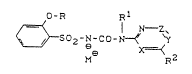Note: Descriptions are shown in the official language in which they were submitted.
2 ~
The invention relates 1o a new process for
preparing sulphonylurea salts, to new sulphonylurea salts
and to their use as herbicides.
It is known that sulphonylurea salts are obtained
when solutions of sulphonylureas in halogenohydrocarbon
solvents are reacted with alkali metal hydroxides or
alkaline earth metal hydroxides and the solvent is dis-
tilled off, if appropriate, after filtration (cf. EP-A
304,382).
Since in this procedure the product generally
does not crystallize from the solution, it is necPssary
for the solvent to be distilled, which requires large
amounts of energy, and the chances of obtaining a purifi-
cation effect are low.
3-Substituted-1-(2-halogenoalkoxy-benzene-
sulphonyl)-3-heteroaryl-(thio)ureas are known as herbi-
cides (cf. EP-A 251,079). However, in aqueous medium,
these compounds are suhject to relatively rapid hydroly-
tic degradation. It has not yet been possible to prepare
salts of these compounds, which should be more stable,
of sufficient quality.
A process has now been found for preparing
sulphonylurea salts of the general formula (I)
O-R R1
N--Z~
~S02-N-CO-N~ `Y ( I )
M R2
Le A 27 329 - 1 -
2 ~ ~, 2 ~ 3
in which
M~ represents an ~lkali metal ion or the counterion,
formed by protonation, of a basic organic nitrogen
compound,
R represents halogenoalkyl,
R1 represents optionally substituted radicals from the
series comprising alkyl, alkenyl, alkinyl or aral-
kyl,
RZ represents hydrogen, halogen, alkyl, halogenoalkyl,
10alkoxy, halogenoalkoxy, alkylthio, halogenoalkyl-
thio, amino, alkylamino or dialkylamino,
X represents nitrogen or a -CH group,
Y represents nitrogen or a -CR3 group where
R3 represents hydrogen, halogen, cyano, alkyl,
15formyl, alkyl-carbonyl or alkoxy carbonyl, and
Z represents nitrogen or a -CR4 group where
R4 represents hydrogen, halogen, alkyl, halogeno-
alkyl, alkoxy, halogenoalkoxy, alkylthio,
alkylamino or dialkylamino,
which is characterized in that
sulphonylureas of the general formula (II)
O-R R1
~ SO2-NH-CO-N ~ ~ (II)
in which
R, R1, R2, X, Y and Z have the abovementioned meanings
Le A 27 329 - 2 -
are reacted with alkali metal hy~droxides or wl~t~ bas
organic nitrogen compounds in the presence of hydro-
carbons as diluents at temperatures between 0C and
100C, and the products which are obtained in crystalline
form in this procefis are isolatedl by customary methods.
Furthermore, it has been found that the sul-
phonylurea salts of the formula (:[) to be prepared by the
process according to the invention are distinguished by
a powerful herbicidal activity.
The sulphonylurea salts of the formula (I) in
which Y represents nitrogen, to be prepared by the
process according to the invention, are new and, as new
substances, likewise a subject of the present invention.
It is to be regarded as surprising that the
sulphonylurea salts of the formula (I) are obtained by
the process according to the invention in a very simple
manner in very good yields and in high purity, since a
smooth reaction of the polar reaction components in
hydrocarbons as non-polar solvents was not to be
expected.
As comparison experiments have shown addition-
ally, an analogous reaction in polar solvents results in
the formation of a more or less large proportion of
degradation products which virtually do not occur in the
reaction of the process according to the invention.
Compared with the corresponding ~free~ sulphonyl-
ureas, the new sulphonylurea salts show, in particular,
the advantageous property of considerably more stable
storage and use forms (formulations and spray liquors).
The process according to the invention preferably
Le A 27_329 - 3 -
rel~tes to preparing sulphonylurea salts and - if Y
represents nitrogen ~ new sulphonylurea salts of the
formula (I) in which
M~ represents a lithium, sodium or potassium ion, a
tri-(Cl-C4-alkyl)-ammonium ion, a N-(C3-C6-cyclo-
alkyl)-NlN-di-(Cl-C4-alkyl)-ammonium ion or in each
case the counterion, formed by protonation, of N-
(Cl-C4-alkyl)-pyrrolidine, N-(Cl-C4-alXyl)-piperidine,
N-(Cl-C4-alkyl)-morpholine, N,N'-di-(Cl-C4-alkyl)-
piperazine, N,N-di-(C1-C4-alkyl)-benzylamine, 1,5-
diazabicyclo-[4.3.0]-non-5-ene (DBM), 1,8-diazabi-
cyclo-[5.4.0]-undec-7-ene (DBU) or 1,4-diazabicyclo-
[2.2.2]-octane [DABCO],
R represents halogeno-Cl-C4-alkyl,
R1 represents Cl-C6-alkyl (which is optionally substi-
tuted by fluorine, cyano, C1-C4-alkoxy or C1-C4-
alkylthio), or represents C3-C6-alkenyl and C3-C6-
alkinyl twhich are optionally substituted by fluor-
ine or chlorine), or represents phenyl-C1-C2-alkyl
(which is optionally substituted in the phenyl
moiety by fluorine, chlorine, nitro, cyano, methyl,
methoxy or C1-C2-alkoxycarbonyl),
R2 represents hydrogen, fluorine, chlorine, bromine,
methyl, ethyl, trifluoromethyl, methoxy, ethoxy,
difluoromethoxy, methylthio, ethylthio, amino,
methylamino, ethylamino, dimethylamino or diethyl-
amino,
represents nitrogen or a -CH group,
~ represents nitrogen or a -CR3 group where
3C R3 represents hydrogen, fluorine, chlorine,
Le A 27 329 - 4 -
f~ 3 ~ 3 ~
bromine, methyl, formyl, acetyl, methoxy-
carbonyl or ethoxycarbonyl,
and
Z represen~s nitrogen or a -C~4 group where
R4 xepresents hydrogen, fluorine, chlorine,
bromine, methyl, ethyl, trifluoromethyl,
methoxy, ethoxy, propoxy, isopropoxy, difluoro-
methoxy, methylthio, ethylthio, methylamino,
ethylamino, dime~hylamino or diethylamino.
In particular, the process according to the
invention relates to preparin~ sulphonylurea salts and -
if Y represents nitrog~n - new sulphonylurea salts of the
formula (I)
in which
N~ represents a sodium ion or a potassium ion, or
represents a trimethyl-, triethyl-, tripropyl- or
tributylammonium ion, or a N,N-dimethyl-, N,N-
diethyl- or N,N-dipropyl-cyclopentyl = onium ion, a
N,N-dimethyl-, N,N-diethyl- or N,N-dipropyl-cyclo-
hexylammonium ion, or in each case the counterion,
formed by protonation, of N-methyl-, N-ethyl- or N-
propyl-pyrrolidine, N-methyl-, N-ethyl- or N-propyl-
piperidine, N-methyl-, N-ethyl- or N-propyl-
morpholine, N,N'-dimethyl-, N,N'-diethyl- or N,N'-
dipropyl-piperazine, N,N-dimethyl-, N,N-diethyl- or
N,N-dipropyl-benzylamine, 1,5-diazabicyclo-[4.3.0]-
non-5-ene (DBN), 1,8-diazabicyclo-[5.4.0]-undec-7-
ene (DBU) or 1,4-diazabicyclo-~2.2.2]-octane
(DABC0),
30 R represents difluoromethyl or trifluoromethyl,
Le A 27 329 _ 5 _
~t~32~ ~
Rl represents methyl,
R2 represents hydrogen, chlorine, methyl, ethyl,
trifluoromethyl, methoxy, ethoxy, difluoromethoxy,
methylthio, ethylthio, methylamino, ethylamino or
dimethylamino,
X represents nitrogen,
Y represents nitrogen or a CH group ~very particularly
preferably nitrogen), and
Z represents a C-R4 group where
R4 represents hydrogen, chlorine, methyl, ethyl,
trifluoromethyl,methoxy, etho~y, difluorometh-
oxy, methylthio, ethylthio, methylamino,
ethylamino or dimethylamino.
If, for example, 3-(4,6-dimethoxy-pyrimidin-2-
yl)-3-methyl-1-(2-difluoromethoxy-phenylsulphonyl)-urea
and trimethylamine are used as starting substances, the
course of the reaction in the process according to the
invention can be outlined by the following equation:
OCHF2 CH3 OCH3
SO2-NH-CO-N ~ ~ I N(CH3)3
OCH3
(for example OCHF2 fH3 OCH3
- ~ 02-N-CO-N--~
toluene) N
HN( CH3)3 OCH3
Le A 27 329- 6 -
2~32~8
Formula (II) provides a general definition of the
sulphonylureas to be used as starting substances in the
process according to the invent:ion for preparing com-
pounds of the formula (I)~
In formula (II), R, R1, R2, X, Y and Z preferably,
or in particular, have those meanings which have already
been mentioned above in connection with the description
of the compounds of the formula (I) accord.ing to the
invention as being preferred, or particularly preferred,
for R, R1, R2, X, Y and Z.
Examples of the starting substances of the
formula (II) are listed in Table 1 below.
O-R R1
~ SO2-NH-CO-N ~ ~ (II)
Table 1: Examples of the starting substances of the
formula (II)
R Rl R2 X Y Z
. .
CF3 . CH3 OCH3 N CH C-OCH3
CF3 CH~ CH3 N N C-OCH3
CF3 CH3 OCH3 N CH C-C1
CF3 CH3 C2H5 N CH C-OCH~
CF3 CH3 CH3 N CH C-OC2H5
CF3 CH3 CH3 N CH C-OCH3
CF3 CH3 CH3 N CH C-CH3
CF3 CH3 CH3 N CH C-C1
CF3 CH3 CF3 N CH C-OCH3
CHF2 CH3 OCH3 N CH C-Cl
Le A 27 329 _ 7 _
~JI~L~L~.J~
Table 1 - continuation
R R1 R2 X Y Z
CHF2 CH3 OCH3 N CH C-OCH3
CHF2 CH3 CH3 N N C-CH3
CHF2 CH3 CH3 N N C-OCH3
CHF2 CH3 OCH3 N N C-OCH3
CHF2 CH3 CF3 N CH C-OCH3
CF3 ~H3 CH3 N N 5-CH3
CF3 CH3 C2H5 N N C-OCH3
CF3 CH3 CH3 N N C-OC2H5
CF3 CH3 OCH3 N N C-OCH3
CF3 CH3 C2H5 N N C-OC2H5
CF3 CH3 OCH3 N N C-NHCH3
CF3 CH3 OCH3 N N C-NHC2H5
CF3 CH3 C2H5 N N C-NHCH3
CF3 CH3 CH3 N N C-SCH3
CF3 CH3 OCH3 N N C-SCH3
~e A 27 329 - 8
2~
The starting substances of ~he formula (II) are
known and/or can be prepared by processes knvwn per se
(cf. EP-A 251,079).
The process according to the invention is carried
out using alkali metal hydroxides or basic organic
nitrogen compounds. Preferred substances are lithium
hydroxide, sodium hydroxide and potassium hydroxide,
tri-(C~-C4-alkyl)-amine, N-(C3-C6-cycloalkyl)-N,N-di-
(Cl-C4-alkyl)-amine, N-(Cl-C4-alkyl)-pyrrolidine, N-(Cl-C4-
alkyl)-piperidine, N-(Cl-C4-alkyl)-morpholine, N,N'-di-
(Cl-C4-alkyl)-piperazine, N,N-di-(C1-C4-alkyl)-benzylamine,
1,5-diazabicyclo-[4.3.0]-non-5-ene (DBN), 1,8-diazabi-
cyclo-[5.4.0]-undec-7-ene (DBU) and 1,4-diazabicyclo-
[2.2.2]-octane (DABCO).
Particularly preferred substances are sodium
hydroxide and potassium hydroxide, trimethyl-, triethyl-,
txipropyl- and tributylamine, N,N-dimethyl-,N,N-diethyl-
and N,N-dipropyl-cyclopentylamine, N,N-dimethyl-, N,N-
diethyl- and N,N-dipropyl-cyclohexylamine, N-methyl-l N-
ethyl- and N-propyl-pyrrolidine, N-methyl-, N-ethyl- and
N-propyl-piperidine, N-methyl-, N-ethyl- and N-propyl-
morpholine, N,N'-dimethyl-, N,N'-diethyl- and N,N'~
dipropyl-piperazine, N,N-dimethyl-, N,N-diethyl- and N,N-
dipropyl-benzylamine, 1,5-diazabicyclo-[4.3.0]-non-5-ene
(DBN), 1,8-diazabicyclo-[5.4.0]-undec-7-ene (DBU) and
1,4-diazabicyclo-[2.2.2]-octane (DABCO).
The process accordiny to the invention is carried
out in the presence of hydrocarbons as diluents. Diluents
which are preferred in the process according to the
invention are aromatic, in particular benzoidal
Le A 27 329 - 9 -
3 2 ~
hydrocarbons, which contain, if appropriate, 1 to 3 alkyl
substituents, each of which has 1 to 3 carbon atoms.
Examples of these which may be mentioned are: benzene,
toluene, o-, m- and p-xylene, ethylbenzene, propyl-
benzene, cumene, 1,2,3-trLmethylbenzene, 1,2,4-trLmethyl-
ben~ene and I,3,5-trimethylbenzlene. Toluene is very
particularly preferred as a diluent in the process
according to the invention.
In the process according to the invention, the
reaction temperatures can be varied within a substantial
range7 In general, the process is carried out at tempera-
~ures between 0C and 100C, preferably at temperatures
between 10C and 50C.
In general, the process according to the inven-
tion is carried out under atmospheric pre sure. However,
it is also possible to carry out the process under
increased or reduced pressure.
For carrying out the process according to the
invention, between 0.9 and 1.5 moles, preferably between
1.0 and 1.3 moles, of an alkali metal hydroxide or a
basic organic nitrogen compound are generally employed
per mole of sulphonylurea of the formula (I).
The reactants can be combined in any desired
sequence. In a preferred embodiment of the process
according to the invention, the sulphonylurea of the
formula (I) is first stirred with the hydrocarbon
diluent, and an alkali metal hydroxide or a basic organic
nitrogen compound is then added. The reaction mixture is
stirred until crystallization of the product is virtually
complete; the product is subsequently isolated by
Le A 27 329 - 10 -
~,~332~
filtration with suction.
The active compounds of the formula (I) can be
used as defoliants, desiccants, agents for destroying
broad-leaved plants and, especially, as weed-killers. By
weeds, in the broadest sense, there are to be understood
all plants which grow in locations where they are
undesired. Whether ~he substances according to the
invention act as total or selective herbicides depends
essentially on the amount used.
The active compounds according to the invention
can be used, for example, in connection with the follow-
ing plants:
Dicotyledon weeds of the genera: Sinapis,
Lepidium, Galium, Stellaria, Matricaria, Anthemis,
Galinsoga, Chenopodium, ~rtica, Senecio, Amaranthus,
Portulaca, Xanthium, Convolvulus, Ipomoea, Polygonum,
Sesbania, Ambrosia, Cirsiu~, Carduus, Sonchus, Solanum,
Rorippa, Rotala, Lindernia, Lamium, Veronica, Abutilon,
Emex, Datura, Viola, Galeopsis, Papaver, Centaurea,
Trifolium, Ranunculus and Taraxacum.
Dicotyledon cultures of the aenera: Gossypium,
Glycine, Beta, Daucus, Phaseolus, Pisum, Solanum, Linum,
Ipomoea, Vicia, Nicotiana, Lycopersicon, Arachis,
Brassica, Lactuca, Cucumis and Cucurbita.
Monocotyledon weeds of the genera: Echinochloa,
Setaria, Panicum, Digitaria, Phleum, Poa, Festuca,
Eleusine, Brachiaria, Lolium, Bromus, Avena, Cyperus,
Sorghum, Agropyron, Cynodon, Monochoria, FLmbristylis,
Sagittaria, Eleocharis, Scirpus, Paspalum, Ischaemum,
Sphenoclea, Dactyloctenium, Agrostis, Alopecurus and
Le A 27 329 - 11 -
J ~ 9~
Apera.
Monocotyledon cultures of the genera: Oryza,
Zea, Triticum, Hordeum, Avena, Secale, Sorghum, Panicum,
Saccharum, Ananas, Asparagus and Allium.
However, the use of the active compounds accord-
ing to the invention is in no way restricted to these
genera, but also extends in the same manner to other
plants.
The compounds are suitable, depending on the
10 concentration, for the total combating of weeds, for
example on industrial terrain and rail tracks, and on
paths and squares with or without tree plantings.
Equally, the compounds can be employed for combating
weeds in perennial cultures, for example afforestations,
15 decorative tree plantings, orchards, vineyards, citrus
groves, nut orchards, banana plantations, coffee planta-
tions, tea plantations, rubber plantations, oil palm
plantations, cocoa plantations, soft fruit plantings and
hop-fields, on lawn, turf and pasture-land, and for the
20 selective combating of weeds in annual cultures.
The compounds of the formula (I) according to the
invention are particularly suitable for selectively
combating dicotyledon weeds in monocotyledon cultures as
a pre-emergence or post-emergence method.
~he active compounds can be converted into the
customary formulations, such as solutions, emulsions,
wettable powders, suspensions, powders, dusting agents,
pastes, soluble powders, granules, suspension-emulsion
concentrates, natural and synthetic materials Lmpregnated
30 with active compound, and very fine capsules in polymeric
Le A 27 329 - 12 -
2 ~
substances.
These formulations are produced in a known
manner, for example by mixing th~e active compounds with
extenders, that is liquid solvents and~or solid carriers,
optionally with the use of surface-active agents, that is
emulsifying agents and/or dispersing agents and/or foam-
forming agents.
In the case of the use of water as an extender,
organic solvents can, for example, also be used as
auxiliary solvents. As liquid solvents, there are suit-
able in the main: aromatics, such as xylene, toluene, or
alkylnaphthalenes, chlorinated aromatics and chlorinated
aliphatic hydrocarbons, such as chlorobenzenes, chloro-
ethylenes or methylene chloride, aliphatic hydrocarbons,
such as cyclohexane or paraffins, for example petroleum
fractions, mineral and vegetable oils, alcohols, such as
butanol or glycol as well as their ethers and esters,
ketones, such as acetone, methyl ethyl ketone, methyl
isobutyl ketone or cyclohexanone, strongly polar sol-
vents, such as dimethylformamide and dimethyl sulphoxide,as well as water.
As solid carriers there are suitable: for example
ammonium salts and ground natural minerals, such as
kaolins, clays, talc, chalk, quartz, attapulgite, montmo-
rillonite or diatomaceous earth, and ground syntheticminerals, such as highly disperse silica, alumina and
silicates, as solid carriers for granules there are
suitable: for example crushed and fractionated natural
rocks such as calcite, marble, pumice, sepiolite and
dolomite, as well as synthetic granules of inorganic and
Le A 27 329 - 13 -
~ g~
organic meals, and granules of organic material such as
sawdust, coconut shells, maize cobs and tobacco stalks;
as emulsifying and/or foam-forming agents there are
suitable: for example non-ionic and anionic emulsifiers,
such as polyoxyethylene fatty acid esters, polyoxy-
ethy ene fatty alcohol ethers, for example alkylaryl
polyglycol ethers, alkylsulphonates, alkyl sulphates,
arylsulphonates as well as albumen hydrolysis products;
as dispersing agents there are suitable: for example
lignin-sulphite waste liquors and methylcellulose.
Adhesives such as carboxymethylcellulose and
natural and synthetic polymers in the form of powders,
granules or latexes, such as gum arabic, polyvinyl
alcohol and polyvinyl acetate, as well as natural phos-
pholipids, such as cephalins and lecithins, and syntheticphospholipids, can be used in the formulations. Further
additives can be mineral and vegetable oils.
It is possible to use colorants such as inorganic
pigments, for example iron oxide, titanium oxide and
Prussian Blue, and organic dyestuffs, such as alizarin
dyestuffs, azo dyestuffs and metal phthalocyanir.e dye-
stuffs, and trace nutrients such as salts of iron,
manganese, boron, copper, cobalt, molybdenum and zinc.
The formulations in general contain between 0.1
and 95 per cent by weight of active compound, preferably
between 0.5 and 90%.
For combating weeds, the active compounds accord-
ing to the invention, as such or in the form of their
formulations, can also be used as mixtures with known
herbicides, finished formulations or tank mixes being
Le A 2? 329 - 14 -
possible. ~y~
Suitable herbicides for the mixtures are known
herbicides, such as, for example, l-amino-6-ethylthio-3-
(2,2-dimethylpropyl)-1,3,5-triazine-2,4(1H,3H)-dione
(AMæTHYDIONE) or N-(2-benzothiazolyl)-N,N'-dimethyl-urea
(METABENZTHIAZURON) for combating weeds in cereals; 4-
amino-3-methyl-6-phenyl-1,2,4-triazin-5(4H)-one
(METAMITRON) for combatin~ weeds in sugar beet, and 4-
amino-6-(l,l~dimethylethyl)-3~methylthio-1,2,4-triazin-
5(4H)-one (METRIBUZIN) for combating weeds in soya beans;
furthermore also 2,4-dichlorophenoxyacetic acid (2,4-D);
4-(2,4-dichlorophPnoxy)-butyric acid (2,4-DB); 2,4-
dichlorophenoxypropionic acid (2,4-DP); N-(methoxy-
methyl)-2,6-diethyl-chloroacetanilide (ALACHLOR); 2-
chloro-4-ethylamino-6-isopropylamino-1,3,5-triaæine
(ATRAZINE); 3-isopropyl-2,1,3-benzothiadiazin-4-one 2,2-
dioxide (BENTAZONE); methyl 5-(2,4-dichlorophenoxy)-2-
nitrobenzoate (BIFENOX); 3,5-dibromo-4-hydroxy-benzo-
nitrile (BROMO~YNIL); 2-chloro-N-{[(4-methoxy-6-methyl-
1,3,5-triazin-2-yl)-amino]-carbonyl}-benzenesulphonamide
(CHLORSULFURON); N,N-dimethyl-N'-(3-chloro-4-methyl-
phenyl)-urea (CHLORTOLURON); exo-l-methyl-4-(1-methyl-
ethyl)-2-(2-methylphenyl-methoxy)-7-oxabicyclo-(2,2,1)-
heptane (CINMETHYLIN); 3,6-dichloro-2-pyridinecarboxylic
acid (CLOPYRALID); 2-chloro-4-ethylamino-6-t3-cyanoprop-
ylamino)-1,3,5-triazine (CYANAZINE); 2-[4-(2,4-dichloro-
phenoxy)-phenoxy]-propionic acid, its methyl ester or its
ethyl ester (DICLOFOP); S-ethyl N,N-di-n-propylthiocar-
bamate (EPTAME); 4-amino-6-t-butyl-3-ethylthio-1,2,4-
triazin-5(4H)-one (ETHIQZINE); 2-{4-[(6-chloro-2-benz-
Le A 27 3?9 - 15 -
~ J~'3
oxazolyl)-oxy]-phenoxy}-propanoic acid, its methyl ester
or its ethyl ester (FENOXAPROP); 2-[4-(5-trifluoromethyl-
2-pyridyloxy)-phenoxy]-propanoic acid or its butyl ester
(FLUAZIFOP); l-methyl-3-phenyl-5-(3-trifluoromethyl-
phenyl)-4-pyridone ( FLURIDONE); [( 4-amino-3,5-dichloro-
6-fluoro-2-pyridinyl)-oxy]-acetic acid or its l-methyl-
heptyl ester ( FLUROXYPYR); 2-~4-[(3-chloro-5-(trifluoro-
methyl)-2-pyridinyl)-oxy]-phenoxy}-propanoic acid or its
ethyl ester (HALOXYFOP); 3-cyclohexy1-6-dimethylamino-1-
methyl-1,3,5-triazine-2,4-dione (HEXA~INONE); methyl 2-
[4,5-dihydro-4-methyl-4-(1-methylethyl)-5-oxo-lH-
imidazol-2-yl]-4(5)-methylbenzoate(IMAZAMETHABENZ);3,5-
diiodo-4-hydroxybenzonitrile (IOXYNIL); N,N-dimethyl-N'-
(4-i~opropylphenyl)-urea (ISOPROTURON); (2-methyl-4-
chlorophenoxy)-acetic acid (MCPA); (4-chloro-2-methyl-
phenoxy)-propionic acid (MCPP); N-methyl~2-(1,3-benzo-
thiazol-2-yloxy)-acetanilide (MEFENACET); 2-chloro-N-
(2,6-dimethylphenyl)-N-[(lH)-pyrazol-l-yl-methyl]-acet-
amide (NETAZACHLOR); 2-ethyl-6-methyl-N-(l-methyl-2-
methoxyethyl)-chloroacetanilide (METOLACHLOR); 2-{[~((4-
methoxy-6-methyl-1,3,5-triazin-2-yl)-amino)-carbonyl]-
amino]-sulphonyl}-benzoic acid or its methyl ester
(METSULFURON); (2-chloro-4-trifluoromethylphenyl)-(3-
ethoxy-4-nitro-phenyl) ether (OXYFLUORFEN); N-(l-ethyl-
propyl3-3,4-dimethyl-2,6-dinitroaniline (PENDIMETHALIN);
2-chloro-N-isopropylacetanilide (PROPACHLOR); 0-(6-
chloro-3-phenyl-pyridazin-4-yl) S-octyl thiocarbamate
(PYRIDATE); ethyl 2-[4-(6-chloro-quinoxalin-2-yl-oxy)-
phenoxy]-propionate(QUIZALOFOP-ETHYL);2-chloro-4,6-bis~
(ethylamino)-1,3,5-triazine (SIMAZINE); 2,4-bis-[N-
Le A 27 329 - 16 -
~3J ~ J ~ ~
ethylamino]-6-methylthio-I~3l5-tria~ine (SIMETR~NE); 4-
ethylamino-2-t-butylamino-6-methylthio-s-triazine
lTERBUTRYNE); methyl 3-[[[[(4-methoxy-6-methyl-1,3,5-
triazin-2-yl)-amino]-carbonyl]-2~mino]-sulphonyl]-thio-
phene-2-carboxylate (THIAMETURON); S-(2,3,3-trichloro-
allyl) N,N-diisopropylthiocarbamate (TRI-ALLATE) and 2,6-
dinitro-4-trifluoromethyl-N,N-dipropylaniline (TRIFLUR-
ALIN). Surprisingly, some mixtures also show synergistic
action.
Mixtures with other known active compounds, such
as fungicides, insecticides, acaricides, nematicides,
bird repellants, plant nutrients and agents which improve
soil structure, are also possible.
The active compounds can be used as such, in the
form of their formulations or in the use forms prepared
therefrom by further dilution, such as ready-to-use
solutions, suspensions, emulsions, powders, pastes and
granules. They are used in the customary manner, for
example by watering, spraying, atomizing or scattering.
The active compounds according to the invention
can be applied either before or after emergence of the
plants. They can also be incorporated into the soil
before sowing.
The amount of active compound used can vary
within a substantial range. It depends essentially on the
nature of the desired effect. In general, the amounts
used are between l g and 1000 g of active compound per
hectare of soil surface, preferably between 5 g and 500 g
per ha.
The preparation and use of the active compounds
Le A 27 329 - 17 -
~ ~ e~ ~ 3 ~ g
according to the invention can be seen from the following
examplPs .
Preparation Examples:
Examplel
OCF3 OCH3
~S 2 ~ N - C O - N~ N
Na CH3 OCH3
4.4 g (O.010 mol) of 3-(4,6-dimethoxy-s-triazin-
2-yl)-3-methyl-1-(2-trifluoromethoxy-phenylsulphonyl)-
urea are stirred with 15 ml of toluene, and 0.5 g
(O.0125 mol) of sodium hydroxide powder are then added.
The reaction mixture is stirred for 60 minutes at 20C to
25C, which results in the formation of a viscous crys-
talline paste which i~ kept stirrable by adding a total
of 30 ml of toluene. When the reaction is complete, the
crystalline product is isolated by filtration with
suction.
4.0 g (87 % of theory) of the sodium salt of 3-
(4,6-dimethoxy-s-triaæin-2-yl)-3-methyl-1-(2-trifluoro-
methoxy-phenylsulphonyl)-llrea are obtained; melting point
224C.
Example 2
OCF3 OCH3
6~ 7~<
K CH3 oc~3
Le A 27 329 - 18 -
r~ l^J ~ ~ ~
4.4 g (0.010 mol) of 3-(4,6-dimethoxy s-triazin-
2-yl)-3-methyl-1-(2-trifluoromethoxy-phenylsulphonyl)-
urea are stirred with 50 ml of toluene, and 0.8 g of 80 %
potassium hydroxide powder (0.0114 mol of KOH) is then
added. The reaction mixture is stirred for 15 hours at
20C to 25C, and the product which is obtained as
crystals is isolated by filtration with suction.
4.1 g (86 % of theory) of the potassium salt of
3-(4,6-dimethoxy-s-triazin-2-yl)-3-methyl-1-(2-trifluoro-
methoxy-phenylsulphonyl)-urea are obtained; melting point
193~C (decomposition).
Other examples which can be prepared analogously
are the compounds of the formula (I) listed in Table 2
below.
O-R R1
~ 5O2-N-CO- ~ ~2 (I)
Le A 27 329 - 19 -
~3239~
t, _
CJ~ o
C -- ~o ~, E N E O` N c~
C 1:~` N
O C~
Z
~ N ~ r 1 ~ Z
:~: Z Z ~-) Z Z _ ~
H
E ~r U o O u U ~ ~
U O O O O O O O
O~`~ ~ ~ U ~ U
C
,~ Z Z Z Z Z Z Z
O
Z Z Z ~ Z Z :Z ~
o
N ~ 'q 5 St~
.~ ~ ~ O U O O O O
1~ ~ U ~ 5 ~ 51:~
N N
.. ~ ~ k ~ ~ L h
CJ
E~~ O r~ O
Le A 27 329 - 20 -
~i.S~3~3~
c -
~ N N ~
~ y ~)
1~5 t~ tr~
_I
6 o o O o
O
.C
~ ~ Z Z Z Z
o
X Z Z Z Z
c
O ~ X r~
~I; O O
O
I~; U
E
X
~ IL ~ IL
.. o ~ U
CJ ¦ --~
E~ I X o
Le A27 329 - 21 -
~ ~ é~
For example, the compound given in Table 2 as Example 5
can be prepared as follows:
OCF3 OCH3
-~-CO-N~
( C2H5 ) 3NH CH3 OCH3
8.8 g (0.02 mol) of 3-(4,6~dimethoxy-S-triazin-2-yl)-3-
methyl-l-(2-trifluoromethoxy-phenylsulphonyl)-urea are
dissolved in 88 ml of toluene by heating to approximately
40C; 2.2 g (0.021 mol) of triethylamine are subsequently
added at 35C to 40~C. The product starts to crystallize
after a few minutes. The mixture is stirred for another
approximately 15 hours at 20C, and the crystalline
product is then isolated by filtration with suction.
8.8 g (82 ~ of theory) of the triethylammonium
salt of 3-(4,6-dLmethoxy-s-triazin-2-yl)-3-methyl-1-(2-
trifluoromethoxy-phenylsulphonyl)-urea are obtained;
melting point: 125DC (decomposition).
Le A 27 329 - 22 -
2~ 8
Use Examples:
ExamPle A
Post-emergence test
Solvent: 5 parts by weight of dimethylformamide
Emulsifier: 1 part by weight of alkylaryl polyglycol
ether
Additive: 0.1 % Renex-36 (= polyoxyethylene(5)
tridecyl ether; wetting agent)
To produce a suitable preparation of active
compound, 1 part by weight of active compound is mixed
with the stated amount of solvent, the stated amount of
emulsifier and additive is added and the concentrate is
diluted with water to the desired concentration.
Test plants which have a height of 5 15 cm
are sprayed with the preparation of active compound in
such a way as to apply the particular amounts of active
compound desired per unit area. The concentration of the
spray liquor is 80 chosen that the particular amounts of
active compound desired are applied in 1,000 1 of
water/ha. After three weeks, the degree of damage to the
plants i8 rated in ~ damage in comparison to the develop-
ment of the untreated control. The figures denote:
0~ = no action (like untreated control)
100% = total destruction
In this test, for example, the compounds of
Preparation Examples 1, 2, 4, 5, 8, 9, 10, 11 and 12 have
a very powerful action against weeds combined with a very
good tolerance by wheat, barley and maize.
Le A 27 329 - 23 -
~ é,~ J
Example ~
Pre-emergence test
Solvent: 5 parts by weight of acetone
Emulsifier: 1 part by weight of alkylaryl polyglycol
ether
To produce a suitable preparation of active
compound, 1 part by weight of active compound i8 mixed
with the stated amount of solvent, the stated amount of
emulsifier is added and the concentrate is diluted with
water to the desired concentration.
Seeds of the test plants are sown in normal
soil and, after 24 hours, watered with the preparation
of the active compound. It is expedient to keep constant
the amount of watex per unit area. The concentration of
the active compound in the preparation is of no impor-
tance, only the amount of active compound applied per
unit area being decisive. After three weeks, the degree
of damage to the plants is rated in % damage in com-
parison to the development of the untreated control. The
figures denote:
0% = no action (like untreated control)
100% = total destruction
In this test, for example, the compounds of
Preparation Examples 1, 2, 4 and 5 have a very powerful
action against weeds combined with a very good tolerance
by wheat, barley and maize.
Le A 27 329 - 24 -