Note: Descriptions are shown in the official language in which they were submitted.
2 2 ~
-- 1 --
SLURRY_HYDROTREATING PRO OESS
BACKGROUND OF IIHE INVENTION
This invention relates to the use of a
catalyst slurry for hydrotreating heavy fossil fuel
feedstocks such as vacuum gas oils or heavy gas oils.
High catalyst activity is maintained by circulating the
catalyst between a hydrotreating zone and a hydrogen
stripping reactivation zone.
The petroleum industry employs hydrotreating
to process heavy vacuum gas oils, particularly coker
gas oils, in order to improve their quality as fluid
catalytic cracker (FCC) feeds. Hydrotreating accom-
plishes the saturation o~ multi-ring aromatic compounds
to one-ring aromatics or completely saturated
naphthenes. This is necessary to assure low coke and
high gasoline yields in the cat cracker. Multi-ring
aromatics cannot be cracked effectively to mogas and
heating oil products, whereas partially hydrogenated
aromatics or naphthene~ can be cracked to premium
products. Hydrotreating is further capable of removing
sulfur and nitrogen which is detrimental to the crack-
ing process.
Hydrotreating employs catalysts that tend to
become poisoned by organic nitrogen compounds in the
feed. Such compounds become adsorbed onto the catalyst
and tie up naeded hydrogenation sites due to the slow
kinetics or turnover for hydrodenitrogenation. Higher
temperatures may be utilized to overcome this problem.
However, at high temperatures thermodynamic equllibrium
2~252~0
tends to favor the preservation o~ undesirable multi-
ring aromatic compounds.
It is an object of the present invention to
circumvent both the kinetic and equilibrium limts
encountered in conventional hydrotreating processes
which employ fixed bed catalysts. It is a further
object of the present invention to provide an improved
hydrotreating process employing a catalyst slurry. It
is a still further ob~ect of the present invention to
accomplish reactivation of the catalyst employed in the
present process by hydrogen s~ripping the catalyst in
an essentially continuous cyclic process.
In comparison to the present process, hydro-
gen stripping with a conventional fixed bed reactor has
been found to provide only a temporary gain in catalyst
activity, which gain is guickly lost in a few days.
Therefore, frequent and expensive shut downs would be
required for hydrogen strippin~ to be effective in a
fixed bed hydrotreating process.
Hydrotreating processes utilizing a slurry of
dispersed catalysts in admixture with a hydrocarbon oil
are generally known. For example, Patent No. 4,557,821
to Lopez et al discloses hydrotreating a heavy oil
employing a circulating slur~y catalyst. Other patents
disclosing slurry hydrotreating include U.S. Patent
Nos. 3,297,5Ç3; 2,912,375; and 2,700,015.
Various problems in operating the slurry
processes disclosed in the prior art have apparently
hindexed commercialization. For example, according to
the process disclosed in Patents Nos. 4,557,821;
2~2~2~
-- 3
2,912,375 and 2,700,015, it is necessary to reactivate
the catalyst by air oxidation. ~owever, air oxidation
is expensive since depressurization of th~ catalyst
environment between the hydro~reating reactor and the
reactivator, requiring expensive locX hoppers, is
necessary before combusting of the contaminants on the
catalyst. Furthermore, expensive equipment is neces-
sary to avoid air contamination and possible explo-
sions.
BRI F DESCRIPTION OF THE INVENTION
The present invention is directed to a method
of maintaining high catalyst activity in a slurry
hydrotreating process for heavy fossil fuels wherein a
hydrotreating catalyst of small particle size is
contacted with heavy petroleum or synfuel stocks for
hydrogenation of heavy aromatics and removal of nitro-
gen and sulfur. The catalyst is circulated between a
hydrotreating reaction zone and hydrogen stripping
reactivation zone.
These and other objects are accomplished
according to our invention, which comprises:
(1) reacting the heavy fossil fuel in a hydro-
treating zone with hydrogen in the presence
of a hydrotreating catalyst;
(2) separating the catalyst from the product of
the hydrotreating zone;
2~'2522~
-- 4 --
(3) reactivating the catalyst in a reactivation
zone by subjecting the same to hydrogen
stripping; and
(4) recycling the reactivated catalyst to the
hydrotreating zone.
BRIEF rDESCRIPTION OF THE DRAWINGS
The process of the invention will be more
clearly understood upon reference to the detailed
discussion b~low upon reference to Fig. 1 (Sole Fig.)
which shows a schematic diagram of one process scheme
according to this invention comprising a slurry hydro-
treating step and hydrogen reactivation stripping step.
DETAILED DESCRIP~ION OF THE INVENTION
Applicants' process is directed to a slurry
hydrotreating process in which the ca~alyst used in a
hydrotreating zone is reactiuated by hydrogen stripping
in a cyclic, preferably continuous process.
The catalyst is reactivated in a separate
reactivation zone and recycled back to the hydro-
treating zone. In additionj fresh or reactivated
(regenerated) catalyst can be continually added while
aged or deactivated catalyst can be purged or reacti-
vated. Because the catalyst is being regularly reacti-
vated according the present process, the slurry hydro-
treating step can be operated at more severe conditions
(which otherwise tend to deactivate th~ catalyst) than
used in conventional fixPd bed hydrotreating. A
conventional fixed bed hydrotreater typically operates
202~22~
5 --
for about ~ or 2 years before it is necessary to shut
it down in order to replace the catalyst. An advantage
of the present slurry process in combination with
catalyst reactivation is increased activity o~ the
catalyst compared to a fixed bed.
It is noted that the permanent deactivation
of the catalyst which occurs in conventional fixed bed
hydrotreating is reduced in the present hydrotreating
process by hydrogen reactivation. This permanent
deactivation is believed to occur by the presence of
coking, resulting ~rom polymerization reactions and
metal deactivation, caused by the presence of organic
metal compounds present in the feedstocks. These
polymerization reactions are prevented by periodic
hydrogen reactivation which trips adsorbed feed from
the catalyst.
The slurry hydrotreating process of this
invention can be used to treat various feeds including
fossil fuels such as heavy cataiytic cracking cycle
oils (HCC0), coker gas oils, and vacuum gas oils (VG0)
which contain significant concentrations of multi-ring
and polar aromatics, particularly large asphaltenic
molecules. Similar gas oils derived from petroleum,
coal, bitumen, tar sands, or shale oil are suitable
feeds.
Suitable feeds for processing according to
the present invention include those gas oil fractions
which are distilled in the range of 500 to 1200F,
pre~erably in the 650 to 1100F range. Above 1200F it
is difficult or impossible to strip all of the feed off
the catalyst with hydrogen and the catalyst tends to
2Q2~2~
coke up. Also, the presence of concarbon and
asphaltenes deactivate the catalyst. The feed should
not be such that more than 10% boils above 1050F. The
nitrogen content is normally greater than 1500 ppm. The
3+ ring aromatics content of the feed will generally
represent 25% or more by weight. Polar aromatics are
generally 5% or more by weight and concarbon con-
stitutes 1% or more by weight.
Suitable catalysts for use in the present
process are well known in the art and include, but are
not limited to, molybdenum (Mo) sulfides, mixtures of
transition mçtal sulfides such as Ni, Mo, Co, Fe, Wl
Mn, and tha like. Typical catalysts include NiMo, CoMo,
or CoNiMo combinations. In general sulfides of Group
VII metals are suitable. (The Periodic Table of
Elements referred to herein is given in Handbook_ of
ChemistrY and Physics, published by the Chemic~l Rubber
Publishing Company, Cleveland, Ohio, 45th Edition,
1964.) These catalyst materials can be unsupported or
supported on inorganic oxides such as alumina, silica,
titania, silica alumina, silica magnesia and mixtures
thereof. Zeolites such as USY or acid micro supports
such as aluminated CAB-O-SIL can be suitably composited
with these supports. Catalysts formed in situ from
soluble precursors such as Ni and Mo naphthenate or
salts of phosphomoly~dic acids are suitable.
In general the catalyst material may range in
diameter from 1 ~ to 1/8 inch. Preferably, the cata-
lyst partiales are 1 to 400 ~ in diameter so that intra
particle diffusion limitations are minimized or elimi-
nat~d during hydrotreating.
2~2522~
In supported catalysts, transition metals
such as Mo are suitably present at a weight percent o~
5 to 30%, preferably 10 to 20%. Promoter metals such
as Ni and/or Co are typically present in the amount of
1 to 15%. The surface area is suitahly about 80 to 400
m2/g, preferably 150 to 300 m2/g.
Methods of preparing the catalyst are well
known. Typically, the alumina support is formed by
precipitating alumina in hydrous form from a mixture of
acidic reagents in an alkaline aqueous aluminate
solution. A slurry is formed upon precipitation of the
hydrous alumina. This slurry is concentrated and
generally spray dried to provide a catalyst support or
carrier. The carrier is then impregnated with cataly-
tic metals and subsequently calcined. For example,
suitable reagents and conditions for preparing the
support are disclosed in U.S. patents Nos. 3,770,617
and 3,531,398, herein incorporated by reference. ~o
prepare catalysts up to 200 microns in average dia-
meter, spray drying is generally the preferred method
of obtaining the final form of the catalyst particle.
To prepare larger size catalysts, for example about
1/32 to 1/~ inch in average diameter, extruding is
commonly used to Xorm the catalyst. To produce cata-
lyst particles in the range of ~00 ~ to 1/32 inch, the
oil drop method is preferred. The well known oil drop
method comprises forming an alumina hydxosol by any of
the teachings taught in the prior art, for example by
reacting aluminum with hydrochloric acid, combining the
hydrosol with a suitable gelling agent and dropping the
resultant mixture into an oil bath until hydrogel
spheres are formed. The spheres are then continuously
withdrawn from the oil bath, washed, dried, and
- 8 - ~ ~2~220
calcined. This treatment converts the alumina hydrogel
to corresponding crystalline gamma alumina particles.
They are then impregnated with catalytic metals as with
spray dried particles. See for example, U.S. Patents
Nos. 3,745,112 and 2,620,314.
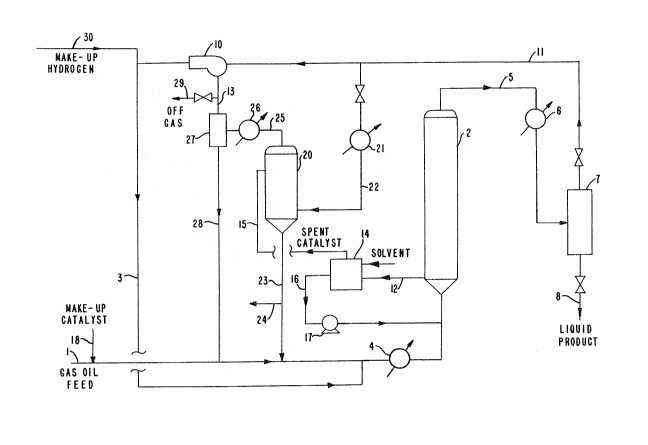
Referring to FIG. 1, a feed stream 1, con-
sisting for example of gas oil feed, is introduced into
a slurry hydrotreating reactor 2. Before being passed
to this reactor, the feedstream is typically mixed with
a hydrogen containing gas in stream 3 and heated to a
reaction temperature in a furnace or pr~heater 4. A
make-up hydrogen stream 30 may be introduced into the
hydrogen stream 3, which in turn may be either com-
bined with the feed stream or alternatively mixed in
the hydrotreating reactor 2. The hydrotreating reactor
contains a catalyst in the form of a slurry at a solids
weight percent of about 10 to 70 percent, preferably 40
to 60 percent. In the embodiment shown in the ~igure,
the feed enters through the bottom of the reactor and
bubbles up through an ebulating or fluidized bed.
Depending on the size o~ the catalyst parti-
cles, the hydrotreating reactor may have filters at the
entrance and/or exit orifices to keep the catalyst
particles in the reactor. Alternatively, the reactor
may have a flare (increasing diameter) conPiguration
such that when the reactor is kept at minimum fluidi-
zation velocityj the catalyst particles are prevented
from escaping through an upper exit orifice.
Although a single slurry hydrotreating
reactor may be used in the present process, it is
preferred for greater efficiencies that the slurry
2 2 ~
g
hydrotreating process be operated in two or more
stages, as disclosed in copending U.S. Application No.
414,175, hereby incorporated by re~erence. Accord-
ingly, a high temperature stage may be followed by one
or more low temperature stages. For example, a two
stage process might process fresh feed in a 760F stage
and process the product from the first stage in a 720F
stage. Alternatively, several stages can be operated
at successively lower temperatures, such as a 780F
stage followed by a 740F stage followed by a 700~F
stage. Such an arrangement provides fast reaction
rates in the first stage and lower e~uilibrium multi-
ring aromatics levels (hence greater kinetic driving
forces) in the final stage or stages. Staging is espe-
cially advantageous in the present slurry process as
compared to a fixed bed process because the initial
stages can be operated at higher temperatures, heat
transfer is better and diffusion does not limit reac-
tion rates.
Referring again to FIG. 1, an effluent from
the hydrotreating reac~or 2, containing liquids and
gases and substantially no catalyst solids, is passed
via stream 5 through a cooler 6 and introduced into a
gas-liquid separator or disengaging means 7 where the
hydrogen gas along with ammonia and hydrogen sulfide
by-products from the hydrotreating reactions may be
separated from the liquid product in stream 8. The
separated gases in stream 11 are recycled via com-
pressor 10 back for reuse in the hydrogen stream 3.
The recycled gas is usually passed through a scrubber
to remove hydrogen sulfide and ammonia because of their
inhibiting effects on the kinetics o~ hydrotreating and
also to reduce corrosion in the recycle circuit.
2 2 ~
-- 10 --
In many cases, the liquid product in stream 8
is given a light caustic wash to assure complete
removal of hydrogen sulfide. Small quantities of
hydrogen sulfide, if left in the product, will oxidize
to free sulfur upon exposure to the air, and may cause
the product to exceed pollution or corrosion specifi-
cations.
In ordex to reactivate the catalyst in the
hydrotrea~ing reactor 2, an exit stream containing
catalyst solids is removed from the reactor as stream
12 and enters a separator 14, which may be a filter,
vacuum flash, centrifuge, or the like to divide the
effluent into a catalyst stream 15 and a liquid stream
16 for recycle via pump 17 to the hydrotreating
reactor 2.
The catalyst stream 15 from separator 14
comprises suitably 30 to 60 percent catalyst. Option-
ally this catalyst stream may be diluted with a lighter
liquid such as naphtha to fluidize the catalyst and aid
in the transport of the catalyst, while permitting easy
separation by dis~illation and recycle. In any case,
the catalyst material is transported to the stripper
reactor or reactivator 20. A hydrogen stream 22,
pre~erably heated in heater 21, is introduced into
reactivator 20 where the catalyst is hydrogen stripped.
The reactivator yields a reactivated catalyst stream
23 for recycle back to the hydrotreating reactor 2.
Spent catalyst may be purged from stream 23 via line 24
and fresh make-up catalyst introduced via line 18 into
the feed stream. The reactivated catalyst from the
202~22~
reactivator 20 is suitably returned to the hydro-
treating reactor 2 at a rate of about 0.05 to 0.50 lbs
reactivated catalyst to lbs gas oil feed, preferably
0.1 to 0.3.
The reactivator 20 also yields a top gas
stream 25 which is subsequently passed through cooler
26, gas-liquid separator 27 and via stream 13 combined
with the hydrogen recycle stream 11. Off gas may be
purged via line 29. Stripped liquids from tha separa-
tor 27 may be returned to the hydrotreater reactor 2
via stream 28.
The process conditions in the process depend
to some extent on the particular faed being treated.
The hydrotreating zone of the reactor is suitably at a
temperature of about 650 to 780F, preferably 675 to
750F and at a pressure of 800 to 4000 psiy, preferably
1500 to 2500 psig. The hydrogen treat gas rate is 1500
to 10,000 SCF/B, preferably 2500 to 5000 SCF/B. The
space velocity or holding time (WHSV, lb/lb of cata-
lyst-hr) is suitably 0.2 to 5.0, preferably 0.5 to 2Ø
The reactivating zone is suitably maintained
at a temperature of about 650 to 780F, preferably 675
to 750~F, and a pressure of about 800 to 4000 psig,
preferably 1500 to 2500. The strip rata (SCF, lb
catalyst-hr) is suitably about 0.03 to 7, preferably
0.15 to 1.5.
EXAMPLE 1
To illustrate a slurry hydrotreating process,
according to the first step of the present invention,
2~2~2~
- 12 -
the following experiment was conducted. A commercial
hydrotreating catalyst, KF-840, was crushed and
screened to 32/42 mesh size. Catalyst properties are
shown in Table I. This crushed catalyst was then
sulfided overnight using a 10% H2S in H2 gas blend. A
10.3 gram sample of the presulfided catalyst was added
to a 300 cc stirred autoclave reactor along with 100
cc's of a heavy feed blend comprised of heavy vacuum
gas oils, heavy coker gas oils, coker bottoms and heavy
cat cracked cycle oil. Properties of the feed are
listed in Table II.
Table I
Catalyst Properties
Nio, Wt% 3.8
MoO3, Wt% 19.1
P20s, Wt% 6.4
Surface Area, m2/gm 175
Pore/volume, cm3/gm 0.38
2~2~22~
- 13 -
Table II
Feedstock ProPerties
Sulfur, Wt% 1.63
Nitrogen, Wt% 0.39
Carbon, Wt% 87.63
Hydrogen, Wt% 9.60
Gravity, API 9.2
Wt% Aromatics by HPLC
Saturates ~6
1 Ring g
2 Ring 10
3+ Ring 43
Polar Aromatics 12
GC Distillation, F
5% 665
~0% 753
50% 882
80% 1004
95% 115~
The autoclave was heated to 720F under 1200
psig hydrogen pressure. The autoclave was operated in
a gas flow thru mode so that hydrogen treat yas was
added continuously while gaseous products were taken
off. Hydroqen was added over the course o~ the run so
that the initial hydrogen charge plus make-up hydrogen
was equivalent to 3500 SC~/B of liquid charged to the
autoclave. After two hours at reaction conditions, the
autoclave was quenched or cooled quic~ly to stop
reactions. The autoclave reactor was de-pressured and
the catalyst was filtered from the liquid products.
These products were then analyzed to determine the
sxtsnt o~ HDS (hydrodesulfurization), HDN (hydro-
denitrogenation), and aromatics hydrogenation. The
results are shown in Table III below.
2~2522~
- 14 -
In another run, at a higher catalyst loading,
a 30.9 gram of the same presulfided catalyst was added
to a 300 cc sample stirred autoclave reactor along with
100 cc's of the same heavy feed blend. The autoclave
was run as the same conditions as in the previous
experiment. The results of this run are also shown in
Table III.
Table III
Slurry Catalyst Loading
and Feed Fresh, Sulfided Fresh, Sulfided
Product QualityProperties Catalyst Catalyst
Slurry Catalyst Loading
Wt% Catalyst on FF. 0 10.5 31.5
Slurry Product Quality
Wt% Sulfur 1.63 0.32 0.10
Wt% Nitrogen 0.39 0.22 0.093
Wt% Sats + lR AR 34 55 66
Wt% 3+ R AR & Polars 55 28 18
Wt% Polar AR 12 4.1 1.2
From these results, it can be concluded that
the fresh catalyst slurry was very effective for
removing organic ~ulfur and organic nitrogen compounds
from the heavy feed blend. With only 10% catalyst on
fresh feed (FF), only 20% of the organic sulfur, 55% of
the organic nitrogen, and half the 3+ ring aromatics
contained in the raw feed remained. Only a third of
the heavies~/ polar aromatic compounds remained. With
a higher catalyst loading, 31~ on fresh feed, even
higher levels of contaminant removal were obtained.
Only 6% of the organic sulfur, a fourth of the organic
nitrogen, and a third of the heavy aromatics remained.
Polar aromatics were reduced to 10% of the feed valuQ.
202~22~
-- 15 --
EXAMPLE 2
-
To illustrate the second step of the inven-
tion, involving hydrogen catalyst reactivation, the
following experiment was conducted. Catalyst dis-
charged from an autoclave experiment at the same
conditions of the first two runs o~ Example 1 was
stripped with an H2S/H2 blend for 18 hours at 650F.
After hydrogen stripping, the catalyst disc~arged from
the first autoclave pass was laden with 3.6% "coke" or
adsorbed hydrocarbons. A 32.0 gm sample of this coke
laden catalyst, containing 30.9 gms of the NiMo/alumina
catalyst was charged to a 300 cc autoclave with 100
cc's of the same feed used in Experiment 1. The
autoclave was run at the same conditions as Experiment
1. The catalyst was filtered from the products and
hydrogen stripped again for use in a subsequent run.
This procedure was repeated until the product analyses
had leveled off. Product analyses are shown in Table
IV.
Catalyst discharged from an autorlave run at
the same conditions as in Experiment 1 was filtered and
charged to the autoclave with the same feed as the
previous runs. The same filtered catalyst was recycled
in the autoclave several times in order to line out
catalyst performance. The results of these runs are
shown below.
2~2~220
- 16 -
Table IV
Recycled,
Slurry Catalyst Loading Hydrogen Recycled,
and Stripped Filtered
Product Quality Catalyst Catalyst
Slurry Catalyst Loading
Wt~ Catalyst on FF 31.5 31.5
Slurry Product Quality
Wt~ Sulfur 0.10 0.12
Wt% Nitrogen o.os3 0.18
Wt~ Sats + lR AR 64 61
Wt% 3+ R AR ~ Polars 18 23
Wt~ Polar AR 1.2 2.7
From the above results, it can be concluded
that the recycled catalyst was still highly active for
nitrogen and sulfur removal, as well as aromatics
hydrogenation. Although, catalyst activity for HDN and
heavy aromatics removal were diminished somewhat,
hydrogen stripping restored catalyst to nearly fresh
activity.
EXAMPLE 3
To further illustrate a hydrogen stripping
catalyst reactivation process, the following experiment
was conducted. Another lot of the same commercial
catalyst used in the previous experiments was used in a
fixed bad reactor for several hundred hours on oil.
Prior to discharging, the catalyst was stripped with
hydrogen at 700F for several hours. After the cata-
lyst was discharged from a fixed bed reactor, a portion
of it was crushed and screened to 32/42 mesh size.
This catalyst was laden with 21.2% coke or adsorbed
~02a2~
- 17 -
hydrocarbons. A 39.2 gram sample of this coked cata-
lyst, containing 30.9 grams of NiMo/alumina catalyst,
was charged to the autoclave with the same feed as the
previous examples. The catalyst was filtered from the
products and recycled in an autoclave run several times
in order to line-out catalyst performance. The results
of these runs with the hydrogen stripped, aged catalyst
and the filtered, aged catalyst are shown in Table IV.
Table IV
Hydrogen Recycled,
Slurry Catalyst Loading Stripped, Filtered,
and Aged Aged
Product QualityCatalyst Catalyst
Slurry Catalyst Loading
Wt% Catalyst on FF 31.5 31~5
Slurry Product Quality
Wt% Sulfur 0.20 0.25
Wt% Nitrogen 0.14 0.27
Wt~ Sats + lR AR 62 56
Wt% 3+ R AR ~ Polars 25 29
Wt% Polar AR 3.6 5.2
From the above results, it can be concluded that
although the hydrogen stripped catalyst was less active
than fresh, it was substantially more active than the
catalyst which was recycled without hydrogen stripping.
On the other handl without hydrogen stripping, the aged
catalyst lost much of its activity.
The process of the invention has been des-
cribed generally and by way of example with reference
to particular embodiments for purposes of clarity and
illustration only. It will be apparent to those
skilled in the art from the foregoing that various
2~2~22~
- 18 -
modifications of the process illustrated herein can be
made without departure from the spirit and scope of the
invention.