Note: Descriptions are shown in the official language in which they were submitted.
2031631
DECYANATION APPARATVS AND
PROCESS FOR PURIFYING WATER
FROM CYANIDE USING THE SAME
Technical Field
The present invention relates to the purification
of water, particularly to an apparatus for removing
cyanide from liquid waste - a decyanation apparatus
; and a process using the same for purifying polluted
-`- water.
, .i
Background of the Invention
, .
It is well known that hypertoxic cyanides exist
in liquid waste from existing production processes of
ore dressing, metallurgy, coking and electroplating
etc. and methods universally adopted for removing
cyanide such as ionic exchange, ozonization or direct
electrolytic oxidation are not efficient. For example,
in electrolytic oxidiation the current efficiency is
not stable, therefore harmful gases are generated in
the process and the processing cost is high. During
the operation of the niobium anode cyanide processor,
explosive gases such as hydrogen and chloramine as well
as toxic gases such a nitrogen trichloride, cyanhydrin
and chloride acid escaped may cause secondary pollution.
In alkaline chlorine process, a chloric oxidant
(chlorine, liquid chorihe sodium subchlorate, or
bleaching power etc.) is added to the cyanide containing
water so as to oxidize and decompose cyanides under
-- 1 --
2os~l6~l
alkaline condition. Since availibe chlorine may degrade
during the storage of chloric oxidants, it reacts
chemically with cyanic ions in the process, generating
harmful gases such as cyanic acid and cyanogen chloride.
In addition, chloric oxidants are easy to leak out
during the transportation, therefore, secondary
pollution might be caused. The chlorinecyanogen
equivalent wright is not easy to control in the process,
which may produce excessive chlorine or cause the
cyanogen content to exceed discharge standard. Meanwhile
the cost is high.
Ionic exchange is usually used in the desalination
of drinking water, and the processing of heavy metal
ions and radioactive elements. It is effective in pro-
cessing liquid waste containing less than 50PPM of
cyanogen and not suitable to process liquid waste con-
taining more than 200PPM of cyanogen.
Summary of the Invention
The object of the present invention is to provide
a decyanation apparatus which can remove hypertoxic
cyanide from the liquid industrial waste and therefore
solve the problems existed in the prior art apparatus.
Another object of the present invention is to
provide a novel process for purifying liauid industrial
waste using said apparatus in combination with the prin-
ciples of electrochemical reaction so as to remove
cyanide through electrolytic purification. The gases
2031631
exhausted from the process are repurified, thus the
problem of secondary pollution is solved.
The objects of the present invention are realized
in a manner that three storage tanks containing HCl,
NaOH and Nacl respectively supply said materials via
electromagnetic valves and pipes to an electrolyser
cell with their amount controlled by a flowmeter; the
liauid waste is pemped into the cell of an electrolyser
from a water collecting sump; the pH value in the
electrolyser cell is controlled and displayed on a
'~e.
controlling board via a pH sensor and an
oxidation-reduction potentiometer (ORP) provided within
the electrolyser; a fan outside the electrolyser blows
air into the electrolyser cell for stirring which is
further promoted by a three-dimentional eddy current
generated by spray nozzles installed vertically and
horizontally in the electrolyser; a set of electrode
plates provided within the electrolyser cell reverse
polarities under the control of a inverter; the CN bond
of the cyanide is completely destroyed in the
7:,
electrolysis and the gases exhausted from the elec-
trolyser are sent to a purifying tower by a fan; there
stored within the tower liquid alkali .. which is
sprinkled by nozzles of a sprinkling unit; the gases
prinkled with liquid alkali pass through a PN filler
(Paul ring) layer in the middle section of the tower
and are transformed into CO2 and N2 which are then
exhausted. There is ~no secondary pollution casused by
-- 3
2~3163~
gases that have been processed thus and so.
Brief Description of Drawing
Fig. 1 is an embodiment of the decyanation apparatus
according to the present invention;
Fig. 2 is the front view of an embodiment of the elec-
trolyser;
Fig.3 is the front view of an embodiment of the
electrode plate set;
Fig.4 is the contour of an electrode plate of the elec-
trode plate sets in Fig.3;
Fig.6 is the sectional view of the wiring flange for
the electrode plate set;
Fig.7 is the sectional view of the supporting frame
of the electrode plate set;
Fig.8 is the front view of the purifying tower;
Fig.9 is the schematic diagram of the upper and lower
supporting plates;
Fig.10 is the front view of an embodiment of the water-
gas separating member of the purifying tower;
Fig.11 is the top view of the water-gas separating
. ~
'~i member of the purifying tower;
Detailed Description of the Preferred Embodiment
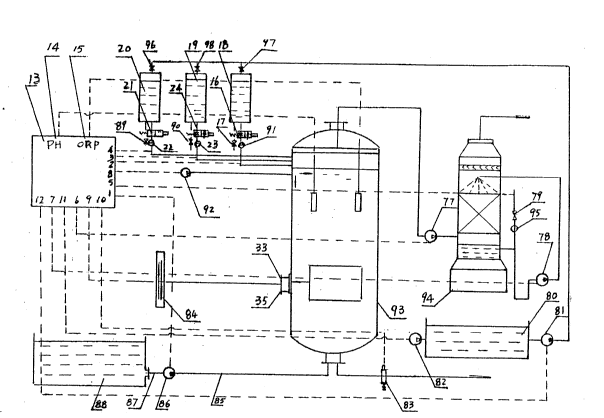
Fig.l shows an embodiment of the decyanation
apparatus according to the present invention.
A pH acidimeter (14) and an oixdation-reduction
potentiometer (ORP~ (15) are provided on an control
2031631
board (13). A set of 6 keys on the right side of the
board functions as follows: key (l) connected to the
inlet pump of the electrolyser (93) controls the opera-
tion of the pump; key (2) is connected to an
electromagnetic valve (16) and a flowmeter of a
hydrochlorlc acid tank (18); key (3) is connected to
an electromagnetic valve (24) and a flowmeter of a
alkali tank (19); key (4) is connected to a
electromagnetic valve (21) and a flowmeter of salt tank
(20); the three keys (2, 3, 4), according to the display
of pH acidimeter t14), control the addition of materials
in the acide, alkali: and salt tanks (18, l9, 20) into
the cell of the electrolyser (93) so as to match the
pH value with a given valve; key (5) is connected to
a electromagnetic valve (79) and a flowmeter (95) for
controlling the flow of liguid alkali. into purifying
tower; key (8) connected to a fan of the electrolyser
(93) controls the fan to blow air into the electrolyser
for stirring. Another set of 6 keys on the lower part
of the control board is provided, among them, key (10)
connected to the discharge electromagnetic valve (83)
on a flange below .the electrolyser (93), controls the
discharge of water; silicon rectrifying switch (9)
controls the thyristor (84) having a phase-inverter,
the output terminal of which is connected to the
positive and negative electrode wiring board (33, 35)
of the electrolyser (93) to reverse the polarity of
the electrodes at regular intervals so as to speed up
2031631
electrolysis; kev (6) is connected to a fan (77) in
the passage between electrolyser (93) and the purifying
tower, for controlling the fan to blow waste gases
exhausted from the electrolyser (93) to the purifying
tower (94) and maintain a continued purifying action;
key (11) is connected to a fan (82) for agent tank (80)
to control the fan to give a stir in the agent tank
(80); key (7) is connected to a alkali~ pump (78) of
the purifying tower (94) and controls the pump to pump
liquid alkali from the purifying tower (94) into a
sprinkling pipe (72) on the upper section of the
purifying tower, the liquid alkali. is then sprinkled
via a number of nozzles, generating a purifying action
on the waste gases filtered by a filtering layer; key
(12) is connected to a agent pump (81) for tank (80)
to control the pump to pump off acid, alkali. and salt
.~ in tank (80).
In Fig. 2 the electrolyser of the apparatus
according to the present invention comprises an
- electrolyser cover (27), a water-gas separating member
.. (28), a cylindrical body (27) and a set of electrode
plates etc. Electrolyser cover (27) is a pot-shaped
covering member with its central top stretched upward
forming a flange which is connected with another flange
(26) by bolts (49) or other means. Flange (26) is
connected with an exhaust pipe (25). A connection socket
for a pH sensor and a connection socket for a ORP sensor
are mounted in opposition to each other on cover (27).
203~631
On the cylindrical body (37), a blower (30) is
provided between the body and an air inlet pipe (29)
to the body, and connected with a plurality of
horizontal and vertical blowing nozzles (31, 32) inside
the body. A positive wiring means (33) and a negative
wiring means (35) are fixed on a flange (34) by means
of screws or the like. Flange (34) is connected fixedly
with a flange projected outward from the body and having
an opening through which a set of electrode plates
defining an anode and a cathode is inserted into the
lower part of the body defining a electrolyser cell.
The electrode plates are connected fixedly to each other
by means of bolts (44), nuts (45) and washers (52) all
painted with a layer of anticorrosive PTFE. The
electrode plate set is supported by a frame connected
integrally with the body and projected~ in ward in the
body. The body has a contour at its bottom similar to
cover (27). i.e. a pot-shaped contour with its central
part projected downwardly forming a flange connected
with a flange (40) by bolts (39). A liquid waste inlet
,,.
pipe (38) and a discharge pipe (41) are connected to
extend through flange (40) in opposition to each other.
A pH acidimeter (42) and an ORP sensor (43) are disposed
within the body above the blowing nozzles. A
floating-ball fluviograph is installed on the body to
control the liquid level in the body.
Fig.3 is the front view of the electrode plates
set. The positive plates (56) (anode) and the negative
20~163.~
plates (57) (cathode) are e~ual in number and arranged
positive alternating with negative with spacings between
each pair of plates being preferably 5mm and filled
with insulation blocks (53). Each e]ectrode plate has
two holes provided at i two ends respectively; within
each hole a insutating ring (54) is placed. Each
insulation block (53) also has a central hole so that
the connecting bolts (44) can be inserted through which
and the hole at the end of the plate to fix plates (56,
57) onto a supporting frame (55) and wiring flanges
(34). In order to avoid corrosion, bolts (44), nuts
(45) and washers (52) are preferably made of stainless
steel or other sort of steel painted with PTFE.
Fig. 4 is the lateral view of the electrode plate
set. Two rectangular copper plates (one as positive
and the other as negative) are spaced apart in
opposition to each other. On each of which openings
equal in number to the positive or negative plates are
provided to receive positive or negative plates in a
manner that positive electrode plates (56) are inserted
fixedly into the openings on positive copper plate and
negative electrode plates into negative copper plate.
The electrode plates are cut to the shape shown in Fig.
S so that the possibility of coming into contact with
two copper plates by a electrode plate is eliminated.
With the action of a phase-inverter, the polarities
of the electrode plates reverse in regualar intervals,
thus the inactivation of electrodes is effectively
2031~3~
avoided while the bath voltage is stabized.
Fig. 6 is the sectional view of the wiring flange
(34). Flange (34) is shaped into a rectangular sleeve
- so that the electrode plate set can be placed therein.
A pair round holes perpendicular to the rectangular
opening of the sleeve and in opposition to each other
on the sleeve are also provided on the flange to fix
the electrode plates (56, 57) with insulation blocks
therebetween onto the flange.
Fig. 7 is the sectional view o~ the supporting
frame (55). Supporting frame (55) is also in the form
of a rectangular sleeve into which the electrode plate
set is placed. At its upper and lower ends, holes are
provided perpendicular to the rectangular opening of
the sleeve so as to supportedly fix the electrode plates
with insulation blocks there between into the frame.
~~ Now referring to Fig.ll. A water-gas separating
member (28) is provided between the cover (27) and the
body of the electrolyser, which is made up of two per-
forated plastic plates sandwiched therebetween a th,n
layer of fiber material for water-gas separation. A
plurality of ventilating holes are provided on the side
wall of the member, thus the density of waste gases
may be diluted during air exhaust and the possible
explosion ignited by high density hydrogen and back-flow
caused by the negative pressure owing to the
insufficient amont of air from blower are prevented.
Fig. 8 is the front view of the waste gas purifying
2031~31
tower (94). Purifyiny tower (94) is a cylindrical body
constucted of three sections. The upper section is con-
nected at is top with a taper cover forming a water-
gas separating member (73) of the purifying tower; a
flange formed thereon is connected with a flange (74)
by bolts (59); through the central portion of flange
(74) an exhaust pipe (58) for exhausting purified ron-
toxic gases C~2 and N2 is hermetically inserted into
the body. A liguid alkaline sprinkling pipe (72) with
4-5 sprinkling nozzles (71) is provided within the upper
section of the body by extending the same hermetically
therethrough, so that a sprinkling chamber (70) is
formed. The middle section of the cylindrical body is
filled wlth polyhedral PN particles (Paul Ring) (68)
forming a gas-liquid reaction chember (69) for changing
the liquid phase of the waste gases and hydrolyzing
and oxidizing the waste gases. A flange-like porous
lower supporting plate (75) is provided between the
lower saction and the middle section, a similar upper
supporting plate (76) is installed between the middle
section. The lower section of the cylindrical body is
divided into two parts; the upper part has a flange
projected outwardly thereform which is connected with
a waste gas inlet pipe (64) having a flange at its end;
and the lower part forms a liquid alkali storage tank
(65) which also has a flange projected outwardly
therefrom and connected a pipe (66) to a liguid alkali
pump, the bottom of the liquid alkali storage tank
- 10 -
203163~
(65) is taper shaped to increase the stability and
capacity of the tower.
Fig. 9 is the schematic diagram of the upper and
lower supporting plates. The upper and lower supporting
plates (76, 75) a-e in the form of disc with a plurality
of apertures for filtration.
The purifying process of the present invention
is now described.
Firstly, the liquid waste in a precipitating pool
is pumped into the cell of electrolyser (93), Hcl (30%),
NaOH (16%) and Nacl are piped from three storage tanks
via electromagnetic valves into the electrolyser cell
in which the pH value of the liquid waste is adjusted
to 10.5 according to the display of acidimeter (1~)
by adding acid or alkali into the cell. The adoption
of pH acidimeter for automatically monitoring,
controlling and adjusting the pH valve of the liquid
waste in the electrolyser cell shortens the reaction
process, increases current efficiency and reduces the
operation cost. Next, Nacl of 180g/1 is added into the
electrolyser cell under the control of a electromagnetic
valve, a flowmeter and a timer in a manner that the
Nacl/liquid waste is controlled to 0.15g/1^~3g/1
according to the concentration of the liguid waste.
Then air is blown into the electrolyser cell by
blower(30) to give a stir to t~le liquid therein. The
introduction of air-stir in the electrolyser cell
prevents the precipitation of metal cyanide and the
- 11 --
2031S31
formation of flocculus substance which may adsorb cyanic
ions, hence reduces concentration difference so that
the undissolved chlorine accelerates the decomposition
of CN. Liquid NaOH is piped from the alkali tank via
electromagnetic valve (24) into the storage tank within
the purifuing tower and then sprinkled onto the waste
gases to be purified. The waste gas then passes through
a layer of PN fillers. The main reactions in the
purifying tower are
NaOH+HCN ~NaCN+H20
CN +C12+20H~ CNO +2Cl+H20
2CHO +3CLO +H20 Co2t+N2t+oN +3Cl
CNCL+2NaOH ~NaCNO+NaCL+H20
2NaCNO+3NaCLO+H20 ~2C02t+N2~+2NaOH+3NaCl.
; The li~uid waste containing cyanide is
electrolytically oxidized. The waste gas produced from
electrolysis is decomposed and purified in the purifying
tower, where the CN bond is destroyed completely, and
the metal ions are separated out on the negative plates.
The reactions on anode in the electrolyser cell
are
~-~ CN +20H -2e ~ CNO +H20
2CHO+40H -6e ~ 2Co2t+N2~+2H2o
. _
The reaction on cathode is 2H +2e ~ H2~ with heavy
` metals are reduced and separated out; the secondary
reaction is
CNO +2H20 NH4 +C03
In order to accelerate the oxidlzation and decompo-
- 12 -
2~31g31
sition of cyanide in the electrolyser cell, NaCl is
added. The reaction on the anode is
CL-e ~(C1)
and secondary reactions are
2Cl-2e ~C12
20H +CL2- OCl +Cl +H20
CN +OCL +2H20--~CNCL+20H
CNCl+20H ~CNO +Cl+H20
2CNO +30Cl+H20 ~2Co2~+N2t+3CL +H20
and HOCl~ HCl+(O)
~' To overcome the problems existed in existing
process of decyanide by electrolysis, i.e. instability
of current efficiency, generation of harmful gases and
`- high processing cost, experiments have been made by
:~ the inventor on the condltion of electrolytical
oxidization, current efficiency and the correlation
3 among the related quantities in the process. The
prerequisite for a solution to the existing problems
is found to be the materal of electrode. Hence, on the
basis of the research on titanium electrode by Dt-Nora
., .
( Italy), Damond (USA), ICI (UK), a new electrode DSA5
is developed, with which high current density
electrolysis can be realized and the separating-out
of nascent oxygen and nascent chlorine is promoted.
Furthermore, the current efficiency is increased.
The apparatus according to the present invention
is provided with insoluble electrodes of same material
in a manner that the posi~tive and negative electrodes
- 13 -
203~631
are equal in number and arranged in an assembly with
small spacings therebetween. The electrodes used in
the apparatus according to the present invention are
capable of resisting high-density current and changing
polarities automatically. Due to the salt adhered on
the negative plates caused by the products on the
electrodes, the deposite of calcium and magnesium ions
on negative electrode, the concentration of products
and electrolyte in the solution, the electrodes exhibit
different overpotentials for different CL and H , hence
the bath resistance in the electrolyser cell is
increased resulting in boosting of bath voltage and
reduction of current efficiency. The adoption of
phase-reversion effectively prevents the inactlvation
of electrodes, increases the conductivity, reduces
voltage drop, therefore stabilizes the bath voltage
and maintains a low overpotential for CL (a low EMF
for separating out chnorine). In the process, no sludge
is procuded and secondary pollution is prevented
accordingly.
The adoption of high density current ( 55A/dm2),
small spacing between electrodes and phase-reversion
at. regular intervals (every 3-8 minutes) facilitates
decomposition of cyanide at both positive and negative
electrodes; while the addition of sodium chloride
(0.5-3g/L) results in the generation of sodium
subchlorate with remaining cyanogen being oxidized by
chlorine at a certain pH value (10.5).
- 14 -
203163~
During the electrolysis, CN is continually
destroyed and the complexing e~uillibrium of metal
cyanide complexions is des-troyed forming indoluble metal
cyanide which then precipitates, and at the same time
forming flocculus substance which adsorbs a few cyanagen
ions. The formation of insoluble metal cyonide and
flocculus substance prevents oxidization of cyancgen
by available chlorine. To solve this proble air-stirring
is introdued, which results in the reduction of
differential concentration and accelerates decomposition
' of CN in combination with undisselved chlorme. Comparing
with mechanic stirring, air-stirring is advantageous
to the dissociation of cyanide. In the electrolyser
cell, sodium subchlorate and chlorine are generated
because of the addition of NaCl thereinto. The reaction
is :
NaCN+NaClO ~NaOCN+NaCl
NaCN+Cl2~+2NaOH ~NaOCNt2NaCl+H20
It has been found through experiments that the reaction
finishes instantaneously when pH712 and the critical
1 pH value is 10.5, but the primary product is hypertoxic
cyanogen chloride no matter how much the pH value is
NaCN+NaClO+H20 ~CNC12+2NaOH
For pHc10.5, the following hydrolytic process occurs:
CNCl+2NaOH -NaCl+NaCNO+H20
while NaOCN (cyanate) is completely oxidized into
nitrogen, i.e.
2NaOCN+3C12t6NaOH ~2NaHC03+N2~+6NaCl+2H20
- 15 -
2~63~
where the critical pH is just the same as that in the
process of cyanide transforming into cyanate, i.e. 10.5.
The oxide content in the process of reaction varies
from a few milligrams per liter up to thousands of mil-
ligrams per liter with industries. Therefore, a
oxidizing reducing potentiometer (ORP) is employed to
automatically monitor the oxide content so that
electrolysis can be carried out without testing the
oxygen content in the liquid waste separately. ORP is
also used to monitor the equivalent potential of
chlorine cyanogen. As the reading of ORP reaches 350mv
tterminal potential) the liquid waste that has been
purified is discharged. This significantly simplifies
the procedure of lab testing of the content of chlorine
and cyanogen and the feeding metering of chlorine and
cyanogen and feeding metering of the reagents.
`i The apparatus according to the present invention
is capable of treating 0.1-1000 tons of liquid waste
a day with the concentration of the water to be purified
being 1-8000mg/L .
J
-'
- 16 -