Note: Descriptions are shown in the official language in which they were submitted.
c ~ ¦ f~
13-BROMO- AND 13,14-DIBROMOERGOLINES, THEIR
PRODUCTION AND USE IN MEDICINAL AGENTS
Backqround of the Inventio_
The invention relates to 13-bromo- and 13,14-
dibromoergolines, their production and use in medicinalagents, as well as to intermediate products for their
preparation.
It is known from DE-A-3,824,661.9, which corresponds
to U.S. Serial No. 07/380,352, that longer-chain
hydrocarbon residues in the 6-position enhance the
dopamine-agonistic activity of ergolines. This effect is
surprisingly also retained in ergolines brominated in the
13-position or in the 13- and 14-positions At the same
time, the metabolic stability of the compounds is
improved, thus attaining increased bioavailability.
Summary of the Invention
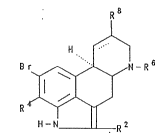
The invention relates to compounds of Formula I
R B
H-N R 2
wherein
:
-
R2 is C16-alkyl, C26-alkenyl or CH2-O-C1~-alkyl,
R4 is hydrogen or bromine,
R6 is C26-alkyl, C36-alkenyl or C35-cycloalkyl-
Cl 2-alkyl,
R8 is ~-NII-CX-R3, ~-NH-So2-NR5R7~ CH2-Y, B-CO-NH-
optionally substituted phenyl, B-Co-NR9-co-NHR
wherein
X is oxygen or sulfur,
R3 is hydrogen, C16-alkyl, -O-(CH2) n N(CH3)2
or -N(C14-alkyl)2 or Cl4-alkoxy-substituted
cl~6-alkYl,
Rs and R7 each means hydrogen or C14-alkyl,
Y is hydro~en, OH, O-C16-acyl, CN, SCH3
or CONH2,
R9 and R10 each means C14-alkyl or -(CH2)n-N(CH3)2, and
n stands for l, 2, 3 or 4, and
C8---C9 ~ means a single or double bond wherein, if
R8 is CH3, CH20H or CH2-O-C16-aCYl,
C8 = C9 means a double bond and if
R8 is CH2-CN, CH2-SCH3 or CH2-CONH2,
C~___C9 iS a single bond and R8 is in the
B-position.
The acid addition salts of compounds of Formula I
are also included in the compounds provided.
The invention also relates to methods of producing
compounds of Formula I by (a) brominating compounds of
Formula II, (b) substituting, in the 2-position,
compounds of Formula III or (c) alkylating or
alkenylating compounds of Formula IV. The intermediates
of Formula III are also provided. Formulas II-IV are
defined in the discussion which follows.
The invention also relates to pharmaceutical
preparations which comprise a compound of Formula I, or
an acid addition salt thereof, and a pharmaceutically
compatible vehicle.
c~ c - ~ y l ~
Methods for treating Parkinson's disease and
providing dopamine agonistic activity in a host are also
provided wherein compounds of Formula I or acid addition
salts thereof, are adminlstered to a host.
S The terms used herein to deEine substituents of
Formula I have the following exemplary meanings. Alkyl
means in each case a straight-chain or branched alkyl
residue, such as, for example, methyl, ethyl, n-propyl,
isopropyl, n-butyl, isobutyl, sec-butyl, tert-butyl,
pentyl, hexyl, heptyl, 2,2-dimethylpropyl, 2-methylbutyl,
3-methylbutyl, l-ethylbutyl, isopentyl, isoheptyl, 1-
methyl-l-ethylpropyl.
In cases where R2 and R6 means an alkenyl residue,
the latter preferably contains only one double bond,
wherein the double bond in residue R6 cannot be proximate
to the nitrogen atom. Examples of suitable alkenyl
residues are: vinyl, l-propenyl, 2-propenyl, 1-methyl~2-
propenyl, l-butenyl, methallyl.
Suitable C16-acyl groups are derived from aliphatic
carboxylic acids, e.g., alkanoyl, such as, for example,
formic acid, acetic acid, propionic acid, butyric acid,
caproic acid.
The substituent of the phenyl residue, such as that
of R8, can be in the o-, m- or p-position; suitable are
Cl4-alkyl or -alkoxy, e.g., methyl, methoxy, or halogen,
such as fluorine, chlorine, bromine or iodine.
The residues R9 and R10 are preferably different.
Hydrocarbons of up to 4 carbon atoms are to be
considered preferred embodiments for R6 and R2.
The compounds of this invention can occur as E-
isomers or Z-isomers or, if a chiral center is present in
residue R2, as diastereomers and as mixtures thereof. The
isomers and isomer mixtures are also encompassed by the
present invention. The physiologically compatible acid
addition salts are derived from the known inorganic and
organic acids, such as, for example, hydrochloric acid,
r ~
sulfuric acid, hydrobromic acid~ citric acid, maleie
acid, fumaric acid, tartaric acid, etc.
The compounds o~ this invention as well as their
acid addition salts exhibit, in particular, central
dopaminergic effectiveness and therefore can be used as
medieinal agents.
The dopamine-agonistie aetivity was determined with
the aid of the method of automat:ie reeording of
stereotypies in rats, diselosed by Horowski (Arzneim.
lo Forseh. 12:2281_22~6, 1978): Direetly after
intraperitoneal administration of the test eompound and,
respeetively, the vehiele, male Wistar rats (90-120 g)
are plaeed singly into restraining cages of aerylie
glass. By way of an eleetrodynamie reeording system
mounted in front of the animal's head, the number of
eontaets with a steel beaker with a eentral metal rod, as
a eonsequenee of stereotypie ehewing, lieking and gnawing
motions, is reeorded for a period of 60 minutes. The
average values + S.E.M. of the number of eontaets during
60 minutes are ealculated for the various treatment
groups, each encompassing 12 animals, and the
signifieanee of the differenees among the average values
of the various doses of test eompound as eompared with
the vehiele-treated eontrol group is determined with the
aid of simple varianee analysis in conjunction with the
Dunnett test.
The results are set out in the table below.
T A B L E
Triggcring of Stereotypies in Rats Aftcr
Intraperitoneal Treatment lith Vehicle and, Respcctivcly
Various Doses of Ergoline Urea Derivatives
(x: p ~ 0.05, xx: p < 0.01, Variance Analysis/Dunnett Test versus Control; n = Number of
Animals)
.. . . . ........... .. . . ... ...
Stereotypies (Counts per 60 Minutes)
(Avera~e Value ~ S.E.M.)
Dose of Test compound (3(13-bromo-6-n-ethyl-2-methyl-ergolinyl)1,1-
diethvl urea (mqtk~ body ~ei~ht)
n Control 0.025 0.1 0.39 1.56 6.25
4 U 12 2137 ~ ô49 1945 ~ 472 2470 ~ 548 7499 ~1342xx 8066 ~ 1248xx 7381 ~ 1336xx
Since the compounds of this invention are
distinguished especially by their dopamine-agonistic
activity without the occurrence of strong ~-adrenergic
effects, they are particularly well suited for the
treatment of Parkinson's disease. The compounds of this
invention can be administered at dosages of from 0.00001
to 0.1 mg/kg of host, per day, preferably from 0.001 to
0.1 mg/kg of host per day, analogously to the known agent
Bromocryptin.
In order to utilize the compounds of this invention
as medicinal agents, they are brought into the form of a
pharmaceutical preparation containing, besides the active
agent, pharmaceutical organic or inorganic, inert
vehicles suitable for enteral or parenteral
administration, such as, for example, water, gelatin, gum
arabic, lactose, amylose, magnesium stearate, talc,
vegetable oils, polyalkylene glycols, etc. The compounds
are administered at a dosage within the range of 0.001 to
10 mg~with a pharmaceutically compatible vehicle. The
pharmaceutical preparations can be present in solid form,
e.g., as tablets, dragees, suppositories, capsules, or in
the liquid form, e.g., as solutions, suspensions or
emulsions. Optionally, they contain moreover auxiliary
materials, such as preservatives, stabilizers,
surfactants or emulsifiers, salts for varying osmotic
pressure, or buffers.
The compounds of this invention can be produced
pursuant to methods known per se.
For example, compounds of this invention can be
obtained by
(a) brominating a compound of Formula II
f~ J,
H
N
H-- R~
wherein
R6, R8, ~a___C9 have the above meanin~s and
R2 is C1.6--alXyl; or
(b) substltuting, in the 2-position, a compound o~
Formulz~ III or its quaternary salt
R ~
r ~ ~N -
~
H-N CH2 N /O
wherein R6, ~8 and CB ~=C9 ha~e the above me~ning~; or
tc) alkylating or alkenyla~ ng a compound of
Formula IV
. . .
;J ~ !i i ', 'Y,
H ~:~
D r ~ "N-11
H-N R2
wherein R2, R8 and C~ C9 have the above meanings, and,
if desired, subsequently thiolating a carbonyl group
and/or forming the acid addition salts.
The compounds of Formula II are brominated according
to method (a) in a strongly acidic solution, for example
in trifluoroacetic acid or glacial acetic acid. Suitable
brominating agents are elemental bromine, pyridine
hydrobromide perbromide, or pyrrolidone hydroperbromide;
optionally, chlorinated hydrocarbons, such as chloroform,
methylene chloride or ethers, such as tetrahydrofuran,
dioxane and isopropyl ether can be added as solvents.
The bromination is conducted at temperatures of -20C to
80C, preferably at room temperature, and is finished
after about 15 minutes up to one hour.
When adding molar amounts of brominating reagent,
13-bromo derivatives are mainly obtained; with the use of
2 moles of brominating reagent, 13,14-dibromo derivatives
are isolated.
Introduction of the substituents in the 6- or 2-
position can take place prior to or after bromination.
In accordance with method (b), the substituent R2 is
introduced in accordance with the procedure described in
German Patent Application P 38 24 661.9. In this
process, the Mannich base of Formula III is substituted
in nucleophilic fashion or is oxidized to the 2-formyl
derivative which is subsequently reacted in a Wittig
reaction to the desired compound of Formula I.
'J
The nucleophilic exchange takes place optionally
after quaternization of the aminomethyl group in an inert
solvent, such as alcohols, polar aprotic solvents,
ethers, or chlorinated hydrocarbons at room temperature
5 or elevated temperature; alcoholates can be utilized as
nucleophilic anions which can be subsequently converted
into the CH2-OH group, if desired. For preparing the 2-
methyl derivative, the quaternary salt can be reduced in
polar solvents, such as alcohols, with sodium
borohydride.
Oxidation to a 2-CHO compound can take place
analogously to the process described in R.A. Jones et
al., Synthe-tic Communications 16:1799 (1986) with
pyrolusite or tert~butyl hypochlorite in inert solvents
at room temperature. Conversion of the 2-formyl
compounds to compounds of Formula I wherein RZ means an
alkenyl residue can take place in a Wittig reaction, such
as, for example, with alkyl triphenylphosphonium
halogenide in polar solvents, such as cyclic and acyclic
ethers, chlorinated hydrocarbons, dimethylformamide or
dimethyl sulfoxide at temperatures of -50C up to the
boiling temperature of the reaction mixture; for
producing the ylene, strong bases are added, such as
alkali alcoholates, lithium organyl, and others.
If the substituent R2 contains a hydroxy group, the
latter can be reduced, e.g., by reaction with NaBII4 in
glacial acetic acid to the corresponding 2-alkyl
derivative, or it can be dehydrated with introduction of
a double bond.
The production of substituents R2 hydroxylated in the
l-position can be conducted, for example, by
Grignardization or lithium alkylation of the 2-aldehydes
or ketones. Grignardization can take place with the
customary Grignard reagents, such as alkyl magnesium
halides in an aprotic solvent, such as cyclic and acyclic
C~' . ~, .; ,~ . i:!
ethers at low temperatures (-70C to 0C). The reaction
with alkyllithium takes place under analogous conditions.
Substitution in the 6-position according to method
(c) can be performed, for example, in accordance with the
process described in ~. Cerny et al., Coll. Czech. Chem.
Comm. 49:2828 (1984) or the procedure disclosed in EP-
21,206, by reacting the 6H compound of Formula IV with
the corresponding R6-halogenides (bromides, chlorides,
iodides). Suitably, the reaction is carried out in an
inert solvent, such as dimethyl sulfoxide,
dimethylformamide, acetonitrile or nitromethane in the
presence of bases, such as alkali hydroxides or
carbonates.
Conversion of the amides and urea dsrivatives into
the thioamides and thiourea derivatives can take place,
for example, according to the procedure disclosed in EP-
A-217,730 by reaction with phosphorus oxychloride and
thiolating agent, or with Lawesson's reagent (2,~-bis(4-
methoxyphenyl-1,3-dithiophosphetane-2,4-disulfide))
according to Fieser and Fieser, "Reagents for Org.
Synth." IX, 49. The compounds of Formula I are isolated
either as the free bases or in the form of their
physiologically compatible acid addition salts.
For the formation of salts, a compound of Formula I
is dissolved, for example, in a small amount of methanol
or methylene chloride and combined with a concentrated
solution of the desired acid.
The isomer mixtures can be separated according to
the usual methods, such as, for example, crystallization,
chromatography or salt formation, into the diastereomers
and/or trans/cis isomers.
The invention also encompasses the compounds of
Formula III representing valuable intermediates for the
preparation of pharmacologically effective compounds.
The conversion of the intermediate products takes place
according to the methods described hereinabove.
6 ~ ' 7 ~ r
-- 10 --
The starting compounds are either known or can be
prepared analogously to known compounds or analogous to
methods described herein. For example, the bromination
of the starting compounds can be effected in accordance
with the process described in E:P-A-217,734, and the 2-
morpholinomethyl group can be introduced pursuant to the
method disclosed in DE-A-3,824,661.9.
The examples set forth below are to explain the
process of this invention.
-- 11 --
Without further elaboration, ik is believed that one
skilled in the art can, using the preceding description,
utilize the present invention to its fullest extent. The
following preferred specific embodiments are, therefore,
to be construed as merely illustrative, and not
limitative of the remainder of the disclosure in any way
whatsoever.
In the foregoing and in the following examples, all
temperatures are set forth in degrees Celsius and unless
otherwise indicated, all parts and percentages are by
weight.
The entire disclosures of all applications, patents
and publications, cited above and below, and of
corresponding application Federal Republic of Germany
P 39 31 819.2, filed September 20, 1989, are hereby
incorporated by reference.
From the foregoing description, one skilled in the
art can easily ascertain the essential characteristics of
this invention, and without departing from the spirit and
scope thereof, can make various changes and modifications
of the invention to adapt it to various usages and
conditions.
,. ..
.
12 ~ ~ J^~;d ~ ,~
Example 1
3-(13-Bromo-2-me-thyl-6-n-propyl-8~-ergolinyl)-
l,l-diethylurea
____________________________________ _________
382 mg of 1,1-diethyl-3-(2-methyl-6-n-propyl-
8~-ergolinyl)urea (1 mmol) is dissolved in 20 ml of
trifluoroacetic acid and, at room temperature, 1 ml of
a l-molar solution of bromine in dichloromethane is
added dropwise. The mixture is stirred for 30 minutes,
then combined with ice, made alkaline with concen-trated
ammonia solution, and extracted with dichloromethane.
The organic phases are dried with sodium sulfate and
evaporated. The residue is chromatographed on silica
gel with dichloromethane/methanol 95:5. Yield:
259 mg (56~ of theory); after crystallization from
ethyl acetate, 146 mg ~31~ of theory~, [~]D = -11
(0.5% in chloroform).
Analogously, the following 13-bromo derivatives
are prepared from the respective starting compounds:
3-(13-bromo-2-ethyl-6-n-propyl-8a-ergolinyl)-1,1-
diethylurea, yield 42%;
3-(13-bromo-6-ethyl-2-methyl-8~-ergolinyl~-1,1-
diethylurea, yield 26%, [~]D = -17 (0.5% in chloroform);
N-(13-bromo-2-me-thyl-6-n-propyl-8~-ergolinyl)formamide,
yield 47%;
N-(13-bromo-2-ethyl-6-n-propyl-8~-ergolinyl)formamide,
yield 35%;
N-(13-bromo-2-methyl-6-n-propyl-8~-ergolinyl)-2-methyl-
propionamide, yield 28%, [~]D = +13 (0.5% in chloroform);
- :L3 - ~3 i., ~ 7 . ~
N-(13-bromo-2-methyl-6-n~propyl-~-ergolinyl)-2-
methyl-2-ethylbutyrylamide, yield 47%;
N-(13-bromo-2-methyl-6-n-propyl-8~-ergolinyl)methoxy-
acetamide, yield 32~, [~]D = ~~7 (0-5% in chloroform);
N-(13-bromo-6-ethyl-2-methyl-8~-ergolinyl)formamide,
yield 48~;
3-(13-bromo-2-methyl-6-n-propyl-8a-ergolinyl)-1,1-
diethylthiourea, yield 14%, [~]D = -~20 (0.5% in
chloroform);
10 (13-bromo-2-methyl-6-n-propyl-8~-ergolinylamino)-
(dimethylamino)sulfone, yield 41%;
13-bromo-2-methyl-8~-methylthiomethyl-6-n-propylergoline,
yield 16%, [~]D = -46 (0.1% in chloroform);
13-bromo-6-cyclopropylmethyl-2-methyl-8~-methylthio-
15 methylergoline, yield 49%, [a]D = -68 (0.1% in
chloroform);
13-bromo-6-ethyl-2-methyl-8~-methylthiomethylergoline,
yield 29%;
13-bromo-6-cyclopropylmethyl-2-ethyl-8~-methylthio-
20 methylergoline, yield 31%, 1~]D = -60 (0.1% in
chloroform);
13-bromo-2-ethyl-8~-methylthiomethyl-6-n-propylergoline,
yield 42%, [~]D = ~57 (0.1% in chloroform);
(13-bromo-2-methyl-6-n-propyl-8~-ergolinyl)aceto-
25 nitrile, yield 65%, [~]D = -42 (0.5% in chloroform~;
(13-bromo-6-ethyl-2-methyl-8~-ergolinyl)acetonitrile,
yield 47%;
f~/ ~ ~ J _ i
- 14 -
(13-bromo-2-ethyl-6-n-propyl-8~-ergolinyl)acetonitrile,
yield 40%, [a]D = -40 (0.5% in chloroform);
(13-bromo-2-methyl-6-n-propyl-8~-ergolinyl)acetamide,
yield 36~o, [a]D = -47 (0.5~i in pyridine);
(13-bromo-6-ethyl-2-me-thyl-8f~i-ergolinyl)acetamide,
yield 52%;
(13-bromo-2-ethyl-6-n-propyl-8~-ergolinyl)acetamide,
yield 20%, [a]D = -28 (O.l~i in pyridine);
13-bromo-2-methyl-6-n-propyl-8~-ergolinecarboxylic acid
(4-fluoroanilide), yield 45%, [a]D = -102 (0.1% in
pyridine);
13-bromo-8,9-didehydro-2,8-dimethyl-6-n-propylergoline,
yield 38%, [a]D = -51 (O.l~i in chloroform);
13-bromo-8,9-didehydro-6-ethyl-2,8-dimethylergoline,
lS yield 11%, [a]D = -36 (0.1% in chloroform);
13-bromo-8,9-didehydro-2,6-diethyl-8-methylergoline,
yield 19%;
13-bromo-8,9-didehydro-8-hydroxymethyl-2-methyl-6-n-
propylergoline, yield 43%, [a]D = -51 (0.1% in
pyridine);
13-bromo-8,9-didehydro-6-ethyl-8-hydroxymethyl-2-
methylergoline, yield 37%.
-- 1 5 -- f~ J J ,J) ~
Example 2
3-(13,14-Dibromo-2-methyl-6-n-propyl-8~-ergolinyl)-
l,l-diethylurea
____________________________._______ _______________
382 mg of 1,1-diethyl-3-12-me-thyl-6-n-propyl-
8~-ergolinyl)urea (1 mmol) is dissolved in 20 ml of
trifluoroacetic acid and, at room temperature, 2 ml of
a l-molar solution of bromine in dichloromethane is
added dropwise. The mixture is stirred for 30 minutes,
then combined with ice, made alkaline with concentrated
ammonia solution, and extracted with dichloromethane.
The organic phases are dried with sodium sulfate and
evaporated. The residue is chromatographed on silica
gel with dichloromethane/methanol 95:5, yield: 56%,
[~]D = -14 (0.5% in chloroform).
The following compounds are prepared
analogously:
3-(13,14-dibromo-6-ethyl-2-methyl-8~-ergolinyl)-
l,l-diethylurea, yield 49%~ [~]D = ~ (0-5% in
chloroform);
N-(13,14-dibromo-6-ethyl-2-methyl-8~-ergolinyl)form-
amide, yield 63%;
N-(13,14-dibromo-2-methyl-6-n-propyl-8~-ergolinyl)-
methoxyacetamide, yield 67%, [~]D = +2 (0.5% in
chloroform);
13,14-dibromo-6-ethyl-2-methyl-8~-methylthiomethyl-
ergoline, yield 54%;
13,14-dibromo-2-methyl-6-n-propyl-8~-methylthiomethyl-
6-n-propylergoline, yield 36%;
~ .
- 16 - ~ 3 ,~
6-cyclopropylmethyl-13,1~-dibromo-2-ethyl-8~-
methylthiomethylergoline, yield 62~;
(13,14-dibromo-6-ethyl-2-methyl-8~-ergolinyl)acet-
amide, yield 43%;
13,14-dibromo-8,9-didehydro-2,8-dimethyl-6-n-propyl-
ergoline, yield 51%.
Example 3
3-(13-Bromo-6-n-propyl-2-vinyl-8~-ergolinyl)-
l,l-diethylurea
________ __ _________________________________
By heating to 100 C, 4.47 g of 3-(13-bromo-6-
n-propyl-8~-ergolinyl)-1,1-diethylurea (10 mmol), 8 g
of morpholine hydrochloride (63 mmol) and 1.5 g of
paraformaldehyde (50 mmol) are dissolved in 70 ml of
anhydrous dimethylformamide. After 30 minutes, the
mixture is cooled, poured on ice, made alkaline with
concentrated ammonia solution, and extracted with
toluene. The organic phases are dried with sodium
sulfate and evaporated, the residue is dissolved in
30 ml of trifluoroacetic acid and heated for 30 minutes
to 60 C. This reaction mixture is again poured on ice,
gently made alkaline with concentrated ammonia solution,
and extracted by sha~ing with dichloromethane. The
organic phases are dried as above and concentrated by
evaporation, the residue is chromatographed on silica
gel with dichloromethane/methanol, thus obtaining
3.11 g of oily 3-(13-bromo-2-morpholinomethyl-6-n-
propyl-8~-ergolinyl)-1,1-diethylurea (57~ of theory).
This crude produc-t is dissolved in 150 ml of tetra-
hydrofuran, the solution is cooled -to -40 C, combined
with 3 ml of triethylamine, and then a solution of 1 ml
of tert-butyl hypochlorite in 15 ml of tetrahydrofuran
is quickly added dropwise. After 30 minutes of agita-
tion, the mixture is poured on ice, made alkaline with
.
.
25~ str~ J~ all~lllollia~ ancl extracted l)y shalcincJ with
ethyl acetate. lrlle orgallic pil~lses are dried wi-th
sodium sulfate and the solvent removecl by c1istillation;
the resicl~le is crude 3-(13-bromo-2-formyl-6-n-propyl-
8~-ergolinyl)-1,1-diethylurea, yield 2.0 g.
10 g of methyltriphenylphospllonium bromide
(2~ mmol) is s~ls~)ellded in 100 m] oE anhydrous tetra-
hydrofuran, cooled to -70 C, and 3.45 g of potassium
tert-buty]ate is added. ~fter L5 minutes of agitation,
a solution of -the crude product in 50 ml of anhydrous
tetrahydrofuran is added dropwise and the mixture is
allowed to warm up to 0 C wi-thin three hours. Then
the mixture is combined with satura-ted sodium chloride
solution and extracted with ethyl acetate. The organic
phases are dried and evapora-ted, the residue is
chroma'.ographed on silica gel with dichloromethane/meth-
anol. Yield: 1.52 g. Crystalliza-tion Erom ethyl
acetate/diisorpopyl ether yields 1.03 g of the final
product (21% of theory).
From 3-(13-bromo-6-ethyl-8~-ergolinyl)-1,1-
diethylurea, 3-(13-bromo-6-ethyl-2-vinyl-8a-ergolinyl)-
l,l-diethylurea is analogously produced in a 27% yield.
r~
- 18 -
From the foregoing description, one skllled in the
art can easily ascertain the essential characteristics of
this invention, and without departing from the spirit and
scope thereof, can make various changes and modifications
of the invention to adapt it to various usages and
conditions.