Note: Descriptions are shown in the official language in which they were submitted.
` 2028293
METHOD FOR TREATING ZINC-CONTAINING SULFATE SOLUTION
This invention relates to a method for treating zinc-
containing sulfate solutions for the recovery of zinc and
sulfuric acid.
BACKGROUND OF THE INVENTION
In the process for the recovery of zinc from minerals,
mineral concentrates may be treated in a variety of ways
that include the roast-leach-electrowinning and the
pressure leach-electrowinning processes. During
processing, many zinc-containing sulfate solutions are
encountered from which it is often desirable to recover
both the zinc and the sulfuric acid. Such solutions may
contain from relatively low to relatively high amounts of
zinc, sulfuric acid or both. For example, in the
electrowinning of zinc from zinc sulfate electrolyte,
purified electrolyte is subjected to electrolysis, zinc is
deposited on the cathodes and recovered, and a portion of
the circulating electrolyte is purged as spent acid to
control the accumulation of impurities. Other solutions
encountered in hydrometallurgical zinc processes, for
example, are ones that contain zinc, sulfuric acid and
other metal values and impurities. Such solutions are
often treated first for the recovery of metal values and
the residual solutions must then be treated before
discarding to prevent ecological damage. Similarly,
solutions containing small amounts of zinc and
concentrations of certain impurities must be treated prior
to discharge to the environment. *
202~293
_ 2
BRIEF DESCRIPTION OF THE PRIOR ART
Numerous processes exist for the recovery of metal values
and for the removal of acid and impurities from solutions.
Aside from conventional precipitation, crystallization and
evaporation processes, such processes include ion exchange,
solvent extraction and membrane processes such as membrane
electrolysis, reverse osmosis, dialysis and
electrodialysis.
Many methods have also been developed specifically for
treating the purge of spent electrolyte for the recovery of
values and for minimizing effects on the ecology when
solutions are discarded. The methods include electrolysis
for stripping a portion of the contained zinc,
concentration for the precipitation and recovery of zinc
sulfate, membrane electrolysis or electrodialysis for the
recovery of acid which may include the cathodic deposition
of zinc, ion exchange, dialysis, solvent extraction of
zinc, usually with organic phosphoric phosphonic or
phosphinic acid-type extractants, and solvent extraction of
acid with amine-type extractants.
In general, these methods for treating zinc-containing
sulfate solutions do not provide the desired separation of
zinc sulfate from sulfuric acid and, in many cases, leave
a residual solution that must be further treated before
being discarded.
2~1~8`~93
SUMMARY OF THE I NVENT I ON
I have now discovered that zinc-containing sulfate
solutions may be successfully treated to remove the major
portion of the sulfate content as a solution of sulfuric
acid and to recover a substantially pure acid zinc sulfate
solution. The sulfuric acid solution and the zinc sulfate
solution may be returned to the zinc recovery process. An
effluent solution is formed that is substantially free of
zinc, is low in acid and contains impurity elements such as
magnesium and manganese.
More specifically, zinc-containing sulfate solutions
containing sulfuric acid are subjected to an acid removal
step for the formation of an acid solution and a solution
low in acid and containing substantially all the zinc and
the impurity metals. The acid removal is carried out by a
membrane process such as dialysis or electrodialysis alone
or in combination. The acid-reduced solution which
contains substantially all the zinc from the zinc-
containing sulfate solution, is then subjected to solvent
extraction for the extraction of zinc and acid, and for the
formation of a residual solution substantially free of zinc
but containing the impurity metals such as magnesium and
manganese, and having a low acid content. The residual
solution is removed from the process.
When using dialysis, zinc-containing sulfate solution as
feed solution is fed into the dialyzate compartments of a
dialysis unit containing alternating dialyzate and
diffusate compartments separated from each other by anionic
2028293
_ 4
membranes. The diffusate, withdrawn as product, is a
solution of sulfuric acid substantially free of zinc. The
dialyzate which contains substantially all the zinc and
impurity metals that are present in the feed solution, is
passed to solvent extraction.
When using electrodialysis, zinc-containing sulfate
solution as feed solution is fed to the diluate
compartments of an electrodialysis unit comprising
alternating anionic and cationic membranes between a
cathode and an anode. Concentrate is circulated through
the concentrate compartments, and a portion of the
circulating concentrate is removed as a sulfuric acid
solution concentrated in acid as compared to the feed
solution. Diluate from the diluate compartments is
circulated to the diluate compartments, and a portion of
the circulating diluate is removed and passed to solvent
extraction. The removed diluate has a reduced acid content
and contains substantially all the zinc from the feed
solution. Electrodialysis may be carried out in one or
more stages, as necessary to achieve the desired degree of
sulfuric acid removal.
According to another embodiment, zinc-containing sulfate
solution is subjected to dialysis for the formation of a
dialyzate relatively low in acid and containing
substantially all the zinc and impurity metals from the
feed solution. The dialyzate is passed to the subsequent
solvent extraction. The diffusate is passed to the diluate
compartments of an electrodialysis unit and a portion of
`` 202~2~3
the circulating diluate is returned to the diffusate
compartments of the dialysis unit. Preferably, the
concentration of acid in the returning diluate is
maintained low by the addition of water or other solution
in order to increase the driving force for the dialysis.
In the electrodialysis, a concentrate is formed which is
recycled to the concentrate compartments. A portion of the
circulating concentrate is removed as concentrated acid
product substantially free of metal cations.
The dialyzate from dialysis or the diluate from
electrodialysis is subjected to a dual-circuit, side-by-
side, simultaneous solvent extraction of zinc sulfate and
sulfuric acid. The pH in the zinc extraction is controlled
in the range of about 1.3 to 5. In the one circuit, acid
is extracted with a suitable extractant from feed solution
and from the raffinate from the other circuit for the
extraction of zinc. Separated organic phase from the acid
extraction is subjected to stripping with water or an
alkaline substance for the recovery of sulfuric acid or a
sulfate. The raffinate from the acid extraction is passed
to the zinc extraction step where zinc is extracted with a
suitable extractant. The raffinate from the zinc
extraction, which also contains sulfuric acid generated in
the zinc extraction, is passed to the acid extraction step.
Separated organic phase is subjected to stripping with an
acid solution, and a zinc sulfate solution suitable for
return to the hydrometallurgical process for the recovery
of zinc is recovered.
2~2~2~
_ 6
The solvent extraction may be carried out in one or more
side-by-side, simultaneous extraction stages in a
countercurrent, cross-current or co-current fashion.
Suitable extractants for sulfuric acid include primary,
secondary, tertiary and quaternary amines, and suitable
extractants for zinc sulfate include organic phosphoric,
phosphinic, phosphonic and thiophosphinic acids.
According to the main embodiment of the invention there is
provided a method for treating zinc-containing sulfate
solution containing zinc sulfate, sulfuric acid and
impurity metals which method comprises the steps of feeding
zinc-containing sulfate solution to an acid removal for the
formation of a first solution containing sulfuric acid,
said first solution being substantially free of zinc and
impurity metals, and for the formation of a second solution
having a relatively low concentration of sulfuric acid and
substantially containing the zinc and impurity metals;
recovering said first solution; passing said second
solution to dual-circuit, side-by-side simultaneous solvent
extraction for the extraction of sulfuric acid in an acid
extraction circuit with an organic extractant suitable for
the extraction of sulfuric acid forming an acid extract and
for the extraction of zinc in a zinc extraction circuit
with an organic extractant suitable for the extraction of
zinc forming a zinc extract, and the formation of residual
solution; recovering sulfate from said acid extract as a
compound chosen from the group consisting of sulfuric acid,
sodium sulfate and ammonium sulfate; and recovering zinc
from said zinc extract as a zinc sulfate solution.
2~28293
_ 7
It is an aspect of the present invention to provide a
method for treating zinc-containing sulfate solutions.
It is another aspect to provide a method for removing
sulfuric acid from zinc-containing sulfate solutions by
dialysis, electrodialysis or both.
It is a further aspect to provide a method for
simultaneously removing zinc sulfate and sulfuric acid from
zinc-containing sulfate solutions by solvent extraction.
It is yet another aspect to provide a method for the
removal of acid from zinc-containing sulfate solutions by
a membrane process and a simultaneous, dual-circuit, side-
by-side solvent extraction of zinc and sulfuric acid from
the acid-reduced solution.
BRIEF DESCRIPTION OF THE ACCOMPANYING DRAWINGS
The aspects of the present invention will become apparent
from the following detailed description with reference to
the accompanying drawings wherein:
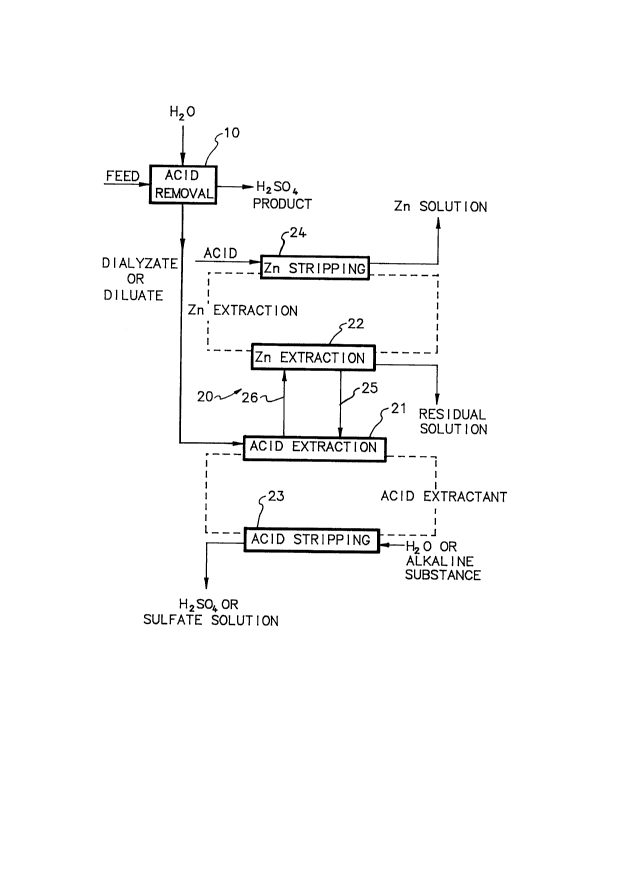
Figure 1 is a schematic flowsheet of the main embodiment of
the method according to the invention; and
Figure 2 is a schematic flowsheet of a preferred embodiment
of the acid removal step of Figure 1.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
According to the method of the present invention, zinc-
containing sulfate solutions are treated for the recovery
`- 2~2~g3
of values, which include zinc sulfate and sulfuric acid.
The zinc-containing sulfate solutions may also contain
impurities such as, for example, magnesium, manganese,
sodium, potassium, chloride and fluoride as well as
relatively small amounts of other dissolved metals such as,
for example, Cd, Cu, As, Sb, Fe, Pb, Tl and Ge. The small
amounts of other metals do not interfere with the recovery
of a relatively pure acid and a relatively pure zinc
sulfate solution. The impurities and other metals are
generally removed in a residual solution, as will be
described. If larger amounts of other metals are present,
a major portion of such metals should be removed prior to
processing the solution according to the instant invention.
Impurities and other dissolved metals will be referred to
as impurities hereinafter. The concentrations of zinc,
acid and impurities in the zinc-containing sulfate solution
depend on the composition of the zinc concentrates and on
the process for the recovery of zinc. Spent electrolyte
may contain, for example, from 10 to 100 g/L Zn, from 50 to
200 g/L sulfuric acid and from a few to about 20 g/L
impurities. A typical spent electrolyte may contain, for
example, 50 g/L Zn, 150 g/L H2SO4, 7 g/L Mg and 2 g/L Mn.
According to the main embodiment of the invention, zinc-
containing sulfate solutions are fed to an acid removal for
the formation and the recovery of a first solution
containing sulfuric acid and being substantially free of
zinc and the impurities, and for the formation of a second
solution having a relatively low concentration of sulfuric
202~293
~_ g
acid and substantially containing the zinc and the
impurities. The second solution is passed to a two-
circuit, side-by-side simultaneous solvent extraction for
the extraction of sulfuric acid in an acid extraction
circuit with an organic extractant suitable for the
extraction of sulfuric acid and for the extraction of zinc
in a zinc extraction circuit with an organic extractant
suitable for the extraction of zinc, and the formation of
residual solution substantially containing the impurities.
Sulfate is recovered from the acid extract as sulfuric acid
or a sulfate, and zinc is recovered from the zinc extract
as zinc sulfate solution.
With reference now to Figure 1, zinc-containing sulfate
solution is fed as feed solution to an acid removal 10 for
the formation of an acid solution substantially free of
zinc and impurities, and of a solution having a reduced
acid content and substantially containing the zinc and the
impurities contained in the feed solution. Acid removal 10
is carried out by a membrane process. The membrane process
may be dialysis, electrodialysis or dialysis integrated
with electrodialysis. The feed solution is preferably
filtered to remove substantially any contained solids prior
to acid removal.
Dialysis is carried out in a dialysis unit divided into
alternating dialyzate compartments and diffusate
compartments separated by suitable anion permselective
membranes. Suitable membranes are, for example, SelemionTM
DMV or DSV membranes. Other suitable membranes may be
2028~93
._, 10
used. The suitable membranes are substantially impermeable
to other dissolved species other than anions, in this case
sulfate ions. Protons, however, easily transfer due to
their small size and high mobility, thereby satisfying the
requirement for electron neutrality.
Feed solution is fed into the dialyzate compartments at a
rate of about 0.7 to 2.5 L/h.m2. Water or an aqueous
solution is fed into the diffusate compartments. Sulfate
and bisulfate ions as well as hydrogen ions pass from the
dialyzate compartments through the membranes into the
diffusate compartments forming a dialyzate (second
solution) in the dialyzate compartments and having a
reduced acid content, and substantially containing the zinc
and the impurities from the feed solution. A diffusate
(first solution) containing sulfuric acid substantially
free of zinc and impurities is formed in the diffusate
compartments. The dialyzate is passed to a solvent
extraction, generally indicated with 20, to be described.
The diffusate is removed from the process as a product.
The dialysis is carried out at ambient temperatures
preferably at a temperature in the range of about 20 to
40C, with the feed solution and water or other aqueous
solution being fed countercurrently by gravity flow. The
flows of feed solution and water are selected to give the
desired acid recovery and concentration in the diffusate.
Means for providing a desired temperature are provided.
When dialysis is used by itself, the feed and water flows
are normally selected to be about equal. The ions migrate
through the membranes under the driving force created by
` 2028293
11
the concentration gradient of sulfate ions across the
membranes. The dialysis may be carried out in one or more
stages.
Electrodialysis is carried out in an electrodialysis unit
comprising a multiplicity of alternating suitable cation
permselective exchange membranes and suitable anion
permselective exchange membranes arranged in a stack
between an anode and a cathode positioned at opposite ends
of the unit in an anode compartment and a cathode
compartment, respectively. Alternating diluate
compartments and concentrate compartments are formed
between the membranes. Suitable cationic membranes are,
for example, SelemionTMCMV-A and CMR membranes, and suitable
anionic membranes are, for example, SelemionTMAAV membranes.
Other similarly suitable membranes may be used. The
cathode is made of, for example, stainless steel and the
anode is made of, for example, platinum-plated titanium.
Other suitable electrode materials may be used.
The feed solution of zinc-containing sulfate solution is
fed into the diluate compartments. During electrodialysis,
acid anions in the feed solution to the diluate
compartments pass from these compartments to the
concentrate compartments through the anionic membranes.
Acid-reduced solution is withdrawn as diluate from the
diluate compartments. At least a portion of the withdrawn
diluate may be recirculated to the diluate compartments.
The remaining diluate is passed to solvent extraction 20,
to be described. The acid-reduced diluate (second
`` 2~282~
~ 12
solution) substantially contains the zinc and the
impurities from the feed solution. A minor loss of zinc
occurs to the concentrate, i.e. solution concentrated in
sulfuric acid (first solution). The loss of zinc to
concentrate in electrodialysis is somewhat higher than the
loss to the diffusate product obtained with dialysis. A
concentrate is withdrawn from the concentrate compartments
and at least a portion of the withdrawn concentrate may be
circulated as feed to the concentrate compartments. The
recirculation of diluate and concentrate is done mainly to
ensure turbulent conditions in the compartments. If
desired, an amount of water or other solution may be fed to
the concentrate compartments to control the acid
concentration. Concentrate containing sulfuric acid and
substantially free of zinc and impurities is recovered.
The electrode compartments are rinsed with separate rinse
solutions or a common rinse solution. Rinse solutions are
chosen from dilute sulfuric acid and acidic sodium sulfate
solution, are maintained at a pH in the range of about 0 to
4, and are circulated at flow rates in the range of about
25 to 90 L/h.m2. Gases evolved at the electrodes are
carried from the electrode compartments in rinse solution.
The flows of concentrate, diluate and rinse solution
through the respective compartments are adjusted such that
the differential pressure across the membranes does not
exceed about 150 kPa.
The electrodialysis is carried out at temperatures in the
range of from 0C to about 60C and at direct currents
` _ 13 2~28293
applied between the electrodes equivalent to a value of the
current density in the range of about 10 to 1000 A/m2,
preferably in the range of about 400 to 1000 A/m2. Solution
is fed to the electrodialysis at a rate in the range of
about 2 to 40 L/h.m2 per membrane pair. The flows through
the compartments should be turbulent and substantially
balanced across the membranes. Electrodialysis may be
carried out in one or more stages. In order to obtain a
high degree of acid removal or high acid concentrations in
the product, two or more stages of electrodialysis may be
required.
Either dialysis or electrodialysis is satisfactory in
producing a sulfuric acid product and a solution reduced in
acid content and substantially containing the zinc and the
impurities from the feed solution to the acid removal 10.
The removal of acid in acid removal 10, however, is
improved when both dialysis and electrodialysis are used in
an integrated fashion, as shown in Figure 2. When dialysis
alone is used, best results are achieved at feed and water
rates of 0.7 to 1.4 L/h.m2 at temperatures of from about 10
to 25C, and up to 2.5 L/h.m2 at 40 to 45C. The
concentration of acid in the diffusate is usually lower and
may only be slightly higher than that of the feed solution.
When electrodialysis is used by itself as acid removal
step, zinc losses occur which detract from the desired
efficiency, and multiple stages may be required to improve
the separation. However, when both dialysis and
electrodialysis are used in an integrated fashion,
increased feed and water rates to the dialysis can be used,
` 2~2~29~
_ 14
and a more highly-concentrated acid product containing a
negligible amount of zinc can be produced, while forming a
low acid metal-containing solution for feeding to solvent
extraction 20.
With reference now to Figure 2, which shows another
embodiment of acid removal 10, zinc-containing sulfate
solution as feed solution is fed to a dialysis unit 11 of
acid removal 10. Dialysis unit 11 is constructed and
operated as described with reference to Figure 1. Feed
solution is fed into the dialyzate compartments 13, sulfate
ions pass through the membranes 14 into the diffusate
compartments 15 forming a diffusate. Dialyzate reduced in
acid content and substantially containing the zinc and the
impurities from the feed solution is passed to solvent
extraction 20, to be described. Dialyzate is removed from
dialyzate compartments 13 at a rate substantially equal to
the rate of addition of feed solution, which is in the
range of about 1 to 5.0 L/h.m2. These feed rates are
increased to about twice the commonly used rates for a
process using dialysis only. A solution, to be described,
is fed to diffusate compartments 15. Diffusate discharged
from diffusate compartments 15 is passed to the diluate
compartments of electrodialysis unit 12 of acid removal 10.
Electrodialysis unit 12 is a unit constructed and operated
as described with reference to Figure 1. Diffusate from
dialysis 11 is fed to and passed through the diluate
compartments, generally indicated with D, of
electrodialysis unit 12, and diluate withdrawn from the
20282~3
diluate compartments D is recirculated. A portion of the
circulating diluate is passed through diffusate compartment
15 of dialysis 11. Circulating diluate has an acid content
lower than that of the feed solution, and passing diluate
through diffusate compartments 15 provides the necessary
driving force for the dialysis. Before entering diffusate
compartments 15, an amount of acid-receiving solution may
be added to increase the driving force further.
The acid-receiving solution may be dilute sulfuric acid,
salt solution or water. The portion of circulating diluate
and a quantity of acid-receiving solution, if any, is fed
to diffusate compartments 15 at a rate in the range of
about 2 to 10 L/h.m2 of membrane surface area. The amount
of acid-receiving solution is chosen to balance any water
losses to the dialyzate and concentrate streams leaving
dialysis 11 and electrodialysis 12, respectively.
Concentrate is removed from the concentrate compartments,
generally indicated with C, and is recirculated through
these compartments. A portion of the circulating
concentrate is recovered as acid product.
As stated, either the dialyzate from the dialysis or, when
only electrodialysis is used, the diluate from the
electrodialysis is passed from acid removal 10 to dual-
circuit, side-by-side simultaneous solvent extraction 20,
as shown in Figure 1. In extraction 20, sulfuric acid and
zinc are each separated by a suitable extractant in one or
`` 202~29;3
16
more extraction stages, and the zinc extraction raffinate
25 from each zinc extraction stage in the zinc extraction
circuit is exchanged with the acid extraction raffinate 26
from a corresponding acid extraction stage in the acid
extraction circuit. Substantially pure sulfuric acid or a
sulfate solution and substantially pure zinc sulfate
solution are stripped from the respective extracts, while
the withdrawn residual solution is substantially free of
zinc and acid, and substantially contains the impurities.
Again, with reference to Figure 1, dialyzate or diluate,
i.e. second solution from acid removal 10 reduced in acid
content and substantially containing the zinc and
impurities contained in the feed solution to the process,
is fed to acid extraction 21. The concentration of acid in
solution fed to solvent extraction 20 is usually below
about 50 g/L, but should preferably be as low as possible
in order to reduce the need for acid removal during solvent
extraction. In the acid extraction circuit, acid is
extracted from the raffinate 25 from zinc extraction 22, to
be described, and combined with second solution from acid
removal 10. The combined aqueous solution is mixed with an
organic extractant suitable for extracting sulfuric acid,
and an acid extract and an acid extraction raffinate 26 are
formed. Suitable extractants for sulfuric acid are amine-
type extractants including primary, secondary, tertiary and
quaternary amines such as, for example, AlamineTM336
(tricaprylamine) or PrimeneTM JMT (a 16-22 carbon atom, tert
alkyl primary amine). The acid extractant is mixed with a
suitable diluent to give an amine concentration of about 5
2~282~3
_ 17
to 30% by volume. If desired, a modifier may be added at
a concentration of, for example, about 1 to 8~ by volume.
The acid extract, i.e. the acid-loaded organic phase, is
passed to acid stripping 23, and the acid extraction
raffinate 26 is passed to zinc extraction 22 of the zinc
extraction circuit.
In acid stripping 23, sulfuric acid is stripped from the
acid extract, and stripped organic phase is returned to
acid extraction 21. The sulfuric acid is stripped with a
compound chosen from water and suitable alkaline
substances. Stripping with water makes it possible to
recover sulfuric acid as such, while stripping with an
alkaline substance forms a sulfate. Stripping with alkali
is more efficient than stripping with water, and is
preferred. One of a number of alkaline substances such as
alkali metal hydroxide or ammonium hydroxide solutions may
be used, but the use of ammonium hydroxide is preferred
because a saleable byproduct is obtained. The formed
sulfuric acid or sulfate solution, preferably ammonium
sulfate solution, is recovered. Both acid extraction 21
and acid stripping 23 are carried out at ambient
conditions, and may be carried out in one or more stages.
The raffinate 26 from acid extraction 21 is passed to zinc
extraction 22, where zinc is extracted from the solution
with a suitable organic extractant. Suitable extractants
are suitable organic phosphoric acids such as di-2-
ethylhexyl phosphoric acid (D2EHPA) or mono-2-
ethylhexylphosphoric acid (M2EHPA) or mixtures thereof,
2a28293
_ 18
suitable phosphonic acids, suitable phosphinic acids, and
suitable thiophosphinic acids such as bis - 2, 4, 4-
trimethylpentylmonothiophosphinic acid such as, for
example, CyanexTM 302 or bis-2, 4, 4-
trimethylpentyldithiophosphinic acid, such as, for example
CyanexTM 301. The use of CyanexTM 302, D2EHPA or a mixture
of M2EHPA and D2EHPA is preferred. The organic extractants
are used with a diluent such as, for example, ExxsolTM D80
to form an organic phase containing extractant in the range
of about 5 to 40%, by volume, the balance being diluent.
Modifiers may be used. The organic phase is mixed with
acid extraction raffinate from acid extraction 21. The
mixing is carried out for a time sufficient to achieve
substantially equilibrium zinc extraction into the organic
phase, i.e. the zinc extract, and with the formation of a
zinc extraction raffinate and residual solution. The pH in
zinc extraction 22 is maintained in the range of about 1.3
to 5.0, and preferably in the range of about 1.7 to 3.0 by
the addition of an appropriate amount of alkali such as
sodium hydroxide or ammonium hydroxide. The zinc
extraction 22 may be carried out in one or more stages.
Zinc extraction raffinate from zinc extraction 22, which
also contains the acid generated during zinc extraction, is
passed to acid extraction 21. Residual solution is
removed from the process as substantially zinc-free
raffinate. Residual solution is substantially free of acid
and zinc but contains substantially all the impurities,
especially magnesium and manganese, that were contained in
the zinc-containing sulfate feed solution to the process.
`` 2~28293
_. 19
Residual solution may be passed to further treatment for
the removal of environmentally harmful substances before
being discarded.
The zinc extract from zinc extraction 22 is passed to zinc
stripping 24 for the removal of zinc from the zinc extract
and for the recovery of a concentrated zinc sulfate
solution and a stripped organic phase. Although one stage
is shown, zinc stripping 24 may be carried out in one or
more stages. Stripping 24 is done with sulfuric acid.
Preferably, the sulfuric acid is acid recovered from acid
removal 10. Depending on the concentration of the acid
recovered from removal 10, the acid for stripping may be
used as it is recovered from acid removal 10 or may be
fortified by the addition of an amount of concentrated
sulfuric acid. The strength of the acid used in zinc
stripping 24 is dependent on the zinc concentration desired
in the zinc sulfate solution to be recovered from zinc
stripping 24. The concentration of zinc in the recovered
solution may, for example, be the same as that used in
electrolyte used in the electrowinning process of zinc,
such as for example, about 60 to 160 g/L zinc as zinc
sulfate, preferably about 100 to 160 g/L. When 140 to 160
g/L zinc in the product solution is desired, the acid used
for stripping should contain about 220 to 250 g/L sulfuric
acid. Removal of traces of organic extractants in the
recovered zinc sulfate solution may be desirable, and may
be done, for example, by a treatment with activated carbon
(not shown).
`` 2~2~2~3
~ 20
The stripped organic phase is recycled from zinc stripping
24 to zinc extraction 22, and, if desired, may be subjected
to scrubbing before being added to extraction 22. Fresh
organic phase may be added to make up for any losses that
may have occurred.
The invention will now be illustrated by the following non-
limitative examples.
Example 1
A solution of spent zinc electrowinning electrolyte
containing 100 g/L H2SO4, 5.0 g/L Zn and 7.4 g/L Mg was fed
at a rate of 43.4 L/h.m2 into the diluate compartments of
an electrodialysis unit having `alternating SelemionTM
CMV-A and AAV membranes. Electrodialysis was carried out
at 1000 A/m2, at 41C for 24 hours with water being added
to the concentrate compartments at a rate of 2 L/h.m2.
Diluate and concentrate were recycled through their
respective compartments at a linear velocity of 5 cm/sec.
The final diluate contained 85 g/L H2S04, 5.1 g/L Zn and
7.5 g/L Mg, and the final concentrate contained 216 g/L
H2SO4, 0.2 g/L Zn and 0. 2 g/L Mg.
Example 2
Using the same electrodialysis unit as in example 1, the
test of example 1 was repeated under the same conditions,
but instead of feeding water to the concentrate
compartments, a portion of the feed solution was used at a
rate of 2.9 L/h.m2. The final diluate contained 85 g/L
2~8~93
21
H2SO4, 5.3 g/L Zn and 7.5 g/L Mg, and the final concentrate
contained 220 g/L H2SO4, 3.8 g/L Zn and 5.9 g/L Mg.
The results of Examples 1 and 2 show that single stage
electrodialysis provided satisfactory separation of the
acid and the metals in the feed solution. However, the
acid concentration in the diluate was only reduced by 15%
from that of the feed.
Example 3
If the zinc-containing sulfate solution has a high sulfuric
acid content, two stages of electrodialysis are required to
reduce the sulfuric acid concentration to a desired low
value. A spent electrolyte containing 150 g/L H2SO4, 49 g/L
Zn and 8.2 g/L Mg was fed at a rate of 5.5 L/h.m2 to the
diluate compartments of a first electrodialysis unit having
alternating SelemionTM CMV-A and AAV membranes. Water was
fed to the concentrate compartments at a rate of 1.15
L/h.m2. Electrodialysis was carried out at 52C and 1000
A/m2. Diluate from the first unit was fed to the diluate
compartments of a second unit, similar to the first unit,
and concentrate from the second unit, containing 118 g/L
H2SO4 and 28.5 g/L Zn, was fed to the concentrate
compartments of the first unit. The second electrodialysis
unit was operated at a current density of 575 A/m2. Diluate
from the second unit was recovered as product and found to
contain 29 g/L H2SO4 and 31 g/L Zn. Concentrate from the
first unit was recovered as concentrated solution
containing 178 g/L H2SO4 and 44. 5 g/L Zn. It is noted that
the zinc losses in the concentrated acid product were
~2~2~3
22
rather high. Zn losses with the concentrate could be
reduced by stripping the zinc from the solution.
Example 4
A dialysis unit was assembled to consist of 19 sheets of
S SelemionTM anionic membranes with a total effective membrane
area of 3970 cm2. An acidic sulfate solution containing
zinc and impurities was fed to the dialyzate compartments
and water to the diffusate compartments of the dialysis
unit. The process conditions and results for four tests
are given in Table I.
TADLE I
Flow rate q/L
l/h m2~25~Zn Mo Mn
Test 1
Selemlon DSV membrane~; 22C
Feed solutlon 0 95150 50 8 1 5
Water
Dl~fusate 0 95120 2 0 0 31 0 06
Dialyzate 1 029 45 7 7 3 1 4
Test 2
Selemlon DSV membrane~ 21C
Peed solutlon 0 91234 20 7 9 1 1
W~ter 0 83
Dlffusate 0 76214 1 6 0 54 0 1
Dlalyzate 0 98 51 17 7 0 0 93
Test 3
Selemlon DMV membranes Z0C
Feed Jolutlon 0 92151 50 8 1 5
W~ter
Dlffusate 0 84 122 1 0 0 13 0!02
Dlalyzate 1 035 46 7 8 1 47
Test 4S
sS-l-mlon DMV ~embrane~
Feed ~olutlon 0 77 234 20 7 9 1 1
Water o 79
Dlffu~ate 0 80 182 0 4 0 13 0 03
D~alyzat~ 0 76 45 19 8 7 9 1 1
2~2~2~3
_ 23
These results show that dialysis may be used to remove acid
efficiently and selectively from an acidic zinc sulfate
solution containing impurities, but the acid concentration
of the diffusate acid product was lower than that of the
feed solution in all tests.
Example 5
This example illustrates the use of more than one stage
dialysis for achieving an increased acid removal. A
dialysis unit equipped with Selemion DSV membranes was used
to treat a spent electrolyte from a zinc production plant.
The results are given in Table II.
TABLE II
Flow Rate g/L
L/h.m2 H2SO4 Zn Mg Mn
Feed Solution0.91 234 22.2 8.5 1.1
Water 0.83 - - - -
Diffusate 0.76 216 1.4 0.48 0.07
Dialyzate 0.98 51 19 8.0 0.95
The acid-reduced dialyzate was further treated by feeding
to a second stage dialysis unit at 0.83 L/h.m2 with water
fed countercurrently at 0.81 L/h.m2. The final dialyzate
was found to contain 10.6 g/L H2SO4.
Example 6
This example illustrates the effect of temperature on the
efficiency of acid removal. Using a dialysis unit equipped
with Selemion DMV membranes, tests were carried out at
~82g3
_ 24
various controlled temperatures. The results are given in
Table III.
TABLE III
Acid
Temp. Feed Rate g/L H2SO~ Remov2al
C l/h.m2 Feed Dialyzate Diffusate g/h.m
25 0.99 170 49 125 120
40 0.92 170 30 139 142
40 1.4 170 48 116 171
It follows that the acid removal efficiency increases with
increasing temperature.
Example 7
This example shows that dialysis integrated with
electrodialysis yields a concentrated acid substantially
free of zinc and impurity metals, and yields an acid-
depleted solution substantially containing the zinc and
impurity metals. Using the units of Examples 1 and 4,
respectively, the test was carried out according to the
scheme shown in Figure 2. Dialysis was carried out with an
increased concentration gradient by adjusting the acid
concentration of 11 g/L H2SO4 in the diluate from
electrodialysis to 7.5 g/L H2SO4 prior to feeding the
diluate to the diffusate compartment of the dialysis.
Solutions were at 40C. The current density in the
electrodialysis was 500 A/m2. Flow rates and analyses are
given in Table IV.
2G2829~
_ 25
TABLE IV
stream rate Zn Mg H2SO4
L/h.m2 g/L g/L g/L
feed to dialysis 1.7 53 9.2 145
dialyzate 2.2 52 8.6 38
diffusate 4.3 1.2 0.2 46
solution to diffusate4.8 1.2 0.2 7.5
compartment
diffusate to electro- 6.7 1.2 0.2 46
dialysis
diluate 5.6 - - 11
concentrate 1.1 <1 <0.5 220
Repeating this test using electrodialysis at 750 A/m2 and
with a feed rate of 11.6 L/h.m2 yielded a diluate at 10 g/L
H2SO4 and an acid product (concentrate) containing 250 g/L
H2S04 .
Example 8
This example illustrates the extraction of zinc from
sulfate solution with various extractant solutions and at
various pH values. Zinc extraction tests were carried with
an aqueous feed solution containing 20 g/L Zn, 7.7 g/L Mg
and 1.1 g/L Mn obtained form the dialyzate of a dialysis
treatment of an acidic sulfate solution.
Portions of the extractant solution were contacted with
three fresh portions of the aqueous feed at an o/a (organic
to aqueous) volumetric phase ratio of 1, with equilibrium
pH values maintained by the addition of sodium hydroxide.
The separated aqueous phases were analyzed.
The extractants tested were 20% by volume solutions in
ExxsolTM D80 of D2EHPA: di-2-ethylhexylphosphoric acid,
2~282~3
_ 26
EHPA: a mixture of mono- and di-2-ethylhexylphosphoric
acid, or MEEPA: mono-2-ethylhexyl-2-ethylhexylphosphonic
acid. The pH values and test results are given in Table V.
TABLE V
EQUILIBRIUM pH: 0.93
Loading in Extract Phase, g/L
Extractant Zn Mg Mn
D2EHPA 1st contact: 2 0.1 0
2nd contact: 2 0.1 0
3rd contact: 2 0.1 0
EHPA 1st contact: 2 0.4 0.1
2nd contact: 2 0.5 0.2
3rd contact: 2 0.7 0.2
MEEPA 1st contact: 0 0 0
2nd contact: 0 0 0
3rd contact: 0 0 0
EQUILIBRIUM pH: 1.54
D2EHPA 1st contact: 8 0.1 0
2nd contact: 10 0.1 0
3rd contact: 11 0.1 0
EHPA 1st contact: 5 0.8 0.2
2nd contact: 7 0.9 0.3
3rd contact: 8 0.9 0.3
MEEPA 1st contact: 5 0.1 0.1
2nd contact: 6 0.1 0.2
3rd contact: 7 0.1 0.2
EQUILIBRIUM pH: 2.52
D2EHPA 1st contact:17.5 0.2 0.15
2nd contact:21.5 0.1 0.25
3rd contact:21.5 0.0 0.40
EHPA 1st contact:16.2 0.7 0.44
2nd contact:21.2 0.3 0.40
3rd contact:24.0 0.0 0.40
MEEPA 1st contact:16.1 0.3 0.20
2nd contact:18.1 0.1 0.30
3rd contact:20.1 0.0 0.45
.` 2Q~2~3
_ 27
The results show only little extraction at the low pH of
0.93 and with some improved extraction of zinc at pH 1.54.
At pH 2.52, zinc extraction greatly improved to more than
twice what could be achieved at pH 1.54. Moreover, zinc
extraction was selective over magnesium and manganese
extraction. D2EHPA and EHPA were slightly superior to
MEEPA.
Example 9
This example illustrates the use of alkylthiophosphinic
acids as extractants for zinc. Portions of 15% solutions
of CyanexTM 301 and CyanexTM 302 in ExxsolTM D80 were
conditioned by contacting with two fresh portions of 225
g/L H2SO4 solution at an o/a ratio of 2. The organic phase
was separated and then contacted at an o/a ratio of 2 with
a dialyzate solution pre-adjusted to pH 2.7 with sodium
hydroxide addition. No further adjustment in pH was made.
The aqueous phase was separated and analyzed. The
extractant phase was then stripped by contacting with two
fresh portions of 225 g/L H2SO4 solution at an o/a ratio of
7. The aqueous phase was separated and analysed in each
case. The results of the extraction and stripping are
given in Table VI.
` 2~293
28
TABLE VI
AQUEOUS FEED: 45.0 g/L Zn, 7.2 g/L Mg
Extraction: Loading in Extractant Phase, g/L
Zn Mg
CyanexTM 301: 11.0 0.4
CyanexTM 302: 5.1 0.28
Stripping: % Zn Stripped g/L in strip liquor
After After Maximum
1st Contact 2nd Contact Achieved Possible
CyanexTM 301 28.2 35.8 21.7 77
CyanexTM 302 80.4 85.2 28.7 35.7
In comparing CyanexTM 301 and 302, the results show that
while CyanexTM 301 gave superior zinc extraction, it gave
inferior stripping. Increasing the temperature of the
stripping step to 44C gave a slight improvement.
Example 10
This example illustrates acid extraction from zinc-
containing solutions by amine extractants. An acidic
solution containing 20 g/L Zn and 30 g/L H2SO4 was contacted
with 20% (by volume) solutions of amines in ExxsolTM D80 at
an o/a phase ratio of 1. In each contact, the phases were
allowed to separate, and the aqueous phase was analyzed.
In all cases, no significant change in the zinc
concentration was noted. The extraction of acid by two of
the amines are given in Table VII.
` ~ 29 202~2~3
TABLE VII
Extractant Loading after Loading after
1st contact 2nd contact
(g/L H2SO41 (g/L H2SO4)
20% PrimeneTM JMT 25 31
20% AlamineTM 336 25 33
Using a feed solution containing 47 g/L Zn and 39 g/L H2SO4,
AlamineTM 336 was used to determine the effect of extractant
concentration. Results are given in Table VIII.
TABLE VIII
Loading after Loading after
1st contact 2nd contact
Extractant (g/L H2SO4) (g/L H2SO4)
10% AlamineTM 336 16 17
15 20% AlamineTM 336 29 35
No apparent difference in phase disengagement rate was
observed for the 10% and 20% extractant solutions.
Therefore, the use of a 20% solution is preferred for its
higher capacity for acid removal.
Example 11
This example illustrates the use of 20% D2EHPA in ExxsolTM
D80 for the efficient extraction of zinc. Efficient and
selective zinc extraction required pH control during
extraction. Various extractions were carried out with the
equilibrium pH adjusted to 2.52 by sodium hydroxide
addition. The aqueous feed solution containing 21 g/L Zn
was derived from a dialysis treatment of an acidic zinc
sulfate solution from a zinc production plant. The
2028~9~
equilibrium distribution of zinc derived from a series of
six contacts at pH 2.52 is given in Table IX.
TABLE IX
g/L Zn in Raffinateg/L Zn in Extract
1.5 8.3
3 0 16.5
4 5 18.0
6.0 18.3
12 20.0
18 22.0
The equilibrium data can be used to show that a feed
solution containing 21 g/L Zn can be treated in two
countercurrent extraction stages at an o/a phase ratio of
3:2 in order to reduce the zinc concentration to about 1.5
g/L or less in the raffinate.
Example 12
This example illustrates the efficient stripping of zinc
from a loaded 20% DEHPA in ExxsolTM D80 extractant. An
extract containing 22 g/L Zn was stripped in contacts with
a 225 g/L H2SO4 solution simulating two countercurrent
stages.
A zinc concentration of 135 g/L was obtained in the
resulting strip liquor. By difference, it was calculated
that zinc stripping from the extract was over 99% complete,
with the residual zinc in the organic phase reduced to 0.07
g/L.
` 2028293
_ 31
Thus zinc was efficiently stripped from the 20% DEHPA
solution.
Example 13
This example illustrates how dialysis and solvent
extraction can be integrated in the treatment of a zinc-
containing acid sulfate solution. Spent electrolyte from
electrolytic zinc production containing 234 g/L H2SO4, 22
g/L Zn, 8.5 g/L Mg and 1.1 g/L Mn was filtered to remove
any solids and fed at 0.82 L/h.m2 to the dialyzate
compartments of a dialysis unit equipped with SelemionTM DSV
membranes. Water was fed to the diffusate compartments at
0.85 L/h.m2. A diffusate stream was withdrawn at 0.76
L/h.m2 and was found to contain 212 g/L H2SO4, 1.6 g/L Zn,
0.54 g/L Mg and 0.08 g/L Mn. The dialyzate solution,
withdrawn form the dialyzate compartments at 0.91 L/h.m2,
was found to contain 42 g/L H2SO4, 20 g/L Zn, 7.9 g/L Mg and
0.95 g/L Mn. The dialyzate was fed to the first stage of
acid extraction of a dual-circuit, side-by-side, zinc and
acid extraction. Two stages for each extraction were
provided using four mixer-settlers. The organic in the
acid extraction circuit was a 20% by volume solution of
AlamineTM 336 in ExxsolTM D80, and 20% D2EHPA in ExxsolTM D80
was used in the zinc circuit. With respect to fresh
aqueous feed flow, the acid extractant flow was in the
ratio of 4 to 1, and the zinc extractant flow was in the
ratio of 1.5 to 1. The zinc extraction and the acid
extraction were operated with counter-current and cross-
2~2~293
_ 32
current flows respectively. The raffinate from
corresponding acid and zinc extraction stages were
exchanged continuously, thus maintaining a raffinate pH at
1.7 to 3 with simultaneous zinc and acid extraction. A
final residual raffinate containing 0.2 g/L Zn, 7.8 g/L Mg
and 0.92 g/L Mn and at a pH of 1.9 was withdrawn from the
second zinc extraction stage.
The acid extract from the acid extraction was treated in a
single stage with an ammonium hydroxide solution to strip
the loaded acid, giving an ammonium sulfate solution at 450
g/L (NH4)2SO4. The addition of ammonium hydroxide was
controlled to maintain the stripping at pH 8 in the aqueous
phase. The stripped acid extractant was scrubbed in a
single stage with a dilute ammonium sulfate solution
derived by feeding water and a portion of the strip liquor
from the acid stripping stage. The scrubbed acid
extractant was then recycled to the acid extraction.
The zinc extract was treated in a two-stage counter-current
stripping using a 240 g/L H2SO4 solution fed at an aqueous
to organic ratio of 1:11. A zinc sulfate solution
containing 144 g/L zn was produced. The stripped zinc
extractant was recycled to the zinc extraction.
Example 14
This example illustrates the use of integrated dialysis and
electrodialysis for acid removal followed with solvent
extraction for the recovery of zinc.
2Q~293
33
A feed solution containing 145 g/L H2SO4, 53 g/L Zn, 7.5 g/L
Mg and 1.5 g/L Mn was fed at 1.7 L/h.m2 to the dialyzate
compartments of a dialysis unit equipped with SelemionTM DMV
membranes. Water was added to a portion taken from a
recirculating diluate stream from an electrodialysis unit
to give a solution containing 7.5 g/L H2SO4, 1.2 g/L Zn, 0.2
g/L Mg and 0.03 g/L Mn. This solution was fed at 4.8 L/h.m2
to the diffusate compartments of the dialysis unit. The
dialysis unit was operated at 25C. Acid was removed from
the feed solution giving a dialyzate which contained 38 g/L
H2SO4, 41.2 g/L Zn, 5.8 g/L Mg and 1.2 g/L Mn. The
dialyzate was withdrawn at 2.2 L/h.m2. A diffusate stream,
withdrawn at 4.3 L/h.m2, contained 46 g/L H2SO4, 1.2 g/L Zn,
0.16 g/L Mg and 0.03 g/L Mn. The diffusate was fed to the
diluate compartments of the electrodialysis unit at 6.7
L/h.m2. The electrodialysis was carried out at 500 A/m2 and
45C. A portion of the recirculating diffusate, withdrawn
at 5.6 L/h.m2, was found to contain 11 g/L H2SO4, 1.3 g/L
Zn, 0.2 g/L Mg and 0.04 g/L Mn. The withdrawn diluate
solution was used in making a feed solution for the
diffusate compartments of the dialysis unit as described
above. A portion of the recirculating concentrate was
withdrawn at 1.1 L/h.m2 as the acid product containing 220
g/L H2SO4, 0.7 g/L Zn, 0.1 g/L Mg and 0.02 g/L Mn.
The dialyzate product was fed to a dual-circuit, side-by-
side, zinc and acid extraction process similar to that
described in Example 13. The acid extractant was 20%
AlamineTM 336 in ExxsolTM D80 fed at organic to aqueous feed
ratio of 4. The zinc extractant was 30% D2EHPA in EXXSO1TM
202~29~
34
D80 fed at an organic to aqueous ratio of 3 to 1. The
solvent extraction was carried out as described for Example
13, except that a spent acid containing 145 g/L H2SO4, 53
g/L Zn, 7.5 g/L Mg and 1.5 g/L Mn was used for zinc
stripping. A residual (raffinate) solution was withdrawn
which contained 0.15 g/L Zn, 5.8 g/L Mg, 1.1 g/L Mn and at
pH 2.1.
Zinc stripping, carried out at an organic to aqueous ratio
of 6.7 to 1, gave a strip liquor containing 145 g/L Zn, 7.5
g/L Mg, 1.5 g/L Mn and 6 g/L H2SO4. Acid stripping with
ammonium hydroxide at pH 8 gave an ammonium sulfate
solution containing 445 g/L (NH4)2SO4.
It is understood that changes and modifications may be made
in the embodiments of the invention without department from
the scope and purview of the appended claims.