Note: Descriptions are shown in the official language in which they were submitted.
2023502
~0~61/7000
W~/dp~
8/s~so
17368
TMPROVED ELECTROL~YTIC MET~OD FOR
COLORING ~ODIZED A~UMINUh~
BACK~ROU~JD OF THE INV~TION
.
The color~ng of alumtnum by fo~mation o~ anodic
cxide films and electrolytlc de~ositio~ of ino~g~nic
particle~ thereln ha6 been known for many years and
compr~ses several well-defl~ed ~te~. F1rs~,
s~odization of alumlnum or other light metal
produaes a porous oxide film (porous 2nodic layer)
on th~ metal under alt~rna~lng or direct curr~nt
flow in an acid b~th ln which th~ metal is
suspendQd. The b~h gener~lly coneains suluric,
oxal~c, phos~horic or chromlc acid.
ln the s~bse$uent el~ctrocolorlng process,
lnorgan~c material, usually a metal, is deposited in
the potes of th~ anodic oxide ~ilm by the passage o~
an el~ctric curren~, usually alternating cur~ent,
between ~he anod~zed aluminum s~b~tra~e and a
counterolectrode, which counterelectro~ ugually
consists o gr~phl~e or stainle~s ste~l, although
nickel, copper, and ~in electrodes can also be
used. The depo~ltion of inorganic materl~l
~unctions to giv~ ~he metal a colored appeara~e,
the a~a~ent aolor due to optical inter~erence
efec~s. In a porou5 anotic aluminum oxide film,
the pore~ are evenly ~paced apa~ and ther~ is a
barrier layer o~ al~lmlnum oxide ~ett~reen ~he ~ottom
_ .................................... .
~2~2
B0561~7000
WAK/dpv
8/9/gO
1736a
of the pore and the ~ur~ace of the metal. Ino~ganic
metallic p~gments d~posited ln the pores of the
anodic film result in light ~eing scattQred both
from the lower ends of the indlvidual deposits and
from the aluminum/al~minum oxide interface. ~he
color pxoduced depends upon the dlfference i~
optical pa~h langth r~sultlng from separation of the
two ligh~ scattering surfaces. e.g. the ends of th~
deposits and the al~minum/aluminum oxide lntsrface.
~he pore di~meter ant bdrrier layer thickness are
directly related to the applied anod~zin~ voltaga.
Increase in the size o~ the ~eposits and
ch~nges ln the colors produced can be achleved by
modi~ication of the porzs adjacent to the bsrriex
layer. ~n order to o~tain coloring by optical
~nterference e~fec~s, however, i~ i8 nece~6ary to
~rovide anodised me~al tn which the deposited
particles are constra~ned to have an average size of
at least 260 A at a 3eparation di~tance f~om t~e
alumi~um/aluminu~ oxite lnterface of the or~er of
300-700 A.
Although slectrolytic coloring pe~mit6 colors
to be obtained, the repertoire of colors produced i~
often limlted to bronzes, blacks and reds,
Furthermore, it is often necess~ry ~o have a
coloring bath for ~ach color. ~herefore, t~e
ma~ority of procedures for anodizing aluminUm pleces
2023502
B0561/7000
W~K/dpv
8/9/90
1736a
--3--
only pro~uce lim~ted co10r6 due to the higher C08tS
lnvolved in having multiple color~ng ~a~hs. In
addit~on, mos~ conventional ~nodlzlng p~ocedures use
a double anod~zlng proces6, exemplifled by methods
~tillzlng both 6ul~huric acld and phospho~ic acid
~nodizing solutlons tO modify the pore~ of the
bs~rier layer, ~se of a s~con~ ~cidic bath
~olution, such a5 phos~ho~ic acid, i5
disadvantageous because it i~creases the likelihood
of contamina~ion by phosphate io~s in the
slectrocolo~ing proce~s, Contami~ation by
phos~horic acid in this mann~ may prevent the
Qffectlve sealing of the finAl ~oduct and lead to
the g~adual loss of color th~ough weathering.
Other electroly~lc procedures use complex wave
forms such ~s a~ymmetric ~ine wAves to incre~s~ thQ
guality of the final product ~y producing more
consi~tent colors, but these wave forms raquire
expensiv~ equlpment.
~ t ls impo~tant, therefore, to devalop an
electrolytic proce~ that can produce a wide vs~i~ty
o~ colo~s quic~ly and eiciently wit~out the use of
unnecessa~y baths ~nd sophisticated electrical
~quipment which maXe ~he process mo~e compl~c~ted
and sub~tantially increage the C08t.
The maln ob~ect of the lnvention i~ to ob~ain
the range of colors of tho visible spectrum by
,
2023~02
.
B0561/7000
WAK/dp~
8/9/g o
1736a
ele~troly~ic colo~ng of aluminum or ot~er metal~ in
a simple and unifo~m manner witho~t contamln~ting
the anodizing and electrocoloring bath~ with
~hosphoric acl~. A furthe~ ob~ect of tho ~nvention
ls to provlde an improved proce~ fo~ modifying th~
anodic ~arrier usin~ as f~w sepa~ate ~aths ~s
posstble.
~ ~art~cular, one objQct of thi~ inv~nt~on
provide6 ~or barrier layer modification ln the
electrocolor~ng ~ath 50 that all thQ colors are
obtained in ~ha same tank, thus elimi~at~ng a second
anodizing treatment.
$UMMARY OF THE tNVEN~IO~
This inv~ntion per~ains to an im~rovad process
for the electrolytic colo~ing o~ a metallic
substra~ such as aluminum or alum~num alloys. It
has ~een discovered that ~he a~plication of direct
and al~ a~ing cu~rent in a defined sequence will
modify ~he ~srrier film ~nd the pores, to produc~
bo~h a wider range ant increased bri~htness of
colors. The u~e of a defined sequence o~ direct a~d
al~e~na~ing current to modify the pores of the
anodic aluminum ox~de fil~ and ba~ie~ ~ilm is
designed to mo~e precisely control the dimQn~ions of
~aid f~lms without th~ need for a second anodizing
202~02
Bo561/7000
WAX/dpV
8/9/so
1736~
bath of phosphor~c acid. The improved ~e~hod uses
inexpen~ive and ea~ily o~tained equlpmen~.
In one aspect of the invention, a process for
the Qlectrolytic coloring of metallic substrat~ 15
provi~ed by ~he ~ollow1ng steps:
~ a) developing a porou~ anodic f11m on ~he
substra~e ln a sulphuric acid electrolytic b~th;
(b) mo~fying the anodic b~rrler film ~y
sequentially applying to the substrate a ser~es of
volta~es; a firs~ vol~age o~ direct current, a
secon~ ~oltage of alt~rnating curr~nt, and,
optionally, a ~hird voltage of dlrect current;
(c) ~lectrolytically depogiting an amount of
tnorga~ic materlal in the pores prevlously modlfied
in step (b).
In a ~r~errad embodiment, the alternat~ng
current uæed in the modifying method i8 rymmetrical
80 that the peak voltage of the ~08itiVQ half-waYe
i8 equal to the peak ~oltage of the n~gative
half-wave. The ftnal direct current applt~ation i~
dasigned to redissolve any electrolytically-
deposit~d inorgan~ o material an~ to i~sure
uniformity of the barrler film.
In another as~ect of th~ vention, the
modlflc~sion s~ep as outlined above can be performed
in either the sulphuric acid ano~izi~g bath, ln a
~eparate modific~tion b~th, or Fre~erably, in the
elect~ocoloring bat~ itself.
_.
, .
2~3~02
B~561/7000
WAK~dpv
~/9/~o
1736a
In a ~urther ~SpQCt of this in~ention, direct
current i~ eptlonally ~pplled to the metsl substrate
af~er ~he electrocoloring step to radis~ol~e the
inorganlc deposit6. Thls procedure allow~ for fine
scale ad~ustment of ~he color ~one, resulting in
more pr~cise control of th~ colors of the final
alumlnum or other metalllc product.
An aluminum article having an anod~c oxlde
coat~ng on it~ surfa~e ls also descr~bed, said
article produced according to the following process:
a. d~velopng a porou~ anodic film on the
subs~rat~ in a sulphuric w id ele~trolytic bath:
b. modifying th~ anodic ~arrier film by
segu~ntially applying to the substrate a f~rst
volta~e of direct cuxren~, a s~cond volt4ge of
altexnating current and a thlr~ voltage of d~ct
current:
c. ~lectrol~tically depositing an amou~t of
inorganic metallic matQrial in an elèctrocoloring
bath, a ma~erlal deposited w~hin the pore~ of the
oxid$zing layer.
Brief~Descri tion of the Drawln~s
FIG. 1 is a sch~ma~lc illustration o the
~nodic laye~ formed on ~he su~tra~e du~ing the
a~odizatlon StQp.
' ' ~
.
2~35~2
B0561~7000
WAK/dpv
8/s/so
1736~
-7-
FI~. 2 illustrate~ the effect o the
altarnat~ng curr~nt treatment of th~ modificatlon
s~ep, serving to increase the d~ame~r of the pore
by forming a cavity.
~ illustrst~ depo~ltion of inor~an~c
material in ~he pores of the anod~c la~er during the
elactrocoloring step.
DETAt~EI) DESCRIPT~ON OF THE INV~TION
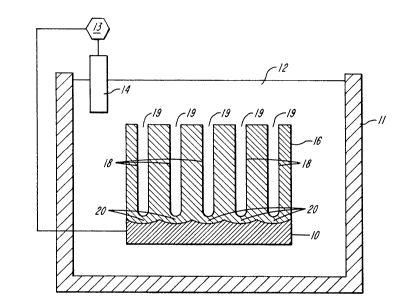
Refarring to FIGS. 1-3. the anodizing process
and the improved me~hod o~ the p~e8~nt in~ntlon are
schematically illustrated . Befo~e tha metal
subst~ate 10 is ~u~jectad to the ano~izing process,
it i~ preparQd ~sinq co~ventional metho~s ~or
achi~v~n~ a uniform, smooth and ~tt~active finish.
I~itial trea~men~s can compri6~ d~greasing, matting,
polishing, ~i~sing and neutraliz~ng,
Thc prQpared piece 16 then ~po~tted in the
anod~c ox~dation tank 11 which tank gonera}ly
contalns an acid ~olution comprl~ng sulphuric acid
12. ln ~ome cases, addl~i~e~ can be used in th~
sulphuric acid bath to dlminlsh ~h~ dtssolu~ion
strength of the elect~olytQ. Other acids or acid
mlxtures such as a mixture 4~ sulphuric acid and
chromic acid can al60 be us~d in the con~entio~al
anodizing bath.
2023~02
Bo561/7000
WAK/dpv
a~s~o
173~a
T~e su~strate lo is then sub~ected to an
anodizing ~low of direct 13 curront wherein the
~ubstrate is the posit1v~ el~ctrode (anod~) ~nd
el~ct~odes 14 m~de o~ aluminum, carbon, lead,
stainless ~teel and the llke, are the negative
electrode ~ ca~hode) .
In ~hls anodizing step, if the substrate 10 is
aluminum, an anodic l~yer 16 i8 formed on the
substr~te (~IG. 1). The layQ~ 16 i5 pO~OUS,
containing a plu~ality of eveAly spaced pore~
the distance between the bottom of a pore 18 and the
subctr~te lo, ~eing defined as the ~arrier film 20.
The thickness of the laye~ ~6 an~ t~e length and
depth of t~e pores 18 will vary dependi~g on many
variables such as time, which will determ~ne the
pore thickness: voltage, which will determine t~e
barrier film 20 size; temperature, which will
determine the diameter of the pore in additio~ to
the dis~olution rate of the anodic laye~, and
current ~ens~ ty .
The ty~cs of current used tc develop t~a anodic
layer 16 a~e n~t critical to the function~ng of this
in~ention. Direct current, alterna~ing current; or
alternating current w~ th dirQct cu~rent components,
either in sine, sqUarQ, or ~ulsed waves, in any of
their fre~uencie~, ca~ b~ employed in this
conventional anodizin~ st~p. ln gene~al, di~ect
2023~2
~0561~7000
WAK/dpv
~/9 /9 0
1736a
_g_
curr~nt voltages ~n the range of 16-22 volts axe
u~ed in sulfuric acid-based electrolyt~s dep~nding
upon the strength and t~mperature of the acid.
~ene~ally, the thickness of the r~sulting ~arrier
film ~ ~ on the order o 10 A per volt ~ppli~d,
Typicslly, in sulfurio acid anodizing bath 12 the
electrolyte contains 15-20% ~y w~lght) sulfuric
acid at a temperasure of 20aC and a voltage of 17-18
volts. In no~mal sulfuric acid anodizng, ~e pore
diameters 19 are in the rango of 1S0-180 P~
(15-18nm). The barrier f~lm thickness 20 is
typically about e~ual to ~he por~ diam~ter lg in the
anodiza~lon step. Thes~ same conditions hold true
with mixed sulfuric acid-oxal~c acid ~lectrolytes 12.
The operatlng range~ which m~y ~e used mogt
effectlvely in thi~ anod~sng step are tho~e in
which the ~ulphuric acid el~ctrolyte ha~ a
concentration of 50 to 250 g~l, tem~e~atur~s ~ange
from -5 to 40C, and D.C. voltages range from 5 to
S0 vol~s with preferred voltages of 15-20 volts
D.C. The time during whlch the curr~nt 18 applied
may v~y ~om 1 to 100 m~nut~s.
It 16 essential tha~ ths anodlc laye~ 16 has a
conslstent thic~ness, height of barrier film 20, and
diameter of pore 19. There~ore, esta~lished
cond~tlons mus~ be ma~nta~ned wsthin a narrow
tolerance ~ange. A~y va~ation may induc~ a
~23~2
Bo561/7000
WAX/dpv
a/s/so
1736a
--10--
differ~nt color from that de~ired. T~is will result
bacause ~he color depends upon the th~cXness of the
anodic lay~r 16 and, especially, on the thicknos~ of
the ~ar~ier film 20, i.e., the d~etance betwoen the
su~st~ate 10 and the bottom of the pore 18. Whan
inorganic material i6 deposited wi~h~n the pores 18
under alternating currQnt condlt~on~ by
alectro~olo~ing of thQ porou~ ahodic layer 15 (FIG.
Sl, the barrier film 20 d~st~nce ~ill dir~ctly
influence the wa~elQngth of visible light, thus
producing a wa~elength corre~ponding to a qiven
color of the visible spectrum by opt~cal
i~terference.
Mod~ficatio~ of the anodlc layer 16 (FIG. 2) is
performed i~ an a~id elect~olyte 12 whi~h pexmi~s
the flow of the current through the bar~iar film 20
a~d ~ub~e~uent ~o~mation of hydrogen within the
poras 18. Generally, th~s is ~one 'oy modifying the
pore wall~ sur~ounding the ba~rier film 20 to form a
ca~ity 22 with a volume and dimensions proportional
to the temperatu~, co~centratlon, voltaga,
treatment time, and the l~ke. When the cavity 22 is
formed, enlarging of the ~ol~mo o~ the bo~tom of ~he
pore 18 determines the level o~ color which ~ay be
obtained when inorganic material~ are deposited on
the pores 18 in the subsequent ~lectrocoloring
step. ~or example, in a ~mall ca~ity, the barrier
. _ .
. .
-
,
:.
2~23~02
30S61/7000
wAx/dp~
s/9~9o
1736a
film ~0 b~tween the inorganic ma~erials daposited
~nd the metalllc subs~rate 10 i~ small, r~aultin~ in
a short wavelen~th with the ~ppearance of
violaceous colo~. If the above distance i5
incrsased, other colors will begin to emerge.
The barri~r film 20 i8 ~ semi-conduc~or which
rests~s the passage o~ the current. This re~stance
will ~e d~rectly proportional to the thicXnQ~ of
the layer.
It has b~en discovered that the app~ication of
di~ect and alternating current 17 in a deflnod
seguenoe will modify ~he barrier film 20 and the
~ores 18, to produce both a wi~er range and
inCrQaSed ~rlg~tAe85 of colors. The use of a
defined saquence of dir~ct and alternatlng current
to modify the pores 18 of the anodic layer 16 and
barrier film 20 is deslgned to more pr~clsely
control the dimensions of said components withou~
the need for a s~cond anodizing bath of phosphoric
acid. Us~ of phosphoric acid is problema~c since
lt c~n be ca~ied over as a ~ontaminant into
subs~guent treatments and maXes thQ metallic
substra~e more difficult to 8eal. Thus,
weather-resistance can be impaired. The lmpro~ed
method us~s inexpensive and easily o~tainsd
eguipmenS.
The term "~lternaSing current" d~no~es a type
o current ~ary~ng between poslti~e and n~g~tive
_ _.
. .
2023~02
so56l/7ooo
WAX/dpv
9/~/90
1736a
-12-
polarity, ~vin~ a po6i~ivo and nega~lve cycle
alternately. Alternatin~ current can be a pure sl~e
wave or it can ~e modifi~d in any other wsve form.
In a particularly preferr~d embodiment, the A.C.
voltage is symmetrlcal. Th~ term "symm~trical"
refer~ to the mede of ap~lication of alternating
current as to well as the ~alues thereof. The term
is meant to deno~e an alt~nating currant ir. wh~ch
the peak voltage of the negative half-wa~e i8 equal
~o the peak voltage of ~he ~06itive half-wav~.
In a partl~ularly prsferr~d embod~ment of this
m~thod, the anodic layer 16 a~d bar~ier film 20 are
modi~ied by sequentially applying to the ~ubst~ate
1~ a tripa~tite voltage sequence comprisi~g a fi~st
voltage of dixect cur~ent, a second voltag~ of
symmetrical alternating current, followed ~y a th~rd
voltage of direcS cur~ent.
The efsct of ~he diferent voltage treatments
on mod~ication of the anod~c ~ilm i5 not yot fully
under~tood. It ls probable that the firss direct
current application prov~de~ for a un1form barrlsr
film thickness, while the alternat~ng current ser~es
to inc~e~se the ~iameter of the ~ore l9 at the
bottom of the pore lB by formation of a cavity ~2.
Formation of the cavity tends to reduce the size of
~he barrler Çilm 20. Typically, alternati~g current
tre4tment~ in conventional procodures lea~ to
2023~Q2
BOs61/7000
WAKJdpv
8/s~go
1736~
-13-
ele~ated temperatureY in ~he bath. This, in ~urn,
tncreases the rates of reaction. Htgher temp~ratu~e
w~ll result ln variable cond~tions wlthi~ the
anodizing lay~r so tha~ the ~ore d~amete~s, thQ
cavity ~lm~nsio~s, and the barrier film thickness
may not be entirely uniform. The ~inal DC cu~rant
t~eatmen~ ~s a~pli~d for a tim~ ~uffi~nt to ad~u~t
the thickness of the bar~ier ~llm 20 to the ex~ent
necessary to ~orm a film appro~r~ate for the chosen
color and to ~n6ure uniformity o~ barriQr ~ilm
thickness. ~ho uniformity of the barrier ilm i6
dirQctl~ rolated to the uniormity of ~he color once
~norga~ic ma~erlal8 are depo~ite~ in the pores
(Figure 3). 3y not pro~id~ng a final D.C. t~atment
to ensu~e uniformlty of barrier f~lm thickness, the
result~ng colored metalll~ subgtra~e will often have
a spQckled appearance wi~h ralnbow-llke patchee
interspersed throughout a color~d bac~ground. The
du~a~ion of D.C:. t~oa~ment i~ preferably le~s than
about 20 mlnutos. ~he duratlon i8 8t~0ngly
dependent o~ the voltage; low voltages will re~u~re
a long tlme to adjust the barrier fllm 2a~ Shorter
times and high~r voltages re~ult i~ a thi~ner
barrler f~lm ~0 and A corre~pondingly ~hort~
wa~reler~gth of light ~roduoed by optical
interferencQ~ U~e of a ~eguential treatmen~ of
diroct and alternating current s~rves ~reclsQly to
2023~02
~as6l/7000
~AK~dpv
8/s/so
1736a
--14--
control the barrier fll~ distance 20, thu~ enabling
more control of the final ~olor~ when inorganic
materials are deposlted in ~he ~lec~rocoloring step
(F~G. 3). The best results can be obtainod by
ollow~ng a trlpartite seguence as d~scribe~ abova.
Other combinations o~ direct an~ alternating
current~ can be u~ed, p~ovide~ that the altarnatinq
current is symmetrlcal. ~he ~inal D.C. trsatment
can be eliminated, bu~ the resultlng colo~ may not
be unlform. In all embodiments o~ the improved
modiica~ion step6 de~cribed herein. the D.C.
voltage i~ les~ than 20 volts. The alteznating
cu~rent is al 80 les~ than 25 volts.
I~ ls impor~ant ~o maintain a con~ant
t~mperature wlth a varlation o~ le~ than about
2-34C during the motlficatlon proc~ss. A
temperature of a~out 20~C ~s pref~rred. At
temperatu~es m~ch higher than about 30C, ~he a~odic
layer 1~ ls rapldly dissolved du~ to the hlgher
chemical activity at the higher tempe~a~ures. The
pores are then enlarged and mors metal w~ll be
depo~ited i~ the sub~eqyent el~ctrocoloring step.
Thi~ re~ults in darXer color~ which may be less
de~irable under certain ci~cumstance~.
The time o~ each voltage treatment will dep~nd
on the temperature and o~her parameters b~t ~hould
be pre~erably lcss than 20 minutes, ~ince at t~s
2023502
B0561/7000
WAK/dpV
8/9 ~9 0
36a
-15-
beyond ~h~ point, the process becomes less
e~flcie~t ~nd co~seguently more expensive.
In a ~ur~h~r embodiment of this mod~fication
s~ep, 6p~C~ al ~oltage characteristics are cho~en to
overcom~ the electrical resistance of the barrier
fllm. A3 mentioned previously, the barrl~r film 20
is a semi-conductor, and ~5 the barrie~ ~ilm
1ncrea~es ln size, the ~lectrlcal resistance o~ the
a~odic layer l~ also increases concommitantly.
Therefore, a pr~ferred method o~ applying tho direct
c~rrent and $ymmetric al~er~atlng current i8 to
apply the ~olta~ in a linearly lncrea61ng ~nner,
in o~her word~, in a "~amped" configurat~on. This
ramping may be par~icularly important tur~n~ t~e
A.C. treatment ~e~nce, since the i~creasing
resistance of the barrier film ar the f~lm ~nlarges
~Qnd8 tO distort t~Q ~ymmetric s~e wsve input.
Another impo~tant ~eatur~ of the ~n~ention is
that these co~trolled direct and alternating curren~
tr~atmen~s can be p~r~ormed in t~e ano~izing bath
(FIG. l) a~ well as in the elect~ocoloring b~t~
(FIG. 3).
Where the anodizing ~lectrolyte is
6ubstantially free o~ metall~c salts typically used
in el~ctrocoloring, m~tallic deposits cannot form
during the modifica~ion 5tep. Wher~ th~ anodic
l~yer an~ barrl~r fil~ are mod~fied by the mothod of
, .
2023~02
B0s61~7000
~AXJdpv
8/g/so
1736a
-16-
this lnven~on in the electrocoloring bath itself,
the ele~troly~e is ~Ot su~t~ntially free of metal
salts a~d pigme~tary deposits can form under
altern~ting current conditions. Thus, whon the
improved modiflcation step o this invention is
performed in thc elQctrocoloring bath, pore
modification can ~ommence sim~ltaneously w~th
formation of lnorganic depo~its . The spe~lf ~ o
volta~e sequence3 d~cribed hersin can, howe~er, b~
employed to mor~ precisely control the barr~er ~ilm
th~cknsss and eliminata metal deposition prior to
actual electrocolorlng.
~ he al~ernating cur~a~t volt~ge treatment of
the modification 8~0p, which treatment would
normally deposit unwanted ~etallic pl~ments in an
electrocoloring bath, is ~hosen ~o that the exten~
of metallic depos~tlon is Xept to an absolute
mi~imum. One w~y to accomplish thi~ i~ to apply
~lternating current so as to provide for an
extremely thin barr~ar fllm.
The final DC tr~atment of the modi~icatio~ st~
will cause slight redlssolution of any metallic
depo6its that ~ave been in~dvartently formed durin~
the alte~natin~ curr~n~ ~reatment of the
modification s~ep ~n the electrocoloring oath. ThiY
step ls advantageous because it pro~id~ a more
precise control o~er the barr~er fllm dopth pr~or ~o
202~02
~as6l/7000
WAK~dp~t
8 ~ 0
11'36a
actual elect~ocoloring, thus leading to more
definitlon ln the final color produc~ion when
electrocoloring does take place under altern~ting
curren~. Whe~ the modificatlon step is performed in
the electrocoloring bath. the proceture el~minat~s
the need for sqp~rata modlfication an~
elec~rocoloring baths and morQ efflclently ~ses
available chemic~ls and elec~rical equipment.
In yet another embodiment, the ~mproved
modiflcstion step can also be per~orm~d in a
completely 6epa~ate ba~h. This separate bath i8
typ~cally an ACi~iC electxolyte containing a
carboxylic acld, sulp~ona~ed organic, or inorganic
mineral ~c~d such a6 sulphuric acid, oxalic acid,
tartaric acid and t~e like.
once the pore~ 18 and the he~ht of the ba~rier
f~lm 20 are modified according to the improved
p~OCe~5 of this in~en~lon, inor~anic materials are
deposited in the thus-enlargBd e~d ~egio~ o~ the
pores ln the electrocolor~ng ~tep (FIG, 3).
The goneral procedures used ~n th0
electrocolortng step are convQntional. Inorqanlc
materlal 24 ~ con~ained with~n a p~gmented acid~c
sal~ 26 wherein the material is a m0tal selected
from one or more of tin, nlc~el, cobalt, copper,
silver, cad~ium, i~on, lead, manga~ese, ~nd
mol~bdenum. Preferably, electrolytlc coloring is
.
.
. - 2023~02
.
B05~1/7000
WAK~dpv
~/9/9o
1736a
-18-
perfo~med in a solution o~ mqtal 8alt ~6 having ~
concentration r~tio to sulfuri~ acid of less than
10~ counter-electrode 27 is tmmers~d in the
metal salt ~a~h 2~ and connected to the *lt~rnatin~
current BOU~Ce 30, The counter-electrodes can vary
with the type of metal sal~ u6sd, In typ~
applica~ions, gr~phl~e, carbon, ni~kel, or stainle~
stael can be used. Sinc~ the color produced ~epen~
on the difference ln optical path rasulting from
separatton of the two light scatter~ng surfaces, the
separatio~ will depend upon the barrier fllm
d~stance 20. To obtain colors in the visible rang~,
~epa~ation between the surfaces o~ the teposits 24
and the subst~ate 10 5ho~1d be in the ran~ o~ about
300-700 A. Resulting colors range from blue-violet
due to interfer~nco effects at the shorter
wavelsngths and dark gr~en due to interference
effects at the longer wavelengths. ln th~ pre~r~d
electrocoloring steps, alternating current ~o~t
efficiently deposits inorganic piqment 2~ from
metallic sal~ ~olut~ons to the bottom of ~he ~ores
1~. Typically, alternati~g ~urrent of le~s th~n 20
vol~s is preferred.
The alternating ~ur~ent will deposit the metal
oxide t~ obt~in a desired color, the ~olo~ depend~ng
on th~ hQi~ht of the barrier ~llm ~0. ~he t~me
durlng wh~ch th~ altarna~ing curren~ treatment is
-~
20235~2
305~1/700
WAX/dpv
sJ~/so
1736a
appl~ed will determine the tone of ~he color, and
the barrier film distance 20 w~ll determine the
actual color ~tself. For example, a one minute
treatment with an alts~nating current ~ill gl~ a
lighter tone of color than a fi~o minute treatment,
th~ d~fference in tone belng primarily a funct~on o~
the amount of deposit~d i~organic pigmen~od material.
It has bo~n show~ ~hat. after fo~mation of
init~al pigment de~o~t~, ther~ ls some increase in
re~is~ancQ of ~he barrier f~lm leading to a change
in chemical condt~ion6 within the pores that m~y
favor the growth of an addltional anodi~ lAyer.
The growth of a further anodic layer ln the
alectrocolorinq ~ath i5 a func~ion of the pH value
of the electrolyts w~ich must be set at a level
which res~lts ln an appropriate rat~ of la~er
without excessl~e redissolut~on of the depo~tad
~igment material. Although the pR of the
electrocoloring solutlon plays an important role,
and should under all circumstances be maintained
abcve 0.8, the oxact p~ is not critical to this
modif~cation ~tep of the invention. Pr~f,orably, the
electrocoloring solution eléctrolyte h~s ~ pH from
0.5 to 2.3.
~ n a further embodiment of this invention, a
short anod~c dl~ct current treatment can be
employed after t~e alternating current treatment in
23~2
BO561/7000
WAX/dp~
8~/90
173~a
-20-
the electrocolorir.g bath. ~is Ghort DC ~eatment
functions to yield th~ same effect as wo~ld a short
treatment in the modification step, whgn said
step is performed in th~ el~ctro~olorin~ ba~h. The
purpo5e o~ this final DC treatmen~ after
electrolytic d~positton of plgmen~ under AC
condition~ is to retuce the ln~ensity of the color
by redissolving the metal oxide deposlts. ~ho DC
treatment is contlnued for a shor~ time (about 1/2
to 3 minutes). Tho current is at a voltag~ less
than ~bout 25 volts. This procedure allows for a
fine scale adjustment of the fina~ barrier fllm
thiokness and amount o~ depo~lts, r~sulting ln m~ted
colors and a more precise control of the flnal
aluminum or other metallic colored ~roduct.
The invention is ill~st~ated further by the
followlng exampl~s:
EXAMPLE 1
This ~xam~le illustrates modification of the
anodic lay~r in a separate b~t~ using a tripartite
se~uence o d1 rec~ current, alternating curren~, and
di~ect current.
Two p~ece~ of 6063 alum~num alloy ~er~ cleaned
in a soa~ solution, etched in 5 ~erCent Caustic soda
at 60C, desmutted in nitri~ acid solution (1:1) a~d
: . .
2023S02
Bos51/7000
WA~C/dpv
8/9J90
1736a
--21--
anodized in a sulphuric acld bath at a temperaturQ
of 20~C w~th a direct curreIIt chsrge density of
2 . SAJdm for 30 minutes . Thi~ re~ul~ed in an anodic
porous lay~r of at leas~ 18 roicrons.
one of the pieces wa~ rinsed and transf~rred to
a modification bath havin~ ~ulphur~c acid ~50 g/l)
at 2DC. $he aluminum ~iece was used as a positi~e
cathode and lead electrodes were the negative
anode. A 3.~. current o~ 16 volts was appli~d for 3
mlnu~Qs. Following this the pi~ce was sub~ected to
a ~ymmetrical alte~a~ing currcnt of 4 volts for 3
minut~s, followed by a D.C. current of 3 vol~ for 4
minutes .
The piece was then r~nsed and tran~ferred to an
electrocolorin~ bath containing 16 g/l tin sulphatQ~
17 g/1 6ulphuric acid, 2 g/1 phenosulphuric acid.
~e electrode~ ~ere stainle~s 8teel and the aluminum
was subjected S4 an alterna~ing current of 18 volts
for 4 ~inutes. A b~ight green colo~ was obtalned
(Table 1 ) .
Ta~le 1 fur~her sets ouS the dlfferen~ ~olors
that c~n bQ o~ta~ned whQn the D.C. curren~ of the
modification s~ep i8 temporally varied in accordance
with the impro~ed modification procedure o~ the
inve~t ion .
,
~023~02
Bos61/7000
WA~pv
8/sJso
1736~
-22-
~ABLE
. _
Film Barrier film mod~ 1catlon RQsultin~
Helq~ts ~A) under DC or ~C curr~nt ~minute~) ~olors
400 to 415 2 grey
41S to 490 3 blue
4~0 to 560 4 green
S60 to ~80 S yellow
S80 to 660 6 red
650 to 700 ~ purple
The second of the two anodlz~d pieces wa~
treated ldentically except that t~e three part
modlfication step wa~ eliminated. A bronzs color
was obta~ned.
.
EXAMPLE 2
This Example lllustrates modification of the
anodic layer in the elec~rocoloring ba~h using a
dual se~uence of direct current and seguence wa~a
alternating current.
An al~minum p~ce w~ degreased in ~n ~lkalin~
cleaner and desmutted for lO minutes in a 10~ sodium
2023~
~05~1/7000
WAK~dpv
8/9/90
1736a
-23-
hyd~oxide solutlon at 60~C. It was then rin$ed,
ne~tralized, and then anod~ zed ln a bath comprising
1~0 g/l ~ulphur~c acid and lS gf l alu~inum sulphate
at a te~p~raturQ of lg + 0.5C and a direct current
of 3 A/~m or ~0 mlnutes with a positive charge.
The ~lect~odes ~ere carbon.
~ he piece was rinsed and transferred to an
electrocol~ring bath contalnlng 16 g/l stannou6
sulphate, ~0 g/l n~ckel sulphatQ, 2S g/l sulphur~c
acid, 2 g/l phenosulphu~ic acid ~nd 2 g/l c~tric
a~ld. The aluminum piece as the positl~e pole was
treated to a.direct current of 0.4 A/dm for 3
m~nutes. Stainlsss steel el~ctrode~ we~e t~e
negativ~ ~ole. Next, the piece w~s sub~ected to a
symmetrical square wave alternAtlng current hav~ng a
current dens~ty of ~.5 A/dm for 4 minutes and th~n
to a sy~m~trical 6inusoidal alternating current of
18 volts for 3 ~inute~. A green color w~s produced.
~XAMPLE,3
This Example illu~trstes modiflcation of the
anodic lay~r in the electrocolorlng bath us~ng a
dual ~equence of direct current and symmetrical
alternating current.
A 6063 aluminum alloy piece was introduce~ into
an anodizing bath havin~ an electrolyt~ containlng
- ~23502
~30561/7000
WAK/dpv
8Js/so
1~36a
--24--
155 5~1 sul~huric acid, 3 g~l boric acid, 2 g~l
glycerin, at a temperature of 24 ~ ~.5C. Lead
electrodes were u~ed under ~ulsating direct curr~n~
of 4 A/dm for 40 minutss. ~he plece was then
transferred to ~ modifying ~ath and a direct curr~nt
of 0.5 A/dm was applied for 5 minutes, ~he piece
having a positive charge, ~n an electrolytQ
conta~ni~g 20~ g/l s~lphuric acid. ~h~ plece was
next treated by applying a symmetrical alternati~g
curren~ mder curren~ den~ity of ~.8 A~dm ~o~ 2
minutes. Flnally, the piece wa~ rinsed and colored
in an el~ctrolyte containing 18 g/l stannous
sulphate, 1 g/l ascorbic acld, 2 g~l ci~ric acid
with ~in elec~rodes and sub~ected to alte~nating
curzent at a voltage o~ 18 volts for S minu~es un~il
the de~i~ed ~olor gray was obtalned.
Even ~hough the t~vent~on has ~een tescri~éd
and shown in con~ection w~th speclfic embodtment~
thereof, lt is und~rst~o~ by tho~e sXilled in the
art that modlflcatlons may be made to the invention
itself or to any of its applicat~ons mentlonQd
hereln and that the ~ame are encompa~sed within the
spi~it and 6cop~ of the invention, as defined ln t~e
following c}alms.