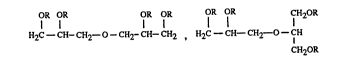Note: Descriptions are shown in the official language in which they were submitted.
2~2~2
1 -
A-17774/=
Hvdroxvphenvlcarboxylic acid esters as stabilizers
The present invention relates to novel hydroxyphenylcarboxylic acid esters, the organic
materials stabilized against thermal, oxidative and actinic degradation using these
compounds and the use of the hydroxyphenylcarboxylic acid esters as stabilizers.
DiaLIcyl-hydroxyphenylcarboxylic acid esters of di- and tripentaerythritol and their use as
stabilizers are described, for exarnple, in US-A-3,642,868. DiaL~cyl-hydroxyphenyl-
carboxylic acid esters are furthermore disclosed in EP-A-366,040 and Derwent abstract
87-105824/15.
The present invention relates to compounds of the formulae Ia andlor Ib
OR fR OR OR OR fR CH~OR
H2C--CH--CH2--0--CHr CH--CH2 , H2C--CH--- CH2--O--CH
CH20R
(Ia) (Ib)
in which the radicals R are identical or different and are a group of the formula II
--C--X {~OH
R2
in which X is a direct bond, methylene, ethylene or a group --CH2--cl H--and Y is
Cl-C8alkyl,
Rl and R2 independendy of one another are Cl-CI8alkyl, Cs-CI2cycloaL~yl,
Cs-C7cycloalkyl which is substituted by Cl-C4alkyl, or are phenyl or CTCIOphenylalkyl,
and R2 is additionally hydrogen.
2~2~ 2C~
-2-
Cl-C8all~yl Y is, for example, methyl, ethyl, propyl, butyl, pentyl, hexyl, heptyl or octyl.
Straight-chain alkyl, in particular methyl, is preferred.
Cl-CI8alkyl Rl and R2 are, for example, methyl, ethyl, propyl, isopropyl, butyl, isobutyl,
tert-butyl, pentyl, hexyl, heptyl, octyl, nonyl, decyl, undecyl, dodecyl, tridecyl, tetradecyl,
pentadecyl, hexadecyl, heptadecyl or octadecyl. Cl-C4alkyl, in particular methyl and
tert-butyl, are preferred. Rl and R2 are particularly preferably tert-butyl.
Cs-CI2cycloaL~cyl Rl and R2 are, for example, cyclopentyl, cyclohexyl, cycloheptyl,
cyclooctyl, cyclononyl, cyclodecyl cycloundecyl or cyclododecyl. Cs-C7cycloaL~cyl, in
particular cyclohexyl, is one of the preferred meanings of Rl and R2.
C5-C7cycloaLkyl Rl and R2 which are substituted by Cl-C4alkyl, preferably methyl, are,
for example, methylcyclohexyl.
C7-CIOphenylalkyl Rl and R2 are, for example, b en z y 1 o r p h eny 1 e thy 1 .
Compounds of the formulae Ia and Ib in which the radicals R are identical are preferred.
X is preferably a direct bond, methylene or ethylene, in particular ethylene.
Compounds of dhe formulae Ia and Ib in which Rl and R2 independently of one another
are Cl-Cl2al~yl, Cs-C7cycloalkyl, Cs-C7cycloalkyl which is substituted by Cl-C4aLkyl, or
are phenyl~ benzyl or phenylethyl and R2 is additionally hydrogen are of interest.
Compounds of the formulae Ia and Ib in which X is a direct bond, medhylene or ethylene,
Rl and R2 independendy of one another are Cl-C4alkyl, cyclohexyl, cyclohexyl which is
substituted by Cl-C4aL~cyl, or are phenyl or benzyl and R2 is additionally hydrogen are
likewise of interest.
Compounds of the formulae Ia and Ib in which X is ethylene, Rland R2 independently of
one anodher are Cl-C4alkyl and R2 is addidonally hydrogen are pardcularly prefe~
2Q~2~ .
Examples of compounds of the formulae Ia and Ib are:
1 ,2,6,7-tetrakis[(3',5'-di-tert-butyl-4'-hydroxyphenyl)propionyloxy]-4-oxaheptane,
1 ,5,~tris[(3',5'-di-tert-butyl-4'-hydroxyphenyl)propionyloxyl-2-[(3',5'-di-tert-
butyl-4'-hydroxyphenyl)propionyloxymethy1]-3-oxahexane,
1,2,6,7-tetrakis[(3'-methyl-5'-tert-butyl-4'-hydroxyphenyl)propionyloxy]~oxaheptane,
1 ,5,6-tris~(3 '-methyl-5 '-tert-butyl-4'-hydroxyphenyl)propionyloxy]-2-~(3 '-methyl-5 '-tert-
butyl-4'-hydroxyphenyl)propionyloxymethyl]-3-oxahexane.
The compounds of the formulae Ia and Ib are suitable for stabilizing organic materials
against thermal, oxidadve and acdnic degradation.
Examples of such materials are:
1. Polymers of monoolefins and diolefins, for example polypropylene, polyisobutylene,
polybut-1-ene, polymethylpent-l-ene, polyisoprene or polybutadiene, and poly ners of
cycloolefins, for example of cyclopentene or norbornene; and furthermore polyethylene
(which can be non-crosslinked or crosslinked), for example high density polyethylene
(HDPE), low density polyethylene (I,OPE) and linear low density polyethylene (LLDPE).
2. Mixtures of the polymers mentioned under 1), for example mixtures of polypropylene
with polyisobutylene, polypropylene with polyethylene (for example PP/HDPE or
PP/IDPE) and mixtures of various types of polyethylene (for example LDPE/HDPE).
3. Copolymers of monoolefins and diolefins with one another or with other vinyl
monomers, for example, ethylene-propylene copolymers, linear low density polyethylene
(LLDPE) and mixtures thereof with low density polyethylene (lDPE),
propylene-but-1-ene copolymers, propylene-isobutylene copolymers, ethylene-but-1-ene
copolymers, ethybne-hexene copolymers, ethylene-methylpentene copolymers,
ethylene-heptene copolymers, ethylene-octene copolymers, propylene-butadiene
copolymers, isobutylene-isoprene copolymers, ethylene-alkyl acrylate copolymers,ethylene-alkyl methacrylate copolymers, ethylene-vinyl acetate copolymers or
ethylene-acrylic acid copolyrners and salts thereof (ionomers), as well as terpolymers of
ethylene with propylene and a diene, such as hexadiene, dicyclopentadiene or
ethylidenenorbornene; and furthermore mixtures of such copolymers with one another and
with the polymers mentioned under 1), for example polypropylene/ethylene-propylene
copolymers, LDPE/ethylene-vinyl acetate copolymers, LDPElethylene-acrylic acid
2 ~ 'i 2'~
- 4-
copolymers, LLDPE/ethylene-vinyl acetate copolymers and LLDPE/ethylene-acrylic acid
copolymers.
3a. Random or alternating copolymers of a-olefins and carbon monoxide.
3b. Hydrocarbon resins (for example Cs-Cg), including hydrogenated modificationsthereof (~or example tackifying resins).
4. Polystyrene, poly-(p-methylstyrene) and poly(a-methylstyrene).
5. Copolymers of styrene or a-methylstyrene with dienes or acrylic derivatives, for
example styrene-butadiene, styrene-acrylonitrile, styrene-aL~cyl methacrylate,
styrene-butadiene-alkyl acrylate, styrene-maleic anhydride and
styrene-acrylonitrile-methyl acrylate; mixtures of high irnpact strength of styrene
copolymers and another polymer, for example a polyacrylate, a diene polymer or an
ethylene-propylene-diene terpolymer; and block copolymers of styrene, for example
styrene-butadiene-styrene, styrene-isoprene-styrene, styrene-ethylene/butylene-s~rene or
styrene-ethylene/propylene-styrene.
6. Graft copolymers of styrene or ~-methylstyrene, for exarnple styrene on polybutadiene,
styrene on polybutadiene-styrene copolymers or polybutadiene-acIylonitrile copolymers,
and styrene and acrylonitrile (or methacrylonitrile) on polybutadiene; styrene, acrylonitrile
and methyl methacrylate on polybutadiene; styrene and maleic anhydride on
polybutadiene; styrene, acIylonitrile and maleic anhydride or maleimide on polybutadiene;
styrene and maleimide on polybutadiene, styrene and aL~cyl acrylates or alkyl
methacrylates on polybutadiene, styrene and acrylontrile on ethylene-propylene-diene
terpolymers, styrene and acrylonitrile on polyalkyl acrylates or polyalkyl methacrylates,
styrene and acrylonitrile on a~rylate-butadiene copolyrners, and mixtures ~hereof with the
copolymers mendoned under 5), such as are known, for exarnple, as so-called ABS, MBS,
ASA or AES polymers.
7. Halogen~ontaining polymers, for example polychloroprene, chlorinated rubber,
chlorinated or chlorosulfonated polyethylene, copolymers of ethylene and chlorinated
ethylene and epichlorohydrin homo- and copolymers,in particular polymers of
halogen-containing vinyl compounds, for example polyvinyl chloride, polyvinylidene
chloride, polyvinyl fluoride and polyvinylidene fluoride; and copolymers thereof, such as
202~ 3 22
vinyl chloride-vinylidene chloride, vinyl cbloride-vinyl acetate or vinylidene
chloride-vinyl acetate.
8. Polyrners whlch are derived from c~,~-unsaturated acids and derivatives thereof, such as
polyacrylates and polymethacrylates, polyacrylamides and polyacrylonitriles.
9. Copolymers of the monomers mentioned under 8) with one another or with other
unsaturated monomers, for example acrylonitrile-butadiene copolymers,
acrylonitrile-aL~cyl acrylate copolymers, acrylonit~ile-alkoxyalkyl acrylate copolymers,
acrylonitrile-vinyl halide copolyrners or acrylonitrile-alkyl methacrylate-butadiene
terpolyrners.
10. Polymers which are derived from unsaturated alcohols and amines, or acyl derivatives
or acetals thereof, such as polyvinyl alcohol, polyvinyl acetate, stearate, benzoate or
maleate, polyvinyl butyral, polyallyl phthalate and polyallylmelamine; and copolymers
thereof with the olefins mendoned in point 1).
11. Homopolymers and copolymers of cyclic ethers, such as polyaL~cylene glycols,polyethylene oxide, polypropylene oxide or copolymers thereof with bisglycidyl ethers.
12. Polyacetals, such as polyoxymethylene, and those polyoxymethylenes which contain
comonomers, for example ethylene oxide; polyacetals which are modified with
thermop1astic polyurethanes, acrylates or MBS.
13. Polyphenylene oxides and sulfides, and mixtures thereof with styrene polymers or
polyamides.
14. Polyurethanes which are derived from polyethers, polyesters and polybutadienes
having terminal hydroxyl groups on the one hand and aliphadc or aromadc
polyisocyanates on ~e other hand, and precursors thereof.
15. Polyamides and copolyarnides which are derived from diamines and dicarboxylic acids
and/or aminocarboxylic acids or the corresponding lactarns, such as polyarnide 4,
polyamide 6, polyamide 616, 6/10, 6/9, 6/12 or 4/6, polyamide 11, polyamide 12 and
aromadc polyamides starting from m-xylene, diamine and adipic acid; polyarnides
prepared from hexamethylenediarnine and isophthalic and/or terephthalic acid and if
2Q~ 22
desired an elastomer as modifier, for example poly-2,4,4-trimethylhexamethylene
terephthalamide, poly-m-phenylene-isophthalamide; block copolymers of the
abovementioned polyamides with polyolefins, olefin copolymers, ionomers or chemically
bonded or grafted elastomers; or with polyethers, for example with polyethylene glycol,
polypropylene glycol or polytetramethy1ene glycol; and furthermore polyarnides or
copolyamides modified with EPDM or ABS; and polyamides which are fused during
processing ("RIM polyamide systems").
16. Polyureas, polyimides, polyamide-imides and polybenzimidazoles.
17. Polyesters which are derived from dicarboxylic acids and dialcohols andlor from
hydroxycarboxylic acids or the corresponding lactones, such as polyethylene
terephthalate, polybutylene terephthalate, poly- 1 ,4-dimethylolcyclohexane terephthalate
and polyhydroxybenzoates, as well as block polyether esters derived from polyethers
having hydroxyl end groups; and furthermore polyesters modified with polycarbonates or
MBS.
18. Polycarbonates and polyester carbonates.
19. Polysu1fones, po1yether sulfones and polyether ketones.
20. Crosslinked polymers which are derived from aldehydes on the one hand and phenols,
urea or melamine on the other hand, such as phenol-formaldehyde resins,
urea-formaldehyde resins and melamine-formaldehyde resins.
21. Drying and non-drying alkyd resins.
22. Unsaturated polyester resins which are derived from copolyesters of saturated and
unsaturated dicarboxylic acids with polyhydric alcohols, and also vinyl compounds as
crosslinking agents, such as also halogen~ontaining modifications thereof of lowflammability.
23. Crosslinkable acrylic resins which are derived from substituted acrylic acid esters, for
example from epoxy acrylates, urethane acrylates or polyester ylates.
24. Alkyd resins, polyester resins and acrylate resins which are crosslinked with melamine
2~2~22
resins, urea resins, polyisocyanates or epoxy resins.
25. Crosslinked epoxy resins which are derived from polyepoxides, for example from
bisglycidyl ethers or from cycloaliphatic diepoxides.
26. Naturally occurring polymers, such as cellulose, natural rubber, gelatin andpolymer-homologous chemically modified derivatives thereof, such as cellulose acetates,
propionates and butyrates, and the cellulose ethers, such as methylcellulose, as well as
colophony resins and derivatives.
27. Mixtures (polyblends) of the abovementioned polymers, for example PP/EPDM,
polyamide/EPDM or ABS, PVC/EVA, PVCtABS, PVC/MBS, PCtABS, PBTPtABS,
PC/ASA, PCtPBT, PVCtCPE, PVClacrylates, POh~lthermoplastic PUR, PC/therrnoplastic
PUR, POMtacrylate, POM/MBS, PPO/H[PS, PPO/PA 6.6 and copolymers, PA/HDPE,
PAtPP and PA/PPO.
28. Naturally occurring and synthetic organic substances which are pule monomeric
compounds or mixtures of these, for example mineral oils, animal or vegetable fats, oils
and waxes, or oils, waxes and fats based on synthetic esters (for example phthalates,
adipates, phosphates or trimellitates), as well as blends of synthetic esters u~th mineral
oils in any weight ratios, such as are used, for example, as spinning preparations, and
aqueous emulsions thereof.
29. Aqueous emulsions of naturally occurring or synthetic rubbers, for example natural
rubber latex or latices of carboxylated styrene-butadiene copolyrners.
The invendon ~us furthcrmore relates to composihons containing an organic material
which is sensidve againts oxidative, thermal or actinic degradahon and at least one
compound of thc formulae Ia andlor Ib, as well as the use of compounds of the formulae Ia
and Ib for stabilizing organic mate~ial against oxidative, thermal or acdnic deg~adadon.
Preferred organic matcrials are polymers, for cxample synthedc polymers, in particular
thermoplastic polymers. Pardcularly p~cfeITed organic materials are polyolefins, for
examplc those mendoned above under points 1 to 3, in particular polyethylene andpolypropylene.
2~2~)~2~
- 8 -
The compounds of the formulae Ia and Ib are in general added to the material to be
stabilized in amounts of 0.01 to 10 %, preferably 0.01 to 5 %, in pardcular 0.01 to 2 %,
based on the total weight of the material to be stabilized.
In addidon to the compounds of the formulae Ia and/or Ib, the compositions according to
the invention can addidonal1y contain conventional additives, for example those
mentioned below.
1 Antioxidants
1.1. ~lkvlated monoPhenols, for example 2,~di-tert-butyl-~methylphenol,
2-tert-butyl-4,~dimethylphenol, 2,~di-tert-butyl-4-ethylphenol,
2,6 di-tert-butyl-4n-butylphenol, 2,~di-tert-butyl~i-butylphenol,
2,~di-cyclopentyl-4-methylphenol, 2-(a-methylcyclohexyl~4,6 dimethylphenol,
2,6 di-octadecyl-4-methylphenol, 2,4,~tri-cyclohexylphenol,
2,~di-tert-butyl-4-methoxymethylphenol and 2,~di-nonyl-~methylphenol.
1.2. ALlcvlated hvdroquinones, for example 2,6 di-tert-butyl-~methoxyphenol,
2,5~i-tert-butylhydroquinone, 2,5-di-tert-amylhydroquinone and
2,~diphenyl-4-octadecyloxyphenol.
1.3. Hvdroxvlated diphenYI thioethers, for example
2,2'-thio-bis-(~tert-butyl ~- methylphenol), 2,2'-thi~bis-(4-octylphenol),
4,4'-thio-bis-(~tert-butyl-3-methylphenol) and
4,4' -thio-bis-(~tert-butyl-2-methylphenol).
1.4. AlkYlidene bisDhenols, for example 2t2'-methylene-bis-(6-tert-butyl-4-methylphenol),
2,2'-methylene-bis-(~tert-butyl-4-ethylphenol), 2,2'-methylene-bis-~4-methyl-
~(a-methylcyclohexyl)-phenol], 2,2'-methylene-bis-(4-methyl-~cyclohexylphenol),
2,2'-methylene-bis-(~nonyl-4-methylphenol), 2,2'-methylene-bis-(4,~di-tert-
butylphenol), 2,2'-ethylidene-bis-(4,~di-tert-butylphenol), 2~2'-ethylidene-bis-(~
tert-butyl-4-isobutylphenol), 2,2'-methylene-bis-[~(a-methylbenzyl)4-nonylphenol],
2,2'-methylene-bis-[~(a,-dirnethylbenzyl)4-nonylphenol], 4,4'-methylene-bis-(2,~di-
tert-butylphenol), 4,4'-methylene-bis-(~tert-butyl-2-methylphenol), 1,1-bis-(5-tert-
butyl-4-hydroxy-2-methylphenyl)-butane, 2,~bis-(3-tert-butyl-5-methyl-2-hydroxy-benzyl)-4-methylphenol, 1,1,3-tris-(5-tert-butyl-4 hydroxy-2-methylphenyl)-butane,
2 ~ 2 2
g
1,1-bis-(5-tert-butyl4-hydroxy-2-methylphenyl)-3-n-dodecylmercaptobutane, ethylene
glycol bis-[3,3-bis-(3'-tert-butyl-4'-hydroxyphenyl)-butyrate],
bis-(3-tertbutyl-4-hydroxy-S-methylphenyl)~icyclopentadiene and bis-[2-(3'-tert-butyl-
2'-hydroxy-5'-methylbenzyl)-~tert-butyl-4-methylphenyl] terephthalate.
1.5. Benzvl comPounds, for example 1 ,3,5-tris-(3,5-di-tert-butyl~hydroxy-benzyl)-2,4,~
trimethylbenzene, bis-(3,5-di-tert-butyl~-hydroxybenzyl)-sulfide, isooctyl 3,S-di-tert-
butyl-4-hydroxybenzylmercaptoacetate, bis-(4-tert-butyl-3-hydroxy-2,6-dimethylbenzyl)-
dithiolterephthalate, 1,3,5-tris-(3,5-di-tert-butyl 4 hydroxybenzyl)-isocyanurate,
1,3,5-tris-(4-tert-butyl-3-hydroxy-2,~dimethylbenzyl)-isocyanurate, dioctadecyl
3,5-di-tert-butyl-4-hydroxybenzylphosphonate, the Ca salt of 3,5-di-tert-butyl~
hydroxybenzylphosphonic acid monoethyl ester and 1,3,5-tris-(3,5-dicyclohexyl-
4-hydroxybenzyl) isocyanurate.
1.6. Acvlaminophenols, for example 4-hydroxylaurylanilide, 4-hydroxystearylanilide,
2,4-bis-(ocylmercapto)-~(3,5-di-tert-butyl-4-hydroxyanilino )-s-triazine and octyl
N-(3,5-di-tert-buyl-4hydroxyphenyl)-carbamate.
1.7. Esters of B-(3.5-di-tert-butvl~-hydroxvPhenvl~propionic acid with mono- or
polyhydric alcohols, for example with methanol, octadecanol, 1,~hexanediol,
neopenylglycol, thiodiethylene glycol, diethylene glycol, triethylene glycol,
pentaerythritol, tris-(hydroxyethyl) isocyanurate and N,N'-bis-~hydroxyethyl~oxalic acid
diamide.
1.8. Esters of ~-(5-tert-butyl~hvdroxv-3-methvlphenyl)-propionic acid with mono- or
polyhydric alcohols, for example with methanol, octa-decanol, 1,~hexanediol,
neopentylglycol, thiodiethylene glycol, diethylene glycol, triethylene glycol,
pentaerythritol, tris-(hydroxy)ethyl isocyanurate and N,N'-bis-(hydroxyethyl)-oxalic acid
diamide.
1.9. Esters of B-(3.5-dicYclohexYI-4-hvdrox~rphenvl)-~roPionic acid with mono- or
polyhydric alcohols, for example with methanol, octadecanol, 1,~hexanediol,
neopenylglycol, thiodiethylene glycol, diethylene glycol, triethylene glycol,
pentaerythritol, tris-(hydl~oxy)ethyl isocyanurate and N,N'-bis-(hydroxyethyl~oxalic acid
diamide.
202~X2
- 10
1.10. Amides of ~-(3,5-di-tert-butvl-4-hvdroxv~henyl)-propionic acid, for example
N,N'-bis-(3,5-di-tert-butyl-4-hydroxyphenylpropionyl)-hexarnethylenediamine,
N,N'-bis-(3,5-di-tert-butyl-4-hydroxyphenylpropionyl)-trimethylenediamine and
N,N'-bis-(3,5-di-tert-butyl-4-hydroxyphenylpropionyl)-hydrazine.
2. W absorbers and li~ht stabilizers
2.1. 2-(2'-HvdroxvPhenY!)-benzotriazoles, forexarnple the S'-methyl, 3',5'-di-tert-butyl,
S'-tert-butyl, 5'-(1,1,3,3-tetramethylbutyl), S-chloro-3',5'-di-tert-butyl,
S-chloro-3'-tert-butyl-S'-methyl, 3'-sec-butyl-S'-tert-butyl, 4'-octoxy, 3',5'-di-tert-amyl
and 3',5'-bis-(,a-dimethylbenzyl) derivatives.
2.2. 2-HvdroxvbenzoPhenones, for example the 4-hydroxy, 4-methoxy, 4-octoxy,
4-decyloxy, 4-dodecyloxy, 4-benzyloxy, 4,2',4'-trihydroxy and
2'-hydroxy-4,4'-dimethoxy derivatives.
2.3. Esters of substituted or unsubstituted benzoic acids, for example 4-tert-butyl-phenyl
salicylate, phenyl salicylate, octylphenyl salicylate, dibenzoylresorcinol,
bis-(4-tert-butylbenzoyl)-resorcinol, benzoylresorcinol, 2,4-di-tert-butylphenyl3,5-di-tert-butyl-4-hydroxybenzoate and hexadecyl 3,5-di-te~t-butyl~hydroxybenzoate.
2.4. Acrvlates, for example ethyl or isooctyl a~-cyano-,~"B~iphenylacrylate, methyl
-carbomethoxycinnamate, methyl or butyl cc-cyano-~-methyl-p-methoxycinnamate,
methyl c~-carbomethoxy-p-methoxycinnamate and N-(~-carbomethoxy-~-cyanovinyl)-2-methylindoline.
2.5. Nickel comPounds, for example nickel complexes of 2,2'-thio-bis-[4-(1,1,3,3-
tetramethylbutyl~phenols], such as the l: l or 1:2 complex, if desired with additional
ligands, such as n-butylamine, triethanolamine or N-cyclohexyldiethanolamine, nickel
dibutyldithiocarbamate, nickel salts of ~hydroxy-3,5~i-tert-butylbenzylphosphonic acid
monoalkyl esters, such as of the methyl or ethyl ester, nickel complexes of ketoximes,
such as of 2-hydroxy-4-methylphenylundecylketoxime and nickel complexes of
l-phenyl-4-lauroyl-S-hydroxy-pyrazole, if desired with additional ligands.
2.6. Stericallv hindered amines, for example bis-(2,2,6,6-tetramethylpiperidyl) sebacate,
bis-( 1 ,2,2,6,~pentamethylpiperidyl) sebacate, bis-(l ,2,2,6,6-pentamethylpiperidyl)
202~ ;~2~
- 11 -
n-butyl-3,5-di-tert-butyl-4-hydroxybenzylmalonate, the condensation product of
1-hydroxyethyl-2,2,6,6-tetramethyl-4-hydroxypiperidine and succinic acid, the
condensation product of N,N'-bis-(2,2,6,6-tetramethyl-4-piperidyl)-hexarnethyl-
enediamine and 4-tert-octylarnino-2,6-dichloro-1,3,5-s-triazine, tris-(2,2,6,6-tetrarnethyl-
4-piperidyl)-nitrilotriacetate, tetrakis-(2,2,6,6-tetrarnethyl 1 piperidyl)-
1,2,3,4-butanetetraoate and 1,1'-(1,2-ethanediyl)-bis-(3,3,5,5-tetramethyl-piperazinone).
2.7. Oxalic acid diarnides, for example 4,4'~ioctyloxyoxanilide, 2,2'-dioctyloxy-S,S'-
di-tert-butyloxanilide, 2,2'-di-dodecyloxy-S,S'-di-tert-butyloxanilide,
2-ethoxy-2'-ethyloxanilide, N,N'-bis-(3-dimethylarninopropyl)-oxalamide,
2-ethoxy-S-tert-butyl-2'-ethyloxanilide and a mixture thereof with
2-ethoxy-2'-ethyl-5,4'-di-tert-butyloxanilide, and mixtures of o- and p-methoxy- and of o-
and p-ethoxy-disubstituted oxanilides.
2.8. 2-(2-HYdroxYPhenYl)-l~3~s-triazines~ for exarnple 2,4,~tris(2-hydroxy-4-octyloxy-
phenyl~-1,3,5-triazine, 2-(2-hydroxy-4-octyloxyphenyl) ~I,~bis-(2,4-dimethylphenyl)-
1,3,5-triazine, 2-(2,4~ihydroxyphenyl)4,~bis-(2,4-dimethylphenyl)-1,3,5-triazine,
2,4-bis-(2-hydroxy-~propyloxyphenyl)-~(2,4-dimethylphenyl)-1,3,5-triazine,
2-(2-hydroxy-4-octyloxyphenyl)4,~bis-(4-methylphenyl)-1,3,5-triazine and
2-(2-hydroxy-4-dodecyloxyphenyl)-4,~bis-(2,4-dirnethylphenyl)- 1 ,3,5-triazine.
3. Metal deactivators, for example N,N'-diphenyloxalic acid diarnide, N-salicylal-N'-
salicyloylhydrazine, N,N'-bis-(salicyloyl)-hydr~zine, N,N'-bis-~3,5-di-tert-
butyl-4-hydroxyphenylpropionyl)-hydrazine, 3-salicyloylamin~1,2,~triazole and
bis-(benzylidene)-oxalic acid dihydrazide.
4. Phosphites and phosphonites, for exarnple triphenyl phosphite, diphenyl alkylphosphites, phenyl dialkyl phosphites, tris-(nonylphenyl) phosphite, trilauryl phosphite,
trioctadecyl phosphite, distearylpentaerythritol diphosphite, ~is-(2,4-di-tert-butylphenyl)
phosphite, diisodecylpentaerythritol diphosphite, bis-(2,~di-tert-butylphenyl)-
pentaerythritol diphosphite, tristearylsorbitol triphosphite, tetrakis-(2,4-di-tert-
butylphenyl)4,4'-biphenylene diphosphonite, 3,9-bis-(2,4-di-tert-butylphenoxy)-
2,4,8,1~tetraoxa-3,9-diphosphaspiro[5.5~undecane.
5. Peroxide-destro~/in~ compounds, for example esters of ~-thiodipropionic acid, for
example the lauryl, stearyl, myristyl or tridecyl ester, mercaptobenzimidazole, the zinc salt
202~ 1 22
of 2-mercaptobenzimidazole, zinc dibutyldithiocarbamate, dioctadecyl disulfide and
pentaerythritol tetrakis-(,B-dodecylmercapto)-propionate.
6. Polvamide stabilizers, for example copper salts in combination with iodides and/or
phosphorus compounds and salts of divalent manganese.
7. Basic costabilizers, for example melamine, polyvinylpyrrolidone, dicyandiamide,
triallyl cyanurate, urea derivatives, hydrazine derivadves, amines, polyarnides,polyurethanes and alkali metal salts and alkaline earth metal salts of higher fatty acids, for
example Ca stearate, Zn stearate, Mg stearate, Na ricinoleate, K palmitate, andmony
pyrocatecholate or tin pyrocatecholate.
8. Nucleatin agents, for example 4-tert-butylbenzoic acid, adipic acid and diphenylacetic
acid.
9. Fillers and reinforcin~ a~ents, for example calcium carbonate, silicates, glass fibres,
asbestos, talc, kaolin, mica, barium sulfate, metal oxides and hydroxides, carbon black and
graphite.
10. Other additives, for example plasticizers, lubricants, emulsifiers, pigments, fluorescent
brighteners, flameproofing agents, antistatic agents and blowing agents.
The conventional additives are added, for example, in concentrations of 0.01 to 10 %,
based on the total weight of material to be stabilized.
The compounds of the formulae Ia and Ib and if desired other additives are incorporated
into the organic material by known methods; for example, the incorporation into the
polymeric material can be caIried out before or during shaping or by applying the
dissolved or dispersed compounds to the polymeric material, if desired with subsequent
evaporation of the solvent. The compounds of the formulae Ia and Ib can also be added in
the form of a masterbatch, which contains these, for example, in a concentration of 2.5 to
25 % by weight, to the materials to be stabilized.
The compounds of the formulae Ia and Ib can also be added before or during the
polymerization or before the crosslinking.
2 ~ 2 2
- 13 -
The compounds of the formulae Ia and Ib can be incorporated in the pure forrn or in a
form encapsulated in waxes, oils or polymers into the material to be stabilized.
The materials stabilized in this way can be used in widely varying forms, for example as
films, fibres, tapes, moulding composidons or profiles, or as binders for surface coatings,
adhesives or putty.
The compounds of the formulae Ia and Ib can be prepared by processes analogous to
known processes, for example by reacdng a diglycerol with a compound of the formula m
uo~x-~l-Z (m)
o
R2
in which Rl, R2 and X are as defined above and Z is, for example, Cl-C4alkoxy, hydroxyl
or chlorine, in the presence of a catalyst.
If Z is Cl-C4alkoxy it is advantageous to use dibutyltin oxide, dtanium tetraisopropoxide,
LiNH2, LiH, LiOA, KOA or NaOA, in which A is, for example, hydrogen, Cl-Cloalkylor a phenyloxy group which is unsubstituted or substituted by 1 to 3 Cl-Cloalkyl radicals,
as catalysts. In this case, the reacdon is preferably carried out in an organic solvent, for
example benzene, toluene, xylene, dimethylformamide and the like. It is furthermore
advantageous for the reactants to be introduced into the reacdon vessel in an inert gas, for
example nitrogen or argon, and for the alcohol fonned during the reacdon to be distilled
off. The reacdon mixture can be further heated, for example, at 1 80-220C under a
pressure of 10-1013 mbar for 8-20 hours to bring the reacdon to completion.
If Z is hydroxyl, an acid, for example p-toluenesulfonic acid, is advantageously used as
the catalyst. If Z is chlorine, it is advantageous for the reaction to be carried out in the
presence of an acid acceptor, for example pyridine, triethylamine, NaOH or KOH. In this
case, the reaction can be carried out, for example, at a temperature of -10 to 150C.
The compounds of the formula m are known and, if they are not commercially available,
2 0 2 ~ , 2 ~J
- 14-
can be prepared by processes analogous to known processes, such as are described, for
example, in EP-A-219,459.
If a mixture of 1,2,6,7-tetrahydroxy-4-oxaheptane and 2-hydroxymethyl-l,S,~trihydroxy-
3-oxahexane is employed as the diglycerol, a mixture of the compounds of the forrnulae Ia
and Ib, which can be separated by chromatographic methods, is obtained.
The invention thus furthermore relates to a mixture of the compounds of the forrnulae Ia
and Ib.
The invention also relates to a mixture of the compounds of the formulae Ia and Ib which
is obtainable by reaction of diglycerol with an ester of the formula m
R
HO ~X--C--Z
~
R2
in which Rl, R2 and X are as defined in claim 1 and Z is Cl-C4alkoxy, hydroxyl or
chlorine, in the presence of a catalyst.
The ratio of the compounds Ia/Ib in the mixture is, for example, 100/1 to 1/100, preferably
100/1 to 60/10 and in particular 90/10 to 80/10.
The following examples illustrate the invention in more detail. The parts and percentage
data in these examples relate to the weight, unless stated otherwise.
xamDle 1: Preparation of 1,2,6,7-tetrakis[(3',5'-di-tert-butyl-4'-hydroxy-
phenyl)-propionyloxy]-4-oxaheptane
C(CH3)3
OR OR OR OR ,--~/
HzC--CH- -CHz--0--CHrCH--CH2 whe R _ ICl--CHZ--CH2{~OH
C(CH3)3
2t~7~ ,~2
121.6 g (0.42 mol) of methyl 3-(3',5'-di-tert-butyl-4'-hydroxyphenyl)propionate and
13.3 g (0.08 mol) of diglycerol are initially introduced into a 350 ml sulfonating flask and
are heated to 90C under nitrogen. 0.16 g of dibutyltin oxide (= 0.8 mol %, based on the
diglycerol employed) is then added as a catalyst and the reaction mixture is further heated
to 180C, methanol distilling of After 75 minutes, the apparatus is slowly placed under a
vacuum (13.3 mbar) and is heated at 185C for 12 hours. The reaction mixture is cooled
and the excess methyl 3-(3',5'-di-tert-butyl-4'-hydroxyphenyl)propionate employed is
distilled off under a high vacuum (2.67 x 10-5 bar) at 123-127C. Purification of the crude
product by column chromatography gives 77.5 g (= 83 % of theory) of a colourlessamorphous powder having a melting point of 57C.
Elemental analysis:
Calculated: 73.60 % C; 9.18 % H
Found: 73.83 % C; 9.15 % H
ExamDle 2: Preparation of 1,2,6,7-tetrakis[(3'-methyl-5'-tert-butyl-4'-hydroxy-
phenyl)-propionyloxy]4-oxaheptane
OR OR OR OR = ~~ CH3
H2C--CH--CHz--0--CHr CH--CH2 where R - ICI--CH2 CH~OH
C(CH3)3
45 g (0.18 mol) of methyl 3-(3'-methyl-5'-tert-butyl4'-hydroxyphenyl)propionate and
6.65 g (0.04 mol) of diglycerol are inidally introduced into a sulfonadng flask and heated
to 100C 0.1 g of dibutyldn oxide (= 1 mol %, based on the diglycerol employed) is then
added as a catalyst. The reacdon mixture is heated to 180C and kept at this temperature
in vacuo (133 mbar) for 13 hours. Working up is ca~ied out analogously to the method
described in Example 1. 28.2 g (= 68 % of theory) of an arnorphous powder which has a
meldng point to 55C are obtained.
Elemental analvsis:
Calculated: 71.65 % C; 8.34 % H
Found: 71.31 % C; 8.53 % H
2 ~ 2 /~J
Example 3: Stabilization of polypropylene
100 parts of polypropylene powder containing 0.1 % of calcium stearate are mixed with
0.3 % of dilauryl thiodipropionate and 0.1 % of the stabilizer shown in Table 1 and the
mixture is then kneaded at 200C for 10 minutes in a Brabender Plastograph. The
composidon thus obtained is pressed to sheets 1 mm thick in a press at a surfacetemperature of 260C, and strips 1 cm wide and 10 cm long are cut from these sheets. One
or more such strips from each sheet are suspended in a circulating air oven heated to
135C or 149C and observed at regular intervals of dme. Oxidadve decomposition of
these strips can be recognized by a yellow discoloration starting as a circle. The duration
of dme in days before decomposidon occurs is a measure of the stability of the sample.
Table 1:
Compound Number of days of oven ageing before decomposition
from Example 135C 149C
- 1 1
241 103
2 189 ~0