Note: Descriptions are shown in the official language in which they were submitted.
2~2~6
LAMlNATEn ORG~NIC PHOTOSENSITIVE MATERIAL
BACKGROUND OF THE INYENTION
Field of the Invention
This invention relates to a laminated organic photo-
sentive material which has a charge producing layer and acharge transporting layer formed on an electroconductive
support and which has in particular a high sensitivity to
a semiconductor laser region wavelength so as to be suitable
for use as a photosentive material for a laser beam printer.
Description of the Prior Art
A composite or laminated type organic photosensitive
material has been developed and put to practical use in
recent years. This type of organic photosensitive material
is disclosed in, for example, Japanese Patent Publications
Nos. 42380/1980 and 34099/1985. It comprises an electro-
conductive support, and a charge producing layer and a
charge transporting layer formed on the support. For
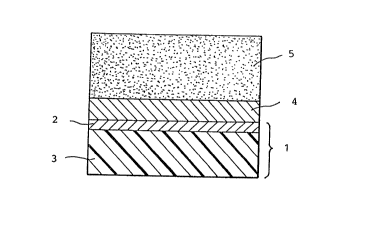
instance, such a composite photosensitive material has an
electroconductive support 1 of aluminum layer 2 deposited
on a polyester fil0 3, a charge producing layer 4 formed
on the aluminum layer, and a charge transporting layer 5
formed on the charge producing layer, as illustrated in
Fig. 2
The charge producing layer is formed by, for example,
preparing a dispersion of a charge producing substance
together with an organic solvent, a binder resin, and if
necessary a plasticizer, applying the dispersion onto the
support, and drying to a thin film. The charge trans-
porting layer is formed by, for example, dissolving a charge
~ ~ 2 '~ ~ 4 6
transporting substance in an organic solvent together with
a binder resin, and if required a plasticizer, applYing the
solution onto the charge producing layer, and drying to a
thin fiIm. A charge transporting laYer may be first formed
on the support, and then a charge producing layer on the
charge transporting layer.
There are alreadY known a number of laminated type
organic photosensitive materials containing a variety of
charge producing substances and charge transporting
substances in the charge producing layer and charge
transporting layer, respectively. For example, there is
described in Japanese Patent Laid-Open No. 60-255854, a
photosensitive material which contains a hydrazone
compound represented by the formula:
~ CHz
~ CHz ~ CH3
as a charge transporting substance, and a metal phthalo-
cyanine compound such as copper phthalocyanine or a
nonmetal phthalocyanine such as tetramethylphthalocyanine
or dialkylaminophthalocyanine as a charge producing
substance. However, these known photosensitive materials are
still unsatifactory in charging properties or sensitivity.
As above set forth, it is already known that a metal
phthalocyanine and a nonmetal phthalocyanine are photo-
conductive, and in particular, a single layer photosensitive
material which employs the X-type nonmetal phthalocyanine
as a photoconductive material is disclosed in U.S. Patent
No. 3,816,118. However, this known photosensitive material
has a very low sensitivitY.
Meanwhile, there has been a demand for a laminated
3 ~ ~
organic photosensitive material for use in a laser beam
printer which is sensitive to a long wavelength region from
about 750 nm to about 850 nm, and for such a purpose the
use of a variety of charge producing substances and charge
transporting substances have hitherto been proposed.
However, any of these known laminated organic photosensitive
materials which employ the beforementioned specific
hydrazone compound as a charge transporting substance has
a low sensitivity to a semiconductor laser region long
wavelength from about 750 nm to about 850 nm.
SUMMARY OF THE INYENTION
The present inventors have made an extensive investi-
gation to solve the problems as above set forth involved
in the known laminated organic photosensitive materials.
in particular to obtain a laminated organic photosensitive
material having a high sensitivity to the long wavelength
region of 750-850 nm. ~s results the inventors have found
that the co-use of the aforementioned specific hydrazone
compound as a charge transporting substance and X-type
nonmetal phthalocyanine as a charge producing substance
together with a halogen-containing resin as a binder resin
for a charge producing layer provides a laminated organic
photosensitive material very sensitive to the laser region
long wavelength of 750-850 nm.
In accordance with the invention, there is provided
a laminated organic photosensitive material which comprises
an electroconductive support, a charge producing layer
and a charge transporting layer formed thereon wherein the
charge producing layer contains X-type nonmetal phthalo-
cyanine as a charge producing substance and a halogen-
containing resin as a binder resin for the layer, and the
charge transporting layer contains a hydrazone compound of
the formuia:
4 h.i ~ 2 l~ 3 4 6
CHz
~ CHz ~ CH3
as a charge transporting substance and a halogen-containing
resin as a binder resin for the charge producing layer.
BRIEF DESCRIPTION OF ~HE DRAWINGS
Fig. 1 is an X-ray diffraction diagram (CuK ~,
powder method) of X-type nonmetal phthalocyanine used as a
charge producing substance in the laminated organic photo-
sensitive material of the invention, and
Fig. 2 is a section of a known laminated organic
photosensitive material.
The laminated organic photosensitive material of
the invention contains X-type nonmetal phthalocyanine as a
charge producing substance. It is represented by the
formula:
~
N N N
~ NH HN
N
N 4 ~ N
~,.D
3 ~ ~
Tn the production of the laminated organic photo-
sensitive material of the invention. a dispersion or a
solution of the nonmetal X-type phthalocyanine as a charge
producing substance, a halogen-containing polymer as a
binder resin, and if necessary a plasticizer, is coated
on an electroconductive support and dried to form a charge
producing layer, and then a solution of the hydrazone
compound as a charge transporting substance and a binder
resin, and if necessary a plasticizer, is coated on the
charge producing layer and dried to a form a charge
transporting layer. The charge producing layer and the
charge transporting layer may be laminated in the reverse
order, that is, the charge transporting layer may be first
formed on the support, and then the charge producing layer
on the charge producing layer.
The halogen-containing resin as a binder resin for
the charge producing layer is exemplified by polyvinyl
chloride, vinyl chloride-vinyl acetate copolymer, ethylene-
vinyl chloride copolymer or vinyl chloride-vinyl acetate-
maleic anhydride copolymer. The copolymer may be a graftcopolymer. When a vinyl chloride copolymer is used, it is
preferred that the copolymer contains a vinyl chloride
content of not less than 20 % by weight.
The smaller the content of the binder resin in the
charge producing layer, the better, but it is usually in
the range of about 5-50 % by weight based on the layer.
The charge producing layer has a thickness usually of about
0.05-20 microns, preferably of 0.1-10 microns.
The charge transporting substance used in the
invention is a hydrazone compound as rePresented by the
formula hereinbefore given. The hydrazone compound is
contained in the charge transporting layer usually in an
amount of 10-60 % by weight based on the laye, and the
layer has a thickness usually of 5-100 microns.
The binder resin for the charge transporting layer
is of the type which is soluble in an organic solvent and
is highly compatible with the charge transporting substance
so that a stable solution thereof may be prepared easily.
Moreover, it is preferable to use a resin which is
inexpensive and can form a film of high mechanical strength,
transparency and electrical insulation. Preferred examples
of the binder resin may be exemplified by, for example,
polycarbonate, polystyrene, styrene-acrylon.trile copolymer,
polyester resin or polyvinyl chloride.
The organic solvent used for the preparation either
of the charge transporting layer or of the charge producing
layer is not specifically limited, but it may include, for
example, chloroform, 1,2-dichloroethane, 1,1,2,2-tetra-
chloroethane, tetrahydrofuran or dioxane,
The invention will now be described more specifically
with reference to examples, however, the invention is not
limited thereto.
ExamPle
Polyvinyl chloride having an average polymerization
degree of 800 (PVC-SR from Chisso K.K.) was purified by a
reprecipitation method using tetrahydrofuran and n-hexane
as solvents therefor and then vacuum dried.
An amount of 1.6 parts by weight of the polyvinyl
chloride and 2.2 parts by weight of X-type nonmetal
phthalocyanine (8120B from Dainippon Ink Kagaku Kogyo K.K.)
were milled together in 96.2 parts by weight of tetrahydro-
furan to prepare a dispersion.
The dispersion was applied by a doctor blade onto an
aluminum film deposited on a polyethylene terephthalate
film, allowed to dry at room temperature and then dried by
heating at 80C for 60 minutes, to form a charge producing
layer having a thickness of 0.6 microns.
An amount of 10 parts by weight of polycarbonate
~2~3~
~Yupiron E-2000 from Mitsubishi Gas Kagaku Kogyo K.K.) an~
8 parts by weight of the aforesaid hydrazone compound as a
charge transporting substance were dissolved in 82 parts
by weight of chloroform to prepare a solution. ~he
solution was applied onto the charge producing layer by a
doctor blade having a clearance of 100 microns, allowed to
dry at room temperature and then dried by heating at lOO'C
for 60 minutes to form a charge transporting layer having a
thickness of 20 microns, whereby a laminated organic
photosensitive material was obtained.
Example 2
~ laminated photosensitive material was prepared in
the same manner as in the Example 1 using vinyl chloride-
vinyl acetate copolymer having a vinyl acetate content of
15 % by weight (from Nippon Kayaku K.K.) as a binder resin
for the charge producing layer.
Example 3
A laminated photosensitive material was prepared in
the same manner as in the Example 1 using vinyl chloride-
vinyl acetate graft copolymer having a vinyl chloride
content of 50 mol % (Graftmer R-5 from Nippon Zeon K.K.)
as a binder resin for the charge producing layer.
Example 4
~ laminated photosensitive material was prepared in
the same manner as in the Example 1 using vinyl chloride-
vinyl acetate-maleic anhydride copolymer (Esleck MF-10 from
Sekisui Kagaku Kogyo K.K.) as a binder resin for the charge
producing layer having a thickness of 0.3 microns.
Comparative ExamPle
A laminated photosensitive material was prepared in
the same manner as in the Example 1 using N,N-diethylamino-
benzaldehyde diphenylhydrazone as a charge transportingsubstance.
Comparative ExamP!e 2
A laminated photosensitive material was prepared in
the same manner as in the Example 1 using the same
polycarbonate as before mentioned as a binder resin for
the charge producing layer.
Comparative Example 3
A laminated photosensitive material was prepared in
the same manner as in the Example 3 using N,N-diethylamino-
benzaldehyde diphenylhydrazone as a charge transporting
substance.
The laminated photosensitive materials prepared as
above set forth were each evaluated for electrostatic
charging characteristics by use of an electrostatic
charging testing device (Model EPA 8100 from Kawaguchi
Denki Seisakusho~.
The surface of photosensitive material was negatively
charged with a charge corona of -6 KV, and the surface
potential was measured as an initial potential. Then, after
the photosensitive material was left standing in the dark
over a period of five seconds, the surface potential was
measured as a charge retention rate.
Then the surface was irradiated at an illuminance of
5 lux with a halogen lamp, and the length of time was
measured until the point at which the surface potential
3 ~ ~
dropped to a half of its initial value, and the half-time
exposure El~2 (lux) of the photosensitive material to that
point of time was determined as its photosensitivity.
Further, the surface was irradiated with a monochro-
matic light having a wavelength of 750 nm and a luminousintensity of 0.5 ~ W/cmZ. The length of time was measured
until the point at which the surface potential dropped to
a half of its initial value, and the half-time exposure
El~z (~ J/cm2) of the photosensitive material to that point
of time was determined as its photosensitivity.
The surface potential after five seconds from the
irradiation of light was also measured as a residual
potential in eiher cases above.
As the results are shown in Table 1, the photo-
sensitive material of the invention has a small half-time
exposure E,~z when irradiated with either white light or
monochromatic light (750 nm), and has a high photo-
sensitivity.
lo ~43~
_
~r ~ ~ u~
C~
o
~ o ~ . ~ CO ~ C`l ~ o~ ~ ~
~ s ~ .~ ~ ~ u~ ~O ~ ~ a~ ~
~ ~
~1~
~1
E ~
~ tl) ~ c~
_~ S~ C~C`lC~C`~ ~
O
CC) C~ U~ O ~D C~
S~ ~ ~ ~ . . . . .
L~ ~ ~ U~ ~ cr~
~- Q) ~ ~ CC CX: 00 a~
~:
.~ co c~a ~ o co cr~
- a~ ~~ ~ O O ~ cr~
~ ~ ~ ~ ~ ~ .~
_ O ~ l l I
~ C`~ C~ ~ . _ E
~ O
Q. Q 1~)
X E
*