Note: Descriptions are shown in the official language in which they were submitted.
O.Z. 0050/41291
ubstituted azolylmethy~.s~C~2~ ~-a~ols and funqicides
containina them
The present invention relates to novel azolyl-
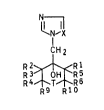
methylcycloalkanols of the seneral formula I
N~
I-X (I)
CH2
R2~Rl
R3 OH R5
R 4~ g T~ ~R 6
where
R1 and R5 are identical or different and are each hydrogen
or C1- to C4-alkyl;
Rl and Rs together with the carbon atom on which they are
substi~uents are >C=CH-R7 or >CH-Z-R7,
where Z is CH2, O, S, SO, SO2 or N-Ra;
R7 is Cl- to C8-alkyl, phenyl, biphenyl, naphthyl, hetero-
aryl, benzyl or C3- to C8-cycloalkyl, where each of these
radicals may be monosubstituted to trisubstituted by
halogen, nitro, phenoxy, amino, Cl- to C4-alkyl, Cl- to C4-
alkoxy or Cl- to C4-haloalkyl, or i9 tetrahydropyranyl,
or, where R1 and R5 together with the carbon atom on which
they are 3ubstituents are >CH Z-R7, R7 may additionally be
hydrogen;
RZ and R3 are each hydrogen or Cl- to C4-alkyl, or, where
R1 and R5 together with the carbon atom on which they are
~ubstituent~ are >C=CH-R7, R2 and R3 together with the
carbon atom on which they are substituents may addition-
ally be >CaCH-R7;
R4 and RA are each hydrogen or Cl- to C4 alkyl, or
R3 and R4 togekher with the carbon atoms on which they are
substituents are a saturated or unsaturated 5-membered or
6-membered carbocyclic riny, or
R2, R3, R4 and R9 together with the carbon atoms on which
they are sub~tituents are an unsubstituted or
- 2~ 2 ~J ~ '~ O. Z . 0050/41291
halogen-substituted phenyl ring, or
where Rl and R5 together with the carbon atom on which
they are substituents are >C=CH-R7, R2 and R3 together
with the carbon atom on which they are substituents may
additionally be >C=CH-R7;
R6, R9 to Rl2 are each hydrogen, C~ to C~-alkyl, phenyl,
biphenyl, naphthyl, heteroaryl, benzyl, dioxolanyl or C3-
to Ca-cycloalkyl, where each of these radicals may be
monosubstituted to trisubstituted by halogen, nitro,
phenoxy, amino, Cl- to C4-alkyl, Cl- to C4-alkoxy or Cl- to
C4-haloalkyl;
T is (CH2)n, =CR11Rl2, O or S;
n is an integer from 1 to 5, with the proviso that Rl to
Rl2 are not sLmultaneously hydrogen, and with the proviso
that Rl and R5 together are not
=CH-CH2 ~ Cl or =CH-~CI
when R2 to R4 and R6 to Rl2 are hydrogen, and
X is CH or N,
and plant~tolerated acid addition salts and metal com-
plexes thereof.
The present invention furthermore rela~es toprocesses for the preparation of these compounds, inter-
mediates used, fungicidal and growth-regulating agents
which contain the novel azolylmethylcycloalkanols as
~5 active substances, and methods for controlling fungi and
methodY for regulating plant growth with the aid of these
compounds.
European Patent 324,646 discloses azole-
substitu~ed cycloalkanol derivativeR which carry a
,CH-CH 2~3c 1-
group as the radical -Y-Rl, and their use as fungicides.
However, the fungicidal ac~ions are not sa~isfactory in
all cases.
It i~ an object of the present invention to
2 2 ~ -~
~ 3 - o.z. 0050/41291
provide novel azole compounds having better fungicidal
and growth-regulating actions.
We have found that this object is achieved by the
azolylmethylcycloalkanols of the general formula I,
defined at the outset, and plant-tolerated acid addition
salts and metal complexes thereof.
We have furthermore found processes for the
preparation of these compounds, intermediates used,
fungicidal and growth-regulating agents which contain the
novel azolylmethylcycloalkanols as active substances, and
methods for controlling fungi and methods for regulating
plant growth with the aid of these compounds.
The azolylmethylcycloalkanols of the general
formula I are obtained in general in the form of racem-
ates or as dia~tereomer mixtures. These isomers can beseparated in a conventional manner, for example on the
basis of their solubility or that of their salts or by
column chromatography, and can be isolated in pure form.
Pure enantiomers can be obtained from such an isolated
diastereomer by known methods.
Both the individual dia~tereomers or enantiomers
and mixture3 thereof generally obtained in the ~ynthesis
can be used as fungicidal active ingredients.
With regard to the fungicidal action, compounds
I in which R1 and R5 together with the carbon atom on
which they are substituent~ are >C=CH- are particularly
preferred.
If R1 and R5 together with the carbon atom on
which they are substituents are >CH-Z-, preferred com-
pound~ are thos2 in which Z is O or S.
Where R1 and R5 together with the carbon atom onwhich they are ~ubstituen~s are >C=CH- ~7 is bonded to the
CH group; if Rl and R5 togethex with the carbon atom on
which they are subR~ituents are >CH-Z-, there 1~ a bond
between R7 and Z.
Example-~ of each radical are:
Cl-C8-alkyl, preferably Cl-C4-alkyl, in particular methyl,
2 ,.~
- 4 - O.Z. 0050/41291
ethyl, n-propyl, isopropyl, n-butyl sec-butyl or tert-
butyl; among the Cl-C8-alkyl radicals of more than 4
carbon atoms, n-pentyl and neopentyl are preferred;
phenyl and halogen-substituted phenyl, ~uch as 2-chloro-
phenyl, 3-chlorophenyl, 4-chlorophenyl, 2-fluorophenyl,
3-fluorophenyl, 4-fluorophenyl, 2-bromophenyl, 3-bromo-
phenyl, 4-bromophenyl, 2,3-dichlorophenyl, 2,4-dichloro-
phenyl, 2,5-dichlorophenyl, 2,6-dichlorophenyl, 2-chloro-
4-fluorophenyl or 2-chloro-6-fluorophenyl;
phenyl which is monosubstituted by nitro, phenoxy, amino
or C1-C4-alkyl, such as 3-nitrophenyl, 4-nitrophenyl, 3-
phenoxyphenyl, 4-phenoxyphenyl, 3-aminophenyl, 4-amino-
phenyl, 4-ethylphenyl, 4-isopropylphenyl or 4-tert-
butylphenyl;
phenyl which is substituted by two or three different
radicals from among those mentioned above, eg. 2-chloro-
6-methylphenyl;
phenyl which is monosubstituted or disubstituted by C1-
C4-alkoxy, such as 2-methoxyphenyl, 3-methoxyphenyl, 4-
methoxyphenyl, 4-tert-butoxyphenyl, 2,4-dimethoxyphenyl
or 3,4-dimethoxyphenyl;
methylphenyl which is trisub~tituted by halogen, such as
2-trifluoromethylphenyl, 3-trifluoromethylphenyl or 4-
trifluoromethylphenyl;
p-biphenyl;
1-naphthyl or 2-naphthyl;
hetaryl having 5 or 6 ring atoms, in particular 6-
membered rings having up to three nitrogen atoms, such as
2-pyridyl, 3-pyridyl or 4-pyridyl, and 5-membered rings
preferably having one or two of the hetero atoms O, S and
N, in particular 2-furyl, 2-thienyl, 3-thienyl, 4-
oxazolyl, 4-thiazolyl, 4-i50xazolyl, 5-isoxa~olyl or 5-
imidazolyl;
benzyl; halobenæyl;
C3_CB_CYC10a1kY1, preferably cyclopantyl or cyclohexyl.
Wh~re R1 and R5 together with the carbon atom on
which they are sub~tituents are >CH Z--, the novel
~J ~ ~J 2 ~ 3 !~
- 5 - o.z. 0050/41291
compounds of the general formula I in which R7 is hydrogen
are al~o preferred.
Nhere Rl and Rs together with the carbon atom on
which they are substituents are >C=CH-, the compounds in
which R7 is 4-tetrahydropyranyl are also preferred.
R2 and R3 independently of one another may be
identical or different and are each preferably hydrogen
or n-alkyl of 1 to 4 carbon atoms, in particular methyl.
Where Rl and R5 together with the carbon atom on which
they are substituents are >CH-Z-, the novel compounds I
in which R2 and R3 are identical are preferred. If Rl and
R2 together with the carbon atom on which they are substi-
tuents are >C=CH-, the azolylmethylcycloalkanols I in
which R2 and R3 together with the carbon atom on which
they are substituents form >C=CH-R7 are particularly
noteworthy. For example, the radicals stated for R7 are
then applicable, preferably tert-butyl, phenyl, 2-chloro-
phenyl, 4-chlorophenyl, 4-fluorophenyl, 2~bromophenyl, 4-
bromophenyl, 2,4-dichlorophenyl, 2-methylphenyl, 4-
methylphenyl, 2-methoxyphenyl, 4-methoxyphenyl, 2-triflu-
oromethylphenyl, 4-trifluoromethylphenyl, 2-furyl,
cyclopentyl or cyclohexyl.
Suitable acid addition salts are the plant-
tolerated salts of acids which do not adversely affect
the ~ungicidal action of I, eg. the hydrochloride~,
bromides, sulfate~, nitrate~, pho~phates, oxalates and
dodecylbenzenesulfonate~. However, since the activity
of the 8alts i~ due to the cation, the anion i~ not in
general important. The novel active ingredient sal~s are
ad~antageously prepared by reacting the azolylmethyl-
cycloalkanols I with suitable acids.
Metal complexe~ of the novel compounds I or of
their salts are preferably formed with metals of main
group II, such as magnesium or calcium, of main groups
III or IV, such a~ aluminum, tin or lead, or with me~als
of subgroup~ I to VIII, the subgroup elements of the
fourth period being par~icularly p~eferred, especially
2~ o Z. 0050/41291
copper, zinc, manganese, iron, cobalt and nickel. For
this purpo~e, the azolylmethylcycloalkanols are reacted
with the corresponding metal ~alts.
Preferred azolylmethylcycloalkanols are those of
the general formula
N
~N--X
CH 2
~C~
where
R1 and R5 together with the carbon atom on which they are
substituents are >C=CH-R7 or ~CH-Z-R7, where ~ is O, S,
S ~ S2 or N-R7;
R7 is C~- ~o C8-alkyl, phenyl, biphenyl, naphthyl, hetero-
aryl, benzyl or C3- to C8-cycloalkyl, where each of these
radicals may be monosubstituted to trisubstituted by
halogen, nitro, phenoxy, amino, Cl- to C4-alkyl, C,- to C4-
alkoxy or C,- to C4-haloal~yl, or is tetrahydropyranyl,
or, where R1 and R5 together with the carbon atom on which
they are substituents are >CH-Z-, R7 may additionally be
hydrogen,
RZ and R3 are each hydrogen or C,- to C4-alkyl, or, wher~
R1 and R5 together with the carbon atom on which they are
substituents are >C=CH-, R2 and R3 together with the
carbon atom on which they are substituent~ may addition-
ally b0 >C=CH~R7;
n is an integer from 2 to 5~ and
~ is CH or N,
and plant-tolerated acid addition salt3 and metal com-
plexes thereof.
A~olylethanol derivatives of the general
formula I
- 7 - O.Z. 0050/41291
~11
N~N
CH2 (I)
R 23~R I
R4 T~CH2
where
R1 and R5 together with the carbon atom on which they are
substituents are >C=CH-R7 or >C~-CH2-R7;
R7 is Cl~C~-alkyl, phenyl, biphenyl, naphthyl, heteroaryl,
benzyl or C~-C8-cycloalkyl, where each of these radicals
may be monosubstituted to trisubstituted by halogen,
nitro, phenoxy, amino, Cl-C4~alkyl or Cl-C4-haloalkyl;
R2, R3 and R4 are identical or different and are each
hydrogen or Cl- to C4-alkyl, or
10 R3 and R4 together with the carbon atoms on which they are
substituents are a saturated or un~aturated 5-membered or
6-membered carbocyclic ring, or
R2, R3 and R4 together with the carbon atoms on which they
are substituents are an unsubstituted or halogen-
substituted phenyl ring, orwhere R1 and R5 together with the carbon atom on which
they are sub~tituents are >C=CH-R7-, R2 and R3 together
with the carbon atom on which they are substituents may
additionally be >C=CH-R7;
T is CH2, O or S, and
X is CH or N,
and plant-tol~rated acid addition salts and metal com-
plexes thereof are also preferred.
Other preferred azolylmethylcycloalkanols are
tho~e of the general formula I
N~X
N~
~R 6
where
Rl and R5 are identical or dif~erent and are each hydrogen
:;
;
_h~ 3 ?~ ~J ~ ~3 O. Z . 005n/4l29l
or Cl- to C4-alkyl;
R6 i5 Cl- to C8-alkyl, phenyl, biphenyl, naphthyl, hetero-
aryl, benzyl, dioxolanyl or C3- to Ca-cycloalkyl, where
each of the~e radicals may be monosubstituted to trisub-
stituted by halogen, nitro, phenoxy, amino, Cl- to C4-
alkyl, Cl- to C4-alkoxy or Cl~ to C4-haloalkyl, and
X is CH or N,
and plant-tolerated acid addition salts and metal com-
plexes thereof.
10Azolylmethylcyclohexanols of the general
formula I ~x~
N~ ~ ,R 7
R 9~R I O
where
Y is > C=CH- or ~ CH-CHz-;
15R4 and R9 to R12 are each hydrogen, Cl- to C8-alkyl, C3- to
C6-cycloalXyl or phenyl, where each of these radicals may
be monosubstituted to trisubstituted by halogen, nitro,
phenoxy, amino, Cl- to C~-alkyl, Cl- to C4-alkoxy or Cl- to
C4-haloalkyl, with the proviso that R4 and R9 to Rl2 are
not simultaneously hydrogen;
R7 is Cl- to C9~alkyl, phenyl, biphenyl, naphthyl, hetero-
aryl, benzyl or C3-to C8-cycloalkyl, where each of these
radicals may be monosubstituted to trisubstituted by
halogen, nitro, phenoxy, amino, Cl- to C4-alkoxy or Cl- to
C4 haloalkyl, and
X is CH or N,
and plant-tolerated acid addition salts and metal com-
plexes thereof are also preferred.
Compounds of the general formula I where T is CH2
and R4 and R9 have the abovemen~ioned meanings, with the
proviso that R4 and R9 are not both simul~aneously hydro-
gen and the remaining radic~ls have ~he abovementioned
meanings are also preferred.
The novel azolymethylcycloalkanols I are
C~ ~ 3 2 h ~ ~
- 9 - O.Z. 0050/41291
generally prepared ~y reacting a compound of the general
formula II with a compound of the general formula III.
Preferred compounds III are those in which ~e is
hydrogen or an alkali metal atomr in particular sodium or
S potas s iUlTl .
If Me is hydrogen, a weight ratio of III to II of
from 2 : 1 to 6 : 1, in particular about 3 : 1, is
advantageously maintained. The reaction is carried out
in the presence or absence of an inert solvent or dilu-
ent, advantageou~ly with the addition of an inorganic or
organic base and with or without the addition of a
reaction accelerator.
The preferred solvents and diluents include
ketones, such as acetone, methyl ethyl ketone or cyclo-
hexanone, nitriles, such as acetonitrile or propio-
nitrile, alcohols, 4uch as methanol, ethanol, isopropan-
ol, n-butanol or glycol, esters, such as ethyl acetate,
methyl acetate or butyl acetate, ethers, such as tetra-
hydrofuran, diethyl ether, dimethoxyethane, dioxane or
diisopropyl ether, amides, such as dimethylformamide,
dimethylacetamide or N-methylpyrrolidone, sulfolane or
corresponding mixtures.
Suitable bases, which may also be use~ as acid
acceptors in the reaction are, for ex~mple, alkali metal
hydroxides, ~uch as lithium hydroxide, sodium hydroxide
or potassium hydroxide, alkali metal carbonates, such as
sodium carbonate, potassium carbonate, sodium bicarbonate
or potassium bicarbonate, pyridine or 4-dimethylamino-
pyridine. Howe~er, it is also possible to use other
conventional bases.
Preferred reaction accelerators are metal hal-
ides, such as sodium iodide or potassium iodide, quater-
nary ammonium salts, such as tetrabutylammonium chloride,
bromide or iodide, or crown ethers, such as 12-crown-4,
15-crown~5, 18-crown-6 or dicyclohexano-18-crown-6.
The reaction is generally carried out at from 10
to 150C, in particular from 20 to 120C, under
:
'~ :
~ ~ ~ rJ 2 ~ o.Z. 0050/41291
atmospheric or ~uperatmo~pheric pressure, continuously or
batchwise.
If Me is a metal atom, a weight ratio of III to
II of from 1 : 1 to 3 : 1, in particular 1 : 1, is
preferred. The reaction is carried out in the presence
or absence of a solvent or diluent and with or without
the addition of an inorganic or organic base. The
preferred solvents and diluents include amides, such as
dimethylformamide, diethylformamide, dLmethylacetamide,
N-methylpyrrolidone, hexamethylphosphorotriamide, sul-
foxides, such as dimethyl sulfoxide, and finally
sulfolane.
Suitable bases, which may also be used as acid
acceptors in the reaction are, for example, alkali metal
hydrides, such as lithium hydride, sodium hydride and
potassium hydride, alkali metal amide~, such as sodium
amide and potassium amide, and sodium tert-butylate and
potassium tert-butylate.
The reaction is carried out in general at from
-10 to 120C, preferably from 20 to 80. In the presence
of a solven~, the reaction is advan~ageously carried out
at the boiling p~int of the particular solvent.
The compounds II can be prepared in a simple
manner by known method~ from the ketones of the formula
IV
Il (IV~
R 9~T R 10
for example by reaction with trime~hylsulfonium methyl-
sulfate (cf. Corey and Chaykov~ky, J. Am. ChemO Soc. 64
(1962), 3782).
The compounds IV can be prepared by generally
known methods. Where Rl and R5 form >CH-Z-, Can~acuzene
and Tordeux, Can. J. Chem. 54 (17) (1976), 2659-2766 may
be mentioned; if Rl and R5 form >C=CH-, reference may be
madP to Houben-Weyl-Nuller, Method~n der organischen
~ J3~ $ ~ O.Z. 0050/41291
Chemie, Georg-Thieme Yerlag, Stuttgart 1972, Vol. Vlb.
Examples of the compounds I~ are shown in Tables
1 and 2.
The compounds of general formula I and their
salts and metal complexes are suitable as fungicides and
are well tolerated by plant~.
Preparation Examples
Method A1:
2-(4-Chlorophenoxy)-cyclohexanone
~CI
23.3 g (0.181 mol) of 4-chlorophenol were added
to a solution of 5.4 g (0.225 mol) of sodium hydride (50~
strength dispersion in mineral oil) in 100 ml of N,N-
dimethylformamide. Thereafter, 20 g (0.151 mol) of 2-
chlorocyclohexanone were added and stirring was carried
out for 24 hours at room temperature. After the addition
of 100 ml of water and extraction several times with
methyl tert-butyl ether, the organic phase was washed
with water, dried over sodium sulfate and evaporated down
under reduced pressure.
Yield: 32.9 g (97~)
Melting point: 102-104C.
Method Bl:
3-(4-Chlorophenoxy)-l-oxabicyclo~ n .2.5]octane
~CI
22.7 g (0.121 mol) of trime~hylsulfonium methyl-
sulfat~ and 30 g of concentrated sodium hydroxide ~olu-
tion were added to a solution of 12.3 g (0.055 mol) of
2-(4-chlorophenoxy)-cyclohexanone in 100 ml of methylene
chloride. After the reaction mix~ure had been stirred
for from 12 to lS hours at room ~emperature (20C), 100 ml
~ 1~ ? ~ ~ 3 0 z. 0050/41291
of water were added to ~he solution and the organic phase
was separated off, washed twice with water, dried over
sodium sulfate and evaporated down.
Yield: ll.S g (88%)
S Method B2:
3-(4-chlorobenzylidene)-l-oxabicycloEo.2.s]octane
o~7
~cl
The procedure d~scri~ed in Method B1 was fol-
lowed, 23.5 g (0.108 mol) of 2-(4-chlorobenzylidene)-
cyclohexanone in 100 ml of methylene chloride, 24 g
(0.128 mol) of trimethylsulfoni~un methyl~ulfate and 50 ml
of 50% strength by weight sodium hydroxide solution being
used.
Yield: 24 g (95%)
EXAMPLE 1
1-(1,2,4-Triazol-l-ylmethyl)-2-(~-chlorophenoxy)-cyclo-
hexan-l-ol
~N~
N~ --¦
~CI
3.4 g of 50% strength by weight sodium hydroxide
solution were added to a solution of 3.5 g ~0.051 mol) of
1,2,4-tria201e in 50 ml of N,N-dlmethylformamiAe and the
mixture was heated at 50C for 30 minutes. Thereafter,
.0 g (0.033 mol) of 3-t4-chloropheno~y)-1-oxabicyclo-
[0.2.5]octane, which had been dissolved in 20 ml of N~N
dimethylfonmamide, were added dropwi~e at room tempera-
ture. After the reaction mix~ure had been s~irred for 15
hours at room temperature, 100 ml of water were added to
the solution and the mixture was extracted se~eral tLmes
by shaking with methyl tert-butyl ether. The isolated
organic phase was washed twice with water~ then dried
~3 ~ 3O.z. 0050/41291
over sodium sulfate and evaporated down. Crystallization
of the residue from methyl tert-butyl ether/n-hexane gave
the product as a 2 : 1 diastereomer mixture.
Yield: 6.5 g (63~)
Melting point: 120-122C
--r4 - O.Z. 0050/41291
EXAMPLE 2
1-tl,2,4-Triazol-l-ylmethyl)-2-(4-chlorophenylidene)-
cyclohexan-l-ol
N~
N
~CI
6.8 g of 50~ strength by weight sodium hydroxide
solution were added to a solution of 6.2 g (0.091 mol) of
1,2,4-triazole in 100 ml of N,N-dimethylformamide and the
mixture was heated for 30 minutes at 50C, similarly to
Example 1. After the reaction mixture had been cooled
to room temperature, lO.S g (0.045 mol) of 3-(4-chloro-
benzylidene)-1-oxabicyclo[0.2.5]octane, which had been
dissolved in 50 ml of N,N-dimethylformamide, were added
dropwise to the solution and the mixture was stirred for
12 hours at room temperature. Thereafter, 100 ml of
water were added, the mixture was extrac~ed several times
with methyl tert butyl ether and the organic phase was
washed with water, dried over sodium sulfate and evapora-
ted down under reduced pressure.
Yield: 10.5 g (77%).
The compounds listed in Tables 1 and 2 can be
prepared according to ~ethods B1 and B2, and those in
Tables 3 and 4 can be prepared according to Examples 1
and 2.
~ ly! 3 ~
- 15 - O. z . ~050141291
_ _ _ _
c~ ~ a~ cO
z ~ ~ E E
D 0 ~ ~t r~ 0
0~ D O
_u~
~y~c ~ o o o o o o o o o o o o o o ~ o
~ \ ~
o /~
tr
~rl T
I S ~ I T I T _ T T :1: I T I ~,
r
1~I T ~ T T T T T ~ I T
~ ~ T _ . I 1 3 ~ S ;t
y y ~ ~ ~ s ~o ~ ~ y ~
- l
` 3 O . Z . 0 0 5 0 / 412 91
E
O ~
Z ` ~
~O O
r~l OOOOOOO OOOOOOOOOOOOOOOOO
T S ~ S S T T ~T r S S 5: S ~ 9' T S T I I T T
T S S ~ ~r . S S ~ T T T S T T S T T
e ~ ~ L ~ ~ ~ ~ L L L L L ~ = L U ~ L L L
x ~
},~ ?~ o z 0050/41291
E
z - -
'D
E ~ ~
oooooooooooooooooooooV~
T S . T T ~ ' T ~ T T _ T _ T T T T T
Y T S S ~ ' T 1 ~r S ~ X T ~ S T S ~ T ~ ~ ~-
;~ 2 S
.~ ~O O O ~ ~:t C ~ - s P~ S U~ X X ~ ~
~1 S ~ ~ la C~ Q. O., C~ g. U C.3 0 0 L ;_ I I
~Z ~ ~ ~ ~ S -- ~ L c9 a~ Z a~ 2 ~
C~ ~ ~ ~ ~ ~ ~ ~ V ~ ~ I ~1 U t ~ ~ t~ t~ U ~ 3
-
¢ E ...... .. .. .. .. .. .. ... .. .
~" '"1~ ~J ~J ,~ ~3
- i8 - O. Z . 0050/412gl
E
0
~ O
T
E ~ o~
~ ~r S -- T T T T _ , S 5 T S T S S I T 'r T T T
c: s s r :r: T T _ T S ' . ~ T -- ~ ~ S T S T T :S I ~ T
~ ~ t~ ~ ~ 3 ~~:t ~ S T S T S T ~D ~
~ , ~ T TS ~ID ~O ~
C I ~ ~.) Y ~ I Y 1 ~1 ~ ~1 ~ <~ ~ X -e T
~ I I I I L L L L S S S ~ L) t_~ J
-
~1 a)
X
2 ~ 3
- 19 - O.Z. 0050/41291
T z O O O O O O O O O O O O O O O O O
~ 2 -- r ~ _ S T T I ~ T :1: X r r T 3 2 ~' S r
a: ~ ~ , , ~ T ~ ~ S X T T r = 1 = = =
C ~ S ~ ~ ~ ~ ~ 3 ~ T
C O t_) t~ L L I I V ~J I I ~ t,~ O V
T -- S~ , I I L L 1
a~ ~ o- o cn ~ a- ~ ~ o o o o o o o o o o ~
- 20 - O. Z . 0050/41291
E
-
ooooooooooooooooooo
~ T 2 2 2 ~, . æ s _ r
t:~ 2 S ~ S 2 S 3 -- ' 2 -- T r -- -- 3 2
t'7 e~
C ,~, S ~ t ~ T
l ~ 'D S T ~ ~ S
o ~ ~ Y I -- _ I I Y ~ L
_I
e~
x
~ ~. 3 ~
- 21 - O.Z. 0050/41291
S ~ ~_ _
CC S CO~ ~ Cr. ` '` '
` ~ o V~
~" ~r~ _ O-D
,
_u~
~Y ~
/~
U T S ~ 2 ' T
_ T T S S S T S -~ T
') ~ ~ V , T T
~ ~_ CO ~ O
C- X
'. ~'~ "
- 22~ ?t ~ ~ o . z . OO50 /4 12 9 1
-
-
Z ~
S _ ._
o
_ _
E ~D r`
_ ~
T T ~ S T ~ T T :1:
L L
I l ~ I S X T
T
~ T T T T
~ ~ ~ ~ r . T
L L ~.. ~ 3: S
-
~ :"
22 ~Sd ~ 2 ~
_- 3 _ O.z. 0050/41291
:~
~o
r~
o
~D
~ 0
q 1`
S ~ ~ _ T T T T T ~
T
S T
11
:1: ~' S S r T T r T
~ ~ ~ ~ ~ S '~
T I :1: S S
C ~.) V ~ 1 1 V ~ ~
S ~ I~ ~ S S ~ ~ ~ ~ L L i. L L
-- tJ 4 ~1 t~ t.~ O O O O Z Z 1-- L ~ r, O. i:L 1
-
`' q~
X
2 ~J ,i3 ~
- 24 - O.Z. 0050/41291
~,
o o
o
~,
Vl _
Z ~o
S ` _ o~
--
E ~o ~ ~
~ ~ O ~D
Cl: T '_ ~ ~ S S ~ ~ ~
~1 S r
12: ~ I T ~ ~ :C I I S T '~ = T
~ S
C ~ C ~ C C ~C ~ X ~ S I '~
c ~ ~ o o o o o o o o l l ~
-25 - O. Z . 0050/41291
~ _ _ _
~ ~ o
T '` _ _ _
~ oo o al ~r
e r~ o ,~ ~
C ~ ~-
T T I T
C~ ~ ~ ~ ~ ~ T T T
C~ 2 2 T . T T 2 r
aJ ~ ~ T T ~ ~
~ T T ~0 ~O (O X
.,1 ~ y I I I ~ y S
1~ T
C T T
O ~ C~ C~ O T O 1~ t,~ 1
?~ 3
26 - O.Z. 0050/41291
a
o~
. . ~
o
_.,
* ~
L _ ~
~ E--~ o
V
E o ~ ~
Y ~ ~ 1--
U- ~q o :~
Y ~ ~ ~ ,_
C ~ o ~
c~
CJ
S S T ~
3 X z ~ z z ~ 1 ~ z z z z z z z z z z
-
~_S_ ~ O S
J o o o o o o O O O O o O O O O O O
1'~ , ~ T
S T ~ ~ T T ~ T T C.
~, t.~ S ~ , T ~ r T 2
X S T
T ~ S T r i :~: S T S I I I
V V
..) ~ ~ ~ ~ ~ 1 T S 1 ~ ~ 3
f ~ g ~-j O . Z ~ 0050/4129 1
. .
~ r~
E
,_ ~
:~
0
~ _.
~ ZZZXZZ~ZZZZZZ~ZZZZZ~ZZZ-'ZZ
i~ OOOOOOOOOOOOC~OOOOOOOOOOOOO
-- ~T ~ . T T T T ~ S T ~' 3 , r r r ~ 2 ~: r
~ T
J T ~ ~ ~ T t l T ~ T _ T ~: ~ r ~ T r
T r
C ~ T T _ ,* ,;~ ~ ~ S ~ ~ 1 ~ T r T T
~ L L L L S T ~ T lV ~J LL,
~ o .~ o r~ a ~ ~ o _ ~ ~ ~ In ~ r~ c~ ~ o .
- ~#8~ O . Z . 0 05 0 / 4 1 2 9
E
Ul
_.
a
-
~c ZZZZZZZZZZZZZZZZZZZZZZZZZZ
O
T T T T T S ~ T T ~ r I S S S ~ T = S I T T
~': S ~ ' S T ~ T I X :S: S T T ~: I
t~ T
C ~ ~ ~V ~ O O O
C ~ ~ ~ ~ ~ S :~ -- ~ ~ ~ ~ S ~ ~ a. ~.
o ~ ~ I I ~ C~ O O -- S ~ 'D It~
O O O Z Z Z Z C.~ ~ ~1 ~ S T
~ ~1 ~ U') ~0 ~ Cl) ~ O ~ ~1 ~r~ ~ b'~ ~D 1~ ~;0 (~ O -- ~1 ~1 ~ IS'I ~0 1-- 0 CJ
;~
2~ ~J 1 2 ~ ~ ~3 o. z . 0050/41291
X X
E E
E O O -
C o C~
E ~ ~ a c:
u
o o o o
o c~
~- ~ O o
~ Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z t~ Z Z ~ 2 ~ z c~ z c~ z
o o o o o o o o o o o o o ~ ~
T S ~ ~ T S 1 S S T = T t~ ~ t.) T
S T ~--
C~ ~ T I ~: ~r 3 T S ~ T T T T ~ S X: r
~1
~ ~ ~ ~ T ~ T ~ T ~r T ~
C C~. ~ Q. ~ ~ ~.) O O ~ O ~ L L I I I I I I I I V
U Z Z 3i (~ U ~ O. ~ T X T T O O V V V V V V
~, O ~ D 1` c a~ o ~ ~ ~ ~ u~ ~o r` GO cn c~ ~ ~ ~ ~ u
30 ~ Pp ~ ~d ~ ~ . Z . 0050/41291
Il J ~
c:~ ,a X
fi
E
-
-
~ .
o o
~ u~ O
~ _
~ I I
Ei o ~
_ _
S z S z z z S
T T a~ r :~ S S-- S -- _ T S T I r I T , T I I S
(~C ' T T ~ T S T 2 r T T I 1 T I ~ T = '~ T ~ I T
S ~ ~ ~ ~ ~ ~ 3 I I
~ ~ ~ .a~ .:t ;t' ~ -~ ~ T T `r ~1 T S
C I I I ~ s ~ ~ ~t ~ ~ * S T
~ ~ m ~ 43 C~ S ~ I ~
-
~ ,.. ~ ~D r~ oo ~ O O o o C~ o o 0 0 ~
E~
. . ,
~322~ :3 o.z. 0050/41291
E
o
-
-
Z Z Z Z ~ X c~ Z 2 Z Z æ z z z z z z z z z z z z z ~
S I C_) t.) 2 T ~ C.~ :C C~ I ~ r ~ I C~
ZZZZZ;!:ZZZZZZZZZZZ
1~ T T T .~ S S S T ~ 2 -- r T I T 2 T T ~ I T I T
~Y T T T S T S T S S S S S S S S I I T T T T ~ I I T
~ m ~ ~ T T T T ~ ~ ~ ~ ~ T
rl al ~ -r S -- S ~ O ~ ~ ~ T
X O O ~ L I I I I t~
~f~3?~t~c~
- - 32 - O.Z. 0050/4129
E
U~
E
X
r~
a-
ZZZZZZZZZZZZZZZZZZZZZZZZZZ
''zzzzzzztn
~ _ T S ~ ~ T S Z S T S T S ~ T S T T = ~' r
Q T ~ S S ~ T ~ 1 T ' = S T T T T = = = T T
;t S T ~ I T
S ~ ~ T T ~ ~ 3 ~ '*
e O ~ U ~ C ~ s ~ ~ , = = =r ~ = = = =
:: :
~2~
-33 - O. Z . 0050/41291
.
Cl Q C~
X
_.
CO
6 o ~
t,l a~ co
o oo C~
X Z Z :~ Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z z Z Z Z T I
.
O O O ~ O o O O
0~ S S I X T ~ ' S S ~ T ~' T T I T S r
~ T S '1~ T r S ~ ~ T X S S T S ~r ~r ~ I T
C ~ ~ = s ~ ~ ~ ~ ~ ~ ~ ~ U ~ ~
r~
_ 34F~ ~ 3 2 ~ ~ ~3 - Z 0050/41291
~_
~o ~
~o . .
~`
__ __
3 a ~ 3 ~
c
,
3 x z ~ Z Z X Z Z
_u~
~ ~: C
r~
Z \ ~ ~ T T T ~ T
~ 3 3
Il ll 11
~: T T ~ ~ ~ -r ~ ~ I
T T ~ S ~ O
~r aJ
E~
2 ~ ~ ~3
- 35 - O. Z . 0050/41291
-
S o
z I I :1: r
T S ~ 3 T ~ I I ~J T ~_)
T S ' 2 T
I' T ~ T
Il 11 11 11 11 11 11 11
1 ~ ~ X ~r ~ T ~
a) ~ 3
r
.,1 CJ ~
C ~ ~ r T i ~r T T S r
d'
~ o ~
,a
~i ~
36~ S~ o.z. 0050/412~1
E
V
-- _
oO ~`
r `D
~ '~ ~
X Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z
S T
r s s ~ s ~: s~
:' :
_ 37 - O.z. 0050/41291
-
Z '' _ '' ~
~ o ~ ~ o
X ~ Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z
S T r ~ ~ T X ~' ~ ~ T
-~e
~: r ~- T S S ~ S T ~ C.~ T
,~ S V~ ~ .,~ T S S
C ~ ~ L L
~ 3 ~ ~ ~ ~ 3
(~ 5~ 2 `~ g ~ o.z . 0050/41291
S S
a~ S S 2 2 T S '~ 5~ S
C~ CO
L ~ 3 T
~ t~ ~
X :E S ~ T S
C ~ S S ~ 9 S
L ~ ~ O O ~ ~r S S T '
Q)
IY
.
_ 39~~ t3 -Z- 0050/41291
3,
E
~0
Co
S .
V
3 ~
~ 2 Z r Z Z r 2 Z ~ Z V Z Z ~ Z
X V T T T-- T X ~ r~ ~ I
:1: T T ~
~I T ~ T 1 1 l l
V I S --CJ T . tJ
~U S ~ T ~ T ~ T
C v
~1 1 1 1 1 1 1 1 1 1 1 1 1 1 1
C ~ ~ ~ V ~ ~ 1 ~ I ~ r
O 1~ 1 1 1 1 11 1 1 1 11 1 '
3 ;1~ 3`;r
c~ ~ 0 0 o~ 0 a~
~3 X ~ ~ ~ ~ ~ 3
- 40 - ~ ~ O. Z . 0050/41291
~U~J tJL~
E
~ O
. ~
. ~
I~ _
.
-- V -- o o
x z z ~ z z ~.~ Z S Z Z Z S Z z
CIC I T ~ ~ ~r T ~ ~ r ~,~
I~ ~ ~
3 ~
I
T S ~'
Q: ~ S ~ S C~
::J S ~ ~ T T 3 ~ r ~- T T ~ T 1
C ~ ~ ~ ~ ~ V
~r
~o r~ oo ~ o ~ ~ O
~ ~ cr ~ o o~ c~ o ~ o o o o o o o .--
E~ X ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~
- 41 - O. Z . 0050/41291
C~
C~
o
,
~ZZZ~: Z ZZ~ ZZZZZZZZZZZ
~: T T ~ ~ T i '1 ~ T T T S r -r S S S
~ ~ ~ 11
S r
~: s æ v ~ _ T S ~r ~ S ~ S 1~ T T T T
-;t ;~` ~:t -;t S S
S ~: T T ~ S:1: S'D
C I I I I I I I I o o a~ ,J ~ ~ T i-
T S T ~-- S T ~ T 1 ~ ~ CL 0. O. a. ~ C ~
O O O O ~ a~ m z z: ~ z z
.
- 42 - O. Z . 0050/41291
o
V~
r ~O
v
o
E ~ ~
~CZ Z Z ZZZZZZZZZZ ZZ ZZZ
-- T ~ ~ T T T
1~ T T ~ T :C X S t_~ ~ S T ~ T T
O O O
V
C ~-- ~-- ~-- U ~ ~
.,~ L :~ L :~ L ~ '~ 'L 'L 'L 'L L L ~ t L ~ L L
o r~ ~ L 1-- L Q"
-
- 43 - O. Z . 0050/~1291
L
O
w
Z:
~ O
_4 CO O
O
,r) oo
E ~ n
_.
XZZZZZZ Z ~ZZZ ~Z~Z~ZZ Z
C ~ ~ ~ ~ ~ t~ t~ ~,~ ~ ~ t~ ~ t
r,r~ ~ r~
T S ~ T _ _ T ~ I t~ ~ T
?
~ ~ T C ~ T r
r~
~ -- ~ ~ ~ ~ O ~ ~ ~ ~ C ~ X X ~ X
.-1 C C ~ ~ ~ L L O (I) Q~ L)
~ ,_ ,~ ,~ ,~ 1, 1,1, c~ o ~ Q. ~ 3 C~ S .C .~: t- '-
c S a ~
O ~ L L ~ C.~ > U V ~ ~ U ~
E- X ~ ~ ~ , ~ ~ $ ~ ~ 3 ~t~ ~ ~ ~ `J ;l~ ~ ;l ~ ;t
_ 44 _ o.z. 0050/412gl
T T V Z 2 Z V Z V Z V Z ~) Z Z Z V
T T T ~ T T T T T I T T S
~VV~V~V~VVVC~VVVVVVVVVV
T S 1~~1
C ~ ) V r T ~ D
''I V V ~ V_, _ 25~ ~ ,o I I V ~_~ V V V V V
V C~ V V VC ~1~ IL I I I I '`I '`
O V ~ V L O T
Q~
~ X ~ ~ ~ ;t ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ 3 ~ ~ `:t 2 ~ ~ ~
- 45 - O.Z. 0050/41291
o 2 ~ ~3 ~
X Z C,~ Z~ Z ~ ;! S Z ~ Z S Z I j T T ~
X T ~ ~ ~S T i C S T T T T S T T T I T I I T
æ T ~ '~ S ~ 2
TT ~ X S C ~ ~ .1 C S U U~
"~~.7 ~~'1 jv~ r~ T T T C ~ O C~ Q. Q 1~.
O t~ O O O O U7 U7 ~ t~l Z Z Z Z
~ 0 ~ ~ o o- ~ o cr cr~ o- a- o o o o o o o o o o
~ X ~ ~ ~ ~ ~ ~ 3 ;~ P 3 ~ 3 ~ ~ ~ ~ ~ ~ ~ 3 3
.
. ' . ,
.
_ d~6 - O.Z. 0050/41291
~ 2 i.` 3 ~ 2 ~ ~
~ Z V Z V Z ~ Z ~ Z ~ Z ~ Z t-- Z ~ Z ~ Z
a: T ~ T T I T T S ~ . r ~ _ T S
f~
tJ ~ ~ V ~ ~ ~ ~ V ~ ~ , ~ ~ S X ~ ~ ~ ~ S
O O cn a~
L ~ e S
V ~ C C C C
~ C ~ C L L L L L L L L ~ O O O O
o L ~ L '~ ~ ~ 3 ~ ,5: .C S
~r
- - 47 ~ Z. 0050/41291
L C`J
E ~ 2C~d
C~
V~
_.
C~
s ~D
z
o o o o o o o
0 ~ ~ ~ -- C~ O` ~
T T T T T T -r ~ S ~: S ~ S I S S :!:
X ~ ) ~ Zt~ Z ~ Z C~ Z t_)
~r7 S I I ~' T T T I -r I S S T
~1: I S I T . . ~ t O ~ ~ T ~
T
t~J T S I I I ~ S T I ~ ~ T T I -r
,~,
I T
T ~ ~7
o ~ ~ o ~o
~' I C.~ T I ~:t ~ ~ S S I ~ r C T :C I
, _1 ~ ~ I ~O ~ S r~ ~ r t ~O ~ S I ~ t ~D tO D
_ I I ~ I I ~ _' _ I I I I L r t ~ L
a~
~ I_ ~ ~ ~ O --
- 48 - O.Z. 0050/41291
u- ~J ' l ~ 2 ~ ~ ~
_.
e
x ~ > t~ ~ V Z: ~ Z ~ Z C. Z c.~ Z ~I Z Z ~ 2 C.~ Z
~ S I~ r ~ 3~ I I I ~ r ~ r
tt.~ V V ~ t~ ~ X ~ S :e I T 1 T
I
~a o o
c ~ ~~ y ~ c~ c:
~ ~ ~7 S .C S L :~ L
T S ~1~ V X ~ ~ ~ ~ RJ ~ ~ ~ L L ~ L .
o ~ ~ o o o m ~ z z 1-- L 1-- L ~. I:L 0. LL 1~ 1-- 1~ 1--
~ X ~ ~ 3 ~ ~
~a
- 4g - O.Z. 0050/41291
L ,~ V ~
,_
S
O o o o o
O O
~ X ~
X <_~ Z ~C,~ Z I Z S Z I -r
O
I T T j~
E
i + + + + +
0
~ I T
T ~ T I T
C L L L ~0 ~ ~ ~ o I I I O
O O O OO L I I
O 1'~ ~ ~ L
~1~ 11
t 3 ~ ~ ~ ~:t 3 ~ ~ ~ ~ '`J ~
~ 50 ~~ s~ ~ ~-Z 0050/41291
Method B3
2-(4-Chlorobenzylidene)-spiro-(trans-decalin-1,2-oxirane
(compound No . 5 .1 )
~CI
4 5 . 5 g of (O.24 mol) of trimethyl 8Ul fonium methylsulfate
and 42 ml of concentrated sodium hydroxide solution were
added to a solution of 30.2 g (0.11 mol) of 2-(4-chloro-
benzylidene)decal-l-one in 200 ml of methylene chloride.
After the reaction mixture has been stirred at room
temperature (20C) for 12 hours, 100 ml of water were
added to the solution and the organic phase was separated
off. The organic phase was washed twice with water and
dried over sodium sulfate and evaporated down.
Yield: 29 g (91 ~
Melting point: 102-105C
EXAMPLE 3
1-(1,2,4-Triazol-l-ylmethyl)-2-(4-chlorobenzylidene)-
decan-1-ol (compound No. 8.1)
~7
N~N~
~CI
5.2 ml of 50 % strength by weight sodium
hydroxide solution were added to a qolution o 6.~ g
(0.1 mol~ 1,2,4-triazole in 50 ml of N,N-dLmethyl-
formamide and the mixture was heated at 50C for 30
minutes. 14.4 g of (o.os mol~ of 2-(4-chlorobenzylidene)-
spiro(~rans-decalin-1,2-oxirane), which had been dis-
solved in 30 ml of N,N-dime~hylformamida, were then added
dropwise at room temperature. After the reaction mixture
had ~een stirred at room temperature for 15 hours, 100 ml
of water were added ~o the solution, which was extracted
several times when shaken with methyl ter~-butyl ether.
The isolated organic phase was washed twice with water
and then dried over sodium sulfate and evaporated down.
- 51 - O.Z. 0050/41291
3J ~J 3 ~'
By crystallizing the residue from methyl tert-butyl
ether/n-hexane, the product was obtained as an enantiomer
mixture.
Yield: 13.3 g (63 ~)
Melting point: 180-186C
The compounds listed in Tables 5, 6 and 7 can be
prepared according to B3 and the compounds in Tables 8,
9 and 10 can be prepared according to Example 3.
- -52 ~ U ~V ~"J ~'J ~J '~3 0 . Z . O OS O / 4 12 9 1
o
O o
~ ~0
l-- ~ ~ ~ t ~ O O O O O u~ ~ O
T = ~ J = S ~ Z
T ~ ~ S S r
u~
E~
_ 53 ~ 3 O . Z . 0050 /4 129 1
s
:r: s s s S T ~ S T S ~ S ~ S S S T T T T 1 T
r S S ~ ~ T t~ T t,~ S
T 11ll I 11 I 11 S 11 11 Il T 11 ~ 11 T 11 T T S
~ S . T S S S T 3 ~ T ~ T T T T S T = T = S :~ T
V T
. ~ ~ T S ~ T T T U~
r S ~ S ~ ~ L L -- o o o
~ ~ ~ ~ T T
V ,~ ~ I I ~ ~ ~ I ,~. , S. I ~ -t ~ ~ U ~ ~7
u~
~ ~ L~
-- 54 - O. Z . 0050/41291
a. i
E ~n
~D
O C~ ~> O O t~ ~ ~ O ~ ~ O ~ ~ t~
, S ~ 2 T ~ ~ I 2
e~ T ~ ~ 'S T I T I ~ ~t -:t ~ 2
C
T I :~ T ::1 I X T ~ 1 T T T
T ~ T T ~) C l ~ V
a)
~ O ~ O
2 ~)J ~ ~
- 5-5 -- O. Z . 0050/41291
T T S . S ~ T T` = T T ~ I r T T
,,~ 2 r ~ ,~ , T ~ ~ T I ~ r r r
11 11 I 11 T 11 T 11 T 11 T ~ 11 T ll ll 11 T ll 11 11 A ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ /~ ~\ ~ ~ ~ ~ A
~ ~ ~ 3 T ~ ~ T 3 ~ 3 '~ T ~
C ~ ~ I r T C~ I I T T -r
.,~ ~ r ~ ~ -- -- T _ 'r y t~ ~ y ~ ~ y
C I I I Y I Y I Y Y I Y ,.. ,~ ~. IL I I I I T S T
~D
i~ X
- 5 6~ ' S~ ~- ' O . Z . 0 0 5 0 / 4 1 2 9 1
T
E
~ S ~ ~ U ~ ~
U7 T I T ,. r
3: ~ T T T C ) ~r T T ~ T T T ~ ~ T T ~ I T
I ~ Y ~ I 11 ~ ~' s '' ~ ~ r ~ ~ ~) r ~
y ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ A ~ ~ t~ ~
T ~ ~ S T ~ ~ 'C T .* `;t
-- _ -- C C C~ ~ ~J V
L l_ L L L L L L ~ -- O O O O
~D
~' ~ 3
- 57 - O. Z . 0050/41291
V
O O U~ V) O O V~ O O O O O ~ ~ O O O ~
U- T ~ . ~ T S ~ T ~ S r ~ T T ~ r ~.
Y~ ~ U ~
Il ~S 11 T 11 S 11 11 T 11 T 11 11 T ll 11 I 11 r
o: ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ /~ ~ A A
~ ST ~ T T S S T ~ T 3 . _ T r T r
C~
,- u ~ sr s T T S T ~ T T T r 5: ~ I r
O /)~( ~`J I S S S T S S t~ T r s ~ s 3: r T
:~: r ~ r
:s: s s s ~ q s s s ~
y ~ . y ~ y ~ _ _
T I T ~ ~ U ~ ~ 3
r
1~ 0
J 2 ~ ? 1
58 - O . Z .0050/4 12 9 1
S o
E ~D
~ o o oo o o o o o o o V~
y~-- S S S T T ~ C ) T T ~_) r
' -- S '` TS ~: ' X 1 r
cl: s ~ ~ ~ r r ~ r r r
~_ S= ~ ~3 _ T = T ~ I
-
S S U~
S ~ I~ X S ~ ~ ~
. ~~ ~ ~ ~ O _
, '
'~ ' ' ' ,
;
t~
- 59- - O. Z . 0050/41291
~ o o o o o o o o o o o ~ o o o
~ T:C ~ T T ~ S ~ T S C~ ~ S T ~
~ ~ ~') V 1111 S 11 1~ 1
Cl T T T1 S ~ S ~ I T T
Cl: T . t~ T T ~ T T ~J ~ I
S S
c~: r ~ -- ~ ~ T ~ S S T T r T
C ~ ~r T S S -e T X T .-- --
~ ~ ~ ~ S S . ~r S
I~ I I
I~
a3
Z~
- 60 ~ J?~ O.Z. 0050/41291
T
0~
E
~ o o o o o o o o o o
~ <`. .~,
Cl T ~ TS ~
T1l1l T 1l 1l T
T T I T S T T T T
C~: S ' T T T ~e
~ ~Jz
~ r I _ s s s T ~ T
-
_ _ _
~ ~ X X
S_ L L. S~ O O O
O ~ ~ 3 ~ . _ _ _
r~
~¢ ~a . . . . . . . . . .
~s~$ 2 ~ ~ ~ z . 0050/4l29l
o o o o
o
co l o
~, o ,9 o o
X Z S~ Z Z ~ 2 Z Z ~ Z Z Z Z Z Z Z Z Z Z
T T -- ~ (~l ~1 ~
O O O O O C~ O V~ l t.,) o
S 2 ~ I S S = ~ S S
X--Z~
T _ ~ T T T ~ S T T ~ ~ T
-
T T T ~ ~ T ' T S T S r T r
V ~ ~ ~ ~ ~ ~ U ~ ~ ~ ~ ~ I T 1 T
¢~
k~
- ~6`~ O . Z . 0050/41291
ZZZZZZZZZZZZZZZZZZZZZZZZ
1- o ~ ~ ~ ~ o ~ t~ ~ ~ o o u~ ~ ~ ~ o o ~ o
u S I S S S
~ T 11 11 11 S 11 ~ 11 11 11 T 11 ~ 11 11 11 T 11 I 11 11 I 11 11
~: T S S ~ ~ T T T ~C '~ T T S ~ ~r T T I ~ I S I T
-
a~ T T S :C S r
~ D ~ ~ ~ ~ ~ ~ s ~: S r ~-- ~ ~= T T
S S C.
X 0 ~ C3 CC~ 0 01~
: ' :
., .
~ . .
~2~
-- ~3 - O.Z. 0050/41291
x z Z Z Z Z Z Z Z Z Z Z Z Z Z ~;! 2 Z Z Z Z Z Z Z Z
~- O ~ ~ U O O ~ ~ O O ~ ~ O O ~ ~ O O ~ ~ O O ~ t~
A A ~ ~ ~ A ~ <~ ~ CAJ C ~ I T
T ~ r . T T T ~ T T r T T = T ~ T r T
T T S T S -- ~ -- S -- T X T ~ ~, ~, ~, ~, ~, ~,
o I ~ ~ S I ~ x ~ ~ ~ z z æ z z Z
m _I
C~ ~ ~ 0 ~ x ~ a~ x ~ o~ ~o 0 ~o
- 64 ~ 30.z. 0050/~12~1
~ ZZZZZZZZZZZZZZZZZZZZZZZZ
1--0 0 ~ C~ O O ~ ~ O O ~ C~ O O ~ _~ O O ~ ~ O O ~ ~
U T T S T T T r T I r
~Y _r ~ T ~ T ~ ~ S~) T ~ T t_~ T ~ T ~ T r~
~ Ir~ Ir~ I ~ I r~ I ~ I
Il T11 S 111 U T11 T11 T 1l ~11 T 11 ~ 11 2 U T: il T
Y ~ X ~ ~ A A A ~ X X X ~ ~ ~ A X A ~ A ~ ~ ~ ~ A
Cc T T ~ T S S T T T ~ ~ T T ~ T T T T r I T 1 T
tU _ _ _ _ _ _ T T T T T ~ T T
C~ ~c C C C e ~
~ t~ ~ ~' Q~ S S S ~ X ~ ~ L L S_L. L L
x ~ ~ I ~ 1 5 ~ 5 5 ~ ~ ~ I ~, S ~ ~ ~ ~ ~ ~ ~ ~
cj ~ S;) r~
--65 - O.Z. OOSO/41291
E
X ZZZZZZZZZZZZZZZZZZZZZZ
- o o ~ ~ o O ~, ~ O O ~S ~ O O r S O s
r ~, I C~ ~ ~ S ~ S U S ~ S ~ T
~IC e S S ' T T ~ T S ~ ~ T S S ~ T T . S I S I
_ _ _ _
_ _ _ _ _ _ T S C T ~ ~ .~
C _ ~ ,, ~ ~ ~ ~ c c e x x x
U ~ V V V t~ V ~
GO
O ~ ~ O .--
~' ~ ~ ~ a- ~ o- ~7 C~' _ _ O o o
,
.
- 66 - O. Z . 0050/41291
o o
o o~
~ _.
o ~
~ _.
Z ~ Z Z 2 C_~ Z Z 2 Z Z Z 2 Z Z Z Z Z
~ C~ ~ O ~ O ~ > O t~ ~ O t~ ~ O U')
tlC T S T ~ ~ ~ ~ ~ S T t_~ I T ~ I T
~ A A A A A A A ~ A A A A ~ A ~ ~ ~ A
_ U7
\ / T
~_ '0~ _ _ _ _ _
X--~ ~ ~ ~
L2 ~ s I T T S T , :,, ~, ~ ~ ~ T T :C S ~ ~
T ~ T T I T ~ I S T I I
C~ e~ O ~ ~ O ~ T i I ~ T
r~
--~ ~ rr ~ ~ '.D
- 6 7 ~ 'J '~ ) O ~ Z ~ 0 0 5 0 / 4 1 2 9 1
X Z~ZZZZZZZZZZZZZZZZZZZZZZ
T ' 2 T 2 S 2 ~ 2 2 1: = = ~ I =
l-- O ~ ~ ~ O C.~ ~) O ~ 1 0 t,~ O ~ t.~ O ~ ~.)
2 _ C,~ ~ S ~ = C,~ T = ~
11 11 11 11 11 2 1111 S 11 ~ 11 11 1) 11 T 11 ~ 11 T
~: A X X ~ ~ A ~ ~ ~X X ~ X X ~ ~ X ~ ~ ~ ~ ~ A A
-- S S ~ r- S ~
C ~ S9 ~ S T
a~
- 6 8 -~ 3 O . Z . 005 ~) / 4 12 9 1
y
E
2 Z Z 2 2 Z Z Z Z Z Z Z Z Z Z 2 Z 2 Z Z Z 2 Z Z
- o ~ O S ~ O S ~ = ~ O ~ ~ O S ~ X r O I
~ l T 11 11 T ~ l T 11 11 I 11 r 1l ll T
~ ~ ~ ~ X ~ t ~ '~ ~ ~ ~ ~ ~ T -r I 3
C S ~ ~ ~t ~ ~ ~ ~ t ~ r ,~ r I r T I I T ~
IIIIIIIIIIIIIIIIIIIIIIII
,.~
U~ ~O ~` ~ C~ O _ ~ ~ ~ ~ ~ r~ 0 ~ O _ ~ f'l ~ u ~D
- 6!~ ~ CJ F~ s?J ~ -i 0. Z . 0050/41291
D~
e
x z z z z Z Z 2 Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z
~ O C~ O C.~ ~ O ~ ~ t ~ O ~ O C~ ~ ~
Ul I T T S T T T I :~:
v Y ~ y ~ ~ , s y s ~) ~ " y ~ y ~ ~ ~ r y
11 0 T 11 ~ ll11 T111l I 11 T 11 11 T 11 11 T 11 I 11 11 T
CC X X X A X X ~ X X X X X X X A X X X X X X X ~ X
_ _ _ _ _
~C ~ ~ ~ T T 2 ~ ~ ~ ~ ~ S 1 ' ~ ~ ~ ~ ~ ~- T T
aJ
C S T T I S S T S S T S S S T T S
r C ~ ~ ~:
O S ~: S S ~ S S I S :e T 't- Z Z Z Z Z
~1
.
7 0~ O . Z . O 0 5 0 / 4 12 9 1
x Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z 2 z
1-- o ~ iI~ t ~ i,S) o ~S I o i~ C~ ~1 ~ o ~ i~ o i~ i~ ~ o ~J i~
I T i.~ I i~ T ~ t,.l T S t~ ~ ~ T ~ T i~
i11 1I S 1I S 11~I S ~ ~ 11 3 1l 1I S 11 11 1I T 11 11 I
~1: ~ ~ ~ ~ ~ ~ A ~ ~ ~ ~ ~ A A A A A A ~ ~ ~ A ~ ~
_ _ _ _ _ _ _ _
'- ~ ~ ~ S ~ t S ~ t ;t I T X ~ ~
t ~ 3 ;1
I -- _ T I ~ T
~ _ C ,C C C S C S C ): C ~, ~ C C ~U ~ i V I
i~ a~ 2 Z Z Z Z Z 2 Z ~ Z C~l Cl:l a~ i, ~ i~ y i, ~D O O y y
p~ Q~ ~ i~ i~ ~ i~ ~o r~ i~o i~ g o o c, i~ > o o c~ o _~
W
t~ ';; O . Z . 0050/41291
x z z 2 Z Z Z z Z z z z Z Z Z Z Z Z Z Z Z Z Z Z Z
~ O r C~ S ~ O ~ ,~ O S ,~ ~ ~ O S :C O r t~ O r tJ
t I T ~ I t ) S r t~ T r ~, T ~ 2 T ~) r T C~ T ~ I T t )
~: A A ~ A ~ ~ A ~ ~ ~ A A A ~ ~ ~ ~ ~ ~
_ ,.~
~s ~ ~ ~ r T ~ ~ ~ ~ ~ ~ 2 T ~ ~ t 3 ~ T T ;t 3
r T T 2 T T r r T T
r ~ r r~ U~ r
C T 2 S S S T T S S C ~ L L L L
C,~ O O O O O O O O O O O ~ ~ ~`J ~ ~ (~ t~l ~I tJL tl 1:~ t~ 0_
r~ I I I I I I I I I I I T S ' :1 T T T ~ I I I I I
t ~ t~ ~ C~ ~ ~ ~ tr~ tr) t~ t-'l
F
t~ t.~ a~ t~ t~ a~ ~ a~ cr ~ ~ o~ tJl ~ ~ tJ t~ t~- t~ tJ~
- :~22~'2~ O.Z. 0050/41291
X ZZZZZZZZZZZZZZZZZZZZZZZZ
S I 2 S~C S S T S 2 2 I 2 T . 2
l-- O t~ ~ ~ ~ O t.~ O ~ t.~ ~ ~ O ~ ~ O ~ C.~ O ~ t_~
Cl T 2 ~ ~ ~ T~ T ~ ~ ~ T 2 t~J 2 ~ S ~ ~J T ~~ 11 1I T 11 2 11 2 11 ~ 11 2 11 11 2 11 11 ~ 2 11 2
CC ~ ~ A A ~ ~ A ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ A A
~ t 2 ~ ~ ~ 2 ~ ~t ~:t ~ ~ I T T
C ---- -- _ _ _ _ _ _ _ r _ _ _ _
,1~ ~ ~ ~ r ~ -- C C C C C C ~ C C ~ ~ C C
L L L L L L L L L L L ~
O ~ ~ C~ a ~ ~ a ~ ~ a a ~ c c c ~ 1: c c c s
a~
_1 ~ O _ ~ ~ ~ ~ ~ I~ o~ ~ o _ ~ ~ ~ ~ ~ o o o
E~ cr at a. ~ '
S:~
J '`J ~ ~ O . ~ . 0 0 5 0 / 4 1 2 9 1
X ZZZZZZZZZZ;~ZZZZZZZZ
T -r ~ S 5 T T T T :t T ~r I I
~ C~ ~ ~ ~ ~ O ~ ~ O ~ ~ ~ ~ O ~ O ~
U-T T S _ S 1: I
Cl:T ~ . _ ~ S T ~ r ~ T ~ S T T ~ :C ~.)
~ 1I T 11 11 ~ 11 1I T 1I T 11 11 1I T 11
a: ~')XXXXXX~ X~XX~X~
_ _ _ _ _ _
. . T T ~ ~ ~ ~ ~
~ ~ ~ ~ ~ ~ ~ ~ ~ 3 ~ X X X ~ X X
1~ L ~ L L L L L ~ ~ U U ~ ~ ~
0 ~ o ~ D 1` ~ Ot O _
E~ ~
7 4 ~ ",. O . Z . O 0 5 0 / 4 12 9 1
o ~J o
o ~> oo
O~ ~
E ~ ~ In
Z Z Z Z Z Z Z Z Z Z Z Z
o o ~n o o o v~ o o o ~ o
C ~ S t.~ ~: Z t.l S I
~11 T 111l 11 11 11 11 T 11 11
Ct: X X A ~ A ~ ~ ~ X ~ ~ ~
2: T T S T 1 ~ T T :~: S T I
t~ S ---- ~n :1 -- T I T
~3 S T ~ I~ S T
X--Z
Il ~ /\
~ , ~ T ~ T :I
C~ V
T S T S
_I
~ a)
~ ~ O --' ~
3 ~
X ~ ~ ~ o ~
'
- 7S~ 3?,2~3 ' Z' 0050/41291
C4
x Z Z Z Z Z 2 Z Z Z Z Z Z Z Z Z
1- 0 0V~ O O O~7 0 0 0 ~ O O O V~
U- T
2 y ~ y T ~ = S = y
Cl: T T T T I T T T T T T T I I T
C~: T I T ¦ T T T ¦ I T = I = :1:
QC T T T T T .: _ _ = T ~ = T
~ 11 11 11
2 ' S
T ~ 1 T ~ ~ S
_I
o O O ~ O O O o O O O O O ~ o
~ ~ s~
cc
2 ;! Z Z 2 Z Z
o o o In o o o ~ o o o v~ o
T S ~ ~ T :C ~.) S X :~: ~ S ~--
.~ Il 11 X ll li 11 T ll ll 11 T 11 11
~Y s x ~ s ~ r ~ I ~ T I ~ r
I~ I
~: ¦ T X ~ T :C T ~ S I :1:
~ ~ r s :s I I T r
C ~ ~ ~ S 1' =
~,) LL LL LL ~ IL X 1 ~ . T S X
O ~ ~ ~ '`J ~ ~ ~ ~ ~ ~ ~ ~ t,~l
t.~ ~ ~ t.`~ ~ ~ ~D r~ 0~ O O
O O ~ O O O O O O O O O O
7 7 ~ r) r O . Z 0 0 5 0 / 412 9 1
~c zzzzzzzzzzzzz zzz z zz
1-- o o u~ O O ~ O O ~n o o ~ o o o ~ o o o
y ~ T S t~ ~ S t~ T t.. ~ ~1 X ' ~ T S '1
C~: A ~ ~ ~ A ~ ~ ~ ~ ~ ~ ~ ~ ~\
T S 2 T T . T S S T S T T X
r
r S ~ ~C ~ T ~ T T T X T :~ T T T T T I
~
~ I _ _ _~
~ ~ ~ Y ~ ~ T ~ T T
a~
"~ T T _ T S ; T T
~ 5 ~ r ,C r C C C ~ ~ y C~ ~) ~ ~ ~ T
z z z z z z ~ D m I T , , , , T I I ~
_I
a,~
ooooooooo~ooo ooo o oo
X ~
- 78 - O.~. ~050/~1291
_.
X Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z
1-- ~ o o o v) o o o n o o o ~ o o o v~
U U U S ~ U ~~ S 11 11 U ~ 1' U U U U
t~C T S T S T S I S S T r I S I ~ S
u~
C~ I S S S S T T T T
OC T 11 r s s: s ~: ~ ~ T . T U
-
.t ) .
a~ a
S r ~ ~ ~ , ~ T T
~ U L L ~ 1 ~ S ~_ C ~_ L L
O ~ ~ ~ ~ ~ ~ ~
O O O O O O O O ~ O O O O O O O O
- 79 - Q.Z. 0050/41291
~ ~ s? ~ G~
X Z Z Z Z Z
,_ o o o V~ o
m T ~ T
Il 11 ~ 11 11
Y I T T
C T ~ I
+
~I T
C ~ ~
O . l~ O O O
~, t ~ U
O
0
O O O C~ O
E- X
.
.:
- 82 ~ ~ ,$ ~ O.Z. 0050/41291
Method B4
4-(4-Chlorophenyl)-l-oxabicyclo[0.2.5]octane (compound
No.1, Table 1) O
Cl
54.4 g (0.30 mol) of trimethylsulfonium methylsulfate and
50 ml of 50 % strength of sodium hydroxide solution were
added to a solution of 30 g (0.14 mol) of 3-(4-chloro-
phenyl)cyclohexanone in 100 ml of methylene chloride.
After the reaction mixture had been stirred for 12 hours
at room temperature (20C), 100 ml of water were added to
the solution and the organic phase was separated off. The
organic phase was washed twice with water, dried over
sodium sulfate and evaporated down.
Yield: 28.2 g (91 %)
EXAMPLE 4
1-(1,2,4-Triazol-l-yl-methyl)-3-(4-chlorophenyl)-cyclo-
hexan-1-ol (compound No. 12.1, Table 12)
F~
N~N~
~CI
12.4 g of S0 % strength by weight sodium hy-
droxide solution were added to a solution of 11.3 g
(0~16 mol) of triazole in 100 ml of N,N-dimethylformamide
and the mixture was heated at 50~C for 30 minutes. 18.2
g of (0.08 mol) of 4-(4-chlorophenyl~ oxabicyclo-
~0.2.5]octane, which had been di~olved in 20 ml of N,N-
dimethylformamide, w~re addad dropwise at room tempera-
ture. After the reaction mixture had been stirred at room
temperature for 15 hours, 100 ml of water were added to
the solution~ which was extrac~ed several ~imes by
shaking with methyl tert-bu~yl ether. The organic phase
was washed ~wice with water and dried over sodium sulfate
; <..v' rld f~J ~ o Z 0 0 5 0 / 412 91
and evaporated down.
Crystallization of the residue from methyl tert-butyl
ether/n-hexane gave the product as a 2:1 diastereomer
mixture.
Yield: 20.3 g (87 %)
Melting point: 128-130C
The compounds listed in Table 11 can be prepared
according to Method B4 and the compounds listed in Table
12 according to Example 4.
- 82 - O . 2 . D050/41291
Z E
,4 ~ ~
U~ U~
Ir7 T t I t ~ V t~ T ~ T V ~ T ~ t_) r
_ D
T .- T ~ ~ r S V r ~ ~, _ T ~ = T ~ I ~)
S 2 T
T T T -r T ~ 1 3 ~ = I T
I I I I I U~ U U I I I ~ ~ Y Y ~
_I
. . . ~ . ~ ~ ~ ~ -- ~ ~ ~ ~ ~ ~ ~ ~ ~ o
- 83 - O . Z . 0050/4 129 1
2 s ~
~ E E
o o
U~
~:
S ~ ~ ~
~ _ _
~ ~ o
u~ ~ ~ ~ , S ~ I ~ ~ T
_~ _ 1 T ~ ~ ~ T S X ~: S ~ S ~ c~
~ S
~ S = S = ~ S S = ~ S S ~ = ~ = ~ U
) r~ S 8 'i ' Z ' S O / 412 91
~D .
~:
z E
U~
D
1~ _ S ~ S ~ S ~ ~ T C ~
~ ~ ~ t-~ ~ ~ ~ ~ ~ ~
._ ~ S ~ T ~ ~ ~ 'C X C t S ~
_ _
C t~
C ~ ~ o o ~ T I r .
~ ~ ~ ~ O O -- ~ C C C ~
~ ~. ~ o ~ ~ ~ v Q~ " 0 _ ,
o ~ ~ ~ ~ s
~ ~o Z Z Z Z ~ L ~ ~ u
_l
_1
~ ~ u~ ~O r~ co ~ o -- ~ ~ ~ ~ ~o 1~ oo Ot O --
e
E~ _ ~
~/3
.
- 85 - O. Z . 0050/41291
22 i,
$
L
E o o
U _ _
~ o o
C~ ~
o o
o
~ ~7 o
o
o
x z ~ z z ~ ~ z z z c~ z t.~ 2 Z ~ Z r Z Z
~ ~ T ~ ) T S t ) ~ T ~ ~ ~ ~ T CJ
U7 _ 10
~--~
L Z ~ s s ~ ~ T ~ t_~ t~ T S T ~ ~ S ' T~
t'~ ~ ~ 1
-t 3 ~t ~t - t ~ ~ ~ 3 ~ ~D
I ~ T T ~ T Y
O
C~ o .~
m ,~ .~ 0 ~
E~ ~ ~
x
- 86 - O. Z . 0050/412gl
'~'.`2?.'3"~
~ ,.
o a
E ....
o ~ _
~ o o
~D .
c~: ~ _
u~ ~
a) -
~t S S S T T T T ~ ~
In t~ , T ~ ~ S T ~ J T I C~ T I ~
_. ~ S ~ T ~ S X ~ ~ ~r S S C.~ ~ 1 T ~ ~J ~ ~ I
C I L ~ J LO ~ C ~ LO LO ~ ~ L ~ U ~ U L
o _ ~ ~ ~ ~ o ~ 0 ~ O
~3
- 87 - O. Z . 0050/4129
L 11 11 11 11
E o c~ O O
~ c~ o Q
X o~ o o O
X ~ _. ,n
~4 ~ ._
~ oo o a7
E ~ ~ ~r
_ ~ ~r I r I ~
~ T ~~ I ~ = _ T ~ 3 ~ I ''
._ u ~) S ~' S t.~ ~,1 S S S ~ C ~r S ~ t~ I S :1: ~ t.) T --
~ ~ ~ ;~
T _ r
cr a~
o ~ ~ c.7 ~ c~ ~ ~ l y y y y ~ l t~ ~ ~ ~ ~ ~ ~ +~
~I aI ~ J ~ v ~ S ~ S t~ ~ L L -r
1 ~ u7 ~9 ~ ~ o ~, ~ ~ ~ tn ~D ,5,~ ~ ~ O ~ ~o ~o 3 U') ~D
- 88 - O.Z. 0050/41291
3 ~ r2 ~ ~i J
le
E o _. ~
U- t~ ~ ~
Y
,C,~
~'
,~ z ~ r Z ~ Z Z V Z ~, Z z s z z r z ~ r z ~,
In ~ v 3 s s ~ ~ ~ T a ~ ~ V I T ~J ~ T T ~ ~
_~ ~ t I S ~= S t.~ ~ V t~ 1 T t~ ~ ~ T ~
~ _ _
= = ~ s = ~ r s
s s s = = = = s ~ o. ~. c ~ ~ ~ ~ ~ c c = = . .
a~ 'I ~o ~O o _ ~ ~ ~ ~n ~ I~ ~D ~ O -- ~ ~7 o5 ~ ~~ CO ~ O
- 89 - O.Z. 0050/41291
6 ~
X T ~' ~' T T Z I Z S Z ~ Z ~ 2 C_) Z ~
~1 ~ ~) (~7 ~ ~ ~) T T I Y
~ ~ ~ ~S: T C,~ ~ S ~S: t.) ~ S I ~ ~ S ~ T ~ ~ I T
__
~ I
~ x _ _ C ~ C ~ e C e e ~ ~ ~ v
C~ L ~ ~ a ~ ,, V v ~-
~ o ~ ~ ~ ~ u~ o -- ~ ~ ~
D~ O~ a~ ~ a. cn ~D o, a) O, O o o o o o c~ o o o ~ ~ _ _ _,
'
- 9~0f;~ ~ f ~ ` O . Z . 0 0 5 0 / 4 12 9 1
z v Z v 2 ~
e
~ , O
~ .
~ ~ T ~ t ~ ,
~, o o o o o o 3
Q: V C~ ~, C ) ., ~
a,l ~ ~ o O
~ ~ ~ ~ ~ ~ ..
- 91 - ~.Z. 0050/41291
~ ~ ~ 3 2 2 g ~3
Method A2 ^~
2-(4-Chlorobenzylidene)-3,5,5-trimethylcyclohexanone
H 3C ~=`C 1
10 g of boron trioxide were added to a solution
of 763 g of (5.45 mol) of 3,3,5-trimethylcyclohexanone
and 140.5 g (1 mol) of 4-chlorobenzaldehyde, and water
was separaked off at a reaction temperature of 190C over
5 hours. In a subsequent distillation of the reaction
mixture, 181 g (69 ~) of 2-(4-chlorobenzylidene)-3,5,5-
trimethylcyclohexanone were obtained at 0.2 mbar and
168~.
Method B5
3-~4-Chlorobenzylidene)-4,6,6-trimethyl-1-oxabicyclo-
~0.2.5]octane O
H 3C~C 1
63 g (0.335 mol) of trimethylsulfonium methylsul-
fate and 70 g of concentrated sodium hydroxide solution
were added to a solution of 40 g (0.15 mol) of 2-(4-
chlorobenzylidene)-3,5,5-trimethylcyclohexanone in 200 ml
of methylene chloride. After the reaction mixture had
been stirred at room temperature (20C) for from 12 to lS
hours, 200 ml of water were added to the solution and the
organic phase was separated off. The organic pha e was
washed twice with water, dried over sodium sulfate and
evaporated down.
Yield: 37.4 g (89 %)
EXAMPLE 5
1-(1,2,4-Triazol-l-yl-methyl)-2 (4-chlorobenzylidene)-
3,5,5-trimethylcyclohexan-1-ol
~x~
N~N~
H3C~Cl
~ 2t ~ 2 ~ ~ 3 Z~ 0050/41291
9.8 g of 50 ~ strPngth by weight sodium hydroxide solu-
tion were added to a solution of 8.8 g (0.128 mol) of
1,2,4-triazole in 100 ml of N,N-dimethylformamide and the
mixture was heated at 50C for 30 minutes. 27.1 g
(0.098 mol) of 3-(4-chlorobenzylidene~-4,6,6-trLmethyl-
1-oxabicyclo[0.2.5)octane, which had been dissolved in
50 ml of N,N-dimethylformamide, were then added dropwise
at room temperature (20C) and the mixture was stirred
for 12 hours at room temperature. Thereafter, 200 ml of
water were added, the mixture was extracted several times
with methyl tert-butyl ether and the organic phase was
washed with water, dried over sodium sulfate and evapor-
ated down under reduced pressure.
Yield: 4.0 g (12 %)
Melting point: 88-90~C
The compounds listed in Table 13 can be prepared
according to Method B5 and the compounds listed in Table
14 according to Example 5.
C 1~ j O - Z 0 0 5 0 / 4 12 91
tl: _
Z ~
I
_~
r~
~:v~
C~
~D
+ T
~ T ~ D ~ ~ rv
Cl -- _ ~ T y -- I I I ~ ~ ~ Q T
~ ~ T V ~ Z ~D O
C::
t_~ ~ ~ T I T I -- I ~ I T I I I I T I I
I ~ ~ ~ ~ ~ ~ I ~ ~ ~ V ~ C~
11 I 11 11 11 11 11 11 I 11 11 11 11 11 11 11 11 11
ll A ~ A ~
C~ I I T I I I TI I I I I I I T I
o o r~
~ ~ ~I T I I I = T I I T
O Ct: I :1: I T T T I I T I I I I 3 r T T I
--
I = T T I T I I T I ~ I I = T T ' I
I ~ = :~ I = I I I T I
r-~ ~ ~ t~l ~) I~ I~ I I ~ ~ S I ~ r T
O ~ ~ ~ ~ n ~ I_
rr~ r.~ r.
E~ X ~
E~
j $ ~ z . o o 5 0 / 4 12 9 1
~:
I
E
~ ~ ~ ~ .$ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ S ~ D S :~ ~
S T S r S ~ T T '~ S T _ _ _ ~D 'D ~ ~ C_~ 3 ~O ~D
D ~ ~ T T ~ D
I ~ D I I t~
t~
I ~_1 T . S 1 S t ~ ~ S T ~ _ _ T _ ~_)C ) -- T T i- r
11 --1111 11 11 11 11 11 S11 11 S 11 11 11 11 ~_) I -- 11 11 11 11 11
A ~ ~ ~'~ A
r~ S ~ ~ ~ ~ ~
~3 ~3 T~3 T I T T T T S 1 ~3 ~ ~3 ~3 v S S~e I = T 2 S
~_
S~ ~c
~3 ~
I s
u~ n ~ u~
O ~ ~-~ T _ L ~J SD ~ ~
_ ~3 ~ ~ ~ 1~ ~ T S S 2 r ~ ~ S
~ ~) ~ ~ ~ ~
_ S -- _ S S T T T T ~3 ~ t~ 3 S
.n ~n
,_ T r S r ~: S 'S~ ~3 ~~,3 S ~ S X ~ T I ~r1 C S 1 ~
o ~ ~ ~ ~ ~ ~ ~ ~ ~.3~3 ~ ~ 3 ~3 ~ T S I X T -r
T S T ~ S X ~ S ~ 3 St,~ C ~ ~ 2 S S Y ~ T I ~r
_J ~ O ~ 3 1~ O _ ~J ~ ~ ~n ~ a l (~ O -- ~I ~1
X
'
`
- - 95 ~ 2~ .Z. 0o5o/4l2gl
~,
T
I T ~ T
~ D ` T Y ~ ~ ' T I .~ ~ r
2 T S T S ~ S S S S T ~ T 2 r 2 2 T = T = I ~ T T r
S S S T S T ~ r S S s S X S S ~ S = ~: T S
12:
o I ~: S T S :~ rS TT. T T ~: S I S S T T ~ r T S
C~:
_ = I = _
~1 'r S S S C ~ S T ~ S 2 I ~ r S X I T S ~,) t.) ~ t_l t.
C~
_ T S S :~ S X X ~ X ~'C
_ T T cr~ ~ ~ ~ Y Y Y Y Y I 1~ ; C c
_ ~D ~0 'D ~ T S ~ S ' ' ' ' O O O O O
L L L ~ ~ ~ ~ = S S
<~ T T ~ T S T -r T 3 T ~ ~ T T :1 T ~ ~ ~ S T T r
~' S ' T T T ~ T T T ~ I ' 1 T I T ~ I r T
:
- 96 - O.Z. 0050f41291
- ~J~,?J~
S
T I X T T ~
y I :~ T y S: I y y I T ~ I T U
~0 T y y ~ ~O T ~ y t~ y T ~ ~0 T
_ I T l ~
t~
, , T T I
11 ll 11
J A ~ ~ ~ ~ ~ ~ ~ A
T I T T ~ T T r ~ T ' ~ S r . T
O T S 1 T ~ S T C,~ U ~
~C
T ~ T . S S S T S S ~ r T ~ ~ ~ S T
Y
_. I S ~ S r S S ~ S I S r ~ 2 ~
~Jt '~ S ~ ~S ~ S ~ ~ S T T ~S T T T T ~ T
V C~ ~ ~ V ~ C~ V ~ ~ V V ~ V
I ~r T S S ~ _ T S S
a~
~I --~ ~ ~ 3 ~ X a- O _ ~I ~ ;t ~ D r- a) O C:~ _
r~ r-- I~ I~ I`~ I~ r~ 1~ 1~ C~ CO CO Ot~ 0 0 ~) 0 0 a~ cr a c
,
,:
.
- 97 _ O. Z . 0050/41291
f~ ~f~
CC t~ C~
_ ~ o o
Y ~ O ~_
~- _ ~
U
c~:
~:
T ~9 ~ 3 T I T T T
t_) T
. ~ S T I _ Y _ ~ ) T ,~ I
Il T 4 T I T T = ~ T T T t~ I T T T-
11 T l~ 11 T T 11 U
~ ~ A ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~
o
a~ I T:C T I T T T T T T T T T T I T
O
Q
~7~ ~ = r c, w~ I T T = T I = T
# ~
--Z ~ T I = T T T = T T = T I T = I T T
~ I T = I = T ~ = I T T T T T T
o~ ~ S = I S ~ X ' T I I
~I -- T I I S '~ I 3: S X I I T T T T
_l
;~ ~O ~ ~ ~ 3 L~l lD 1--
F~ ~J ~r 3 ~r ~r ~ 3 :~r ~ ~ J ~ ;t ~ ~t ~ ~ :t
X
:'
'
'` `' ' ~.'. ~ " '
,
` ~:
:
- 98 - O. Z . 0050/41291
c. ~
~Y
_
E
x r z z r :~
T
U~ _ _ T
T ~ o S ~ D T = T ~ ~ T ~
I 1~ 1 ~
S __ T T S r s r r S ~ ~ T . T ~ ) r T S tJ ~
~ T 11 l ~ T S 11 11 T r
~ ~ ~ ~ ~ ~ A A ~ A ~ ~ ~ A ~ ~ ~ ~ ~ A ~ ~ A
U~ -- ~ S ~
C: S _ T ~ y S ~ S ~ ~ St.J S I S S T _ r T T S T
S~ :~
O ~ ~ ~ C~ S ~ I r
_ s _ _ ~ _ s , T S ~ ~ ~ _ s ~ I S S
IL~
_~ S -- _ S _ ` T _ T :~C Y _ T T T _ ~ S S ~ _ I C~
_~ ~ ~r
_ T S . T ~ T S X S ~r ~ s T ~ ~ C.~ C~
T ` _ S S T y X 3 S S . 2 S S S S I ~ 3 ~S ~ ~
~ T S ~ S '5 '~; X ~ ~ S i ~ S T T r T ~ T I r s I
- 99 - O.z. 0050/41291
~ L~ J ~
~ ~ .
_ O
~ C ~
.
`_ Q; ~ .
I S z S S
T
r T T r T ~D (~ ~ T ~ ~ D I ~ T
_ _ _ _ _ _ _ 1,~ T I ~ ~ I T IL. U~
~ C_~ 1~ S T T T
S ~1 I S S T S I I T
s s ~ s s r ~ s ~ T I T ~ ~ ~ ~ ~ C~ I T S I T S S ~
llll I ll ll ll T ll ll ll ll 11 T ~r ~ I T Il ll ll ll ll ll ll S
A A ~ A A A A ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ .'\ ~ ~ A ~ ~
~C> T S t~ ~ ~ ~ ~ 2 S S S S :~ T :1: ' S S I T S
o ~ ~ ~ ~'t ~ ~ ~) t~ ~ ~ ~1
_ -r I r S ~: S c ~ ~ r ~ _ T T I S r :~: s s s s
_ ~ ~ T ~ t,;l ~ ~ U ~.1 T T T I S S -- S ~ '~ S T S
_ ~ s r s s r s c ~ ~ r s ~ ~ r s r s r ~ s I
U ~ ~ ~ ~ t~ S S ~ ~: S 1: T ~i S I S r T T r
~ ~ ~ T T S S T S T T 2 S S T I r T
a.~
X ~
. ~ :
. :
- - 100 - O.Z. 0050/41291
J i'J~ ~3
3: o o
~ o ~
. ~
~ ~,~
x Z Z Z Z Z C~ Z U Z 2 Z Z Z C_~ Z ~_) Z Z Z Z Z c ~ Z U 2 Z
t.~ I T ~ ~ ;I' ~ ~ r X ~ 3 ~ ~ ~ T ~ ~ ~ ~ T
I ~ tD ~9 T ~ I T ~ 9 T ~r T ~ I ~ ~D ~D I I T I ~ ~Q
~ T ~ D ~ T ~ J T U ~
I T T S T S U U T ' = I = S U ~.? T ~ ~ I T T 3 ~ T T
~o ~ A~A
~: T T T T T T S ~ 1 T 1- T S e T T T S T T T T 2 ~ I T
C~: S ~: S ~ S T T T T T . T T ~ S T = 1 T T T T T
a 3 i T T _ , T T T T S S T T T T T ' T = = I T
~ T T T J T ~, ~
~ ~ V ~ X
-- r T 1 T ~ ~ ~ ~ ~ ~ ~ ~ I I ~ I ~ I I I ~ r~ .C ~ ~ r
~1 V ~ ~ ~ ~ ~ ~ U
~: S -S S T ~ T S T ~ T S S ~ S S S ~ T I T '~
T X X S T I T ~ T S T ~ X T T ~ T T I T T S
C~
- 101 - O. Z . 0050~41291
~ ~ ~J ?J ~ ~ Li
Y
_
I I z z S z z z z z ~ z z z z z
T ~ I S
S ~ ~ ~ ~ T ~ S ~ ~, S e~ I I S ~ D I I S ;t ~ 0 I S
1,~ 1 _ _ _ _ I I '-- -- I S I ~
T
S ~S S ~ ~ S. SS S S :~: S S ~' S ~ S
~D
Cl ~- S ~ S S ~: S S X S S 2 S T T T I S T T T ~: S ~ T
O trl ~) ~r) (~ (~') (r
_ 1 T S S ~e S T S S 2 T S T ~ ~ T T T ~ ~ ~
_ S X S S S T S I ~ S ~ T r . S T I T T T
_ _ _
~C X ~S
_ O O O S :C i S S S 2 ':C S T S S S r S I T S
V ~ U
T T T ~ i S T T T S ~ V V ~ ~ V ~ ~ ~ ~ ~
T
1~ 1 ~ T X ~ ' ~ S S ~ S T T ~ . S ~ Y ~ :IC T T ~
_~ o ~ as o ~ o ~ O
r~ ~ cr o o o o o o o o o o
1~ ~ ~ ~ ~ ;1~ ~ ~ ~ ;t -* ~ ~ ~ ~ ~ ~ 3 3 ~t ~ ~ 2
.
- 102 - O.Z. 0050/41291
~J 2 ~ 3
C~:
_,
x ~J 2 Z Z Z Z t_) Z Z ;C Z
T ~ ~ T T
~ ~ T ~ ~ ~ ~
_ I I _ -- I IL
l~ l l l l ~ l l l l l
S ~ S
11 11 11 11 11 11 I~ 11 I~ 11 11
A ~ ~ A ~ ~ A ~ ~ ~ ~
T T T J-- . ~ ~ ~ ~ ~ ~,)
O
_. S C,~ S S ~: S
_ C S T T T T ~ S
_ ~S T T r S r T T
~t ll T S S T T X 1
V ~
T T ~r r S ~ ~ x
_ ~ ~ ~ ~ ~ I` a~ o~ o
X ,~
~ J2~
The azolylmethylcycloalkanols have excellent
activit~ against a broad spectrum of phytopathogenic
fungi, in particular from the class consisting of the
Ascomycetes and Basidomycetes. Some of them have sys-
temic activity and can be used as foliage and soil
fungicides.
They are particularly important for controlling
a multiplicity of fungi on various crops, such as wheat,
rye, barley, oats, rice, corn, grass, cotton, soybean,
coffee, sugar cane, grapevines, fruit and ornamental
plants and vegetable plants such as cucumber, beans and
cucurbitaceae, and on the seeds of these plants.
They are specially suitable for controlling the
following plant diseases:
Erysiphe graminis (powdery mildew) in cereals,
Erysiphe cichoracearum and Sphaerotheca fuliginea on
cucurbitaceae,
Podosphaera leucotricha on apples,
Uncinula necator on grapevines,
Puccinia species on cereals,
Rhizoctonia species on cotton and lawns,
Ustilaso species on cereals and sugar cane,
Venturia inaequalis (scab) on apples,
Helminthosporium species on cereals,
Septoria nodorum on wheat,
Botrytis cinerea (gray mold) on strawberries and grape-
vines,
Cerco~pora axachidicola on peanuts,
Pseudocercosporella herpotrichoides on wheat and barley,
~0 Pyricularia oryzae on rice,
Phytophthora infestans on potatoes and tomatoes,
Fusarium and Verticillium species on various plants,
Plasmopara viticola on grapevines and
Alternaria species on vegetables and fruit.
The compounds are used by spraying or dusting the
plants with the active ingredients or treating the sePds
of the plants with the active ingredients. Application
- 104 ~ ~ Z 0050/41291
tJ .~
is effected before or after infection of the plants or
seeds by the fungi.
They can be converted into the conventional
formulations, such as solutions, emulsions, suspensions,
dusts, powders, pastes and granules. The application
forms depend on the intended uses; they should in any
case en~ure fine and uniform distribution of the azolyl-
methylcycloalkanol. The formulations are prepared in a
known manner, for example by extending the active in-
gredient with solvents and/or carriers, if desired withthe use of emulsifiers and dispersants, and other organic
solvents may also be used as auxiliary solvents when
water is used as a diluent. Suitable assistants for this
purposs are essentially: solvents, such as aromatics (eg.
xylene), chlorinated aromatics (eg. chlorobenzenes),
paraffins (eg. mineral oil fractions), alcohols (eg.
methanol and butanol), ketones (eg. cyclohexanone),
amines (eg. ethanolamine and dimethylformamide) and
water; carriers such as ground natural minerals (eg.
kaolins, aluminas, talc and chalk) and ground synthetic
minerals ~eg. finely divided silica and silicates);
emulsifiers, such as nonionic and anionic emulsifiers
(eg~ polyoxyethylene fatty alcohol ethers, alkyl-
sulfonates and arylsulfonates) and dispersants, such as
ligninsulfite waste liquors and methylcellulose.
The fungicides contain in general from 0.1 to 95,
preferably from 0.5 to gO, % by weight of active
ingredients.
The application rates are from 0.02 to 3 kg of
active ingredient per ha, depending on the type of effect
desired. The novel compounds can also be used in mater-
ial protection (wood preservation), for example against
Paecilomyces variotii.
The agents and the ready-to-use formulations
prepared therefromt such a~ solutions, emul~ions, suRpen-
sions, powders, dusts, pastes or granules, are used in a
known manner, for example by spraying, atomi~ing,
- 152 ~J3 ~ f'J ~ ~ 3 O.Z. 0050/41291
dusting, broadcasting, dressing or pouring.
Examples of such formulation~ are:
I. a solution of 90 parts by weight of compound No.
3.1 or 4.1 and 10 parts by weight of N-methyl-~-
S pyrrolidone, which is suitable for use in the
form of very small drops;
II. a mixture of 20 parts by weight of compound No.
3.2, 80 parts by weight of xylene, 10 parts by
weight of the adduct of from 3 to 10 moles of
ethylene oxide with l mole of oleic acid N-
monoethanolamide, 5 parts by weight of the
calcium salt of dodecylbenzenesulfonic acid, 5
parts by weight of the adduct of 40 moles of
ethylene oxide with 1 mole or castor oil; by
finely distributing the solution in water, a-
dispersion is obtained;
III. an aqueous disper~ion of 20 parts by weight of
compound No. 3.84, 40 parts by weight of cyclo-
hexanone, 30 parts by weight of isobutanol and 20
parts by weight of the adduct of 40 moles of
ethylene oxide with l mole of castor oil;
IV. an aqueous dispersion of ~0 parts by weight of
compound No. 3.85~ 25 parts by weight of cyclo-
hexanol, 65 parts by weight of a mineral oil
2.S fraction boiling within a range from 210 to 280C
and 10 parts by weight of the adduct of 40 moles
of ethylene oxide with 1 mole of castor oil;
V. a mixture milled in a hammer mill consi~ting of
80 parts by weight of compound No. 3.8S, 3 parts
by weight of the sodium salt of diisobutyl-
naphthalene-~-sulfonic acid, 10 parts by weight
of the sodium salt of a lignin~ulfonic acid
obtained from a ~ulfite waste liquor and 7 parts
by wPight of silica gel powder; by finely di~
tributing the mixture in water, a spray liquor is
ob~ained;
VI. A thorough mixture of 3 parts by weight of
~P~ 2 ~ 3 o . z . 0050/41291
compound No. 3 . 90 and 97 parts b~ weight of
finely divided kaol in; this dusting agent con-
tains 3% by weight of ac~ive ingredient;
VII. a thorough mixture of 30 parts by weight of
compound No. 4.2, 92 parts by weight of silica
gel powder and 8 parts by weight of liquid
paraffin, which has been sprayed onto the ~urface
of the silica gel; this formulation Lmparts good
adhesion to the active ingredient;
VIII. a stable aqueous dispersion of 40 parts by weight
of compound No. 4.7, 10 parts by weight of the
sodium salt of a phenolsulfonic acid/urea/formal-
dehyde condensate, 2 parts by weight of silica
gel, 4 8 parts by weight of water, which can be
lS further diluted,
IX. a stable oily dispersion of 20 parts by weight of
compound No. 4. 8, 2 parts by weight of the
calcium salt of dodecylbenzenesulfonic acid, 8
parts by weight of a fatty alcohol polyglycol
ether, 20 parts by weight of the sodium salt of
a phenolsulfonic acid/urea/formaldehyde conden-
sate and 6 8 parts by weight of a paraffinic
mineral oil.
The novel agents may also be present in these
application forms together with other active ingredients,
for example with herbicides, insecticides, growth regula-
tor~ or fungicides, or with fertilizers.
The ~zolylmethylcycloalkanols of the formula I
can influence virtually all developmen~ s~age~ of the
plant in various ways and are therefore used as growth
regulator~. The variety of actions of the plant grow~h
regulators depends in particular
a) on the plant species and variety,
b) on the time of application, based on the development
stage of the plant, ~nd on the season,
c) on the place and method of application (seed dress-
ing, soil treatment or foliage application),
:,
- 10 7 ~r u ~d 2 ?A ~ ~ o . z . 0050/41291
d) on climatic factors, for example temperature and
amount of precipitation, and also on the length of
day and light intensity,
e) on the soil characteristic~ (including fertilizer
application),
f) on the formulation or application form of the active
ingredient and finally
g) on the concentrations in which the active substance
is u~ed.
From the number of different possible methods of
application of the novel plant growth regulators in
cultivation, some are mentioned below.
A. The compounds which can be used according to the
invention can be employed for pronounced inhibition of
the vegetative growth of the plants, which manifests
itself in particular in a reduction in the length of
growth. The treated plants accordingly exhibit squat
growth; furthermore, a dull foliage coloration is
observed.
A practical advantage is, for example, the
reduction in the growth of grass along road edges,
hedge~, canal banks and on lawn areas, such as parks,
sports complexes, orchards, ornamental lawns anA air-
fields, so that the labor-int~nsive and ~xpensive cutting
of grass can be reduced.
The increase in the strength of crop3 susceptible
to lodging, such as sereals, corn, rice, sunflowers and
soyb~an, i8 also of economic interest. The re~ulting
shortening and strengthening of th~ stem reduce or
eliminate the danger of lodging (bending) of plants under
unfavorable weather conditions prior to harvesting.
The use of growth regulator~ for inhibiting the
length of growth and for altering the period of ripening
of cotton is also important. ~his permits complately
mechanized harvesting of thi~ impor~ant crop.
By u~ing yrowth regulators, it is also possible
to increase or inhibit lateral branching of the plants.
1 O 8f '~t 5 ; ,~ r ~ t O z 0 0 5 0 / D~ 1 2 9 1
This is of interest when, for example in the case of
~obacco plants, the formation of side shoots is to be
inhibited in favor of leaf growth.
Growth regulators can also be used to achieve a
considerable increase in the frost resistance, for
example in the case of winter rape. On the one hand, the
length of growth and development of a leaf or plant mass
which is too luxuriant (and hence particularly suscep-
tible to frost) are inhibited. On the other hand, the
young rape plants are inhibited in the vegetative stage
of development after sowing and prior to the onset of the
winter frosts, despite favorable growth conditions. This
also eliminates the frost risk of such plants, which tend
to exhibit premature cessation of the inhibition o
blooming and a transition to the generative phase. In
other crops too, for example winter cereals, it is
advantageous if, by treatment with novel compounds in the
fall, the stocks are well tillered but do not ~tart the
winter with too luxuriant a growth. This makes it
possible to prevent increased sensitivity to frost and,
owing to the relatively small leaf or plant mass, attack
by various diseases tfor ex~mple fungal diseases). The
inhibition of vegetative growth also permits denser
planting of the soil in many crops, so that a higher
yield, based on the soil area, can be achieved.
B. The novel agent~ can be used to achieve higher
yields bo~h of plant parts and of plant ingredients. For
example, it i~ po3sible to induce the growth of larger
amounts of buds, blooms, leaves, fruits, seeds, roots and
tubers, to increa e the content of sugar in sugar beet,
sugar cane and citrus fruits and the protein content in
cereals or soybean or to stimulate rubber trees to
produce greater latex flow.
The azolylmethylcycloalkanols of the formula I
can result in increased yi~lds through intervention in
the plant metabolism or by promotion or inhibition of the
vegetative and/or of the generative growth.
~ ~ ~ 2 ,~
- 109 - O.Z. 0050/41291
C. Finally, plant growth regulators can be used to
achieve both a shortening or lengthening of the stages of
development and an acceleration or retardation of the
ripening of the harvested plant parts before or after
harvesting.
For example, facilitating harvesting is of
economic interest, this being permitted by concentrated
dropping or a reduction in the adhesion to the tree in
the case of citrus fruits, olives or other types and
varieties of pomes, drupes and hard-shelled fruit. The
same mechanism, ie. the promotion of ~he formation of
abscission tissue between the fruit or leaf part and the
shoot part of the plant is also essentia~ for readily
controllable defoliation of crops.
D. Growth regulators may furthermore be used to
reduce the water con~umption of plants. This is par-
ticularly Lmporkant for agricultural areas which have to
be artificially irrigated at great expense, for example
in arid or semiarid regions. By using the novel
substances, it is possible to reduce the intensity of
irrigation and hence to carry out more economical farm-
ing. The influence of growth regulators results in
better utilization of the available water, because, inter
alia,
the extent of opening of the ~tomata is reduced,
a thicker epidermis and cuticula are formed,
the root penetration of the soil is improved and
the microclimate in the stock of plants is advantageously
affected by more compact grow~h.
The active ingredients to be used according to
the invention can be fed to the crops both via the seed
(as a seed dressing) and via the soil, ie. through the
roots or via the foliage.
Because of the high toleration by plants, thP
application rate can be greatly varied~
In the ca~e of seed treatment, in general amounts
of active ingredien~ of from 0.001 to 50, preferably from
S ;'~J ' 1 & ~j
- 110 - O.Z. 0050/~1291
0.01 to 10, g per kilogram of seed are required.
For foliage and soil treatment, in general doses
of from 0.01 to 10, preferably from O.OS to 3, kg/ha are
regarded as sufXicient.
The formulations or the ready-to-use preparations
produced therefrom, such as solutions, emulsions, suspen-
sions, powders, dusts, pastes or granules, are applied in
a known manner, for example by the preemergence method or
the postemergence method or as dressings.
Use Examples
The comparative active ingredient chosen was
1-(1,2,4-triazol-1-ylmethyl)-2-~4-chlorobenzyl)-cyclo-
hexan-l-ol, which is disclosed in European Patent
324,646.
EXAMPLE I
Activity against Pyrenophora teres
Barley seedlings of the Igri variety, in the two-
leaf stage, were sprayed to run-off with the aqueous
suspensions which contained 80~ of active ingredient and
20% of emulsifier, the percentages being based on dry
substance. After 24 hours, the plants were inoculated
with a spore suspension of the fungus Pyrenophora tere~
and placed for 48 hour~ in an air-conditioned chamber
with high atmospheric humidity at 18~C. Thereafter, the
plar.ts were cultivated in a greenhouse at from 20 to 22C
and 70% relative humidity for a further 5 days. The
extent of development of the symptoms was then
determined.
s~ ?
~ ~ '.,' I f t`l f J l_ J
~ O.Z. 0050/41291
Rating: Infested leaf area in %
Active ingredient Infestation of the leaves after
from Example application of 0.05% strength
aqueous active ingredient formulation
Ratings ~ ~ infestation
3.90 0 0
4.1 l 5
4.7 1 5
4.8 1 5
Comparative active
ingredient 3 25
Untreated 4-5 65
EXAME'LE II
Activity against cucumber mildew
Young cucumber plants of the Chinesische Schlange
variety, in the two-leaf stage, were sprayed with an-
aqueous conidia suspension of cucumber mildew (Erysiphe
cichoracearum and Sphaerotheca fuliginea). On the
following day, these plants were sprayQd to run-off with
an aqueous spray liquor which contained 80~ of active
ingredient and 20% of emulsifier, the percentages being
based on the dry substance, and were placed in a green-
house at from 20 to 22C and from 70 to 30% humidity. 20
days after application of the active ingredient, the
extent of fungal infestation was determined.
Active ingredient Infestation of the leaves after
from Example application of 0.025% strength
aqueou~ active ingredient formulation
Rating~ ~ % infestation
4.1 1 5
Comparative active
ingredient 4 70
Untreated 5 100