Note: Descriptions are shown in the official language in which they were submitted.
HOECHST A~TIENGESELLSCHAFT - HOE 89/F 297 Dr.WE/St
2a2~7~
Description
Plant-protection agents containing N-arylhydrazones, and
novel N-arylhydrazones
The invention relates to ~afeners or antidotes which,
when combined with herbicides, can reduce the phytotoxic-
ity of the herbicides in crop piants.
The invention relates to plant-protection agents which
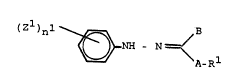
contain N-arylhydrazones of the formula (I) or ~alts
thereof
(Z1)nl ~ A Rl (I)
where
Z1 is halo~en, nitro, cyano or alkyll alkoxy, alkylthio
or cycloalkyl, the last four radicals mentioned
being unsubstituted or monosubstituted or polysub6-
tituted by alkoxy, hydroxyl or halogen, furthermore
is amino, mono- or dialkylamino, phenyl or phenoxy,
phenyl or phenoxy being unsubstituted or monosubsti-
tuted or polysubstituted by halogen or haloalkyl,
Rl is alkyl, alkenyl, alkynyl or cycloalkyl, the last
four radicals mentioned being unsubstituted or
monosubstituted Dr poly6ubstituted by radical6 from
the group comprising alkoxy, alkylthiot ~ono- or di-
alkylamino, alkoxycarbonyl, alkylcarbonyloxy, cyano
and halogen, i6 furthermore phenylalkyl or phenyl,
each of which is unsubstituted or monosl~stituted or
polys~b&tituted in t~.~ phenyl radical by r2dicals
from the group comprising alkyl, alkoxy, alkylthio,
mono- and dialkylamino, alko~ycarbonyl, cyano and
halogen,
nl is an integ~r from 0 to 5,
A i O or S,
B is B radical of the formula 2 ~ ~ s ~
-CO - R2 or ~ (Z2)n2
where
z2 has the meaning mentioned for 1, and
n2 is an integer from 0 to 5, and
R2 is OH or alko~y, alkenyloxy, alkynyloxy, alkylthio
or cycloalkoxy, the la~t five radicals mentioned
being unsubstituted or mon~ub~tituted or polysub-
stituted by radicals from the group comprising
alkoxy, alkylthio, mono- and dialkylamino, cyano and
halogen,
is furthermore trialkylsilylmethoxy, a radical of
the formula
-N- ~ or -C ~ R
R O
where in each case R is hydrogen or al~yl, Z3 has
the meaning mentioned for zl, and n3 i~ an integer
from 0 to 5,
R2 is furthermore mono- or dialkylamino, cycloalkyl-
amino, pyridino, morpholino, dimethylmorpholino, a
radical of the formula
R3
- O - N = C
R4
where R3 and R4 in~ependently of one another are
alkyl radicals, or R3 and R4 together with the carbon
atom linking them form a cycloalkyl radical,
in combination with customary auxiliaries for formula-
tions of plant protection agents.
In formula (I), al~yl, alkoxy and alkylthio radicals as
_ 3 _ 2.~
well as the corresponding unsaturated radicals can be in
each case straight-chain or branched. Halogen iB fluor-
ine, chlorine, bromine or iodine, preferably fluorine,
chlorine or bromine, in particular fluorine or chlorine.
In the case where R2 = OH, the compounds of the formula
(I) can form salt6. Salts which can be employed according
to the invention are those which can be u3ed in agricul-
ture. Examples of suitable salts are metal salt~ such as
alkali metal salts or ~lkaline earth metal salts, in
particular sodium or potassium ~alts, salt~ with am-
monium, mono-, di-, tri- or tetra-(Cl-C4)alkylammonium, or
with mono-, di-, tri- or tetra-(Cl-C4)alkanolammonium.
Formula (I) furthermore also embraces all stereoisomers
and mixtures thereof. Stereoisomers can occur mainly when
there is an asymmetrical carbon atom or suitably 6ub-
stituted double bonds in the formula (I).
Plant-protection agents according to the inv~ntion which
are of particular interest are those containing N-
arylhydrazones of the formula (I) or salts thereof,
where
Z~ radicals independently of one another are halogen,
nitro, ~yano, (Cl-C4)alkyl, (C1-C4)slkoxy, (Cl-C4)-
alkylthio, the alkyl, alkoxy and alkylthio groups
being unsubstituted or monosubstituted or polysub-
stituted by halogen atoms, in particular fluorine or
chlorine, or are (C3-C6)cycloalkyl which i5 unsub-
~tituted or substituted by (Cl-C4)alkyl, or are
amino, ( C,-C4 )alkylamino, di(Cl-C4)alkylAmino,
hydroxymethyl, (C1-C4)slkoxymethyl, phenyl or
phenoxy, phenyl and ~henoxy being unsubstituted or
monosubstituted or polysubstituted by halogen or
monosubstituted by trifluoromethyl or substituted by
one or more halogen substituent~ and one trifluoro-
methyl,
n1 is 0, 1, 2 or 3,
A is S or 0,
~ 4 ~ 2~f~ 37i~
Rl iS ( C3-C~ ) CyC loalkyl, (Cl-C6)alkyl, (C2-C6)alkenyl,
(C2-C6)alkynyl, the four radicals mentioned being
unsubstituted or monosubstituted or polysubstituted
by radicals from the group comprising (Cl-C2)alkoxy,
mono- or di(Cl-C6)alkylamino, (Cl-Csjalkoxycarbonyl,
(Cl-C6)alkylcarbonyloxy, (Cl-C2)alkylthio, cyano and
halogen, is ~urthermore phenyl (Cl-C6)alkyl or
phenyl, each of which is unsub6tituted or monosub-
~tituted or polysubstituted $n ~he phenyl radical
by radicals from the group compri ing (Cl-C4)alkyl,
(Cl-C4)alkoxy, (Cl-C4)alkylthio, mono- and di(C~-C6)-
alkylamino, (Cl-C6)alko~ycarbonyl, cyano and halogen,
B is a radical of the formula
-co-R2 or
(Z )n2
where Z2 has the meaning mentioned above for Zl and
n2 is 0, l, 2 or 3,
R2 is hydroxyl, -QCH2Si(CH3)3, (C3-C6)cycloalkoxy,
phenyl~Cl-C6)alkoxy, phenoxy, (~2-~6 ) alkenyloxy ,
(C2-C6)alkynyloxy,(Cl-C6)alkoxy,(Cl-C~)alkylthio,the
alkoxy or alkylthio group being un~ubstituted or
monosubstituted or disubstituted by (Cl-C2)alkoxy,
mono- or di(Cl-C6)alkylamino, (Cl-C2jalkylthio or
cyano, or monosubstituted or polysubstituted by
halogen, or
R2 i~ a radical of the formula
R ~ or -C ~ R
where in each case R i5 hydrogen or (Cl-C~)alkyl, Z3
has the meaning mentioned for z1, and n3 is o~ l~ 2
or 3,
R2 i8 furthermore mono- or ditCl~C4)alkylamino, (C5-C6)-
cycloalkylamino, piperidino, morpholino or 2,6-
5 - ~ ~ 2 ~
dimethylmorpholino or 8 radical of the formula
R3
- O-N = C ~
~ R4
where
R3 and R4 independently of one annther are (Cl-C4)-
alkyl, or R3 and R4 together with the carbon atom
linking them form a 5-, 6- or 7-membered cycloalkyl
radical.
Preferred plant-protection agents according to the
invention containing N-arylhydrazones of the formula (I)
or salts thereof are those where
Z1 radicals independently of one another are halogen,
in particular fluorine or chlorine, nitro, (C1-
C4)alkyl, (Cl-C4)alkoxy or trifluoromethyl,
A is oxygen,
n1 is 0, 1 or 2,
R1 is phenyl which is unsubstituted or monosubstituted
to trisubstituted by radical~ from the (Cl-C4)alkyl
group, in particular methyl, (Cl-C4)alkoxy and
halogen, or is (Cl-C4)alkyl,
B is a radical of the formula
-CO - R2 or ~
(Z2)n2
where
R2 is h~droxy or ( C~-C4 )alkoxy,
Z3 iS halogen, in particular chlorine, or (Cl~Cq)~
alkyl, and
n3 is O ~ 1 or 2.
Some of the compounds of the formula (I) are known ~rom
scientific publications ~Bull. Chem. Soc. Japan 48, 365-
366 (1975), J. Chem. S~c. ~C) 368 (1970); Tetrahedron 28,
5903 (1972); Tetrahedron Lett. 31, 3211 (1972); Can. J.
- 6 - 2 Q 2 ~ ~ 7 ~
Chem. 91, 4115 (1973)). However, their ~afener effect has
not been recognized to date.
The present invention therefore also relates to the novel
compounds of the formula ~I) or salts thereof which have
not been described to date. These are the above-defined
compounds of the formula (I) or ~alts thereof, with the
exception of those compounds in which
a) Rl is unsub~tituted or sub6tituted phenyl,
A is oxygen and
B is a radical of the above-defined formula
~ ~Z2)n2
and with the exception of those compounds in which
b) Z1 is 4-CH3, 4-Br, 4-Cl, 4-NO2, 3-CH3,
n' is O or 1,
Rl is unsub~tituted phenyl, 4-methylphenyl or 3-
methylphenyl,
A is oxygen and
B is CO2C2H5-
The present invention al80 relates to a process for the
preparation of the novel compounds of the formula (I) and
fialts thereof, which comprises reacting a compound of the
formula (II)
~ -NH - N ~ (II)
(Zl)nl
where (Z)nl and B ha~e the meanings given above and ~al
is Br, Cl or I,
with alcohols or thiol of the formula ~A-Rl
where A and R1 have the abovementioned meanings, and with
a base ~uch as, for example, NaOEt, ROCzH5~ NH3 and
202~ ~' 7~3
NaOCOCH3.
Suitable solvents are unpolar organic solvents, for
example ethers ~uch as diethyl ether or THF, or the
alcohols themselves required for the reaction.
The compounds of the formula (II) are known from the
literature (for example Bull. Chem. Soc. Jpn 48 365-366
(1975)) or they can be prepared analogously to the known
compounds.
The compounds of the formula (I) reduce or prevent
phytotoxic secondary effects of plant protection agents,
in particular of herbicides, which can occur when these
ayents are employed in crops.
The compounds of the formula (I) can be applied in
succession or together with the herbicidally active sub-
stances. They are then capable of le~sening or completelyelLminating noxious secondary effects of the herbicides
in crop plants, without Lmpairing the effectiveness of
these herbicides against noxious plants.
By virtue of this, the field of application of conven-
tional plant protection agent~ can be widened to a very
considerable extent. Such compoundc which pos6ess the
property of protecting cr3p plants against phytotoxic
damageby herbicides are called "an idotes" or "6afenersN.
Examples of herbicides whose phytotoxic ~econdary effects
can be reduced by means of the compounds of the formula
(I) are carbamates, thiocarbamates, halo~ tanilîdes,
substituted phenoxy-, naphthoxy- and phenoxyphenoxy
~arboxylic acid derivative6 as well as heteroaryloxyphen-
oxycarboxylic acid derivati~es, such as quinolyloxy-,
quinoxalyloxy-, pyridyloxy-, benzoxazolyloxy- and ben-
zothiazolyloxy-phenoxy-carboxylic acid e~ters, and
furthermore dimedone oxime derivative~. Preferred com-
pounds amongst these are phenoxyphenoxy- and heteroaryl-
-- 8 --
20~!~,,7~3oxy-phenoxycarboxylic acid esters. Suitable es~ers 1n
this connection are, in particular, lower alkyl, alkenyl
and alkynyl esters.
The following herbicides may be mentioned by way of
example but without impo6ing any restriction:
A) Herbicides of the type of the (Cl-C4)alkyl, (C2-C~)-
alkenyl and ( C3-c4 )alkynyl phenoxyphenoxy- and heteroaryl-
oxy-phenoxycarboxylates, such as methyl 2-(4-(2,4-
dichlorophenoxy)phenoxy)propionate,
methyl 2-(4-(4-bromo-2-chlorophenoxy~phenoxy)propionate,
methyl 2-(4-(4-trifluoromethylphenoxy~phenoxy)propionate,
methyl 2-(4-(2-chloro-4-trifluoromethylphenoxy)phenoxy)-
propionate,
methyl 2-(4-~2,4-dichlorobenzyl)phenoxy)propionate,
ethyl 4-t4-(4-trifluoromethylphenoxy)phenoxy)pent-2-
enoate,
ethyl 2-(4-(3,5-dichloropyridyl-2-oxy)phenoxy)propionate,
propargyl 2-(4-(3,5-dichloropyridyl-2-oxy)phenoxy~propio-
nate,
ethyl 2-(4-(6-chlorobenzoxazol-2-yloxy)phenoxy)propion-
ate,
methyl 2-(4-(3~chloro-5-trifluoromethyl-2-pyridyloxy)-
phenoxy)propionate,
butyl 2-(4-(5-trifluoromethyl-2-pyridyloxy)phenoxy)pro-
pionate,methyl 2-(4-(3-fluoro-5-chloropyridyl-2-oxy)phenoxy)pro-
pionate,
propargyl 2-(4-(3-fluoro-5-chloropyridyl-2-
oxy)phenoxy)propionate,
methyl 2-(4-(6-chloro-2-quinoxalyloxy)phenoxy)propionate,
methyl 2-(4-(6-fluoro-2-quinoxalyloxy)phenoxy~propiona~e,
methyl 2-(4-(6-chl~ro-2-quinolyloxy)phenoxy)propionate,
B) Chloroacetanilide herbicides, such as
N-me*hoxymethyl-2,6-diethylchloroacetanilide,
N-(3'-methoxyprop-2'-yl~methyl-6-ethylchloroacetanilide,
N-(3-methyl-1,2,4-oxdiazol-5 ylmethyl)-2,6-dimethyl-
9 ~ ;7i~
chloxoacetanilide,
C) Thiocarbamates, such a~S-ethyl N,N-dipropylthiocarbamate or
S-ethyl N,N-diisobutylthiocarbamate
D) Dimedone derivatives, such as
methyl 3-(1-allylo~yimino)butyl-4-hydroxy-6,6~dimethyl-
2-oxocyclohex-3-enecarboxylate
2-tN-etho~ybutyrimidoyl)-5-(2-ethylthiopropyl)-3-hydroxy-
2-cyclohexen-1-one,
2-(N-ethoxybutyrLmidoyl)-5-(2-phenylthiopropyl)-3-hyd-
roxy-2-cyclohexen-1-one,
2-(1-allyloxyLminobutyl)-4-methoxycarbonyl-5,5-dLmethyl-
3-oxocyclohexenol,
2-(1-(3-chloroallyloxy)iminobutyl)-5-(2-ethylthio)-
propyl)-3-hydroxycyclohex-2-enone,
2-(1-(ethoxyimino)butyl)-3-hy~roxy-5-(thian-3-yl)cyclo-
hex-2-enone or
2-(1-ethoxyiminopropyl)-5-(2,4,6-trimethylphenyl)-3-
hydroxy-2-cyclohexen-1-one.
The ratio of ~afener : herbicide can vary within wide
lLmits, within the range of between 1 : 10 and 10 : 1, in
particular between 2 : 1 and 1 . 10. The amounts of
herbicide and safener which are ideal in each case depend
on the type of the herbicide used or on the safener used
as well as on the nature of the plant stand to be
treated, and they can be determined for each individual
case by appropriate experiments.
The ~afener~ are mainly employed in particular in cereal
crops (whe~t, rye, barley, oats3, rice, maize, sorghum,
but also in cotton, ~ugar beet, ~ugar cane and soya bean.
Depending on their properties, the ~afeners of the
formula ~I~ can be us0d for pre-treating the seed of the
crop plant (seed treatment)~ or ~hey can be incorporated
in the seed furrows prior to sowing, or u~ed together
with the herbicide prior to, or after, plant emergence.
Pre-emergence ~reatment includes both the ~reatment of
the area under cultivation prior to sowing and the
treatment of the areas under cultivation where seed has
been sown but growth of the crop plants has not yet tsken
place. Application together with the herbicide i6 prefer-
red. Tank mixes or readymixes can be employed for this
purpose.
The application rates which are required of the compounds
of the formula (I) can vary within wide limit~, depending
on the indication and the herbicide used, and they
generally vary between 0.01 and 10 kg of active ~ubstance
pex hectare.
The present invention therefore also relates to a method
of protecting crop plants against phytotoxic secondary
effects of herbicides, which comprises applying an
effective amount of a compound of the formula (I) before,
after, or simultaneou~ly with, the herbicide.
Moreover, the compounds according to the invention
exhibit growth-regulating properties in crop plants. ~hey
engage in the plant metabolism in a regulating manner and
can thus be employed for facilitating the harvest, for
example by provoking desiccation, abscission and stunted
growth. Moreover, they are also fiuitable for generally
monitoring and inhibiting undesired vegetative growth,
without at the same tLme destroying the plants. Inhibi-
tion of vegetative growth is very important in many
monocotyledon and dicotyledon crops since lodging can be
reduced, or completely inhibited, by this means.
The compounds of the formula (I) or a combination thereof
with one or more of the mentioned herbicides, or ~roups
of herbicides, can be formulated in various ways, depend-
ing on the given biological and/or physico~hemical
parameters. The following are examples of suitable
formulation types: wettable powders (WP), emul~ifiable
concentrates (EC), aqueous 801ution5 (SL~, concentra~ed
emulsions such as oil-in-water nnd water-in-oil emulsions
(EW), sprayable solutions or emulsion6, disper~ions based
on oil or water (SC), dusts (DP~, seed-treatment aqents,
absorption granules for ground and spreading application (FG), water-
dispersible granules (WG), ULV formulation~, micro-
capsules or waxes.
These individual types of formulation~ are known in
principle and are described, for example, in: Winnacker-
Kuchler, ~Chemische ~echnologie" tChemi~al Technology~
Volume 7, C. Hau~er Verlag Munich, 4th Ed. 1986; vsn
Valkenbur~, "Pesticides Formulations", ~arcel Dekker
N.Y., 2nd Ed. 1972-73; R. Marten~, "Spray Drying Hand-
book~, 3rd Ed. 1979, G. Goodwin Ltd. London.
The formulation auxiliaries reguired, such as inert
materials, surfactants, solvents and ~urther additives
are likewise kr.own and hre des~ribed, for example, in:
Watkins, ~Handbook of Insecticid Du t Diluents and
Carriers , 2nd Ed., Darland Books, Caldwell N.J.; H.v.
Olphen, "Introduction to Clsy Colloid Chemi~try"; 2nd
Ed., J. Wiley ~ Sons, N.Y.~ ~Solvents Guide~, 2nd Ed.,
Interscience, N.Y. 1950; ~cCutcheon~s, nDetergents and
Emulsifiers Annual", ~C Publ. Corp., Ridgewood N.J.;
Sisley and Wood, "Encyclopedia of Surface Active Agentsn,
Chem. Publ. Co. Inc., N.Y. 1964; Sch~nfeldt, ~Grenz-
fl~chenaktive ~thylenoxidaddukten ~Surface-active
Ethylene Oxide ~dduct6], Wi~. Verlagsge~ell., Stuttgart
1975; Winn~cker-RUchler, ~Chemi~che Technologie"
[chemical Technology], Volume 7, C. ~user Verlag ~unich,
4th Ed. 1986.
On the basi~ of these fonmulation~, it is al~o pos6ible
to prepare c~bin~tion~ with other pe~ticidally w tive
substance6, fertilizers ~nd/or grow~h regulators, for
example in the form of a readymix or as a tankmix.
Wettable powers ar~ preparation~ which are uniformly
- 12 - 2~ 3
dispersible in water and which, besides the active
substance, also contain wetting agen~s, for example
polyoxethylated alkylphenols, polyoxethylated fatty
alcohols, alkanesulfonates or alkylarylsulfonates, and
S dispersing agents, for example sodium ligninsulfonate,
sodium 2,2~-dinaphthylmethane-6,6~-disulfonate, sodium
dibutylnaphthalenesulfonate, or alternatively ~odium
oleoylmethyltaurinate, in addition to a diluent or inert
substance.
Emulsifiable concentrate~ are prepared by dis601ving the
active substance in an organic solvent, for exEmple
butanol, cyclohexan~ne, dimethylf~rmamide, xylene and
also higher-boiling aromatic compounds or hydrocarbons,
with the addition of one or more emulsifiers. Examples of
emulsifiers which can be used are. calcium salts of an
alkylarylsulfonic acid, such as Ca dodecylbenzenesul-
fonate, or non-ionic emulsifiers, such as fatty acid
polyglycol esters, alkylaryl polyglycol ethers, fatty
alcohol polyglycol ethers, propylene oxide/ethylene oxide
condensation products (for example block polymers), alkyl
polyglycol ethers, sorbitan fatty acid esters, polyox-
ethylene sorbitan fatty acid esters or polyoxethylene
sorbitol esters.
Dusting agents can be obtained by grinding the active
2S substance with finely divided ~olid sub6tance6, for
example talc or natural clay~, ~uch as kaolin, bentonite,
pyTophyllite or diatomaceous earth.
Adsorption granules for ground and spreadmg application can be
produced either by ~praying the active ~ubstance onto
adsorpti~e, granulated inert material or by applying
active substance concen~rates onto the xurface of car-
riers, 6uch as sand, kaolinites or granulated inert
material, by means of binders, for example polyvinyl
alcohol, sodium polyacrylate or, alternatively, mineral
oils. Suitable active substances can also be ~ranulated
in the manner which is conventional for the production of
- 13 - 2 ~ 2 ~ , 3 ~ ~3
~ fertilizer granules, if de ired in a mixture with fer-
tilizers.
In general, the agrochemical preparations contain 0.1 to
99 percent by weight, in particular 0.1 to 95% by weight,
of active substance of the formula (I) or of an antidote/
herbicide mixture of acti~e substance, 1 to 99.9~ by
weight, in particular 5 to 99.8% by weight, of a solid or
a liquid additive and 0 to 25% by weight, in particular
0.1 to 25~ by weight, of a surfactant.
The active substance concentration in wettable powders
is, for example, about 10 to 90~ by weight; the remainder
to 100% by weight comprises conventional formulation
components. In the ca~e of ~mulsifiable concentrates, the
active 6ubstance concentration can be about 1 to 80~ by
weight, preferably 5 to 80% by weight. Formulations in
the form of dusts usually contain 1 to 25% by weight,
preferably 5 to 20% by weight of active substance, spray-
able solutions about 0.2 to 25% by weight, preferably 2
to 20% by weight of active ~ubstance. In the case of
water-dispersible granules, the active ~ubstance content
depends partly on whether the active compound is liquid
or 601id. In general the water-di~persible granules have
a content of between 10 and 90% by weight.
In addition, the active ~ubstance formulations mentioned
contain, if appropriate, the adhesives, wetting agents,
disper~ing agentfi, emulsifiers, penetrant , ~olvents,
fillers or carriers which are conventional in each case.
For use, the formulations, present in commercially avail-
able form, are diluted, if appropriate, in a customary
manner, ~or e~.~mple using water in the case of wettable
powders, emulsiiiable concentrates, dispersion~ and
water-disper~ible granules. Preparations in the form of
dusts, or adsorption granules for gra md and spreadin~ application and
also sprayable ~olutions are u~ually not further diluted
with other inert substances before use.
- 14 _ 2~a~7~
The application rate required, of the compounds of the
formula (I), varies with the external conditions, ~uch
as, inter alia, temperature, humidity and the type of
herbicide used. It can vary within wide limits, for
example between 0.005 and 10.0 kg/ha or more of active
in~redient; preferably, however, it i8 between 0.01 and
5 kg/ha.
The examples which follow are intended to illustrate the
invention:
A. Formulation E~ample~
a) A dustinq agent i8 obtained by mixin~ 10 parts by
weight of a compound of ~he formula (I) and 90 parts
by weight of talc as inert ~ubstance, and comminut-
ing the mixture in a hammer mill.
b) A wettable powder which i~ readily disper~ible in
water is obtained by mixing 25 parts by weight of a
compound of the formula (I), 64 part~ by weight of
kaolin-containing guartz as the inert substance, 10
parts by weight of potas~ium ligninsulfonate and 1
part by weight of sodium oleoylmethyltaurinate as
the wetting and dispersing agent, and grinding the
mixture in a pinned disk mill.
c) A dispersion concentrate which i8 readily
di~persible in water is obtained by mixing 20 parts
2S by weight of a compound of the formula (I) with 6
parts by weight of alkylphenol polyglycol ether
(~Triton X 207), 3 part~ by weight of isotridecanol
polyylycol ether ~ EO) and 71 parts by weight of
paraffinic mineral oil (boiling range, for example,
about 255 to above 277C), and grinding the mixture
in a ball mill to a fineness of below S microns.
d) An emul~ifiable concentrate is obtained from 15
parts by weight of a compound of the formula (I), 75
~ 15 -
parts by weight of cyclohexanone as the solvent and
10 parts by weight of oxethylated nonylphenol a~ the
emul~ifier.
e) Water-dispersible granules are obtained by mixing
75 parts by weight of a compound of the formula I,
10 " of calcium ligninsulfonate,
5 " of sodium lauryl sulfate,
3 ~ of polyvinyl alcohol and
7 " of kaolin,
grinding the mixture in a pinned di6k mill, and
granulating the powder in a fluidized bed by spray-
ing on water as the granulation liquid.
f) Water-dispersible granules are also obtained by
homogenizing, in a colloid mill,
25 partC by weight of a compound of the formula ~I),
5 " of sodium 2,2'-dinaphthylmeth-
ane-6,6'-disulfonate,
2 ~ of ~odium oleoylmethyltaurinate,
1 part by weight of polyvinyl alcohol,
17 parts by weight of calcium carbonate ~nd
50 " of water,
precomminutin~ the mixture, 6ub5eguently grinding
the mixture in a bead mill, and atomizing and drying
the resulting suspen~ion in a spray tower by means
of a single-fluid nozzle.
B. Chemical e~2mple~
~thyl 2~ ~ethylph~nylo~)-2-(4-~itrop~enylhydrazono)-
acetate ~Example No. 42)
O.69 g of ~odium i~ dissolved in 100 ml of ab801ute
e~hanol. To thi~ there are added 3.24 g of 4 methylphenol
and stirring is continued for 15 minutes at room
- 16 ~ J ~
temperature. The mixture is subsequently cooled to 0C,
and 8.16 g of ethyl 2-chloro-2-(4-nitrophenylhydrazono)-
acetate are added. Stirring is continued for 18 hours at
room temperature. The product is filtered off with
~uction and extracted by stirring with ethyl acetate.
After drying, 7.2 g (70~ of theory) of product of melting
point 158~C are obtained.
Ethyl 2-phenylo~y-2-phenylhydrazonoacetate (~sample No.7)
To a ~olution of 0.68 g of sodium ethanolate in 40 ml of
absolute ethanol there i8 added 0.94 g of phenol, and the
mixture is stirred for 15 minutes. 2.26 g of ethyl 2-
chloro-2-phenylhydrazonoacetate are subsequently added at
0C, and stirring i8 continued for 18 hours at room
temperature. The product is filtered off with suction and
recrystallized from ethanol. In this manner, 1.7 g (60%)
of product of melting point 82-83C are obtained.
Ethyl 2-methoxy-2-(2,4-dichlorophenylhydrazono)acetate
(~xample No. 27)
To 147.8 g of ethyl 2-chloro-2-~214-dichlorophenylhydra-
zono)acetate in 100 ml of tetrahydrofuran there are added
dropwise at 0C 50.59 g of triethylamine. After 15
minutes at 0C, 40 ml of methanol are added, and the
mixture is stirred for S hours at room temperature. The
mixture is evaporated completely on a rotary evaporator,
and the product is subsequently chromatographed on silica
gel (eluent: n-heptane/ethyl acetate 1:1). In this
m~nner, 21.82 g ~15~ of theory) of product of melting
point 119-122C are obtained.
~thyl 2-etho y-2-l2,4-dichlorophenylhydrazo~o)acetate
(B~ampl~ 153
29.5 g of ethyl 2-~hloro-2-~2~4-dichlorophenylhydrazono)-
acetate and 8.2 g of sodium ~cetate are refluxed for 5
hours in 100 ml of 95% pure ethanol. The reaction mixture
is subsequently evaporated on a rotary evapor~tor, the
product is taken up in C~2Cl2, a~d the mixture i5 ex-
tracted with water. The orqanic phase i~ dried with MgS04
1 7 ~
and evaporated on a rotary evaporator. Subsequent column
chromatography gives 13.7 g of product of melting point
47 CC .
Table I which follows lists the abovementioned examples
together with further examples which can be prepared in
an analogous manner:
18 -- ~ r~
Table I
N ~ ( I )
(Z)nl A Rl
No. (Z)nl -A-Rl B ~5.p. C
2- CL - O- C6Hs CO2C2Hs
2 4-Cl
3 2,4-Cl
4 3,5-C
2, 6- C
6 4- N02 17 ~I
7 H ll It 82- 83
8 4- OCH
9 2- CH
2,4- (CH3)2
11 4- F 71
12 3- CF
13 2- Cl - O- C2H5
14 4- Cl l' ll
2,4-C12 ll ll 47
16 2,5-Cl
17 2,6-Cl
18 4- NO
1 9 H
4~ OCH3
21 2-CH3
22 2,4- (CH3)2
2 3 4- F
24 3- CF3 ~I ll
2 5 2- Cl _ O- CH3 C02C2H5
2 6 4- CL
27 2, 4- Cl2 ~ 119- 122
-- 19 -- ~ r/ ;_7
(Table I, continuation)
No. (Z)nl A R1 B M.p. C
_
28 3,5-C12 " ~
29 2,6-C12 " "
4-NO2 1~ ~
31 H " "
32 4-OCH3 " ~I
33 2-CH3 " "
34 2,4-(CH3)2
4-F " "
36 3-CF3 " n
37 2-Cl -O-C6H4-CH3(P)
38 4-Cl " "
39 2,4-C12 " "
3,5-C12 " "
41 2,6-C12 " "
42 4-NO2
43 H
44 4-OC~3 " "
2-CH3 " "
46 2,4-(CH3)2
47 4-F " "
48 3-CF3 " "
49 2-Cl -C2H5 -&6H5
4-Cl " "
51 2,4-C12 " "
52 3,5-C12 " "
53 2,6-C12 " "
54 4-NO2 " "
H " " 168-169
56 4-OCH3 " "
57 2-CH3 " "
- 20 -
(Table I, ~ontinuation)
No. (Z)nl A ~l B M.p. C
58 2,4-~CH3)2C2H5 -C6H5
59 4-F " "
3-CF3 " "
61 2-Cl O-C3H7(n) -CO2Et
62 4-Cl " "
63 2,4-C12 - ,.
64 3,5-Cl2 " "
2,6-Cl2 " "
66 4-NO2 " "
67 H " "
68 4-OCH3 " "
69 2-CH3 " "
2,4-(CH3)2
71 4-F " "
72 3-CF3 " "
73 2-Cl -OCH3 ~6H3-2,4-Cl2
74 4-C1 " "
2,4-Cl2 " "
76 3,5-Cl2 " "
77 2,6-Cl2 " "
78 4-NO2
79 H " "
4-OCH3 " "
81 2-CH3
82 2,4-(CH3)2
83 4-F " "
84 3-CF3 ~ " "
2-Cl -C2H5
86 4-Cl " n
-- 21 --
(Table I, continuation)
No. (Z)nl _A R1 B ~l.p. C
87 2, 4- C12 C2HS - C6H3- 2, 4~ C12
88 3, 5- C12 " "
89 ~, 6- C12 " "
9 O ~- NO2 " "
91 H " "
92 4- OCH3 " "
9 3 2 - CH 3 " "
94 2,4- (CH3)2
4-F n
96 3- CF
Biolt~gical E~amples
Example 1
Wheat and barley were grown in plastic pots in a green-
house until they had reached the 3-4 leaf tage, and then
treated in succession with the compounds according to the
invention and the herbicides te~ted, u~ing the post-
amergence method. For this purpo , the herbicides and
the compounds of the formula (I) were applied in the form
of aqueous ~upensions or emulsions, at an application
rate of water of 800 l/ha (converted~. 3-4 weeks after
the treatment, the plants were scored vi6ually for any
type of damage cau~ed by the herbicides applied, the
extent of sustained growth inhibition, in particular,
being taken into account. The asses~ment wa~ expressed in
percentages in comparison with untreated controls.
The results from Table II illustrate that the compounds
according to the invention are capable of effecti~ely
reducing ~evere herbicidal damage on the crop plants.
2P~ ?i
- 22 -
Even when fsr too high doses of the herbicide H had been
applied, severe damage which occurs in the crop plants is
markedly reduced, and lesser damage is eliminated com-
pletely. Mixtures of herbicides and compounds according
to the invention are therefore outstandingly suitable for
selectively controlling weeds in cereal crops.
Table II
Safener action of the compounds according to the inven-
tion
Example No. Dose in kg Damage to crop plants (%)
of a.i./ha TRAE HOW
, _
H 2.0 75
0.2 - 80
H + 3 2.0 + 2.5 40
0.2 + 2.5 - 35
H ~ 27 2.0 + 1.0 0
2.0 + 0.25 10
0.2 ~ 1.0 - 20
0.2 + 0.25 - 22
Abbreviations:
TRAE - Triticum aestivum
HOW = Hordeum ~ulgare
a.i. = active ingredient5 H = ethyl 2-(4-(6-chlorobenzoxazol-2-yloxy)phenoxy
propionate (Fenoxaprop-ethyl)