Note: Descriptions are shown in the official language in which they were submitted.
- 1 -
A-17885/+
UV Absorbers and li~ht-sensitive or~anic material containing same
.
The present invention relates to novel UV absorbers of the anthrone, acridone and
xanthone type, and to light-sensitive organic material containing same.
" A process for protec~ing light-sensitive materials from the harrnful influence of light,
especially of U'~l rays, in which l-hydroxyanthraquinones are used, is disclosed in
~:~ CH-A-379 760.
: . It has now been found that certain anthrones, acridones and a further class of xanthone
~ compounds can be successfully used for this purpose.
:'
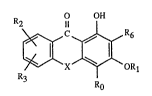
Specifically, the invention relates to compounds of forrnula
O OH
R2 11
Ro
; wherein X is 0, NH or CH2 and R~ is hydrogen or a radical of formula -(CH2)nCO2R7
: wherein n is 1 or 2 and R is alkyl of 1 to 18 carbon atoms or tCH2CH2~m ~, wherein m
. is 1 to 12, Rl is alkyl of 1 to 18 carbon atoms or alkyl of 4 to 18 carbon atoms which is
substituted by hydroxyl and/or may be inten upted by oxygen atoms, or is -COR4, wherein
.` ~
R4 is alkyl or alkenyl, each of 2 to 12 carbon atoms, -(CH2)y~CRs or
-OCH2CH(OH)CH20-CR,, or Rlis -(CH2)yO-IlRs or -(CH2)y~0Rs, wherein R5is alkyl
of 1 to 12 carbon atoms or alkenyl of 2 to 12 carbon atoms, and y is 1 to 12, and
R2 and R3 are each independently of the other hydrogen, alkyl of 1 to 12 carbon atorns,
.. .
2 ~ 7 ~
:
- 2 -
alkenyl of 2 to 12 carbon atoms, -ORl, wherein Rl has the given meaning, or arc chloro,
and R6 is hydrogen or alkyl of 1 to 4 carbon atoms.
; The invention also relates to a process for the preparation of these compounds, to
light-sensitive organic material which contains at least one of said compounds, wherein X
` is O, as well as to a method of protecting light-sensitive organic matenals by using the
r compounds of formula (1), wherein X is O.
:.
In the compounds of formula (1), the substituent Ro is, in addition to hydrogen, a radical
of formula -(CH2)n-C02R. In this formula, R is an alkyl radical of 1 ~o 18 carbon atoms
~5 such as methyl, ethyl, propyl, butyl, hexyl, octyl, decyl, undecyl, dodecyl, hexadecyl and
octadecyl, and corresponding branched isomers, or is a radical of formula
tCH2CH20tm H, wherein m is an integer from 1 to 12. The index n is 1 or 2. Rl is alkyl
of 1 to 18 carbon atoms, i.e. typically methyl, ethyl, propyl, butyl, pentyl, hexyl, octyl,
decyl, dodecyl, hexadecyl or octadecyl, and may also suitably be corresponding branched
alkyl radicals. The alkyl radicals Rl, if they contain 4 to 18 carbon atoms, may be
substituted by one or more hydroxyl groups. They may also be interrupted by one or more
oxygen atoms and may additionally contain one or more hydroxyl groups. Illustrative
examples of such alkyl radicals are groups such as -(CH2)x-O-(CH2)yH and -(CH2)X-O-
:; -(CH2)y~0~(CH2)zH~ wherein the sum of x and y or x, y and z is 4 to 18, and
-CH2 IcHcH2(o)r(cH2)sH1 wherein r is 0 or 1 and s is 1 to 15.
OH
. .
Rl is also a radical of formula -COR4, wherein R4is alkyl or alkenyl each of 2 to 12 carbon
atoms. Exemplary of such alkyl radicals are those cited above. Exemplary of alkenyl
radicals are ethenyl, propenyl, butenyl, hexenyl, octenyl, nonenyl and dodecenyl. The
alkenyl radicals Rl may also be polyunsatura~ed. Rl may also denote the corresponding
1~
branched isomers. R4 also denotes the radicals of formulae -(CH~)y~CRs and
S)
11
-OCH2CH(OH)CH20-CR5, wherein R5 is alkyl of 1 to 12 carbon atoms or alkenyl of ~ to
12 carbon atoms (examples of which are cited above) and y is 1 to 12.
"
' :,- , ~ , . ' ' ,:
.
7 ~
Rl is furtherrnore a radical of formula -(CH2)yO-~Ks or -(CH2)yll-o~s, wherein K5 and y
! have the given meanings.
The substituents R2 and R3 are each independently of the other hydrogen, alkyl of 1 to 12
carbon atoms or alkenyl of 2 to 12 carbon atoms (examples of which are cited above),
wherein Rl has the given meaning, or chloro.
.
X is a divalent radical and is CH2, NH or 0.
';
In the compounds of formula (1), the substituents R2 and R3 a}e preferably hydrogen or
methyl.
A further group of preferred compounds of formula (1) comprises those compounds
wherein Rl is alkyl of 4 to 12 carbon atoms which may be substituted by hydroxyl andlor
interrupted by oxygen, or is -COR4 or -(CH~)yCORs, wherein R4 is aLtcyl of 4 to 8 carbon
atoms, y is 1 to 4, and Rs is alkyl of 1 to 4 carbon atoms.
Among these compounds, those compounds are particularly preferred wherein Rl is alkyl
of 1 to 8 carbon atoms
Further preferred compounds of formula (1) are those wherein Ro is a radical of formula
-CH2CH2CO2R, wherein R is aLl~yl of 1 to 8 carbon atoms or tCH2CH20 m}H and m is 6
to 8. Light-sensitive organic materials can be protected in the practice of this invention
from the harmful influence of UV radiation by providing said materials with a protective
coadng, for example a paint or varnish composition, which contains at least one
compound of forrnula (1), wherein X is 0, or by incorporating such a compound inconventional manner in said organic material. In like manner, it is possible to use
compounds of formula (1), wherein X is NH or CH2.
Light-sensitive organic materials are, in the practice of this invenlion, free from sterically
hindered amines or hydroxyphenylbenzotriazole derivatives.
Illustrative examples of light-sensitive organic materials which can be protected in the
`'
"
~ 2~3~7~
,; a,
. .
practice of this invention from the harmful influence of light are:
1. Polymers of monoolefins and diolefins, for example polypropylene, polyisobutylene,
polybut-l-ene, polymethylpen~-l-ene, polyisoprene or polybutadiene, as well as polymers
of cycloolefins, for example of cyclopentene or norbornene, polyethyl~ne (which can be
uncrosslinked or crosslinked), for.example high density polyethylene (HDPl~), low density
polyethylene (LDP:E~ and linear low density polyethylene (LLDPE).
2. Mixtures of the polymers mentioned under 1), for example mixtures of polypropylene
with polyisobutylene, polypropylene with polyethylene (for example PP/HDPE,
PP/LDPE) and mixtures of different types of polyethylene (for example LDPE/HDPl~).
3. Copolymers of monoolefins and diolefins with each other or with other vinyl
monomers, for example etllylene/propylene copolymers linear low density polyethylene
(LLDPE) and mixtures thereof with low density polyethylene (LDPE),
propylene/but- l-ene copolymers, ethylene/hexene copolymers, ethylene/methylpentene
copolymers, ethylene/heptene copolyrners, ethylene/octene copolymers,
propylene/butadiene copolymers, isobutylene/isoprene copolymers, ethylene/alkyl acrylate
copolymers, ethylene/alkyl methacrylate copolymers, ethylene/vinyl acetate or
ethylene/acrylic acid copolymers and their salts (ionomers), as well as terpolymers of
ethylene with propylene and a diene such as hexadiene, dicyclopentadiene or
ethylidenenorbornene; and also mixtures of such copolymers with each other and with
polymers mentioned in 1) above, for example polypropylene/ethylene propylene
copolymers, LDPE~EVA, LDPE/EAA, LLDPE/EVA and LLDPE/EAA.
.
3a~ Hydrocarbon resins (for example Cs-Cg), including hydrogenated modificationsthereof (for example tackifiers).
4. Polystyrene, poly-(p-methylstyrene), poly-(oc-methylstyrene).
5. Copolymers of styrene or oc-methylstyrene with dienes or acrylic derivatives, for
example styrenel[ch]butadiene, styrene/acrylonitrile, styrene/alkylmethacrylate,styrene/butadiene/alkylacrylate, styrene/ maleic anhydride, styrene/acrylonitrile/methyl
acrylate; mixtures of high impact strength from styrene copolymers and another polymer,
for example from a polyacrylate, a diene polymer or an ethylene/propylene/diene
. terpolymer; and block copolymers of styrene, for example styrene/butadiene/styrene,
. .
,
,:
---. :. . ~ . ~ . :;:,
;, .. . ~ . :, . ..
. ~
~32~
~.
s
styrene/isoprene/styrene, styrene/ethylene/butylene/styrene or
styrene/ethylene/propylene/styrene.
6. Graft copolymers of styrene or c~-methylstyrene, for example styrene on polybutadiene,
styrene on polybutadiene/styrene or polybutadiene/acrylonitrile; styrene and acrylonitrile
(or methacrylonitrile) on polybutadiene; styrene and maleic anhydride or maleimide on
polybutadiene; styrene, acrylonitrile and maleic anhydride or maleimide on polybutadiene;
styrene, acrylonitrile and methyl methacrylate on polybutadiene, styrene and alkyl
acrylates or methacrylates on polybutadiene, styrene and acrylonitrile on
ethylene/propylene/diene terpolymers, styrene and acrylonitrile on polyalkylacrylates or
polyalkylmethacrylates, styrene and acryloni~rile on acrylate/butadiene copolymers7 as
well as mixtures thereof with the copolymers listed under 5), for example the copolymer
mixtures known as ABS, MBS, ASA or AES polymers.
7. Halogenated polymers such as polychloroprene, chlorinated rubbers, chlorinated or
sulfochlorinated polyethylene, copolymers of ethylene and chlorinated ethylene,
epichlorohydrin homo- and copolymers, preferably polymers of halogenated vinyl
compounds, for example poly- vinylchloride, polyvinylidene chloride, polyvinyl fluoride,
polyvinylidene fluoride, as well as copolymers thereof, for example vinyl
chloride/vinylidene chloride, vinyl chloride/vinyl acetate or vinylidene chloride/vinyl
acetate copolymers.
8. Polymers derived from a"l~-unsaturated acids and derivatives thereof, such aspolyacrylates and polymethacrylates, polyacrylamides and polyacrylonitriles.
9. Copolymers of the monomers mentioned under 8) with each other or with other
unsaturated monomers, for example acrylonitrile/butadiene copolymers, acrylo-
nitrile/alkylacrylate copolymers, acrylonitrile/alkoxyalkylacrylate or acrylonitrile/vinyl
halide copolymers or acrylonitrile/alkylmethacrylate~butadiene terpolymers.
10. Polymers derived from unsaturated alcohols and amines or the acyl derivatives or
acetals thereof, such as polyvinyl alcohol, polyvinyl acetate, polyvinyl stearate, polyvinyl
benzoate, polyvinyl maleate, polyvinylbutyrate, polyallyl phthalate or polyallylmelamine;
as well as their copolymers with the olefins mentioned in 1) above.
11. Homopolymers and copolymers of cyclic ethers such as polyalkylene glycols,
,
, ' .
~2~7~
.,
polyethylene oxide, polypropylene oxide or copolymers thereof with bisglycidyl ethers.
12. Polyacetals such as polyoxymethylene and those polyoxymethylenes which contain
ethylene oxide as a comonomer; polyacetals modified with thermoplastic polyurethanes,
acrylates or MBS.
13. Polyphenylene oxides and sulfides and mixtures thereof with polystyrene or
polyamides.
. .
14. Polyurethanes which are derived from polyethers, polyesters or polybutadienes
carrying terminal hydroxyl groups on the one hand and aliphatic or aromatic
polyisocyanates on the other, as well as precursors thereof.
15. Polyamides and copolyamides which are derived from diamines and dicarboxylicacids andl[ch]or from aminocarboxylic acids or the corre- sponding lactams, such as
polyamide 4, polyamide 6, polyarnide 6/6, 6/10, G/9, 6/12 and 4/6, polyamide 11,polyamide 12, aromatic polyamides obtained by condensation of m-xylene, diamine and
adipic acid; polyamides prepared from hexamethylenediamine and isophthalic and/or
terephthalic acid, with or without an elastomer as modi~1er, for example poly-2,4,4,-
trimethylhexamethylene terephthalarnide or poly-m-phenylene isophthalamide; block
copolymers of the aforementioned polyamides with polyolefins, olef1n copolymers,ionomers or chemically bonded or grafted elastomers; or with polyethers, for example
with polyethylene glycol, polypropylene glycol or polytetramethylene glycol; and also
polyamides or copolyamides modified with EPDM or ABS, and polyamides condensed
during processing ~RIM polyamide systems).
16. Polyureas, polyimides and polyamide-imides and polybenzimidazoles.
;`
1~. Polyesters derived from dicarboxylic acids and diols and~or from hydroxycarboxylic
acids or the corresponding lactones, such as poly-ethylene terephthalate, polybutylene
terephthalate, poly-1,4-dimethylolcyclohexane terephthalate, polyhydroxybenzoates as
well as block-copolyether esters derived from hydroxyl-terrninated polyethers; and also
polyesters modified with polycarbonates or MBS.
18. Polycarbonates and polyester carbonates.
`:
.`
, ~ ;
, .
,
. .
: . ., , ,:. .
. . . . . ..
. .
2~3~7 ~
.
7 -
19. Polysulfones, polyether sulfones and polyether ketones.
20. Crosslinked polymers which are derived from aldehydes on the one hand and phenols,
ureas and melamines on the other hand, such as phenol/formaldehyde resins,
. urea/formaldehyde resins and melamine/formaldehyde resins.
21. Drying and non-drying alkyd resins.
22. Unsaturated polyester resins which are derived from copolyesters of sa~urated and
unsaturated dicarboxylic acids with polyhydric alcohols and vinyl compounds as
crosslinking agents, and also halogen-containing modifications thereof of low
flammability.
23. Crosslinkable acrylic resins derived from substituted acrylic esters such as epoxy
acrylates, urethane acrylates or polyester acrylates.
24. Alkyd resins, polyester resins or acrylate resins which are cross-linked with melamine
resins, urea resins, polyisocyanates or epoxy resins.
25. Crosslinked epoxy resins which are derived from polyepoxides, for example frorn
bisglycidyl ethers or from cycloaliphatic diepoxides.
26. Natural polymers such as cellulose, rubber, gelatine and chemically modifiedhomologous derivatives thereof such as cellulose acetates, cellulose propionates and
cellulose butyrates, or the cellulose ethers, such as methylcellulose; as well as rosins and
their derivatives.
'~ :
-. 27. Mixtures (polyblends) of the aforementioned polymers, for example PP/EPDM,
Polyamide 61EPDM or ABS, PVC/EVA, PVS/ABS, PVC/MBS, PC/ABS, PBTP/ABS,
PC/ASA, PC/PBT, PVC/CPE, PVC/acrylates, POM/therrnoplastic PUR, PC/thermoplasticPUR, POM/acrylate, POM/MBS, PPE/HIPS, PPE/PA 6.6 and copolymers, PA/HDPE,
PA/PP, PA/PPE.
` In addition to containing the compounds of forrnula (l), the light-sensitive organic
materials may also contain further conventional stabilisers and additives, for example:
. ~ ' ' .
` ~ ' `'
. .
Z3~71
- 8-
1. Antioxid~nts
1.1. Alkylated monophenols, for example 2,6-di-tert-butyl-4-methylphenol, 2-tert-
butyl-4,6-dimethylphenol, 2,6-di-~ert-butyl-4-ethylphenol, 2,6-di-tert-butyl-4-n-
butylphenol, 2,6-di-tert-butyl-4-isobutylphenol, 2,6-dicyclopentyl-4-methylphenol,
2-(o~-methylcyclohexyl)-4,6-dimethylphenol, 2,6-dioctadecyl-4-methylphenol, 2,4,6-tri-
cyclohexylphenol, 2,6-di-tert-butyl-4-methoxymethylphenol, 2,6-di-nonyl-4-methyl-
phenol,
'
1.2. Alkylated hydroquinones, for example 2,6-di-tert-butyl-4-methoxyphenol, 2,5-di-
tert-butylhydroquinone, 2,5-di-tert-amylhydroquinone, 2,6-diphenyl-4-octadecyloxy-
phenol.
1.3. Hvdroxylated thiodiphenvl ethers, for example 2,2'-thiobis(6-tert-butyl-4-methyl-
phenol), 2,2'-thiobis(4-octylphenol), 4,4'-thiobis(6-tert-butyl-3-methylphenol),4,4 ' -thiobis(6-tert-butyl-2-methylphenol).
1.4. Alkvlidenebisphenols, for example 2,2'-methylenebis(6-tert-butyl-4-methylphenol),
2,2'-methylenebis(6-tert-butyl-4-ethylphenol), 2,2'-methylenebis[4-methyl-6-(o~-methyl-
cyclohexyl)phenol], 2,2'-methylenebis(4-methyl-6-cyclohexylphenol), 2,2'-methylene-
bis(6-nonyl-4-methylphenol)j 2,2'-methylenebis(4,6-di-tert-butylphenol), 2,2'-ethylidene-
bis(4,6-di-tert-butylphenol), 2,2'-ethylidenebis(6-tert-butyl-4-isobutylphenol), 2,2'-
` methylenebis[6-(oc-methylbenzyl)-4-nonylphenol], 2,2'-methylenebis[6-(a,o~-dimethyl-
benzyl)-4-nonylphenol], 4,4'-methylenebis(2,6-di-tert-butylphenol), 4,4'-methylene-
bis(6-tert-butyl-2-methylphenol), 1,1-bis(S-tert-butyl-4-hydroxy-2-methylphenyl)butane,
2,6-bis(3-tert-butyl-S-methyl-2-hydroxybenzyl)-4-methylphenol, 1,1 ,3-tris(S-tert-
butyl-4-hydroxy-2-methylphenyl)butane, 1,1-bis(5-tert-butyl-4-hydroxy-2-methyl-
phenyl)-3-n-dodecylmercaptobutane, ethylene glycol bis~3,3-bis(3'-tert-butyl-4'-hydroxy-
phenyl)butyrate], bis(3-tert-butyl-4-hydroxy-S-methylphenyl)dicyclopentadiene, bis~2-
(3'-tert-butyl-2'-hydroxy-S'-methylbenzyl)-ff~tert-butyl-4methylphenyl] terephthalate.
` l.S. Benzyl compounds, for example 1,3,5-tris(3,5-di-tert-butyl-4-hydroxybenzyl)-2,4,6-
``.` trimethylbenzene, bis(3,5-di-tert-butyl-4-hydroxybenzyl) sul~lde, isooctyl 3,5-bis(tert-
butyl-4-hydroxybenzylmercaptoacetate), bis(4 tert-butyl-3-hydroxy-2,6-dimethylbenzyl)
dithiolterephthalate, 1,3,5-tris(3,5-di-tert-butyl-4-hydroxybenzyl) isocyanurate,
1,3,5-tris(4-tert-butyl-3-hydroxy-2,6-dimethylbenzyl) isocyanurate, dioctadecyl 3,5-di-
~ ,;
~32~7~
tert-butyl-4-hydroxybenzylphosphonate, calcium salt of monoethyl 3,5-di-tert-butyl-
4-hydroxybenzylphosphonate, 1,3,5-tris(3,5-dicyclohexyl-4-hydroxybenzyl)isocyanurate.
i
1.6. Acylaminophenols, for example 4-hydroxylauranilide, 4-hydroxystearanilide, 2,4-
bis(octylmercapto)-~-(3,5-di-tert-butyl-4-hydroxyanilino)-s-triazine, octyl ~-(3,5-di-tert-
butyl-4-hydroxyphenyl)carbamate.
1.7. Esters of e-(3.5-di-tert-butyl-4-hydroxvphenyl)propionic acid with mono- orpolyhydric alcohols, e.g. with methanol, octadecanol, 1,6-hexanediol, neopentyl glycol,
triethylene glycol, pentaerythritol, tris(hydroxyethyl) isocyanurate, thiodiethylene glycol,
N,N'-bis(hydroxyethyl)oxalodiamide.
1.8. Esters of ~-(S-tert-butyl-4-hvdroxv-3-methvlphenvl)propionic acid with mono- or
polyhydric alcohols, e.g. with methanol, diethylene glycol, octadecanol, triethylene glycol,
1,6-hexanediol, pentaerythritol, neopentyl glycol, tris(hydroxyethyl) isocyanurate,
thiodiethylene glycol, N,N'-bis(hydroxyethyl)oxalodiamide.
`:
1.9. Esters of ~-(3,5-dicvclohexYI-4-hvdroxvphenYl)propionic acid with mono- or poly-
hydric alcohols, e.g. with methanol, diethylene glycol, octadecanol, trie~hylene glycol,
1,6-hexanediol, pentaerythritol, neopentyl glycol, tris(hydroxyethyl) isocyanurate,
thiodiethylene glycol, N,N'-bis(hydroxyethyl)oxalodiamide.
1.10. Amides of ~-(3~5-di-tert-butYI-4-hYdroxyphenyl)propionic acid e.g. N,N'-
bis(3,5-di-tert-butyl-4-hydroxyphenylpropionyl)hexamethylenediamine, N,N'-bis(3,5-
di-tert-butyl-4-hydroxyphenylpropionyl)trimethylenediamine, N,N'-bis(3,5-di-tert-
butyl-4-hydroxyphenylpropionyl)hydrazine.
2. UV absorbers and li~t stabilisers
2.2. 2-Hydroxybenzophenones. for example the 4-hydroxy, 4-methoxy, 4-octoxy,
4-decyloxy, 4-dodecyloxy, 4-benzyloxy, 4,2',4'-trihydroxy and 2'-hydroxy-4,4'-
dimethoxy derivative.
2.3. Esters of substituted and unsubstituted benzoic acids, for example, 4-tert-butylphenyl
salicylate, phenyl salicylate, octylphenyl salicylate, dibenzoylresorcinol,
bis(4-tert-butylbenzoyl)-resorcinol, benzoylresorcinol, 2,4-di-~ert-butylphenyl
,
`, ~
.
: . .
: . ' .
2~2~ ~
- 10 -
3,5-di-tert-bwtyl-4-hydroxy- benzoate and hexadecyl 3,5-di-tert-butyl-4-hydroxybenzoate.
2.4. Acrylates, for example ethyl a-cyano-,B"B-diphenylacrylate, isooctyl a-cyano-
~,~-diphenylacrylate, methyl a-carbomethoxycinnamate, methyl oc-cyano-,B-methyl-p-
methoxy-cinnamate, butyl ol.-cyano-,B-methyl-p-methoxy- cinnamate, methyl a-carbo-
methoxy-p-methoxycinnamate and N-(~-carbomethoxy-~-cyanovinyl)-2-methylindoline.
2.5. Nickel compounds~ for example nickel complexes of 2,2'-thio-bis[4-(1,1,3,3-tetra-
methylbutyl)phenol], such as the 1:1 or 1:2 complex, with or withowt additional ligands
such as n-butylamine, triethanolamine or N-cyclohexyldiethanolamine, nickel dibutyl-
dithiocarbamate, nickel salts of 4-hydroxy-3,5-di-tert-butylbenzyl-phosphonic acid
monoalkyl esters, e.g. of the rnethyl or ethyl ester, nickel complexes of ketoximes, e.g. of
2-hydroxy-4-methyl-phenyl undecyl ketoneoxime, nickel complexes of 1-phenyl-4-
lauroyl-S-hydroxypyrazole, with or without additional ligands.
2.6. Oxalyl diamides~ for example 4,4'-dioctyloxyoxanilide, 2,2'-dioctyloxy-5,5'-
di-tert-butoxanilide, 2,2'-didodecyloxy-S,S'-di-tert-butoxanilide, 2-ethoxy-2'-
ethyloxanilide, N,N'-bis(3-dimethylaminopropyl)oxalamide, 2-ethoxy-S-tert-butyl-2'-ethyloxanilide and its rnixture with 2-ethoxy-2'-ethyl-5,4'-di-tert-butoxanilide and
mixtures of ortho- and para-methoxy-disubstituted oxanilides and mixtures of o- and
p-ethoxydisubstituted oxanilides.
. .
2.7. 2-(2-Hydroxyphenvl)-1,3,5-triazines, for example 2,4,6-tris(2-hydroxy-4-~c~loxy-
phenyl)-1,3,5-triazine, 2-(2-hydroxy-4-octyloxy-phenyl)-4,6-bis(2,4-dimethylphenyl)-
1,3,5-triazine, 2-(2,4-dihydroxy-phenyl)-4,6-bis(2,4-dimethylphenyl)-1,3,5-triazine,
2,4-bis(2-hydroxy-4-propyloxyphenyl)-6-(2,4-dimethylphenyl)- 1 ,3,5-~riazine,
2-(2-hydroxy-4-octyloxyphenyl)-4,6-bis(4-methylphenyl)-1,3,5-triazine, 2-(2-hydroxy-
4-dodecyloxyphenyl)-4,6-bis(2,4-dimethylphenyl)- 1 ,3,5-triazine.
3. Metal deactivators, for example N,N'-diphenyloxalyl diamide, N-salicylal-
N'-salicyloylhydrazine, N,N'-bis(salicyloyl)hydrazine, N,N'-bis(3,5-di-tert-
butyl-4-hydroxyphenylpropionyl)hydrazine, 3-salicyloyl- amino-1,2,4-triazole,
bis(benzylidene)oxalic dihydrazide.
4. Phosphites and phosphonites, for example triphenyl phosphite, diphenylalkyl
phosphites, phenyldialkyl phosphites, tris(nonylphenyl) phosphite, trilauryl phosphite,
~.:
. , .
. ~. ' ' '
'"'~'~ ' ' "
.-" ' '
~3~J~7 ~
.
trioctadecyl phosphite, distearyl pentaerythritol diphosphite, tris(2,~-di-tert-butylphenyl)
phosphite, diisodecyl pentaerythritol diphosphite, bis(2,4-di-tertbutyl~henyl) penta-
erythritol diphosphite, tristearyl sorbitol triphosphite, tetrakis-(2,4-di-tert-butylphenyl)
4,4'-biphenylene diphosphonite, 3,9-bis(2,4-di-tert-butylphenoxy)-2,4,8,10-
tetraoxa-3,9-diphosphaspiro[S.S]undecane.
5. Peroxide scavenPers~ for example esters of ~-thiodipropionic acid, for example the
lauryl, stearyl, myristyl or tridecyl esters, mercaptobenzimidazole or the zinc salt of
2-mercaptobenzimidazole, zinc dibutyldithiocarbamate, dioctadecyl disulfide,
pentaerythritol tetrakis(,B-dodecylmercapto)propionate.
6. Polvamide stabilisers~ for example, copper salts in conjunction with iodides and/or
phosphorus compounds and salts of divalent manganese~
7. Basic co-stabilisers, for example, melamine, polyvinylpyrrolidone, dicyandiamide,
triallyl cyanurate, urea derivatives, hydrazine derivatives, amines, polyamides,polyurethanes, alkali metal salts and alkaline earth metal salts of higher fatty acids for
example calcium stearate, zinc stearate, magnesium stearate, sodium ricinoleate and
potassium palmitate, antimony pyrocatecholate or zinc pyrocatecholate.
8. Nucleating a~ents, for example, 4-tert-butylbenzoic acid, adipic acid, diphenylacetic
acid.
9. Fillers and reinforcin~ a~ents~ for example calcium carbonate, silicates, glass fibres,
asbestos, talc, kaolin, mica, barium sulfate, metal oxides and hydroxydes, carbon black,
graphite.
10. Other additives~ for example~ plasticisers, lubricants, emulsifiers, pigments,
fluorescent whitening agents, flameproo~lng agents, antistatic agents and blowing agents.
Among the organic materials which can be protected in the practice of this invention from
light-induced degradation, organic polymers are to be singled out for special mention,
preferably synthetic polymers. It is therefore preferred to protect thermoplastic polymers
and, more particularly, surface-coating compositions in which the polymer binder will
preferably be stabilised against the influence of light.
,
.~ ,, , ~ .,,
. ~ ~
,
2~?J~7~
Surface-coating compositions, i.e. paint or varnish compositions, which contain the
compounds of formula I may be, for example, pigmented or unpigmented paint or varnish
compositions or metallic paints They may contain an organic solvent or be solvent-free, or
they may be water-based paints.
The paint or varnish compositions may contain as binder a polymer selected from those
cited previously. Illustrative examples of paint or varnish compositions containing special
binders are the following:
1. paint or varnish compositions based on cold- or hot-crosslinkable alkyd, acrylate,
polyester, epoxy or melamine resins or mixtwres of said resins, to which an optional acid
curing catalyst is added;
2. two-component polyurethane paint or va~nish compositions based on hydroxylated
acrylate, polyester or polyether resins and aliphatic or aromatic polyisocyanates;
3. single component polyurethane paint or varnish compositions based on blocked
polyisocyanates which are deblocked during stoving;
4. two-component paint or varnish compositions based on (poly)ketimines and aliphatic or
aromatic polyisocyanates;
5. two-component paint or varnish compositions based on (poly)ketimines and an
unsaturated acrylate resin or a polyacetoacetate resin or a methyl acrylamidoglycolate
methyl ester;
6. two-component paint or varnish compositions based on carboxyl or amino group
containing polyacrylates and polyepoxides;
7. two-component paint or varnish compositions based on anhydride group containing
acrylate resins ad a polyhydroxy or polyamino component;
8. two-component paint or varnish compositions based on (poly)oxazolidines and
anhydride group containing acrylate resins or unsaturated acrylate resins or aliphatic or
aromatic polyisocyanates;
9. two-component paint or varnish compositions based on unsaturated polyacrylates and
polymalonates;
10. thermoplastic polyacrylate paint or varnish compositions based on thermoplastic
acrylate resins or not self-crosslinking acrylate resins in conjunction with etherified
melamine resins;
11. paint or varnish systems based on siloxane-modified acrylate resins; and
12. paint or varnish systems based on fluorine-modified acrylate resins.
. ,~
~"`;
~.
,
: . ~ .. . .
.~` ` .- , :
.
~3~7 ~
- 13-
The paint or varnish compositions may also be photocwrable compositions, in which case
the binder consists of monomer or oligomer compounds which contain ethylenic double
- bonds and which are converted by actinic light or with electron beams into a crosslinked
high molecular weight forrn. The binder is usually a mixture of such compounds.
The paint or varnish compositions may be applied as single layer or two-layer systems, in
which case the stabilisers of this invention are preferably added to the topmost layer.
The paint or varnish compositions can be applied to the substrates (metal, plastic, wood
and the like) by the conventional techniques, for example by brushing, spraying, coating,
immersion or electrophoresis.
Paint or varnish compositions which are applied to motor cars (automotive lacqers) are
especially preferred.
Provided the light-sensitive organic materials of this invention are paint or varnish
compositions, these latter will preferably contain no alkyd resins as binders.
The compounds of formula (1) may be incorporated in the paint or varnish compositions
as well as other light-sensitive organic materials by conventional known methods. The
`- amounts are usually in the range from 0.01 to 5.0 % by weight, preferably from ().02 to
3.0 % by weight, based on the organic material. The amount of UV absorber to be chosen
will be dependent on the nature of the material and the requirements made of its stability.``:
The components of stabiliser combinations can be added singly or in admixture to the
organic material. The addition of compounds of formula (1) and additional optional
stabilisers is preferably made before or during the shaping of the material if it is, for
example, a thermoplastic. The addition can, however, also be made during polymerisation
or before polymerisation to the starting monomers.
The compounds of formula (1) can be prepared by methods which are known pe se. For
example, by heating a mixture of the cornpounds of formulae
'``
-
:`
.
.
. :
`:: ` :
2~)3~,~7~
- 14-
OH
R2 11 ~R6
(2) ~ and (3) HXJ~OH-
R3 Ro
wherein Ro, R2, R3, R6 and X have the given meanings, it is possible to obtain the
` intermediate of ~rmula
.
O OH
.' R2 11
j~C ~ R6
R3
Ro
`'`
which, by reaction with, for example, Rl-Cl or R1-Br, wherein Rl has the given meaning,
` can be converted into the compound of formula (1).
The invention is illustrated in more detail by the following Examples in which parts and
percentages are by weight, unless otherwise stated.
., ~
Preparatorv Examples:
~` Example 1: 22.8 g of 1,3-dihydroxyxanthone (prepared according to Grover, J. Chem. Soc.
``~` 1955,3982) togetber with 15.2 g of K2CO3 and 21.2 g of 1-bromooctane are refluxed for
~` 15 hours in 250 ml of methyl ethyl ketone. The salt is isolated by filtration from the cooled
; ~ reaction mixture and the methyl ethyl ketone solution is concentrated by evaporation
~ under vacuum. Recrystallisation of the residue from heptane gives 1-hydroxy-3-octoxy-
", xanthone with a melting point of 98C.
Example 2: 22.8 g of 1,3-dihydroxyxanthone (prepared according to Grover, J. Chem. Soc.
1955, 3982) are charged to 300 ml of absolute ethanol. Then 11.2 g of potassium
` ~ tert-butylate, and afterwards 0.5 g of potassium iodide, are slowly added dropwise. Then
` ~ 12.3 g of ethyl chloroacetate are added dropwise at room temperature over ca. 15 minutes
and the reaction mixture is thereafter heated for 6 hours under reflux. The reaction mixture
~,
~ .~
~32~7~
: - 15-
is poured into 500 ml of water and acidified with 20 ml of glacial acetic acid. The
precipitate is isol~ted by filtration and dried. Recrystallisation from hexane gives the
compound of formula
:
O OH
OCH2coC2Hs
with a melting point of 165C.
Example 3: 22.8 g of 1,3-dihydroxyxanthone (prepared according to Grover, J. Chem. Soc.
` 1955, 3982) are heated in 200 ml of xylene to 120C. After the addition of 1.5 g of
tetrabutylammonium bromide, 20.5 g of 2-ethylhexyl glycidyl ether are added dropwise
and the reaction mixture is thereafter stirred for 24 hours at 120C. Then S g of Tonsil AC
are added to the reaction solution, which is stirred for S minutes at 110C and clarified by
filtration. The filtrate is concentrated by evaporation, the residue is taken up in 300 ml of
hexane, and 50 g are filtered over silica gel. The ~lltrate is concentrated by evaporation to
give the compound of formula
:
`~ O OH
e~OCH2CHCH20CH2CH-C4Hg
~'"................................ I I
;` OH C2H5
` in the form of a yellowish resin which slowly crystallises. Melting point: 56-58C.
Use Example 1: The UV absorbers of the invention are tested in a two-layer metallic
paint.
.~
"~ A clear varnish of the following cornposition is prepared:
~, Uracron~ 2263 XB (50 %)5 9. 2 parts
. Cymel~ 327 (90 %) 11.6 parts
Baysilon@) A (1 % in xylene) 1.0 part
butyl glycol acetate S.S parts
.
.
2 ~ 7 ~
- 16-
xylene 19.4 parts
butanol 3.3 parts
100.0 parts
.
To the above composition is added a solution of 1 part of the compound of Example 1 in
10 parts of xylene.
This clear varnish composition is diluted with a mixture of xylene (13 parts), butanol
(6 parts) and butyl glycol acetate (1 part) to a sprayable consistency and sprayed on to a
prepared aluminium sheet (coil coated, automotive filler, silver metallic prirner lacquer
based on polyester/cellulose acetate butyrate/melamine resin) and stoved for 30 minutes at
130C. The resultant finish has a dry layer thickness of 40 to 50 ~lm. For comparison
purposes, a similarly prepared aluminium sheet is coated with the above clear varnish
composition from which the UV absorbers are excluded.
The specimens so obtained are subjected to weathering and then tested for gloss retention.
Compared with the specimen without UV absorber, the stabilised specimens exhibit a
markedly better resistance to weathering. The results are reported in the following Table 1.
, .
Table 1: Evaluation of gloss retention after weathering according to DIN 67 530
~` (20C gloss~
.: _ _ _ _
. UV Absorber 20 gloss after
~; 0 400 800 1200 hours
,~
~ none 86 75 45 16
: according to
Example 1 84 78 74 81
~:~ according to
~`~ Example 2 85 75 68 74
according to
~, Example3 86 80 80 80
` according to
~` formula (S)85 73 70 74
, . .
.
.
. `.~
,
- , . :~
-
:- , ,, . , :-
.
~32~7~
- 17 -
o OH
(formula (5) ~ OCH3
cycle: 8 hours UV (UVB-313), 70C; 4 hours cond., 50C (UVCON).
. .
Use Example 2: The procedure of Use Example 1 is repeated, except that 0.5 part of the
compound of formula (6)
.~ H3C~3
CH3-N~ o-Co-(cH2)4---- is additionally used. The results are reported in
. H3C CH3 _ 2
Table 2.
: `:
. ` Table 2: Evaluation of gloss retention after weathering according to DIN 67 530
(20C gloss)
. _ , _ _
: UV Absorber 20 gloss after
. ~ 0 400 800 1200 hours
'.. ~ _
none 86 71 52 36
1 part
according to
Example 1 +
~' 0.5 part of (6) 86 70 61 56
1 part according
to Example 3 +
0.5 part of (6) 86 74 66 57
cycle: VDA method C (Xenotest 1200)
~`~;.,
. ~
`:"```
.
.
:
. :' :` ~' ' ' ' '
, . . .. . .