Note: Descriptions are shown in the official language in which they were submitted.
~- 203102~
METHOD FOR PR03UCING ZI~C B~ MEA~IS OF IRON MELT REDUCTION
The invention relates to a method for producing zinc from
oxidic zinc raw materials by reducing them in carbonaceous
iron melt. In this method the zinc ra~ material is inject-
ed, simultaneously with the carbonaceous reducer, into the
iron melt, so that the oxidic zinc is reduced and volatized
as metallic vapour. The vapour is discharged from the iron
melt together with the carbon monoxide, which is released in
the reduction reaction, and the carrier gas, and is con-
ducted out of the reactor into a separate condenser, where
the zinc is recovered by condensing.
,
Nowadays the production of zinc is mainly carried out by
roasting sulphidic zinc concentrate in a fluidized bed fur-
nace at a temperature of roughly 950C, so that an oxidic
zinc raw material, i.e. the calcine, is formed. The zinc
oxide of the calcine is then dissolved into a slightly
acidic electrolyte, wherefrom the zinc is recovered in
electrolysis. In the roasting process there is created an
amount of zinc ferrite, corresponding to the amount of iron ~i
contained in the concentrate, which ferrite does not dis- ~
solve in the first so-called weak acid leaching. Therefore ~ ~ -
the precipitate left undissolved at the first stage is dis- ~ -
solved at the second stage in a so-called (hot) acid leach-
ing. There is now also dissolved part of the iron, which
must be precipitated prior to conducting the solution into
electrolysis. The iron is precipitated for instance by
means of a jarosite or goethite process, where the amount of
produced iron residue is remarkable with respect to the
slight iron content. The said iron residues are nowadays
stored in waste ponds which form a potentlal environmental
hazard owing to their heavy metals and other harmful compo-
nents. The process is continued with solution purification
and electrolysis. The amount of energy consumed in the
process is relati~ely larger than the energy consumed in the
production of other ordinary metals, and about twofold with ~ ~ ~
, ~ ,
, .
: . ;, . :,: : . ~ ,
. :i . , , , , ~ ~
2~310~
respect to the theoretical energy deMand. The ma~or part of
this energy required is the electric energy consumed in the
electrolysis.
The major part of the pyroMetallurgically produced zinc is
nowadays manufactured in the so-called Imperial Smeltiny
process from zinc and lead concentrates. In this method the
sulphidic cGncentrate is si.nter-roasted into oxide in a
proc~ss which renders a lump product suitable to be used as
feed in a shaft furnace. In connection with the sintering
process, there are created large amounts of dusts conta.ining
lead/zinc, which are unhealthy when inhaled. The dusts and
gases ccntaining S02 also form a remarkable environmental
hazard. The sintered roast is fed, together with hard met-
allurgical coke, into a shaft furnace, where the roast is
heated while passing down through the shaft. The heat re-
quired by the process is produced in the bottom part of the
shaft furnace by burning part of the carbon contained in the
coke by means of preheated air, while part thereof serves as
a reducer in the process. Oxidic zinc and lead are reduced
in the lower part of the shaft furnace, and the lead forms a
separate phase at the bottom of the furnace. Gangue and the
possibly added fluxes as well as the coke ashes form a mol-
ten slag on top of the lead. A prerequisite for the process
is that the iron oxide contained in the slag is not reduced
into iron. This sets a limit to the employed reduction
potential, and certain amounts of heavy metals remain unre-
duced in the slag; these may limit the use of the said slag
for instance as earth filling. The zinc reduced in the
lower part of the furnace is volatized and lifts, together
with the CO, C02, H20, H2, N2 gas created in the process,
countercurrent with respect to the batch, up to the top part
of the furnace, wherefrom the gas is in most cases conducted
to a lead splash condenser. In this condenser the gas is
quickly chilled while meeting the cooler metal splash
curtain, so that the reoxidation of zinc is kinetically
prevented owing to the effect of the H20 and C02
. .
" , ~,
-,
. - ,.
,, , - . . , ~ , . .
~, - . : :, : :
~ ,; : :,
- - ~, ,, , -
-" 2~3~2~
of the gas, and metallic zinc is disso].ved into the lead.
The lead is circulated out of the furnace and chilled, so
that the solubility of zinc thereto is reduced and the mol-
ten zinc is separated on top of the lead layer, wherefrorn it
is recovered. Because lead is capable of dissolving only a
small amount of zinc, the amount of lead to be circulated in
the process per a given time unit must be about 400 times as
much as the produced amount of zinc.
The plasmazinc method described in the articles Bjorkling,
G., Faldt, I., Santen, S.: Das PLAS~ZINC-Verfahren fur die
Verwertung geringwertiger Rohstoffe; Erzmetall 34(1981)2,
77-81, and Eriksson, SO: The Plasmazinc process for recovery
of zinc from primary and secondary materials; Zinc '85,
827-840, differs from the Imperial Smelting method mainly in
that the heat required by the shaft furnace process is
brought into the process in the form of plasma gas developed
by means of electric energy. Thus the flue gases created in
the process are more reducing, and theoretically the zinc
can be condensed directly in a zinc bath. However, the
energy consumption and costs are relati~ely high. ~
In the patent application GB-A 2,048,309 there is introduced ~:
a zinc production method where the sulphidic concentrate is
smelted into a sulphidic, low-oxygen matte, wherefrom the
zinc is volatized by circulating the sulphidic matte through
a vacuum. According to the said patent application, the
required energy is brought to the process by means of oxi-
dizing part of the sulphur contained in the sulphide in a
different part of the furnace, which makes it possible to
produce even other metals, for instance copper. The weak
spot in the process is the need to use a vacuum.
,~
In the method of the US patent 4,741,770, the sulphidic zinc
raw material is first smelted by injecting it in oxidizing
conditions into a slag bath, so that the zinc is oxidized
and remains in the slag. The ~inc-bearing slag thus created
, - ::' . ~,:
, .
2~3~0~
is then reduced by means of the injection method in the
second step of the process, SG that the zinc is volatized
and is recovered by condensing from the gas phase. A method
of the same type is introduced in the patent publication AU
86 615~7, but there the zinc is allowed to reoxidize into
fume, which is then treated in a separate process in order
to produce metallic zinc.
In the articles Abramowitz, H., Rao, Y. K.: Direct reduction
of zinc sulphide by carbon and lime, Trans. Instn. Min.
Metall. Section C 87C(1987) C180-188 and Ueda, Y., Nakamura,
T., Noguschi, F.: Direct reduction of zinc sulphide, Met.
Rev. of MMIJ, 1(1984)2, 70-83 there is described a process
where sulphidic zinc concentrate is allowed to react in a
raised temperature together with carbon and lime. The lime
reacts with the sulphur of the zinc sulphide and there is
created calcium sulphide. The created zinc oxide reacts
with the carbon, and is reduced and volatized as gas to-
gether with the carbon monoxide created in the reaction, ;
from which gas the zinc is easily condensed without danger
of reoxidation. Among the drawbacks of the method let us
point out the need for external, indirectly produced energy
in order to obtain heat, and the gypsum created as a side
product from the reaction, the storage whereof creates a
potential environmental hazard.
In the latter article, among others, there are shown calcu~
lations which prove that it is theoretically possible to -~
reduce and volatize zinc with a good yield into a gas phase
directly from the sulphidic raw material by allowing it to
react, with a precisely defined proportion of constituents,
together with carbon and oxygen. Problems arise from the
difficulty to condense the zinc as metallic, with a suffi-
ciently good efficiency, from the SO2+CO2+CO gas; so far
this has not succeeded.
. ..
~: ., .-, ~, : : . , . , , :
- ~ - ,
~03~ 9
For example in the article Azakami, T., Itoh, S.: Fundamen-
tal studies of zinc extraction by the iron-reduction dis-
tillation process, Metallurgical Review of MMIJ, 5(1988)1,
55-69, there are described processes where the zinc frorn ar
oxidic zinc raw material, which in the former case consists
mainly of metal industry dusts, and in the latter case of
molten slag, is reduced by means of metallic, solid iron.
In the Azakami process, a mixture of iron oxide and zinc
oxide is first reduced at the temperature of 1000 - 1150 K
by carbon monoxide, so that the iron oxides are reduced.
Thereafter the temperature is raised up to 1370 K, where the
oxidized iron can reduce the zinc oxide, and the zinc is -
volatized as metallic gas, which then is condensed. The
drawback of these methods is the indirect use of energy for
maintaining the relevant temperatures, and the controlling
of the amount of solid iron required in the reactions.
The US patent 4,514,221 introduces a method where a zinc
roast is injected, together with carbon which is used as a
reducer and energy source, and with oxygen-enriched air to a
slag phase, so that the zinc oxide is reduced and volatized
as metallic vapour together with the the CO~CO2+N2 gas, ;
wherefrom it is condensed either in a lead or zinc splash
condenser. The creation of metallic iron must be avoided in
this process, and therefore the zinc content of the slag
phase remains fairly high, so that the slag phase requires
further treatment, for instance slag fuming prior to its
storage in the residue area, in order to avoid the environ-
mental hazards caused by the dissolution of zinc.
In the above described injection-based zinc production pro-
cesses the injection of the oxidic material and the reducer
takes place into the slag melt. In the method of the
present invention, the carbonaceous reducing material is
injected, together with the oxidic zinc-bearing material,
into iron melt by means of a carrier gas. Iron melt as a
medium differs remarkably from slag melt, among others due
"
,:
,, ~ ., ,
,: ., . : ` " , - ~ ,
, .,
, -
~ 20310~9
to its better heat transfer and lower viscosity. Moreover,the carbon dissolved in the iron serves as a reducer and
also as a carbon buffer, which stabili2es the reduction
reactions if there are disturbances in the feeding. By
employing iron melt as a medium there is achieved a higher
smelting capacity than while u5ing slag melt. In the method
of the invention, the reduction potential can be adjusted
remarkably lower than in the Imperial Smelting process,
because there is no danger of the reoxidation of zinc.
In coal gasification processes the use of iron melt is a
known method. The finely divided carbonaceous material,
with a typical particle size below 0.1 mm, is injected by
means of a carrier gas to the iron melt together with oxy-
gen. The coal is dissolved in the iron melt and reacts with
the oxygen, thus creating CO gas. The gasification of coal
takes place very rapidly owing to the high temperature and
carbon content of the melt. The ashes and sulphur contained
in the coal form a slag on the surface of the iron melt,
and part of the sulphur is discharged along with the gases.
The slagging can be improved by means of fluxes. While
employing oxygen as the injection gas, a large amount of -~
heat is released in the coal burning reactions.
In the process developed by the KHD Humboldt Wedag Ag (Mol-
ten Iron Puregas Process), finely divided coke is injected ~ i
to iron melt while the carrier gas is nitrogen. At the same
time oxygen is blown, together with water vapour, and they
react with the carbon dissolved in the iron. The obtained
product is a gas which is mainly composed of carbon monoxide
and hydrogen. The ashes and sulphur of the coke, together
with the flux, form a slag on the surface of the iron melt.
The injection can be carried out either by means of bottom-
blowing or top-blowing technique. The reported injection
capacity of coke into iron melt is 250 - 500 kg/h per tn Fe.
. ~ , ~ . - -
` !
2~1029
The CGS process described in the DE publication 2,952,4differs from the K~D process with regard to the way of
feeding the material. Here the finely divided solids, car-
bon and lime (CaO) used as a slag-~o~ming agent, are blown
together with the oxygen and water vapour onto the surface
of the iron melt at a high velocity.
In the articles Axelsson, C.-L., Torssell, K., Torneman, B.:
Coal gasification in an iron bath, Scad. J. Metallurgy
16(1987), 214-219 and Axelsson, C.-~., Sato, K., Torssell,
K., Torneman, B.: The P-CIG Process for coal gasification,
Scand. J. Metallurgy 17(1988), 242-247, there are described
two coal gasification processes, one of which is carried out
in normal pressure (Coal Iron Gasification Process) and the
other in a 3 bar overpressure (Pressurized-Coal Iron Gasi-
fication Process). In the CIG process, the finely divided
coal, oxygen, slag-forming agents and water vapour are in-
jected into the iron melt either by top blowing or bottom
blowing technique, and the obtained product is mainly CO and ~-
hydrogen gas. In the P-CIG process the injection takes
place as a bottom blowing. By employing overpressure, the
capacity can be raised and the produced gas can be used
directly in certain pressurized projects. The employed
slag-forming agents in the process are lime (CaO) or dolo-
mite.
In the above described processes, solid coal is processed,
by employing iron melt as a medium, mainly into CO gas and
thermal energy, which are used in some other process. In
the method of the present invention, the carbonaceous mate-
rial or gaseous reducer reduces the oxidic zinc raw material
in iron melt into metallic zinc, and the heat required by
the process is obtained, when necessary, ~rom the same pro-
cess by burning an excessive amount of coal with respect to
the oxides reduced in ~he reactor. At least part of the
thermal energy required in the process can also be produced
:. :-
,: '''.: .'' ~, :', . ', . ? -
: ~. . : ~ '
.
~031~9
externally, either by burning the created CO gas, or corn-
pletely by means of an external source of energy.
In the method of the present invention, the zinc raw mate-
rial which is at least partly oxidic is reduced, by rneans of
solid or gaseous reducers, by injecting them into molten
iron by means of a carrier gas. The metallic 7inc is re-
moved from the reduction step in gaseous form, along with
the flue gases, and is recovered in the ~lue gas line in a
separate condenser, by means of zinc or lead washing. The
essential novel features of the invention are apparent from
the appended patent claim 1.
During the reduction process, in the reactor there is formed
at least three phases which are separated ~rom each other in
equilibrium: a metallic melt, mostly iron, an oxidic slag
and a gas phase. Depending on the composition of the feed,
a sulphidic matte phase may also be formed in the reactor.
The raw material of the reduction process is a finely di-
vided material, typically made of zinc concentrate by means
of roasting. In the said method it is also possible to
treat oxidic or partly oxidized metallic residue and side
product materials, which are crushed to a suitable particle
size. The employed reducer is typically finely divided,
i.e. <0.1 mm, coke, coal dust or peat coke. The use of
gaseous reducers, typically hydrocarbons such as natural
gas, is also possible.
Depending on the mainly oxidic, non-reducible gangue brought
to the process by the feed materials, which are oxidic zinc
raw material and reducer, suitable fluxes can be added to
the feed in order to adjust the fluidity and other proper-
ties of the resulting slag. Such additives are typically
lime (CaO) and sand (SiO2).
... . .
. . :: . . ,
- . ~, . -- . .. ..
- . , , : - ..
~o~s~
The process is carried out in a reactor of the closed type,
whereto the raw materials are injected, solid materials by
means of a carrier gas. The injection is advantageoUSly
carried out below the sur~ace of the iron melt, either by
using a separate injection lance, or by intermediation of
the tuyeres of the reactor. The iron melt serves as a me-
dium for the heat and material transfer in the reduction
reactions, and the iron contained in the feed, as well as
the non-volatile side cornpone~ts which are reduced in the
reaction, are dissolved to the iron melt in the stat~onary
state of the process. Part of the iron is let out of the
reactor in batches. The slag phase formed in the reaction
is treated in similar fashion.
Because the dissolution of carbon to iron lowers its melting
point, the reduction process of zinc oxide can be carried
out below the melting point of pure iron. The reduction
temperature is limited by the molten range of the iron-
carbon system, which is largest with high carbon activities,
aCgraf >0.5, and thus the process is typically operated
within the temperature range 1300 - 1500C. -~
,
The heat energy required by the process is produced either
by means of indirect heating, or by burning in the reducing
reactor an excessive amount of coal or other reducer with
respect to the reducible oxides of the feed.
The recovery of gaseous zinc, or zinc vapour, is carried out
in a flue gas line by means of a conventional lead or zinc
washer, where the zinc is dissolved in a known fashion to a
lead or zinc suspension which is colder than the gas flow.
In certain conditions the zinc can also be condensed by
direct cooling.
Because the reduction of the iron oxides in the method of
the present invention does not limit the reducing capacity
of the process, the modest amount of slag created in the
~ . . : : : - :. :: . : .
.:: . -, , . . ~ :,
.: . :
. . .
. ~ .
2031~J2~
process contains only a slight amount of heavy metals. The
said heavy metals are in the slag bound in a ylassy phase,
and are there~ore in a form only slightly soluble to ~ater,
and the said slag can be used for instance as land filling
material. Thus the process does not produce residues which
might cause potential environmental hazards, such as the
jarosite from the electrolytic zinc production.
In the method of the present invention, the raw materials
need not be sintered in order to create good strength. Thus
the process does not result in such dust and sulphur hazards
to the environment as are typical of the sintering process-
es.
In the following the method is described with reference to
two examples.
EXAMPLE 1
The method of the present invention was applied to labora-
tory-scale experiments for manufacturing metallic zinc. A
finely divided zinc roast was injected, together with coke
powder, into carbon~saturated iron melt. The zinc oxide
contained in the roast was reduced in the iron melt into
metallic zinc, and was simultaneously volatized. The vola-
tized zinc was recovered from the gas in a separate con- `~
denser, and the obtained product was metallic zinc.
':
The major equipment in the experiment comprised a feed ap- `
paratus for the solid material, a melting crucible as well
as a condenser connected thereto by means of gas piping.
The melting crucible was located in an induction furnace,
whereby the energy need~d in the process was produced. The
iron melt, mass 2.3 kg, was kept at the temperature of
1400C, and the employed melting crucible was an A12O2 cru-
cible.
,
. . : : :
~31~
The injection of the roast/coke mixture into the iron melt
was carried out by means of a graphite lance with an inte-
rior diameter of 8 mm, and the employed carrier gas ~as
nitrogen (5 dm3/min). The amount of injected roast was 200
g, and the amount of injected coal dust was 30g, the injec-
tion period being 60 min. The analyses of the feed materi-
als ars presented in table I.
Table I: Analyses of ~he feed materials
Roast Coke
% % "
Zn 57.0
Fe 8~0
Pb 1.6
2 3 0.34 2.1
CaO 0.57 0 26
MgO 0.46 0.13
SiO2 1.1 4.1
S 2.1
H2O 0.10
Cfix 89.7
Volat. 1.7
The composition of the iron melt during injection remained
nearly unchanged, and the dissolution of zinc thereto was
extremely slight. The composition of the melt before and
after the injection is presented in table II.
:. . -. .' ' :.
:
--` 2~3~02~
Table II:_The comp sltion o iron melt before (I) and after
(II) the ~niection
(I) (II)
% %
Fe 89.0 87.0
Zn <0.01 <0.01
Pb 0.01 0.02
C 4.0 4.2
As a result from the reduction reactions, the zinc was vo-
latized lnto the gas phase. The gas was conducted, along
the gas piping, into the condenser, where the gas was cooled
down to 500C, so that the zinc was condensed and could be -
separated from the gas. The obtained final product was
metallic zinc. The composition of the metallic zinc was 98% -
Zn. In addition to this, the zinc contained as impurities
some dissolved lead and small amoun~s of oxidic components, -
which in this laboratory-scale experiment were brought to
the product along with dusts.
,.
.
The non-reduced components contained by the roast formed a
thin slag layer on top of the iron melt. The main component
in the slag was SiO2, about 45-60%. Other components in the
slag were CaO, Fe, FeO, A12O3, MgO, MnO and Cr2O3. The zinc
content in the slag was 0.1-1.5 % Zn, and the lead content
was <0.1 % Pb.
EXAMPLE 2
::
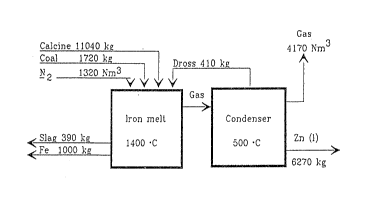
Example 2 presents the material balance for the process of
the present invention. This material balance is based on a
yearly output of 50,000 tons of zinc. The employed carrier
gas in the injection was hydrogen (1 Nm3/10 kg solids). The
approximated zinc yield in the condensing is 95%, while the
rest goes into the oxidic dross, which is recirculated to
: :~ : . : -; , . - , "
- , : . - : : : . , :: :::
~3~0~
the process. The dross is mainly composed of the oxides of
zinc and lead, as well as the iron entered into the process
along with dust. The initial materials are the same as in
example l. The material balance is calculated according to
feed per hour. The material flows are illustrated in a flow
sheet:
Gas
Ca]cine11040~ 9170~m3
Coa] 1720~a ¦ Dross410 ka A
N 1320~m3 1 I - l
-2 V V ~ / ~ I
Gas ~ _
Iron me]l Condenser
~Fe 1000~ 1$00 C 500 C Zn(1)
6270~g
Iron oxides, which form the ma~or part of the gangue in the
roast, are reduced in the process, and therefore the pro-
duced amount of slag is very small, only about 3.5% of the
amount of fed roast. The amount of carbonaceous iron cre-
ated in the process is lO00 kg/h. The slag and iron compo-
sitions according to the thermodynamic balance are the fol-
lowing:
. ~ ..: . - .
: .
: . - . , , :, ~
. : . ~., .
.. -
~n3~r~
14
Slag Iron %
%
CaO 19.3 Fe 88.3
MgO 15.2 C 2.7
23 21.1 Si 1.6
SiO2 44.4 Zn 0.4
FeO 0.1
ZnO 0.05
The obtained zinc product contains about 1.9% Pb, wherefore
it must be further raffinated in order to produce SHG zinc,
for instance by means of a distlllation process. The gas -
discharged from the condenser contains about 68% carbon
monoxide CO, while the rest of the gas is mainly nitrogen
whlch is used as carrier gas in the injection. Owing to its
high thermal value, the said gas can be used for producing
energy for instance in the preheating of the batch, or for
indirect heating of the reactor.
. :: . . ................. ~ ~ .......... , -:
~, ., ... , ~