium or right
ventricle and an R-wave sensing electrode or pair of
electrodes at the catheter tip in the right ventricle.
The hemodynamic information derived from an arterial
line, Swan-Ganz catheter (already present in the
intensive/cardiac care unit patients), or even an
automated mechanical blood pressure cuff could be
integrated together with the electrocardiogram to
provide a temporary automatic antitachycardia system.
Cardioversion-defibrillation could be administered
using externally applied patches: Even a noninvasive
hemodynamically responsive antitachycardia system is
potentially feasible using doppler technology for
pressure measurements. The PDF (narrow window of
function) and the rate/pressure sensing algorithm could
be used simultaneously such that if the rate/pressure
criteria are satisfied (indicating hemodynamically
significant SVT or VT) the device cardioverters and if
the PDF criteria is satisfied indicating (VF)
defibrillation results. This pulse delivery system
could also be incorporated into a single catheter.
It is to be appreciated that when the pressure
criteria is not met, but the rate criteria indicates
tachycardia is present, an antitachycardia pacemaker
could be enabled in an effort to correct the
malfunction.
The rate/pressure sensing algorithms could also
help integrate a cardioverter-defibrillator with an
antitachycardia pacemaker. The hemodynamic function
would determine which of these devices to engage. For
example, when a hemodynamically significant tachycardia
is detected the cardioverter-defibrillator would be
used to terminate the arrhythmia. When a
hemodynamically stable tachycardia is sensed the
antitachycardia pacemaker would attempt to terminate
the arrhythmia using such methods as overdrive, burst,
~ (T
~~~~eej
or extra stimulus pacing, incremental or decremental
scanning, or ultra-high frequency stimulation. If the
tachycardia was accelerated, this would be detected by
the rate/pressure sensing algorithm and cardioverted or
defibrillated. With a pacemaker present, a bradycardia
failsafe could be built into the system.
The adaptation of a hemodynamic parameter to the
sensing system of antitachycardia devices appears to be
a logical improvement to its present function. RVSP,
RVPP and RVEDP are easily determined parameters (via
the transvenous route) and appear to relate important
hemodynamic information. A rate/pressure sensing
algorithm, designed either in series or parallel, could
be integrated with the PDF system such that
hemodynamically significant SVT, VT, and VF would be
detected. The rate/pressure sensing algorithm could
also be applied to a combined cardioverter-
defibrillator and antitachycardia pacemaker.
In its apparatus aspect, the invention can be seen
as being in a system for treating a malfunctioning
heart of the type which includes storage means for
storing electrical energy and electrode means for
electrically coupling the storage means to the heart.
Determining means are provided for determining at least
one hemodynamic parameter. Means provide a first
signal representative of baseline level for the
parameter. Means responsive to output from the
determining means develop a second signal representing
current level of the parameter over a period of given
duration. Means responsive to output from the means
for providing the first signal and output from the
means for developing the second signal charge and
enable discharge of the electrical energy stored by the
storage means across the electrode means upon change in
the current level of the parameter of at least a
predetermined amount from the representative baseline
_g_
CA 02022018 2000-OS-08
level for the parameter.
The means providing a signal representative of
baseline level for the parameter may be constituted by
means providing a signal representative of a fixed
baseline level for the parameter.
The means for providing a first signal
representative of baseline level for the parameter may
be constituted by means for developing a variable first
signal representative of baseline for the parameter
over a period of predetermined duration which is
greater than th,e.period of given duration.
Stated differently, the invention can be seen as a
system for treating a malfunctioning heart which
includes providing a representation of baseline for a
hemodynamic parameter and determining current level of
the parameter over a period of given duration.
Therefore, the system involves delivering
cardioverting/defibrillating electrical energy to the
heart in response to change of at least a predetermined
magnitude in the current parameter from the baseline
for the parameter.
The step of providing a representation of baseline
for the parameter may be constituted by providing a
fixed representation of baseline for the parameter.
The step of providing a representative of baseline
for the parameter may be constituted by providing a
varying representation of baseline for the parameter
over a period of predetermined duration which is
greater than the period of given duration.
The novel features that are considered
characteristic of the invention in its various
aspects are set forth with particularity in the
appended claims. The invention itself, however, both
as to its organization and its method of operation,
together with other objects and advantages thereof is
to be understood from the following description of
_g_
,,
illustrative embodiments, when read in conjunction with
the accompanying drawings, wherein like reference
numerals refer to like components.
BRIEF DESCRIPTION OF THE DRAWINGS
FIG. 1 is a diagrammatic, generalized illustration
of an exemplary, implanted hemodynamically responsive
system for treating a malfunctioning heart.
FIG. 2A is an illustration of one catheter
positioned within a heart, a pressure responsive sensor
forming part of the catheter being shown positioned
inside the right ventricle.
FIG. 2B is an illustration of a second catheter
positioned within a heart, a pressure responsive sensor
forming part of the catheter being shown positioned
within the right atrium.
FIG. 2C is an illustration of a third catheter
positioned within a major vein feeding into the
superior vena cava or in the vena cava itself.
FIG. 2D is an illustration of a fourth catheter
positioned within the left side of the heart, a
pressure responsive sensor being shown positioned
within the left ventricle.
FIG. 2E is an illustration of the fourth catheter
positioned within the left side of the heart, a
pressure responsive sensor a pressure responsive sensor
being shown positioned within the left atrium.
FIG. 2F is an illustration of the fourth catheter
positioned within the left side of the heart, a
pressure responsive sensor being shown positioned at a
point in the arterial system.
FIG. 2G is an illustration of a variant in which
an external blood pressure cuff is provided to sense '
arterial pressure, from which MAP can be derived.
FIG. 2H is an illustration of a fifth catheter
positioned within the left side of the heart, a
-10-
~' ,.;) ~,, .~ s:
S.tg f~ . d ''%r i fv
pressure responsive sensor being shown positioned
within a pulmonary artery between the heart and at
least one lung.
FIG. 2I is an illustration of a sixth catheter
positioned within the left side of the heart, a
pressure responsive sensor being positioned with a
pulmonary vein between at least one lung and the heart.
FIG. 2J is an illustration of a fifth catheter
positioned within the left side of the heart, a
pressure responsive sensor being positioned to effect
sensing of pulmonary capillary wedge pressure.
FIG. 3 is a pictorial illustration of an exemplary
implantable controllable cardioverting/defibrillating
electrical energy generator which may be used in
practicing the present invention, the housing of the
generator being partially broken away to show
positioning of major components thereof.
FIG. 4 is a partially block, schematic diagram of
a hemodynamically responsive system for treating a
malfunctioning heart which is pressure responsive.
FIGS. 5A and 5B constitute a first exemplary
flowchart of a series of actions or steps which may be
carried out by the system illustrated in FIG. 4 and
effect achievement of a corresponding method.
FIG. 6 is a partially block, schematic diagram of
a further hemodynamically responsive system for
treating a malfunctioning heart which is pressure and
rate responsive.
FIGS. 7A and 7B constitute a second exemplary
flowchart of a series of actions or steps which may be
carried out by the system illustrated in FIG. 6 and
effect achievement of a corresponding method.
FIG. 8 is a partially block, schematic diagram of
hemodynamically responsive system for treating a
malfunctioning heart which is a variant of the circuit
of FIG. 6.
-11-
FIGS. 9A and 9B constitute a third exemplary
flowchart of a series of actions or steps which may be
carried out by the system illustrated in FIG. 8 and
effect achievement of a corresponding method.
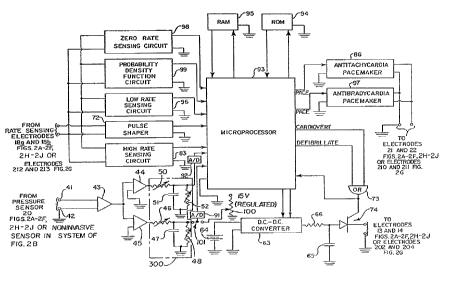
FTG. 10 is a partially block, schematic diagram of
a hemodynamically responsive system for treating a
malfunctioning heart which provides a microprocessor
implementation in accordance with preferred embodiments
of the present invention, as well as those illustrated
in FIGS. 4, 6 and 8.
FIGS. 11-13 are respective graphical
representations along a time axis of a rate wave
(R-wave), mean arterial pressure (MAP) and mean right
atrial pressure (MRAP) of a canine subject respectively
under high right atrial pacing, right ventricle apex
pacing and in ventricular fibrillation, useful in
understanding the present invention.
FIG. 14 is a graphical representation along a time
axis similar to the graphical representation of FIG.
13, the time base having been expanded to show the
affects on the R-wave, the MAP and MRAP which result
from successful defibrillation.
FIG. 15 is a partially block, schematic diagram of
a hemodynamically responsive system for treating a
malfunctioning heart in accordance with an exemplary
embodiment of the invention which is pressure
responsive.
FIGS. 16A and 16B constitute an exemplary
flowchart of a series of actions or steps which may be
carried out by the system of the present invention
illustrated in FIG. 15 and effect achievement of the
invention in its method aspect.
FIG. 17 is a partially block, schematic diagram of
a hemodynamically responsive system for treating a
malfunctioning heart in accordance with a further
exemplary embodiment of the invention which is pressure
-12-
and rate responsive.
FIGS. 18A and 18B constitute a further exemplary
flowchart of a series of actions or steps which may be
carried out by the system of the present invention
illustrated in FIG. 17 and effect achievement of the
invention in its method aspect.
FIG. 19 is a partially block, schematic diagram of
hemodynamically responsive system for treating a
malfunctioning heart which is a variant of the circuit
of FIG. 17.
FIGS. 20A and 20B constitute an additional
exemplary flowchart of a series of actions or steps
which may be carried out by the system of the present
invention as illustrated in FIG. 19 and effect
achievement of the invention in its method aspect.
FIGS. 21-23 and 24A, 24B are respective simplified
block diagrams of'signal processing circuits which may
be used in the circuits of the present invention to
determine respectively RVSP, RVDP, RVEDP and RVPP, in
accordance with the present invention.
FIG. 25 is a graphical representation useful in
understanding the present invention which shows the
respective variations of hemodynamic parameters, in
particular pressure parameters, collected during a
study of a number of patients.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
As shown in FIG. 1, an exemplary embodiment of an
automatic implantable cardioverter-defibrillator system
is designated generally by the numeral IO and
illustrated diagrammatically as being implanted within
a human subject 9. The cardioverter-
defibrillator system 10 includes an implanted housing
12 within which major circuit components of the system
are housed. A first electrode 13 is positioned within
-13-
~
, cT, Cy n, ~" C"~
3"7 Yd F..a ~J . >o.
the heart 11 of the subject 9, the details of placement
and nature of the first electrode being more
specifically shown in FIGS. 2A-2F and 2H-2J to which
reference is to be made below. A second electrode,
illustrated as a patch electrode 14 is positioned on
the outside of the heart 11 at the apex thereof. The
pair of electrodes 13, 14 are provided for the purpose
of delivering D.C. cardioverting/defibrillating energy
from within the housing 12 to the heart 11 under
control of circuitry within the housing, a pair of
insulated leads 16 and 15 respectively being provided
for this purpose. A pair of rate sensing electrodes 18
are provided within the heart 11, these electrodes
being positioned in tissue and being conductively
coupled to circuitry within the housing 12 via an
insulated cable 17. A further pair of leads extend
from a pressure responsive pressure-to-voltage
transducer 20 to circuitry within the housing 12 via an
insulated cable 19. It is to be understood that the
insulated leads 15 and 16, the insulated cable 17 (or
the pair of leads therein), and the insulated cable 19
(or the pair of leads therein) can all be incorporated
into a single cable, the electrode 13, the rate sensing
electrodes 18 and the pressure transducer 20 being
carried by and forming parts of a catheter.
Pacemaking circuitry within the housing 12 may be
provided to produce antitachycardia pacemaking signals,
to a pair of pacing electrodes 21 and 22, illustrated
as being fixed in tissue on the right-side of the
heart. The pacing electrodes 21 and 22 are connected
by respective conductive leads within a cable 23 which
communicates with circuitry within the housing 12.
Turning to FIG. 2A, a more detailed illustration
of the heart 11 of a subject, shows the heart in
somewhat more detail and in section so that placement
of parts of the system within the heart 11 can be seen
-14-
;I,. G'y G~1~ ~-,. :i e':
'V FJ hl ~'v .~.
in more detail, albeit diagrammatically. The heart 11
as illustrated includes a right ventricle 26, a right
atrium 27, a left atrium 28 and a left ventricle 30.
The electrode 13 is positioned within the superior vena
cava. It is to be understood that the patch electrode
14, which cooperates with the electrode 13, could also
be modified into a different form so it too could be
positioned within the heart. The electrode 13 could be
replaced with a patch electrode so that it also could
be positioned on the surface of the heart, without
departing from the present invention. The electrodes
13 and 14, in cases not involving implantation, could
be replaced with conventional paddle electrodes or
other external, body engaging electrodes, again without
departing from the present invention. Thus, the
invention could be used as a temporary measure for
patient care in intensive care units and the like.
As illustrated in FIG. 2A, the pacing electrodes
21 and 22 are shown as being positioned on the exterior
wall of right ventricle 26 fox the purpose of
illustration; these pacing electrodes could be placed
elsewhere on or within the heart 11 in accordance with
the needs of individual patients, taking into account
the best particular location most suitable for
correcting or overcoming the particular malfunction
involved, the condition of the individual patient and
his or her heart being taken into account.
Heart rate wave (R-wave? sensing electrodes 18a
and 18b are illustrated as being positioned near the
apex of the heart 11 within the right ventricle 26, for
purposes of illustration. Other locations are equally
well suited; again, the selected location being chosen
with the condition of the particular patient and his or
her heart in mind. The electrodes 18a and 18b are
conductively connected to the circuitry within the
housing 12 via leads 17a and 17b within the cable 17.
-15-
J FE l .t.. iJ
The pressure-to-voltage transducer 20, as
illustrated in FIG. 2A, is positioned within the right
ventricle 26. Two conductive leads 19a and 19b within
the cable 19 (FIG. I) provide electrical communication
from the pressure responsive transducer 20 to circuitry
within the housing 12 (FIG. 1). Thus, a D.C. voltage
signal representative of the actual, instant pressure
within the right ventricle 26 is fed to the circuitry
within the implanted housing 12 (FIG. 1).
IO As illustrated in FIGS. 2B-2F and 2H-2J the heart
11, as well as the components of the system of the
present invention, other than the pressure-to-voltage
transducer 20, correspond to the heart 11 and the
system components as shown in FIG. 2A. The placement
of the transducer 20 differs, in each of FIGS. 2B-2F
and 2H-2J. As shown in FIG. 2A, the transducer 20
provides, as its output, a variable D.C. voltage
representative of the varying pressure within the right
ventricle 26. As shown respectively in FIGS. 2B-2F and
2H-2J, the transducer 20 is positioned within and
produces a variable D.C. voltage which represents
respectively the pressure within the right atrium 27,
within the central venous system (in particular, a
major vein 29) the left ventricle 30, the left atrium
28, the arterial system (in particular, an artery 31
remote from the heart 11), a pulmonary artery, a
pulmonary vein and a point to sense pulmonary capillary
wedge pressure.
In FIG. 2H, a fifth catheter is shown positioned
in the right side of the heart. A pressure responsive
sensor 20, in this case, is positioned in one of the
pulmonary arteries extending toward one of the lungs.
The sensor 20 could, if desired, be positioned more
upstream in the layer pulmonary artery which carries
blood to both lungs. The sensor 20 could be positioned
in one of the smaller arteries which carries blood to
-16-
~? ~J~ f..°s f), ." i'..
i 6,~ r3 1.~~ ~.
one or another of the lobes of one lung. The other,
components of the catheter fifth correspond to those
illustrated in FIGS. 2A-2C.
It is also within the contemplation of the present
invention to place the pressure sensor 20 within a
pulmonary vein (feeding into left side of heart), as
shown diagrammatically in FIG. 2I; in this case the
conductive leads 19a, 19b and the cable 19 are
positioned in the vicinity of the vein, with the leads
19a and 19b extending through the wall of the vein. In
this case, the other components of the sixth catheter
correspond to those of the catheters shown in FIGS.
2D-2F.
Referring to FIG. 2J, as sensor 20 is shown
positioned within a small blood vessel (being fed from
a minor pulmonary artery) for the purpose of measuring
pulmonary capillary wedge pressure. In a realized
study conducted by applicant, pulmonary capillary wedge
pressure was sensed using a dual lumen transvenous
ballon tip catheter which was placed into the right
heart chambers through the internal jugular vein and,
thence, into the blood vessel. The other components of
the catheter shown in FIG. 2J correspond to those shown
in FIGS. 2H and 2I.
In FIG. 2G a portion of a noninvasive system for
sensing heart rate and pressure of the type which may
be used in an intensive care unit (ICU), a recovery
room, coronary care unit (CCU), and/or in a routine
care patient facility is illustrated. The system of
3n FIG. 2G can be considered a system which can be
substituted for the invasive systems shown in FIGS. 1,
2A-2F and 2H-2J. A patient 200 is shown in a reclined
posture on a bed 201. A pair of pulse-delivering
electrodes 202 and 204 (substitutes for electrodes 13,
14; FIGS. 2A-2F and 2H-2J) are positioned respectively
on the anterior and posterior chest of the patient 200
-17-
rs .i r)
~~'a~~"~_
for the purpose of coupling cardioverting/-
defibrillation energy pulses to the patient, respective
insulated leads 205 and 206 (substitutes for leads 15,
16; FIGS. 2A-2F and 2H-2J) and a cable 203 being
provided to conduct the pulses to the patient, from a
pulse-generating apparatus 208 (substitute for the .
circuitry within housing 12; FIG. 1). The leads 205
and 206 and electrodes 202 and 204 are to be used in
place of the cardioverting/defibrillating electrodes 13
1~ and 14 (FIGS. 1, 2A-2F and 2H-2J), were the system of
the present invention to be used in a noninvasive
stand-alone or portable or patient-carried
configuration, instead of in an implantable
configuration as illustrated in FIGS. 1, 2A-2F and
2H-2J. Positioned concentrically about the respective
electrodes 202 and 204 and insulated therefrom, are
respective pacing electrodes 210 and 211 (substitutes
for 21, 22; FIGS. 1, 2A-2F and 2H-2J). A pair of
respective rate (R-wave) sensing electrodes 212 and 213
(substitutes for electrodes 18, FIG. 1; 18a, 18b, FIGS.
2A-2F and 2H-2J) are provided centrally within and
insulated from the electrodes 202 and 204,
respectively. The pair of rate-sensing electrodes 212,
213 are connected respectively via respective insulated
leads 214, 215 and a cable 216 to the apparatus 208.
The pair of pacing electrodes 210, 211 are connected
respectively via respective insulated leads 217, 218
and a cabJ.e 219 to the apparatus 208.
Moreover, rather than an invasive pressure
transducer of the type illustrated in FIGS. 1, 2A-2F
and 2H-2J, the system may be modified to sense, in a
noninvasive fashion, arterial pressure using a
conventional cuff 207 removably fixed to, as shown, the
right upper arm of the patient 200, the sensed
pressure-related electrical signals being produced by a
conventional transducer within the apparatus 207. A
-18-
~'', r'~. f°;~ n ~ ,r,
~..I <JL,J?J~i.J
pneumatic tube or conduit 209 is provided both to
supply automatically and intermittently compressed air
to the cuff 20? and to receive either audible sounds
(which are processed within the apparatus 208 to derive
MAP representing data) or an electrical output from a
transducer positioned within the cuff 207. The
transducer produces electrical output signals which
appears on a pair of conductive leads within the
conduit 209. The cuff 207 is supplied, as is
conventional, intermittently with compressed air via
the air conduit 209. The components illustrated in
FIG. 2G are used to monitor arterial blood pressure
intermittently, for example once for a short period
every 30 seconds. The pressure data so developed can
be used to develop long-term mean baseline
pressure-related signals and short-term (current) mean
pressure-related signals. Such intermittently
developed inputs can, as will be readily understandable
by persons skilled in the art, be used in place of the
inputs provided from the pressure sensing transducer 20
(FIGS. 1, 2A-2F and 2H-2J) to derive pressure- and
heart rate-representing input signals fir use in
conjunction with the circuits discussed hereinbelow.
The apparatus 208 may be provided with a heart rate
display 220, baseline MAP display 221, and a current
MAP display 223. An EKG strip recording 222 could be
produced by the apparatus from a connection electrode
arrangement (now shown) which could include the rate
(R-wave) sensing electrodes 212 and 213. It is to be
appreciated that the present invention can be realized
using pressure transducers which may be implanted to
sense arterial pressure. The pressure transducer may
be arranged about a selected artery, for example.
One possible general implantable configuration of
the housing 12 is shown in FIG. 3. The housing 12
includes a case 32, made of titanium, and a header 33,
-19-
~~ 9~ ;: r ~'.t !) ..~
~d ~j rJ F~i 'ii ~..i. a.j
formed of an epoxy material, fixed to the case 32, all
external components being hermetically sealed and
biocompatible for human implantation. Within the case
32 is a battery pack or battery 34, an energy storage
capacitor 35 and an electronic module 36 in or on which
circuit components, other than the battery pack or
battery 34 and the capacitor 35, are positioned.
Detailed embodiments of exemplary circuits which are in
or on or connected to the module 36 are illustrated in
FIGS. 4, 6, 8 and 10, to which reference is made
hereinbelow. A plurality of pairs of receptacles 37-40
are shown in the header 33 for receiving corresponding
pairs of leads which are respectively within the
insulated cables 15, 16 and 17 and 19 and 23 (FIG. 1).
Turning to FIG. 4, an exemplary embodiment of the
circuit components, which may be positioned within the
housing 12 (FIGS. 1 and 3) or the bed-side apparatus
208 (FIG. 2G), includes a pair of input terminals 41,
42 which receive the variable D.C. voltage output
signal representing pressure from the pressure
responsive transducer 20 (FIGS. 1, 2A-2F and 2H-2J) or
noninvasive transducer (in system of FIG. 2G), the
terminal 42 being connected to a point of circuit
reference potential (ground). The terminals 41, 42 are
connected to an amplifier 43, which amplifies the
pressure representing D.C. input signal and feeds the
same to respective buffer amplifiers 44 and 45. The
circuit of FIG. 4 is suitable for treating a
malfunction heart using a pressure-only criteria. It
is to be understood that the portion of the circuitry
designated 300 can be considered to be a signal
processing circuit which may, in preferred embodiments,
be replaced by the respective circuits shown in FIGS.
21-24.
The output from the buffer amplifier 45 is
supplied to an RC circuit constituted by an adjustable
-20-
:~: 6~ Q?.. !:i o
l
~.i e~~ ~h7 ~~.i _Z. ~~
resistor 46 connected to ground via a series connected
storage capacitor 47 having a large adjustable resistor
48 connected in parallel therewith. The time constants
(charging and discharging) of these circuit components
are such that the D.C. voltage across the capacitor 47
represents the mean pressure sensed by the transducer
20 (FIGS. 1, 2A-2F and 2H-2J) or a noninvasive
transducer (in system of FIG. 2G) aver a relatively
long period, fox example during the preceding fifteen
(15) minutes or even longer (for example a number of
hours) or shorter (for example one hundred twenty (120)
seconds) being suitable in some cases. The resistors
46 and 48 may be set by a medical professional to suit
the particular patient involved, so far as what the
most suitable period length (period of predetermined
length) fox baseline data acquisition appears to be
most suitable. The D.C. voltage (first signal) which
appears across the capacitor 47 thus represents a long
term mean baseline pressure. The term "mean" as used
herein is broad and includes the average value as well
as values near the average. The output from the buffer
amplifier 44 is supplied to a second RC circuit
constituted by an adjustable resistor 50 connected to
ground via a capacitor 51, which has an adjustable
resistor 52 connected in parallel therewith. The time
constants (charging and discharging) of these circuit
components are such that the D.C. voltage (second
signal) which appears across the capacitor 51
represents the short term mean pressure sensed by the
transducer 20 (FIGS. 1, 2A-2F and 2H-2J) or the
noninvasive transducer (in system of FIG. 2G) over a
relatively short period, for example, during the
preceding fifteen (15) seconds or longer (for example
60 seconds) or shorter (for example six seconds). The
resistors 50 and 52 may be set by a medical
professional to suit the particular patient involved,
-21-
y C~s. :~' r. .? l~ $."'.
! ~"
~~e ki' ~ ~G:a 'eu
so far as what the most suitable period length (period
of given length) for current data acquisition appears
to be most suitable.
As illustrated the long term (baseline) and short
5 term (current) D.C, voltage signals which appear across
the respective capacitors 47 and 51 are fed
respectively to the inverting and noninverting
terminals of an operational amplifier 53, a difference
D.C. voltage signal appearing as the output from the
operational amplifier 53. As shown, the inverting and
noninverting terminals of the operational amplifier 53
are connected as they would be were the sensed or
determined hemodynamic parameter expected to increase
during hemodynamic compromise. Were the sensed (or
determined hemodynamic parameter expected to decrease,
the terminals would be reversed. The D.C. output
signal from the operational amplifier 53 is fed to a
first input terminal of a first comparator 54, the
second input terminal of the comparator 54 is connected
2U to the wiper of a potentiometer 55 which is connected
between ground and a point of fixed D.C. potential,
illustrated as being +15 volts, from an internal power
supply bus.
Whenever the voltage supplied to the comparator 54
from the operational amplifier 53 exceeds the voltage
supplied via the wiper from the potentiometer 55, a low
(ZERO) level on the output terminal from the comparator
54 goes high (ONE), the ONE signal being coupled as an
enabling input to a gate 56 and to a sample-and-hold
circuit 57 which receive, at their respective signal
input terminals, the voltage representing current mean
pressure appearing across the capacitor 51 and the
voltage representing mean baseline pressure appearing
across the capacitor 47.
A D.C. output from the sample-and-hold circuit 57
is stored in a storage circuit, for the purpose of
-22-
s n .! r~
~~~'~~.~.a
illustration shown as a capacitor 58. This stored
voltage signal (stored first signal) representing mean
baseline (long-term) pressure is supplied to the
inverting input terminal of an operational amplifier 60
which has its noninverting input terminal connected to
the output terminal of the gate 56, which when enabled,
passes the D.C. voltage signal appearing across the
capacitor 51 and representing current (short-term) mean
pressure to the operational amplifier 60. As
illustrated, the inverting and noninverting terminals
of the operational amplifier 60 are shown as they would
be connected were the hemodynamic parameter expected to
increase. Were the hemodynamic parameter selected
expected to drop, the terminals would be reversed. The
output from the operational amplifier 60 is supplied to
an input terminal of a comparator 61, which has its
other input connected to the wiper of a potentiometer
62 connected between ground and the +15 volt power
supply bus. Whenever the voltage supplied to the
2U comparator 6l from the operational amplifier 60 exceeds
the voltage supplied from the potentiometer 62, an
indication of hemodynamic compromise, the output
terminal of the comparator 61 goes from low (ZERO) to
high (ONE) which signal is passed to the enable
terminal of a D.C.-to-D.C. converter 63. It is to be
understood that the wipers of the potentiometers 55 and
62 are independently adjustable; consequently, the
wiper on the potentiometer 62 may be positioned so that
the pressure difference which causes its output to go
from ZERO to ONE is slightly greater than pressure
difference which causes the comparator 54 to initiate
the enabling functions. The D.C.-to-D.C. converter 63,
when enabled, receives current from a low voltage
battery pack or battery 64 and converts it into a high
D.C. voltage, for example a voltage of 720 volts, which
is used, when the converter is enabled, to charge an
-23-
6 t > ;~ c",g ~~ .t f;,a
' ' C.'~
H x ' ~ l.~ ~.~..
energy storage capacitor 65, via a resistor 66 towards
the high voltage. The capacitor 65 is of such size
that it will store energy levels sufficient to produce
the desired cardioverting/defibrillation pulses. The
desired pulse is a truncated exponential pulse of about
25 Joules delivered approximately 17 seconds from onset
of the hemodynamic compromise. The pulse could,
especially when defibrillation is being undertaken
after a failed attempt to cardiovert, be delivered
somewhat later and with a higher energy level.
Once the capacitor 65 is charged to a sufficiently
high D.C. voltage level to provide sufficient energy to
effect cardioversion, as determined by a comparator 67,
which receives on one input terminal a voltage
proportional to the increasing D.C. voltage across the
capacitor 65, a highly resistive voltage divider 68
being in parallel'to the capacitor 65. The second
input terminal of the comparator 67 is connected to the
wiper of a potentiometer 70 which is connected between
ground and the +15 volt bus. When the voltage across
the energy storing capacitor 65 is sufficient to supply
a cardioverting energy pulse to the malfunctioning
heart, the voltage supplied to the one input terminal
of the comparator 67 exceeds the voltage supplied to
its other input terminal from the potentiometer 70 via
its associated wiper. Under these conditions, the
output from the comparator 67 goes from low (ZERO) to
high (ONE), which ONE signal effects an enabling of an
analog gate 71. The gate 71 has its signal input
connected to receive an output from a pulse shaper 72,
which receives an input from the rate sensing
electrodes 18a, 18b (FIGS. 1, 2A-2F and 2H-2J) or from
the rate sensing electrodes 212, 213 (FIG. 2G) and
produces a pulse train in synchronism with the R-wave
supplied from the electrodes 18a, 18b or electrodes
212, 213. If the pulse train from the pulse shaper 72
_2~_
~, ~'~,. 6'; 6'.. I ~ ..' f ;
~d ~~L~~ ~~x~ rw' 'v tv
is present, these pulses are passed, via the gate 71,
to an OR circuit 73 and thence to the gate electrode of
an SCR 74. The first of these pulses which, if
present, appears on the gate electrode fires the SCR 74
thereby discharging the energy then stored on the
capacitor 65 into the malfunctioning heart, via the
electrodes 13 and 14 (FIGS. 1, 2A-2F and 2H-2J) or the
electrodes 202 and 204 (FIG. 2G) in an effort to effect
cardioversion, the discharge being in synchronism with
the R-wave.
In the event that the pulse shaper 72 does not
produce a pulse to fire the SCR 74 because of the
absence of an R-wave, the ONE signal from the
comparator 67 is passed, via a delay circuit 75, which
provides a delay of about three seconds or more and
enables a pulse generator 76 causing it to produce an
output pulse to initiate defibrillation which is
supplied, via the OR circuit 73, to the gate electrode
of the SCR 74 causing the SCR to fire. The energy
storage capacitor 65, which by then has charged to a
higher level discharges, via the SCR 74 and the
electrodes 13 and 14 (FIGS. 1, 2A-2F and 2H-2J) or the
electrodes 202 and 204 (FIG. 2G), into the
malfunctioning heart in an effort to effect
defibrillation, the energy level being higher than
would have been the case had the capacitor been
discharged three seconds earlier. The delay circuit
may be composed of an RC circuit connected to the
comparator 67 so that the capacitor thereof charges
toward the ONE level slowly; for example the capacitor
may take about three (3) seconds or more as indicated
above to achieve the ONE level, allowing time to
receive one or more synchronizing pulses from the pulse
shaper 72, if present.
The sample-and-hold circuit 57 is reset whenever
the comparator 61 output goes from ONE to ZERO, which
-25-
'aae~'';.'1.U
occurs when the difference between the stored signal
representing baseline mean pressure and the signal
representing current mean pressure returns to an
acceptable level, indicating that the hemodynamic
compromise has been overcome. The resetting is
accomplished by an inverter 77 and a differentiating
circuit constituted by a capacitor 78 and a resistor 80
connected in series in the denominated order from the
output terminal of the inverter 77 to ground, a
~0 positive going spike appearing across the resistor 80
each time the input to the inverter 77 from the
comparator 61 goes from ONE to ZERO.
In the event the first pulse delivered to the
heart fails to effect a correction in the pressure
(which would cause the output of the comparators 54 and
61 to become ZERO, removing the enable signals from the
sample-and-hold circuit 57 and the converter 63), the
capacitor 65 is recharged and discharged a number of
additional times, for example three more times in an
effort to correct the malfunction. The number of
discharges is sensed by a counter 8l, which has its
input connected to the output of the OR gate 73. If
the counter 81 reaches a count of four within the given
time period, for example a period of three minutes, its
output goes from ZERO to ONE, which is applied to the
converter 63 as a disabling (OFF) signal. An internal
timer within the converter 63 holds the converter OFF
for a given period so that the patient will not receive
more shocks during this given period. At the end of
the period the converter 63 returns to a READY
condition and is again able to respond to an ENABLE
signal from the comparator 61. The counter 81 resets
itself to zero whenever it either reaches its maximum
count of four or fails to reach the count of four
within the given time period.
It is to be appreciated that the circuit of FIG. 4
described above may be considered, at least in part, to
-26-
5'"a P, Z, ~7 n !'; s ' i
r
~d 1f i.,s ie~ us . iri
be a controller or processor, which could be realized
as a microprocessor, the processor being identified by
the numeral 82. The processor 82, with its associated
components, in effect carries out the steps set out in
the flowchart of FIGS. 5A and 5B.
The circuit of FIG. 4 could be associated with an
antitachycardia pacemaker and/or an antibradycardia
pacemaker, if desired.
Turning to FIG. 6, a further exemplary embodiment
of the circuit components, which may be positioned
within the housing 12 (FIGS. 1 and 3) or the apparatus
208 (FIG. 2G) includes a pair of input terminals 41, 42
which receive the variable D.C. voltage output signal
representing pressure from the pressure responsive
transducer 20 (FIGS. 1, 2A-2F and 2H-2J) or the
noninvasive transducer (in system of FIG. 2G), the
terminal 42 being connected to a point of circuit
reference potential (ground). The terminals 41, 42 are
connected to an amplifier 43, which amplifies the
pressure representing D.C. input signal and feeds the
same to respective buffer amplifiers 44 arid 45. The
circuit of FIG. 6, with associated components, is
suitable for practicing the present invention in which
both pressure and beating rate criteria are to be taken
into account. The rate criterion is examined first
and, if met, the pressure criteria axe then considered.
The output from the buffer amplifier 45 is
supplied to an RC circuit constituted by an adjustable
resistor 46 connected to ground via a series connected
storage capacitor 47 having a large adjustable resistor
A8 connected in parallel therewith. The time constants
(charging and discharging) of these circuit components
are such that the D.C. voltage (first signal) across
the capacitor 47 represents the mean pressure sensed by
the transducer 20 (FIGS. 1, 2A-2F and 2H-2J) or the
noninvasive transducer (in system of FIG. 2G) over a
-27-
f . y'~ ~~. ~ f?, -° f''.
~~i~al~sh~.,~~f
relatively long period, for example during the
preceding fifteen (15) minutes or even longer (for
example a number of hours) or shorter (for example one
hundred twenty (120) seconds) being suitable in some
cases. The D.C. voltage (first signal) which appears
across the capacitor 47, thus represents a long term
mean baseline pressure. The term "mean" as used herein
is broad and includes the average value, as well as
values near the average. The output from the buffer
lU amplifier 44 is supplied to a second RC circuit
constituted by an adjustable resistor 50 connected to
ground via a capacitor 51, which has an adjustable
resistor 52 connected in parallel therewith. The time
constants (charging and discharging) of these circuit
~5 components are such that the D.C. voltage (second
signal) which appears across the capacitor 51
represents the short term mean pressure sensed by the
transducer 20 (FIGS. 1, 2A-2F and 2H-2J) or the
noninvasive transducer (in system of FIG. 2G) over a
20 relatively short period, for example, during the
preceding fifteen (15) seconds or longer (for example
60 seconds) or shorter (for example six seconds).
As illustrated the long term (baseline) and short
term (current) D.C. voltage signals which appear across
25 the respective capacitors 47 and 51 are fed
respectively to the signal input terminal of a
sample-and-hold circuit 57 and to the signal input
terminal of a gate 56. A rate sensing circuit 83 is
arranged to receive a beating rate (R-wave) signal from
30 the rate sensing electrodes 18a, 18b (FIGS. 1, 2A-2F
and 2H-2J) or from the rate sensing electrodes 212, 213
(FIG. 2G). Whenever the rate exceeds a given rate, for
example 155 beats per minute, indicating tachycardia,
the output terminal of the rate sensing circuit 83 goes
35 from low (ZERO) to high (ONE). The ONE signal (first
control signal) is supplied as an enabling input to the
_28_
s.,.. ru ., < ~,
F.~ 4t~~ t~e ~1~ ~. C3
gate 56 and to sample-and-hold circuit 57. The D.C.
voltage representing current mean pressure appearing
across the capacitor 51 is fed via the enabled gate 56
to the noninverting input terminal of an operational
amplifier 60. The D.C. voltage representing mean
baseline pressure appearing across the capacitor 47 is
transferred to the sample-and-hold circuit 57,
appearing across its associated capacitor 58. This
stored D.C. voltage representing mean baseline pressure
is supplied to the inverting input terminal of the
operational amplifier 60 which has its noninverting
input terminal connected to the output terminal of the
gate 56 which, when enabled as noted above, passes the
D.C. voltage signal appearing across the capacitor 51
and representing current mean pressure to the
operational amplifier 60. As illustrated, the input
terminals of the operational amplifier are connected as
they would be to receive signals representative of a
sensed or determined hemodynamic parameter expected to
increase during hemodynarnic compromise. Were the
sensed or determined hemodynamic parameter expected to
decrease, the terminals would be reversed.
The output from the operational amplifier 60 is
supplied to an input terminal of a comparator 61, which
has its other input connected to the wiper of a
potentiometer 62 connected between ground and the +15
volt power supply bus. Whenever the voltage supplied
to the comparator 61 from the operational amplifier 60
exceeds the voltage supplied from the potentiometer 62,
an indication of hemodynamic compromise, the output
terminal of the comparator 61 goes from low (ZERO) to
high (ONE) and the signal (second control signal) is
passed to the enable terminal of a D.C.-to-D. C.
converter 63. The D.C.-to-D. C. converter 63, when
enabled, receives current from a low voltage battery
pack or battery 64 and converts it into a high D.C.
-29-
t~ ;m F.e v .~.
voltage, for example a voltage of 720 volts, which is
used, when the converter is enabled, to charge an
energy storage capacitor 65, via a resistor 66 towards
the high voltage. The capacitor 65 is of such size
that it will store energy levels sufficient to produce
the desired cardioverting/defibrillation pulses. The
desired pulse for cardioversion is a truncated
exponential pulse of about 25 Joules delivered
approximately 17 seconds from onset of the hemodynamic
compromise.
Once the capacitor 65 is charged to a sufficiently
high D.C. voltage level to provide sufficient energy to
effect cardioversion, as determined by a comparator 67,
which receives on one input terminal a voltage
proportional to the instant D.C. voltage across the
capacitor 65, a resistive voltage divider 68 being in
parallel to the capacitor 65. The second input
terminal of the comparator 67 is connected to the wiper
of a potentiometer 70 which is connected between ground
and the +15 volt bus. When the voltage across the
2U energy storing capacitor 65 is sufficient to supply a
cardioverting energy pulse to the malfunctioning heart,
the voltage supplied to the one input terminal of the
comparator 67 exceeds the voltage supplied to its other
input terminal from the potentiometer 70 via its
associated wiper. Under these conditions, the output
from the comparator 67 goes from low (ZERO) to high
(ONE), which ONE signal effects an enabling of an
analog gate 71. The gate 71 has its signal input
connected to receive an output from a pulse shaper 72,
3p which receives an input from the rate sensing
electrodes 18a, 18b (FIGS. 1, 2A-2F and 2H-2J) or from
the rate sensing electrodes 212, 213 (FIG. 2G) and
produces a pulse train in synchronism with the R-wave
supplied from the electrodes 18a, 18b or from the
electrodes 212, 213. If the pulse train from the pulse
-30-
~~ y, ~h ~, ~" -.'3
~.~ i.,i f~ fJ ~ 1~
shaper 72 is present, these pulses are passed, via the
gate 71, to an OR circuit 73 and thence to the gate
electrode of an SCR 74. The first of these pulses
which, if present, appears on the gate electrode fires
the SCR 74 thereby discharging the energy stored on the
capacitor 65 into the malfunctioning heart, via the
electrodes 13 and 14 (FIGS. 1, 2A-2F and 2H-~2J) or the
electrodes 202, 204 (FIG. 2G) in an effort to effect
cardioversion, the discharge being affected in
synchronism with the R-wave.
In the event that the pulse shaper 72 does not
produce a pulse to fire the SCR 74 because of the
absence of an R-wave, the ONE signal from the
comparator 67 is passed, via a delay circuit 75, which
provides a delay of about three seconds or more, and
enables a pulse generator 76 causing it to produce
output pulse to initiate defibrillation. The pulse is
supplied, via the OR circuit 73, to the gate electrode
of the SCR 74 causing the SCR to fire. The energy
storage capacitor 65, which during the elapsed three
seconds has charged to a higher level, discharges, via
the SCR 74 and the electrodes 13 and 14 (FIGS. 1, 2A-2F
arid 2H-2J) or electrodes 202 and 204 (FIG. 2G), into
the malfunctioning heart via the electrodes 13 and 14
(FIGS. 1, 2A-2F and 2H-2J) or electrodes 202 and 204
(FIG. 2G) in an effort to effect defibrillation, the
energy level being higher than it would had been had
discharge been effected three (3) or more seconds
earlier. The delay circuit may be composed of an RC
circuit connected to the comparator 67 so that the
capacitor thereof charges toward the ONE level slowly;
for example the capacitor may take about three (3)
seconds or more to achieve the ONE level, allowing time
to receive one or more synchronizing pulses from the
pulse shaper 72, if present.
The sample-and-hold circuit 57 is reset whenever
the comparator 61 output goes from ONE to ZERO, which
-31-
~T
occurs when the difference between the baseline mean
pressure and current mean pressure returns to an
acceptable noncompromising level. The resetting is
accomplished by an inverter 77 and a differentiating
circuit constituted by a capacitor 78 and a resistor 80
connected in series in the denominated order from the
output terminal of the inverter 77 to ground, a
positive going spike appearing across the resistor 80
each time the input to the inverter 77 from the
~0 comparator 61 goes from ONE to ZERO.
In the event the first pulse delivered to the
heart fails to effect a correction in the pressure by
overcoming the hemodynamic compromise (which would
cause the output of the comparator 6l to become 2ER0,
. 15 removing the enable signal from the converter 63), the
capacitor 65 is recharged and discharged a number of
additional times,~for example three more times in an
effort to correct the malfunction. The number of
discharges is-sensed by a counter 81, which'has its
20 input connected to the output of the OR gate 73. If
the counter 81 reaches a count of four within the given
time period , for example a period of three minutes; its
output goes from ZERO to ONE, which is applied to the
converter 63. as a disabling (OFF) signal. The counter
25 81 resets itself to ZERO count whenever it either
reaches i,ts maximum count of four or fails to reach the
count of our within the given time period. An
internal timer within the converter 63 holds the
converter OFF for a given period so that the patient
3p will riot receive more shocks during this given period.
At the end of the period the converter 63 returns to a
READY condition and is again able to respond to an
ENABLE signal from the comparator 61.
As can be seen from the foregoing description of
n of the circuit of FIG. 6, cardioverting/-
ti
35 o
the opera
defibrillating D.C. pulses are delivered to the
-32-
~: . r, u'~ .u, .~, i
r .r '
t,G l.i ya' '~~ _,_ ;~
malfunctioning heart only when the rate criterion is
first satisfied and, thereafter, the pressure criteria
also satisfied. This can be viewed as a series
rate-pressure algorithm.
In the event the rate criterion is met, but the
pressure criteria are not; that is to say no
hemodynamic compromise presents, the circuit of FIG. 6
nevertheless acts to enable an antitachycardia
pacemaker 86 which supplies pacing signals to the pair
of pacing electrodes 21, 22 (FIGS. 1, 2A-2F and 2H-2J)
or the pair of pacing electrodes 210, 211 (FIG. 2G).
To enable the pacemaker 86, two signals must be
supplied to an AND circuit 85, the first being a ONE
signal from the rate sensing circuit 83, the second
being a ONE signal supplied to the AND circuit 85 via
an inverter 84 from the output terminal of the
comparator 61. When no hemodynamic compromise
prevails, the output terminal of the comparator 61 has
a low (ZERO) output. This ZERO output is inverted by
the inverter 84 and appears as a ONE on the second
input terminal of the AND circuit 85. Thus, when both
inputs to the AND circuit 85 are ONE, the
antitachycardia pacemaker 86, which may be any one of a
number of conventional types is energized.
It is to be appreciated that the circuit of FIG. 6
described above may be considered, at least in part, to
be a processor, which could be realized as a
microprocessor, the processor being identified by the
numeral 82. The processor 82, with its associated
3U components, in effect carries out the steps set out in
the flowchart of FIGS. 7A and 7B.
It is to be understood that the system of FIG. 6
could be associated with a failsafe antibradycardia
pacing system, if desired.
Turning to FIG. 8, an additional exemplary .
embodiment of the circuit components, which may be
-33-
L~ '~, C':.. ' .f'~ .°, i..
~1i' ~ !d ~tJ''~ t.J
positioned within the housing 12 (FTGS. 1 and 3) or the
apparatus 208 (FIG. 2G) includes a pair of input
terminals 41, 42 which receive the variable D.C.
voltage output signal representing pressure from the
pressure responsive transducer 20 (FIGS. 1, 2A-2F and
2H-2J) or the noninvasive transducer (in system of FIG.
2G), the terminal 42 being connected to a point of
circuit reference potential (ground). The terminals
41, 42 are connected to an amplifier 43, which
1.0 amplifies the pressure representing D.C. input signal
and feeds the same to respective buffer amplifiers 44
and 45. The circuit of FIG. 8 can be used in
practicing 'the present invention using both rate and
pressure criteria. In this case the rate and pressure
criteria must exist simultaneously to start the
sample-and-hold function.
The output from the buffer amplifier 45 is
supplied to an RC circuit constituted by an adjustable
resistor 46 connected to ground via a series connected
2U capacitor 47 having a large adjustable resistor 48
connected in parallel therewith. The time constants
(charging and discharging) of these circuit components
are such that the D.C. voltage across the capacitor 47
represents the mean pressure sensed by the transducer
20 (FIGS. 1, 2A-2F and 2H-2J) or the noninvasive
transducer (in system of FIG. 2G) over a relatively
long period, for example during the preceding fifteen
(15) minutes or even longer for example a number of
hours) or shorter (for example one hundred twenty (120)
seconds being suitable in some cases. The D.C. voltage
(first signal) which appears across the capacitor 47
thus represents a long term mean baseline pressure.
The term "mean" as used herein is broad and includes
the average value, as well as values near the average.
The output from the buffer amplifier 44 is supplied to
a second RC circuit constituted by an adjustable
-34-
~',~~:.=,~a,-,f;.~
Y'd ttl .'~.! ICd ~'.l J~_ s~~
resistor 50 connected to ground via a capacitor 51,
which has an adjustable resistor 52 connected in
parallel therewith. The time constants (charging and
discharging) of these circuit components axe such that
the D.C. voltage (second signal) which appears across
the capacitor 51 represents the short term mean
pressure sensed by the transducer 20 (FIGS. 1, 2A-2F
and 2H-2J) or the noninvasive transducer din system of
FIG. 2G) over a relatively short period, for example,
lU during the preceding fifteen (15) seconds or longer
(for example 60 seconds) or shorter (for example six
seconds).
As illustrated the long term (baseline) and short
term (current) D.C. voltage signals which appear across
the respective capacitors 47 and 51 are fed
respectively to the inverting and noninverting
terminals of an operational amplifier 87, a difference
D.C. voltage signal appearing as the output from the
operational amplifier 87. As illustrated, the input
terminals of the operational amplifier 87 are connected
as they would be were the selected, sensed or
determined hemodynamic parameter expected to increase
during hemodynamic compromise. Were the selected
hemodynamic parameter expected to decrease, the
terminals would be reversed. The D.C. output signal
from the operational amplifier 87 is fed to a first
input terminal of a comparator 88. The second input
terminal of the comparator 88 is connected to the wiper
of a potentiometer 89 which is connected between ground
and a point of fixed D.C. potential, illustrated as
being +15 volts, from an internal power supply bus.
Whenever the voltage supplied to the comparator 88
from the operational amplifier 87 exceeds the voltage
supplied via the wiper from the potentiometer 89, a low
(ZERO) level on the output terminal from the comparator
88 goes high (ONE), the ONE signal being coupled to a
-35-
r. ~.'; !'~ .~? .~ j.
r
~~~t~ J 4c~ '..i ..~. v.j
first input terminal of an AND circuit 90 which has its
other input terminal coupled to the output terminal of
a rate sensing circuit 83, which produces a ONE signal
on its output terminal whenever the heart rate exceeds
a predetermined value, for example 155 beats per
minute. When the AND gate 90 receives ONE signals on
both its input terminals, its output goes high (ONE)
which enables a gate 56. The ONE signal from the AND
gate 90 is also fed as an enabling input to a
sample-and-hold circuit 57. The voltage representing
current mean pressure appearing across the capacitor 51
is fed to the noninverting input terminal of an
operational amplifier 60. The voltage representing
mean baseline pressure appearing across the capacitor
47 is fed to the sample-and-hold circuit 57. Were the
selected hemodynamic parameter expected to decrease,
the input terminals of the operational amplifier 87
would be reversed.
A D.C. output from the sample-and-hold circuit 57
is stored in a storage circuit, for the purpose of
illustration shown as a capacitor 58, This stored
voltage is supplied to the inverting input terminal of
the operational amplifier 60 which has its noninverting
input terminal connected to the output terminal of the
gate 56, which when enabled, passes the D.C. voltage
signal appearing across the capacitor 51 and
representing current mean pressure to the operational
amplifier 60. The output from the operational
amplifier 60 is supplied to an input terminal of a
3U comparator 61, which has its other input connected to
the wiper of a potentiometer 62 connected between
ground and the +15 volt power supply bus. Whenever the
voltage supplied to the comparator 61 from the
operational amplifier 60 exceeds the voltage supplied
from the potentiometer 62, an indication of hemodynamic
compromise, the output terminal of the comparator 61
-36-
G~ a~ '~ ' ;
~~ J
~~ vv f
goes from low (ZERO) to high (ONE) which signal is
passed to the enable terminal of a D.C.-to-D. C.
converter 63. It is to be appreciated that the wipers
of the potentiometers 89 and 62 can be adjusted
independently. Thus, one can set the wiper of the
potentiometer 62 so that the hemodynamic compromise
must get worse than it was when the sample-and-hold
circuit 57 is enabled before the output from the
comparator 61 enables the D.C.-to-D.C. converter 63.
The D.C.-to-D. C. converter 63, when enabled, receives
current from a low voltage battery pack or battery 64
and converts it into a high D.C. voltage, for example a
voltage of 720 volts, which. is used, when the converter
is enabled, to charge an energy storage capacitor 65,
via a resistor 66 towards the high voltage. The
capacitor 65 is of such size that it will store energy
levels sufficient to produce the desired
cardioverting/defibrillation pulses. The desired pulse
for effecting cardioversion is a truncated exponential
pulse of about 25 Joules delivered approximately 17
seconds from onset of the hemodynamic compromise.
Once the capacitor 65 is charged to a sufficiently
high D.C. voltage level, as determined by a comparator
67, which receives on one input terminal a voltage
proportional to the D.C. voltage across the capacitor
65, a resistive voltage divider 68 being in parallel to
the capacitor 65. The second input terminal of the
comparator 67 is connected to the wiper of a
potentiometer 70 which is connected between ground and
the -X15 volt bus. When the voltage across the energy
storing capacitor 65 is sufficient to supply a
cardioverting energy pulse to the malfunctioning heart,
the voltage supplied to the one input terminal of the
comparator 67 exceeds the voltage supplied to its other
input terminal from the potentiometer 70 via its
associated wiper. Under these conditions, the output
-37-
ro .~
~''V. '..~. . . ~ , . "r ('.;
lw ,i' f .r G,~~ v<:' .:._ f~
from the comparator 67 goes from low (ZERO) to high
(ONE), which ONE signal effects an enabling of an
analog gate 71. The gate 71 has its signal input
connected to receive an output from a pulse shaper 72,
which receives an input from the rate sensing
electrodes 18a, 18b (FIGS. 1, 2A-2F and 2H-2J) or the
rate sensing electrodes 212, 213 (FIG. 2G) and produces
a pulse train in synchronism with the R-wave supplied
from the electrodes 18a, 18b or the electrodes 212,
213. If the pulse train from the pulse shaper 72 is
present, these pulses are passed, via the gate 71, to
an OR circuit 73 and thence to the gate electrode of an
SCR 74. The first of these pulses which, if present,
appears on the gate electrode fires the SCR 74 thereby
~5 discharging the energy stored on the capacitor 65 into
the malfunctioning heart, via the electrodes 13 and 14
(FIGS. 1, 2A-2F arid 2H-2J) or the electrodes 202 and
204 (FIG. 2G) in an effort to effect cardioversion, the
discharge being affected in synchronism with the
R-wave.
In the event that the pulse shaper 72 does not
produce a pulse to fire the SCR 74 because of the
absence of an R-wave, the ONE signal from the
comparator 67 is passed, via a delay circuit 75, which
provides a delay of about three seconds or more, and
enables a pulse generator 76 causing it to produce an
output pulse which is supplied, via the OR circuit 73,
to the gate electrode of the SCR 74 causing the SCR to
fire. The energy storage capacitor 65, which by then
has been charged to a higher level, discharges, via the
SCR 74 and the electrodes 13 and 14 (FIGS. 1, 2A-2F and
2H-2J) or the electrodes 202 and 204 (FIG. 2G), into
the malfunctioning heart in an effort to effect
defibrillation. The delay circuit 75 may be composed
of an RC circuit connected to the comparator 67 so that
the capacitor thereof charges toward the ONE level
-38-
Gd 1i ~:r (;a ~;' '. 'va
slowly; for example the capacitor may take about three
(3) seconds or more to achieve the ONE level, allowing
time to receive one or more synchronizing pulses from
the pulse shaper 72, if present.
The sample-and-hold circuit 57 is reset whenever
the comparator 61 output goes from ONE to ZERO, which
occurs when the difference between the baseline mean
pressure and current mean pressure returns to an
acceptable noncompromising level. The resetting is
1~ accomplished by an inverter 77 and a differentiating
circuit constituted by a capacitor 78 and a resistor 80
connected in series in the denominated order from the
output terminal of the inverter 77 to ground, a
positive going spike appearing across the resistor 80
each time the input to the inverter 77 from the
comparator 61 goes from ONE to ZERO.
In the event'the first pulse delivered to the
heart fails to effect a correction in the pressure
(which would cause the output of the comparator 61 to
become ZERO, removing the enable signal from the
converter 63), the capacitor 65 is recharged and
discharged a number of additional times, for example
three more times, in an effort to correct the
malfunction. The number of discharges is sensed by a
Z5 counter 81, which has its input connected to the output
of the OR gate 73. If the counter 81 reaches a count
of four within the given time period, for examgle a
period of three minutes, its output goes from ZERO to
ONE, which is applied to the converter 63 as a
disabling (OFF) signal. The counter 81 resets itself
to zero whenever either it reaches its maximum count of
four or it fails to reach a count of four within the
given time period. An internal timer within the
converter 63 holds the converter OFF for a given period
so that the patient will not receive more shocks during
this given period. At the end of the period the
-39-
t F .-, ~y ry ~ f.">
. (:7
.. ~ ii F'~ ~'d =1,
converter 63 returns to a READY condition and is again
able to respond to an ENABLE signal from the comparator
61.
As can be seen from the foregoing description of
the operation of the circuit of FIG. 8,
cardioverting/defibrillating D.C. pulses are delivered
to the malfunctioning heart only when the rate and the
pressure criteria are simultaneously satisfied. This
can be viewed as a parallel rate-pressure algorithm.
In the event the rate criterion is met, but the
pressure criteria are not; that is to say no
hemodynamic compromise presents, the circuit of FIG. 8
nevertheless acts to enable an antitachycardia
paceraaker 86 which supplies pacing signals to the pair
of pacing electrodes 21, 22 (FIGS. 1, 2A-2F and 2H-2,7)
or the pacing electrodes 210, 211 (FIG. 2G). To enable
the pacemaker 86,'two signals must be supplied to an
AND circuit 85, the first being a ONE signal from the
rate sensing circuit 83, the second being a ONE signal
supplied to the AND circuit 85 via an inverter 84 from
2U the output terminal of the comparator 61. When no
hemodynamic compromise prevails, the output terminal of
the comparator 61 has a low (ZERO) output. This ZERO
output is inverted by the inverter 84 and appears as a
ONE on the second input terminal of the AND circuit 85.
~5 Thus, when both inputs are ONE, the antitachycardia
pacemaker 86 is energized.
It is to be appreciated that the circuit described
above may be considered, at least in part, to be a
controller processor, which could be realized as a
3~ microprocessor, the processor being identified by the
numeral 82. The processor 82, with its associated
components, in effect carries out the steps set out in
the flowchart of FIGS. 9A and 9B.
The circuit of FIG. 8 could be associated with a
35 failsafe antibradycardia pacemaker, if desired.
-40-
~ ,.,, ," .
s~ fa '., ~ : , ~ J
i,Y Fa/ ~ J 1.
Turning to FIG. 15, an exemplary embodiment of
circuit components of the present invention, which may
be positioned within the housing 12 (FIGS. 1 and 3) or
the bed-side apparatus 208 (FIG. 2G), includes a pair
of input terminals 41, 42 which receive the variable
D.C. voltage output signal representing pressure from
the pressure responsive transducer 20 (F7:GS. l, 2A-2F
and 2H-2J) or noninvasive transducer (in system of FIG.
2G), the terminal 42 being connected to a point of
circuit reference potential (ground). The terminals
41, 42 are connected to an amplifier 43, which
amplifies the pressure representing D.C. input signal
and feeds the same to a buffer amplifier 44. The
circuit of FIG. 15 is suitable for practicing the
present invention using a pressure-only criteria.
A D.C. voltage level (first signal) representative
of fixed baseline'pressure appears on the wiper of a
potentiometer 100 which may be set by a medical
professional to suit the particular patient involved.
The potentiometer 100 is connected, as illustrated,
between system ground and a point of +15 volts,
regulated. The medical professional, based on a
patient's condition and history, could set the wiper of
the potentiometer at a suitable patient-specific point,
reflecting an appropriate baseline. It is to be
understood that the point may be selected prior to
implantation. The circuit may be adapted to enable the
patient-specific set point to be changed, the set using
radio and/or magnetic coupling (not shown).
The term "mean" as used herein is broad and
includes the average value as well as values near the
average. The output from the buffer amplifier 44 is
supplied to a RC circuit constituted by an adjustable
resistor 50 connected to ground via a capacitor 51,
which has an adjustable resistor 52 connected in
parallel therewith. The time constants (charging and
-41-
'°
(-~J. 'L. ~ a4d, '~: _,. '4~r
discharging) of these circuit components are such that
the D.C. voltage (second signal) which appears across
the capacitor 51 represents the short term mean
pressure sensed by the transducer 20 (FIGS. 1, 2A-2F
and 2H-2J) or the noninvasive transducer (in system of
FIG. 2G) over a relatively short period, for example,
during the preceding fifteen (15) seconds or longer
(for example 60 seconds) or shorter (for example six
seconds). The resistors 50 and 52 may be set by a
medical professional to suit the particular patient
involved, so far as what the most suitable period
length (period of given length) fox current data
acquisition appears to be most suitable. Were the
device already implanted, conventional radio or
magnetic links could be used to change the setting of
the variable resistors 50 and 51 were a patient's
condition to make~such adjustments desirable.
As illustrated the baseline and short term
(current) D.C. voltage signals which appear
respectively on the wiper of the potentiometer 100 and
across the capacitor 5J. are fed respectively to the
inverting and noninverting terminals of an operational
amplifier 60, a difference D.C. voltage signal
appearing as the output from the operational amplifier
60. As shown, the inverting and noninverting terminals
of the operational amplifier 60 are connected as they
would be were the hemodynamic parameter expected to
increase during hemodynamic compromise. Were the
hemodynamic parameter involved expected to decrease,
the terminals would be reversed. The D.C, output
signal from the operational amplifier 60 is fed to a
first input terminal of a first comparator 61, the
second input terminal of the comparator 61 is connected
to the wiper of a potentiometer 62 which is connected
between ground and a point of fixed D.C. potential,
illustrated as being +15 volts, from an internal power
-42-
f n., ~ ~ $ .~, ,. ('',.
Y.p 'ia ~1~ Fud~ ':it .I_ V
supply bus.
Whenever the voltage supplied to the comparator 61
from the operational amplifier 60 exceeds the voltage
supplied from the potentiometer 62, an indication of
hemodynamic compromise, the output terminal of the
comparator 61 goes from low (ZERO) to high (ONE) which
signal is passed to the enable terminal of a
D.C.-to-D. C. converter 63. The D.C.-to-D. C. converter
63, when enabled, receives current from a low voltage
'10 battery pack or battery 64 and converts it into a high
D.C. voltage, for example a voltage of 720 volts, which
is used, when the converter is enabled, to charge an
energy storage capacitor 65 (or a capacitor pack), via
a resistor 66 towards the high voltage. The capacitor
65 is of such size that it will store energy levels
sufficient to produce the desired cardioverting/-
defibrillation pulses. The desired pulse may be a
truncated exponential pulse of about 25 Joules
delivered approximately 17 seconds from onset of the
hemodynamic compromise. The pulse could, especially
when defibrillation is being undertaken after a failed
attempt to cardiovert, be delivered somewhat later and
with a higher energy level.
Once the capacitor 65 is charged to a sufficiently
high D.C. voltage level to provide sufficient energy to
effect cardioversion, as determined by a comparator 67,
which receives on one input terminal a voltage
proportional to the increasing D.C. voltage across the
capacitor 65, a highly resistive voltage divider 68
being in parallel to the capacitor 65. The second
input terminal of the comparator 67 is connected to the
wiper of a potentiometer 70 which is connected between
ground and the +15 volt bus. When the voltage across
the energy storing capacitor 65 is sufficient to supply
a cardioverting energy pulse to the malfunctioning
heart, the voltage supplied to the one input terminal
-43-
Vii: l hm ";;f ,.b_ firs
of the comparator 67 exceeds the voltage supplied to
its other input terminal from the potentiometer 70 via
its associated wiper. Under these conditions, the
output from the comparator 67 goes from low (ZERO) to
high (ONE), which ONE signal effects an enabling of an
analog gate 71. The gate 71 has its signal input
terminal connected to receive an output from a pulse
shaper 72, which receives an input from the rate
sensing electrodes 18a, 18b (FIGS. 1, 2A-2F and 2H-2J)
or from the rate sensing electrodes 212, 213 (FIG. 2G)
and produces a pulse train in synchronism with the
R-wave supplied from the electrodes 18a, 18b or
electrodes 212, 213. If the pulse train from the pulse
shaper 72 is present, these pulses are passed, via the
gate 71, to an OR circuit 73 and thence to the gate
electrode of an SCR 74. The first of these pulses
which, if present; appears an the gate electrode fires
the SCR 74 thereby discharging the energy then stored
on the capacitor 65 (or the bank of capacitors) into
the malfunctioning heart, via the electrodes 13 and 14
(FIGS. 1, 2A-2F and 2H-2J) or the electrodes 202 and
204 (FIG. 2G) in an effort to effect cardioversion, the
discharge being in synchronism with the R-wave.
In the event that the pulse shaper 72 does not
produce a pulse to fire the SCR 74 because of the
absence of an R-wave, the ONE signal from the
comparator 67 is passed, via a delay circuit 75, which
provides a delay of about three seconds or more and
enables a pulse generator 76 causing it to produce an
output pulse to initiate defibrillation which is
supplied, via the OR circuit 73, to the gate electrode
of the SCR 74 causing the SCR to fire. The energy
storage capacitor 65 (or the bank of. capacitors), which
by then has charged to a higher level discharges, via
the SCR 74 and the electrodes 13 and 14 (FIGS. 1, 2A-2F
arid 2H-2J) or the electrodes 202 and 204 (FIG. 2G),
-44-
f.... rnr ~'!,
into the malfunctioning heart in an effort to effect
defibrillation, the energy level being higher than
would have been the case had the capacitor been
discharged three seconds earlier. The delay circuit
may be composed of an RC circuit connected to the
comparator 67 so that the capacitor thereof charges
toward the ONE level slowly; for example the capacitor
may take about three (3) seconds or more as indicated
above to achieve the ONE level, allowing time to
1~ receive one or more synchronising pulses from the pulse
shaper 72, if present.
In the event the first pulse delivered to the
heart fails to effect a correction in the pressure
(which would cause the output of the comparator 61 to
become ZERO, removing the enable signal from the
converter 63), the capacitor 65 is recharged and
discharged a number of additional times, for example
three more times in an effort to correct the
malfunction. The number of discharges is sensed by a
counter 81, which has its input connected to the output
of the OR gate 73. If the counter 81 reaches a count
of four within the given time period, for example a
period of three minutes, its output goes from ZERO to
ONE, which is applied to the converter 63 as a
disabling (OFF) signal. An internal timer within the
converter 63 holds the converter OFF for a given period
so that the patient will not receive more shocks during
this given period. At the end of the period the
converter 63 returns to a READY condition and is again
able to respond to an ENABLE signal from the comparator
61. The counter 81 resets itself to zero whenever it
either reaches its maximum count of four or fails to
reach the count of four within the given time period.
In the event cardioversion or defibrillation is
successful, the short term mean current pressure (as
reflected by the voltage across the capacitor 51)
-45-
~a'it~~Gi ~'Y_'.3
returns to normal, the output terminal of the
comparator 61 goes low (ZERO) from high (ONE) thereby
removing the enabling input from the converter 63 and
stopping the charging of the capacitor 65. The system
is thus made ready for another sequence in the event
the pressure condition sensed indicates that
hemodynamic compromise is again present. In the event
the short term mean current pressure returns to normal
before the first cardioverting or defibrillating pulse
is delivered, the output of the comparator goes to low
(ZERO), removing the enable signal from the converter
63, thus stopping the charging of the capacitor 65.
It is to be appreciated that the circuit of FIG.
described above may be considered, at least in part,
15 to be a controller or processor, which could be
realized as a microprocessor, the processor being
identified by the numeral 82. The processor 82, with
its associated components, in effect carries out the
steps set out in the flowchart of FIGS. 16A and 16B.
The circuit of FIG. 15 could be associated with an
antitachycardia pacemaker and/or an antibradycardia
pacemaker, if desired.
Turning to FIG. 17, a further exemplary embodiment
of the circuit components of the present invention,
which may be positioned within the housing 12 (FIGS. 1
and 3) or the apparatus 208 (FIG. 2G) includes a pair
of input terminals 41, 42 which receive the variable
D.C. voltage output signal representing pressure from
the pressure responsive transducer 20 (FIGS. 1, 2A-2F
and 2H-2J) or the noninvasive transducer (in system of
FIG. 2G), the terminal 42 being connected to a point of
circuit reference potential (ground). The terminals
41, 42 are connected to an amplifier 43, which
amplifies the pressure representing D.C. input signal
and feeds the same to a buffer amplifier 44. The
circuit of FIG. 17, with associated components, is
-46-
6J, f~~ 4.~, (.'3 ;~.
~d '~.~i~ ~,~.! ~J ~..i .iA_ ~~yi
suitable for practicing the present invention in which
both pressure and beating rate criteria are to be taken
into account. The rate criterion is examined first
and, if met, the pressure criteria are then considered.
A D.C. voltage level (first signal) provided on
the wiper of a potentiometer 100, which is connected
between ground and a regulated +15 volts source,
represents a fixed baseline pressure. The wiper may be
set by a medical professional taking into account the
history and condition of the particular patient, The
potentiometer 100 may be adjusted, possibly using
conventional magnetic or radio links as noted above.
The term "mean" as used herein is broad and
includes the average value, as well as values near the
average. The output from the buffer amplifier 44 is
supplied to a RC circuit constituted by an adjustable
resistor 50 connected to ground via a capacitor 51,
which has an adjustable resistor 52 connected in
parallel therewith. The time constants (charging and
2U discharging) of these circuit components are such that
the D.C. voltage (second signal) which appears across
the capacitor 51 represents the short term mean
pressure sensed by the transducer 20 (FIGS. 1, 2A-2F
and 2H-2J) or the noninvasive transducer (in system of
FIG. 2G) over a relatively short period, for example,
during the preceding fifteen (15) seconds or longer
(for example 60 seconds) or shorter (for example six
seconds). As in the circuit of FIG. 15, the resistors
50 and 51 may be adjusted, taking into account the
patient°s possibly changing condition, possibly using
conventional radio or magnetic links.
As illustrated the baseline and short term
(current) D.C. voltage signals appear respective on the
wiper of the potentiometer 100 and across the capacitor
51. The voltage (second signal) from the capacitor 51
is fed to the signal input terminal of a gate 56. A
-47-
~
T ro .~
~.~°:.afad:~:~_;.U
rate sensing circuit 83 is arranged to receive a
beating rate (R-wave) signal from the rate sensing
electrodes 18a, 18b (FIGS. 1, 2A-2F and 2H-2J) or from
the rate sensing electrodes 212, 213 (FIG. 2G).
Whenever the rate exceeds a given rate, for example 155
beats per minute, indicating tachycardia, the output
terminal of the rate sensing circuit 83 goes from low
(ZERO) to high (ONE). The ONE signal (first control
signal) is supplied as an enabling input to the gate
56. The D.C. voltage representing current mean
pressure appearing across the capacitor 51 is fed via
the enabled gate 56 to the noninverting input terminal
of an operational amplifier 60. The D.C. voltage
(first signal) representing baseline pressure appearing
on the wiper of the potentiometer 10 is supplied to the
inverting input terminal of the operational amplifier
60 whi~:h has its rioninverting input terminal connected
to the output terminal of the gate 56 which, when
enabled as noted above, passes the D.C. voltage signal
z0 appearing across the capacitor 51 and representing
current mean pressure to the operational amplifier 60.
As illustrated, the input terminals of the operational
amplifier 60 axe connected as they would be to receive
signals representative of sensed or determined
parameters which are expected to increase during
hemodynamic compromise. Were the selected hemodynamic
parameter expected to drop during hemodynamic
compromise, the terminals would be reversed.
The output from the operational amplifier 60 is
supplied to an input terminal of a comparator 61, which
has its other input connected to the wiper of a
potentiometer 62 connected between ground and the +15
volt power supply bus. Whenever the voltage supplied
to the comparator 61 from the operational amplifier 60
exceeds the voltage supplied from the potentiometer 62,
an indication of hemodynamic compromise, the output
-48_
;.
4.a it' ~ na c:
terminal of the comparator 61 goes from low (ZERO) to
high (ONE) and the signal (second control signal) is
passed to the enable terminal of a D.C.-to-D. C.
converter 63. The D.C.-to-D. C. converter 63, when
enabled, receives current from a low voltage battery
pack or battery 64 and converts it into a high D.C.
voltage, for example a voltage of 720 volts, which is
used, when the converter is enabled, to charge an
energy storage capacitor 65 (or a pack of capacitors),
via a resistor 66 towards the high voltage. The
capacitor 65 is of such size that it will store energy
levels sufficient to produce the desired
cardioverting/defibrillation pulses. The desired pulse
for cardioversion may be a truncated exponential pulse
of about 25 Jou7.es delivered approximately 17 seconds
from onset of the hemodynamic compromise.
Once the capacitor 65 is charged to a sufficiently
high D.C. voltage level to provide sufficient energy to
effect cardioversion, as determined by a comparator 67,
which receives on one input terminal a voltage
proportional to the instant D.C. voltage across the
capacitor 65, a resistive voltage divider 68 being in
parallel to the capacitor 65. The second input
terminal of the comparator 67 is connected to the wiper
of a potentiometer 70 which is connected between ground
and the +15 volt bus. When the voltage across the
energy storing capacitor 65 is sufficient to supply a
cardioverting energy pulse to the malfunctioning heart,
the voltage supplied to the one input terminal of the
comparator 67 exceeds the voltage supplied to its other
input terminal from the potentiometer 70 via its
associated wipex. Under these conditions, the output
from the comparator 67 goes from low (ZERO) to high
(ONE), which ONE signal effects an enabling of an
analog gate 71. The gate 71 has its signal input
connected to receive an output from a pulse shaper 72,
-49-
6a « ~'<~ ; ~. ;rt' _>. :J
which receives an input from the rate sensing
electrodes 18a, 18b (FIGS. 1, 2A-2F and 2H-2J) or from
the rate sensing electrodes 212, 213 (FIG. 2G) and
produces a pulse train in synchronism with the R-wave
supplied from the electrodes 18a, 18b or from the
electrodes 212, 213. If the pulse train from the pulse
shaper 72 is present, these pulses are passed, via the
gate 71, to an OR circuit 73 and thence to the gate
electrode of an SCR 74. The first of these pulses
which, if present, appears on the gate electrode fires
the SCR 74 thereby discharging the energy stored on the
capacitor 65 into the malfunctioning heart, via the
electrodes 13 and 14 (FIGS. 1, 2A-2F and 2H-2J) or the
electrodes 202, 204 (FIG. 2G) in an effort to effect
cardioversion, the discharge being affected in
synchronism with the R-wave.
In the event'that the pulse shaper 72 does not
produce a pulse to fire the SCR 74 because of the
absence of an R-wave, the ONE signal from the
2~ comparator 67 is passed, via a delay circuit 75, which
provides a delay of about three seconds or more, and
enables a pulse generator 76 causing it to produce an
output pulse to initiate defibrillation. The pulse is
supplied, via the OR circuit 73, to the gate electrode
of the SCR 74 causing the SCR to fire. The energy
storage capacitor 65 (or a pack of capacitors), which
during the elapsed three seconds has charged to a
higher level, discharges, via the SCR 74 and the
electrodes 13 and 14 (FIGS. 1, 2A-2F and 2H-2J) or
electrodes 202 and 204 (FIG. 2G), into the
malfunctioning heart via the electrodes 13 and 14
(FIGS. 1, 2A-2F and 2H-2J) or electrodes 202 and 204
(FIG. 2G) in an effort to effect defibrillation, the
energy level being higher than it would had been had
discharge been effected three (3) or more seconds
earlier. The delay circuit may be composed of an RC
-50-
n i'' "'' ~"1 i~;,
,i'~ isf ~a 't,I .~.
circuit connected to the comparator 67 so that the
capacitor thereof charges toward the ONE level slowly;
for example the capacitor may take about three (3)
seconds or more to achieve the ONE level, allowing time
to receive one or more synchronizing pulses from the
pulse shaper 72, if present.
In the event the first pulse delivered to the
heart fails to effect a correction in the pressure by
overcoming the hemodynamic compromise (which would
cause the output of the comparator 61 to become ZERO,
removing the enable signal from the converter 63), the
capacitor 65 is recharged and discharged a number of
additional times, fox example three more times in an
effort to correct the malfunction. The number of
discharges is sensed by a counter 81, which has its
input connected to the output of the OR gate 73. If
the counter 81 reaches a count of. four within the given
time period, for example a period of three minutes, its
output goes from ZERO to ONE, which is applied to the
converter 63 as a disabling (OFF) signal. The counter
81 resets itself to ZERO count whenever it either
reaches its maximum count of four or fails to reach the
count of four within the given time period. An
internal timer within the converter 63 holds the
converter OFF for a given period so that the patient
will not receive more shocks during this given period.
At the end of the period the converter 63 returns to a
READY condition and is again able to respond to an
ENABLE signal from the comparator 61.
As can be seen from the foregoing description of
the operation of the circuit of FIG. 17,
cardioverting/defibrillating D.C, pulses are delivered
to the malfunctioning heart only when the rate
criterion is first satisfied and, thereafter, the
pressure criteria also satisfied. This can be viewed
as a series rate-pressure algorithm.
-51-
C'. ~"~ ;p ,n. J! i':
v~~ t~s r J '~, ~~ _t"
In the event the rate criterion is met, but the
pressure criteria are not; that is to say no
hemodynamic compromise presents, the circuit of FIG. 17
nevertheless acts to enable an antitachycardia
pacemaker 86 which supplies pacing signals to the pair
of pacing electrodes 21, 22 (FIGS. 1, 2A-2F and 2H-2J)
or the pair of pacing electrodes 210, 211 (FIG. 2G).
To enable the pacemaker 86, two signals must be
supplied to an AND circuit 85, the first being a ONE
signal from the rate sensing circuit 83, the second
being a ONE signal supplied to the AND circuit 85 via
an inverter 84 from the output terminal of the
comparator 61. When no hemodynamic compromise
prevails, the output terminal of the comparator 61 has
a low (ZERO) output. This ZERO output is inverted by
the inverter 84 and appears as a ONE on the second
input terminal of~the AND circuit 85. Thus, when both
inputs to the AND circuit 85 are ONE, the
antitachycardia pacemaker 86, which may be any one of a
number of conventional types is energized.
In the event cardioversion or defibrillation is
successful, the short term mean current pressure (as
reflected by the voltage across the capacitor 51)
returns to normal, the output terminal of the
comparator 61 goes low (ZERO) from high (ONE) thereby
removing the enabling input from the converter 63 and
stopping the charging of the capacitor 65. The system
is thus made ready for another sequence in the event
the pressure condition sensed indicates that
hemodynamic compromise is again present. In the event
the short term current pressure returns to normal
before any cardioverting or defibrillating pulses are
delivered (as in the case of FIG. 15), the enable
signal is revived from the converter 63 and the
charging of the capacitor 65 stops.
It is to be appreciated that the circuit of FIG.
17 described above may be considered, at least in part,
-52-
,.,, ,_ ~~ ~, ~. E-:
~',, 'zt.'.a G~.a ~ .i.. t3
to be a processor, which could be realized as a
microprocessor, the processor being identified by the
numeral 82. The processor 82, with its associated
components, in effect carries out the steps set out in
the flowchart of FIGS. 18A and 188.
It is to be understood that the system of FIG. 17
could be associated with a failsafe antibradycardia
pacing system, if desired.
Turning to FIG. 19, another exemplary embodiment
of the circuit components of the present invention,
which may be positioned within the housing 12 (FIGS. 1
and 3) or the apparatus 208 (FIG. 2G) includes a pair
of input terminals 41, 42 which receive the variable
D.C. voltage output signal representing pressure from
the pressure responsive transducer 20 (FIGS. 1, 2A-2F
and 2H-2J) or the noninvasive transducer (in system of
FIG. 2G), the terminal 42 being connected to a point of
circuit reference potential (ground). The terminals
41, 42 are connected to an amplifier 43, which
amplifies the pressure representing D.C. input signal
and feeds the same to buffer amplifier 44. The circuit
of FIG. 19 can be used in practicing the present
invention using both rate and pressure criteria. In
this case the rate and pressure criteria must exist
simultaneously to enable the system.
A D.C. voltage level (first signal) appears on the
wiper of a potentiometer 100, which is connected
between ground and a regulated +15 volt source, the
signal representing fixed baseline pressure. The wiper
(as in the circuits of FIGS. 15 and 17) may be set by a
medical professional in accordance with needs of a
specific patient and may be adjusted later, if desired,
using radio or magnetic techniques.
The term "mean" as used herein is broad and
includes the average value, as well as values near the
average. The output from the buffer amplifier 44 is
-53-
O1 l,"; .i~ !',. .P ~i1
"it w Gu J -~. LJ
supplied to a RC circuit constituted by an adjustable
resistor 50 connected to ground via a capacitor 51,
which has an adjustable resistor 52 connected in
parallel therewith. The time constants (charging and
discharging) of these circuit components are such that
the D.C. voltage which appears across the capacitor 51
represents the short term mean pressure sensed by the
transducer 20 (FIGS. 1, 2A-2F and 2H-2J) or the
noninvasive transducer (in system of FIG. 2G) over a
relatively short period, for example, during the
preceding fifteen (15) seconds or longer (for example
60 seconds) or shorter (for example six seconds). The
size of the resistors 50 and 51.(as in the circuits of
FIGS. 15 and 17) may be adjusted, a desirable feature
were a patient's condition or needs to change.
As illustrated the baseline and short term
(current) D.C. voltage signals which respectively
appear on the wiper 100 of the potentiometer 100 and
across the capacitor 51 are fed respectively to the
inverting and noninverting terminals of an operational
amplifier 87, a difference D.C. voltage signal
appearing as the output from the operational amplifier
87. As illustrated, the input terminals of the
operational amplifier 87 are connected as they would be
were the hemodynamic parameter sensed or determined
expected to increase during hemodynamic compromise.
Were the sensed or determined selected hemodynamic
parameter expected to decrease, the terminals would be
reversed. The D.C. output signal from the operational
amplifier 87 is fed to a first input terminal of a
comparator 88. The second input terminal of the
comparator 88 is connected to the wiper of a
potentiometer 89 which is connected between ground and
a point of fixed D.C. potential, illustrated as being
+15 volts, from an internal power supply bus.
Whenever the voltage supplied to the comparator 88
from the operational amplifier 87 exceeds the voltage
-54-
F~~ ~~3
supplied via the wiper from the potentiometer 89, a
low
(ZERO) level on the output terminal from the comparator
88 goes high (ONE), the ONE signal being coupled to
a
first input terminal of an AND circuit 90 which has
its
other input terminal coupled to the output terminal
of
a rate sensing circuit 83, which produces a ONE signal
on its output terminal whenever the heart rate exceeds
a predetermined value, for example 155 beats per
minute. When the AND gate 90 receives ONE signals on
both its input terminals, its output goes high (ONE)
which enables a gate 56. The voltage (second signal)
representing current mean pressure appearing across
the
capacitor 51 is fed to the noninverting input terminal
of an operational amplifier 60: The voltage (first
signal) representing fixed baseline pressure appearing
on the wiper of the potentiometer LOO is fed to the
inverting input terminal of the operational amplifier
60. Were the selected hemodynamic parameter expect
to
decrease, the input terminals of~the operational
amplifier 60 would be reversed. A D.C. output,from
the
sample-and-hold circuit 57 is stored in a storage.
The
operational amplifier 60 which has its noninverting
input terminal connected to the output terminal of
the
gate 56, which when enabled, passes the D.C. voltage
signal appeasing across the capacitor 51 and
representing current mean pressure to the operational
amplifier 60. The output from the operational
amplifier 60 is supplied to an input terminal of a
comparator 6l, which has its other input connected
to
the wiper of a potentiometer 62 connected between
ground and the +15 volt power supply bus. Whenever
the
voltage supplied to the comparator 61 from the
operational amplifier 60 exceeds the voltage supplied
from the potentiometer 62, an indication of hemodynamic
compromise, the output terminal of the comparator 61
goes from low (ZERO) to high (ONE) which signal is
-55-
coy>.=,.,ra,u.' i;
~~r ~i ~~ G;e a,' .ta (J
passed to the enable terminal of a D.C.-to-D. C.
converter 63. It is to be appreciated that the wipers
of the potentiometers 89 and 62 can be adjusted
independently. Thus, one can set the wiper of the
potentiometer 62 so that the hemodynamic compromise
must get worse than it was when the gate 56 was opened
before the output from the comparator 61 enables the
D.C.-to-D. C. converter 63. The D.C.-to°D. C. converter
63, when enabled, receives current from a low voltage
battery pack or battery 64 and converts it into a high
D.C. voltage, for example a voltage of 720 volts, which
is used, when the converter is enabled, to charge an
energy storage capacitor 65 (or capacitor pack), via a
resistor 66 towards the high voltage. The capacitor 65
is of such size that it will store energy levels
sufficient to produce the desired cardioverting/-
defibrillation pulses. The desired pulse for effecting
cardioversion may be a truncated exponential pulse of
about 25 Joules delivered approximately 17 seconds from
onset of the hemodynamic compromise.
Once the capacitor 65 is charged to a sufficiently
high D.C. voltage level, as determined by a comparator
67, which receives on one input terminal a voltage
proportional to the D.C. voltage across the capacitor
65, a resistive voltage divider 68 being in parallel to
the capacitor 65. The second input terminal of the
comparator 67 is connected to the wiper of a
potentiometer 70 which is connected between ground and
the +15 volt bus. When the voltage across the energy
storing capacitor 65 is sufficient to supply a
cardioverting energy pulse to the malfunctioning heart,
the voltage supplied to the one input terminal of the
comparator 67 exceeds the voltage supplied to its other
input terminal from the potentiometer 70 via its
associated wiper. Under these conditions, the output .
from the comparator 67 goes from low (ZERO) to high
-56-
z-~ .~,_ . ....
~ :fir n ': !:3
(ONE), which ONE signal effects an enabling of an
analog gate 71. The gate 71 has its signal input
connected to receive an output from a pulse shaper 72,
which receives an input from the rate sensing
electrodes 18a, 18b (FIGS. 1, 2A-2F and 2H-2J) or the
rate sensing electrodes 212, 213 (FIG. 2G) and produces
a pulse train in synchronism with the R-wave supplied
from the electrodes 18a, 18b or the electrodes 212,
213. If the pulse train from the pulse shaper 72 is
present, these pulses are passed, via the gate 71, to
an OR circuit 73 and thence to the gate electrode of an
SCR 74. The first of these pulses which, if present,
appears on the gate electrode fires the SCR 74 thereby
discharging the energy stored on the capacitor 65 into
the malfunctioning heart, via the electrodes 13 and 14
(FIGS. 1, 2A-2F and 2H-2J) or the electrodes 202 and
204 (FIG. 2G) in an effort to effect cardioversion, the
discharge being affected in synchronism with the
R-wave.
In the event that the pulse shaper 72 does not
produce a pulse to fire the SCR 74 because of the
absence of an R-wave, the ONE signal from the
comparator 67 is passed, via a delay circuit 75, which
provides a delay of about three seconds or more, and
enables a pulse generator 76 causing it to produce an
output pulse which is supplied, via the OR circuit 73,
to the gate electrode of the SCR 74 causing the SCR to
fire. The energy storage capacitor 65, which by then
has been charged to a higher level, discharges, via the
SCR 74 and the electrodes 13 and 14 (FIGS. 1, 2A-2F and
2H-2J) or the electrodes 202 and 204 (FIG. 2G), into
the malfunctioning heart in an effort to effect
defibrillation. The delay circuit 75 may be composed
of an RC circuit connected to the comparator 67 so that
the capacitor thereof charges toward the ONE level
slowly; far example the capacitor may take about three
_57-
~, s. e. P
(3) seconds or mare to achieve the ONE level, allowing
time to receive one or more synchronizing pulses from
the pulse shaper 72, if present.
In the event the first pulse delivered to the
heart fails to effect a correction in the pressure
(which would cause the output of the comparator 61 to
become ZERO, removing the enable signal from the
converter 63), the capacitor 65 is recharged and
discharged a number of additional times, for example
three more times, in an effort to correct the
malfunction. The number of discharges is sensed by a
counter 81, which has its input connected to the output
of the OR gate 73. If the counter 81 reaches a count
of four within the given time period, for example a
period of three minutes, its output goes from ZERO to
ONE, which is applied to the converter 63 as a
disabling (OFF) signal. The counter 81 resets itself
to zero whenever either it reaches its maximum count of
four or it fails to reach a count of four within the
given time period. An internal timer within the
converter 63 holds the converter OFF for a given period
so that the patient will not receive more shocks during
this given period. At the end of the period the
converter 63 returns to a READY condition and is again
able to respond to an ENABLE signal from the comparator
61.
As can be seen from the foregoing description of
the operation of the circuit of FIG. 19,
cardioverting/defibrillating D.C. pulses are delivered
to the malfunctioning heart only when the rate and the
pressure criteria are simultaneously satisfied. This
can be viewed as a parallel rate-pressure algorithm.
In the event the rate criterion is met, but the
pressure criteria are not; that is, to say no
hemodynamic compromise presents, the circuit of FIG. 19
nevertheless acts to enable an antitachycardia
_58_
4 ~ ~7 ~> .q ~
'~.~ l:.. ~ '.' a. t~
pacemaker 86 which supplies pacing signals to the pair
of pacing electrodes 21, 22 (FIGS. 1, 2A-2F and 2H-2J)
or the pacing electrodes 210, 211 (FIG. 2G). To enable
the pacemaker 86, two signals must be supplied to an
AND circuit 85, the first being a ONE signal from the
rate sensing circuit 83, the s°cond being a ONE signal
supplied to the AND circuit 85 via an inverter 84 from
the output terminal of the comparator 61. When no
hemodynamic compromise prevails, the output terminal of
the comparator 61 has a low (ZERO) output. This ZERO
output is inverted by the inverter 84 and appears as a
ONE on the second input terminal of the AND circuit 85.
Thus, when both inputs are ONE, the antitachycardia
pacemaker 86 is energized.
In the event cardioversion or defibrillation is
successful, the short term mean current pressure (as
reflected by the voltage across the capacitor 51)
returns to normal, the output terminal of the
aomparator 61 goes low (ZERO) from high (ONE) thereby
removing the enabling input from the converter 63 and
stopping the charging of the capacitor 65. The system
is thus made ready for another sequence in the event
the pressure condition sensed indicates that
hemodynamic compromise is again present.
It is to be appreciated that the circuit of FIG.
19 described above may be considered, at least in part,
to be a controller processor, which could be realized
as a microprocessor, the processor being identified by
the numeral 82. The processor 82, with its associated
components, in effect carries out the steps set out in
the flowchart of FIGS. 20A and 20B.
The circuit of FIG. 19 could be associated with a
failsafe antibradycardia pacemaker, if desired.
Turning to FIG. 10, a fourth exemplary embodiment
of circuit components of a system for treating a
malfunctioning heart, which may be positioned within
-59-
(
~ I~~,~i.U
the housing 12 (FIGS. 1 and 3D or in the apparatus 208
(FIG. 2G) or used in a portable system which may be
' carried on the body of a patient or used in fixed
installation, such as in ICU's, CCU's, hospital rooms
and the like includes a pair of input terminals 41, 42
which receive the variable D.C. voltage output signal
representing pressure from the pressure responsive
transducer 20 (FIGS. 1, 2A-2F and 2H-2J) or the
noninvasive transducer (in system of FIG. 2G), the
terminal 42 being connected to a point of circuit
reference potential (ground). The terminals 41, 42 are
connected to an amplifier 43, which amplifies the
pressure representing D.C. input signal and feeds the
same to respective buffer amplifiers 44 and 45. The
circuit of FIG. 10 can be used in practicing the
present invention using either hemodynamic parameter
(such as pressure) criterion alone or both rate and
hemodynamic parameter criteria (either in parallel or
series). The circuit of FIG. l0~can be used to carry
out the methods, illustrated as algorithms in the
flowcharts of FIGS. 5A, 5B arid 7A, 7B and 9A, 9B, 16A,
16B and 18A, 18B and 20A, 20B. The circuit of FIG. 10
can be considered as a digital, microprocessor-based
version of the hand-wired analogue circuitry shown in
FIGS. 4, 6 and 8, when the single-pole, double-throw
switch 101 is set as shown. In the other position of
the switch 101, the circuit can be considered to be a
digital, microprocessor-based version of the hand-wired
analogue circuitry illustrated in FIGS. 15, 17 and 19.
Of course, the microprocessor-based circuit of FIG. 10
could be programmed to carry out other routines. For
example, were a rate criterion to be satisfied, the
circuit could be arranged (1) simply to monitor
selected hemodynamic parameter (such as pressure), (2)
to effect antitachycardia pacing and/or to cardiovert.
As further examples, were both rate and the selected
-60-
~j.~,.aaf.~
i ~ ~1(
~rA 'i.i fd ~J 'u ~;. ~_i
hemodynamic criteria to be satisfied, the circuit of
FIG. 10 could be programmed (1) to effect
antitachycardi.a pacing and/or (2) to cardiovert/-
defibrillate. Moreover, the selected interventions
could be programmed so that when one is tried and
fails, another is tried and so on. For example, if a
tachycardia were detected regardless of whether or not
hemodynamic compromise is present an antitachycardia
pacemaker would attempt early to revert the arrhythms
to normal and if this fails cardioversion/-
defibrillation would then attempt the same. A detailed
discussion of one possible program is discussed below.
The output from the buffer amplifier 45 is
supplied to an RC circuit constituted by an adjustable
resistor 46 connected to ground via a series connected
storage capacitor 47 having a large adjustable resistor
48 connected in parallel therewith. The time constants
(charging and discharging) of these circuit components
are such that the D.C. voltage (first signal) across
the capacitor 47 represents the mean pressure sensed by
the transducer 20 (FIGS. 1, 2A-2F and 2H-2J) or the
noninvasive transducer (in system of FIG. 2G) over a
relatively long period, for example during the
preceding fifteen (15) minutes or even longer (for
example a number of hours) of shorter (for example 120
seconds) being suitable in some cases. The D.C.
voltage level across the capacitor 47 thus represents a
long term mean baseline pressure. The term "mean" as
used herein is broad and includes the average value as
well as values near the average. The output from the
buffer amplifier 44 is supplied to a second RC circuit
constituted by an adjustable resistor 50 connected to
ground via a capacitor 51, which has an adjustable
resistor 52 connected in parallel therewith. The time
constants (charging and discharging) of these circuit
components are such that the D.C. voltage (second
-61-
6~ ,3':, ~ C's r, ~ ~-,
~:s sue. G,I ~:i .,7. t..i
signal) which appears across the resistor 51 represents
the short term mean pressure sensed by the transducer
20 (FIGS. 1, 2A-2F and 2H-2J) or the noninvasive
transducer (in system of FIG. 2G) over a relatively
short period, for example, during the preceding fifteen
(15) seconds or longer (for example 60 seconds) or
shorter (for example six seconds).
As illustrated the long term (baseline) and short
term (current) D.C. voltage signals which appear across
the respective capacitors 47 and 51 are fed
respectively via respective analogue-to-digital
converters (A/D's) 91 and 92 to respective inputs of a
microprocessor 93. The A/D converters 91 and 92, in
operation, convert the respective analogue signals .
~5 which appear across capacitors 47 and 51 into
corresponding digital signals for processing by the
microprocessor 93, the microprocessor having associated
therewith a ROM 94, which supplies programmed
instructions to the microprocessor, and a RAM 95, which
stores and supplies digital signal representations of
pressure-related signals from and to the
microprocessor.
Another input of the microprocessor 93 is supplied
with high (ONE) and low (ZERO) signals from a high rate
sensing circuit 83, which produces a ONE signal
whenever the heart rate, as sensed by the electrodes
18a and 18b (FIGS. 2A-2F and 2H-2J) or by the
electrodes 212 and 213 (FIG. 2G), exceeds a
predetermined rate, for example a rate of 155 b.p.m.
3p The actual rate selected would, of course, depend on
the individual patient and a professional opinion as to
his or her condition. A pulse shaper 72, which also
receives an input from the rate sensing electrodes 18a
arid 18b (FIGS. 2A-2F and 2H-2J) or from the rate
sensing electrodes 212 and 213 (FIG. 2G), is provided
to supply narrow D.C. pulses to the microprocessor 93;
-62-
if present, these pulses would be used as synchronizing
pulses for cardioversion.
An antitachycardia pacemaker 86 is connected to an
output terminal of the microprocessor 93 to receive
therefrom a pace enable signal to, in effect, enable or
turn on the pacemaker 86 under the command of the
microprocessor 93. Two other output terminals from the
microprocessor 93 provide respective card:iovert and
defibrillate command signals to an OR circuit 73, which
cooperates with a D.C.-to-D. C. converter 63, a battery
64, a charging resistor 66, storage capacitor 65 and a
SCR 74 in the same manner as the corresponding circuit
components having the same reference numerals function
in the hand-wired circuits illustrated in FIGS. 4, 6
and 8. The output of the OR gate 73 is also supplied
to an input terminal of the microprocessor 93,
supplying signals to a counting means within the
microprocessor 93 which corresponds to the counter 81
(FIGS. 4, 6 and 8).
As thus far described, the circuit of FIG. 10 can
carry out the methods defined in the flowcharts of
FIGS. 5A, 5B and 7A, 7B and 9A, 9B, the respective
programs being supplied by the ROM 94. In operation,
the circuit of FIG. 10, with the switch 101 set as
illustrated, can be seen as a microprocessor
realization of the hand-wired analogue circuits of
FIGS. 4, 6 and 8. With the switch 101 set in its other
position, the capacitor 47 and the resistor 48 are
disconnected frorn the input to the A/D converter 91 and
the wiper of the potentiometer 100 connected thereto.
The voltage which appears on the wiper of the
potentiometer 100 thus constitutes the first signal,
representing in this case the fixed baseline pressure.
The circuit of FIG. 10 when so connected on a carry out
the methods defined in the flowcharts of FIGS. 16A, 16B
and 18A, 18B and 20A, 20B. It is to be appreciated
-63-
~d ~L~ F,,! f~° S.i wi i.j
that the circuit of FIG. 10 can be programmed to effect
somewhat different routines and be provided with
additional inputs, as well.
If desired for example, a low rate sensing circuit
96 could be provided, its input being coupled to the
rate sensing electrodes 18a and 18b (FIGS. 2A-2F and
2H-2J) or the rate sensing electrodes 212 and 213 (FIG.
2G). The low rate sensing circuit 96 supplies a high
(ONE) signal to an input terminal whenever the beating
rate, as sensed by the electrodes 18a and 18b or the
electrodes 212 and 213, falls below a given rate, for
example 45 b.p.m., indicative of bradycardia. Under
these conditions (provided the rate were not zero), the
microprocessor 93 would provide a command enable signal
to an antibradycardia pacemaker 97. When enabled, the
pacemaker 97 would supply bradycardia-correcting pacing
signals to a patient's heart via the pacing electrodes
21 and 22 (FIGS. 1, 2A-2F and 2H-2J) or the pacing
electrodes 210 and 211 (FIG. 2G).
If desired, a zero rate sensing circuit 98,
responsive to output from the rate sensing electrodes
18a and 18b (FIGS. 2A-2F and 2H-2J) or the rate sensing
electrodes 212 and 213 (FIG. 2G) can be provided. This
zero rate sensing circuit 98 produces a high (ONE)
output signal whenever. the beating rate is zero,
indicating the heart has stopped beating (sometimes
referred to as going °'flat line"). This may represent
either asystole or fine ventricular fibrillation.
Under this condition, the microprocessor 93 is
programmed to first effect a charging and discharging
of the storage capacitor 65, supplying a ONE signal via
its command defibrillate output connection to the OR
gate 73 and then to effect antibrachycardia pacemaking
after a given number of capacitors) discharges (say 4)
if no hemodynamic improvement is noted. The order of
defibrillation and pacemaking may be programmed in a
-64-
G3 C% ~~~ ~~a r~, r ;,
~ h..~ ~i L:: 'i.' ..;. (.!
reverse manner as desired.
The circuit of FIG. 10 includes, if desired, a
narrow window probability density function circuit 99,
which has its input coupled to the sensing electrodes
18a and 18b or sensing electrodes 212 and 213. The
probability density function circuit may be of the type
disclosed in U.S. Pat. Nos. 4,184,493, 4,202,340 and
4,475,551 of Langer et al. and which produce a high
(ONE) output signal whenever fine ventricular
fibrillation is present. This ONE output is supplied
to an input of the microprocessor 93 which, in
accordance with its program stored in the ROM 94,
effects the charging and discharging of the storage
capacitor 65, supplying via its aommand defibrillate
output a ONE signal to the OR gate 73 to initiate
discharge.
Conventional antitachycardia systems function
primarily as rate-only sensing devices and perform
inadequately in differentiating hemodynamically stable
from unstable tachycardias. Consequently, in the
course of developing the present invention, mean right
atrial (MRAP), mean right ventricular (MRVP), and mean
arterial pressures (MAP) were studied by the applicant
for determining if a basis was present to distinguish
significant arrhythmias and serve as a basis for
improving antitachycardia systems.
Hemodynamic responses to rapid atrial and
ventricular pacing were examined in 10 closed-chest
anesthetized dogs. Pressure monitoring catheters
placed in the femoral artery, high right atrium (HRA),
and right ventricular apex (RVA) measured HIAP, MRAP,
and MRVP at baseline heart rates and after 30 and 60
sec. rapid HRA and RVA pacing. Pressures recorded
during rapid pacing (average of the pressures at 30 and
60 sec. of pacing) at pacing rates of 180, 250, and
280/min. were compared to those recorded initially at
-65-
~~'v i ~ 4.a. .~ .., u::
baseline heart rates.
An exemplary graphical representation of the ECG
wave MAP and MRAP of one dog is illustrated in FIG. 11
along a time base of 15 seconds, the pacing rate in
this case being 250 b.p.m. starting at time zero. The
traces of MAP and MRAP indicate that the changes are
slight; hemodynamic compromise is not indicated. As
illustrated in FIG. 12, when the dog was subjected to a
pacing rate of 280 b.p.m. starting at time zero, in
this case as clearly shown by the traces, the MAP
dropped markedly within two seconds and MRAP increased
markedly within one second. Hemodynamic compromise
prevailed. Thus, it is clear that the selected
criteria can be sensed and properly form the basis of
improved antitachycardia systems and methods. In FIG.
13, traces of MAP and MRAP of a dog whose heart has
been planed in ventricular fibrillation at time zero
clearly shows marked hemodynamic compromise, the traces
of the MAP and MRAP indicating that MAP dropped and
continued to drop to an extremely low level in about
eight seconds, while the MRAP increased considerably
within the same period. As sensing algorithms, a MRAP
algorithm and a combined MRAP-rate algorithm were
tested fn this dog using a hand operated
antitachycardia-defibrillator system. Tn FIG. 14, the
ECG, MAP and MRAP traces of a dog whose heart was
placed into ventricular fibrillation at time zero is
shown for a time period of about 36 seconds, a
defibrillating pulse having been applied after a time
lapse of about 22 seconds. As shown in the MAP and
MRAP traces of FIG. 14, considerable hemodynamics
comprise appears from the onset of fibrillation and
once the defibrillating pulse has been applied, is
reversed. Moreover, normal beating rate was restored
within about three seconds.
Rapid RVA pacing, simulating ventricular
tachycardia, resulted in significant increases in MRAP
-66-
,.~ r -> -.
ia, '~,i s;:~ a.:~ '<.,_ ;:~
(5.5 ~ 0.5 to 12.0 ~ 1.0 mmHg., p 0.001) and MRVP
(11.0 ~ 1.2 to 16.0 ~ 0.9 mmHg., p 0.02) with marked
hemodynamic compromise (MAP decreased from 85 ~ 6 to 50
~ 6 mmHg., p 0.01). These parameters remained stable
during HRA pacing (simulating atrial tachycardia). The
sensing algorithms successfully indicated those
arrhythmias requiring termination, hemodynamically
unstable ventricular tachycardia and fibrillation.
Hemodynamically stable tachycardias were merely
monitored, not manually terminated.
The respective hemodynamic systems for treating a
malfunctioning heart and which respond to a selected
hemodynamic parameter include a signal processing
circuit generally designated by the numeral 300 in
FIGS. 4, 6 and 8. As shown, the signal processing
circuit 300 derives long and short term mean pulmonary
artery pressure (1~IPAP) , .mean pulmonary vein pressure
(MPVP), mean or pulmonary capillary wedge pressure
(MPCWP). Were a different hemodynamic parameter, such
as systolic pressure, diastolic pressure, diastolic end
pressure or pulse pressure selected, different signal
processing circuits may be used, as replacements. Such
circuits are illustrated respectively in FIGS. 21, 22,
23 and 24A, 24B.
Turning to FTG. 21, the replacement signal
processing circuit 300, shown associated with
amplifiers 44, 45 (also shown in FIGS. 4, 6 and 8).
The pressure sensor 20, positioned as shown in FIG. 2A,
would be used to develop a pressure-responsive signal
which would, after amplification in preamplifier 43
(FIGS. 4, 6 and 8), be amplified in the respective
amplifiers 44 and 45 and supplied as signal inputs to
respective gates 302 and 303. The output signal from
the amplifier 44 is also fed to positive slope detector
304 and to zero slope detector 305. Output from the
respective detectors 304 and 305 are fed to an AND
--67-
Y J 5.! i~,I H,d '~"a . ~. L/
circuit 306, the positive slope detector being coupled
to the AND circuit 306 via a delay circuit 307.
The output from the AND circuit 306 is fed to the
enabling inputs of the gates 302 and 303. Output from
the gates 302 and 303 are fed to respective shift
registers 308 and 309, respective signal storage being
provided by storage means, shown diagrammatically as
respective capacitors 310, 311. A clock 312 is
provided for shifting signals provided from the gates
302 and 303 into arid through the respective shift
registers 308 and 309.
Signals from the individual stages of the
respective shift registers 308 and 309 are fed in
parallel to respective arithmetical averaging circuits
313 and 314.
In operation, respective electrical signals
representations of RVP are fed to the gates 302, 303.
So long as the slope of the output signal from the
amplifier 44 is positive, indicating an increasing
instant pressure within the right ventricle, a ONE
output, delayed slightly appears as a ONE signal on an
input to the AND gate 306. So long as the zero slope
detector 305 does not detect a zero slope in the signal
received from the amplifier 44, which would otherwise
be present were the systolic pressure peak present, a
ZERO would appear on the second input to the AND gate
306. When a zero slope condition is detected the
second input to the AND gate 306 becomes ONE and a ONE
appears on the output of the AND gate.
The ONE output from the AND gate 306 enables each
of the gates 302 and the current signal level outputs,
actual real time representation of peak pressure, from
the respective amplifier 44, 45 are supplied to the
respective gates 302 and 303 and temporarily stored in
storage members, shown diagrammatically as the
capacitors 310 and 311. The stored levels are gated
-68-
into the respective shift registers 308 and 309 and
stepped through the stages within the registers under
control of the clock 312. The shift register 309 has
relatively more stages than the shift register 308 so
that long term data is present in register 309 at any
given time and short term data is present in register
308, and the respective averaging circuits 314 and 313
produce outputs representing respectively long term and
short term signal representations of RVSP. Output from
the respective arithmetical averaging circuits 313 and
314 are supplied to other circuit components of the
systems shown in FIGS. 4, 6 and 8 in place of the
signals appearing across the respective RC circuits 51,
52 (FIGS. 4, 6 and 8) and 47, 48 (FIGS. 4, 6 and 8).
Were one to wish to use right ventricular
diastolic pressure (RVDP) as the selected hemodynamic
parameter, the circuit of FIG. 22 could be used as the
signal processing circuit. The circuit of FIG. 22
differs from the circuit of FIG. 21 only in that a
negative slope detector 315 has been substituted for
the positive slope detector 304 (FIG. 21). The
remaining circuit components are the same, albeit these
components are designated by numerals associated with a
prime (' ) sign.
Were one to select right ventricular end diastolic
pressure (RVEDP) as the hemodynamic parameter, the
circuit shown in FIG. 23 could be used. In this case,
the zero slope detector 305 (FIG. 22) would be replaced
by an amplifier or differentiating circuit 316 which
receives its input from the R-wave sensing electrodes
so that the output from the AND circuit 306 would be
ONE only when a delayed output signal from the negative
slope detector 315 and a signal from the amplifier 316,
indicative of the occurrence of an R-wave peak, or the
appearance of the leading edge of the R-wave were one
to elect to use a differentiating circuit as the
-69-
4'g "~"~ ca t~ .R ()
~i.% ~d ~d 't,~ Ji. kj
circuit component 316.
Were right ventricu7.ar pulse pressure (RVPP) to be
the selected hemodynamic parameter, the circuit of
FIGS. 24A, 24B could be used. The circuit of FIGS.
24A, 24B is, in essence, a combination of the circuits
of the circuits of FIGS. 22 and 23, with two
operational amplifiers 317 and 318 added between the
the averaging circuits and the remaining circuitry of
FIGS. 4, 6 and 8. As shown in FIGS. 24A, 24B, outputs
~0 from the respective averaging circuits 313 and 313' are
fed respectively to the noninverting and inverting
inputs of the operational amplifier 317 which produces,
as its output, a signal representation of the
difference between the short term RVSP and the short
term RVDP. Similarly, output from the respective
averaging circuits 314 and 314' are fed to the
respective noninverting and inverting inputs of the
operational amplifier 318 Which produces a signal
representing the difference between long term RVSP and
long term RVDP. The circuits of FIGS. 21, 22, 23 and
24A, 24B could also be used as a replacement for the
~ circuit 300 in the system of FIG. 10.
In the event one wished to apply the teachings of
FIGS. 21, 22, 23 and 24A, 24B to the fixed (possibly
adjustable) baseline, the portion of the circuits of
FIGS. 21, 22, 23 and 24A, 24B involving the short term
determinations would be retained and used as
replacements for the signal processing circuit 301
shown in FIGS. 15, 17 and 18.
The hemodynamic responses to the onset and
termination of sustained ventricular tachyarrhythmias
were further studied by applicant and his coworkers in
20 human patients to determine the ideal parameters)
for a hemodynamically responsive antitachycardia
system. Right atrial, right ventricular, and mean
arterial pressures along with intracardiac electrograms
-70-
~,~~ G~ C.,'~ i~ i~7
ti5 ~d ~d foy .6. ~.J
were continuously recorded. Patients were 57 ~ 3 years
(mean ~ SE) with an average left ventricular ejectian
fraction of 28 t 3~. The underlying heart disease was
coronary artery disease with a remote myocardial
infraction (15 patients), cardiomyopathy (4 patients),
and Ebstein's anomaly (1 patient). As illustrated
graphically in FIG. 25, pressures were measured at
baseline (cycle length of 701 ~ 30 ms); at 5, 15, and
30 seconds after ventricular tachyarrhythmia induction
(19 ventricular tachycardias and 4 ventricular
fibrillations; cycle length of 247 ~ 11 ms); and at 15
and 30 seconds after arrhythmia termination (cycle
length of 688 ~ 30 ms). At 15 seconds of
tachyarrhythmia, when mean arterial pressure (MAP)
decreased from baseline 80 ~ 4 mmHg to 34 ~ 3 mmHg,
mean right atrial pressure (MRAP) increased from
baseline 7 ~ 1 mml~g to 14 ~ 1 mmHg. Right ventricular
systolic pressure (RVSP) decreased from 39 ~ 2 mmHg to
27 t 2 mmHg, but right ventricular diastolic pressure
(RVDP) increased from 7 ~ 1 mmHg to 11 ~ 1 mmHg,
resulting in a right ventricular pulse pressure (RVPP)
decrease from 32 ~ 3 mmFig to 13 ~ 2 mmHg; all changes
from baseline were significant at 95~ by the,Dunnett's
test. Changes in mean right ventricular pressure
(MRVP) during this study did not appear to be as
significant, as are other pressure changes, and is not
now recommended as a preferred parameter. The changes
persisted throughout the 30 seconds of ventricular
tachyarrhythmias. Change in right ventricular end
diastolic pressure (RVEDP) upon termination, all
pressures except mean right ventricular pressure and
right ventricular diastolic pressure returned rapidly
towards baseline. In conclusion, right ventricular
systolic pressure (RVSP) and pulse pressure (RVPP)
appear very useful parameters for sensing. and
determining as hemodynamic parameters for incorporation
-71-
Y ~ /1, f v
~J ~r ~ ' i
4r .i
into the algorithms of a cardioverter-defibrillator and
antitachycardia devices, in general, and in particular
the present invention. Other pressure parameters,
including mean pulmonary artery pressure (MPAP), mean
pulmonary vein pressure (MPVP) and mean pulmonary
capillary wedge pressure (MPCWP) and right ventricular
end diastolic pressure (RVEDP) are also suitable as a
basis for practicing the present invention.
The present invention provides significant
advancements in the treatment of patients having
malfunctioning hearts. The systems of the present
invention operate automatically. The baseline pressure
and permitted deviations therefrom are not based on an
average of a large sampled population or standard;
rather, these parameters are patient-specific.
It is to be understood that the foregoing detailed
description and accompanying illustrations have been
set out by way of example, not by way of limitation.
Numerous other embodiments and variants are possible,
without departing from the spirit and scope of the
invention, its scope being defined in the appended
claims.
_~2_