Note: Descriptions are shown in the official language in which they were submitted.
r.
-1-
A-17718/+
3-Phenylbenzofuran-2-ones
The present invention relates to novel 3-phenylbenzofuran-2-ones, to the
organic material
stabilized by means of them against thermal, oxidative and actinic degradation
and to the
use of these compounds for stabilizing organic materials.
The use of some benzofuran-2-ones as stabilizers for organic polymers is known
from
GB-A 2,042,562 or BE-A 881,496 and also the Derwent Abstracts 86-207 397/32
and
86-214 695/33 and the Research Disclosure 288,28888 (1988). Some benzofuran-2-
ones
and their use in colour photography are described in US T 904,003.
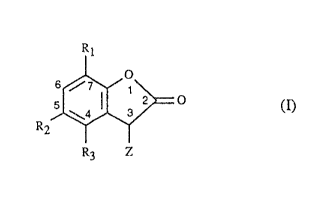
The present invention relates to compounds of the formula I
Ri
a
s /~
z o (I)
R
R3 z
in which R1 is Cl~-C3palkyl, R2 is hydrogen, C1-Clpalkyl, Cg-Cl2cycloallcyl,
CS-C~cycloalkyl which is substituted by Cl-C4alkyl, or is phenyl or C7-
Clzphenylalkyl, R3
is hydrogen or C;-C4allcyl and Z is phenyl, phenyl which is substituted by Cz-
Cgalkyl,
Cl-C4alkox~ or chlorine, a group
O
' I
O.~(~H2) n
in which n is 1 or 2 or a group
i
-2-
A OH
A
A
in which the radicals A independently of one another are Ct-Csalkyl, methoxy
or ethoxy.
Examples of R1 as Ct3-C3oalkyl, preferably Ct3-Cl~alkyl, are tridecyl,
tetradecyl,
pentadecyl, hexadecyl, heptadecyl, octadecyl, nonadecyl and icosyl.
Preferably, alkyl
radicals of this type which are branched in the 1-position are especially
1-(Ct2-Ctbalkyl)ethyl, for example 1-dodecylethyl, 1-tridecylethyl, 1-
tetradecylethyl,
1-pentadecylethyl and 1-hexadecylethyl. 1-Dodecylethyl and 1-hexadecylethyl
are
particularly preferred.
Examples of R., as Ct-Ctoalkyl are methyl, ethyl,,propyl, butyl, pentyl,
hexyl, heptyl,
octyl, nonyl and decyl. R2 is preferably Ct-C4alkyl, in particular methyl and
teat-butyl.
Examples of R~ as CS-Ct2cycloalkyl are cyclopentyl, cyclahexyl, cycloheptyl,
cyclooctyl,
cyclonanyl, cyclodecyl, cycloundecyl or cyclododecyl. R2 is also preferably
CS-C~cycloalkyl, especially cyclohexyl.
Examples of RZ as CS-C~cycloalkyl which is substituted by Ct-C4alkyl are
methylcyclohexyl and tert-butylcyclohexyl.
Examples of RZ as C~-Ct2phenylalkyl are benzyl and 2-phenylethyl.
Examples of R3 as Ct-Cdalkyl are methyl, ethyl, propyl and butyl. R3 is
preferably methyl.
Examples of Z as substituted phenyl are o-, m- or p-methylphenyl, a-, m- or
p-ethylphenyl, o-, m- or p-butylphenyl, o-, m- or p-methoxyphenyl, o-, m- or
p-ethoxyphenyl, o-, m- or p-chlorophenyl and 2,4,6-trimethyl-5-hydroxyphenyl.
Z is preferably phenyl.
Compounds of the formula I in which Rt is Ct3-C2oalkyl and Z is phenyl are
preferred.
~~~~'~"~."~
-3-
Compounds of the formula I in which R1 is C14-Cl8alkyl are also preferred.
Compounds of the formula I in which R2 is hydrogen, C1-C4alkyl, cyclohexyl,
phenyl or
benzyl are also preferred.
Compounds of the formula I in which RZ is hydrogen or Cl-C4alkyl are also
preferred.
It is particularly preferable for R3 to be hydrogen or methyl.
Compounds of the formula I in which R1 is C14-Cl$alkyl and R2 and R3
independently of
one another are hydrogen or C1-C4alky1 are of particular interest.
The following are preferred examples of compounds of the formula I:
H33C16 C'H.. H33C,c CH" H25C,n (''H"
O O O
H3C ~ H3C
H33C. ~ rH., H33Clf cH~
O O
(F13C;3C
3-Phenyl-5-methyl-7-(1'-dodecylethyl)-benzofuran-2-one is a particularly
preferred
compound of the fomnula I.
The compounds of the formula I are suitable for stabilizing organic materials
against
thermal, oxidative or actinic degradation.
The following are examples of materials of this type:
1. Polymers of monoolefins and diolefins, for example polypropylene,
polyisobutylene,
polybutene-l, polymethylpentene-h, polyisoprene or polybutadiene, as well as
polymers of
cyclooleftns, for instance of cyclopentene or norbornene, polyethylene (which
aptionally
can be crosslinked, for example high density polyethylene (HDPE), low density
polyethylene (LDPE) and linear low density polyethylene (LLDPE).
2. Mixtures of the polymers mentioned under 1), for example mixtures of
polypropylene
with polyisobutylene, polypropylene with polyethylene (fox example PP/HDPE,
PP/LDPE) and mixtures of different types of polyethylene (for example
LDPE/HDPE).
3. Copolymers of monoolefins and diolefins with each other or with other vinyl
monamers, for example ethylene/propylene copolymers, linear low density
polyethylene
(LLDPE) and its mixtures with low density polyethylene (LDPE), propylene/but-1-
ene,
propylene/isobutylene, ethylene/but-1-ene, ethylene/hexene,
ethylene/methylpentene,
ethylene/heptene, ethylene/octene, propylene/butadiene, isobutylenersoprene,
ethylene/alkyl acrylate, ethylene/alkyl methacrylate, ethylene/vinyl acetate
or
ethylene/acrylic acid copolymers and their salts (ionomers) and terpolymers of
ethylene
with propylene and a dime, such as hexadiene, dicyclopentadiene or
ethylidene-norbornene; as well as mixtures of such copolymers and their
mixtures with
polymers mentioned in 1) for example polypropylene/ethylene-propylene-
copolymers,
LDPE/ethylene-vinyl acetate-copolymers, LDPE/ethylene-acrylic acid-copolymers,
LLDPE/ethylene-vinyl acetate-copolymers and LLDPE/ethylene-acrylic acid-
copolymers.
3a. Statistical or alternating copolymers of «-olefins with carbon monoxide.
3b. Hydrocarbon resins (for example CS-C9) and hydrogenated modifications
thereof (for
example tackifters).
4. Polystyrene, poly-(p-methylstyrene), poly-(«-methylstyrene).
5. Copolymers of styrene or «-methylstyrene with dimes or acrylic derivatives,
for
example styrene/butadiene, styrene/acrylonitrile, styrene/alkyl methacrylate,
-s-
styrene/butadiene/alkyl acrylate, styrene/maleic anhydride,
styrene/acrylonitrile/methyl
acrylate; mixtures of high impact strength from styrene copolymers and another
polymer,
for example from a polyacrylate, a diene polymer or an
ethylene/propylene/diene
terpolymer; and block copolymers c>f styrene, for example
styrene/butadiene/styrene,
styrene/isoprene/styrene, styrene-ethylene/butylene-styrene or
styrene-ethylene/propylene-styrene.
6. Graft copolymers of styrene or «-methylstyrene, for example styrene on
polybutadiene,
styrene on polybutadiene-styrene or polybutadiene-acrylonitrile; styrene and
acrylonitrile
(or methacrylonitrile) on polybutadiene; styrene, acrylonitrile and methyl
methacrylate on
palybutadiene; styrene and malefic anhydride on polybutadiene; styrene,
acrylonitrile and
malefic anhydride or maleimide on polybutadiene; styrene and maleimide on
polybutadiene, styrene and alkyl acrylates or methacrylates on polybutadiene,
styrene and
acrylonitrile on ethylene/propylene/diene terpolymers, styrene and
acrylonitrile on
polyalkylacrylates or polyalkylmethacrylates, styrene and acrylonitrile on
acrylate/butadiene copolymers, as well as mixtures thereof with the copolymers
listed
under 5), for instance the copolymer mixtures known as ABS-, MBS-, ASA- or
AES-polymers.
'~. Halogen-containing polymers, such as polychloroprene, chlorinated rubbers,
chlorinated
or sulfochlorinated polyethylene, copolymers of ethylene and chlorinated
ethylene,
epichlorohydrin homo- and copolymers, in particular polymers from halogen-
containing
vinyl compounds, for example polyvinyl chloride, polyvinylidene chloride,
polyvinyl
fluoride, polyvinylidene fluoride, as well as copolymers thereof, for example
vinyl
chloride/vinylidene chloride, vinyl chloride/vinyl acetate or vinylidene
chloride/vinyl
acetate copolymers.
8. Polymers which are derived from «,~-unsaturated acids and derivatives
thereof, such as
polyacrylates and polymethacrylates, polyacrylamides and polyacrylonitriles.
9. Copolymers from the monomers mentioned under 8) with each other or with
other
unsaturated monomers, for instance acrylonitrile/butadiene,
acrylonitrile/alkyl acrylate,
acrylonitrile/alkoxyalkyl acrylate or acrylonitrile/vinyl halogenide
copolymers or
acrylonitrile/alkyl methacrylate/butadiene terpolymers.
10. Polymers which are derived from unsaturated alcohols and amines, or acyl
derivatives
~-~~'l
-6-
thereof or acetals thereof, such as polyvinyl alcohol, polyvinyl acetate,
polyvinyl stearate,
polyvinyl benzoate, polyvinyl maleate, polyvinyl butyral, polyallyl phthalate
or
polyallylmelamine; as well as their copolymers with olefins mentioned in 1)
above.
11. IIomopolymers and copolymers of cyclic ethers, such as polyalkylene
glycols,
polyethylene oxide, polypropylene oxide or copolymers thereof with bis-
glycidyl ethers.
12. Polyacetals, such as polyoxymethylene and those polyoxymethylenes which
contain
- comonomers for example ethylene oxide; polyacetals modified with
thermoplastic
polyurethanes, acrylates or MBS.
13. Polyphenylene oxides and sulfides, and mixtures thereof with polystyrenes
or
polyamides.
14. Polyurethanes which are derived from polyethers, polyesters or
polybutadienes with
terminal hydroxyl groups on the one side and aliphatic or aromatic
polyisocyanates on the
other side, as well as precursors thereof.
15. Polyamides and copolyamides which are derived from diamines and
dicarboxylic acids
and/or from aminocarboxylic acids or the corresponding lactams, such as
polyamide 4,
polyamide 6, polyamide 6/6, 6/10, 6/9, 6/12 and 4/6, :polyamide 11, polyamide
12,
aromatic polyamides obtained from m-xylene, diamine and adipic acid;
polyamides
prepared from hexamethylenediamine and isophthalic and/or terephthalic acid
and
optionally an elastomer as modifier, for example poly-2,4,4-
trimethylhexamethylene
terephthalamide or poly-m-phenylene isophthalamide. Block copolymers of che_
aforementioned polyamides with polyolefins, olefan copolymers, ionomers or
chemically
bonded or grafted elastomers; or with polyethers, fox instance with
polyethylene glycol,
polypropylene glycol or polytetramethylene glycol. Further, polyamides or
copolyamides
modified with EPDM or ABS; and polyamides condensed during processing
"RIM-polyamide systems".
16. Polyureas, polyimides, polyarnide-imides and polybenzimidazoles.
17. Polyesters which are derived from dicarboxylic acids and diols and/or from
hydroxycarboxylic acids or the corresponding lactones, such as polyethylene
terephthalate, polybutylene terephthalate, poly-i,4-dimethylolcyclohexane
terephthalate
and polyhydroxybenzoates as well as block polyether-esters derived from
polyethers
having hydroxyl end groups; also polyesters modified with polycarbonates or
MBS.
18. Polycarbonates and polyester-carbonates.
19. Polysulfones, poIyether-sulfones and polyether-ketones.
20. Crosslinked polymers which are derived from aldehydes on the one hand and
phenols,
urea and melamine on the other hand, such as phenol/fozmaldehyde resins,
urea/forntaldehyde resins and melamine/formaldehyde resins.
21. Drying and non-drying allcyd resins.
22. Unsaturated polyester resins which are derived from copolyesters of
saturated and
unsaturated dicarbaxylic acids with polyhydric alcohols, and vinyl compounds
as
crosslinking agents, and also halogen-containing, modifications thereof of low
inflammability.
23. Thermosetting acrylic resins, derived from substituted acrylic esters,
crosslinkable for
example epoxy-acrylates, urethane-acrylates or polyf;ster-acrylates.
24. Alkyd resins, polyester resins or acrylate resins crosslinked with
melamine iesins, urea
resins, polyisacyanates or epoxide resins.
25. Crosslinked epoxide resins which are derived from polyepoxides, for
example from
bis-glycidyl ethers or from cycloaliphatic diepoxides.
26. Natural polymers, such as cellulose, natural rubber, gelatine and
derivatives thereof
which are chemically modified in a polymer-homologous manner, such as
cellulose
acetates, cellulose propionates and cellulose butyrates, or the cellulose
ethers, such as
methylcellulose; and rosins and their derivatives.
27. Mixtures (polyblends) of polymers as mentioned above, for example PP/EPDM,
polyamide/EPDM or ABS, P~IC/EVA, PVC/ABS, PVC/IvIBS, PC/ABS, PBTP/ABS,
PC/ASA, PC/PBT, PVC/CPE, PVC/acrylates, POM/thermoplastic PUR,
PC/thermoplastic
PUR, POM/acrylate, POM/PvIBS, PPO/HIPS, PPO/PA 6.6 and copolymers, PA/II~DPE,
-g_
PA/PP, PA/PPO.
The invention therefore also relates to compositions containing an organic
material which
is sensitive to oxidative, thermal or actinic degradation and at least one
compound of the
formula I, and to the use of compounds of the formula I for stabilizing
organic material
against oxidative, thermal or actinic degradation.
Preferred organic materials are polymers, for example synthetic polymers or
thermoplastic
polymers. Organic materials which are particularly preferred are polyolefins,
for example
polypropylene or polyethylene.
In general, the compounds of the formula I are added to the material to be
stabilized in
amounts of 0.01 to 10 %, preferably 0.01 to 5 % and particularly 0.01 to 2 %,
relative to
the total weight of the material to be stabilized.
As well as the compaunds of the formula I, the compositions according to the
invention
can contain, in addition, conventional additives, for example those indicated
below.
1. Antioxidants
1.1. Alkylated monophenols, for example 2,6-di-tart-butyl-4-methylphenol,
2-tart-butyl-4,6-dimethylphenol, 2,6-di-tart-butyl-4-ethylphenol,
2,6-di-tart-butyl-4-n-butylphenol, 2,6-di-tart-butyl-4-isobutylphenol,
2,6-di-cyclopentyl-4-methylphenol, 2-(«-methylcyclohexyl)-4,6-dimethylphenol,
2,6-di-octadecyl-4-methylphenol, 2,4,6-tricyclohexylphenol,
2,6-di-tart-butyl-4-methoxymethylphenol and 2,6-di-nonyl-4-methylphenoL
1~2. Alkylated hydroquinones, for example 2,6-di-tart-butyl-4-methoxyphenol,
2,5-di-tart-butylhydroquinone, 2,5-di-tart-atnylhydro~uinone and
2,6-Biphenyl-4-octadecyloxyphenol.
1.3. HYdroxylated thiodiphenyl ethers, for example
2,2'-thiobis-(6-tart-butyl-4-methylphenol), 2,2'-thiobis-(4-octylphenol),
4,4'-thiobis-(6-tent-butyl-3-methylphenol) and 4,4'-thiobis-(6-tart-butyl-2-
methylphenol).
1.4. All~lidene bisphenols, for example 2,2'-rnethylenebis-(6-tart-butyl-4-
methylphenol),
-9-
2,2'-methylenebis-(6-tent-butyl-4-ethylphenol),
2,2'-methylenebis-[4-methyl-6-(«-methylcyclohexyl)-phenol],
2,2'-methylenebis-(4-methyl-6-cyclohexylphenol),
2,2'-methylenebis-(6-nonyl-4-methylphenol), 2,2'-methylenebis-(4,6-di-tart-
butylphenol),
2,2'-ethylidenebis-(4,6-di-tart-butylphenol),
2,2'-ethylidenebis-(6-tent-butyl-4-isobutylphenol),
2,2'methylenebis-[6-(«-methylbenzyl)-4-nonylphenol],
2,2'-methylenebis-[6-(«,«-dimethylbenzyl)-4-nonylphenol],
4,4'-methylenebis-(2,6-di-tart-butylphenol),
4,4'-methylenebis-(6-tart-butyl-2-methylphenol),
1,1-bis-(5-tent-butyl-4-hydroxy-2-methylphenyl)-butane,
2,6-bis-(3-tart-butyl-5-methyl-2-hydroxybenzyl)-4-methylphenol,
1,1,3-tris-(5-tart-butyl-4-hydroxy-2-methylphenyl)-butane,
1,1-bis-(5-tent-butyl-4-hydroxy-2-methylphenyl)-3-n-dodecylmercaptobutane,
ethylene glycol bis-[3,3-bis-(3'-tart-butyl-4'-hydroxyphenyl)-butyrate],
bis-(3-tent-butyl-4-hydroxy-5-methylphenyl)-dicyclopentadiene and
bis-[2-(3'-tart-butyl-2'-hydroxy-5'-methylbenzyl)-6-tart-butyl-4-methylphenyl]
terephthalate.
1.5. Ben ~I compounds, for example,
1,3,5-tris-(3,5-di-tart-butyl-4-hydroxybenzyl)-2,4,6-trimethylbenzene,
bis-(3,5-di-tart-butyl-4-hydroxybenzyl) sulfide,
isooctyl 3,5-di-ten-butyl-4-hydroxybenzylmercaptoacetate,
bis-(4-tart-butyl-3-hydroxy-2,6-dimethylbenzyl)-dithiolterephthalate,
1,3,5-tris-(3,5-di-ten-butyl-4-hydroxybenzyl) isocyanurate,
1,3,5-tris-(4-tart-butyl-3-hydroxy-2,6-dimethylhenzyl) isocyanurate,
dioctadecyl 3,5-di-tart-butyl-4-hydroxybenzylphosphonate, the Ca salt of
monoethyl
3,5-di-tart-butyl-4-hydroxybenzylphosphonate and
1,3,5-tris-(3,5-dicyclohexyl-4-hydroxybenzyl) isocyanurate.
1.6. Acylaminophenols, for example 4-hydroxylauranilide, 4-
hydroxystearanilide,
2,4-bis-(octylmercapto)-6-(3,5-di-tart-butyl-4-hydroxyanilino)-s-triazine and
octyl
N-(3,5-di-tart-butyl-4-hydroxyphenyl)carbamate.
1.1. Esters of a-(3 5-di-tart-butyl-4-hydroxyphenyl)-propionic acid, with
monohydric or
polyhydric alcohols, for example with methanol, octadecanol, 1,6-hexanediol,
neopentyl
- 10-
glycol, thiodiethylene glycol, diethylene glycol, triethylene glycol,
pentaerythritol,
Iris-(hydroxyethyl) isocyanurate and N,N'-bis-(hydroxy)ethyloxamide.
1.~. Esters of - 5-ten-butyl-4-hy_droxy-3-methylphenyl)-propionic acid with
monohydric
or polyhydric alcohols, for example with methanol, octadecanol, 1,6-
hexanediol,
neopentyl glycol, thiodiethylene glycol, diethylene glycol, triethylene
glycol,
pentaerythritol, tris-(hydroxy)ethyl isocyanurate and N,N'-bis-
(hydroxyethyl)oxamide.
1.9. Esters of p-(3,5-dicyclohexyl-4-hydroxy~henyl)-propionic acid with
manohydric or
polyhydric alcohols, for example with methanol, octadecanol, 1,6-hexanediol,
neopentyl
glycol, thiodiethylene glycol, diethylene glycol, triethylene glycol,
pentaerythritol,
tris-(hydroxy)ethyl isocyanurate and N,N'-bis-(hydroxyethyl)axamide.
1.10. Amides of ~(3 5-di-tart-butyl-4-hydroxyphenyl)_propionic acid, for
example
N;N'-bis-(3,5-di-tart-butyl-4-hydroxyphenylpropionyl)-hexamethylenediamine,
N,N'-bis-(3,5-di-tart-butyl-4-hydroxyphenylpropionyl)-trimethylenediamine and
N,N'-bis-(3,5-di-tart-butyl-4-hydroxyphenylpropionyl)-hydrazine.
2. UV absorbers and light stabilizers
2.1. 2-(2'-1-iydroxyphenyl)-benzotriazoles, for example the 5'-methyl-, 3',5'-
di-tart-butyl-,
5'-tent-butyl, 5'-(1,1,3,3-tetramethylbutyl), 5-chloro-3',5'-di-tart-butyl, .
5-chloro-3'-tart-butyl-5'-methyl, 3'-sec-butyl-5'-tart-butyl, 4'-octoxy, 3',5'-
di-tart-amyl
and/or 3',5'-bis-(«,«-dimethylbenzyl) derivatives.
2.2. 2-1-Iydrox by enzophenanes, for example the 4-hydroxy, 4-methoxy, 4-
octoxy,
4-decyloxy, 4-dodecyloxy, 4-benzyloxy, 4,2',4'-trihydroxy and
2'-hydroxy-4,4'-dimethoxy derivatives.
2.3. Esters of substituted or unsubstituted benzoic acids,, for example 4-tart-
butylphenyl
salicylate, phenyl salicylate, octylphenyl salicylate, dibenzoylresorcinol,
bis-(4-tent-butylbenzoyl)-resorcinol, benzoylresorcinal, 2,4-di-tart-
butylphenyl
3,5-di-tart-butyl-4-hydroxybenzoate and hexadecyl 3,5-di-ten-butyl-4-
hydroxybenzoate.
2.4. Acr less., for example ethyl or isooctyl «-cyano-~,~-diphenylacrylate,
methyl
a-carbomethoxycinnamate, methyl or butyl «-cyano-~-methyl-p-methoxycinnamate,
-11-
methyl «-carbomethoxy-p-methoxycinnamate and
N-(p-carbomethoxy-p-cyanovinyl)-2-methylindoline.
2.5 Nickel compounds, for example nickel complexes of
2,2'-thiobis-[4-(1,1,3,3-tetramethylbutyl)-phenol], such as the 1:1 complex or
the 1:2
complex, if appropriate with additional ligands, such as n-butylamine,
triethanolamine or
N-cyclohexyldiethanolamine, nickel dibutyldithiocarbamate, nickel salts of
monoalkyl
4-hydroxy-3,5-di-tart-butylbenzylphosphonates, such as the methyl or ethyl
ester, nickel
complexes of ketoximes, such as of 2-hydroxy-4-methylphenyl undecyl ketoxime,
and
nickel complexes of 1-phenyl-4-lauroyl-5-hydroxypyrazole, if appropriate with
additional
ligands.
2.6. Sterically hindered amines, for example bis-(2,2,6,6-
tetramethylpiperidyl) sebacate,
bis-(1,2,2,6,6-pentamethylpiperidyl) sebacate, bis-(1,2,2,6,6-
pentamethylpiperidyl)
n-butyl-3,5-di-tart-butyl-4-hydroxybenzylmalonate, the condensation product
farmed from
1-hydroxyethyl-2,2,6,6-tetramethyl-4-hydroxypiperidine and succinic acid, the
condensation product formed from
N,N'-bis-(2,2,6,6-tetramethyl-4-piperidyl)-hexamethylenediamine and
4-tart-octylamino-2,6-dichloro-1,3,5-s-txiazine, txis-(2,2,6,6-tetramethyl-4-
piperidyl)
nitrilotriacetate, tetrakis-(2,2,6,6-tetramethyl-4-piperidyl) 1,2,3,4-
butanetetraoate and
1,1'-( 1,2-ethanediyl)-bis-(3,3,5,5-tetramethylpiperazinone).
2.7. C?xamides, for example 4,4'-di-octyloxyoxanilide,
2,2'-di-octyloxy-5,5'-di-tart-butyloxanilide,
2,2'-di-dodecyloxy-5,5'-di-ten-butyloxanilide, 2-ethoxy-2'-ethyloxanilide,
N,N'-bis-(3-dimethylaminopropyl)-oxamide, 2-ethoxy-5-tart-butyl-2'-
ethyloxanilide and a
mixture thereof with 2-ethoxy-2'-ethyl-5,4'-di-tart-butyloxanilide, and
mixtures of
o-methoxy- and p-methoxy-disubstituted oxanilides and of o-ethoxy- and
p-ethoxy-disubstituted oxanilides.
2.8. 2-(2-Hydroxyphenyl)-1,3,5-triazines, for example
2,,4,6-txis-(2-hydroxy-4-oetyloxyphenyl)-1,3,5-triazine,
2-(2-hydroxy-4-octyloxyphenyl)-4,6-bis-(2,4-dimethylphenyl)-1,3,5-triazine,
2-(2,4-dihydroxyphenyl)-4,6-bis-(2,4-dimethylphenyl)-1,3,5-triazine,
2,4-bis-(2-hydroxy-4-propyloxyphenyl)-6-(2,4-dimethylphenyl)-1,3,5-triazine,
2-(2-hydroxy-4-octyloxyphenyl)-4,6-bis-(4-methylphenyl)-1,3,5-triazine and
- 12-
2-(2-hydroxy-4-dodecyloxyphenyl)-4,6-bas-(2,4-dimethylphenyl)-1,3,5-triazine.
3. Metal deactivators, for example N,N'-diphenyloxamide,
N-salicylal-N'-salicyloylhydrazine, N,N'-bas-(salicyloyl)-hydrazine,
N,N'-bas-(3,5-di-tert-butyl-4-hydroxyphenylpropionyl)-hydrazine,
3-salicyloylamino-1,2,4-triazole and bas-(benzylidene)-oxalic acid
dihydrazide.
4. Phosphates and ~hosphonites, for example triphenyl phosphate, Biphenyl
alkyl
phosphates, phenyl dialkyl phosphates, tris-(nonylphenyl) phosphate, trilauryl
phosphate,
trioctadecyl phosphate, distearyl pentaerythrityl diphosphite, tris-(2,4-di-
tert-butylphenyl)
phosphate, diisodecyl pentaerythrityl diphosphite, bas-(2,4-di-tert-
butylphenyl)
pentaerythrityl diphosphite, tristearyl sorbitol triphosphite,
tetrakis-(2,4-di-tert-butylphenyl) 4,4'-biphenylenediphosphonite and
3,9-bas-(2,4-di-tert-butylphenoxy)-2,4,8,10-tetraoxa-3,9-
diphosphaspiro[5.5]undecane.
5. Compounds which destroy~eroxides, for example esters of ~-thiodipropionic
acid, for
example the lauryl, stearyl, myristyl or tridecyl ester,
mercaptobenzimidazole, the zinc salt
of 2-mercaptobenzimidazole, zinc dibutyldithiocarbamate, dioctadecyl disulfide
and
pentaerythrityl tetrakis-(~-dodecyimercapto)propionate.
6. Polyamide stabilizers, for example copper salts in combination with iodides
andJor
phospharus compounds and salts of divalent manganese.
7. Basic co-stabilizers, for example melamine, polyvinylpyrrolidone,
dicyandiamide,
triallyl cyanurate, urea derivatives, hydrazine derivatives, amines,
polyamides,
polyurethanes, alkali and alkaline earth metal salts of higher fatty acids,
fox example Ca
stearate, Zn stearate, Mg stearate, Na ricinoleate and K palmitate, antimony
pyrocatecholate or tin pyrocatecholate.
8. Nucleating agents, for example 4-tert-butylbenzoic acid, adipic acid or
diphenylacetic
acid.
9. Fillers and reinforcing~yents, for example calcium carbonate, silicates,
glass fibres,
asbestos, talc, kaolin, mica, barium sulfate, metal oxides and hydroxides,
carbon black and
graphite.
-13-
10. Other additives, for example plasticizers, lubricants, emulsifiers,
pigments, fluorescent
whiteners, fire-retarding agents, antistatic agents and blowing agents.
The conventional additives are added, for example, in concentrations of 0.01
to 10 %,
relative to the total weight of the material to be stabilized.
The incorporation of the compounds of the formula I and, if desired, other
additives into
the polymeric, organic material is effected by known methods, for example
before or
during shaping or by applying the compounds in dissolved or dispersed form to
the
polymeric, organic material, if appropriate with subsequent evaporation of the
solvent.
The compounds of the formula I can also be added to the materials to be
stabilized in the
form of a master batch in which they are present in a concentration of, far
example, 2.5 to
25 % by weight.
The compounds of the formula I can also be added before or during
polymerization or
before crosslinking.
The compounds of the formula I can be incorporated into the material to be
stabilized in
an undiluted form or encapsulated in waxes, oils or polymers.
The compounds of the formula I can also be sprayed onto the polymer to be
stabilized.
They are capable of diluting other additives (for example the conventional
additives
indicated above) or melts thereof in such a way that they can also be sprayed
onto the
polymer to be stabilized together with these additives. Addition by spraying
on during the
deactivation of the polymerization catalysts is particularly advantageous, it
being possible,
for example, to use for spraying the steam used for deactivation.
In the case of polyolefins polymerized in spherical form it can be
advantageous, for
example, to apply the compounds of the formula I by spraying on, if
appropriate together
with other additives.
A preferred embodiment of the present invention is, therefore, the use of
compounds of
the formula I for stabilizing polymers against oxidative, thermal or actinic
degradation, the
compounds of the formula I being sprayed onto the polymer.
The materials thus stabilized can be used in a very wide variety of shapes,
for example as
- 14-
films, fibres, tapes, moulding materials or profiles or as binders for paints,
adhesives or
putties.
The compounds of the formula I can be prepared analogously to known processes;
for
example by condensation of phenols of the formula II
OH
(a)
-R3
R2
in which Rt, Rz and R3 are as defined above with 1-1.3 mol of mandelic acid or
a
mandelic acid derivative, as described in GB-A 2,042,562. Instead of mandelic
acid or a
mandelic acid derivative, it is also possible to use a compound of the formula
III or IV
Z-CH-C°OH Z-CH-C°CI
I II ' i II
X O Ct O
UI) W)
in which Z is as defined above and X is, for example, acetoxy,
tolylsulfonyloxy or
mesitylsulfonyloxy. If a compound of the formula IV is used, it is appropriate
to use the
process described in Example 3 of BE-A 881,496.
The reactions are preferably carried out within a temperature range from 180
to 220°C and
under a pressure of 10 - 1013 mbar.
Insofar as they are not commercially available, the compounds of the formula
II can be
prepared analogously to known processes; for example by catalytic alkylation
of phenols
of the formula
2~2~~.~
-15-
OH
Rs
i
with linear «-olefins (Ct3-C3o)~ Alkylation is mainly carried aut in the
liquid phase without
using a solvent.
The following may be mentioned as suitable catalysts:
a) Friedel-Crafts catalysts, for example proton acids (sulfuric acid,
phosphoric acid,
p-toluenesulfonic acid etc), solid inorganic catalysts (acid-activating
bleaching earths, for
example montmorillonite types, oxides of aluminium and silicon etc) and other
Friedel-Crafts catalysts (boron trifluoride, aluminium chloride,
ferric(III)chloride, zinc
chloride etc),
b) o-selective catalysts, for example aluminium phenates and y-aluminium
oxide, as
described in US-A 3,367,981 and DE-B 1,142,873, and also other modifications
of
aluminium oxide.
o-Selective catalysts are preferred, and the active 7-aluminium oxide
described in the
abovementioned US-A and DE-B is particularly preferred.
In the presence of Friedel-Crafts catalysts, the reaction is appropriately
carried out under
"normal pressure'° within th;e temperature range from, for example, 30
to 150°C. In the
case of alkylation in the presence of o-selective catalysts, it is
advantageous to carry out
the reaction at an elevated temperature (approx. 200-350°C) under
pressure (approx.
20-200 bar).
After the alkylation the catalyst is removed from the crude material, for
example by
extraction by washing or filtration, and the latter can then be purified by
distillation.
The compounds of the formula II can be obtained in the form of a mixture
of isomers which can be separated, for example, by means of chromatographic
methods, in
- 16-
particular gas chromatography and high-pressure liquid chromatography (HPLC).
It is also
possible, however, to employ the mixture of isomers for the preparatian of the
compounds
of the formula I, so that the compounds of the formula I are also obtained as
a mixture of
isomers. This mixture of isomers of the compounds of the formula I can, if
necessary, be
separated or can be used withaut treatment for stabilizing organic materials
against
oxidative, thermal or actinic degradation.
It is also possible to employ commercially available mixtures of olefins
(H2C~CH-CI-I2-A1 in which A1 is, for example, Ct3-CmatkYl, Cl~-C2ialkYl or
C2t-C2~alkyl) for the preparation of the compounds of the farmula II.
The following examples illustrate the invention further. Unless stated
otherwise, parts and
percentages are by 'weight.
Example l: Preparation of the compound
3-phenyl-7-(1'-hexadecylethyl)-benzofuran-2-one.
4.8 g (31.5 mmol) of mandelic acid and 10.4 g (30 mmol) of 1-
hexadecylethylphenol are
heated together at 190-200°C far approx. 8 hours under reduced pressure
(13.3 mbar). In
the course of this the water formed in the reaction is removed by
distillation. When the
reaction is complete, the reaction product is filtered over silica gel
(hexane) and is then
treated with cold hexane. The product has a melting point of 60-62°C
and is in the form of
a white powder. The yield is 7 g (= 50 % of theory).
Elementary anal
Calculated: C 83.06 %; hI 10.02 %
Found: C 83.04 %; H 10.02 %
Examples 2-5: The compounds indicated in Table 1 are prepared analogously to
Example 1.
Table l:
-17-
81
O
R2
Ex- R1 R2 R3 M.p. Elementary analysis
ample
2 1-Hexa- -H -CH3Oil calcd.: C 83.14 %; H
10.15 %
decylethyl found : C 83.11 %; H
10.33 %
3 1-Hexa- -CH3 -H 42C calcd: C 83.14 %;1rI
10.15 %
decylethyl found : C 83.43 %; I-I
10.33 %
4 1-Dodecyi--CH3 -H Oil calccL: C 82.81 %; H
9.59 %
ethyl found : C 82.88 %; H
9.76 %
1-Hexa- -C(CH3y3-H Oil calcti.: C 83.34 %;
H 10.49 %
~ ~ ~ ~ ~
decylethyl found : C 83.47 %; H
10.47
Example 6: Stabilization of polypropylene.
1.3 kg of polypropylene powder (melt index: 3.2 g,~10 minutes, measured at
230°C/2.16 kg) are mixed with 0.05 % of calcium stearate, 0.05 % of
tetrakis-[3,5-di-tart-butyl-4-hydroxyphenylpropionyloxymethylJ-methane and
O.OS, % of
the stabilizer shown in Table 2. This mixture is extruded at 100 revolutions
per minute in
an extruder having a cylinder diameter of 20 mm and a length of 400 mm, the 3
heating
zones being adjusted to 260°C, 270°C and 280°C. 'The
extrudate is drawn through a water
bath to cool it and is then granulated. The resulting granules are extruded
for a second and
third time. After these 3 extrusions, the melt index is measured at
230°C/2.16 kg. The
results are shown in Table 2.
-18-
Table 2:
Compound Melt index*~ [g/10
from mint
Example at 230C/2.16 kg
No.
1'7.2
1 4.6
2 5.9
. 3 n.5
4 d.3
*>Low values mean gofld stabilization.