Note: Descriptions are shown in the official language in which they were submitted.
2~25'7~
C-4201
G-3414
PYROI,YSIS PROCESS AND APPARATUS
Field of the Invention
The present invention relates to a pyrolysis
reaction assembly and method for recovering an
inorganic component from a feed stream having mixed
organic and inorganic components.
Background of the Invention
Typically, polymer wastes or paint sludge
containing a substantial volume of an inorganic filler
component are disposed of via land fill. Disposal by
land fill, however, has created an increasing problem
because of lack of land fill space and ~he inability of
polymers to rapidly degrade at the land fill site. In
some states, paint sludge can not be land filled but
rather must be incinerated at much greater expense. A
known alternative to disposing of filled polymer waste
and paint sludge by land fill is to pyrolyze the
polymer or paint sludge to volatize the organic
component and thus, separate the organic component from
the inorganic component.
Pyrolysis is the thermal decomposition of
organic matter at temperatures sufficient to volatize
or gasify organic matter in the feed in the absence of
oxygen or any oxidizing agent. By using a pyrolysis
reaction, the inorganic components of the feed can he
separated from the organic components and collected for
re-use. The organic components are removed and
disposed of in an environmentally safe manner.
Pyrolysis reaction assemblies are known in the
prior art. In one such pyrolysis assembly, a feed
material is introduced to a pyrolysis reactor through a
- ~
~2~05
reactor inlet having a cooling jacket thereon for
preventing clogging of the inlet. The reaction chamber
is free of oxygen or an~ oxidizing agent and is heated
above the volatization point of any organic components
of the feed material by a furnace as~embly including a
burner. An impeller shaft within the reaction chamber
forces the inorganic and non-volatized residue to an
outlet. The volati~ed organic component is removed by
a vapor outlet and is further process~d. The processed
organic gas has,condensibles removed and is recycled
and used to fuel the burner used to heat the reaction
chamber. The inorganic residue having carbon residue
thereon is cooled and collected for re-use.
number of industries generate significant
quantities of paint sludge and/or filled plastics such
as a sheet molding compounds ~SMC), reinforced reaction
injection molding compounds (RRIM) and the reacting
precursors of the aforesaid. If the inorganic filler
component of such materials could be economically
recycled into substantially the same material from
`, which it emanated, substantial reductions in landfill
requirements and cost, as well as substantial
reductions in the cost of fresh filler materials would
result.
It is an object of the present invention to
provide a method and apparatus for continuously
pyrolytically decomposing the organic component of such
organic - inorganic mixtures as filled polymers and
paint sludge and recovering the inorganic component
thereof in a carbon-free state useful as filler in
substantially the same type of material from whence it
202~70~
emanated.
It is a further object of the present
invention to provide a continuous method and apparatus
by which a Eeed of mixed organic and inorganic material
can be pyrolitically decomposed into a recoverable
inorganic filler material free of any carbon or organic
residue and gaseous organic decomposition products
which can be disposed of in an environmentally safe
manner.
It is a still further object of the present
invention to provide an improved pyrolytic reactor
assembly which includes a means for preventing
pressurization of the reactor assembly during the
pyrolytic reaction.
Other objects and advantages of the present
invention will be readily appreciated as the same
becomes better understood by reference to the following
detailed description thereof considered in connection :
with the accompanying drawings wherein: :
Figures in the Drawings
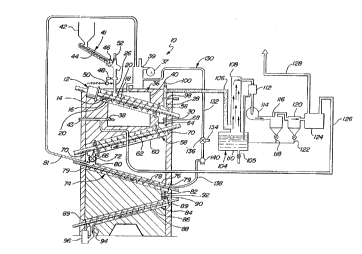
~ igure 1 is a schematic view of the pyrolysis
reaction assembly and materials recovery system of the
present invention; and
Figure 2 is an enlarged cross-sectional view
of the pyrolysis reaction assembly and gas treatment
portion of the materials recovery system.
Summary of the Invention
According to the present invention there is
provided a pyrolysis reaction apparatus of the type for
pyrolytic decomposition of a feed material having an
organic and an inorganic component so as to
.
2 ~
continuously recover the inorganic component in a
carbon-free form useful for recycling back into the
same type of material from whence it e~anated (i.e.,
SMC, RRIM, P~INT) as well as into other materials and
to produce useful decomposition products of the organic
component. The apparatus comprises reactor means ~or
pyrolitically decomposing the feed material into
gaseous decomposition products of the organic component
and a solid residue comprising principally the
inorganic component contaminated with carbon. The
reactor means has an entrance end for receiving the
feed material and an exit end for discharging the
residue. The assembly includes means for heating the
reactor means to a pyrolysis temperature suitable to
the decomposition of the feed material. Feeder means
is provided at the entrance end for supplying the feed
material to the reactor means. Conduit means is also
provided for exhausting the gaseous decomposition
products from the reactor means. Condenser means
associated with the conduit means is provided for
condensing combustible condensable liquids from the
gaseous decomposition products while permitting
combustible, substantially non-condensable, gases to
pass therethrough. Also provided is a means for
collecting the combustible liquid.
The assembly further comprises first cooling
means the entrance end of which is in communication
with the exit end of the reaction means for receiving
and cooling the exitin~ residue in a substantially
non-oxidizing environment to a superambient temperature
suitable for the oxidation o~ the carbon content of the
,: '
2~257~
residue without degradation of the inorganic component
of the residue sought to be recovered. The assembly
further includes oxidation means the entrance end of
which is in direct closed communication with the exit
end of the first cooling means for continuously
receiving the residue from the first cooling means and
reacting the carbon thereof with oxygen to form gaseous
carbon-dioxide and liberate the same from the inorganic
component. The oxidation means has an inlet end for
receiving the residue from the first cooling means and
an outlet end for discharging carbon~free inorganic
components.
The assembly further includes means between
the exit end of the first cooling means and the inlet
end of the oxidation means for transferring the
residue to the oxidation means including means
associated therewith for isolating the cooling means
against invasion of gases emanating from the oxidation
means. The assembly further includes second cooling
means in communication with the outlet end of the
oxidation means for continuously receiving and cooling
the carbon-free inorganic component exiting from the
oxidizer. The assembly further includes means for
collecting the inorganic component for re-use.
Detailed Description of the Drawin~s
A pyrolysis reaction assembly of the type for
pyrolitically decomposing a feed material having an
organic and an inorganic component so as to
continuously recover the inorganic component,
carbon-free, and to produce useful decomposition
products of the organic component is generally shown at
10 in the figures.
The assembly 10 includes a reactor asse~bly
generally indicated at 12 for pyrolitically decomposing
a feed material having organic and inorganic components
into gaseous decomposition products of the organic
component and a solid residue. The solid residue
comprises principally the inorganic component of the
feed which compon~nt is contaminated with carbon.
The feed materials to be pyrolitically
decomposed are filled thermoset/thermoplastic polymers
`~ or paint sludge all of which have a mixed organic
component and an inorganic filler material (e.g., glass
reinforced). Examples of such polymer materials are
: reinforced reaction injected molded (RRIM) thermoset
~ 15 plastic materials which have an organic component that
;` comprises substantially urethanes; unreacted precursors
of the RRIM polymer i.e. polyols and isocyanates; waste
sheet molding compounds (SMC~ which have an organic
,:
component that comprises styrene cross-linked
polyesters; uncured SMC reactants ~commonly called
.~ "purged SMC") comprising styrene and unsaturated
polyesters; and such epoxies, phenolics, silicates,
melamines, diallylphthalates, and polyimides as are
; typically used in reinforced plastics. Paint sludge
has an organic component that may comprise acrylics,
polyesters or urethane, and solvents and cross-linkers
~ therefor.
.~ When the eed comprises SMC, the reusable
inorganic filler component typically comprises calcium
carbonate (CaCo3) and glass which typically comprises
about 48 weight percent and about 27 weight percent
.:
... .
'`
:: :
: ~ ' :;' :'',
:, ' :
. . .
,: ,~,
. 2~7~
respectively of the SMC. When the feed comprises RRIM,
the inorganic filler material to be recovered is glass
which typically comprises about 15 weight percent of
` the RRIM. If paint sludge from an automobile plant
comprises the feed, the inorganic and organic
components comprise about 40% of the feed stream and
the balance H2O. About 75~ of the non-aqueous contents
is the organic and 25% recoverable inorganics, such as
aluminum oxide (A12O3), titanium dioxide (Tio2)~ silica
(SiO2), barium sulphate (BaSO4) and to a lesser degree
;~ metal components.
The feed may be mixed with other plastics
~ which do not have a recoverable filler material. For
; example, reaction injected molded (RIM) plastics do not
have an inor~anic component but the polymer can provide
considerable combustible oils and gases when pyrolzed.
; These plastics may be added to or mixed with a feed
having RRIM, SMC and particularly paint sludge where
; there is a need to ensure sufficient self generated
combustibles to heat the pyrolyzer. In this manner,
the pyrolysis process recovers the organic component
which has heat value which will be described
~; subsequently~ Further, the inorganic filler material
from the RRIM and SMC materials is recovered and can be
; 25 re-used. when mixing plastic material not having
filler (e~g., RIM) with those having a filler material,
it is desirable to keep the inorganic or filler
component to a level of about 33 weight per~ent of the
feed.
In the event that the ~eed comprises a solid
plastic material, it is desirable that it first be cut
or comminuted in to pieces about two-inches long in
order to prevent clogging of the feeder and/or
pyroly~er. In the event that paint ~ludge is the feed
material, no such comminution is necessary.
The reactor assembly 12 includes conveyor
means 14. The conveyor means 14 includes a stationary
reactor housing 16, a central shaft 18 supported for
rotation within the reactor housing 16 and a helically
wound vane forming a plurality of flights 20 fixedly
secured to the ~entral shaft 18 for rotation therewith.
The flights 20 convey the feed material and the solid
residue through the reactor housing 16. As can best be
viewed in Figure 2, the reactor housing 16 has an
entrance end 22 and an exit end 24. The entran~e end
22 includes an inlet 26 for receiving the feed
material. The exit end 24 includes conduit means
comprising a gas outlet 28 for exhausting the organic
gaseous decomposition products from the reactor
housing 16 and a residue outlet 30 for allowing the
solid residue to exit the reactor housing 16.
The flights 20 at the entrance end 22 of the
reactor housing 16 have a smaller amplitude than the
1ights 20 near the exit end 24 of the reactor housing
16. That is, the flights 20 at the entrance end 22 are
spaced radially some di6tance from the walls of the
reactor housing 16 and the flights 20 extend radially
further outwardly from the shaft 18 and very ne~r the
walls of the reactor housing 16 at the exit end 24.
~lso the pitch or length of the flights 20 becomes
progre~sively longer in the direction of the exit end.
This configuration forces the feed material and the
:, . ~ . .:
,~
: . ~ - : .
,... . , -
. .
` 2~7~
residue material through the reactor housing 16 toward
the exit end 24. At least one of the longer flights
20, that is the flights near the exit end 24 of the
reactor housing 16 r has an opening 32 therein. The
opening~s) 32 allow the gaseous decomposition products
which are formed in the pyrolytic reactor housing 16 to
pass therethrough to the gas outlet 28 and thereby
prevent pressurization of the reactor housing 16 by the
decomposition products and reduce the incidence of
plugging or jam~ing of the reactor by the solids
therein. The central shaft 18 is rotated by any
suitable motor assembly 34 (Fi~ure 2).
To urther aid movement of the fèed material
and residual material through the reactor housing 16,
the reactor housing 16 is inclined ~uch that the
entrance end 22 is at a height relatively higher than
the exit end 24. Preferably, the angle of inclination
is about 30. By utilizing an assembly which is
inclined, gravitational forces will aid movement of the
feed material and solid residue through the reactor,
housing 16.
The reactor assembly 12 further includes a
furnace assembly generally indicated at 36. The furnace
assembly 36 i6 for heating the reactor housing 16 to a
predetermined pyrolysis temperature. The reactor
housing 16 and furnace assembly 36 comprise a retort
for pyrolitically decomposing the feed material. The
temperature to which the reactor assembly 12 is heated
by the furnace assembly 36 is determined by the
composition of the feed material being utilized. It is
desirable to operate the reactor assembly at a
2~25'70~
temperature of about 75 F above the vaporization
temperature of the organic component of the feed. By
operating at this temperature, charring of the organic
component is minimized, and hence the amount of carbon
contamination on the solid residue is al60 minimized.
Hence, for example, a suitable pyrolysis temperature
for SMC will typically be 1300 F plus or minus 50 F
and 1450 F plus or minus 50 F for RRIM and paint
sludge.
The fu~rnace assembly 36 includes a burner 38
which preferably burns a gaseous organic material. The
furnace assembly 36 further includes an insulated
furnace wall for retaining the heat of the burner 3~.
Preferably, the burner 38 is initially fired with
natural gas supplied from an outside source through a
conduit 43. The burner 38 is later ~ired with the
recovered gaseous decomposition products from the
pyrolytic reactor 16 (i.e., the uncondensible
pyrogases). That is, once the pyrolysis reaction has
begun and the organic decomposition products have been
recovered, they are sen~ to the burner via conduit 126
and burned in place of outside natural gas or in
admixture therewith. Hence once initiated, the
process can be essentially self sustaining energy wise.
The exhaust gas from the furnace assembly is supplied
to a heat exchanger 39 as diagrammatically shown in
Fig. l. At the heat exchanger 39, heat removed from
the burner exhaust gases is used to initially heat the
inlet air supplied to the oxidation assembly by the
supply conduit assembly generally indicated at 130.
The supply conduit assembly 130 includes a hot air
' ' ' ~' :
i
7 ~ ~
supply conduit 132 and an ambient air supply conduit
134. The hot air supply conduit 132 and ambient air
supply conduit 134 are each connected to a mixing valve
136 whereat the flow from each conduit 132, 134 can be
regulated. An oxidizer supply conduit 138 is also
connected to the mixing valve 136 and receives the
resultant air mixture from the two supply conduits 132,
134. The oxidizer supply conduit 138 includes a means
for forcing air through all of the supply conduit
assembly 130. This means may comprise a compressor 140
or any other device to force the air through the supply
conduit assembly 130 (Figure 1).
The assembly 10 further includes feeder means
generally indicated at 41 at the entrance end 22 of the
reactor housing 16 for supplying the feed material to
the reactor housing 16. The feeder means 41 comprises
a feed hopper 42 into which the feed material is
initially placed. The feed material is transferred
from the feed hopper 42 to the inlet 26 of the
reaction housing 16 by an appropriate conveying
assembly. As shown in Figures 1 and 2, a screw type
conveyer 44 is used to transfer the feed from the feed
hopper 42 to the inlet 26. Depending upon the type of
feed material, different conveying assemblies may be
utilized. For example, when the feed material
comprises SMC, a feed auger as shown in Figure 1 may be
used to convey the feed material from the feed hopper
42 to the inlet 26. To convey semi-solids such as
paint sludge, a ramp feeder, as well known in the art,
is used to transfer the feed material from the hopper
42 to the inlet 26 of the reactor housing 16. If the
11
2~25 70~
feed comprises a liquid, a centrifugal pump may be
used.
The feed material passes through a rotary air
lock 46 prior to entering the inlet 26. The rotary
air lock 46 prevents air from en~ering the inlet 26 and
thereby the reactor housing 16 while allowing the feed
material to pass into the inlet 26. The feed material
also passes through a gate valve 48 prior to entering
the inlet 26.
The i~let 26 further has an inert gas inlet
valve 50 connected thereto for allowing an inert gas to
enter the reactor housing 16. Prior to feeding the
feed material from the hopper 42 to the inlet 26 and
prior to heating the reactor housing 16, air is purged
out from the reactor housing 16 by introducing an inert
gas, preferably nitrogen, through the inert gas inlet
50 to flood the reactor housing 16. The pyrolysis
reaction is carried out in the absence of oxygen so as
to avoid potentially dangerous rapid oxidation and the
potential formation of toxic gases depending on the
composition of the feed. ~y excluding oxygen, this
risk is prevented.
Hence, to pyrolitically decompose the feed
material, the feed material passes through the rotary
air lock 46 and the gate valve 48 into the inlet 26 and
to the reactor housing 16 which is purged of oxygen by
flooding the reactor housing 16 with nitrogen. The
reactor housing 16 has been preheated to a
predetermined temperature by the furnace assembly 36.
The feed ~aterial passes continuously into the
reactor housing 16 where it is volatized or gasified
12
, ',:
:
:
: - :
.
.
^ :
2~7~
13
and decomposed within about 30 minutes. The recoverable
gaseous decomposition products pass through a filter or
screen 56 and to the gas outlet 28. The screen 56
prevents any non-gaseous residual material from passing
to the gas outlet 28.
In order to prevent the inlet 26 from becoming
plugged, an automatic cleaning system comprising a
plunger or ram 52 (see Fig. 2) is automatically
periodically inserted into the inlet 26 at
predetermined time intervals to relieve any clogging
or accumulation of the feed material. Similarly, a~
best viewed in Figuze 2, an auto~atic cleaning assembly
comprising a plunger or ram 100 is positioned over the
gas outlet 28. An automatic force is periodically
applied to the ram 100 to force the ram downwardly into
the outlet 28 to thereby clear any solid restrictions
or accumulation which may build up in the gas outlet
28. The filter 56 is moved during such cleaning so as
to permit solids to be ejected through residue outlet
30.
As was previously stated, the solid residue is
formed during the pyrolysis reaction. The solid
residue comprises the inorganic filler material o~ the
feed contaminated with carbon. The residue is conveyecl
by the flights 20 to the residue outlet 30 of the
reactor housing 16. The residue outlet 30 is
connected to a first cooling means or assembly,
generally indicated at 58. More specifically, the
first cooling assembly 58 is in direct closed
communication with the residue outlet 30 of the reactor
housing 16. The first cooling assembly 58 receives and
13
~257~
14
progressively cools the inorganic residue from the
reactor assembly 16 in a substantially non-oxidizing
environment as it traverses rom one end to the other
of the cooling means 58. By using a clo~ed connection
between the reactor housing residue outlet 30 and the
first cooling assembly 58, ambient air i~ prevented
from contacting the solid residue and entering the
pyrolysis reactor.
Th~ first cooling assembly 58 comprises a
screw type conveyor including a housing 60 and an auger
62 supported for rotation within the housing 60. A
suitable motor (not shown) rotates the auger 62. The
housing 60 of the first cooling assembly 58 has an .:
inlet 64 and an outlet 66 at the opposite end of the
housing 60 from the inlet 64. The inlet 64 of the
first cooling assembly 58 is connected by an air-tight,
expansion joint 68 to the residue outlet 30 of the
reactor housing 16. The inlet 64 of the first cvoling
assembly 58 is elevated to a height relatively higher
than the outlet 66 for gravity assist in moYing the
residue through the housing ~0. Preferably, the angle
of inclination between the outlet 66 and the inlet 64
is about 30. The first cooling assembly 58 further
has removable covers 70 on the ends thereof which allow
easy clean up of the interior of the housing 60.
The first cooling assembly 58 cools the solid
residue material to a superambient temperature less
than that in the pyrolyzer but still suitable to
readily oxidize the carbon content of the solid residue
without degrading the inorganic component of the
residue. Hence, the temperature to which the residue
14
~, . i, .
,' ~
' ~ '' ' `
2~2~7~
is cooled is dependent upon the make-up of the
inorganic material. Preferably, the residue is cooled
to approximately 1150 F, plus or minus 25 F when the
feed comprises SMC, RRIM or paint sludge.
The cooled residue then passes through a
second rotary air lock 72 into an oxidation ~eans or
oxidation assembly generally indicated at 74. The
oxidation assembly 74 is operative to continuously
receive the cooled solid residue and to react the
carbon therein with an oxidizing agent, preferably
oxygen ~e.g. air), to form gaseous carbon dioxide and
liberate the same ~rom the inorganic component. ~he
second rotary air lock 72 allows the solid residual
material to pass from the first cooling housing 60 to
the oxidation assembly 74 without allowing any air or
carbon dioxide to enter the first cooling housing 60.
~y using a first rotary air lock 46 and a second rotary
air lock 72, the reactor housing 16 and first cooling
housing 60 are kept free of any oxygen once they have
been purged by the inert gas.
The oxidation assembly 74 includes a
screw-type conveyer having a housing 76 and an auger 78
supported for rotation within the housing 76. The
housing 76 has removable covers 79 on the ends thereof
to allow easy cleaning of the interior thereof. A
suitable motor (not shown) rotates the auger 78 within
the housing. Further, the oxidation assembly 74 has an
inlet 80 in direct closed communication with the outlet
66 of the first cooling assembly 58 through the rotary
air lock 72. This prevents the residue from contacting
ambient air as it passes from the first cooling housing
,'
.
2~2~
16
60 to the oxidation housing 76. The oxidation assembly
74 further includes an outlet 82 for discharging the
carbon-free inorganic component. The inlet 80 of the
oxidation assembly 74 is elevated to a height greater
than the outlet 82. The preferred angle of inclination
is about 30. This allows a gravitationa:L force to aid
movement of the solid residual/inorganic filler
material through the housing 75 to the outlet 82.
The oxidation assembly 74 is kept at
substantially the same temperature as the residue
exiting the first cooling assembly 58. That is, the
temperature is about 1150~ F, plus or minus 25 F. The
oxidation housing 76 is initially heated to the
reguired temperature by hot air heated by the exhaust
of furnace assembly 36 in the heat exchanger 39. That
is, the mixing valve 136 is opened or regulated such
that the supply of hot air from the conduit 132 and the
supply of ambient air from the conduit 134 results in a
hot air stream in the conduit 138 equal to oxidation
temperature. Initially most o~ the air will be
supplied through the hot air supply conduit 132. After
the oxidation process has begun, heat is given off due
to the exothermic oxidation reaction. Hence after
initiation, cooler air is substituted for the heated
air to maintain the desired temperature in the housing
76. This is conveniently accomplished by regulating
the mixing valve 136 to permit ambient temperature air
or a mixture o~ ambient temperature air and preheated
air to obtain a supply of air to the oxidation housing
76 through the conduit 138 which air is at the desired
temperature.
16
'
2 ~
The air is allowed to be introduced to the
oxidation housing 76 through the conduit 138. This air
is prevented from moving to the first cooling housing
60 by the second rotary air lock 72. The air, and in
particular, the oxygen in the air, reacts or oxidizes
the carbon to form gaseous carbon dioxide which
liberates the carbon from the inorganic filler
materialO The carbon dioxide enriched air removed from
the oxidation housing 76 may be passed to the heat
exchanger 39 t~rough a conduit 81 and the heat removed
therefrom in the heat exchanger 39. Any residual heat
from the CO~-enriched air stream or from the furnace
exhaust ~that not used in the process to provide
preheat air to the oxidizer) can be used elsewhere
(e.g. to form steam for other plant operations). The
CO2-enriched air exiting the oxidation assembly 74 may
further be passed through a milk of lime solution
[i.e., Ca~OH)2] to precipitate CaCO3 and thereby reduce
the amount of CO2 vented to the atmosphere. Where
appropriate, any CaCO3 collected at this location may
be added to that recovered as solid residue from the
oxidation assembly 74 and reused.
As was previously stated, the second ro~ary
air lock 72 prevents air form entering the first
cooling housing 60 through the outlet 66 of the first
cooling housing 60. The second rotary air lock 72
further provides a means disposed between the cooliny
assembly 58 and the inlet 80 of the oxidation housing
76 for transferring the cooled solid residue to the
oxidation housing 76 while isolating the first cooling
assembly 58 against invasion of gases emanating from
17
:
2~5'7~
18
the oxidation housing 76. That is, in addition to :
preventing air from entering the first cooling assembly
58, the rotary air lock 72 prevents the gasified carbon
dioxide from entering the first cooling assembly 58.
A second cooling means or assembly generally
indicated at 84 is provided for continuously receiving
and cooling the carbon-free inorganic filler material
exiting the oxidation housing 76. The second cooling
assembly 84 comprises a screw-type conveyor having a
housing 86 and ~n auger 88 supported for eotation in
the housing 86. The housing 86 has removable covers 89
thereon for allowing easy cleaning of the interior of
the housing 86. A suitable motor (not shown) rotates
the auger 88 within the housing 86. The second cooling
assembly 84 has an inlet 90 connected in direct closed
communication to the outlet 82 of the oxidation housing
76 through an expansion joint 92. Further, the second
cooling housing 86 has an outlet 94 at the opposite end
thereof. The inlet 90 of the second cooling assembly
84 is at a height relatively higher than the outlet 94.
Preferably, the angle of inclination between the outlet
94 and the inlet 90 is about 30. This angle allows
gravity to aid in feeding the inorganic filler material
from the inlet through the housing 86 to the outlet 94.
The carbon free inorganic filler materials
formed in housing 76 are transferred to the ~econd
cooling assembly housinq 86 through the expansion joint
92 wherein they are then cooled to approximately 300
F, plus or minus 25 F. The carbon-free residue
comprises either CaCO3 and/or glass from the SMC, glass
from the RRIM scrap and Tio2~ A12O3, silica (SiO2),
18
7 ~ `5
19
barium sulphate ~BaSO4) and/or other fillers and
extenders from the paint sludge respectively. These
are all inorganic filler materials to be recovered and
re-used depending on which type of feed material is
utilized. The inorganic filler materials are collected
in substantially their original (i.e. chemically) form
in a storage bin 96 which is connected directly to the
outlet 94 of the second cooling assembly 84. SMC/RRIM
residues typically comprise chunks of CaCO3 and glass
fibPrs wherein ~the glass fibers are shorter than in the
original feed stock as a result of being broken by the
impellers of the several screw conveyors. Paint
residue typically comprise about 400 micron powder.
The recoverable organic gaseous materials or
decomposition products formed in the pyrolysis reaction
housing 16 are also treated. The gases formed in the
housing 16 pass through the screen 56, and a gate valve
98 as shown in Fig. 2. After the gases pass through
the gate valve 98, they pass to a gas condenser means
or assembly generally indicated at 104. The gas
condsnser assembly 104 is connected to the reactor
housing gas outlet 28 and receives the gaseous
decomposition products. The gaseous decomposition
products include condensable and non-condensable
organic material. The gas separation assembly 104
separates the condensable organic material from the
non-condensable material by condensing the condensable
organic material to a liquid (hereinafter sometimes
referred to as pyro oil) for discharge through a drain
line 105. This leaves only the non-condensable organic
gaseous material (hereafter sometimes referred to as
19
pyro ga~es).
Initially, the gaseou~ decomposition products
having both con~ennable and non-condensable compoxlentn
are carried through pyroga~ conduit 106 introduced to
the bottom of a con~enning tower 108 having oil therein
compriæing principally cond~nnate from reactor exhau~t
ga~e~. The oil in in counterflow contact Inot shown)
with the incoming ganeous material and remove~ some of
the conden~able material from the organic ga~eous
mixture. That iæ~ the oil flows downwardly through the
upst~n~;ng co~en~ing tower 108 and contacts the gas
which flows upwardly and in the direction of the arrow
o~ Figure 1 and through the tower. The cool oil
co~n~es the co~nQible~ therein and collect~ at the
ba~e 110 of the co~d~nning tower 108. The ga~ then
flows through an impingement ~eparator 112 to further
~eparat~ the con~nnable material fxom the
non-con~nn~hle gaseou~ material. Pyro oil Erom the
ba~e 110 i~ recirculated back to the top of the tower
108. The gas i8 then pas~ed through a blower 114
which in preferably a three ~peed vacuum blower and to
a cooling cyclone 116. The blower can be adjusted 80
a~ to preve~t pres~urization of the reactor housing 16.
In the cooling cyclone 116, the rc -in;ng co~d~n~able
material (i.e. pyro oil) is li~ue~ied and removed from
the non-condQnRable material (i.e., pyro gas) and
drain~ through the drain line 118 to the bane 110 of
the condenning tower 108. A second cooling ayclone :l20
may al~o be provided for removing further co~d~nnable
material from the non-cond~nnable organic ganeou~
matsrial. A second drain line 122 is then re~uired to
drain the cond~n~able material ~rom the cooli~g cyclone
A
.......
21
1~0 to the base 110 of the cond~n~ing tower 108. The
drain line 105 connect6 to the base 110 of the
con~n~ing tower 108 and i5 opened to remove pyro oil
from the system aR it accumulates. The pyro oil so
removed i5 combustible and may be used by itself to
fire appropriate burners or preferably used in
admixture with other oil ~e.g. heating oil) for
recovering the heat value therein.
The non-co~n~ed ga QOUS decomposition products
(i.e., pyro gas) are Rent to a distributor 124. At
the distributor 124, the pyro gases are separated into
two ~treams. The ~ir~t provides an outlet 126 for
allowing the pyro ga~eR to be recycled to the burner 38
of the reactor a~ embly 14 where they are burned to
provide heat to tha reactor hou~ing 16. The æecond
provides an alternati~e outlet 128 which lead~ to a
storage ~aaility (not ~hown) ~o that the pyro ga~es can
be ~tored for later uRe.
The method of recovering an inorganic filler
material ~rom a continuou~ feed o mixed organic and
inorganic material is yenerally as follow~.
Fir~tly, a ~eed material is continuou~ly conveyed
through the pyrolytic reactor housing 16 to
progressively pyrolyze the organia component of the
feed mixture as the feed traver6e~ the reactor housing
16. The pyrolysis reaction ~orms gaseous decompoRition
pro~ucts and a carbon-cont ;n~ted solid residue of
inorganic material. The pyrolyzer, or reaction
as~embly 12 operates at a predetermined ~uperambient
temperature whiah is cho~en depen~;ng upon the type of
~eed material to be used and will generally be at lea~t
2~
~i2~7~
22
about 75 F higher than the vaporization temperature of
the organic component of the feed material.
The hot residue exits the reactor housing 16
and is conveyed through the substantially oxygen-free
first cooling housing 60. The residue is cooled in the
first cooling housing from the first temperature
(pyrolysis temperature) to a second superambient
temperature selected on the basis of the composition of
the inorganic material to permit ready oxidation of the
carbon on the lnorganic material without deleteriously
afecting the inorganic material.
While the residue is at the second
superambient temperature, it is transferred from the
first cooling assembly 58 to the oxidation assembly 74
which is maintained at approximately the second
superambient temperature. The residue is conveyed
through the oxidation assembly 74 in the presence of
oxygen, which oxygen is initially preheated to the
second superambient temperature. The oxygen reacts
with or oxidizes the carbon contamination on the
inorganic filler material to gasify the carbon by
forming carbon dioxide to remove or liberate the carbon
from the inorganic filler material.
More specifically, operation of the assembly
10 is as follows. Solid feed material such as SMC or
RRIM is comminuted into approximately two-inch size
feed stock and placed in the feed hopper 42. The
reactor housing 16 and first cooling housing 60 are
then purged of oxygen by introducing nitrogen through
the inert gas inlet 50. Once the nitrogen has been
added to the reactor housing 16 and the first cooling
22
' ~
'
, ~ ''
-: ~ ,
,,
2~25~'9~
23
housing 60, the burner is fired usin~ natural gas from
an outside source. The reactor housing 16 is brought
to and maintained at the first superambient temperature
of approximately 1200 F to 1500 F, depending upon the
type of feed material. The feed material is then
introduced through the rotary air lock 46 and to the
inlet 26 o the reactor housing 16. Once inside the
reactor housing 16, the feed material is pyrolitically
decomposed into a solid residue having carbon
contamination thereon and a gaseous decomposition
product having condensable and non-condensable
components.
The solid residue is forced through the
housing 16 by the flights 20 within the reactor housing
16. The solid residue passes through the outlet 30 and
an expansion joint 68 and to the inlet 64 of the first
cooling housing 60. In the first cooling housing 60,
the residue is cooled to the second superambient
temperature which is approximately llS0 F, plus or
minus 25 F. The solid residue is forced through the
housing 60 by the auger 62 and to the outlet 66 of the
first cooling assembly. The outlet 66 has a rotary air
lock thereon for allowing the cooled residue to pass to
the inlet 80 of the oxidation housing 76. In thi~
manner, the cooled solid residue is transferred to the
oxidation housing 76, which is immediately adjacent the
first cooling chamber 60 while at the second
superambient temperature, without exposing the cooled
residue to ambient air~
In the oxidation chamber, which is kept at
approximately the second superambient temperature of
23
,
~2~
~ 4
1150 F, plus or minus 25 F, the residue contacts air
and is continuously oxidized by the oxygen in the air
to gasify the carbon by forming gaseous carbon dioxide
to thereby separate the carbon from the inorganic
residue. During oxidation of the residue, the air and
carbon dioxide which is formed during the oxidation
process are prevented from entering the cooling chamber
by the rotary air lock 72. ~ence, the oxidation
reaction turns any residual oarbon on the inorganic
filler material~into gasiried carbon dioxide. The
inorganic filler material passes through the oxidation
housing 76 to the outlet 82 and through an expansion
joint 92 to the inlet 90 of a second cooling assembly.
The carbon dioxide may be vented to the heat exchanger
39 through the conduit 81 to help heat the heat air
initially supplied to the oxidation housing 76.
The inlet 90 of the second cooling assembly
allows the inorganic filler material to enter the
second cooling housing 86. The inorganic filler
material is forced through the housing 86 by the auger
88. In the second cooling housing 86, the inorganic
filler material is cooled to approximately 300, plus
or minus 25 F. The carbon free inurganic filler
material is then discharged through the outlet 94 of
~5 the second cooling housing 86 and to a ~torage bin 96.
The recoverable gaseous decomposition products
formed during the pyrolytic reaction in the reaction
housing 16 are sent to a condensing assembly wherein
the condensable component (i.e. pyro oil) of the
gaseous decomposition products are condensed to leave
only a non-condensable gaseous material ~i.e. pyro
24
. ~ "' ;
2 ~
gases). More specifically, the gaseous decomposition
products pass throuyh the screen 56 and through the
outlet 28 of the reactor housing 16, through a gate
valve 98, and to a cooling tower 106. In the cooling
tower 106, pyro oil is passed over the gaseous material
to separate additional pyro oil from the gaseous
material by liquefying the same. The pyro oil is
recovered in the base 110 of the cooling tower 106.
The remaining gaseous material is sent to an
impingement separator 112 wherein further pyro oils are
removed. Finally, the gaseous material passes through
a blower 114 to at least one cooling cyclone 1l6, 120
wherein the remaining pyro oils are condensed to leave
only non-condensable pyro ~ases. The pyro gases are
sent to a distributor 124 wherein a portion is recycled
through the line 126 to the burner assembly 38 to
eliminate the need to fire the burner 38 by an outside
natural gas source. Finally, a portion of the pyro
gases is sent through the line 128 to storage for
further use.
The carbon-free reside produced from the
process/apparatus of the present invention can be
reused in the same type of material ~i.e. SMC, RRIM).
from which it emanated as well as other applications.
Hence, for example, the CaCO3/glass residue chunks can
be ground into five (5) micron or less-powder and used
to replace up to about 50~ of the CaCO3 that would
otherwise be used in SMC and about 2~ of the filler
otherwise used in RRIM. This same material has been
used with mort~r and found to produce concrete which
has more than 7% greater compressive strength than 100%
.
: '
..
- .
2~257~
26
mortar has. SMC residue ground to less than about 100
microns powder is u~eful as a filler in concrete paint.
Paint sludge residue ground to about 5 microns can
replace up to about ~4~% of the filler content of ~ ~ 9
automotive paint primer, up to about ~% of the filler
content o dark-colored commercial topcoat paint and a
significant amount of the filler in anti-chip
automobile paint.
It will be appreciated that the
proce~s/apparatus of the present invention can be used
to pyrolize many other feed materials having mixed
organic and inoryanic component~, including toxic
materials. For example, it has particul~r value in the
pyrolyzation of polyvinylchloride ~PVC~ compounds in
admixture with SMC. Typically, when PYC compounds are
burned (2900 F) they give off toxic combu~tion
products such as dioxins. ~o avoid this, CaCO3 has
been used heretofore during burnin~ to consume the
chlorine and prevent the formation of dioxins. Mixing
PVC compounds with SMC compounds containing CaCo3 and
pyrolyzing the mixture at a temperature above the
decomposition temperature of CaCO3 (preferably at
1500 F-1600 F) causes the CaCO3 to readily react with
çhlorine and thereby detoxify the exhau6t gases ~he-
CaCl2 formed i6 then 5imply leached from the re~idue.Hence, the pre~ent process can be utilized to di6pose
of many compounds (including toxin6) in an
environmentally ~ae manner.
The invention has been described in an
illustrative manner, and it is to be understood that
the terminology which has been used i5 intended to be
26
,
.:
~2~
27
in the nature of words o description rather than of
limitation.
Obviously, many modifications and variations
of the present invention are possible in light o~ the
above teachings. It is, therefore, to be understood
that within the scope of the appended claims the
invention may be practiced otherwise than as
~pecifically described.
: ` ; - ' '' ~ ~ '
....
.