Note: Descriptions are shown in the official language in which they were submitted.
2501-1
- -- 2024519
1 SOLVENT AND WATER/SURFACTANT PROCESS FOR REMOVAL OF BITUMEN
2 FROM TAR SANDS CONTAMINATED WITH CLAY
4 Backqround
Field of the Invention
6 This invention relates to a process for recovering bitumen
7 from tar sand and, more particularly, to a low temperature,
8 solvent extraction step followed by a water process step for
9 recovering bitumen from tar sands contaminated with clay, the
water process step including the use of a specific surface active
11 agent to preclude contamination of the water with either bitumen
12 residue or clay.
13
14 Related Application
This application is a continuation-in-part application of my
16 copending application Serial Number 07/297,670 filed 17 January
17 1989 for PROCESS FOR REMOVAL OF BITUMEN FROM TAR SANDS
18 CONTAMINATED ~ITH CLAY.
19
The Prior Art
21 The term "tar sand" refers to a consolidated mixture of
22 bitumen (tar) and sand. Alternate names for tar sands are "oil
23 sands" and "bituminous sands", the latter term being more
24 technically correct in that the sense of the term provides an
adequate description of the mixture. The sand constituent of tar
~' ,
2024519
.
1 sand is mostly alpha quartz, as determined from x-ray diffraction
2 patterns, while the bitumen or tar constituent of the tar sands
3 consists of a mixture of a variety of hydrocarbons and
4 heterocyclic compounds. This bitumen, if properly separated from
the sands, may be upgraded to a synthetic crude oil suitable for
6 use as a feedstock for the production of liquid motor fuels,
7 heating oil, and/or petrochemicals.
8 About 65 percent of all of the known oil in the world is
9 contained in tar sand deposits or heavy oil deposits. Tar sand
fields occur throughout the world with the exception of the
11 continents of Australia and Antarctica. Significantly large tar
12 sand deposits have been identified and mapped in Canada,
13 Columbia, Trinidad-Tobago, Venezuela, and the United States. The
14 Canadian tar sand deposits, known as the Athabasca tar sands, are
located in the province of Alberta, Canada and are currently
16 being developed. The reserves of bitumen in the Athabasca tar
17 sands alone has been estimated to be approximately 900 billion
18 barrels.
19 In the United States, approximately 24 states contain known
tar sand deposits. However, about 90 to 95 percent of the mapped
21 tar sand deposits are located within the State of Utah and are
22 estimated to include at least 25 billion barrels of oil. While
23 the Utah tar sand reserves appear small in comparison with the
24 enormous potential of the Athabasca tar sands, the Utah tar sand
reserves represent a significant energy resource when compared to
26 the United States crude oil proven reserves (approximately 31.3
2024519
,,
1 billion barrels) and with the United States crude oil production
2 of almost 3.0 billion barrels during 1976. Utah tar sand deposits
3 occur in six major locations along the eastern edge of the state
4 with the bitumen content varying from deposit to deposit as well
as within a given deposit. Current information available
6 indicates that Utah tar sand deposits average generally less than
7 10 percent bitumen (by weight), although deposits have been found
8 with a bitumen saturation of up to about 17 percent (by weight).
9 Athabasca tar sands and Utah tar sands are both also
characterized by the presence of clay as a contaminant. The clay
11 is finely divided and dispersed throughout the tar sand deposits,
12 so that it represents a significant obstacle to the efficient
13 processing of tar sands. The commercial processing of the
14 Athabasca tar sands has created vast settling ponds where clay-
contaminated water is held to allow the clay to settle.
16 Experience has shown that the clay is so extremely fine that it
17 remains suspended in the water for long periods. A further
18 problem is that significant quantities of bitumen are carried
19 into these settling ponds where it agglomerates and floats on the
surface of the water to represent a pollution hazard,
21 particularly for migratory waterfowl, and the like.
22 Tests have also determined that the Utah tar sands lacks
23 connate water so that the bitumen is bonded directly to the sand
24 grains. This bitumen is also at least one order of magnitude or
at least ten times more viscous than bitumen obtained from
26 Athabasca tar sands. Accordingly, the processing of Utah tar
~ 202~519
. ., ~.
1 sands involves both displacement of the bonded bitumen from the
2 sand grains followed by subsequent phase disengagement of the
3 more viscous bitumen from the residual sand phase. Attempts to
4 use conventional hot water processes that have been successfully
applied to the Athabasca tar sands have been unsuccessful for
6 processing Utah tar sands. This failure is readily apparent in
7 light of the recognized differences in both the physical and
8 chemical nature of the Utah tar sands.
9 A more comprehensive discussion of the athabasca tar sands
may be found in the literature including, for example (1) E.D.
11 Innes and J.V.D. Tear, "Canada's First Commercial Tar Sand
12 Development," Proceedings of the Seventh World Petroleum
13 Congress, Elsevier Publishing Co., 3, p. 633, (1967); (2) F.W.
14 Camp, The Tar Sands of Alberta Canada, 2nd Edition, Cameron
Engineering, Inc., Denver, Colorado (1974); and (3) J. Leja and
16 C.W. Bowman, "Application of Thermodynamics to the Athabasca Tar
17 Sands," Canadian Journal of Chemical Engineerinq, 46 p. 479
18 (1968).
19 Additionally, the following U.S. Pa~ents are a few of the
patents which have been granted for apparatus or processes
21 directed toward obtaining bitumen from tar sands: U.S. Pat. Nos.
22 1,497,607; 1,514,113; 1,820,917; 2,871,180; 2,903,407; 2,927,007;
23 2,965,557; 3,159,562; 3,161,581; 3,392,105; 3,401,110; 3,553,099;
24 3,560,371; 3,556,980; 3,605,975; 3,784,464; 3,847,464; 3,875,046;
3,893,907; 4,096,057; 4,120,776; 4,160,720; 4,337,143; and
26 4,410,417. With the exception of U.S. Pat. Nos. 3,605,975,
"- 2024~19
1 4,120,776, and 4,160,720, each of the foregoing patents have been
2 directed toward processing any tar sand, but, in particular,
3 Athabasca tar sands.
4 From the foregoing it is clear that extensive progress has
been made in separating bitumen from tar sands, particularly with
6 regard to the Athabasca tar sands. To date no commercially
7 feasible process has been used on the Utah tar sands other than
8 the simple mining, crushing, and blending of the Utah tar sands
9 into an asphalt cement which, when combined with a gravel
aggregate, forms an asphalt-based concrete highly suitable for
11 use as a paving material.
12 In view of the foregoing, it would be a significant
13 advancement in the art to provide a process for recovering
14 bitumen from clay-contaminated tar sands. An even further
advancement in the art would be to recover bitumen from clay-
16 contaminated tar sands using a readily available solvent that can
17 be recovered and recycled. Importantly, the process should
18 include a water process step along with the careful use of
19 specific surface active agents to preclude contamination of the
water with residual bitumen or clay. Such a novel process is
21 disclosed and claimed herein.
22
23 Brief Summary and Objects of the Invention
24 This invention relates to a novel, low-temperature process
whereby chunks of clay-contaminated tar sand are treated with a
26 readily available solvent. The solvent breaks down the chunks
2~245 19
. ..
1 and forms a slurry of tar sand and solvent. Upon agitation, the
2 solvent removes a substantial portion of the bitumen from the
3 sand to form a liquid phase containing solvent and bitumen. A
4 significant fraction of the clay is also recovered in the
solvent/bitumen phase. The resulting liquid phase is drawn off
6 and processed by centrifugation to separate the clay from the
7 solvent and bitumen. The solvent is then removed from the
8 bitumen by distillation. The bitumen-contaminated sand phase is
9 washed with water to which a carefully selected surface active
agent has been added in order to recover a second liquid phase of
11 bitumen, solvent, and clay. Advantageously, most of the clay
12 has been removed with the first liquid phase so that very little
13 clay remains to contaminate the water during the water washing
14 process. Great care is exercised in the selection of a surface
active agent that will not emulsify the residual bitumen into the
16 water so that the water remains relatively clear.
17 It is, therefore, a primary object of this invention to
18 provide improvements in the process for recovering bitumen from
19 clay-contaminated tar sands.
Another object of this invention is to provide a novel
21 process for removing clay from tar sand by recovering the clay
22 from solvent used to remove bitumen and clay from the tar sand.
23 Another object of this invention is to provide a novel
24 process for the solvent recovery of bitumen from clay-
contaminated tar sands using a readily available solvent, the
26 process including a water wash for recovering additional bitumen
2024519
1 and solvent from the sand.
2 Another object of this invention is to incorporate a
3 carefully selected surface active agent in the water wash
4 process, the surface active agent having the characteristic of
not emulsifying the residual bitumen into the water.
6 Another object of this invention is to remove residual
7 bitumen from sand grains that are essentially free of connate
8 water without emulsifying the bitumen and thereby trapping
9 residual clay in the residual bitumen so as to maintain a
relatively clear water.
11 Another object of this invention is to provide a low-
12 temperature process for recovering bitumen from a clay-
13 contaminated tar sand.
14 Another object of this invention is to provide a process for
producing a clean sand from a clay-contaminated tar sand.
16 These and other objects and features of the present
17 invention will become more readily apparent from the following
18 description in which preferred and other embodiments of the
19 invention have been set forth in conjunction with the
accompanying drawing and appended claims.
21
22 Brief Description of the Drawinq
23 The drawing is a schematic flow diagram illustrating a
24 presently preferred embodiment of the novel process of this
invention for recovering bitumen from a clay-contaminated tar
26 sand while separating out the clay and producing a clean sand.
- 2024519
1 Detailed Description
2 The invention is best understood by reference to the drawing
3 wherein like parts are designated by like numerals throughout in
4 conjunction with the following description.
s
6 General Discussion
7 Numerous processes for recovering bitumen from tar sand are
8 described in both the patent and technical literature. In
9 general, these references are directed to either the Athabasca
tar sands or the Utah tar sands. The processes range among
11 thermal processes, solvent processes, and water processes or a
12 combination of these processes. Interestingly, few of the
13 available references discuss the presence of clay`in the tar sand
14 although clay is now recognized as the primary reason that many
of these processes are not commercially feasible. For example,
16 any process that relies on the use of water, with or without
17 additives, will result in clay-contamination of the water. This
18 contamination requires extensive separation processes in order to
19 recover and recycle the water since environmental restrictions
prohibit the discharge of clay-contaminated water. A further
21 problem is that surface active agents are designed to cause the
22 bitumen to become dispersed or otherwise emulsified in the water
23 phase. This, in turn, creates significant problems because it
24 not only produces a bitumen/water mixture that is difficult to
separate, but it also liberates the clay from the bitumen into
26 the water phase.
2024519
1 Thermal processes also encounter problems when clay is
2 present in the tar sand to any appreciable amount. In
3 particular, the extremely fine particle size of the clay means
4 that it will be carried over into the gaseous phase, for example,
from any type of thermal, fluidized bed process. Additionally,
6 there is an inherent risk that the clay may sinter on hot
7 surfaces during any coking process involving coked tar sand.
8 Advantageously, the process of the present invention uses a
9 solvent to disintegrate chunks of tar sand, dissolve
substantially all of the bitumen therefrom, while,
11 simultaneously, remove the clay in the bitumen/solvent phase.
12 The resulting solvent, bitumen, and clay phase is processed in a
13 centrifuge to remove the clay so that the remaining
14 solvent/bitumen mixture can be separated through a simple
distillation process. Residual bitumen with entrapped clay and
16 solvent is removed from the sand in a low-temperature process
17 using water to which a specific type of surface active agent has
18 been added. This water wash cycle may be repeated several times
19 to assure essentially complete removal of the bitumen and solvent
from the sand while the particular surface active agent precludes
21 emulsification of the residual bitumen in the water.
22 Importantly, the surface active agent is selected so as to
23 disengage the bitumen from the sand grains without emulsifying
24 the bitumen. This step is particularly critical for Utah tar
sand since they are characterized by the lack of connate water so
26 that the highly viscous bitumen is bonded directly to the sand
202451g
1 grains.
2 The selection of the appropriate surfactant in the water
3 wash step of this process is critical to the success of the
4 process. The wrong surfactant can emulsify residual bitumen
leaving a highly contaminated water with bitumen and clay
6 dispersed throughout. This problem exists in the current
7 processing technology practiced in the extraction of bitumen from
8 the Athabasca deposits. If one were to practice this same
9 technology on Utah tar sands, it would not only be impractical
due to the high water discard rate in a desert environment but
11 also violate all the laws dealing with clean waters.
12 Importantly, the surfactant or surface active agent must be
13 able to separate bitumen from sand grains that are characterized
14 by the absence of connate water without simultaneously
emulsifying the bitumen into the wate~ phase. Additional
16 surfactant requirements include its being nontoxic to plant
17 growth so that residual surfactant entrained in the spent sand
18 will permit the sand to be ecologically revegetated during
19 reclamation of the excavation site. A further feature is that
the surfactant must be biodegradable and the degradation products
21 must not be toxic to plant life for the reasons stated
22 hereinbefore.
23 Surfactants can be categorized in three general categories:
24 Cationic, Anionic, and Nonionic. I have discovered that a very
narrow range of nonionic surfactants provides the necessary
26 characteristics that make this process feasible. Table I sets
`` ` 2~245i9
~ .
1 forth a run of experiments on residual tar sands that had been
2 cleaned with solvent to remove ninety percent of the bitumen.
3 The purpose of the experiment was to demonstrate how this
4 material was wetted by water alone and water to which had been
added surface active agents from various types of surface active
6 agents.
7 Table II outlines my discovery that a surfactant selected
8 from primary or linear alcohols of the ethoxylate family with a
9 narrow range of carbon atoms in the primary alcohol chain
provides optimal separation. The number of ethoxy groups on the
11 carbon atoms in the chain are also selected within a relatively
12 narrow range since the greater the number of ethoxy groups on the
13 surfactant molecule, the more soluble the bitumen will be in
14 water. This must be balanced with the fact that the higher
number of ethoxy groups causes an increased rate of disengagement
16 of the bitumen from the sand grains.
17 Correspondingly, the lower range of carbon atoms in the
18 surfactant provides a faster release of bitumen from the sand
19 grains. For example, a surfactant with eight carbon atoms
results in a very fast release of bitumen from the sand, much
21 faster than a surfactant with 12 or 15 carbon atoms. However, an
22 undesirable feature is that surfactants of this type also form
23 emulsions between the released bitumen and the water, an event
24 that must be avoided in order to make this process economically
feasible.
26
11
- 2~2~51~
.
Table I
Aqueous Medium Results
1. Water (only) Not wetted
2. Water with base Wetted slowly
3. Water with 0.5% Not wetted
Cationic surfactant
4. Water with anionic Wetted slowly
surfactant (wetting due to
solution being basic)
5. Water nonionic Wetted immediately
surfactant
(alcohol ethoxylate)
2024519
.
1 Another important limitation is the amount of the surfactant
2 in the water phase. For example, a surfactant of this invention
3 having eight carbon atoms and three ethoxy groups in a
4 concentration range of three to four percent will produce a
complete emulsion. I have found that the maximum allowable
6 concentration of surfactant suitable for the practice of this
7 invention must not exceed about one-half percent, by volume.
8 This must be carefully monitored during the recycle of the water
9 so that the makeup stream of surfactant does not create excess
surfactant. This is important since a certain fraction of
11 surfactant will be lost with the bitumen phase and some will be
12 carried away by the damp sand.
13 The conclusion to be derived from an analysis of the results
14 displayed in Table II is that the two to three ethoxylate units
provide a superior surface active agent as long as the clay
16 floaters do not present interface separation problems during
17 continuous processing. The advantage of this surfactant range is
18 that there is no water contamination problem.
19 Ethoxylate units in the six to eight range present clean
interfaces but requires at least ten minutes settling time before
21 the water can be reused. This time requirement may or may not
22 adversely affect the continuous processing strategy. Greater
23 than eleven ethoxylate units renders the surfactant unusable.
24 Another study was conducted to determine the rate at which
the bitumen/solvent residue separates from the sand phase during
26 the water-wash cycle. Comparisons were made using alcohols with
13
2024519
Table II
Behavior of Nonionic Primary Alcohol
- Ethoxylates (E.O. Units)
with Varying Surfactant Concentrations
2 E.O. units The water layer Lots of clay
regardless of clear, no color floaters at both
concentration interfaces
3 E. O. units Same as above Same as above
regardless of
concentration
6 E.O. units
.5% Water layer Settles out,
light brown few clay floaters
.3% Water layer colorless Settles out,
few clay floaters
7 E.O. units
.5% Water layer Very few clay
light brown floaters1
.3% Water layer colorless Very few clay
floaters
8 E.O. units
.5% Water layer At end of 10 minutes
light black no clay floaters2
.3% Water layer At end of 10 minutes
dark brown no clay floaters2
11 E.O. units Water layer is black- Too dark to tell
black, with no observed
change within one hour
1 Settled out leaving a clear solution within 5 minutes settling
time.
2 The black layer in the water contains oil and clay. A layer of
fine, tan clay settles out as the solution clears up. This
clearing takes place within 10 minutes.
14
2024519
1 eight, twelve and fifteen carbon atoms, C-8, C-12, and C-15,
2 respectively, and with ethoxylate units ranging between three and
3 eight. The studies found that the C-12 and C-15 alcohols were
4 identical with both three and seven ethoxylate units, the seven
ethoxylate units being faster. Surprisingly, the C-8 alcohol
6 produced the fastest and cleanest separation with the greater
7 number of ethoxylate units. The results of this study are
8 summarized in Table III.
9 In conclusion, the C-8 alcohol with six to eight ethoxylate
units appears to be the ideal surface active agent for this
11 process. This surfactant gave the best rate of recovery, a clean
12 separation of phases with no clinging clay/bitumen in the
13 water/bitumen interface. Additionally, this surfactant gave the
14 highest percentage of bitumen recovery with the least number of
process steps. However, great care must be taken to assure that
16 even this surface active agent is maintained at less than 0.5
17 percent, by volume, since even at three percent, by volume, this
18 surfactant produces a complete emulsion.
19 The action involved with this surfactant appears to be the
displacement or phase disengagement of the bitumen from the sand
21 grains with the water/surfactant solution. Importantly, the
22 absence of connate water between the sand grains and the bitumen
23 appears to be the primary requirement for the specificity of the
24 surface active agent particularly when this requirement is
coupled with the equally important requirement that the surface
2024519
Table III
Comparison of Carbon Atoms
in Alcohol Chain Length
with Number of Ethoxylate Units
% of Bitumen left
on sand after l
Alcohol Ethoxylate minute surfactant
Chain Units Results wash
C-8 6 Very large oil 14%
drops (1/4 in.)
Separation
completè in
30 seconds
C-8 8 Very large oil 18%
drops (1/4 in.)
Separation
complete in
30 seconds
C-15 7 Oil drops (1/8 in.) 33%
work way out for
4-5 minutes
C-lS 3 Oil drops (1/8 in.) 36%
without agitation
still coming out
after 10 minutes
,, i
- 202~519
1 active agent does not cause an emulsification of the bitumen into
2 the water phase.
4 The Preferred Embodiment
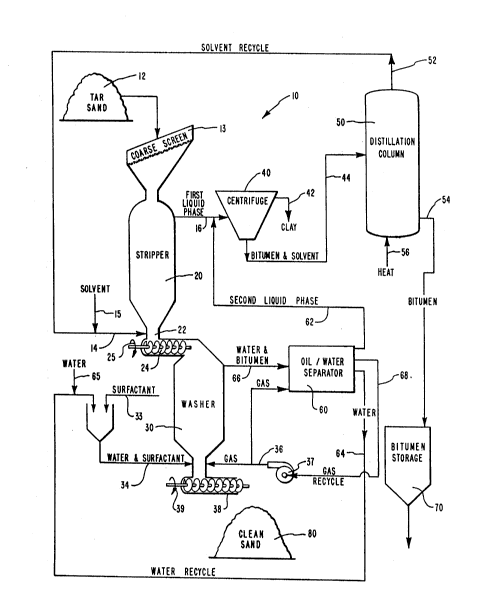
Referring now more particularly to the drawing, a schematic
6 of the basic elements of the novel progress of this invention is
7 shown generally at 10 and includes a stripper 20, a water washer
8 30, a centrifuge 40, a distillation column 50, an oil/water
g separator 60 and a bitumen storage 70. Tar sand 12 is introduced
into stripper 20 through a coarse screen 13 which prevents
11 excessively large pieces of tar sand 12 from entering stripper 20
12 where they could possibly damage an auger 24 at the bottom of
13 stripper 20. The lower end of stripper 20 is constricted by a
14 throat 22 which directs downwardly falling sand into auger 24.
Auger 24 is rotated as indicated by arrow 25 to transfer sand
16 from stripper 20 to water washer 30.
17 Solvent 14 is an organic solvent such as hexane, pentane,
18 gasoline, or the like, and is introduced into stripper 20
19 adjacent throat 22. Solvent 14 is added in the ratio of about 20
to 30% by weight of-Utah tar sand, and as such, becomes saturated
21 with bitumen upon vigorous agitation. Solvent 14 flows upwardly
22 through tar sand 12 where it not only breaks up the agglomerated
23 pieces of tar sand 12 but also dissolves bitumen which, in turn,
24 carries away clay that is interspersed in the bitumen. Solvent
14 dissolves the bitumen so that the resulting first liquid phase
26 16 includes bitumen dissolved in solvent and suspended clay.
17
-- 202451g
1 Stripped sand from stripper 20 is directed by auger 24 into
2 water washer 30 where it falls downwardly and is agitated by an
3 upwardly flowing stream of water/surfactant 34 and gas 36 from a
4 compressor 37. Gas 36 is selected from any suitable gaseous
medium that does not contain oxygen. Suitable gases include
6 nitrogen, carbon dioxide, methane, or the like. The combination
7 of water/surfactant stream 34 along with gas 36 creates a highly
8 agitated slurry of sand inside water washer 30. The result is
9 that residual bitumen is removed by water and surfactant 34 and
carried upwardly into an oil/water separator 60 as a
11 water/bitumen stream 66.
12 A surfactant 33 is mixed with water 32 to produce the
13 desirable ratio of surfactant to water in water/surfactant stream
14 34. Importantly, surfactant 33 is carefully selected from a
range of surface active agents so as to provide a surfactant that
16 is nontoxic to plant growth so that spent sand from this process
17 can be readily revegetated with no adverse environmental effects.
18 It is also important that surfactant 33 is biodegradable for the
19 same reasons. However, the most important feature of surfactant
33 is that it will not emulsify oil in water but is an oil
21 surfactant that helps displace the residual bitumen and bitumen-
22 solvent residue from the sand without emulsifying the bitumen.
23 Surfactant 33 also aids in the formation of bitumen/solvent
24 droplets within the water~surfactant phase 34. These droplets
are large enough that they will rise out of the sand and water
26 phase. The more active the surfactant 33 the larger the droplets
18
~o~519
1 and the faster the separation process.
2 The sequence involved in water washer 30 may be repeated
3 in additional stages if it is determined that a single pass
4 through water washer 30 is insufficient to remove residual
bitumen. An auger 38 at the base of water washer 30 is turned as
6 indicated by arrow 39 to remove clean sand 80 from water washer
7 30.
8 The mixture of gas and water/bitumen stream 66 is introduced
9 adjacent the lower end of oil/water separator 60 where it is
agitated by additional gas 36. Residual bitumen and solvent are
11 separated float to the surface where it is removed from oil/water
12 separator 60 as a second liquid phase 62. The second liquid
13 phase 62 joins the first liquid phase 16 where they are processed
14 by centrifuge 40. Water 64 is removed from oil/water separator
60 and recycled as water 34 back into water washer 30 after being
16 supplemented with make up water 65 and surfactant 33.
17 First liquid phase 16 is joined by second liquid phase 62
18 and they are both introduced into centrifuge 40. Suspended clay
19 is separated out as clay 42 while the resulting solvent/bitumen
stream 44 is directed to distillation column 50.
21 Distillation column 50 is a simple distillation process for
22 separating solvent 52 from bitumen 54. Heat 56 provides
23 sufficient thermal energy for distillation column to cause
24 solvent 52 to become separated from bitumen 54. Solvent 52 is
recycled back to stripper 20 where it is blended with make up
26 solvent 15, if any is required to compensate for solvent carried
19
202~51g
1 over with bitumen 54, to become solvent stream 14. Bitumen 54 is
2 directed to bitumen storage 50.
3 The following examples are merely illustrative of the
4 process of the present invention and are not intended to be
restrictive in any manner in setting forth the novel process of
6 this invention.
7 Example 1
8 Approximately one kllogram of tar sand obtained from the
9 Asphalt Ridge tar sand deposit near Vernal, Utah, was placed in a
two liter vessel. The tar sand was in rough chunks, each chunk
11 about 10 cm in diameter. Solvent in the amount of 210 grams was
12 added to the vessel after which the vessel was sealed. The
13 solvent completely disintegrated the chunks of tar sand in about
14 35 seconds without agitation. The resulting slurry was then
vigorously agitated by shaking the vessel after which the liquid
16 phase was decanted. The liquid phase consisted of 89 grams
17 solvent, 77 grams bitumen and 76 grams clay for a total weight of
18 242 grams.
19 Water (400 ml.) containing 0.5% (by weight) of the specially
selected surfactant of this invention was added to the sand
21 residue in the vessel. The vessel was shaken vigorously to
22 agitate the sand and water slurry. At this point the sand
23 appeared to be clean although there were some residual oil
24 droplets that worked their way out of the sand if the sand were
lightly agitated so as to remain loose. There were three
26 distinct, separable layers having very definite interfaces. The
2024519
l top layer consisted of solvent and bitumen with a limited
2 quantity of clay, a middle layer of water slightly cloudy from
3 small amounts of clay and a lower layer of sand containing a
4 limited amount free oil (bitumen/solvent).
The sand was washed again using an additional 100 ml of
6 water and agitated by bubbling a gas through the mixture. The
7 sand at this stage appeared clean and was analyzed to reveal that
8 at least 94.5% of the bitumen had been removed. The remaining
9 bitumen was present as small droplets interspersed in the sand
and could be removed by subsequent washing.
11 The solvent-rich bitumen from each of the prior steps was
12 combined and introduced into a centrifuge where it was processed
13 at 5,000 g's for lO minutes. The clay portion was removed
14 through this centrifugation process so that the bitumen (after
distillation to remove the solvent) contained only 0.7% clay (by
16 weight).
17 Example 2
18 To provide a preliminary indication of the effect of
19 temperature upon this process, all materials were cooled in ice
water so that the temperature of the process was between 4C and
21 6C. The same process was used as in Example 1, above. Large,
22 approximately 10 cm, chunks of tar sand (1058 grams) were used to
23 fill the stripper chamber. Solvent, 234 grams, was added to the
24 chamber where it took 2.3 minutes for the solvent to soak into
the chunks causing them to collapse into a stirrable slurry. The
26 container was agitated by shaking it for thirty seconds. A
21
2024519
1 portion (106 grams) of the solvent-bitumen-clay mixture was
2 removed and was found to contain 44 grams solvent, 34 grams
3 bitumen and 28 grams clay.
4 Chilled ice water (400 ml) containing 0.5~ (by weight) of
the specially selected, nonfoaming surfactant was added and the
6 vessel shaken vigorously for one minute. The sand phase was
7 relatively clean and contained numerous small oil droplets which
8 required three additional washing cycles to produce a clean sand
9 free of oil droplets. The bitumen was 94.7% (by weight) removed
during this experimental run. Importantly, the surface active
11 agent was carefully selected so as to disengage the bitumen from
12 the sand grains without emulsifying the bitumen into the water
13 phase.
14 The partial removal of solvent from the first step left
extra clay which could not be removed easily. The layer of
16 suspended clay particles was partially in the solvent/bitumen
17 phase and partially in the water phase. This clearly
18 demonstrates the importance of removing the major portion of the
19 clay with the solvent phase.
The foregoing combined liquid phases were removed from the
21 vessel and an additional aliquot of solvent was added to the sand
22 residue. The mixture was agitated by shaking slightly and in
23 about thirty minutes the remaining clay particles had settled to
24 the bottom resulting in a very clean separation between the
solvent-bitumen phase and the water phase. The water phase was
26 very cloudy due to the extra clay present. After several hours
22
- 202~519
without agitation the clay had settled out of the cold water
2 leaving a slightly cloudy water layer over a clean layer of clay
3 on the bottom.
4 The solvent-bitumen phases from the first and second steps
were combined and processed by centrifugation at 5000 g's for ten
6 minutes. The bitumen contained residual clay of 0.5% (by
7 weight).
8 Example 3
9 Using the same equipment, 1143 grams of 13% (by weight)
10 bitumen-content, Utah tar sands were contacted with 234 grams of
11 solvent. After 135 seconds the tar sand had collapsed into a
12 slurry in the absence of agitation. The mixture was then
13 vigorously agitated for one minute by shaking the vessel. The
14 solvent-bitumen-clay phase (215 grams) was decanted and analyzed
15 to contain (by weight) 57% solvent, 31% bitumen, and 12% clay.
16 Water (2100 ml) containing the carefully selected surface
17 active agent 0.5% (by weight) was added to the vessel and
18 agitated vigorously by shaking for one minute. Excellent
19 separation into clean sand and water was obtained upon standing.
20 Medium size oil droplets of solvent-dissolved bitumen were
21 observed interspersed in the sand. The bitumen droplets were too
22 heavy to float to the surface of the water. The addition of 40
23 grams of solvent gave the droplets of bitumen sufficient upward
24 mobility to float on the surface of the water. The resulting
25 layers of solvent-bitumen on top of the water layer had a very
26 clean separation. Importantly, the surface active agent was
2Q2~519
1 carefully selected so that it effectively disengaged the bitumen
2 from the Utah tar sand without emulsifying it into the water.
3 The oil/water mixture was poured off the sand and allowed to
4 stand to provide an excellent, separable interface. The
resulting solvent-bitumen phase contained (by weight) 56%
6 solvent, 37% bitumen, and 7% clay.
7 A second washing with 100 ml of ice water was shaken
8 vigorously with the sand and resulted in the medium-size oil
9 droplets being broken into numerous smail droplets. These small
droplets remained in the sand and did not float to the surface of
11 the water. The addition of 0.5 ml of the specific surface active
12 agent of this invention followed by shaking created the formation
13 of large droplets of oil. Light agitation of the sand using gas
14 bubbles allowed these large droplets to move out of the sand and
float to the surface where they could be skimmed from the water.
16 A repeat washing and agitation of the sand resulted in
17 additional oil being recovered. A third washing produced no
18 additional oil recovery. The bitumen recovery rate was 97% (by
19 weight). The solvent recovery was 88% since no steps were taken
to recover solvent carried over in the sand or to preclude losses
21 through evaporation.
22 The solvent used in this process was obtained from the
23 numerous natural gas wells in the vicinity of the tar sand
24 deposits. The solvent was condensate collected at the well head
and is frequently referred to as "casing head gas" or "drip gas".
26 This solvent consists generally of about 20% pentane, 70% hexane,
24
2~2~519
1 and 10~ heavier compounds (by weight) ranging between C5 to Cg
2 hydrocarbons.
3 The present invention may be embodied in other specific
4 forms without departing from its spirit or essential
characteristics. The described embodiments are to be considered
6 in all respects only as illustrative and not restrictive. The
7 scope of the invention is, therefore, indicated by the appended
8 claims rather than by the foregoing description. All changes
9 which come within the meaning and range of equivalency of the
claims are to be embraced within their scope.
11
12
13
14
16
17
18
19
21
22
23
24
26