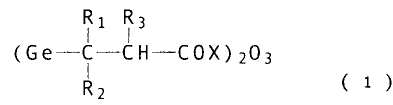Note: Descriptions are shown in the official language in which they were submitted.
~0~'~~~~
SPECIFICATION 72057-14
AGENT FOR PREVENTING AND TREATING OPACITY OF LENS
BACItGROUND OF THE INVENTION
(FIELD OF THE INVENTION]
The present invention relates to an agent for prevent-
ing and treating opacity of lens, more specifically, the
present invention relates to a highly effective agent f.dr
preventing and treating opacity of lens, the agent compris-
ing a combination of specific organic germanium compound
and phenoxazine derivatives.
[PRIOR ART]
Lens of eyes is composed of as principle components
about 65% of water and about 350 of protein, the ratio of
protein contained in lens being higher than other tissues
do. Under a variety of bialogical controls, the protein in
a high concentration, along with water inside cells, forms,
and maintains lens in hydrophilic colloidal state to retain
transparency thereof. If lens which should be transparent
happens to get opaque by some etiology, however, the quan-
tity of light to reach retina decreases. Thus, visual acu-
ity through the lens generally deteriorates, depending on
the degree of opacity induced.
As the etiology of the incidence of opacity in lens is
diversified, it is quite difficult to discuss generally.
One of the proposed mechanism is such that the water-solu-
ble, the membrane and the water-insoluble lens proteins de-
1
scribed above, contain vast amounts of the SH group (thiol
group), which are transformed into a S-S bond through bio-
logical oxidative reaction, to form insoluble aggregated
products, leading to the opacification of lens. The another
most reliable explanation is that the aforementioned pro-
teins react with sugars non-enzymatically and irreversibly
to form reaction mixture called Amadori-products, of which
reactions are general reactions between amino groups of
proteins and carbonyl groups of sugars, known as Mailard
Reaction. And this Mailard Reaction is considered to be key
reaction leading to aging. Such explanation is considered
to be one of the etiology for opaque lens. ,
The typical example of the disease associated with
opaque lens is known as cataract, which is classified into
congenital cataract and acquired one. The latter is further
classified into senile cataract, trawnatic cataract and di-
abetic cataract and others. In any type of cataracts, the
formation of Amadori-products is cansidered to be a cause
of opaque.
PROBLEMS THAT THE INVENTION IS TO SOLVE
One thing which is definitely clear in this field is
that there has not yet been established any therapeutic
treatment to prevent opacity of lens or reduce the opacity
if it might happen.
That is, many problems have not yet been solved re-
garding opacification of lens, including cataract as ex-
plained above, so that specific agents for preventing or
treating opacity of lens have not been developed. Thus, the
2
2~~'~~~
72057-14
agents for exerting significant effects only on recovering
visual acuity or blocking the progress of opacification are
now currently used.
SUi~IARY OF THE INVENTTON
An object of the present invention is to provide
an agent capable of efficiently preventing and treating
opacity of lens.
Another object: of the present invention is to pro-
vide an agent without toxicity or side effects, because
such agents should be administered for a long period.
In order to achieve the above objects, the present
invention is constructed to provide an agent for preventing
and treating opacity of lens, the agent containing as the
effective component the organic germanium campound repre-
rented by the formula;
R1 Rs
(Ge - C - CH - COX )2O3 (1 )
L
[wherein Ri to R3 represent hydrogen atoms, lower
alkyl groups each of which may be the same or different and
selected from the group consisting o~ methyl group, ethyl
group, etc., or phenyl groups substituted or unsubstituted;
X represents a hydroxyl group, an O-lowsr alkyl group, an
amino group or O Y+ [Y represents a metal such as sodium,
potassium, etc., or a compound having a basic group such as
lysozyme and basic amino acid, etc.}] and phenoxazine
derivatives.
3
CA 02027665 2000-12-28
72057-14
BRIEF DESCRIPTION OF DRAWINGS
FIGs. 1 through 6 show the percentage distribution of
lens symptoms in each eye drop group.
FIGS. 1 through 3 show the results of administration
of the eye drop containing the present agent to the mice aged 1
month and thereafter.
FIGS. 4 through 6 show the results of administration
of the eye drop containing the present agent to the animals
aged 5 months and thereafter.
DETAILED DESCRIPTION OF THE INVENTION
The present invention will now be explained in detail
hereinafter. The term "agent" employed in this specification
should be understood to mean "pharmaceutical composition".
The agent for preventing and treating opacity of lens
contains as the effective component the specific organic
germanium compound represented by the formula (1);
R1 R3
(GeCCH-COX) z03
RZ
Explanation about the compound will be made firstly.
The principle structure of the compound is composed
of germylpropionic acid where germanium atom is bonded to a
propionic acid derivative having three substituents R1 to R3
and a functional group containing oxygen, i.e. OX, and the
germanium atoms of the principle structure and the oxygen atoms
are bonded in the ratio of 2:3.
4
Each of the substituents Ri to R3 herein represents a
hydrogen atom, a so-called lower alkyl group such as methyl
group, ethyl group, propyl group, butyl group, etc., or a
phenyl group substituted or unsubstituted; the substituent
X represents a hydroxyl group, an O-lower alkyl group, an
amino group or O Y~ representing the salt of carboxylic
acid, individually. Eubstituent Y represents a metal such
as sodium, potassium or the like (the metal is not neces-
sarily monovalent), or a basic compound represented by ba-
sic amino acid and the like, such as lysozyme or lysine.
The substituents Ri and Rz, and the substituent Rs
are bonded to the germanium atom at a and ~ positions, re-
spectively. Thus, examples of organic germanium
the the
compound to be used accordance the present
in with inven-
tion are illustrated follows;
as
( Ge - CHa- CHz - COOH )zOs ( 1- 1 )
CH3
( Ge - CH - CHz COOH )2O3 ( 1- 2 )
-
CHs
I
(Ge - CHz- CH COOH )2O3 (1- 3 )
-
CH3
( Ge - CH - CH COOH ) 2O3 ~ ( 1 - 4 )
I -
CH3
CH3
I
( Ge - C CHz COOH )2O3 ( 1 - 5 )
- -
CH3
~0'~~
(Ge - CH - CHa - COOH )a0~ ( 1- 6 )
C6H5
CH3
( Ge - CH - CH - COOH ) 2O3 , ( 1- 7 )
CsHs
( Ge - CHa - CHa - COOCH~ ) a 03 ( 1- 8 )
( Ge - CHa - CHa - CONHa ) aO3 ( 1- 9 )
( Ge - CHa - CHa - COO Na+ )a03 ( 1-10 )
The organic germanium compounds having the above
structure may be produced in various processes.
For examples, the compound wherein X is OH in the for-
mula (1) may be produced as shown in the following reaction
formula;
Reaction formula
Ri Rs Ri Rs
I I H20 I I
ClsGe - C - CH - COOH ~ (Ge - C - CH - COOH)aOs
R2 (2) RZ
That is, trihalogermylpropionic acid in which the sub-
s~ituents- Ri to Rs are preliminary introduced,, such as
trichlorogermylpropionic acid (2) and the like, may be hy-
drolyzed.
On the other hand, the compound wherein X is O-lower
alkyl group may be obtained, for example, by reacting
thionyl chloride, etc. with the above compound (2) to
transform the compound into the corresponding acid halide
and subsequently reacting alcohol corresponding to the
above 0-lower alkyl group, followed by hydrolysis of the
resulting compound. And the compound of the formula (1)
6
~~~'~~~
7?057-1~I
'wherein X is NH2 may be obtained, for example, by reacting ammonia
to the above acid halide, followed by the hydrolysis.
The compound of the formula (1) containing COO-Y+
group as substituent X, wherein Y is a metal may be obtained, for
example, by reacting metal hydroxide with the above compound (1),
while the compound containing a basic group as Y may be subjected
to known acid-base reaction.
The organic germanium compounds obtained in the above
manner are subjected to instrumental analysis for nuclear magnetic
LO resonance (NMR) and infrared absorption (IR) spectra, and the
results strongly support that the above compound can be represented
by the above formula (1).
The above formulas represent the organic germanium
compounds in crystalline state, which are hydrolyzed in aqueous
solution at germanium-oxygen bonding. For example, the above
compound (1-1) transforms its original structure into the following
structure;
OH
HO - Ge - CHI - CH2 - COOH
OH
Among the above compounds, the compound (1-1) may be
preferable because of its easy availability.'
The phenoxazine derivative to be used in accordance
with the present invention is represented by the following prin-
ciple structural formula and may have one or more substituents such
as a lower alkyl group, a lower alkoxy group, a hydroxyl group, a
carboxyl group and a lower alkoxycarbonyl group:
_ 7 _
72057-14
N
Nw. \
\ \\
~O V O
and more specifically, 1-hydroxy-5-oxo-5H-pyrido(3,2-
a)phenoxazine-3-carboxylic acid (general name; pyrenoxine)
of the following formula;
HO / ~ COOH
N
Na \
''~ . \ ~~
or its salt with. metal such as sodium, potassium and the
like may be preferable.
i
This compound has been conventionally used for treat-
ment of cataract. The outcome of such treatment has been
reported in academic papers (For example, see Japanese
Clinical Ophthalmology 11:272, 1957).
The other phenoxazine derivatives represented by the
above principle structural formula are publicly disclosed
in Japanese Patent Publication No.10570/1980.
The agent for preventing and treating opacity of lens
in accordance with the present invention contains as the
effective component the organic germanium compound synthe-
sized in the above manner with the phenoxazine derivative
described above; they rnay be prepared into eye drop,
preferably, together with known components such as boric
8
CA 02027665 2000-12-28
72057-14
acid, sodium chloride, sodium hydroxide, or benzalkonium
chloride, E-aminocaproic acid, methyl p-oxyaminobenzoic acid,
and chlorobutanol, in addition to water.
The solubility of the organic germanium compound and
the like as the effective components of the present invention
may increase in a basic eye drop.
Since the organic germanium compound as the effective
component of the present invention has characteristic features
such as extremely low toxicity and little side effects, the
amount thereof to be used in eye drop may be determined in
relatively free manner. And phenoxazine derivatives have been
used for treatment of cataract over a long period and
therefore, the safety thereof has been evaluated and verified.
For example, the eye drop containing as the principle agents
the organic germanium compound and the phenoxazine derivative
may be prepared so that 5-500 mg of the organogermanium
compound and 0.005-0.5 mg of the phenoxazine derivative may be
contained in 1 ml of the eyed drop.
Furthermore, the agent containing the organic
germanium compound and the agent containing a phenoxazine
derivative may be separately prepared into formulations in
advance, and then they may be mixed to prepare the agent of the
present invention as needed basis.
ADVANTAGES OF THE INVENTION
The testing of the effects of the agent of the
present invention in senile accelerated mice susceptible to
opacity of lens, indicates such effects as the decrease in
number
9
72057-14
of opaque eyes and the increase in number of transparent
eyes, due to the eye drop containing the agent of the pre-
sent invention.
EXAMPLES
The present invention will now be explained in the
following embodiments.
As for experimental animals, senile accelerated mice
aged 1 month (12 mice) and 5 months (12 mice) were used.
Both of the age groups were divided into three groups, in-
dividually; Group 1 was given the solution of 0.05 mg/m1 1-
hydrcxy-5-oxo-5H-pyrido(3,2-a)phenoxazine-3-carboxylic acid
(general name; pyrenoxine) (the solution was ref erred to as
pyrenoxine solution hereinafter); Group 2, both of the 40
organic germanium compound (1-1) solution (referred to as
Ge solution) and pyrenoxine solution; Group 3 as the con-
trol group, distilled water containing a suppressive agent
on surface activity as a placebo solution. The administra-
tion was carried out through eye drop, 4 times daily, 6
days /week. Before the initiation of eye drop, and 30, 60,
9O and 120 days after the initiation, mydriasis was induced
using Midorin P (trade-mark) in each subject under the
anesthesia of 0.7m1/kg of sodium nembutal intraperitoneally
injected, before subjecting to lens observation under a
stereomicroscope.
The lens observation in senile accelerated mice may be
roughly classified into 4 items, i.e. transparency, forma-
tion of concentric circles, distortion and cortical opacity
in this example. By the term "transparency" is meant no ab-
2~~'~~
normal symptom in lens so that retinal vessel can be ob-
served through; by the term "formation of concentric cir-
cles" is meant 2 to 5 rings are observed in concentric cir-
cles in a lens; by the term "distortion" is meant the inci-
dence of distortion in image on eyegrounds, because of ab-
normal refraction of lens; by the term "cortical opacity"
is meant the incidence of wedge-like or diffuse opacity.
Two of such symptoms, formation of concentric circles and
distortion were occurred in some lenses and in that cases,
the symptom showing stronger change was selected as an ab-
normal symptom.
1 The gye drox~ experiments startino at 1 month since
birth
The eyes of senile accelerated mice of age 1 month
were examined prior to the administration of the eye drop.
Most of the eyes including those of the control group had
transparent lenses without any abnormal symptoms; mild cor-
tical opacity and no eye anophthalmia was simultaneously
observed in one subject of Group 2 (a simultaneous adminis-
tration group of Ge solution and pyrenoxine solution).
The examination which was carried out on day 30 after
the initiation of eye drop demonstrated that the cortical
opacity observed prior to the initiation of eye drop di.sap-
geared. However, there occurred ring-like concentric cir-
cles in one eye of Group 1 (pyrenoxine administration
group), one eye of Group 2 (a simultaneous administration
of Ge solution and pyrenoxine solution) and two eyes of
Group 3 (as the control group). The examination on day 60
after the initiation of eye drop demonstrated that abnor-
11
. .
mality in lens such as formation of concentric circles and
distortion was observed in one eye of Group 1 and four eyes
of Group 3.
The examination on day 90 after the initiation of eye
drop demonstrated that the six eyes with transparent lens
of Group 1, showed formation of concentric circles (in 4
eyes) and distortion (in two eyes), which indicated the oc-
currence of cataract at initial stage. Of Group 3, four
eyes with distorted lenses did not propose any change from
the symptoms observed at the prior examination. On the con-
trary, lenses of Group 2 were in good condition so that the
lenses were thus diagnosed transparent.
The examination on day 120 after the initiation of eye
drop demonstrated that distortion which was observed in two
eyes of Group 1 on day 90 disappeared. Distortion disap-
peared in one of the four lenses of Group 3, which had been
diagnosed to be in distorted state. Such results indicate
that distortion in lens may not generally be fixed. Of
Group 2, one animal died during the experiments, but all of
the other remaining mice were judged normal at this exami-
nation.
The results heretofore mentioned are shown in TABLE 1
below: The ratios of each symptom to total are shown in
FIGS. 1 through 3.
It might be said that these experiments verify the
preventive effect of the agent of the present invention.
12
TABLE 1
Symptoms
Before eye drop On day 30 On day 60
initiation
Dose group 1 2 3 1- 2 3 1 2 3
Number of eyes 6 7 4 6 7 4 6 7 4
Transparency 6 6 4 5 6 2 5 7 0
Formation of
concentric 0 0 0 1 1 2 0 0 0
circles
Distortion 0 0 0 0 0 0 1 0 4
Cortical 0 1 0 0 0 0 0 0 0
opacity
Symptoms
On day 90 On day 120
Dose group 1 2 3 1 2 3
Number of eyes 6 7 4 6 5 4
Transparency 0 7 0 0 5 0
Formation of
concentric 4 0 0 6 0 1
circles
Distortion 2 0 4 0 0 3
Cortical 0 0 0 0 0 0
opacity
2 The e~ drop experiments startina at 5 months
since birth
A trace of concentric circles in one or two rings be-
gan to appear around lenses in the eyes of senile acceler-
ated mice of age 5 months, but the transparency of their
eyegraunds were judged good. As a result, they were tem-
porarily judged to be transparent lenses before the initia-
tion of eye drop.
13
The examination which was carried out on day 30 after
the initiation of eye drop demonstrated the clear concen-
trio circles in two lenses of Group 2 and four lenses of
Group 3, but the eyes of Group 1 did not show any change.
The examination on day 60 after the initiation of eye
drop demonstrated that Groups 1 and 2 showed good progress
such that all eyes of the mice were judged transparent, and
that formation of concentric circles accompanied with dis-
tortion were induced in four eyes of Group 3.
The examination on day 90 after the initiation of eye
drop demonstrated concerning Groups 1 and 2, the occurrence
of severe change inducing formation of concentric circles
(in 12 eyes) and distortion (in 2 eyes). Of Group 3, four j,
eyes were diagnosed to have distortion and intense change
in the lenses thereof.
The examination on day 120 after the initiation of eye
drop demonstrated the formation of concentric circles in
all of the lenses of Groups 1 and 2 (14 lenses); in partic-
ular, the tendency of exacerbation accompanied by distor-
tion was observed in 4 eyes of Group 1 (pyrenoxine adminis-
tration group). On the other hand, of Group 3 as the con-
trol group, distortion disappeared in one of the four eyes
accompanied by distortion. However, the occurrence of cor-
tical opacity was observed in a different eye, indicating
the further progress of cataract.
The results heretofore mentioned are shown in TF~BLE 2
below. The ratios of each symptom to total are shown in
FIGS. 4 through 6.
14
~~~'~6~
It might be said that these experiments verify the ef-
fects of the agent of the present invention, on preventing
the progress and the treatment.
TABLE 2
Symptoms
Beforeeye drop On day30 On day 60
initiation
Dose group1 2 3 1 2 3 1 2 3
Number 8 4 6 8 4 6 8 4
of eyes
6
Transparency6 8 4 6 6 0 6 8 0
Formation
of
concentric0 0 0 0 2 4 0 0 0
circles
Distortion0 0 0 0 0 0 0 0 4
'
Cortical 0 0 0 0 0 0 0 0 0
opacity
Symptoms
On day 90 On day 120
Dose group 1 2 3 1 2 3
Number of eyes 6 8 4 6 8 4
Transparency 0 0 0 0 0 0
Formation of
concentric ~ 8 0 2 8 1
circles
Distortion 2 0 4 4 0 , 2
Cortical 0 0 0 0 0 1
opacity
As is clearly demonstrated by above described Example,
the effects on preventing or terminating the progress of
cataract were observed in senile accelerated mice in Groups
1 and 2, compared with those of Group 3. Of the group given
pyrenoxine solution alone, however, there were observed a
small number of mice in which cataract was in advanced
stage. That is, the above effects are not necessarily des-
ignated absolute effects. On the contrary, of Group 2,
namely the group given a combination of Ge solution and
pyrenoxine solution, all the lenses remained transparent
over a long period. Additionally to the above effects,
there was obtained remarkable improving effects in that the
lenses with severe symptoms such as cortical opacity recov-
ered transparency.
Tn the above Example, even the other compounds except
the compound (1-1), when administered, showed approximately
identical effects as those described above.
Th present invention is as has been described and
thus, the agent of the present invention is excellent as an
agent for preventing and treating opacity of lens.
16