Note: Descriptions are shown in the official language in which they were submitted.
2~22~7
-1- T1063
THE THERAPEUTIC USE OF 8UB~TIT~TED BEN~ENES, FORMULA~IONS
T~EREO~ AND NOVEL ~UB,STITUTED BENZENE8
The present invention relates to a class of
substituted benzene compounds which stimulate central
muscarinic acetylcholine receptors and therefore are
useful~in the treatment of neurological and mental
illnesses whose clinical manifestations are due to
cholinergic deficiency. Such diseases include presenile
and senile dementia (also known as Alzheimer's disease
and senile dementia of the Alzheimer type respectively~,
Huntington's chorea, tardive dyskinesia, hyperkinesia,
mania and Tourette Syndrome. Alzheimer's disease, the
most common dementing illness, is a slowly progressive
neurological disorder characterised by marked deficits in
cognitive functions including memory, attention, language
and visual perception capabilities.
Published European Patent Application No.
239309 discloses a class of oxadiazol~e compounds having a
substituent of low lipophilicity, which are useful in the
treatment of neurodegenerative disorders. In J. Med.
Chem. (1981), 24 (12), 1475-82 is disclosed 3-hydroxy-3-
~3-methoxyphenyl)quinuclidine and in ~elv. Chim. Acta
(1957), 40, 2170 is disclosed 3-(4-methoxyphenyl)
quinuclidine. No therapeutic activity is ascribed to
either compound. In Khim-Farm. Zh. (1982), 16 (3), 307-
311 are disclosed 3-hydroxy-3-(4-methoxyphenyl)-
quinuclidine; 3-hydroxy-3-(2-methoxyphenyl)quinuclidine
and 3-(4-chlorophenyl)-3-hydroxy quinuclidine; the
authors describe how these compounds did not display
pharmacological activity. Indeed, as reported in the J.
Med. Chem. article mentioned above, related compounds
were tested for dopaminergic activity and found to be
essentially inactive.
.
' :
, ' ' ~.
' , .:,. .
.
2~22~7
-2- T1063
It was therefore unexpected to find that a
class of benzene compounds do exhibit therapeutic
activity and have been found to stimulate cholinergic
transmission.
It is possible that t:he enhancement of
cholinergic transmission demonstrated by the compounds
according to this invention is achieved either directly
by stimulating postsynaptic receptors, or indirectly by
potentiating acetylcholine release.
The compounds according to the present
invention are benzenes, substituted on one of the ring
carbon atoms thereof with a non-aromatic, non-fused
l-azabicyclic ring system and independently substituted
on each of the other ring carbon atoms with a substituent
of low lipophilicity or a hydrocarbon substituent
provided that at least one such substituent contains a
heteroatom and further provided that such substituent is
other than hydroxy; and salts and prodrugs thereof.
Examples of suitable heteroatoms are 0,N,S and halo (eg.
F, Cl, Br).
Accordingly, the present invention provides the
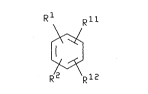
therapeutic use of~a~compound of formula (I):
:
ll
R 2 \R 12
<~
or a salt or prodrug thereof; wherein
l represents~a non-aromatic, non-fused l-aza-
bicyclic ring system; and
; R2, Rll and R12 independently represent
~ hydrogen, halo, -CF3, -oR6, -NR6R7, -NHOR6, -NHNH2, -CN,~
:~
.
' : ~ . -: ' ~. ,
.
, ~ .
2~2~ s'
-3- T1063
COR8, or a substituted or unsubstituted, saturated or
unsaturated hydrocarbon group, provided that at least one
of R2, R11 and R12 is other than hydrogen or a
hydrocarbon group, or R2 and Rl1 or R12 taken together
form a C1_6 alkylenedioxy ring, wherein R6 is C1_6 alkyl,
C2_6 alkenyl or C2_6 alkynyl, R7 is hydrogen, Cl_6 alkyl
or -COCH3, and R8 represents OH, -oR6, NHR7 or -NR6R7.
The present invention also provides a
pharmaceutical composition comprising a compound of
formula (I) as defined above or a pharmaceutically
acceptable salt or prodrug thereof and a pharmaceutically
acceptable carrier therefor other than wherein the
compound of formula ~I) is 3-hydroxy-3-(4-methoxyphenyl)-
quinuclidine; 3-hydroxy-3-(2-methoxyphenyl)quinuclidine
or 3-(4-chlorophenyl)-3-hydroxy quinuclidine.
The present invention also provides a novel
compound of formula (I) as defined above or a salt or
prodrug thereof provided that when Rl is optionally
substituted l-azabicyclot2.2.1]heptanyl, 1-azabicyclo-
[3.3.1]heptanyl or 1-azabicyclot2.2.2]octanyl, then at
least two of R2, Rll and R12 are other than hydrogen with
the exception of
3-(2-chlorophenyl)-3-hydroxyquinuclidine;
3-(3-methoxyphenyl)quinuclidine;
3-hydroxy-3(3-trifluoromethylphenyl)quinuclidine;
3-~3-trifluoromethylphenyl)quinuclidine;
3-(3-chlorophenyl)-3-hydroxyquinuclidine;
3-hydroxy-3-(3-methoxyphenyl)-I-azabicyclo[2.2.1]heptane;
3-(3-halophenyl)quinuclidines, and
3-3-methoxyphenyl-1-azabicyclo[2.2.1~heptane.
In ~ormula (I), preferably, R2, Rl1 and R12 are
each independently selected from hydrogen, halo (F, Cl,
Br, I), and oR6 provided that at least one of R2, Rl1 and R12
is other than hydrogen. More preferably, R6 is selected
: `
- ~ ~
;~
2~2~
-4- T1063
from C1_6 alkyl and C2_6 alkenyl and R7 is C1_6 alkyl.
The azabicyclic ring system is a non-aromatic
ring system containing one nitrogen atom as the sole
heteroatom. Suitably, the ring system contains up to 10
ring atoms, preferably up to 8 ring atoms. Preferably,
the ring system contains a tertiary amino nitrogen atom
in a caged structure. The bicyclic systems may be spiro
or bridged. Preferably, the nitrogen atom is at a
bridgehead. Examples of suitable ring systems for the group
1~ R1 include the following:
R~ Q~
R 3~ ~
wherein the broken line represents an optional chemical
bond; and
the substituents R3 and R4 may be present at
any position, including the point of attachment to the
benzene ring, and independently represent hydrogen, Cl_4
alkyl, halo, Cl_4 alkoxy, hydroxy, carboxy or Cl_4
alkoxycarbonyl, or R3 and R4 together represent carbonyl.
.; ' ' ` .
. .
, , : '
.
2~22~7
-5- T1063
Any substituent on the nitrogen atom of the
azabicycle (designated R5) is hydrogen or C1_4 alkyl.
It will be appreciated that the nitrogen atom
in the azabicyclic ring will carry a lone pair of
electrons.
Suitably, the group R3 is hydrogen or methyl;
and R4 is hydrogen, C1_4 alkoxy, Cl_4 alkyl, C1_4
alkoxycarbonyl, halo or hydroxy; preferably methoxy,
methyl, ~luoro, chloro, hydroxy or methoxycarbonyl.
Preferably, one or both of R3 and R4 is hydrogen. More
preferably, R4 is hydroxy or hydrogen when R3 is
hydrogen.
Preferably, the group R5 represents hydrogen or
methyl.
Suitably, the azabicyclic ring system is
azanorbornane, quinuclidine, l-azabicyclo[2.2.23Octene
(dehydroquinuclidine) or 1-azabicyclo[3.2.1]octane, any
of which may in particular be either unsubstituted or
substituted with methyl, hydroxy, fluoro, chloro or
methoxycarbonyl. Preferably, R1 is quinuclidine, 2,3-
(dehydro)quinuclidine, l-azabicyclo[2.2.1]heptane or 1-
azabicyclo[3.2.1]heptane, especially quinuclidine or 2,3-
(dehydro)quinuclidine, optionally substituted with
hydroxy.
When the groups R2, R11 and/or R12 are
hydrocarbon substituents, they may be C1_15 alkyl, C2_15
alkenyl, C2_I5 alkynyl, aryl or aralkyl. The alkyl,
alkenyl or alkynyl groups may be straight, branched or
cyclic groups. Suitably, the alkyl group comprises from
1 to 6 carbon atoms. The hydrocarbon group(s) may carry
one or more substituents. Suitable substituent groups
include halo, -oR6, -CF3, -NR6R7, -No2, optionally
substituted aryl, keto, -SR6, -SOR6, -SO2R6, -CO2R6 and
-CoNR6R7; wherein R6 and R7 are as defined with respect
.
. ' :- ' .
' . , '
': .
2~22~87
-6- T1063
to formula (I) above.
Substituents most suit:able for the aryl group
include chloro, bromo, methoxy, C1_6 alkyl,
methoxycarbonyl, trifluoromethyl, nitro and -NR6R7.
Preferably, the groups R2, Rll and R12
independently represent hydrogen, halo, -CF3, -OR6,
-NHR6, -CN, -COR8, phenyl(Cl_3)alkyl, C3-6 cycloalkyl,
adamantyl, C2_6 alkenyl, C2_6 alkynyl, Cl_6 alkyl, or
Cl_6 alkyl substituted with -oR6, -NHR6, -SR6, -CO2R6,
-CON(R6)2 or halo. Particular values of the groups R2,
Rll and/or R12 are hydrogen, chloro, fluoro, bromo,
methyl, ethyl, isopropyl, cyclopropyl, benzyl, adamantyl,
amino~ dimethylamino, methoxy, ethoxy, isopropoxy, n-
butoxy, allyloxy, propargyloxy, methoxycarbonyl and
ethoxycarbonyl. Particularly preferred values are
methoxy, chloro and CF3, especially methoxy and chloro
for R2 when Rll and R12 are each hydrogen. Suitably, R2
and Rll may be the same when R12 is hydrogen.
A particularly preferred subclass of compounds
of formula (I) or salts or prodrugs thereof are those
wherein:
Rl is a 7- or 8-membered, non aromatic, non-fused,
l-azabicycle, optionally substituted by hydroxy;
R2, Rll and R12 are each independently selected from
hydrogen, halo (especially chloro~, -CF3, -oR6
(especially -OCH3), or R2 and Rll or R12 taken together
form a Cl_6 alkylenedioxy ring (especially 3,4-methyl-
enedioxy or 3,4-ethylenedioxy).
Especially preferred is wherein:
Rl is l-azabicyclo[2.2.2]octanyl, 2,3-dehydro-1-
azabicyclo[2.2.2~octanyl, 1-azabicyclo[3.2.1.]octanyl,
2,3-dehydro-1-azabicyclo[3.2.1]octanyl, 1-azabicyclo-
[2.2.1]heptanyl and 2,3-dehydro-1-azabicyclo[2.2.1]-
heptanyl, each of which may be optionally substituted by
~ ' . ' -
:
. . . . . .
' ' ' . ' ' . ~ - ' -
.
2~22~87
-7- T1063
hydroxy.
Most of the compounds of this invention have at
least one asymmetric centre and often more than one; and
can therefore exist both as enantiomers and as
diastereoisomers. In particular, those compounds
possessing an unsymmetrical azabicyclic ring system may
exist as exo and endo diastereoisomers. It is to be
understood that the invention covers all such isomers and
mixtures thereof.
One group of prodrugs of compounds of this
invention have a substituent on the benzene ring which is
hydrolysable in ViYo to an amino group.
Groups which are hydrolysable in vivo to an
amino group on the compounds of this invention may be
readily ascertained by administering the compound to a
human or animal and detecting, by conventional analytical
techniques, the presence of the corresponding compound
having an amino substituent in the urine of the human or
animal. Examples of such groups include, for example,
amido and urethane substituents, in particular a group o~
formula -NH.Q, wherein Q represents CHO, COR or C02R, and
R represents an optionally substituted hydrocarbon group.
In this context, the hydrocarbon group R
includes groups having up to 20 carbon atoms, suitably up
to 10 carbon atoms, convenlently up to 6 carbon atoms.
Suitable groups R include~C1_9 alkyl, C2_6 alkenyl, C2_6
alkynyl, C3_7 cycloalkyl, C3 7 cycloalkyl(Cl 6)alkyl,
aryl and aryl(Cl 6)alkyl. The alkyl group R may be
straight or branched chain and may contain, for example,
up to 12 carbon atoms~,~suitably from 1 to 6 carbon atoms.
In particular, the group may be substituted methyl,
ethyl, n- or iso-propyl, n-, sec-, iso- or tert-butyI, n-
or iso-heptyl, or n- or iso-octyl. Suitable cycloalkyl
groups include cyclopentyl and cyclohexyl. The aryl
', ` ` - ' ,
`
. ~ , ,
2~2~7
-8- T1063
group R includes phenyl and naphthyl optionally
substituted with up to five, preferably up to three,
substituent groups.
Also included within the scope of the present
invention are salts of the novel compounds. It will be
appreciated that salts of the compounds for use in
medici~e will be non-toxic, pharmaceutically acceptable
salts. Other salts may, however, be useful for the
preparation of the compounds of the invention or their
non-toxic, pharmaceutically acceptable salts. Acid
addition salts, for example, may be formed by mixing a
solution of the compound with a solution of a
pharmaceutically acceptable, non-toxic acid such as
hydrochloric acid, oxalic acid, fumaric acid, maleic
acid, succinic acid, acetic acid, citric acid, tartaric
acid, carbonic acid or phosphoric acid. Preferred acid
addition salts are the hydr~gen oxalate, hydrochloride,
sesquioxalate, hydrogen maleate, oxalate and hydrogen
tartrate salts. Where the compound (I) carries a
carboxylic acid group, the invention also contemplates
salts thereof, preferably non-toxic, pharmaceutically
acceptable salts thereof, such as the sodium, potassium
and calcium salts thereof.
Salts of amine groups may also comprise the
quaternary ammonium salts in which the amino nitrogen
atom carries an alkyl, alkenyl, alkynyl or aralkyl group.
Such quaternary ammonium derivatives penetrate poorly
into the central nervous system and are therefore useful
as peripherally selective muscarinic agents, useful for
example as antispasmodic agents, agents to reduce gastric
acid secretion, agents to block the muscarinic actions of
acetylcholinesterase inhibitors in the treatment of
myasthenia gravis and as agents to co-administer with
muscarinic agonists in Alzheimer's disease.
.
, ~ .
.
.
.
2~22~7
-9- T1063
Specific compounds within the scope of formula
(I) are:
3-hydroxy-3-(3-methoxyphenyl)quinuclidine;
3-hydroxy-3-(4-methoxyphenyl)quinuclidine:
3-hydroxy-3-~2-methoxyphenyl)quinuclidine;
3-hydroxy-3-(4-chlorophenyl)quinuclidine,
3-(4-methoxyphenyl)quinuclidine;
3-(4-chlorophenyl)-2,3-dehydroquinuclidine;
3-(3-methoxyphenyl)-2,3-dehydroquinuclidine;
3-(3,4-dimethoxyphenyl)quinuclidine;
3-(2,4-dimethoxyphenyl)quinuclidine;
3-(2,5-dimethoxyphenyl)quinuclidine;
3-(3-methoxyphenyl)quinuclidine;
2,3-dehydro-3-(2-methoxyphenyl)quinuclidine;
3-(2-chlorophenyl)-3-hydroxyquinuclidine;
3-(2,4-dimethoxyphenyl)-3-hydroxyquinuclidine;
3-(2,5-dimethoxyphenyl)-3-hydroxyquinuclidine;
3-(3,4-dimethoxyphenyl)-3-hydroxyquinuclidine;
3-(3,4-ethylenedioxyphenyl)-3-hydroxyquinuclidine;
2,3-dehydro-3-(2,4-dimethoxyphenyl)quinuclidine;
2,3-dehydro-3-(2,5-dimethoxyphenyl)quinuclidine;
2,3-dehydro-3-(3,4-dimethoxyphenyl)quinuclidine;
2,3-dehydro-3-(3,4-ethylenedioxyphenyl)quinuclidine;
: 3-(3,4-ethylenedioxyphenyl)quinuclidine;
3-hydroxy-3-(3-trifluoromethylphenyl~quinuclidine;
2,3-dehydro-3-(3-trifluoromethylphenyl)quinuclidine;
3-(3-trifluoromethylphenyl)quinuclidine;
3-(3,5-dichlorophenyl)-3-hydroxyquinuclidine;
3-(3-chlorophenyl)-2,3-dehydroquinuclidine;
6-hydroxy-6-(3-methoxyphenyl)-1-azabicyclo[3.2.1]octane;
3-(2,3-dimethoxyphenyl)-3-hydroxyquinuclidine;
2,3-dehydro-3-(2,3-dimethoxyphenyl)quinuclidine;
6-(3-methoxyphenyl)-1-azabicyclo[3.2.1]oct-6-ene;
2,3-dehydro-3-(3 ! 5-dichlorophenyl)quinuclidine;
.
:
, ,
2~22~7
-10- T1063
3-(3-chlorophenyl)-3-hydroxyquinuclidine;
endo- and exo-6-(3-methoxyphenyl)-1-azabicyclo[3.2.1]-
octane;
3-(2,3-dimethoxyphenyl)quinuclidine;
3-hydroxy-3-(3,4-methylenedioxyphenyl)quinuclidine;
3-(3,5-dichlorophenyl)quinuclicline;
3-~3,5 dimethoxyphenyl)-3-hydroxyquinuclidine;
2,3-dehydro-3-(3,5-dimethoxyphenyl)quinuclidine;
3-(3,5-dimethoxyphenyl)quinuclidine;
3-(3,5-bis-trifluoromethylphenyl)-3-hydroxyquinuclidine;
3-(3,5-bis-trifluoromethylphenyl)-2,3-dehydro-
quinuclidine;
3-(3,5-bis-trifluoromethylphenyl)quinuclidine;
2,3-dehydro-3-(3,4-methylenedioxyphenyl)quinuclidine;
3-hydroxy-3-(3-methoxyphenyl)-1-azabicyclo[2.2.1]heptane;
3-(3-methoxyphenyl)-1-azabicyclo[2.2.1]hept-2-ene;
endo-3-(3-methoxyphenyl)-1-azabicyclo r 2.2.1]heptane;
and salts and prodrugs thereof.
The method of treatment of this invention
includes a method of treating Alzheimer's disease, senile
dementia of the Alzheimer type, Huntington's ~horea,
tardive dyskinesia, hyperkinesia, mania or Tourette
syndrome by the administration to a patient in need of
such treatment of a pharmacologically effective amount of
a compound of formula (I), or a salt or prodrug thereof.
In the method of treatment of this invention,
preferably, when R1 is 3-hydroxy-quinuclidin-3-yl, and
R11 and R12 are~hydrogen, then R2 is o- or p-methoxy, or
p-chloro.
This invention therefore provides a compound of
formula (I) for therapeutic use, such as for use in
treating dementia and/or any of the other conditions
referred to above. Preferably, when R1 is 3-hydroxy-
quinuclidin-3-yl, and Rll and R12 are hydrogen,
2~22~7
~ T1063
preferably R2 is other than o- or p-methoxy, or p-chloro.
It may, where appropriate, be advantageous, in
order to reduce unwanted peripherally mediated side-
effects, to incorporate into any composition a
peripherally acting cholinergic antagonist (or anti-
muscarinic agent). Thus the co~mpounds (I) may be
adminiStered together with a peripheral cholinergic
antagonist such as N-methylscopolamine, N-methylatropine,
propant~eline, methantheline or glycopyrrolate.
The compounds (I) can be administered orally,
parenterally or rectally at a daily dose of about 0.01 to
10 mg/kg of body weight, preferably about 0.1 to 1 mg/kg,
and may be administered on a regimen of 1-4 times a day.
When a cholinergic antagonist is administered, it is
incorporated at its conventional dose.
This invention therefore also provides a
pharmaceutical composition comprising a compound of
formula (I) and a pharmaceutically acceptable carrier
therefor.
In the pharmaceutical compositions or
formulations of this invention, when R1 is 3-hydroxy-
quinuclidin-3-yl, and Rll and R12 are hydrogen, then
preferably R2 is other than o- or p-methoxy, or p-chloro.
More preferably, in the compositions of this
invention, when Rl is quinuclidin-3-yl, and Rll and R12
are hydrogen, then R2 is p-methoxy; and when Rl is
3-hydroxy-quinuclidin-3-yl, and R11 and R12 are H, then
R2 is methoxy or p-chloro; and when Rl is 1-azabicyclo-
[2.2.1]heptan-7-yl, and Rl1 and R12 are hydrogen, then R2
is p-methoxy or p-chloro.
Preferablyt when R1 is quinuclidinyl, and R
and Rl~ are hydrogen, then R2 is methoxy or chloro.
The pharmaceutical formulations of this
invention preferably are in unit dosa~e forms such as
;
,
2~22~7
-12- T1063
tablets, pills, capsules, powders, granules, sterile
parenteral solutions or suspensions, or suppositories for
oral, parenteral or rectal administration. For preparing
solid compositions such as tablets, the principal active
ingredient is mixed with a pharmaceutical carrier, e.g.
conventional tabletting ingredients such as corn starch,
lactose, sucrose, sorbitol, talc, stearic acid, magnesium
stearate, dicalcium phosphate or gums, and other
pharmaceutical diluents, e.g. water, to form a solid
preformulation composition containing a homogeneous
mixture of a compound (I), or a nontoxic, pharmaceu-
tically acceptable salt therecf. When referring to these
preformulation compositions as homogeneous, it is meant
that the active ingredient is dispersed evenly throughout
the composition so that the composition may be readily
subdivided into equally effective unit dosage forms such
as tablets, pills or capsules. This solid preformulation
composition is then~subdivided into unit dosage forms of
the type described above containing from 0.1 to about 500
mg of the active ingredient of the present invention.
The tablets or pills of the novel composition can be
coated or otherwise compounded to provide a dosage form
affording the advantage of prolonged action. For
example, the tablet or pill can comprise an inner dosage
`~ 25 and an outer dosage component, the latter being in the
form of an envelope over the former. The two components
can be separated by;an enteric layer which serves to
resist disintegration in the stomach and permits the
inner component to pass intact into the duodenum or to be
delayed in release. A variety of materials can be used
for such enteric layers or coatings, such materials
including a number of polymeric acids or mixtures of
polymeric acids with such materials as shellac, cetyl
alcohol and cellulose acetate.
',
-
: . .
'
2~22~7
-13- T1063
The liquid forms in which the compositions of
the present invention may be incorporated for
administration orally or by injection include aqueous
solutions, suitably flavoured syrups and flavoured
emulsions with edible oils such as cottonseed cil, sesame
oil, coconut oil and peanut oil, as well as elixirs and
simila~ pharmaceutical vehicles. Suitable dispersing or
suspending agents for aqueous suspension include
synthetic and natural gums such as tragacanth, acacia,
alginate, dextran, sodium carboxymethylcellulose,
methylcellulose, polyvinyl-pyrrolidone and gelatin.
The present invention further provides a
process for preparing a pharmaceutical formulation
according to the invention which process comprises
bringing a compound of formula (I) into association with
a carrier therefor such as by mixing.
The compounds of this invention wherein R3 and
R4 are each other than hydroxy- or carboxy- substituted
on the azabicycle at~the point of attachment to the
benzene ring may be prepared by a process~which comprises
the deh~droxylation~or decarboxylation of a compound of
formula (III) (which is a sub-class of the compounds of
formula (I)) or a salt~thereof:
B
/ ~ ~
: V~ :
<I`II)
wherein V represents a benzene ring independently
substituted on each of the remaining ring carbon atoms
:: :
with a substituent~of low lipophilicity and/or a
hydrocarbon substituent as defined above; A represents
- :
~: ', ' .
r~ ~ 2 2 ~ $ ~
-14- T1063
the residue of an azabicyclic ring as defined above; and
B represents hydroxy or carboxy.
When the group B in compound (III) is hydroxy,
it may be removed by chlorination and elimination or by
dehydration to provide compounds of formula (I) wherein
Ri contains unsaturation at the point of attachment to
the benzene ring. When it is desired to prepare a
compound of formula (I) wherein R1 contains saturation at
the point of attachment to the benzene riny, the
unsaturated compound of formula (I) may be hydrogenated
using conventional methods. Dehydration may be
accomplished by treatment with an acid, for example with
trifluoroacetic acid. For example, chlorination and
elimination may be effected by treatment with phosphorus
oxychloride in the presence of triethylamine, or with
thionyl chloride followed, where necessary, by DBN. The
chloride or the unsaturated product may then be
hydrogenated under conventional conditions, such as over
10% palladium/carbon in methanol. Alternatively, the
compound (III) may be dehydroxylated by the use of
thionyl chloride followed by treatment with tributyltin
hydride in a solvent such as tetrahydrofuran in the
presence of a radical initiator such as azabisiso-
butyronitrile.
The compound of formula (III) where B is
hydroxy may be prepared by reaction of a ketone compound
of formula (IV) with a metal derivative of a benzene
compound of formula (V):
~2~7
-15- T1063
~ y/
~ - V
V
( [V) (V)
wherein A and V are as defined above: and M represents a
metal atom, for example magnesium. The metal derivative
for instance may be prepared by reacting the
corresponding halo-substituted benzene compound such as
the Br- or I-substituted benzene compound with the metal.
When the group B in compound (III~ is carboxy
it may be removed by standard decarboxylation techniques
such as heating in aqueous solution made to pHl with
hydrochloric acid.
The compounds of formula (III) where B
represents carboxy may be prepared by reaction of a
compound of formula (VI) with a compound of formula
(VII): 1
R - W Hal - V
(VI) ~VII)
2s
wherein Rl and V are as defined above, Hal represents
halo, and W represents cyano, a carboxylic acid group
or a derivative thereof which activates the adjacent
position, and subsequently, where necessary, converting
the group W to carboxy, preferably by hydrolysis.
Preferably, W represents an alkyl ester group
such as methoxycarbonyl. Preferably, the halo group is
iodo. The reaction between compounds (VI) and (VII) may
'
f~ 2 $ 8 ~
-16- T1063
be carried out in the presence of a strong base such as
lithium diisopropylamide in a solvent such as
tetrahydrofuran.
The azabicyclic moiet:y may be introduced into
the molecules concerned by methods known from the art, in
particular by methods analogous to those described in
EP-A-0339309.
After any of the above described processes is
complete, one substituent can be converted to another.
For example, an amino group may be converted to chloro,
or hydrazo, -NHNH2, via the intermediacy of diazonium,
~-N2). Similarly, alkoxycarbonyl group~ may be
converted, via carboxy, to an amino substituent, -NH2;
and methoxy may ~e converted to hydroxy by treatment with
concentrated hydrobromic acid; thesa groups may then be
further converted to any value of R2 defined above.
In any of the above reactions it ~ay be
necessary and/or desirable to protect any sensitive
groups in the compounds. For example, if the reactants
employed include amino, carboxy, keto, hydroxy or thiol
groups, these may be protected in conventional manner.
Thus, suitable protecting groups for hydroxy groups
include silyl groups such as trimethylsilyl or t-butyl-
dimethylsilyl, and etherifying groups such as tetrahydro-
pyranyl: and for amino groups include benzyloxycarbonyl
and t-butoxycarbonyl. Keto groups may be protected in
the form of a ketal. Carboxy groups are preferably
protected in a reduced form such as in the form of their
corresponding protected alcohols, which may be
subsequently oxidised to give the desired carboxy group.
Thiol groups may be protected by disulphide formation,
either with the thiol itself or with another thiol to
form a mixed disulphide. The protecting groups may be
removed at any convenient stage in the synthesis of the
desired compound according to conventional techniques.
.
'. . : . ~
2(~22~87
-17- Tl063
The following Examples illustrate the
preparation of compounds according to the invention.
Each of the compounds of the Examples demonstrates an
affinity for the muscarinic receptor, having an IC50
(concentration required to displace 50% of specific t3H]-
N-methylscopolamine binding from rat cortical membrane
prepara,tions) significantly lower than lOO~M.
Penetrability into~the central nervous system of
compounds of this invention was assessed by a measurable
displacement of radioligand binding using standard "ex-
vivo" binding techniques (Ref:J. Neurosurg., 1985, 63,
589-592).
In the Examples, all temperatures are in C;
THF is tetrahydrofuran; and ether is diethyl ether.
'
'
,
2~22~7
- 18- T1063
D13SCRIPTIVE EXAMPLE 1
3-Hydrox~-3-(3-methoxyphen~quinuclidine Hydro~en
02calate
A solution of 3-quinuclidinone (10.88g, 0.087mol) in
anhydro~rs diethyl ether (40mL) was added to a solution of 3-
methoxyphenylmagnesium bromide [prepared from 3-
bromoanisole (14.04g, 0.07mol), iodine (2 cr~stals) and
magnesium (2.00g, 0.0825mol) in anhydrous diethyl ether
(30mL)] at 5C over 20 minutes. After 18 hours at room
temperature a saturated aqueous solution of ammonium
chloride (200mL) was added. ~lacial acetic acid was added to
adjust the aqueous solution of pH7 then tha aqueous was
16 separated and washed with ethyl acetate (100mL).
The aqueous solution was basified to pH12 with 2M
sodium hydroxide solution then e~racted with dichloromethane
(5 x 200mL). The combined organics were dried (sodium
20 sulphate) then evaporated to give a cream solid which was
triturated with diethyl ether (2 x 150mL) to afford the title
compound free base as a cream solid (8.00g, 45%), m.p. 142C-
145C.
The hydrogen oxalate salt had mp 202C-204C (dec.)
(propan-2-ol/diethyl ether). Rf = 0.35 in
dichloromethane/methanol (20:1) on alumina plates; lH NMR
2~22~7
- 19- T1063
(360MHz, D2O) ~ 1.64-1.74 (lH, m), 1.92-2.03 (2:H, m) and 2.40-
2.48 (lEI, m, 5 and 8-CH2); 2.65-2.67 (1H, m, 4-CHt; 3.24-3.32
(2H, m) and 3.42-3.47 (2H, m, 6-CH2 and 7-CH2); 3.58 (lH, dd,
J = 2, 14Hz, 2-CH); 3.87 (3H, s, OCH3); 3.98 (lH, dd, J = 14Hz,
6 2-CH); 7.04 (lH, dd, J = 2,8Hz, Ar-H); 7.11 (lH, dd, Jl = J2 =
2Hz, Ar-H?; 7.16 ~lH, broad d, J = 8Hz, Ar-H); 7.45 (lH, dd, J1 =
J2 = 8Hz, Ar-H); MS, m/z 233 for M+ OI ~ree base, HRMS,
Found: M+, 233.1425, C14H1gNO2 requires M, 233.1416.
(Found: C, 61.58; H, 6.96; N, 5.10. C14HlgN02. 0-75 (:2H20~L
require~ C, 61.88; H,6.87; N,4.66%).
DESCRIPTIVE EXAMPIE 2
3-HYdrox~-3-(4-Methox~phen:srl)quinuclidine H~dro~en
16 Oxalate
The title compound free base (7.7g, 44~) was obtained as
described for Example 1. The hydrogen oxalate salt had mp
117-120C (acetone). Rf, 0.33 in dichloromethanetmethanol
(20:1) on alumina plates; lH NMR (360MHz, D2O) ~ 1.63-1.72
(lH, m), 1.91-2.02 (2H, m) and 2.37-2.47 (lH, m, 5 and 8-CH2);
2.65-2.66 (lH, m,4-CH); 3.20-3.32 (2H, m) and 3.34-3.44 (2H, m,
6-CH2 and 7-CH2); 3.57 (lH, dd, J = 2, 14Hz); 3.85 (3H, s,
OCH3); 3.97 (lH, dd, J = 2, 14Hz); 7.07 (2H, d, J = 9Hz,2 x Ar-
H); 7.49 (2H, d, J = 9Hz, 2 x Ar-H); MS, m/z 233 for M~ of free
base; HRMS, ~ound: M~, 233.1411, C14H1gNO2 requires
233.1416. (Found: C, 58.43; H, 6.45; N, 4.31. C14H1gNO2.
.-:
2~2~
- 20 - T1063
C2H204. 0.25 H20 requires C, 58.62; H, 6.61; N, 4.27%).
DESCRIPTIVE EXAMP:LE 3
3-HvdroxY-3-(2-metho~phen~Tl)quinuclidine
A s~lution of 3-quinuclidinone (3.97g, 31.7mmol) in THF
(15mL) was added dropwise to a solution of 2-
methoxyphenylmagnesium bromide [prepared from 2-
lo bromoanisole (3.76mL, 28.9mmol), iodine (2 crystals) and
magnesium (0.77g, 31.7mmol) in THF (lOmL)] cooled in an
ice/wa$er bath. After 3h at room temperature a saturated
aqueou~ solution of ummonium chloride (50mL) was added. The
resulting mixture (pH10) was diluted with H20 (50mL) and
extracted with dichloromethane (4 x 50mL). The combined
organic extracts were washed with brine, dried over magnesiwn
sulphate and evaporated to a lig~t yellow oil. The crude product
was purified by chromatograp y through silica-gel using
dichloromethane/methanol/~lmmonia (89:10:1) as eluent to give
the title compound as a colourless oil (1.02g); lH NMR (360MHz,
CDCl3) ~ 1.45-1.66 (3H, m) and 2.32 (lH, m, 5 and 8-CH2), 2.44
(lH, br, OH), 2.46, (lH, m, 4-CH), 2.81 (2H, t, J = 8Hz) and 2.93
(lH, m) and 3.10 (lH, m, 6 aDd 7-CH2), 3.29 (lH, d, J = 15Hz, 2-
CH), 3.40 (lH, d, J = 15Hz, 2-CH), 3.91 (3H, s, OMe), 6.98 (2H,
m, Ar-H), 7.27 (2H, m, Ar-H); m/z 233 (M+).
: :
~ .
: : :
~ ' .
- ' ~ . ' -
.: ~
2~22~g7
- 21 - T1063
DESCRIPTIVE EXAMPLE 4
3-(4-Chlorophenvl)-3-hvdroxy quinuclidine H~drogen
Oxalate
The title compound ~ee base (4.98g, 31%) was obtained as
describe*for ExamPle 1. The hydrogen o~alate salt had mp
184-187C (propan-2-ol/methanol). Rf = 0.64 in
dichloromethane/methanol (20:1) on alumina plates; lH NMR
(360MHz, D20) ~ 1.62-1.72 (lH, m); 1.93-2.02 (2H, m); 2.40-2.47
(lH, m, 5-CH2 and 8-CH2); 2.64-2.66 (lH, m, 4-CH); 3.22-3.31
(2H, m) and 3.40-3.4G (2H, m, 6-CH2 and 7-CH2); 3.58 (lH, dd,
J = 2, 14Hz, 2-CH); 3.95 (lH, d, J = 14Hz, 2-CH); 7.44-7.56 (4H,
m, 4 x Ar-H); MS, m/z 237 for M+ of free base; HRM3, Found:
16 M+, 237.0921, C13Hl6ClNOrequiresM, 237.0920. (Found: C,
54-98; H, 5-52; N, 4.20. C13H16ClNO. C2H204 requires C,
54.97; H, 5.54; N, 4.27%j.
DESCRIPTIVE EXAMPLE 5
3-(4Metho~vphenvl) quilluclidine Hydroç~en Oxalate
Triethylsilane (3.8ml~ 0.024mol) was added to a st*red
solution of 3-hydroxy-3-(4-methoxyphenyl)quinuclidine (1.Og,
0.0048mol, prepared as~in xample 2) in trifluoroacetic acid
(lOmL). After 20 hours at 50C the solution was cooled to 5C
and basified to pH9 with saturated sodium hydrogen carbonate
solution. The mixture was e~tracted with dichloromethane (3 x
:: :
: ~ -
. . . ~ .
., ' : : ~ ' : '
,
~. -
.
2~.2~7
- 22 - T1063
lOOmL), then the combined organics were dried (sodium
sulphate) then evaporated to dryness. The crude product was
purified by flash chromatography on silica using
dichloromethane/methanol (20:1) to afford the title compound
6 free base (0.86g, 83%). The hydrogen oxalate salt had mp
104C-106C (acetone). Rf = 0.77 in dichloromethane/methanol
(20:1) on alumina plates; lH NMR (360MHz, D20) ~ 1.75-1.87
(lH, m), 1.88-1.92 ~lH, m) and 2.03-2.17 (2H, m, 5-CH2 and 8-
CH2); 2.22-2.24 (lH, m, 4-CH); 3.29-3.63 (6H, m,2-CH2, 6-CH2
0 and 7-CH2); 3.70-3.79 (lH, m, 3-CH); 3.83 (3H, s, OCH3); 7.03
(2H, d, 3 ~ 9Hz,2 x Ar-H); 7.34 (2H, d, J = 9Hz, 2 x Ar-~I); MS,
m/z 217 for M+ of free base. (Found: C, 61.86; H, 6.81; N, 4.50.
Ci4H19NO. C2H20~. 0.25H20 requires C, 61.62; H, 6.95; N,
4.49%).
1~
E~AMPLE 6
3-(4-Chlorophenyl)-2~3-deh~dro-quinuclidine H:ydro~en
Oxalate
Triethylsilane (7.6mL, 0.047mol) was added to a stirred
solution of 3-(4-chlorophenyl)-3-hydroxyquinuclidine (2.0g,
0.0094mol) in tri~luoroacetic acid (20mL) and dichloromethane
(5mL). After 20 hours at gentle reflux the reaction mixture was
worked up as described for Example 5. The hydrogen oxalate
salt had mp 131-133C (propan-2-olldiethyl ether). Rf 0.29 in
dichloromethane/methanol (20:1) on silica plates; lH NMR
2~22~7
- 23 - T1063
(360MHz, D2O) ~ 1.81-1.89 (2H, m) and 2.12-2.20 (2H, m, 5-CFi2
and 8-CH2); 3.14-3.23 (2H, m) and 3.58-3.66 (3H, m, 4-CH, 6-
CX2 and 7-CH2); 6.97 (lH, s, 2-CH); 7.43-7.59 (4H, m, 4 x Ar-
H); MS, m/z 219 for M~ of free base. (Found: C, 57.68; H, 5.17;
6N~ 4-45- C13H14NCl- C2H2O4- 0.25 H2O requires C, 57.33; H,
5.29; N, 4.46%).
E2AMPLE 7
102,3-Dehydro-3-(3-metho~phenvl)quinuclidine HYdro~en
Oxalate
The title compound was prepared as described in Example
6, mp 76-78C (propan-2-olidiethyl ether). Rf = 0.40 in
16dichloromethane/methanol (9:1) on silica; lH NMR (360MHz,
D2O) o 1.83-1.91 (2H, m) and 2.12-2.21 (2H, m) and 3.58-3.66
(3H, m, 4-CH, 6-CH2 and 7-CH2); 3.87 (3H, s, OCH3); 6.97 (lH,
d, J = lHz, 2-CH); 7.09 (lH, dd, J = 2, 8Hz, Ar-H); 7.13 (lH, dd,
Jl = J2 = 2Hz, Ar-H); 7.21 (lH, d, J = 8Hz, Ar-H); 7.45 (lH, dd,
20Jl = J2 = 8Hz, Ar-H). (Found: C, 62.26; H, 6.28; N, 4.50.
C14H17NO.C2H2O4. 0.25HI20 requires C, 62.03; E, 6.34; N,
4.52Yo).
EXAMPLE 8
.
2,3-Dehydro-3-(2-methoxyPhenyl)quinuclidine Hydrogen
Oxalate
- ,
' . . :
. . , . -
- .
- - ~ ..
2022~
- 24 - T1063
Triethylsilane (1.18mL, 7.36mmol) was added to a stirred
solution of 3-hydroxy-3-(2-methoxyphenyl)quinuclidine (0.25g,
0.94mmol) in trifluoroacetic acid (1.3mI.) and dichloromethane
(5mL). After 18h at room temperature the solution was
6 evaporated, and then dissolved in aqueous potassium carbonate
solution (lN, 50mL) and extracted with dichloromethane (4 x
25mL). ~he combined organic extracts were dried over
magnesium sulphate, evaporated and the crude product purif;ed
by chromatography through silica-gel eluting with
dichloromethane/methanoVammonia (84.5:5:0.5) to give the free
base as a colourless oil (0.19g). The hydrogen oxalate salt had
mp 128-134C (methanol/diethyl ether); lH NMR (360MHz,
D2O) ~ 1.84-1.96 (2H, m) and 2.05-2.16 (2H, m, 5 a~d 8-l:!H2);
3.23 (2H, m, 6-CH2); 3.45 (lH, m, 4-CH); 3.60 (2H, m, 7-C~H2);
16 3.90 (3H, s, OMe); 6.89 (lH, s, 2-CH); 7.06-7.18 (2H, m, Ar-H);
7.35-7.56 (2H, m, Ar-H); m/z 215 (M~). (Found: C, 61.04; H,
6-28; N~ 4-45- C14H17N- C2H2O4. 0-5 H20 requires C, 61.14;
H, 6.41; N, 4.45~o).
EXAMPLE 9
3-(~enYI~-3-hvdroxyq~i~uclidine Hydrochloride
The title compound free base (0.94g, 14%) was obtained as
26 described for Example 1. The hydrochloride salt had mp 260-
2 6 5 C ( M e O H / E t 2 O ) . R f = 0 . 6 0 i n
dichloromethane/methanol/ammonia (40:10:1) on silica plates;
.' - - '~''
~'. '
21~22~7
- 25 - T1063
H NMR (360MHz, D2O) ~ 1.95-2.09 (2EI, m, 5-CEI2); 2.13-2.23
and 2.43-2.51 (each lH, each m, 8-CH2); 3.06-3.08 (lH, m, 4-
CH); 3.17-3.50 (4H, m, 6-CH2 and 7-CH2); 3.80 (lH, dd, J - 2,
14Hz, one of 2-CH2); 4.12 (lH, dd, J = 2, 14Hz, one of 2-(:H2);
6 7.33-7.43 and 7.55-7.60 (each 2H, each m, 4 x Ar-H); MS, m/z
238 for (M+H)+ of free base. (Found: C, ~6.36; H, 6.27; N, 4.96.
C13H16ClNO.HCl. 0.2H2O requires C, 56.21; H, 6.31; N,
5.02~Yo).
1~ EXAMPLE 10
3-(3-Metho~phenyl)quinuclidine H:Ydro~en Oxalate
2,3-Dehydro-3-(3 -methoxyphenyl)quinuclidine (1.10g,
16 0.0051mol) was hydrogenated in ethanol (20mL) containing 10%
palladium on carbon ~1~Omg) and glacial acetic acid (0.3mL,
0.0062mol) at 3.~ x 105 N.m~2 (~0 psi). The reaction mixture
was filtered, then evaporated to dryness. The resulting gum
was dissolved in water (l0m~), basffled to pH~10 with sodium
carbonate and extracted with dichlorometha~e (~ x 10mL). The
combined organics were dried (sodium sulphate) then
evaporated to provide the crude free base which was purified by
column chromatography on silica by elution with
dichloromethane/methanol (20:1). The required product was
obtained as a pale yellow oil (200mg). The hydrogen oxalate salt
had mp 94-96C (propan-2-ol/diethyl ether). R~= 0.77 in
dichloromethane/methanol (20:1) on alumina plates; lH NMR
(360MHz, D2O) o 1.74-2.22 (4H, m, ~-CH2 and 8-CH2); 2.28-2.30
.. ~
2~22~87
- 2fi - T1063
(lH, m, 4-CH); 3.28-3.60 (6H, m, 2-CH2, 6-CH2 and 7-CH2);
3.72-3.78 (lH, m, 3-CH); 3.85 (3H, s, OCH3); 6.96 (lH, s, 2-
aromatic H); 6.97 (lH, d, J = 8Hz, 4-aromlatic H); 7.03 (lH, d, J =
8Hz, 6-aromatic H); 7.41 (lH, dd, Jl = J2 = 8Hz, 5-aromatic H);
6 MS, m/z 217 for M+ of free base. (Found: C, 61.84; H, 6.82; N,
4.51. C14HlgNO.C~2H204Ø25H20 requires C, 61.62; H, 6.9~;
N, 4.49%k
EXAMPLE 11
3-(2,4-DimethoxYphen:yl)-3-hvdrox:yquinuclidine
Hydro~en Oxalate
tButyllithinrn (lOOmL of a 1.7M solution in pentane) was
16 added dropwise to a stirred, cooled (-78C) solution of l-bromo-
2,4-dimethoxybenzene (12.9mL, O.O9mol) in anhydrous diethyl
ether (60mL) under a nitrogen atmosphere, keepi~g the
temperature below -65C. After 1 hour at -78C a solution of 3-
quinuclidinone (lO.Og, 0.08mol) in anhydrous diethyl ether
(80mL) was added dropwise, keeping the temperature below
-65C. After addition the reaction mixture was stirred whilst
warming to room temperature for 16 hours. Saturated
ammonium chloride solution (200mL) was added followed by
glacial acetic acid to pH = 6. The aqueous layer was separated,
washed with ethyl acetate (lOOmL), then the aqueous was
basified to pH = :L2 with 2M sodium hydroxide solution and
extracted with dichloromethane (5 x 20mL). The combined
2~22~7
- 27 - T1063
organics were dlied (sodium sulphate) then evaporated to give a
pale yellow gum (15.7g) which was triturated with diethyl ether
(200mL) to afford the title compound ~ree base as a colourless
solid t9.55g, 45%), mp 145-148C. The hydrogen oxalate salt
had mp 125-128C (acetone). R~ = 0.15 in
dichloromethane/methanol (5:1) on silica plates; lH NM}~
(360MHz, D2O) o 1.87-2.13 (3H, m) and 2.37-2.43 (lH, m, 5-CH2
and 8-CH2); 2.92-2.94 (lH, m, 4-CH); 3.12-3.42 (4H, m, 6-CH2
and 7-CH2); 3.63 (lH, d, J = 12Hz, one of 2-CH2); 3.78 (lH, dd, J
= 2, 12Hz, one of 2-CH2); 3.85 (3H, s, OCX33; 3.87 (3H, s,
OCH3); 6.62 (1~I, dd, J = 2.6, 9Hz, 5-aromatic H); 6.72 (1H, d, J
= 2.~Hz, 3-aromatic H); 7.32 (lH, d, J = 9Hz, 6-aromatic H); MS,
CI~, m/z 264 for (M~H)~ of free base. (Found: C, 57.78; H, 6.63;
N~ 4-02- Cl~H21N3- C2H204 requires C, 57.78; ~I, 6.56; N,
3.96~o).
EXAMPLE 12
:
3-~2,5-DimethoxYphenyl)-3-hydroxYquinuclidine
Hydro~en O2~alate
The title compound free base (7.50g, 36%) was obtained
from 3-quinuclidinone and 1-bromo-2,5-dimethoxybenzene as
described for Example 11. The hydrogen oxalate salt had mp
26 138-142C (propan-2-ol/diethyl ether). Rf = 0.15 in
dichloromethane/methanol (5:1) on silica plates; lH NMR
(360MHz, D2O) ~1.92-2.15 (3H, m) and ~.38-2.44 (lH, m, 5-CH2
-
2~22~
- 28 - T1063
and 8-CH2); 2.93-2.94 (lH, m, 4-CH); 3.14-3.43 (4H, m, 6-CH2
and 7-CH2); 3.66 (lH, d, J = 14Hz, one of 2-CH2); 3.81 (3H, s,
OCH3); 3.84 (3H, s, OCH3); 3.85 (lH, dd, J = 2, 14Hz, one of 2-
CH2); 6.98 (lH, d, J = 3Hz, 6-aromatic H); 7.03 (lH, dd, J = 3,
9Hz, 4-aromatic H); 7.11 (lH, d, J = 9Hz, 3-arornatic H); MS, m/z
263 for M+ of free base. (Found: C, 56.12; H, 6.44; N, 4.06.
C15H21~3 C2H24 5H2O requires C, 56.35; H, 6.67; N,
3.87%).
EXAMPLE 13
3 -(3,4^Dimethox~phenYl) -3 -hydrox~q uinuclidine
Hydro~en O~alate
The title compound free base (3.56g, 17%) was obtained
from 3-quinuclidinone and 1-bromo-3,4-dimetho~ybenzene as
described for Example 11. The hydrogen oxalate salt had mp
151-152C (ethyl acetate). R~ = 0.2 in
dichloromethane/methanol (9:1) on silica plates; lH NMR
(360MHz, D2O) o 1.70-1.73 (lH, m); 1.91-2.02 (2H, m) and 2.41-
2.44 (lH, m, 5-CH2 and 8-CH2); 2.65-2.68 (lE, m, 4-CH); 3.24-
3.34 (2H, m) and 3.41-3.46 (2H, m, 6-CH2 and 7-CH2); 3.56 (lH,
dd, J = 2, 14Hz, one of 2-CH2); 3.88 (3H, s, oCH3j; 3.89 (3H, s,
OCH3); 3.99 (lH, d, J = 14Hz, one of 2-CH2); 7.01-7.14 (3H, m, 3
26 x Ar-H); MS, mlz 264 for (M+H)+. (Found: C, 57.85; H, 6.64; N,
4 03 C17H21N3- C2H24 requires C, 57.78; H, 6.56; N
3.96%).
: ,
':
--
2~22~7
- 29 - T1063
EXAMPLE 14
3-(3~ ~ nyl)-3-hydroxyquinuclidine
Eydro~en Oxalate
The title compound free base (12.20g, 47%) was obtained
from 3-q~inuclidinone and 3,4-ethylenedioxybromobenzene as
described for Example 11. The hydrogen oxalate salt had mp
80-83C (propan-2-ol). Rf- 0.15 in dichloromethane/methanol
10~5:1) on silica plates; lH NMR (360MHz, D2O) ~S 1.63-1.72 (lH,
m); 1.92-2.01 (2H, m) and 2.36-2.46 (lH, m,5-aH2 and 6-CH2);
2.60-2.62 (lH, m,4-CH); 3.21-3.32 (2H, m) and 3.37-3.45 (2H, m,
6-CH2 and 7-CH2); 3.53 (lH, dd, J = 2, 14Hz, one of 2-aH2);
3.92 (lH, d, J = 14Hz, one of 2-CH2); 4.32 (4H; s, 2 ~ OCH2);
166.94-7.07 (3H, m, 3 x Ar-H); MS, m/z 261 for M+ of free base.
(Found: C, 55.42; H, 6.37; N,3-68- C15HlgNO3- C2H24- E2O
requires C, 55.28; H, 6.28; N,3.79%).
EXA~lPLE 15
2~3-Dehydro-8-(2,4-dimethoxyph~enYl)quinuclidine
Hydro~en~ Oxalate
3-(2,4-Dimethoxyphenyl)-8-hydroxyquinuclidi~e (8.5g, ~ ~
2~0.032mol, prepared as in Example 11) was heated under reflux ~ ;in dichloromethane (25~mL) containing trifluoroacetic acid
~; ~ (2~mL) for 24 hours. The reaction mixture was evaporated to
:: :
~: :
: :
:. :
~ . ~ -, : - :
20?,2~87
- 30 - T1063
dryness and the residue dissolv~d in water (30mL), basi~led to
pH = 12 with 2M sodium hydroxide solution then extracted with
dichloromethane (3 x 30mL). The combined organics were dried
(sodium sulphate) then evaporated to give a pale yellow gum
which was purified by column chromatography on silica using
dichloromethane/methanol (20:1). The title compound free base
was obtained as a colourless ~um (6.92g, 88%). The hydrogen
oxalate salt had mp 157-158C (propan-2-oVdiethyl ether). Rf =
0.20 in dichloromethane/methanol (9:1) on silica; lH NMR
(360MHz, D2O) o 1.84-1.91 (2H, m) and 2.06-2.14 (2H, m, 5-CH2
and 8-CH2); 3.14-3.22 (2H, m) and 3.56-3.64 (2H, m, 6-(:H2 and
7-CH2); 3.43-3.45 (lH, m, 4-CH); 3.87 (3H, s, OCH3); 3.88 (3H,
s, OCH3); 6.67 (lH, dd, J = 2.5, 8Hz, 5-aromatic H); 6.70 (lH, d,
J = 2.5Hz, 3-aromatic H); 6.86 (lH, s, 2-CH); 7.33 (lH, d, J =
8Hz, 6-aromatic H); MS, rn/z 245 for M~ of free base. (Found: C,
60-20; H, 6-27; N, 4.10. ClsHlgNO2. C2H2O4Ø25H2O requires
C, 60.08; H, 6.38; N, 4.12%).
EXAMPLE 16
2~3-Dehydro-3-(2,5-dimethoxyphenyl)quinuclidine
Sesquioxalate
The title compound ~ree base (1.50g, 27%) was obtained
25 from 3-(2,5-dimethoxyphenyl)-3-hydroxyquinuclidine (as
described in Example 12) by the method described in Example
15. The hydrogen oxalate salt had mp 108-110C (propan-2-
2~22$~7
- 31 - T1063
oVdiethyl ether). Rf - 0.20 in dichloromethane/methanol (9:1)
on silica plates; lH NMR (360MHz, D2O) o 1.89-1.96 (2H, m)
and 2.07-2.15 (2H, m, 5-CH2 and 8-CH2~; 3.16-3.24 (2H9 m) and
3.58-3.67 (2H, m, 6-CH2 and 7-CH2); 3.41-3.43 (lH, m, 4-CH);
6 3.82 (3H, s, OCH3); 3.85 (3H, s, OCH3); 6.90 (lH, s, 2-CE); 6.94
(lH, d, J = 3Hzj 6-aromatic H); 7.06 (lH, dd, J = 3, 9Hz, 4-
aromatic-H); 7.11 (lH, d, J = 9Hz, 3-aromatic H); MS, m/z 245
for M+ of free base. (Found: C, 56.61; H, 5.98; N, 3.82.
C15HlgNO2. 1.5 C2H2O4 reqwres C, 56.84; H, 5.83; N, 3.68%).
EXAMPLE 17
2,3 -Dehydro-3 -(3,4-D_thoxyphellyl)quinuclidine
Hydro~en Oxalate
The title compound free base (1.31g, 49%) was obtained
from 3-(3,4-dimethoxyphenyl) -3 -hydro~yquinuclidine ( as
described in Example 13) by the method described in Example
15. The hydrogen oxalate salt had mp 154-157C (propan-2-
oVdiethyl ether). Rf = 0.20 in dichloromethane/methanol (9:1)
on silica plates; l~I NMR (360MHz, D2O) o 1.83-1.89 ~2H, m)
and 2.13-2.21 (2H, m, 5-CH2 and 8-CH2); 3.16-3.23 (2H, m) and
3.59-3.69 (3H, m, 4-CH, 6-CH2 and 7-CH2); 3.89 (6H, s, 2 x
OCH3); 6.92 (lH, s, 2-CH); 7.10 (lH, d, J = 9Hz, 5-aromatic H);
7.15 (lH, d, J = 2Hz, 2-aromatic H); 7.23 (lH, dd, J = 2, 9Hz, 6-
aromatic H); MS, m/z 245 for M+ of free base. (Found: C, 59.70;
H, 6.36; N, 4.10. ClsHlgNO2. C2H2O4Ø30 H20 requires C~
2~?,2~87
- 32 - T1063
59.92; H, 6.39; N, 4.11%).
EXAMPLE 18
2,3-DehYdro-3-(3 4-ethvlenedio~yphen~l)quinuclidine
Hydro~en Oxalate
.
The title compound free base (6.0g, 59%) was obtained
from 3-(3,4-ethylenedioxyphenyl)-3-hydroxyquinuclidine (as
described in ExamPle 14) by the me$hod described in Example
15. The hydrogen oxalate salt had mp 145-148C (dec.) (propan-
2-ol/diethyl ether). Rf = 0.20 in dichloromethane/methanol (9:1)
on silica plates; lE NMR (360MHz, D2O) o 1.84-1.90 (2H, m)
and 2.15-2.22 (2H, m, 5-CH2 and 8-CH2); 3.16-3.2~ (2H, m) and
16 3.55-3.69 (3H, m, 4-CH, 6-CH2 and 7-CH2); 4.37 (4H, s, 2 x
OCH2); 6.92 (lH, s, 2-C~I); 6.99-7.16 (3H, m, 3 x aromatic-H);
MS, m/z 243 for M~ of free base. (Found: C, 60.00; H, 6.11; N,
4-06- Clf;~17N~- C~H 04Ø5H20 requires C, 59.64; H, 5.89;
N, 4.09%).
EXAMPLE 19
~ `
3-(2,4-Dimeth~ ILea~lh~-n d~ Hydro~en Oxalate
~ ~ :
2~ 2,3-Dehydro-3-(2,4-dlmetho2yphenyl)quinuclidine (5.9g,
0.024mol, Example 15) was hydrogenated at 3.5 x 105 N.m~2 (50
psi) in ethanol (40mL) contammg 10% palladium on carbon
:
`; ~ ' ',, , . :`
.
,.
2~22~7
- 33 - T1063
(0.59g) and glacial acetic acid (1.5mL, 0.024mol). The reaction
mixture was filtered, then evaporated to dryness. The residue
was dissolved in water (30mL), basified to pH~12 with 2M
sodium hydroxide solution then extractedl with dichloromethane
6 (4 ~ 40mL). The combined organics were dried (sodium
sulphate), evaporated to dryness and the crude product purified
by column chromatography on silica using
dichloromsthane/methanol (15:1) to afford the title compound
free base (l.lOg, 19%) as a colourless gum. The hydrogen
oxalate salt had mp 143C (propan-2-oVdiethyl ether). Rf = 0.27
in dichloromethane/methanol (5:1) on silica plates; lH NMR
(360MHz, D2O) ~ 1.78-2.14 (4H, m, 5-CH2 and 8-CH2); 2.25-
2.27 (lH, m, 4-CH); 3.24-3.4~ (5H, m, one of 2-CH2, 6-CH2 and
7-C~I2); 3.60-3.66 (lH, m, 3-CH); 3.72-3.79 (lH, m, one of 2-
16 CH2); 3.84 (6H, s, 2 x OCH3); 6.66 (lH, dd, J = 2, 8Hz, 5-
aromatic H); 6.69 (lH, d, J = 2Ez,3-aromatic H); 7.29 (lH, d, J =
8Hz,6-aromatic ~I); MS, m/z 247 for M~ of free base. (Found: C~
68.20; H, 7.01; N, 4-2~- C15H21N2- C2H24 0 75 H2O
requires C,58.19; H,7.04; N,3.99%).
EXAMPLE 20
3-(2 5-D~methox~henYl)guinuclidine HYdro~en Oxalate
26 2,3-Dehydro-3-(2,5-dimethoxyphenyl)qu~nuclidine (1.26g,
0.0051mol, Example 16) was hydrogenated in ethanol (35mL)
containing 10% palladium on carbon (0.14g) at 3.15 x 105 N.m~2
..
. ~
2~22~7
- 34 - T1063
(45 psi). The reaction mixture was filtered then evaporated to
dryness. The crude product was purified by column
chromatography on silica using dichloromethane/methanol
(20:1) to give the title compound free base (0.81g, 64%) as a
6 colourless oil. The hydrogen oxalate salt had mp 88-92C
(propan-2-ol/diethyl ether). Rf = 0.25 in
dichloromethane/methanol (6:1) on silica plates; lH NMR
(360MHz, D2O) o 1.80-1.89 (lH, m) and 1.9~-2.16 (3H, m, 5-CH2
and 8-CH2); 2.30-2.32 (lH, m, 4-CH); 3.25-3.45 (5H, m, one of 2-
CH2, 6-CH2 and 7-CH2); 3.67-3.73 (lH, m, 3-CH); 3.75-3.82 (lH,
m, one of 2-CH2); 3.83 (6H, s, 2 x OCH3); 6.94-6.99 (2H, m, 4
and 6-aromatic H); 7.07 (lH, d, J = 9Hz, 3-aromatic H); M~, m/z
247 for M+ of free base. (Found: C, 58.37; H, 7.04; N, 4.25.
C15H21N2- C2H24 0-7~ H2O requires C, 58.19; E, 7.04; N,
3.99%).
EXAMPLE 21
3-(3~4-Dimetho~yphenyl)quinuclidine Hydrogen Oxalate
2,3-Dehydro-3-(3,4-dimethoxyphenyl)quinuclidine (0.67g,
; ~ 0.0027mol, Example 17) was hydrogenated in ethanol (30TnT,)
containing 10% palladium on carbon (0.lOg) at atmospheric
pressure. The reaction mixture was filtered then evaporated to
26 dryness. The crude product was purified by column
chromatography on silica using dichloromethane/methanol
(10:1) to afford the title compound free base. The hydrogen
oxalate salt had mp 134-136C~ (propan-2-oVdiethyl ether). Rf =
, ,
2ql22~
35 T1063
0.14 in dichloromethane/methanol (5:1) on silica plates; lH: NMR
(360MHz, D2O) ~ 1.70-1.94 (2H, m) and 2.07-2.14 (2H, m, 5-CH2
and 8-CH2); 2.26-2.28 (lH, m, 4-CH); 3.27-3.59 (6H, m, one of 2-
CH2, 3-CH, 6-CH2 and 7-CH2); 3.71-3.78 (lH, m, one of 2-CH2);
6 3.87 (3H, s, OCE3); 3.88 (3H, s, OCH3); 6.99-7.03 (2H, m, 2-
aromatic H and 6-aromatic H); 7.90 (lH, d, J = 8Hz, 5-aromatic
H); MS, m/z 248 for (M+H)~ of free base. (Found: C, 57.38; H,
6-90; N~ 4-23- C15H21N2- C2H2O4.H2O requires C, 57.45; E,
7.09; N, 3.94%).
EXAMPLE 22
3-(3,4-Ethylenedioxyphenyl?quinuclidine HYdro~en
Maleate
2,3-Dehydro-3-(3,4-ethylenedioxyphenyl)quinuclidine
{5.00g, 0.0206mol, Example 18) was hydrogenated in ethanol
(~;OmL) containing 10% palladium on carbon (0.~0g) at 2.07 x
105 N.m~2 (30 psi). The reaction mixture was filtered then
evaporated to dryness. The crude product was purified by
column chromatography on silica using
dichloromethane/methanol (20:1) to give the title compound free
base (2.86g, 58%) as a pale yellow gum. The hydrogen maleate
salt gave a hygroscopic glass. Rf = 0.22 in
dichloromethane/methanol (5:1) on silica plates; lH NMR
(360MHz, D2O) o 1.75-1.87 (lH, m); 1.88-1.95 (lH, m) and 2.04-
2.17 (2H, m, 5-CH2 and 8-CH2); 2.19-2.22 (lH, m, 4-CH); 3.26-
- ' '
'
- :
2~22~7
- 36 - T1063
3.52 (6H, m, one of 2-CH2, 3-CH, 6-CH2 and 7-CE2); 3.64-3.75
(lH, m, one of 2-CH2); 4.30 (4H, s, 2 x OCH2); 6.35 (1.7H, s,
maleic acid protons~; 6.87-6.96 (3H, m, 3 :K aromatic-H); MS, m/z
245 for M~ of free base. (Found: C, ~7.78; H, 6.46; N, 3.76.
5C15HlgNO2. 0.85 C4H404. 2H20 requires C, 58.16; H, 7.00; N,
3.69%).
EXAMPLE 23
103-Hydroxy-3-(3-trifluorometh:slphenyl)quinuclidine
Hydroen Oxalate
The title compound free base (2.78g, 13%) was obtained
from 3-~uinuclidinone and 3-bromobenzotrilquoride as described
16for Example 11. The hydrogen oxalate salt had mp 244C (dec)
(propan-2-ol/methanol). Rf = 0.23 in dichloromethane/methanol
(5:1) on silica plates; lH NMR (360MHz, D2O) ~ 1.63-1.72 (lH,
m); 1.95-2.05 (2H, m) and 2.43-2.51 (lH, m, 5-CH2 and 8-CH~2);
2.71-2.72 (lH, m, 4-CH); 3.25-3.38 (2H, m) and 3.44-3.49 (2H, m,
6-CH2 and 7-CH2); 3.63 (lH, dd, J = 2, 14Hæ, one of 2-CH2);
4.02 (lH, d, J = 14Hz, one of 2-CH2); 7.63-7.86 (4H, m, 4 x Ar-
H); MS, m/z 271 for M+ of free base. (Found: C, 54.73; H, 5.28;
N~ 4-20- C14H16~3N- 0-75 C2H2O4 requires C, 54.95; H, 5.21;
N, 4.13%). ~ ~
:: :
2~'~2~
37 T1063
13XAMPLE 24
2,3 -Dehydro-3 -(3 -trifluoromethylphen~,Tl)quinuclidine
Hydro~en Oxalate
The title compound free base (1.45g, 60%) was obtained
from 3-hydroxy-3-(3-trifluoromethylphenyl)quinuclidine (as
described in Example 23) by the method described in Example
15, except that the reaction mixture was heated at reflux for 60
hours. The hydrogen oxalate salt had mp 132-133a (propan-2-
oVmethanol). Rf = 0.35 in dichloromethane/methanol (5:1) on
silica plates; lH NMR (360MHz, D20) o 1.85-1.93 (2H, m) and
2.14-2.23 (2H, m, 5-CH2 and 8-CH2); 3.17-3.26 (2H, m) and
3.61-3.69 (3H, m, 4-CH, 6-CH2 and 7-CH2); 7.06 (lH, d, J =
1.5Hz,2-CH); 7.62-7.89 (4H, m,4 x Ar-H); MS, m/z 253 for M+ of
free base. (Found: C, 55.78; H1 4.78; N, 4.03. C14H~ 4F3N.
C2H2O4 requires C,55.~8; H,4.70; N, 4.08%).
; EXAMPLE 25
3-(3-Tr fluoromethyl~nYl)quinuclidine HYdro~en
Oxalate
2,3-Dehydro-3-(3-trifluoromethylphenyl)quinuclidine
(1.175g, 4.6mmol, Example 24) was hydrogenated at 3.5 x
105N.m~2 (50 psi) in ethanol (40mL) containing 10% palladium
on carbon (120mg). The reaction mixture was filtered,
, :
.
. .
2~322~7
- 38 - T1063
evaporated to dryness, then purified by c~lumn chromatography
on silica using dichloromethane/methanol (20:1) to afford the
title compound free base (0.93g, 79%? as a yellow oil. The
hydrogen oxalate salt had mp 122-123C (propan-2-ol/methanol).
Rf = 0.19 in dichloromethane/methanol (S:1) on silica plates; lH
NMR (360MHz, D20) S 1.81-1.92 (2H, m) and 2.09-2.22 (2H, m,
5-CH2 alrd 8-CH2); 2.34-2.35 (lH, m, 4-CH); 3.28-3.47 (4H, m),
3.60-3.66 (2H, m) and 3.76-3.84 (lH, m, 2-CH2, 3-CH, 6-CH2
and 7-CH2); 7.58-7.69 (4H, m, 4 x Ar-H); MS, m/z 255 for M~ of
free base. (Found: C, 55.53; H, 5.29; N, 4.08. C14H16F3N.
C2H2O4 requires C,55.65; H,5.25; N, 4.06%).
EXAMPLE 26
16 3-(3 5-Dichlorophenyl~-3-hYdro~yquinuclidine Oxalat
.
The title compound free base (1.40g, 7%) was obtained
from 3-quinuclidinone and 1-bromo-3,5-dichlorobenzene as
described for Example 11. The oxalate salt had mp > 200C
(dec) (propan-2-ol/methanol). Rf = 0.25 in
dichloromethane/methanol (4:1) on silica plates; lH NMR
(360MHz, D2O) ~ 1.62-1.74 (lH, m); 1.9~-2.01 (2H, m) and 2.36-
2.46 (lH, m, 5-CH2 and 8-CH2); 2.~8-2.62 (lH, m9 4-CH); 3.26-
3.29 ~2H, m) and 3.40-3.44 (2H, m, 6-CH2 and 7-CH2); 3.51-3.57
26 (lH, mj and 3.89 (lH, d, J = 13Hz,2-CH2); 7.52-7.55 (3H, m,3 x
Ar-H); MS, m/z (CI+) 272 for M+ of free base. (Found: C, 52.40;
.
.
,
. : -
: ~ - : '
~!
39- T1063
~ 5-34; N~ 4 32- C13H15C12N 0-6 C2M204 requires C, 52.29;
H, 5.01; N, 4.29~).
EXAM~LE 27
3-(3-Chlorophenyl)-2,3-dehydroquinuclidine Hydro~en
Oxalate
The titlè compound free base (0.418g, 5~%) was obtained
from 3-(3-chlorophenyl)-3-hydroxyquinuclidine (as described in
Example 33) by the method described in Example 15, egcept
that the reaction mixture was heated at reflux for 60 hours. The
hydrogen oxalate salt had mp 150-161C (propan-2-oVmethanol).
Rf = 0.40 in dichloromethane/methanol (4:1) on si~ica plates; MS,
m/z 219 for M+ of free base. (Found: C, 57.88; ~I, 5.21; N, 4.48.
C13H14ClN. C2H204 requires 0, ~8.16; H, ~.21; N, 4.52%.
EXAMPIE 28
6-HydroxY-6-(3-methoxyl~henyl)-1-azabicyclo[3.2. l]cctane
Hvdro~en Oxalate
The title compound free base (3.12g, 22%) was obtained
from l-azabicyclo[3.2.130ctan-6-one (7.90g) and 3-bromoanisole
(8.4mL) as described for Example 11. The hydrogen oxalate salt
had mp 133-136C (pr~opan-2-ol). Rf = 0.17 in
dichloro~ethane/methanol (5:1) on silica plates; lH NMR
,
.
2~2$~7
- 40 - T1063
(360MHz, D20) ~ 1.80-2.22 (4H, m, 3-CH2 and 4-(:~H2); 2.91-2.94
(lH, m, 5-CH); 3.35-3.75 (5H, m, 2-CH2, 7-CH and 8-CH2); 3.85
(3H, s, OCH3); 4.17 (lH, d, J = 13Hz, 7-CH); 7.02 (lH, dd, J =
2,8Hz, Ar-H); 7.12-7.16 (2H, m, 2 x ~r-H); 7.42 (lH, dd, J = 8Hz,
6 Ar-H); MS, rn/z 232 for (M-H)~ offree base. (Found: C, 58.~1; H,
6-53; N~ 4-45- C14Hl9N2- C2H204. 0.25E20 requires C,
58.62; H, ~.61; N, 4.27%).
EXAMPLE 29
3-(2~3-Dimethoxyphen~1)-3-h~droxyquinuclidine
H:s~dro~en Oxalate
nButyllithium (lOOmL of a 1.6M solution in he~ane) was
16 added dropwise to a stirred, cooled (-10C) solution of 2,3-
dimethoxybenzene (20.4mL, 0.16mol) in anhydrous diethyl ether
(lOOmL) under a nitrogen atmosphere, keeping the temperature
below -5C. After addition the mixture was stirred at 0C for 2
hours then cooled to -20C. A solution of 3-quinuclidinone
(20.0g, 0.16mol) in anhydrous diethyl ether (lOOmL) was added
dropwise keeping the temperature below -10C then after the
addition the mixture was stirred at 0C for 2 hours then at room
temperature for 3 hours. Saturated ammonium chloride
solution (200mL) was added and 2M hydrochloric acid added to
pH = 4. The aqueous layer was separated, washed with diethyl
ether (lOOmL) then basified to pH = 12 with 2M sodium
hydroxide solution and extracted with dichloromethane ~3 x
lOOmL). The combined organics were dried (sodium sulphate)
~22~ J
- 41 - T1063
then evaporated to give a yellow gum (17.89g) which was
triturated with diethyl ether (3 x 60mL) to afford a cream solid
(8.82g, 21%), mp > 105C (dec). The hydrogen oxalate salt had
mp > 110C (dec). Rf = 0.15 in dichloromethane/methanol (5:1)
6 on silica plates, MS, m/3 263 for M+ of free base. HRMS, Found:
M+, 263.1530, ClsH21NO3 requires M, 263.1521.
.
EXAMPLE 30
2,3-Dehydro-3-(2,3-dimethoxyphen ~)quinuclidine
Hydro~en Oxalate
The title compound free base (0.81g, 10%) was obtained
from 3-(2,3-dimethoxyphenyl)-3-hydroxyquinuclidine (as
described in Example 29) by the method described in Example
15. The hydrogen oxalate salt had mp 187-189C (propan-2-ol).
Rf = 0.22 in dichloromethane/methanol (9:1) on silica plates; lH
NMR (25ûMHz, D2O) o 1.84-2.01 (2H, m) and 2.06-2.22 (2EI, m,
5-CH2 and 8-CH2); 3.16-3.32 (2H, m, 6-CH2); 3.37-3.39 (lH, m,
4-CH); 3.57-3.70 (2X, m, 8-CH2); 3.80 (3H, s) and 3.91 (3H, s, 2
x OCH3); 6.9û (lH, s, 2-CH); 6.98 (lH, dd, J = 2, 7Hz, Ar-H);
7.18-7.25 (2H, m, 2 x Ar-H); MS, m/z 245 for M+ of free base.
(Found: C, 58.80; H, 6.28; N, 4.19. C16HlgNO2. C2X2O4.
0.75H20 requires C, 68.53; H, 6.50; N, 4.02%).
.
,
.
.
.
.
2~2?~7
- 42 - T1063
E~ E 31
6-(3-Methoxyphenyl?--l-azabicyclo[3.2~ l]oct-6-ene
Hydro~en Oxalate
The title compound free base (0.45g, 22%) was obtained
from 6-hydroxy-6-(3-methoxyphenyl)-l-azabicyclo~3.2.130ctane
~; ~ (as described in Example 28) by the method described in
Example 15. The hydrogen oxalate salt had mp > 100(:~ (dec).
lo MS, m/z 215 for M~ of free base. HRMS, Found: (M-Hj+
214.1180, C14H16N0 requires (M-H), 214.1232.
13XAldPLE 32
16 2~3-Dehydro-3-(3,5-dlchlorophenyl)quinuclidine HYdro~en
calate
The title compound free base (0.67g, 71%) was obtamed
from 3-(3,5-dichlorophenyl)-3-hydroxyquinuclidine (prepared as
described in xal e 23) b~y the method described m Example
15, except that the~reaction m~ture was heated under reflw~ for
2 weeks. The hydrogen oxalate salt had mp 182-184C (propan-
2-ol/methanol). Rf = 0.48 in dichloromethane methanol (4:1) on
silica plates; lH NMR (380M~z, D20) o 1.82-1.89 (2H,; m) and
25~ ` 2.10-2.18 (2H, m, 5-CH2 and~8-CH2); 3.15-8.23 (2H, m, 6-~
3.56-3.67 (3H, m, 4-GH~and 7-CH2); 7.01 (lH, d, J= 1.5Hz, 2-
CH); 7.55 ~3H, s, 3 x-Ar-H) MS~, m/z 253 for M~ of free base.
(Found: C, 52.35; H, 4.34, N, 4.05; Cl, Z0.63. C13H13C12N.
;:
~ ,, : : : - . : : .
2~2~7
- 43 - T1063
C2H2O4 requires C, 52.34; H, 4.39; N, 4.07; Cl, 20.60%).
E~MPLE 33
6 3-(3-ChloroPhenYl)-3-h sTdroxyquinuclidine HYdro~en
OxaIate
The title compound free base (0.925g, 5%) was obtained
from 3-quinuclidino~e and 3-chlorobromobenzene as described
for Example 11. The hydrogen oxalate salt had mp 194-196C
(propan-2-ol/methanol). Rf = 0.17 in dichloromethane/methanol
(3:1) on silica plates; MS, m/z 237 for M~ of free base.
EXAMPLE 34
Endo- and Exo-6-(3-methoxYphenyl)-1-
azabicyclo[3.2.1]octane HYdro~en Oxalate
6-(3-Methoxyphenyl)-1-azabicyclo[3.2.1]oct-6-ene (0.37g.
1.7mmol, Example 31) was hydrogenated at 3.5 x 105 N.m~2 (~0
psi) in ethanol (15mL) containing 10% palladium on carbon
(20mg). The free base mixh~ re of diastereomers was obtained as
a colourless oil (0.088g, 24%). The hydrogen oxalate salt ~ -
(mixture of endo (85%) and exo (15%) had mp 105-110C
(propan-2-ol/diethyl ether). MS, m/z 218 for (M+H)~ of free
base. (Found: C, 61.82; H, 6.84; N, 4.47. C14H1gNO. C2H2O4.
: :
~: :
.
~,
2~2~
- 44 - T1063
0.25H20 requires C,61.62; H, 6.95; N,4.49%).
EXAMPLE 35
3-(2,3-Dimethoxyphenyl)quinuclidine :Hydrogen Oxalate
2,3~Dehydro-3-(2,3-dimethoxyphenyl)quinuclidine (0.55g,
0.0022mol, Example 30) was hydrogenated at 3.5 x 105 N.m~2
(50 psi) in ethanol (20mL) containing 10% palladium on carbon
(60mg). The free base was obtained as a colourless gum (0.17g,
31%). The hydrogen oxalate salt had mp 98-102C (dec)
(propan-2-ol/diethyl ether). Rf = 0.25 in
dichloromethane/methanol (5:1) on silica plates; lH NMR
(360MHz, D20) o 1.7g-1.87 ~lH, m) and 1.96-2.20 (3H, m,5-CH2
16 and 8-CH2); 2.20-2.23 (lH, m, 4-CH); 3.27-3.48 (5H, m) and
3.77-3.84 (2H, m,2-CH2,3-CH, 6-CH2 and 7-CH2); 3.83 (3H:, s)
and 3.90 (3H, s, 2 x OCH3); 7.06 (lH, d, J = 8Ez, Ar-H); 7.10
(lH, dd, J = 1, 8Hz, Ar-H); 7.24 (lH, dd, J = 8, 8Hz, Ar-H); MS,
m/z 247 for M+ of free base. (Found: C, 57.86; H, 7.01; N, 4.19.
C15H21N2 C2H24 H2 requires C, 57.45; H, 7.09; N,
3.94%).
EXAMPLE 36
3-Hy~roxy-_-(3,4-meth:s~lenediox~phenyl)quinuclidine
Hvdroeen O~alate
' `'
.
,
" :
2~28~7
45 T1063
The title compound free base (12.0g, 61%) was obtained
from 3-quinuclidinone and 3,4-methylenedioxybromobenzene as
described for Example 11. The hydrogen oxalate salt had mp
165-166C (propan-2-oVmethanol). MS~ m/z 247 for M~ of free
5base. (Found: Ct 56.77; H, 5.89; N, 4.06. C14H17N03. C2H209
requires C, 56.97; H, 5.67; N, 4.15%).
-
EXAMPLE 37
3-(3,5 DichloroPhen,rl)quinuclidineHydro~en Oxalate
2,3-Dehydro-3-(3,5-dichlorophenyl)quinuclidine (0.50g,
1.97mmol, Example 32) was hydrogenated at atmospheric
pressure in ethanol (5mL) containing platinum oxide (40mg).
The migture was filtered then evaporated and the crude product
purified by column chromatography on silica using
dichloromethane/methanol (9:1) to afford the title compound free
base as a colourless solid (34mg, 7%). The hydrogen oxalate salt
had mp 190-192C (propan-2-ol). Rf = 0.26 in
dichloromethane/methanol (3:1) on silica plates; lH NMR
~360MHz, D20) ~ 1.82-1.88 (2H, m) and 2.08-2.14 (2H, m, 5-CH2
and 8-CH2); 2.31-2.32 (lH, m, 4-CH); 3.30-3.42 (4H, m, 6-CH2
and 7-CH2); 3.48-3.56 (2H, m, 2-CH2); 3.75 (lH, ddd, J1 = 2Hz,
J2 = J3 = 14Hz, 3-~H); 7.36 (2H, d, J = 2Hz, 2 x Ar-H); 7.44 (lH,
26 dd, Jl=J2=2Hz, Ar-H); MS, m/z 255 for M+ of free base. (Found:
C, 51-94; H, 4-98; N, 4.02. Cl3Hlscl2N C2H2O4 requires C~
52.04; H, 4.95; N, 4.05%).
~322~7
- 46 - T1063
EXAMPLE 38
3-(3,5-Dimethoxyphenyl)-3-hydro~rquinuclidine Oxalate
The title compound free base (13.5g, 64%) was obtained
from 3-quinuclidinone and 3,5-dimethoxybromobenzene (M.R.
Detty, B.J. Murray, J. Amer. Chem. Soc., 1983, 105, 883-890) as
described for Example 11. The oxalate salt had mp 250-251C
(propan-2-oVmethanol). Rf = 0.29 in dichloromethane/methanol
(19:1) on alumina plates; lH NMR (360MHz, D2O) ~ 1.66-1.77
(lH, m), 1.92-2.05 (2H, m) and 2.39-2.49 (lH, m, 5-CH2 and 8-
CH2); 2.63-2.70 (lH, m, 4-CH); 3.12-3.52 (4H, m, 6-CH2 and 7-
CH2); 3.56 (lH, dd, J = 2, 14Hz, one of 2-CH2); 3.87 (6H, s, 2 x
OCH3); 3.97 (lH, d, J = 14Hz, one of 2-CH2); 6.65 (lH, dd,
16 Jl=J2=2Hz, Ar-H); 6.74 (2H, d, J = 2Hz, 2 x Ar-H); MS, m/z 263
for M+ of free base. (Found: C, 61.72; H, 7.61; N, 4.68.
C~15H21N3- 0-5 C2H24 0-25H20 requires C, 61.43; H, 7.25;
N,4.48%).
EXAM~LE 39
2,3-Dehydro-3-(3,5-dlmethoxyphenyl)quinuclidine
Hydro~en Oxalate
: :
The title compound free base (lOg, 86%) was obtained
from 3-(3,5-dimethoxyphenyl)-3-hydroxyquinuclidine (as
describedin ~ 3~)by the method describedin
.
'
~2~
47 T1063
15. The hydrogen oxalate salt had mp 140- 42C (propan-2-
ol/diethyl ether). Rf= 0.22 in dichloromethane/methanol (99:1)
OIl alumina plates; lH NMR (360MHz, D20) o 1.81-1.92 (2H, m)
and 2.10-2.22 (2H, m, 6-CH2 and 8-CH2); 3.12-3.25 (2H, m, 6-
CH2); 3.57-3.69 (3H, m, 4-CH and 7-CH2); 3.86 (6~I, s, 2 x
OCH3); 6.66 (lH, dd, Jl=J2=2Hz, Ar-H); 6.76 (2H, d, J = 2Hz, 2
x Ar-H); 6.98 (lH, s, 2-CH); MS, m/z 245 for M+ of free base.
(Found: C, 60.31; H, 6.31; N, 4.05. C1sH1gNO2. C2H2O4.
0.25H20 requires C, 60.08; H, 6.38; N, 4.12%).
EXAMPLE 40
3-(3,5-Dimethoxyphenyl)quinuclidine Sesquioxalate
2,3-Dehydro-3-(3,5-dimethoxyphenyl)quinuclidine (9.5g,
0.039mol, Example 39) was hydrogenated at 3.5 x 105 N.m~2 (50
psi) in ethanol (90mL) containing 10% palladium on carbon
(1.9g). The reaction mixture was ISltered, evaporated to dryness,
then purified by column chromatography on silica using
dichloromethane/methanol (19:1) to afford the title compound
free base as a colourless semi-solid (8.2g, 86%). The
sesquioxalate salt had mp 142-145C (ethanol). Rf = 0.51 in
dichloromethane/methanol (19:1) on alumina plates; lH NMR
(360MHz, D2O) ~ 1.75-2.22 (4H, m, 5-CH2 and 8-CH2); 2.24-2.31
(lH, m, 4-CH); 3.26-3.60 (5H, m, one of 2-CH2, 6-CH2 and 7-
CH2); 3.69-3.80 (2H, m, one of 2-CH2 and 3-CH); 3.84 (6H, s, 2 x
OCH3); 6.54 (lH, dd, Jl=J2=2Hz, Ar-H); 6.58 (2H, d, J = 2Hz, 2
2~22~7
- 48 - T1063
x Ar-H); MS, m/z 247 for M+ of free base. (Found: C, 55.95; H,
6-42; N~ 3-59- C15H21N2- 1-5 C2H2O4. 0.25 H2O requires G,
55.88; H, 6.38; N,3.62~o).
EX~MPLE 41
3-(3,5 -Bis-trifluoromethylphenyl)-3 -hydroxy~uinuclidine
HYdro~en Oxalate
The title compound free base (4.2g, 16%) was obtained
from 3-quinuclidinone and 1-bromo-3,5-bis-
trlfluoromethylbenzene as described for Example 11. The
hydrogen oxalate salt had mp 173-175C (propan-2-ol). Rf =
0.16 in dichloromethane/methanol (9:1) on silica plates. MS,
lB m/z 340 for (M+H)+ of free base. (Found: C, 47.60; H, 4.07; N,
3-29- C15H15F6N C2H2O4 requires C, 47.56; H, 3.99; N,
3.26%).
.
EXAMPLE 42
3-(3,5-Bis-trifluoromethylphenyl)-2,3-deh~droquinuclidine
Hydro~en Oxalate
The title compound free base (0.95g, 33%) was obtained
26 from 3-~3,5-bis-trifluoromethyiphenyl)-3-hydroxyquinuclidine
~as described in Example 41) by the method described in
Exam~, except that the reaction mixture was heated at
reflux for 7 days. The hydrogen oxalate salt had mp 20~-207C
.
2~122~87
- ~L9 - T1063
(propan-2-oVmethanol). Rf = 0.70 in dichloromethane/methanol
(9:1) on silica plates; lH NMR (360MHz, D2O) o 1.87-1.95 (2H~
m) and 2.07-2.25 (2H, m, 5-CH2 and 8-CH2); 3.18-3.29 (2H, m?
6-CH2); 3.62-3.71 (3H, m, 4-CH and 7-CH2); 7.16 (lH, d, J =
1.5Hz, 2-CH); 8.12-8.15 (3H, m, 3 x ArH); MS, m/z 321 for M+ of
free base. (Found: C, 49.74; H, 3.54; N, 3.37. C15H13F6N.
C2H204 ~equires C, 49.65; H, 3.68; N, 3.41%).
EXAMPLE 43
3-(3,5-Bis-trifluoromethYlphenYl)quinuclidine Hydro~en
Oxalate
3-(3,5-Bis-trifluoromethylphenyl)-2,3-dehydroquinuclidine
(0.80g, 2.5mmol, Example 42) was hydrogenated at 3.5 x
105N.m~2 (50 psi) in ethanol (30mL) containing lO~o palladium
on carbon (0.lOg). The hydrogen oxalate salt had mp 140-142C
(propan-2-ol). Rf = 0.20 in dichloromethane/methanol (9:1) on
silica plates; lH NMR (360MHz, D2O) o 1.83-1.88 (2H, m) and
2.11-2.21 (2H, m, 5-CH2 and 8-CH2); 2.41-2.42 (lH, m, 4-CH);
3.32-3.49 (4H, m, 6-CH2 and 7-CH2); 3.63-3.74 (2H, m) and
3.81-3.86 (lH, m, 2-CH2 and 3-CH); 7.94 (2H, s, 2 x Ar-H); 8.04
(lH, s, Ar-H). (Found: C, 49.38; H, 4.15; N, 3.32. (: 15H15F6N.
C2H2O4 requires C, 49.40; Hj 4.15; N, 3.38%).
26
2 ~ 7
- 50 - T1063
EXAMPLE 44
2,3-Dehydro-3-(3 ~4-methYlenedioxYphenYl)quinuclidine
Hydro en Oxalate
The title compound free base (1.05g, 11%) was obtained
from 3-hydroxy-3-(3,4-methylenedioxyphenyl)quinuclidine (as
described in Example 36) by the method described in Example
15, except that the reaction mixture was heated at reflux for 3
days. The hydrogen oxalate salt had mp 168-170C (propan-2-
oVmethanol). Rf= 0.30 in dichloromethane/methanol (9:lj on
silica plates; lH NMR (360MHz, D2O) o 1.81-1.89 (2H, m) and
2.10-2.19 (2H, mj 6-CH2 and 8-CH2); 3.13-3.22 (2H, m, 6-CH2);
3.54-3.64 (3H, m, 4-CH and 7-CH2); 6.02 (2H, s, O(~I2O); 6.86
(lH, d, J = lHz, 2-CH); 6.96 (lH, d, J = 8Hz, Ar-H); 7.09-7.14
(2H, m, 2 x Ar-H); MS, m/z 230 for (M+H)+ of free base. (Found:
C, 69.64; H, 6.60; N, 4.27. C14H16NO2- 1-1 C2H24 requires C~
69.27;~H, 6.28; N, 4.27%).
~AMPL~ 46
3-Hydroxy-3-(3-methoxyphenYl)-l-
azabicYclo[2.2.1lheptane Hydro~en Oxalate
The title compound free base (6.66g, 37%) was obtained
from 3-bromoanisole and 1-azabicyclo[2.2.1]heptan-3-one (J~a~s~ :
Chem. Commun., 1988, 1618-1619j as described for Example 11.
:: :
,
21~22~7
- 51 - T1063
The hydroge~ oxalate salt had mp 1~2-157C (propan-2
oVmethanol). MS, m/z 219 for M+ of i~ree base.
EXAMPLE 46
3-~3-Methox~,rphenyl)- l-azabicYclo[2.2.1]hept-2-ene
Hydro~en-Oxalate
Thionyl chloride (0.82mL, 11.2mmol) was added to a
lo stirred solution of 3-hydroxy-3-(3-methoxyphenyl)-1-
azabicyclo[2.2.1]heptane (0.502g, 2.25mmol, Example 45) im
anhydrous dichloromethane (30mL). After stirring at room
temperature for 1 hour the solution was heated under reflux for
30 minutes. The reaction mixture was cooled, water (20mL)
16 added followed by potassium carbonate (to pH ~ 12) then the
mixture was extracted with dichloromethane (3 x 50mL). The
combined organics were dried (sodium sulphate) then
evaporated to dryness to give an oil (0.60g). The product was
purified by column chromatography on neutral alumina using
methanoVethyl acetate (gradient elution) to afford the title
:~ compound free base (O.llg, 24%) as a pale brown oil. The
hydrogen oxalate salt had ~mp 142-146 C (propan-2-
oVmethanoVdiethyl ether). MS, CI+, m/z 202 for (M~H)~ of ~ ee
base; lH ~MR (250MHz, D20) o 1.76-1.84 (lH, m) and 2.39-2.51
(lH, m, 5-ClI2); 3.20-3.30 (2H, m), 3.43-3.48 (lH, m) and 3.82-
3.92 (lH, m, 6-CH2 and~7-CH2); 3.86 (3H, s, OCH3); 4.07 (lH, d,
J = 3Hz, 4-CH); 7.00 (lH, s,2-CH); 7.06-7.14 (2H, m, 2 x Ar-Hj;
7.22 (lH, d, J = 8Hz, Ar-H); 7.44 (lH, dd, J1=J2=8Hz, Ar-H).
.
.
. ~
,
2 ~ 7
- 52 - T1063
EXA.MPLE; 47
Endo-3-(3-Methoxyphenyl)-1-azabicycIo[2.2.1]heptane
Hvdro~en Tartrate
3-(3-Methoxyphenyl)-l-azabicycloC2.2.1]hept-2-ene ~0.37g,
1.84mmo~, Example 46) was hydrogenated at 3.5 x 105 N.m~2
(50 psi) in methanol (120mL) containing 10% palladium on
carbon (0.211g~. The mixture was filtered and the filtrate
evaporated to dryness to give a solid (0.36g) which was purified
by column chromatography on silica using
dichloromethane/methanoVaqueous a~nmonia solution (70:30:2-
4) to afford the title compound as an oil (0.271g, 73%). lH NMR
(250MHz, CDC13) ~? 1.24-1.48 (2H, m, 5-CH2); 2.48-2.94 (6H, m)
and 3.16-3.40 (2H, m, 2-CE2, 3-CH, 4-CH, 6-~H2 and 7-CH2);
3.80 (3H, s, OCH3); 6.70-6.82 (3H, m, 3 x Ar-H); 7.22 (lH, dd,
J1=J2=8Hz, Ar-H). The hydrogen tartrate salt had mp 145-
152C (ethanol). lH NMR (250MHz, D2O) o 1.58-1.74 (lH, m)
and 1.85-2.04 (lH, m, 5-CH2j; 3.22-3.55 (6H, m) and 3.76-4.00
(2H, m, 2-CH2, 3-CH, 4-CH, 6-CH2 and 7-CE2) overlapped with
3.85 (3H, s? OCH3); 4.50 (2 x CH of racemic tartaric acid); 6.88-
7.00 (3H, m, 3 x Ar-H); 7.41 (lH, dd, J1=J2=8Hz, Ar-H); MS, m/z
203 ~or M~ free base. (Found: C, 57.57; H, 6.63; N, 3.81.
; C13H17NO.C4H~;06 requires~C, 57.78; H, 6.56; N, 3.96~o).
' .
-
. .
2~22~7
- 53 - T1063
PHARMACEUTICAL EXAMPLES
1. Tablets containin~ 1-26 m~ of compound (~ -
Amount-mg
Compound (1) 1.0 2.0 25.0
Microcrystalline cellulose49.26 48.75 37.25
Modified food corn starch49.26 48.75 37.25
Magnesium stearate 0.50 0.50 0.50
2. Tablets containin~ 26-100 m~ of compound (1)
. .
Amount-mg
Compound (1) 26.0 50.0 100.0
Microcrystalline cellulose52.0 100.0 200.0
Modified food corn starch2.21 4.25 8.5
Magnesium stearate 0.39 0.75 ~ 1.5
Compound (1), lactose, and a portion of the corn starch
are mixed together and granulated to a 10% corn starch paste.
The resulting granulation is~ sleved, dried and blended with the
remainder of the corn starch and the magnesium stearate. The
resulting granulation is then compressed into tablets containing
1.0 mg, 2.0 mg, 26.0 mg, 26.0 mg, 60.0 mg and 100.0 mg of
~ compound (1) per tablet.
; : '
; .
.
- - '
. ~