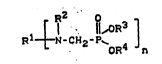Note: Descriptions are shown in the official language in which they were submitted.
- 2027078 ~ ~
, :
, " ~.
Aminomethane phosphonic acid aryl esters and a process
for their preaaration -
.
: .:
This invention relates to aminomethane phosphonic
acid aryl esters of aliphatic and aromatic amines and
polyamines, to a process for their preparation and to -~
their use as flame retardants and as cocondensable -
5 components in oligo and polycondensates based on monomers -~-
containing hydroxyl groups. ~
Compounds containing phosphorus, especially phosphonic ~-
~ ~ acid esters, are highly effective flam~ retardants used
; in f~oams, thermoplasts and duroplasts. One phosphorus~
10~ containing compound commonly used for this purpose $s
dimethyl methylphosphonate (DMMP), as described in
EP~0~108 713, but the use~of DMMP does entail difficulties.
Firstly,~the~substance is a re~atively volatile liquid
bp~.181~-C~)~so thatlt may~get~lo6t by volatilization, espec-
~ially at~elevated temper~atures; secondly, ~ike mostphosphonic~cid~esters~of lower al-iphatic alcohols, it
has~an~al~ylating~aotion in the presence of substances -~
capabIe of being~alkylated, such as amines.
EP 0 149 48~ describes the use of phosphonic acid
20 ~sa?ts of methane phosphonic acid in which the disadvantages ~-
m~ntioned above~are less severe but there are m~ny appli-
catlons for~which~flame retardants in the form of -alts
; cannot~be~used without;~reservations, e.g. in thermoplasts
used in the electrical field.
~ ~ It was an object~of the present invention to pr~ovide
a class of compounds which would be less volatile than
the known classes of~substances and would at the same -~
Le A 27 222
. .
7 ~
time provide good flame retardancY-and would be free
from alkylating properties.
This problem is solved according to the invention
by providing aminomethane phosphonic acid aryl esters
corresponding to the following general formula tI) and
salts thereof:
r, ¦¦,OR3 ]
R1~N-CH2-P~ 4 ~ I )
wherein
R1 ~tands for a monovalent or polyvalent, optionally
substituted, mononuclear or multinuclear aromatic group,
a monovalent or Polyvalent, optionally substituted
aryl~ne alkylene group, an optionally substitued aliphatic
group, an H atom, a group corresponding to the followina
formula
. .
O
¦¦,OR5
I:5 2 ~oR6
an nth part of a group of the formula R7 ~ q ~
n
~:~ a -C- group when n = 2, an optionally substituted amino
: group or a CH2-OH group,
n stands for an integer with a value from 1 to 100,
R stands for an optionally substituted aromatic group,
an optionally substituted heteroaromatic group, an -H-atom,
a group corresponding to the following formula
¦I,ORS
-CH2-P~ 6
Le A 27 222 2
~7078 ::
a -C-R7- group or a CH2-OH group, R3, R4, R5-and R6 denot-
ing, independently of one another, aromatic groups or
substituted aromatic groups,
R7 stands for an H-atom, an optionally substituted
~liphatic group, an optionally substituted aromatic group,
an optionally substituted hetero aromatic group, an option-
~lly substituted amino or imino group or an optionally
~ubstituted aromatic, hetero aromatic or aliphatic group
attached through oxygen or sulphur.
Salts of the aminomethane phosphonic acid aryl esters
corresponding to the general formula (Ij are obtainable
by the quaternization of one or more nitrogen atoms by
means of alkyl;abing substances such as alkyl iodides,
alkyl bromides or alkyl chlorides or phosphorous acLd
esters or phosphoric acid esters or phosphonic acid esters
or phosphin~c acid esters of lower aliphatic alcohols
or by the partial saponification of one or more ester
groups and neutralization with bases such as alkali metal
hydroxides or amines.
;~ 20 ~ Examples of su~table aromatic groups include phenyl
;groups and substituted phenyl groups,
e.q.~alkyl substituted isomeric methyl and dimethylphenyl
groups, the isomeric mo~ ~nd diethylphenyl gn~, ths ~ ric
mono-~nd di-isopropylphenyl groups, the isomeric mono- -
~ ~nd~di-tcrt.-butylphenyl groups, i-octylphenyl, nonylphenyl,
phenylphenyl, isopropylphenylphenyl, dodecylphenyl,
methylenephenylphenyl, I~opropylidenephenylphenyl or
cyclohexylphenyl, or halogenat~d phenyl groups, e.g.
the isomeric~mono- ~nd dichlorophenyl qroups, the isomeric
30;~mono- ~nd dibromophenyl groups or amino substituted phenyl ~-
~roups~such as aminophenyl, or phenyl groups with mixed -
~ubstituents.
The optio~ally sabstituted alkyl groups may be unsub-
stituted and straight chained alkyl groups such as methyl,
ethyl, n-propyl, n-butyl, etc. ~nd isomers thereo, such
Le A 22 i22 3
.
:.,,;~:
~ 7 (~ 7 8
as i-propyl, i-butyl, tert;-butyl, etc.. The substituted
alkyl groups may be monohydroxyl-substituted or poly-
hydroxyl-substituted straight chain or branched groups,
e.g. the CH2-OH group, the CH2-CH2-OH group, the
CH2-CH2-CH2-O~ group, the CH2-CH(OH))-CH3 group; the
CH2-C~(OH)-C2H5 group or the =CH2-CH(OH)-C~2-Cl group;
or mono- or polyamino substituted straiqht chained or
branched groups, e.g. the CH2-NH2 group, the CH2-CH2 NH2
~ro~p, the C~2-CH2-CH2-NH2 qroup or the CH2~CH(NH2)-CH~
group or imino substituted groups, halogen substituted
alkyl groups or mixed substituted alkyl groups.
- The invention further relates to a process fo~ the
preparation of aminomethanephosphonic acid aryl esters
corresponding to the general formula (I)
~5
CH2-~ R~ ~n ~I)
wherein
R1 stands for a monovalent or polyvalent, optionally
substituted, mononuclear or multinuclear aromatic group,
~ . ;
a monovalent or polyvalent, oPtionallv substituted
arylene alkylene group, an optionally substituted aliPhatic
group, an H atom, a group corresponding to the following
formula
i
¦¦pRS
-CH2-~ 6
an nth part of a group of the formula R
n
Le A 27 222 4
2~ 7~78
.
o
a -C- group when n = 2, an optionally substituted amino
group or a-CH2-OH group,
n stands for an integer with a value from 1 to 100,
R2 stands for an optionally substituted aromatic group,
5 an optionally substituted heteroaromatic group, an H :~
atom, a group corresponding to the following formula
o
¦¦,OR5
'
O
a group of the formula -C-R7 or a CH2-OH group,
R3, R4, R5 and R6 denote, independently of one another,
aromat$c or substituted aromatic groups, and
: . R7 stands for an H atom, an optionally substituted aliphatic
group, an optionally substituted aromatic group, an option-
ally substituted hetero aromatic group, an optionally
substituted amino or~imino group or an optionally sub-
stituted aromatic, heteroaromatic or aliphatic group
: a:ttached through bxygen or sulphur
by~the reaction of a nitrogen-containing compound correspond-
:~
: ing to the general formula (II)
]n (II)
20 wherein~ :
R1, R2 and n have the meanings indicated a~ove
with a triarylphosphite corresponding to the general
formula.(III)
R4 ~III)
~ `OR
:
Le A 27 222 5
a~7g
wherein
~3, R4 and R8 stand for identical or different aromatic
groups
in a molar ratio of from 0.01 to 100 mol-~, based on
S the NH functions present, and with paraformaldehyde in
a ratio of from 100 to 600 mol-~ of paraformaldehyde,
based on the triarylph~sphite, at temperatures from 20
to 300C.
The paraformaldehyde may be supplied in the form
of gaseous formaldehyde.
Compounds which are exceptionally rich in phosphorus
may be prepared by using, as nitrogen-containing compound,
a compound containing more than one NH function.
- The reactions proceed particularly smoothly if the
~5 triarylphosphite is used in less than the stoichiometric
quantity, based on all the NH functions present.
The reactions are preferably carried out at temperatures
from 90 to 160C.
~; Processes in which the triarylphosphite used is tri-
phenylphosphite and the nitrogen-containing compound
is urea, ethylene diamine, o-phenylenediamine, m-phenylene
diamine, p-phenylenediamine, isopropylene-bis-p-phenylene
d~amine or derivatives thereof, acrylic acid amide, meth-
acrylic acid amide or derivatives thereof or oligomeric
25 ~to polymeric adducts thereof are particularly preferred.
The optionally substituted nitrogen-containing compound,
the triarylphosphite and paraformaldehyde may be reacted
,together by a single stage reaction.
It has been found particularly advantageous to employ
~-~ 30 a variation of the process in which the optionally substi-
tuted, nitrogen-containing compound and the triarylphosphite
~are first introduced into the reaction vessel and the
paraformaldehyde is 6ubsequently~added thereto.
It has also been found very advantageous to employ
a process variation in which the optionally substituted,
nitrogen.containing compound, the triarylphosphite and
the paraformaldehyde are continuously reacted together.
Le A 27 222 ` 6
~ 7 07 ~
The invention further relates in particular to amino-
methane phosphonic acid aryl esters corresponding to
the general formula (IV)
O ¦¦,OR3
~O~N~~C~~N~ I OR4 (IV)
S wherein - -
R1, R3 and R4 have the.meanings indicated above and
R9 and R10, independently of one another and of R1 and
R2, have the same meaningS as R1, and salts of these com- ~:;
- pounds as well as aminomethane phosphonic acid aryl e.sters
10 corresponding to the general--formula (V) .
..
~ 1lP R~ :
:;: R9~ ,CH2 P~ (V)
O,N --~R--)---N~ 1 OR4
;~:., ~ . ..
wherein
R1, R3 and R4 have the meanings indicated above and
:R9~and R10, which are independent of one another and
lS~ of R~ nd R2, have the ame aean~ s R1 while R rtands
or an organic group~ such as~an~optionally substituted .
. ~lkylene or optlonally substituted arylene, and salts of
these compounds.
The:~minomethane: p osphonic acid ~ryl esters according .~ `:
~.: ,
to the invention are eminently suitable for use as flame
retardants in foams uch as polyurethane foams, polyiso~
~: 20 cyanurate foams or polycarbodi~mide foams based on iso-
cyanates or carbodiimides, thermoplasts such as polyethylene
terephthalate, polybutylene terephthalate, polyamide 6,
`~ polyamide 6,6, polycarbonate, polyvinyl chloride, poly- -
acrylonitrile, polyacrylate, polymethacrylate, polybuta-
diene, polystyrene and copolymers and cocondensates of
various monomers and blends of different thermoplasts,
Le A 27 222 7
~0~7~
e.g. a polycarbonate/acrylonitrile/butadiene/styrene
blend, and ~uroplasts such as phenol formaldehyde resins
based on unsubstituted phenol, cresol, xylenol and higher
homologous phenols, or formaldehyde resins based on
aliphatic or aromatic amines such as urea, thiourea,
dicyandiamide, melamine and the isomeric phenylene
diamines; or resins based, for example, on maleic acid,
phthalic acid, diols or styrene, optionally in combination
with other flame retardants, e.g. triarylphosphates such
as triphenylphosphate, diphenylcresylphosphate or tri-
cresylphosphate, the isomeric isopropyl-phenyl phenyl-
phosphates, the isomeric tert.-butylphenylphenyl-
phosphates and isomeric ~-methylbenzylphenylphenyl-
phosphates;or trialkylphosphates, e.g. triethylphosphate,
15 trichloroethylphosphate, trichloropropylphosphate, tris- ~-
dichloropropylphosphate, ethylene glycol-bis-di-2-chloro-
ethylphosphate, ethylene glycol-bis-di-2-chloropropyl-
phosphate, diethylene glycol-di-2-chloroethylphosphate --
or bis-chloromethyl-propanediol-bis-di-2-chloroethyl- - -
20 phosphate; or phosphonic acid esters, e.g. methane phosphon-
ic acid-dimethylester, methane phosphonic acid diphenyl-
ester or benzene phosphonic acid diphenylester; or phos-
phinic~acid;esters, e.g.;1-methoxy-1-oxophospholene,
1-phenoxy-1~-oxophospholer,~ or triphenylphosphane oxide;
25~ ~or~aliphatic or aromatio phosphanes, e.g. tributylphos-
phane or tr~phenylphosphane; and salts of these compounds
as well as combinations of these compounds. The aminomethane
~i~ `phosphonic acid aryl esters according to the invention
are also eminently suitable as cocondensable components
30 in oligo and polycondensates based on monomers containing
h hydroxyl qroups.
The following ic a general method of preparation
of the compounds according to-the invention:
The nitrogen-containing compound which is to be substituted
35 is brought into contact with a triarylphosphite in a
molar ratio of from 0.01 to 100 mol-~ per NH function
present and with paraformaldehyde in a molar ratio of
Le A 27 222 8
7 0 7 8 - ~
from 100 to 600 mol-~, based on the triarylphosphite
present, at temperatures from 20 to 300C. When the vola-
tile constituents have been distilled off after termina-
tion of the re~ction, the compounds according to the
S invention may be used for the purpose of the invention,
optionally after purification by washing.
In a preferred embodiment, the compounds according
to the invention are prepared as follows: -
The quantities of nitrogen-containing compound and
triarylphosphite corresponding to the required composi:
tion of the product are introduced into a reaction vessel
equipped with stirrer and heated to 140 - 150C. The
quantity of paraformaldehyde required for the desired
composition of the product is slowly added gradua1ly ~-
at this temperature without further heating, at such
a rate that the reaction temperature does not fall below ~-
140C or rise above 150C. When all the paraformaldehyde
has been added and the reaction has been completed, the
volatile constituents are distilled off in a vacuum.
The invention will now be illustrated in more detail .
with the aid of the following Examples (data for purity .
denote percentages of phosphorus unless otherwise indicated). ~ .
. ~ -
;.
Le A 27 222 9
2707~
Examples
Example 1 N-Diphenoxyphnsphonomethyl urea
72 g of product corresponding to a 94~ yield are
prepared with a degree of purity of 91% by the reaction
S of 15 g (0.25 mol) of urea, 77.5 g ~0.25 mol) of triphenyl-
phosphite and 9 g ~0.30 mol) of paraformaldehyde by the
instructions given and removal of 29.5 g of volatile
products by distillation at a temperature of lSO-C ~nd
a pressure of 2 mbar.
Example 2 N,N'-Bis(diphenoxyphosphonomethyl)-urea
127 g of product corresponding to a 92% yield are
prepared with a degree of purity of 91% by the reaction
of 15 g (0.25 mol) of urea, 155 g (0.5 mol) of triphenyl-
phosphite and 18 g (0.6 mol) of paraformaldehyde by the
instructions given and removal of 61 g of volatile
products by distillation at a temperature of 150C and
a pressure of 2 mbar.
ExamPle 3 N,N'-Bis(diphenoxyphosphonomethyl)-1,4-
phenylenediamine
69 g of product corresponding to a 92% yield are
prepared with a degree of purity of 88~ by the reaction
of 13.5 g (0.125 mol) of 1,4-phenylenediamine, 77.5 g
(0.25 mol) of triphenylphosphite and 9 g (0.3 mol) of -
paraformaldehyde by the instructions given and removal
25~ ~of 31 g of volatile products by distillation at a tempera-
ure of 150C and a pressure of 2 mbar.
ExamDle 4 N,N'-Bis(diphenoxyphosphonomethyl)-1,3-
phenylene diamine
68 g of product corresponding to a 91% yield are
prepared with a degree of purity of 87% by the reaction
of 13.5 g (0.125 mol) of 1,3-phenylenediamine, 77.S g
(0.25 mol) of triphenylphosphite and 9 g (0.3 mol) of
paraformaldehyde~by the instructions given and removal
of 32 g of volatile ~roducts by distillation at a temper-
ature of 150C and a pressure of 2 mbar.
.
Le A 27 222 10
,;,.~ " ~.
,~,, ., - , . . ..
7078
Example 5 N,N',N~-Tris(diph~noxyphosphonomethyl)-melamine
203 g of product corresponding t~-a 94~ yield are
prepared with a degree of purity of 90~ ~determined by
31P-NMR, sum of all tbe isomers) by the reaction of 31.5 g
s (0.25 mol) of mel~mine, 232.5 g ~0.75 mol) of triphenyl-
phosphite and 30.0 g (1.00 mol) of paraformaldehyde by
the instructions given and removal of 78 g of volatile
products by distillation at a temperature of 1S0C and
a pressure of 2 mbar.
10 Example 6 N,N'-8is(diphenoxyphosphonomethyl)1,2-diamino- ~- -
ethane
126 g of product corresponding to a 91% yield are
prepared with a degree of purity of 90~ by the reaction
of 15 g (0.25 mol) of ethylenediamine, 155 g (0.5 mol)
Of triphenylphosphite and 20 g (0.66 mol) of paraformal-
dehyde by the instructions given and removal of 64 g
of volatile products by distillation at a temperature
of 150C and a pressure of 2 mbar.
Exam~le 7 N-Diphenoxyphosphonomethylacrylamide
149 g of product corresponding to a 94~ yield are
prepared with a de~ree of purity of 72% by the reaction -
of 35.5 g (0.5 mol) of acrylamide, 155 g (0.5 mol) of
triphenylphosphite, 18 g ~0.6 mol) of paraformaldehyde
and 2 g (0.02 mol) of p-methoxyphenol as stabilizer by
25 the instructions given and removal of 61.5 g of volatile ~ -~
products by distillation at a temperature of 130C and
a pressure of 2 mbar.
It will be appreciated that the instant specification
` 30 and claims are set forth by way of illustration and not
limitation, and that various modifications and changes
may be made without departing from the spirit and scope
of the present invention.
.
Le A 27 222 11
:::