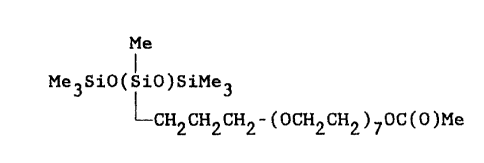Note: Descriptions are shown in the official language in which they were submitted.
2~
POSTEMERGENT HERBICIDE COMPOSITIONS CONTAINING
ACETOXY-TERMINATED SILICONE GLYCOL AND DISPERSANT
It has now been found that a particular silicone
glycol, not specifically disclosed in the art, imparts an
unexpectedly high degree of rainfastness to post emergent
herbicides. The present invention therefore relates to a
composition consisting essentially of:
(I) a postemergent herbicide; and
(II) from about 0.01 to 50 parts by weight, for each part by
weight of said herbicide (I), of a silicone glycol
adjuvant consisting essentially of
(i) from 20 to 95 weight percent of a silicone glycol
having the average structure
Me
Me3SiO(SiO)SiMe3
LCH2CH2CH2 -(OCH2CH2)7OC(O)Me
wherein Me denotes a methyl radical, and
(ii) from 80 to 5 weight percent of a silicone glycol
dispersant having the average formula
Me
Me3SiO(Ii~)xSiMe3
R(OCH2CH2)n~Z
wherein Me has its above defined meaning, R is a
divalent alkylene group having 2 to 6 carbon atoms, Z is
selected from the group consisting of hydrogen, an alkyl
radical having 1 to 3 carbon atoms and an acyl group
having 2 to 4 carbon atoms, n is 8 to 24 and x is 1 to
5.
-- ' --
2~ 29 160
The present invention further relates to a method
for inhibiting the growth of weeds, particularly velvetleaf
plants, comprising contacting at least part of the weed with
a herbicidal formulation, the improvement comprising using as
said herbicidal formulation a homogeneous aqueous dispersion
of the aforementioned composition.
The herbicidal composition of the present invention
is a homogeneous mixturè consisting essentially of (I) a
postemergent herbicide, (II) a silicone glycol adjuvant
consisting essentially of (i) an acetoxy-terminated silicone
glycol having seven ethylene oxide units in its glycol chain
and (ii) a silicone glycol dispersant for silicone glycol
(i) -
The postemer~ent herbicide (I) of the presentinvention is selected from those herbicides well known in the
art to be effective when applied after the emergence of a
plant. Examples of such postemergent herbicides include,
inter alia, 3-isopropyl-lH-2,1,3-benzothiadiazin-4(3H)-one
2,2-dioxide (bentazon) and N-(phosphonomethyl)glycine
(glyphosate). The former herbicide is marketed under the
trademark BASAGRAN3 by BASF Wyandotte Corp., Parsippany, NJ
and the latter herbicide is sold under the trademark ROUNDUP~
by Monsanto Agricultural Products Co., St. Louis, MO.
For the purposes of the present invention, the
herbicide is preferably selected from the diphenyl ether
structures exemplified by the general formula
~ ~ ~3
Specific examples of this class of herbicides include such
compounds as 2,4-dichlorophenyl 4-nitrophenyl ether
(nitrofen); 5-(2-chloro-4-trifluoromethylphenoxy)-2-nitro-
benzoic acid (acifluorofen); ethoxycarbonylmethyl
2-[3-(2,6-dichloro-4-trifluoromethyl-phenoxy)-6-nitro-
A
2~ 29 ~ ~
--3--
phenoxy]propionate; ethoxymethyl 2-[3-(chloro-4-trifluoro-
methyl-phenoxy)-6-nitrophenoxy]-propionate; sodium
5-[2-chloro-4-(trifluoromethyl)phenoxy]-2-nitrobenzoate
(acifluorfen-sodium); methyl 5-(2,4-dichlorophenoxy)-2-
nitrobenzoate (bifenox); and 2-chloro-1-(3-ethoxy-4-nitro-
phenoxy)-4-(trifluoromethyl) benzene (oxyfluorfen). For the
purposes of the present invention, acifluorfen-sodium is a
preferred herbicide.
The silicone glycol (i) of the present invention
has the average structure
Me
Me3SiO(SiO)SiMe3
H2CH2CH2 - ( ~CH2CH2 ) 70C ( O )Me
wherein Me hereinafter denotes a methyl radical. The
silicone glycol (i) may be prepared by coupling the
corresponding allyl-terminated glycol to a bis-siloxane
structure having a hydrogen attached to the central silicon
atom, said structure being
Me
Me3SiO(SiO)SiMe3
Generally, the coupling is accomplished in the presence of a
platinum catalyst. The skilled artisan will recognize that,
in such coupling reactions, a fraction of the allyl-
terminated glycol is not converted and will remain as an
impurity in the final silicone glycol product. Additionally,
as a result of inefficient distillation, the allyl-terminated
glycol employed may contain a minor proportion of molecules
having less than 7, or more than 7, ethylene oxide units.
This, in turn, results in silicone glycols having less than 7
or greater than 7 ethylene oxide units, respectively. The
,~
4 2 ~ 60
herbicide compositions may contain such impurities and still
be within the scope of the present invention.
The silicone glycol dispersant (ii) of the present
invention is similar to the above described silicone glycol
(i) and has the average formula
Me
Me3SiO(SiO)xSiMe3
R(OCH2CH2)n~Z
wherein R is a divalent alkylene group having 2 to 6 carbon
atoms, Z is selected from the group consisting of hydrogen,
an alkyl radical having 1 to 3 carbon atoms and an acyl group
having 2 to 4 carbon atoms, n is 8 to 24 and x is 1 to 5. It
is preferred that x is 1 and n is about 12.
A highly preferred silicone glycol dispersant of
the present invention has the average structure
Me
Me3SiO(SiO)SiMe3
CH2CH2CH2(0cH2cH2)12~
In addition to the aforementioned components, the
compositions of the present invention may also contain other
herbicide adjuvants commonly employed in the art. Examples
of such adjuvants include crop oil concentrate, ORTHO X-77*
spreader, drift control agents, such as LO-DRIFT*, defoaming
agents, such as D-FOAMER,* other compatibility agents, such as
E-Z MIX*, and other adjuvants well known in the herbicide art.
In order to prepare the compositions of the present
invention, from about 20 to 95 weight percent of silicone
glycol (i) is first thoroughly mixed with from about 80 to 5
weight percent of the silicone glycol dispersant (ii) to form
the silicone glycol adjuvant (II). The optimum ratio of
these ingredients is dependent upon the particular silicone
* Trademark (each instance)
-5- 2 0 2 g 1 6 0
glycol dispersant employed and is readily determined through
routine experimentation by the skilled artisan.
The above mentioned silicone glycol adjuvant (II)
is then preferably blended with herbicide (I) to form a
homogeneous dispersion which can then be diluted with water
and sprayed onto plants according to the method of the
present invention, described infra. Alternatively, the
silicone glycol adjuvant (II) may be added directly to a
water solution or dispersion of herbicide (I).
In order to be within the scope of the present
invention, from about 0.01 to 50 parts by weight of the
silicone glycol adjuvant (II) are used for each part by
weight of herbicide (I). Preferably, from about 0.2 to 17
parts by weight of the silicone glycol adjuvant (II) are so
employed.
Preferred embodiments of the present invention
employ silicone glycol (i) and the highly preferred silicone
glycol dispersant, described above, in a weight ratio of
about 2:1 to 9:1, respectively. In a particularly preferred
embodiment, this ratio is 4:1 and about 5 parts by weight of
silicone glycol adjuvant (II) is used for each part by weight
of herbicide (I).
In another aspect, the compositions of the present
invention consist essentially of from about 0.02 to 2.0 parts
by weight of postemergent herbicide (I), from about 0.01 to
50 parts by weight, for each part by weight of said herbicide
(I), of the silicone glycol adjuvant (II) and sufficient
water to provide 100 parts by weight of the total
composition.
The present invention also relates to a method for
inhibiting the growth of weeds, particularly the species
Abutilon theophrasti, hereinafter referred to by its common
name of "velvetleaf." This method comprises contacting at
-6- 202916~
least part of the weed with a homogeneous water dispersion of
a herbicidal composition, as hereinabove described. This
water dispersion is applied to the foliage of the weed by any
of the methods commonly practiced in the art, preferably by
spraying. The amount of the dispersion, and the herbicide
contained therein, to be applied to the velvetleaf may be
varied to a great extent, the optima being determined by such
factors as soil conditions, weather conditions and the type
of crops or other plants growing alongside the weed.
Generally, however, the effective range is about 0.12 to 2
pounds per acre of herbicide formulation.
When the compositions of the present invention are
used according to the above described method, there is
observed a marked improvement in the rainfastness of the
herbicide compositions relative to those containing silicone
glycol adjuvants having (on average) seven ethylene oxide
units in the glycol chain wherein said glycol chain is not
terminated with an acetoxy group. Thus, when compared with
currently used silicone glycol adjuvants, there is provided a
distinct advantage by the instant compositions in that they
permit the use of lower herbicide levels to attain a similar
degree of injury to a weed when there is a reasonable
likelihood of precipitation after broadcasting the herbicide.
Such a reduction in herbicide levels generally results in
reduced insult to adjacent cash crops and is considered
highly desirable.
The following examples are presented to further
illustrate the compositions of this invention, but are not to
be construed as limiting the invention, which is delineated
in the appended claims. All parts and percentages in the
examples are on a weight basis unless indicated to the
contrary.
~ 29 ~ 60
Silicone glycols, having the average structure
Me
Me3SiO(SiO)SiMe3
CH2CH2cH2(0cH2cH2)7Q
wherein Me hereinafter denotes a methyl radical and Q is
defined in Table 1, were prepared by the platinum catalyzed
addition of the appropriate allyl-terminated glycol to an
organohydrogenpolysiloxane having the structure
Me
Me3SiO(SiO)SiMe3
In Table 1, SILICONE GLYCOL C is a commercial silicone
glycol, SILWET~ L-77 (Union Carbide Corp., Danbury, CT), and
is believed to have the above structure wherein the glycol
chain is terminated by a methoxy group.
Table 1
Silicone Glycol Terminal Group Q
SILICONE GLYCOL A -OC(O)Me (Acetoxy)
SILICONE GLYCOL B -OH (Hydroxyl)
SILICONE GLYCOL C -OMe (Methoxy)
SILICONE GLYCOL D-OC(O)CH2Me (Propionate)
SILICONE GLYCOL E-OC(O)CH2CH2COOH (Succinate)
* SILWET~ L-77 (Union Carbide Corp., Danbury, CT)
A silicone glycol having the average structure
Me
Me3SiO(SiO)SiMe3
CH2CH2CH2(0cH2cH2)12
** SILWET~ L-77 is a trademark
~ -8- ~ a ~ ~ ~ 60
was employed as the dispersant for the above described
silicone glycols and will be referred to as DISPERSANT 1
herein.
The herbicide used in the examples was acifluorfen-
sodium marketed by BASF Corporation (Research Triangle Park,
NC) under the trademark BLAZER~.
Examples 1 - lO
Water dispersions of herbicidal compositions were
prepared by first thoroughly mixing the amount of SILICONE
GLYCOL A through SILICONE GLYCOL C indicated in Table 2 with
the amount of DISPERSANT 1. These mixtures were then blended
with BLAZER~ (0.18 gm each) and each blend was diluted with
water to provide 250 ml of total dispersion.
Individually potted velvetleaf plants were grown
under standard greenhouse conditions in BACCTO*professional
potting soil mix. Temperature was controlled at 75 +/-2~F.
Irradiation consisted of normal sunlight supplemented by
high-pressure sodium vapor lamps to provide an added 1,200
,uE/m s at bench level (~E = microeinstein), wherein the
day/night cycle was set at 18 hours and 6 hours,
respectively.
When the plants were 3 to 5 inches tall, they were
sprayed with water dispersions of the herbicide compositions
so as to broadcast herbicide (i.e., BLAZER~) at a rate of 0.03
pounds per acre (0.03 lb/A) along with the adjuvant (i.e.,
silicone glycol plus dispersant, when used). The adjuvant
rate was 3/4 pint/A when 0.94 grams of total adjuvant was
used. Spraying was accomplished by means of a link-belt
sprayer fitted with a TEEJET ~001 E nozzle which delivered
the equivalent of 25 gallons/acre of the herbicide
dispersion. In the spray apparatus employed, the 250 ml
samples, described above, provided the prescribed broadcast
rates.
* Trademark
** Trademark
- - 9-
In addition, the rainfastness of the herbicide
compositions was evaluated by spraying half the plants with
water in order to simulate rainfall. This procedure
consisted of spraying plants from above (8-10 inches above
plant tops) using a TEEJET nozzle which delivered 0.4 gallons
of water per minute. This nozzle was also mounted on a chain
drive and reciprocally moved over four plants at a time, each
such traverse taking about 9-10 seconds. The water spray was
started 15 minutes after application of the herbicide
compositions and was continued for approximately 7 minutes,
at which point the equivalent of one inch of "rain" had
fallen on each plant.
Plant injury was visually determined using a
double-blind experimental mode wherein four replicates were
run for each herbicide composition. Phytotoxicity was ranked
from zero, corresponding to no observable effect, to 100%,
corresponding to total destruction of the plant. These
results were averaged and the values reported using Duncan's
multiple range test to distinguish statistical differences at
the 95% confidence level. As is common practice in the art,
the injury values reported infra include lower case
superscript letters which indicate whether any given set of
values is statistically identical. Thus, for example, when
two injury values have such a superscript in common, this is
an indication that these values are not statistically
different at the 5% level by Duncan's method.
The above described herbicide dispersions were used
to spray velvetleaf plants and the degree of injury, both
with and without rain simulation, was observed seven days
after spraying with the herbicide dispersions of Examples 1 -
3. These results, along with the Duncan statistical
annotations, are presented in Table 2. As a control, four
* Trademark
.~
- 10~
velvetleaf plants were observed which were not sprayed with
any herbicide composition.
Table 2
STI ._ GLYCOL DISPERSANT I Percent Injury to Plant
Example Type Amount (gm) Amount Igm) No Rain With Rain
I A 0.7520.188 d,e 71 e,f
2 A 1.25 -- 60 g 4I j
3 __ __ 0 94 40 5 r~s
4 __ -- 0.752 38 j,k,l r,s
-- -- 0.188 15 0
6 B 0.75Z0.188 83 a,b,c 33 l,m
7 C 0.75Z0.188 88 a 43 i,j,k
8 C 0.94 ~~ 80 b,c,d 44 h,i,j
a,b,c k,l,m
9 C 0.75Z -- 81 36
-- --(BLAZER alone) 0 0
Control -- (No ~LAZER, No adjuvants) 0 0
-11- 202916~
It is apparent from Table 2 that the herbicide
formulation containing the silicone glycol having acetoxy-
terminated glycol chains, in combination with DISPERSANT 1,
provided significantly improved phytotoxicity after exposure
to simulated rain conditions relative to the corresponding
hydroxyl- and methoxy-terminated compounds. Furthermore, it
is seen that DISPERSANT 1, SILICONE GLYCOL B and SILICONE
GLYCOL C did not provide the improved rainfastness as did the
combination of DISPERSANT 1 and SILICONE GLYCOL A when a
total of 0.94 grams of each adjuvant was employed in the
herbicidal composition. SILICONE GLYCOL A was less effective
than the combination even when employed at a higher
concentration (i.e., 1.25 gm).
Examples 11 - 16
Herbicidal composition were prepared and tested
according to the procedures of Examples 1 - 10 wherein only
0.94 grams of the silicone glycols shown in Table 3 were
mixed with 0.14 ml (0.18 gm) of BLAZER (i.e., no DISPERSANT 1
was added). As before, the herbicidal compositions were
applied to velvetleaf at a rate of 0.03 lb/A of BLAZER and
3/4 pint/A of the silicone glycol adjuvant. Table 3
indicates that essentially no improvement in rainfastness is
obtained by varying the glycol chain terminal groups when the
dispersant was omitted.
~ -12- 2029~6a
Table 3
SILICONE GLYCOL Percent Injury to Plant
Example Type End Group No Rain With Rain
11 A Acetoxy 74 a 30 f~g
12 B Hydroxyl 73 a 28 f,g,h
13 C Methoxy 63 b,c 33 e,f,g
14 D Propionate 70 a,b 35 e,f
E Succinate 18 h,i,j o k
16 -- (BLAZER alone) ~~ o k o k
Control -- (No BLAZER, No adjuvants) o k o k