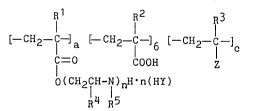Note: Descriptions are shown in the official language in which they were submitted.
ORGANIC SLUDGE DEHYDRATER
BACKGROUND OF THE INVENTION
Field of the Invention:
This invention relates to an organic sludge
dehydrator.
Description of the Prior Art:
Heretofore, the disposal of various plant effluents
and the disposal of sewage and excrements have given rise to
sludge of sedimented particles and excess sludge. As a
dehydrator for the sludge of this type, an organic
flucculant has come to find utility. In the methods for
flocculation and dehydration of sludge, the method which
resorts to exclusive addition of a cationic organic
macromolecular flocculant and the method which resorts to
simultaneous addition of a cationic organic polymeric
flocculant and an anionic organic polymeric flocculant have
been famous.
The method which relies on the sole addition of a
cationic organic polymeric flocculant, however, is not fully
effective in disposing thoroughly of the sludge and bringing
about a satisfactory result in terms of cake content and
speed of filtration, for example.
Further, in case of the method relying on combined
use of a cationic organic polymeric flocculant and an
anionic organic polymeric flocculant, though it possibly
allows improvement in cake content and speed of filtration,
it has a disadvantage that the operation thereof
necessitates installation of a plurality of flocculant
dissolving tanks and flocculant reacting tanks, the
equipment therefor is expensive, and the disposal of sludge
calls for heavy consumption of additives and boosts the cost
of chemicals.
In recent years, a method has been proposed which
uses a cationic organic macromolecular flocculant and an
anionic organic macromolecular flocculant as dissolved
jointly in a solution with the pH of the solution controlled
as disclosed in Japanese Patent Publication SHO 60(1985)-
43,800 and Japanese Patent Laid-Open SHO 58(1983)-216,706.
In the case of this method, however, there is imposed a
restriction on the kind of the cationic organic polymeric
f]occulant to be effectively usable for this method. Then,
in the case of an amphoteric organic polymeric flocculant
using as a cationic component thereof a monomer containing a
tertiary amine or a quaternary salt as disclosed in Japanese
Patent Laid-Open SHO 62(1987)-205,112, a restriction is
imposed on the balance of composition of the flocculant.
When an organic polymeric flocculant containing both
a cationic and an anionic component is used as an organic
sludge dehydrator, the dehydrated cake content is smaller
than when a cationic or an anionic flocculant is used alone
as disclosed in Japanese Patent Publication SHO 60(1985)-
43,800, Japanese Patent Laid-Open SHO 58 (1983) -216,706, and
Japanese Patent Laid-Open SHO 63(1988)-205,112. The use
found for this flocculant, however, is limited.
In the case of an amphoteric organic macromolecular
flocculant having as a cationic component thereof a monomer
containing a tertiary amine, since the balance of
composition is limited, the value of equivalent weight of
cation, that of anion, and the equivalent weight ratio of
cation/anion have their own limits. An amphoterioc organic
sludge dehydrator which combines ability of flocculation and
ability of dehydration remains yet to be developed.
When the dehydraters is in the form of powder, it
necesitales to dissolve them when using. It needs to
require a long time for dissolving, sometimes there is
occurred problems of stability of the polymer, and it also
require a special dilution equipment.
An object of the present invention is, accordingly,
to provide a novel organic sludge dehydrater.
SUMMARY OF THE INVENTION
The object described above is accomplished by an
organic sludge dehydrater of a water-in-oil form amphoteric
copolymer emulsion containing an amphoteric polyelectrolite
represented by the general formula I:
7 ~ R2 IR3
[-CH2- -]a [- CH2-C-]b [-CH2-C-] (I)
O(CH2fH- I )nH n(HY)
R4 R5
wherein n is an integer in the range of 1 to 5, providing
the average value of n is not less than 2, a, b, and c are
proportions such that the sum, a + b + c is 1, or a + b is
1, R1, R2, R3 and R4 are independently hydrogen atom or an
alkyl group, R5 is hydrogen atom, an alkyl group, or an
alkyl group substituted with a ~-hydroxy group, HY is a
monobasic acid, and Z is an amide group represented by the
general formula II:
-CoNR6R7 (II)
wherein R6 and R7 are independently hydrogen atom or an
alkyl group, a hydroxyalkyl group represented by the general
formula m:
--C02CH-CHOH (m)
R8 R9
wehrein R8 and R9 are independently hydrogen atom or an
alkyl group, or a nitrile group represented by the general
formula N:
-CN (N)
or an ester group represented by the general formula ~:
-C02R10 (~)
wherein R10 is an alkyl group, an aromatic group, or an
alicyclic group.
The object is also accomplished by an organic sludge
dehydrater of a water-in-oil type amphoteric polyelectrolite
emulsion, represented by the general formula I, having an
aminoalkyl group and a carboxyl group, which method
comprises emulsifying either at least one anionic monomer
(A) selected from the group consisting of acrylic acid and
methacrylic acid or a mixture of the anionic monomer (A)
with a nonionic monomer (B) in water-in-oil form in the
presence of water, a surfactant, and a hydrophobic organic
solvent, causing an alkylene imine to react on the resultant
water-in-oil form vinylic carboxylic acid emulsion (C)
resulting from the polymerization or copolymerization by the
use of a radical polymerization catalyst thereby
aminoalkylating the emulsion, and subsequently acidifying
the aminoalkylated emulsion with a monobasic acid.
The object is also accomplished by an amphoteric
sludge dehydrater containing an amphoteric polyelectrolite
having aminoalkyl group and a carboxyl group and represented
by the general formula ~:
R1 R2 R3 R10
Z I ]d [ CH2 f - ]e [-CH2-lc-]f [-CH2_ 7,
C=O COOH A B
(CH2CH-N)nH n(HY) (V)
R4 R5
wherein n is an integer in the range of 1 to 5, d, e, f, and
g are proportions such that the sum, d + e + f + g, is 1,
or d + e + f is 1, R1, R2, R3, R4 and R10 are
independently hydrogen atom or an alkyl group, R5 is
hydrogen atom, an alkyl group, or an alkyl group substituted
with a ~-hydroxy group, HY is a monobassic acid, A is an
ester group represented by the general formula ~:
-C02R 1 1 ( vl )
wherein Rll is an alkyl group, an aromatic group, or an
alicyclic group, an unsubstituted or a p-substituted phenyl
group represented by the general formula ~:
~R 12 (V[l)
wherein R12 is hydrogen atom, an alkyl group, or a hydroxy
group, or a nitrile group represented by the general formula
N :
-CN (N)
B is an amide group represented by the general formula II:
-CoNR6R7 (II)
wherein R6 and R7 are independently hyrogen atom or an alkyl
group, a hydroxyalkyl group represented by the general
formula m:
--C02CH-CHOH (m)
R8 R9
wherein R8 and R9 are independently hydrogen atom or an
alkyl group, or a nitrile group represented by the general
formula ~:
-CN (~)
The object is accomplished by an organic sludge
dehydrater of an amphoteric polyelectrolite, represented by
the general formula (V), having an aminoalkyl group and a
carboxyl group, which method comprises either emulsion
polymerizing in water at least one anionic monomer (A)
selected from the group consisting of acrylic acid and
methacrylic acid and a nonionic monomer (D) corresponding to
A in the general formula V to be added for the purpose of
emulsification or effecting in water the emulsion
polymerization in the presence of a nonionic monomer (B)
corresponding to B in the general formula v, causing an
alkylene imine to react on the resultant vinylic carboxylic
acid polymer emulsion (E) thereby aminoalkylating the
polymer emulsion, and subsequently acidifying the
aminoalkylated polymer emulsion with a monobasic acid.
The organic sludge dehydrator of this invention can
be used on organic sludge by the same method as the
conventional cation flocculant formed of the quaternized
dimethylamino methacrylate, for example. It is so effective
as to form tenaceous flocs, which on being dehydrated with a
press produce sludge of a conspicuously lowered water
content. This effect manifests itself particularly
conspicuously when the treatment of dehydration is effected
with a press. During the course of gravitational filtration
in a press dehydrating device, owing to the interaction
between the cation group and the anion group in the polymer,
the speed of filtration is conspicuously heightened, the
water content of filtration residue is notably lowered, and
the peelability of the filtration case from the filter cloth
is improved to a great extent. The dehydrated sludge has
small viscosity and low water content and, therefore, allows
ease of handling and enables the fuel and cost of
incineration to be lowered greatly. The species of organic
sludge which are subjectable to the dehydration treatment
herein include initially sedimented raw sludge occurring in
sewage treatment, excess sludge occurring in activated
sludge treatment and mixtures thereof with other refuses,
digested sludge, and excess sludge produced in the treatment
with activated sludge of various organic substance-
containing waste waters, for example.
EXPLANATION OF THE PREFERRED EMBODIMENT
Also, this invention relat~es to an organic sludge
dehydrator of a water-in-oil form~amphoteric polyelectrolite
emulsion represented by the general formula I possessing an
aminoalkyl group and a carboxyl group, comprising
emulsifying either at least one anionic monomer (A) selected
from the group consisting of acrylic acid and methacrylic
acid or a mixture of said anionic monomer (A) with a
nonionic monomer (B) in water-in-oil form in the presence of
water, a surfactant, and a hydrophobic organic solvent, then
polymerizing or copolymerizing the emulsified monomer with a
radical polymerization catalyst thereby forming a water-in-
oil form vinylic carboxylic acid emulsion (C), then
aminoalkylating said emulsion with an alkylene imine, and
subsequently acidifying the aminoalkylated emulsion with a
monobasic acid.
The anionic monomer (A) is preferable to be acrylic
acid or methacrylic acid. The nonionic monomer (B) is
required to be selected in consideration of the
characteristic of acid dissociation. The neutralization
degree of anionic monomer (A) is from 5-100 mol%, preferably
20-95 mol%.
The nonionic monomer (B) may be any nonionic monomer
copolymerizable with the monomer (A) mentioned above. For
example, a vinylic monomer possessing an amide group
represented by the general formula ~ can be used:
CH2
R3--e C N<R7 (~,
In the general formula X, R3, R6, and R7 are
independently hydrogen atom or an alkyl group. The vinylic
monomers of the general formula X include, for example,
acrylamide, methacrylamide, N,N-dimethyl acrylamide, N,N-
dimethyl methacrylamide, N,N-diethyl acrylamide, and N,N-
diethyl methacrylamide.
A vinylic monomer possessing a hydroxyalkyl group
represented by the general formula X is also usable:
CH2
Il
R8 R9 (X)
,
In the general formula X, R3, R8, and R9 are
independently hydrogen atom or an alkyl group. The vinylic
monomers of the general formula X include, for example,
hydroxyethyl acrylate, hydroxyethyl methacrylate,
hydroxypropyl acrylate, and hydroxypropyl methacrylate.
A vinylic monomer having an ester group represented
by the following general formula XI is also usable:
ICIH2
R3-C - C-O-Rl (XI)
wherein R3 is hydrogen atom or an alkyl group and R10 is an
alkyl group, an aromatic group, or an alicyclic group. The
compounds of the general formula XII include, for example,
methyl acrylate, n-propyl acrylate, iso-propyl acrylate, n-
butyl acrylate, iso-butyl acrylate, 2-ethylhexyl acrylate,
cyclohexyl acrylate, phenyl acrylate, methyl methacrylate,
ethyl methacrylate, n-butyl methacrylate, 2-ethylhexyl
methacrylate, cyclohexyl methacrylate, and phenyl
methacrylate.
The nonionic monomer (B) is used herein for the
purpose of adjusting the molecular weight and ion equivalent
weight of the organic sludge dehydrator.
For the water-in-oil type amphoteric type copolymer
emulsion to be produced by the method of this invention, the
amounts of the anionic monomer (A) and the nonionic monomer
(B) to be used during in the polymerization of the water-in-
oil form vinylic carboxylic acid polymer emulsion (C) must
be fixed so that the produced copolymer emulsion may acquire
a cation equivalent weight value, Cv, in the range of 0.8 to
10.0 meq/g, and an anion equivalent weight value, Av, in the
range of 0.1 to 6.0 meq/g.
When at least one anionic monomer (A) selected from
the group consisting of acrylic acid and methacrylic acid or
a mixture of the anionic monomer (A) with a nonionic monomer
(B) is to be emulsified in water-in-oil form in the presence
of water, a surfactant, and a hydrophobic organic solvent,
the surfactant may be a nonionic surfactant in popular use.
The nonionic surfactants which are usable herein include,
for example, sorbitan monooleate, sorbitan monostrearate,
sorbitan monolaurate, polyoxyethylene sorbitan monostearate,
polyoxyethylene sorbitan monooleate, polyoxyethylene sorbin
monolaurate, polyoxyethylene nonylphenyl ether,
polyoxyethylene lauryl ether, and glycerol monooleate.
These nonionic surfactants may be used either singly or in
the form of a mixture of two or more members.
Optionally, the nonionic surfactant may be used in
combination wit;h an anionic and a cationic surfactant of
ordinary grade.
The hydrophobic organic solvents which are usable
herein include, for example, hydrophobic aliphatic and
aromatic hydrocarbons, vegetable and animal oils, and
modified products of such oils. Typical examples are normal
paraffin, isoparaffin, cyclohexane, naphtene, toluene,
xylene, kerosine, mineral oils, and lamp oil, etc.
The total amount of the anionic monomer (A) and the
nonionic monomer (B) to be used herein is preferable to
account for a concentration in the range of 20 to 80% by
weight, based on the amount of water. The concentration of
the surfactant to be used is preferable to be in the range
of 5 to 30% by weight, based on the amount of the
hydrophobic organic solvent. The ratio of the hydrophobic
organic solvent to the water is in the range of 1 : 10 to 10
:1, preferably l : 5 to 3 : 1.
When the water-in-oil form monomer emulsion obtained
by emulsifying in water-in-oil form at least one anionic
monomer (A) selected from the group consisting of acrylic
acid and methacrylic acid or a mixture of the anionic
monomer (A) with a nonionic monomer (B) in the presence of
water, a surfact;ant, and a hydrophobic organic solvent is to
be polymerized or copolymerized, a redox type or azo type
radical polymerization initiator may be used as occasion
demands. The redox type polymerization initiators include,
for example, ammonium persulfate, potassium persulfate,
hydrogen peroxide, benzoil peroxide, and t-butyl peroxide.
The azo type polymerization initiators usable herein
include, for example, azobis(amidinopropane) Hcl,
azobisisobutyronitrile, azobis(dimethylvaleronitrile), and
azobis(cyclohexanecarbonitrile).
It is also permissible to add to the polymerization
system a well-known chain transfer agent such as isopropyl
alcohol, erythorubic acid, or 2-mercapto ethanol.
The polymerization temperature is preferable to be
externally controlled, during the initial phase of the
polymerization, in the range of 10 to 60C, and, during the
normal phase of the polymerization, in the range of 30 to
1 OOC
Though the polymerization time is variable with the
concentration of monomers, the polymerization temperature,
and the polymerization degree aimed at, for example, it is
generally in the range of 10 minutes to 10 hours, preferably
1 to 7 hours.
Further, preparatory to the aminoalkylation, it is
necessary to fix the amounts of the vinylic carboxylic acid
polymer (C) and the alkylene imine to be used for the
aminoalkylation.
If the cation equivalent weight value, Cv, is less
than o.8 meq/g, the produced amphoteric polyelectrolite
manifests its characteristics only with difficulty. If the
cation equivalent weight value, Cv, exceeds 10.0 meq/g, the
produced amphoteric polyelectrolite does not easily manifest
its characteristics. If the anion equivalent weight value,
Av, is less than 0.1 meq/g, the produced amphoteric
polyelectrolite manifests its characteristics only with
difficulty. Conversely, if the anion equivalent weight
value, Av, exceeds 6.0 meq/g, there arises a disadvantage
that the produced amphoteric polyelectrolite tends to suffer
a decrease in its solubility in water.
-10-
t~
The reaction of aminoalkylation can be effected by
causing an alkylene imine to react on the water-in-oil form
vinylic carboxylic acid polymer emulsion (C).
Preparatory to the aminoalkylation, it is necessary
to fix the amounts of the water-in-oil form vinylic
carboxylic acid polymer emulsion (C) and the alkylene imine.
The aminoalkylation is effected by causing reaction
of the acid group of the vinyl type carboxylic acid polymer
(C) with the alkylene imine as indicated below.
The alkylene imine for exchanging the free carboxyl
group of the vinylic polymer for an aminoester group is a
1,2-alkylene imine (aziridine). Among other 1,2-alkylene
imines, 1,2-propylene imine and ethylene imine prove to be
particularly desirable because of their ready availability
and relatively low prices. Optionally, n-alkyl-substituted
or unsubstituted 1,3-alkylene imines (azetidine) are usable
because their imines, in forming an aminoester group,
exhibit chemical reactivity and other properties similar to
those of 1,2-imines.
The acidification of a suspended aminoalkyl group is
effected with a monobasic acid, which is used in an amount
in the range of 50 to 100 mol% (preferably 60 to 90 mol~),
based on the amount of the added alkylene imine. The
addition of the monobasic acid to the reaction system is
carried out either collectively or peacemeal during the
course of the aminoalkylation. The monobasic acid is
selected from among mineral acids such as hydrochloric acid,
nitric acid, and carboxylic acids such as acetic acid,
formic acid etc.
For the organic sludge dehydrator of water-in-oil
form amphoteric copolymer emulsion to be obtained by the
method of this invention, it is preferable to control
suitably the molecular weight thereof in addition to the
cation equivalent weight and the anion equivalent weight
value mentioned above. With intrinsic viscosity as an index
to molecular weight, the composition of the component
f ~ ~
monomers, the polymerization conditions, etc. are preferable
to be suitably set so that the produced water-in-oil form
amphoteric copolymer emulsion may acquire intrinsic
viscosity [~] in the range of 0.1 to 25, preferably 1 to 15.
Also, this invention relates to an organic sludge
dehydrator of an amphoteric polyelectrolyte represented by
the general formula V having an aminoalkyl group and a
carboxyl group obtained by either emulsion polymerizing in
water at least one anionic monomer (A) selected from the
group cons.isting of acrylic acid and methacrylic acid and a
nonionic monomer (B) corresponding to A in the general
formula V and added for the purpose of emulsification or
effecting said emulsion polymerization in water in the
presence of a hydrophilic nonionic monomer (C) corresponding
to B of said general formula (V), allowing the resultant
vinylic carboxylic acid polymer emulsion to be reacted upon
by an alkylene imine thereby aminoalkylating said vinylic
carboxylic acid polymer emulsion, and subsequently
acidifying said aminoalkylated polymer emulsion with a
monobasic acid.
The anionic monomer (A) to be used herein is as
already described.
The nonionic monomer (D) may be any nonionic monomer
which is emulsifiable and, at the same time, copolymerizable
with the aforementioned monomer (A). The nonionic monomers
which are usable herein include vinylic monomers possessing
an ester group represented by the general formula X I .
Typical examples are as already cited. Further, vinyl
compounds possessing an unsubstituted or p-substituted
phenyl represented by the general formula XII:
CH2
R3-C ~ R12 (XII)
wherein R3 and R12 have the meanings defined above, are also
usable. Typical examples are styrene, p-methylstyrene, and
-12-
p-vinylphenol. Acrylonitrile may be cited as another
example.
The nonionic monomer (D) is used herein for the
purpose of enabling the vinylic carboxylic acid polymer
emulsion (E) to be obtained as an emulsion possessing low
viscosity and allowing an increase in molecular weight.
Generally it is added in an amount of not more than 20 mol%,
based on the amount of the vinylic carboxylic acid polymer
emulsion (E). If this amount exceeds 20 mol%, there arises
a disadvantage that the produced amphoteric polyelectrolite
exhibits inferior solubility in water.
As the nonionic monomer (D), any of the nonionic
monomers which are copolymerizable with the aforementioned
monomers (A) and (B) can be used.
The nonionic monomer (B) is used for the purpose of
adjusting the molecular weight and ion equivalent weight of
the amphoteric polyelectrolite. Generally, it is preferable
to be used in an amount of not more than 70 mol% based on
the amount of the vinyl type caboxylic acid polymer emulsion
(E).
For the amphoteric polyelectrolite to be produced by
the method of this invention, it is necessary to fix the
amounts of the anionic monomer (A) and the nonionic monomers
(B) and (D) to be used in the polymerization of the vinylic
carboxylic acid polymer emulsion (E) so that the produced
amphoteric polyelectrolite may acquire a cation equivalent
weight value, Cv, in the range of 0.8 to 10.0 meq/g and an
anion equivalent weight value, Av, in the range of 0.1 to
6.0 meq/g. The amounts of monomers [the total amount of the
anionic monomer (A) and the nonionic monomers (B) and (D)
(hereinafter referred to as "total amount of monomers")] are
preferable to account for a concentration approximately in
the range of 10 to 80% by weight. If the concentration is
less than 10% by weight, there arises a disadvantage that
the polymerization betrays poor productivity. Conversely,
if the concentration exceeds 80% by weight, there ensure at
-13-
disadvantage that the polymerization generates a large
volume of heat and the polymerization system suffers from
undue rise of temperature.
In emulsion polymerizing in water at least one
anionic monomer (A) selected from the group consisting of
acrylic acid and methacrylic acid and a nonionic monomer (D)
corresponding to A in the general formula V to be added for
the purpose of emulsification with the anionic monomer or in
effecting the emulsion polymerization in the presence of a
hydrophilic nonionic monomer (B) corresponding to B of the
general formula V, it is permissible to use a surfactant for
the purpose of ensuring thorough dispersion of the monomers
(A), (D), and (B). Though the surfactant to be used is not
specifically defined, it is preferable to possess relatively
high hydrophylicity enough for the formation of an 0/W form
emulsion in consequence of the emulsification. The
surfactants which are usable herein for this purpose include
nonionic surfactants such as polyoxyethylene nonylphenyl
ether and polyoxyethylenestearyl ether, anionic surfactants
such as sodium lauryl sulfate and polyoxyethylene
nonylphenyl ether sodium sulfate, and cationic surfactants
such as stearyl amine acetate and stearyl trimethyl ammonium
chloride, for example. The amount of the surfactant to be
used is in the range of 0.01 to 10% by weight, preferably
0.1 to 5% by weight, based on the total amount of monomer~.
In the production of the vinylic carboxylic acid
polymer emulsion (E), it is permissible to use a redox type
or azo type radical polymerization initiator, as occasion
demands. The kinds of the polymerization initiator and the
amount of the polymerization initiator to be used are as
already described.
The polymerization temperature is required to be
controlled externally, during the initial phase of the
polymerization, in the range of 10 to 40C, preferably 20
to 40C, and, during the normal course of the
-14-
:~ `
polymerization, in the range of 30 to 100C, preferably 40
to 80C.
Though the polymerization time is variable with the
concentration of the monomers, the polymerization
temperature, and the polymerization degree aimed at, for
example, it is generally in the range of 10 minutes to 10
hours, preferably 1 to 7 hours.
Preparatory to the reaction of aminoalkylation, it
is necessary to fix the amounts of the vinylic carboxylic
acid polymer emulsion (E) and the alkylene imine.
If the cation equivalent weight value, Cv, is less
than 0.8 meq/g, the produced amphoteric polyelectrolite does
not manifest its characteristics easily. Conversely, if the
cation equivalent weight value, Cv, exceeds 10.0 meq/g, the
characteristics expected of the produced amphoteric
polyelectrolite do not easily manifest themselves. If the
anion equivalent weight value, Av, is less than 0.1 meq/g,
the produced amphoteric polyelectrolite manifests its
characteristics only with difficulty. If this value exceeds
6.0 meq/g, there is a disadvantage that the produced
amphoteric polyelectrolite tends to exhibit inferior
solubility in water.
The aminoalkylation can be carried out by causing
the vinylic carboxylic acid copolymer emulsion (E) to be
acted upon by an alkylene imine.
Specifically, the aminoalkylation is conventional
manner as described above between the carboxylic acid group
of the vinylic carboxylic acid polymer emulsion (E) and the
alkylene imine. The typical examples of the alkylene imine
and the amount of the alkylene imine to be used are as
already described. The conditions for the aminoalkylation
are also as described above.
For the organic sludge dehydrator containing the
amphoteric polyelectrolite by the method of this invention,
it is preferable to control suitably the molecular weight
thereof in addition to the cation equivalent weight value
-15-
and the anion equivalent weight value mentioned above. With
intrinsic viscosity as an index to molecular weight, the
composition of the component monomers, the polymerization
conditions, etc. are preferable to be suitably set so that
the produced amphoteric polyelectrolite may acquire
intrinsic viscosity [~] in the range of 0.1 to 25, preferably
1 to 15.
The organic sludge dehydrator of this invention, in
addition to organic sludge, gives rise to flocs. These
flocs are destined to be dehydrated by the conventional
method. The dehydrating devices which are usable for this
purpose include, for example, screw-press type dehydrating
devices, filter-press type dehydrating devices, belt-press
type dehydrating devices, screw decanters, and centrifugal
devices.
Now, the present invention will be described more
specifically below with reference to working examples. It
should be noted, however, that the present invention is not
limited in any sense by these examples.
Referential Example 1 [Method for production of organic
sludge dehydrator]
In a four-neck flask fitted with a stirrer, a
thermometer, a condenser, a dropping funnel, and a nitrogen
gas inlet tube, 100 g of Isoper M (isoparaffin solvent
produced by Exxon Chemical) was placed and 11.6 g of
sorbitan monooleate was dissolved therein and the resultant
mixture was emulsified by gradual addition thereto of a
mixed solution prepared as an aqueous monomer solution by
the combination of 80 g of acrylic acid, 20 g of acrylamide,
52.9 g of aqueous 28 wt% ammonia solution, and 33.9 g of
deionized water. After the internal gas of the reaction
system had been thoroughly displaced with nitrogen gas, the
reaction mixture was heated to 60C and, in the presence of
0.7 g of azobis(dimethyl valeronitrile) added thereto as a
catalyst, was heated at 60C and, at the same time, stirred
-16-
for 4 hours. Consequently, there was obtained a water-in-
oil form vinylic carboxylic acid polymer emulsion.
Ethylene imine (23.9 g) was added dropwise while
keeping at 50C, and stirring was continued for 30 min. To
the resultant solution, were added dropwise 57.4 g of 61 wt~
of nitric acid, then stirring was continued for 30 min.
Further ethylene imine (76.1 g) were added dropwise, then
stirring was continued for 30 min. Additional 61 wt% of
nitric acid( 110.7 g) were added dropwise, then stirring was
continued for 30 min. give rise to an organic sludge
dehydrator aimed at. The reaction condition are shown in
Table 1.
Referential Examples 2-10
The procedure of Referential example 1 was repeated,
except that the conditions indicated in Table 1 were used.
Referential Example 11 [Production of organic sludge
dehydrator]
In a four-neck flask fitted with a stirrer, a
thermometer, a condenser, a dropping funnel, and a nitrogen
gas inlet tube, 820 g of deionized water and 0. 8 g of sodium
lauryl sulfate were stirred for thorough solution. To the
resultant solution, were added 25.6 g of acrylic acid (AA),
3.2 g of acrylamide (AAm), and 3.2 g of styrene (St). The
reaction mixture was kept stirred and the internal gas of
the reaction system was thoroughly displaced with nitrogen
gas. After the nitrogen displacement, the reaction mixture
was heated to 50C and 0.288 g of ammonium persulfate (APS)
and 0.288 g of sodium hydrogen sulfite were added as
catalyst thereto. Immediately, 102.4 g of acrylic acid
(AA), 12.8 g of acrylamide (AAm), and 12.8 g of styrene (St?
were added dropwise through the dropping funnel to the
reaction mixture over a period of 2 hours, with the
temperature kept at 50C. The resultant mixture was left
-17-
aging for 2 hours. Consequent;ly, there was obtained a
vinylic carboxylic acid polymer emulsion.
The emulsion obtained was kept at 50C throughout
the entire course of reaction and, after dropwise addition
of 38.2 g of ethylene imine thereto, was stirred for 30
minutes. The resultant mixture and 91.8 g of an aqueous 61
wt~ nitric acid solution added thereto were stirred for 30
minutes. Subsequently, the ensuant mixture and 121.8 g of
ethylene imine added thereto were stirred for 30 minutes.
After addition of an aqueous 61 wt% nitric acid solution
(177.2 g), stirring was continued for 30 minutes.
Consequently, there was obtained an amphoteric
polyelectrolite. The reaction conditions and the physical
properties of the reaction product were as shown in Table 1.
Referential Examples 12 to 20
The procedure of Referential Example 11 was
repeated, except that the conditions indicated in Table 1
were used instead.
-18-
Tablel
____ . - . .
Rererential Organic sll1dge dehydration agent
Example Composition in weight
1 El/AAm/AA = 50/10/40
. . El/AAm/AA = 50/15/35
3 El/AA = 50/50
. EI/AAm/AA = 50/20/30
EIMEA/AA = 50/10/40
EVMAm/AN/AA = 50/1515/30
El/AAm/AA = 30128142
El/AAm/AA = 28M3129
. ~ ~ El/AAm/AA -- 24146130
El/AAm/AA = 15/64/21
11 EllAAmlSt/AA = 501515140
12 EIlAAmlSt/AA = 50/10/5/35
13 EIlAAmlBA/AA = 501515140
14 EIMEA/MMA/AA = 50/15/5/30
11~ ET/MAm/MA/AA = 5017.512.5140
16 EVAAm/St~AA = 5017.512.5140
17 ~ ETMEA/MMA/AA--3012V7142
18 ET/AAm/MA/AA = 2813617129
.
__ EllAAm/MAlAA = 2413818130
ET/AAm/AN/AA = 15151/13/21
AA: acrylic acid
AAm: acrylamide
HEA: 2-hydroxyethyl acrylate
AN: acrylonitrile
MAm: methacrylamide
EI: ethylene imine
-19-
St: styrene
MA: methyl acrylate
BA: butyl acrylate
MMA: methyl methacrylate
Examples 1 to 6
Flocs were formed by stirring 150 ml of mixed raw
sludge from a sewage disposal plant (pH 6.1, SS 1.8 wt%,
VSS/SS 76.3~) and an organic sludge dehydrator added thereto
in an amount stated in Table 1 at 300 rpm for 30 seconds.
Into a Buchner funnel draped with a 100-mesh nylon filter
cloth, was poured 100 ml of the flocs of sludge. The amount
of water passed through the filter cloth over a period of 10
seconds was measured. The sludge which passed through the
filter cloth over a period of 5 minutes was nipped between
two filter cloths and squeezed under 0.5 kg/cm2 for 2
minutes. The sludge (cake) resulting from the dehydration
was tested for water content. The results and the physical
properties are shown in Table 2.
Controls 1 to 3
The DAM (N,N-dimethylaminoethyl methacrylate) type
polymers indicated in Table 2 were polymerized by the known
method and then subjected to the same flocculation test as
in Example 1. The results and the physical properties are
shown in Table 2.
-20-
_ a - ~ r ., o r r~ r ~
~' .~ ~ _ __ __ _ _ _ _
_ ,_ o . ~ ,
_ _ r _ ~ _ ~ _
~ ~ ~4;~
O ~ 01 ~ G~ 0~ C~ t- t- C~ .C ;7J ~
_ ~ ~ _ _ _ ___ ~s ~
y E ¦ C E I 9 ~ E '
~ c~ c.~ ~ ~n ~D _~ ~ C'~
';~ ~ E~ ¦ E ¦ E
-21-
Examples 7 and 8
The organic sludge dehydraters indicated in Table 1
were subjected to the same flocculation test as in Example
1, using mixed raw sludge (pH 6.4, SS 1.7 wt~, VSS/SS 73.6~)
from a sewage disposal plant. The results and the physical
properties are shown in Table 3.
Controls 4 and 5
The DAM (N,N-dimethylaminoethyl methacrylate) type
polymers indicated in Table 3 were polymerized by the known
method and subjected to the same flocculation test as in
Example 7. The results and the physical properties are
shown in Table 3.
Examples 9 and 10
The organic sludge dehydraters indicated in Table l
were subjected to the same flocculation test as in Example
1, using mixed raw sludge (pH 6.2, SS 1.4 wt%, VSS/SS 74.6~)
from a sewage disposal plant. The results and the physical
properties are shown in Table 4.
Controls 6 and 7
The DAM (N,N-dimethylaminoethyl methacrylate) type
polymers indicated in Table 4 were polymerized by the known
method and subjected to the same flocculation test as in
Example 7. The results and the physical properties are
shown in Table 4.
~ ;~
~, ~
E E o, c~ _ _ ~J O E E = 3 _ O ~ .
_ __ _ ~ _ __ _ _ e~
Z
r5 _ O Z .E_ E E~ c r Z ~
C~ 5 C~ 5
The cation equivalent weight values, the anion
equivalent weight values, and the intrinsic viscosity
indicated in Tables 2 to 7 were determined by the following
methods.
(1) Cation equivalent weight value
This property was determined by placing 95 ml of
distilled water in a beaker, adding thereto 5 ml of a
solution of 1,000 ppm of a given sample, adjusting the pH
value of the resultant solution to 7.0 by addition of either
1% HCl or 1% NaOH, stirring the solution for about 1 minute,
then adding two or three drops of toluidine blue indicator
solution, and titrating the solution with N/400 PVSK
(polyvinyl sulfate potassium solution) at intervals of 2 ml.
The time at which an interval of at least 10 seconds elapsed
after the color of the sample water had changed from blue to
reddish purple was taken as the end point of this titration.
Cation equivalent weight value (Cv) (meq/g) =
(Amount of titrant [ml] for sample - amount of
titrant [ml] for blank) x F/2 x (concentration of
effective component (ppm) in sample)
The term "effective component" as used herein refers
to the component remaining after removal of neutralizing
acid from the solids of the sample.
(2) Anion equivalent weight value
This property was determined by placing 50 ml of
distilled water in a beaker, adding thereto about 0.3 g of
accurately weighed sample, stirring the resultant solution,
and titrating this solution with a N/10 NaOH solution to
obtain the scale reading of electroconductivity. The scale
reading of titration corresponding to the last (the point at
which neutralization of the whole acid present was
completed) of several points of inflection was taken for
reporting.
(3) Intrinsic viscosity (dl/g)
In 100 parts by volume of water, 0.2 part by weight
of a sample polymer was dissolved and adjusted to pH 4 with
-24-
.~
hydrochloric acid. In a conical flask (200 ml) fitted with
a ground stopper, 50 ml of the resultant solution was placed
and gently stirred with 50 ml of 2N-NaN03 for thorough
solution. Then, the resultant solution was diluted with 1N-
NaN03 to concentrations of 0.02~, 0.04%, 0.06~, and 0.08%,
diluted solutions were adjusted to pH 4.
In a constant temperature bath adjusted to 30C ~
0.1C and fitted with a Canon Fenske viscosimeter, 10 ml of
a sample was placed in the viscosimeter and allowed to flow
down spontaneously. The time required for the sample to
pass through the distance between the vertically separated
marks on the measuring bulb was measured. This procedure
was repeated at least three times to determine the intrinsic
viscosity as the average. A blank test was performed with
an aqueous solution of 1N-NaN03.
This procedure was performed on each of the 0.02 to
o . o8~ solutions mentioned above.
The reduced viscosity was calculated as follows.
Relative viscosity ~rel = t/to
Specific viscosity nlsp = (t - to)/tO ~ ~rel ~ 1
Reduced viscosity ~sp/c
wherein to is the time for downward flow of 1N-NaN03, t is
the time for downward flow of sample solution, ~rel is the
relative viscosity, rlsp is the specific viscosity, and c is
the concentration of sample solution.
On a graph having the horizontal axis graduated for
sample concentration and the vertical axis for reduced
viscosity, the numerical values obtained by the measurement
described above were plotted and straight lines were drawn
across the points. The reading of the vertical axis against
which the sample concentration was O was taken as the
intrinsic viscosity of the sample.
to = the time required for 1N-NaN03
t - the time required the sample soln.
~rel = relative viscosity
rlsp = specific viscosity
-25-
c = the concentration of the sample soln.
Examples 11 to 16
Flocs were formed by stirring 150 ml of mixed raw
sludge (pH 6.7, SS 1.9 wt~, VSS/SS 73.9~) from a sewage
disposal plant and an organic sludge dehydrator added
thereto in an amount stated in Table 1 at 300 rpm for 30
seconds. Into a Buchner fullen draped with a 100-mesh nylon
filter cloth, 100 ml of the flocs was poured. The amount of
water passed through the filter cloth over a period of 10
seconds was measured. Then, the sludge which had passed
through the filter cloth over a period of 5 minutes was
interposed between filter cloths and squeezed under pressure
of 0.5 kg~cm2 for 2 minutes to expel the water. The sludge
(cake) resulting from the dehydration was tested for water
content. The results and the physical properties are shown
in Table 5.
Controls 8 to 10
The DAM (N,N-dimethylaminoethyl methacrylate) type
polymers indicated in Table 5 were polymerized by the known
method and subjected to the same flocculation test as in
Example 11. The results and the physical properties are
shown in Table 5.
-26-
~ ~c~ ----- ----
i~C,
--~ ~
~`
L ~ ~ ¦ c E ~ c~ a .D
L~ Q e
-27-
" ,
Examples 17 and 18
The organic sludge dehydraters indicated in Table 1
were subjected to the same flocculation test as in Example
11, using mixed raw sludge (p~l 6.5, SS 1.2 wt~, VSS.tSS
70.6%) from a sewage disposal plant. The results and the
physical properties are shown in Table 6.
Controls 11 and 12
The DAM (N,N-dimethylaminoethyl methacrylate) type
polymers indicated in Table 6 were polymerized by the known
method and subjected to the same flocculation test as in
Example 11. The results and the physical properties are
shown in Table 6.
Examples 19 and 20
The organic sludge dehydraters indicated in Table 1
were subjected to the same flocculation test as in Example
1, using mixed raw sludge (pH 6.2, SS 1.5 wt%, VSS/SS 71.5%)
from a sewage disposal plant. The results and the physical
properties are shown in Table 7.
Controls 13 and 14
The DAM (N,N-dimethylaminoethyl methacrylate) type
polymers indicated in Table 7 were polymerized by the known
method and subjected to the same flocculation test as in
Example 11. The results and the physical properties are
shown in Table 7.
.~ - - .
a 3 ~ o e c u ~i ~ ~ ~o
~ .c ~ ~ ~ ~
O r ~ = E r
~
~ ~-~9~
-29-