Note: Descriptions are shown in the official language in which they were submitted.
2~3~6
0.2. 0050/41358
Unsaturated cyclohexylacetic acid deri~atives,
and crop-protection aqents containinq same
The present invention relates to cyclohexylacetic
acid derivatives having a fungicidal and in~ecticidal
action, to fungicides and insecticides containing these
compounds, and to a method of combating fungi.
We ha~e found that unsaturated cyclohexylacetic
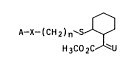
acid derivatives of the formula I
A-X-(CH2)n`S ~ I
H 3CO 2C u
where U i8 =NOCH3, =CHOCH3, =CH2, =CH-CH3, =CX-CH2-CH3,
=N-NH-C~3 or =CH-S-CH3,
n is fxom 0 to 10,
A i~ hydrogen or substituted or unsubstituted alkyl,
cyeloalkyl, aryl or hetaryl, and
X is a single bond in the case where n = O, and oxygen,
sul~ur or a ~ingle bond in the ca~3e where n i~ not 0, and
the plant--compatible acid-additiLon products and ~ase-
addition products thereof have a high fungitoxie and
inseetieidal action and very goocl plant compatibility.
Examples of acids for acid-addition products are
mineral acids, sg. hydrochlorie acid, hydrobromic acid,
phosphoric aeid, sulfurie aeid or nitrie acid, or car-
boxylie acid~, ~uch as formic ac.id, acetic aeid, oxalic
acid, malonie acid, lactic acid, malic acid, succinic
acid, tartarie aeid! citric acid or salicylic acid, p-
toluenesulfonie acid, dodecylbenzenesulfonic acid, or
proton-aeidie compounds in general, eg. saecharin.
Examples of bases for base-addition products are
potassium hydroxide, potassium carbonate, sodium hydrox-
ide, sodium carbonate and ammonium hydroxide.
The novel compounds of the fonmula I may be
produced a~ mîxtures of stereoisomers ~E~Z isomers,
diastereomer~ or enantiomers), which can ~e resol~ed into
the indi~idual components in a conventional manner, for
2 ~
- 2 - O.Z. 005~/41358
example by cry~tallization or c:hromato~raphy. The indi-
vidual i~omers and their mixtures can be us~d as fungi-
cides or insecticides and ar~ included in the invention.
If U can occur in syn/anti-isomers, the invention
5relates to all the isomers, in particular the anti-
isomers.
In particular, the invention relates to all the
diastereomers, in paxticular those having a bis-
equatorial arrangement at the two centers of chixality in
10the cyclohexane ring.
U is =NOCH3, =CHOCH3, =CH2, =C~-CH3, =CH-CHz-CH3~
=N-NH-CH3 or =CH-S-CH3, in particular =O, =NOCH3, =CH-OCH3
or =CH-CH3, preferably =NOCH3.
n i~ from 0 to 10, in particular from 0 to 5,
15prefe~ably 0, 1 r 2 or 3.
A is, for example, a saturated or unsaturated,
straight-chain ox branched hydrocarbon radical having
from 1 to 6, in particular from 1 to 4, carbon atoms or
a C3-CB-cycloalkyl radical, or phenyl, thienyl, fu.ryl,
20pyrryl, pyrazolyl, imidazolyl, oxazolyl, isoxazolyl,
thiazolyl, pyridyl, pyridazinyl, pyrLmidyl, pyrazinyl,
quinolyl, iso~uinolyl, indolyl, benzLmidazolyl, quin-
azolyl, or quinoxslyl, tho cycloalkyl~, alkyls, aryls or
hetaryls mentioned being unsubstituted or suhstituted by
25from 1 to 3 idontical or diferent substituents from the
group comprising halogen, eg. fluorine, chlorine, bromine
or iodine, Cl Ca-alkyl, Cl-Ca-alkylthio, Cl-Ca-alkyl-
sulfinyl, ~yano, hydroxyl, nitro, Cl-C8-alkoximino, Cl-C~-
alkoxy, phenyl, Cl-C4-haloalkyl, Cl-Ca-alkenyloximino,
30formyl, Cl-Cg-alkylcarbonyl or C1-CB-alkoxycarbonyl, or
phenyl which is substituted by from 1 to 3 identical or
different substituents from the group comprising halogen,
eg. fluorine, chlorine/ bromin~ or iodine, Cl-C8-al~yl,
C1-CB-alkylthio, Cl-C8-alkylsulfinyl, cyano, hydroxyl,
35nitro, Cl-C8-alkoximino, C1-CB-alXoxyt phenyl, Cl-Cs-halo-
alkyl ~ cl-cB-alk~nyloxLmino ~ fo~myl or Cl-Cg-alkylcarbonyl-
A i~ preferably a saturat~d or unsaturated,
~3i~5~
- 3 - O.Z. 0050/41358
straight-chain or branched hydrocarbQn radical having
from 1 to 6, in particular from 1 to 4, carbon atoms or
a C3-Cs-cycloalkyl radical, or phenyl, pyridyl or benz-
Lmidazolyl.
5The novel compounds can b~ prepared, for example,
by the following processe~:
Methyl ~-cyclohexen-l-yl-~-oxoacetate 1 (G. Neef
et al., THL 1977 (32), 2825-8~ is reacted with a thiol 2,
if ne~essary with base catalysis, to give a thioether 3
10(Y. Yankar et al., J. Chem. Research 1989, 178-179)
(~cheme 1).
Scheme 1
~lJ IA--X--( CH 2 ) n-SH A--X--( CH 2 ) n`SJ~J
O~CO 2CH 3 H 3C0 2C O
2 3
A and n are as above.
15Substituted methyl ~-oxoacetates 3 are converted
into the aCtiVQ ingred~entq 4 by reaction with CH3-0-NH3Cl
or H2N-NH-CH3 (scheme 2).
Scheme 2
A-X- ( CH 2 ) n`sJ~lA-X- ( CH 2 ) n~s--P
3Co2c o H3C02C ~ u
U is =NOCH3 or -N-NHCH3, and A and n are as abovs .
The methyl ~-octoacetates 3 can be used toprepare
the compounds 5 by a Wittig reaction with (C6Hs)3P+-CH3X-,
( C6H5 ) 3P -CH2-CH3$ ~ ( C6HS ) 3P -CH2-CH2-CH3X (X - halogen3 or
( ~6H5 ) 3P -CH2-0-cH3cl t ( C6~s ) 2P ( =0 )-CH2-O-CH3 or by Peterson
olefin formation u~ing ~CH3)3Si-CHz-O-CH3 tscheme 3~.
Scheme 3
A-X- ( CH 2 ~ n`S~ A--X--( CH 2 ) n`sJ~l
H 3C0 2C O H 3C0 2C U
3 5
~3~1~S
- 4 - O.Z. 0050/41358
U is =CH2, -C~-CH3, -CH-C~2-CH3 or =CHOCH3, and
A and n are a~ above.
The thioenol ethers 7 can be obtained rom the
enol ethers 6 by reaction with methyl mercaptan (EP
5178 826, scheme 4~.
Scheme 4
A-X- ( CH 2 ) n`S~~ A--X--( CH 2 ) n`S~
H 3C0 2C~CHOCH 3 H 3C0 2C~CHSCH 3
6 . 7
A and n are as above.
Th~ Examples below illustrate the preparation of
novel compounds.
EXAMPLB 1
Methyl ~-(2-benzylthioc:yclohexyl)--oxoacetate
(Compound No. 1.15)
2 g (12 mmol) of methyl ~-cyclohexlenyl-~-oxo-
acetate, 1.5 g (12 mmol) of benzyl mercaptan and 1 g
(10 mmol) o triethylamine in 10 ml of methylene chloride
are stirred at room temperature (20C~ for 15 hour~.
After the ~tirring period, water is added to the
batch, and the aqueous phase i~ extracted with methylene
chloride. The organic pha~e i9 extracted with water,
dried over MgSO~ and evaporated.
Chromatographic purification of the crude product
gi~es 2.3 g (66 ~) of the title compound as a pale yellow
oil.
IR (cm1): 2933, 1727, 1277, 1069.
EXAMPLE 2
Methyl ~-(2-benzylthiocyclohexyl)-~-axoacetate O-methyl
oxime
(Compound No. 2.15)
2.3 g (7.9 mmol) of methyl ~-(2-benzylthiocyclo-
he~yl)-~-oxoacetate (E~ample 1) and 0.~6 g (8 mmol) of
methoxyamine hydrochloride in 15 ml of methanol are
stirred at room temperature for 15 hours.
~3~
_ 5 _ o.Z. 0050/41358
Water is added to the reaction mixture~ and the
aqueou~ phase is extracted with ether. The organic phase
is washed with water, dried over MgSO4 and evaporated.
Chromatographic purification of the crude product gives
S 1 g (40 %) of the title compound as a pale yellow oil.
IR (cm~l): 2935, 1726,1151, 1042.
2~3'~
89082~
6 o.Z~ 0050/41358
Table I
A-X-~CH2)n`S~
H3CO2C O
No. A-X-(CH2)n mp. IR: v~cm~1)
5 1. 1 H
1. 2 methyl
1. 3 ethyl
1. 4 isopropyl
1. 5 butyl
10 1. 6 sec.-butyl
1. 7 t-butyl
1. 8 isobutyl
1. 9 pentyl
1.10 pivaloyl
15 1.11 hexyl
1.12 octyl
1.13 decyl
1.14 phenyl oil 2935,1729,1276,1069
1.15 benzyl oil 2933,1727,1277,1069
20 1.16 phenylethyl
1.17 phenylpropyl
1.18 phenylpentyl
I .1 9 1 -naphthy l
1.20 2-naphthyl
25 1.21 2-bromophenyl
1.22 3-bromophenyl
1.23 4-bromophenyl
1.24 2-chlorophenyl
1.25 3-chlorophenyl
30 1.26 4-chlorophenyl
1.27 2-fluorophenyl
1.28 3-fluorophenyl
1.29 4-fluorophenyl
1.30 2-methylphenyl
35 1.31 3-methylphenyl
1.32 4-methylphenyl
1.33 2-ethyl-phenyl
1.34 3-ethyl-phenyl
1.35 4-ethyl-phenyl
890828
7 O.Z. 0050/41358
Table I (contd.)
No. A-X-(CH2)n mp. IR: v(cm~1)
5 1.36 2-iso-propyl-phenyl
1.37 3-iso-propyl-phenyl
1.38 4-iso-propyl-phenyl
1.39 2-tert.-butyl-phenyl
1.40 3-tert.-butyl-phenyl
10 1.41 4-tert.-butyl-phenyl
1.42 4-butyl-phenyl
1.43 4-hexyl-phenyl
1.44 4-nonyl-phenyl
1.45 4-decyl-phenyl
15 1.46 2-methoxy-phenyl
1.47 3-methoxy-phenyl
1.48 4-methoxy-phenyl
1.49 2-trifluoromethyl-phenyl
1.50 3-trifluoromethyl-phenyl
20 1.51 4-trifluoromethyl-phenyl
1.52 2-formylphenyl
1.53 3-formylphenyl
1.54 4-formylphenyl
1.55 2-allyl-O-N=CH-phenyl
25 1.S6 3-allyl-O-N=CH-phenyl
1.57 4-alIyl-O-N-CH-phenyl
1.58 2-nitro-phenyl
1.59 3-nitro-phenyl
1.60 2,4-dichlorophenyl
30 1.61 2,5-dichlorophenyl
1.62 2,6-dichlorophenyl
1.63 3,4-dichlorophenyl
1.64 2,3-dichlorophenyl
1.65 3,5-dichlorophenyl
35 1.66 2,3,4-trichlorophenyl
1.67 2,4,5-trichlorophenyl
1.68 2,4,6-trichlorophenyl
1. 69 2,3,4,6-tetrachlorophenyl
1. 70 2,3,4,5,6-pentachlorophenyl
40 1. 71 2,3,4,5-tetrafluorophenyl
1. 72 2,3,5,6-tetrafluorophenyl
1. 73 2,3,4,5,6-penta~luorophenyl
2~
890828
8 O.Z. 0050/41358
Table I (contd.)
No. A-X-(CH2)n mp. IR: v(cm~l)
5 1. 74 2-chloro, 4-fluorophenyl
1. 75 3-chloro, 4-fluorophenyl
1. 76 2-chloro, 6-methyl-phenyl
1. 77 4-chloro, 2-methyl-phenyl
1. 78 2,4-dichloro, 5-methyl-phenyl
10 l. 79 4-chloro, 2,5-dimethyl-phenyl
1. 80 4-bromo, 2-methyl-phenyl
1. 81 3,5-bistrifluoromethyl-phenyl
1. 82 2,3-dimethyl-phenyl
1. 83 2,4-dimethyl-phenyl
15 1. 84 2,5-dimethyl-phenyl
1. 85 2,6-dimethyl-phenyl
1. 86 3,4-dimethyl-phenyl
1. 87 3,5-dimethyl-phenyl
1. 88 2,4,5-trilnethyl-phenyl
20 1. 89 2,6-diethyl-phenyl
1. 90 2,4-di-tert.-butyl-phenyl
1. 91 2,5-dimethoxy-phenyl
1. 92 3,4-dimethoxy-phenyl
1. 93 2-methyl, 4-terlt.-butyl-phenyl
25 1. 94 2-methoxycarbonyl-phenyl
1. 95 2-ethoxycarbonyl-phenyl
1. 96 2-propoxycarbonyl-phenyl
1. 97 2-butoxycarbonyl-phenyl
1. 98 2-cyano-phenyl
30 1. 99 3-cyano-phenyl
1.100 4-cyano-phenyl
1.101 1-naphthylmethyl
1.102 2-naphthylmethyl
1.103 2-bromobenzyl
35 1.104 3-bromobenzyl
1.105 4-bromobenzyl
1.106 2-chlorobenzyl
1.107 3-chlorobenzyl
1.108 4-chlorobenzyl
40 1.109 2-fluorobenzyl
1.110 3-fluorobenzyl
1.111 4-fluorobenzyl
1.112 2-methylbenzyl
2~3~ ~ ~5
890828
9 o.z. 0050/41358
Table 1 (contd.)
No. A-X-lC~l2)n mp. IR: v(cm~1)
5 1.113 3-methylbenzyl
1.114 4-methylbenzyl
1.115 2-methoxybenyzl
1.116 3-~ethoxybenzyl
1.117 4-methoxybenzyl
10 1.118 4-ethoxybenzyl
1.119 4-butyloxybenzyl
1.120 4-benzyloxybenzyl
1.121 2-trifluoromethyl-benzyl
1.122 3-trifluoromethyl-benzyl
lS 1.123 4-trifluoromethyl-benzyl
1.124 2-formylbenzyl
1.125 3-formylbenzyl
1.126 4-formylbenzyl
1.127 2-allyl-O-N=CH-benzyl
2~ 1.128 3-allyl--O-N=CH-benzyl
1.129 4-allyl--O-N=CH-benzyl
1.130 2-nitrobenzyl
1.131 3-nitrobenzyl
1.132 2,4-dichlorobenzyl
25 1.133 2,6-dichlorobenzyl
1.134 3,4-dichlorobenzyl
1.135 2,3-dichlorobenzyl
1.136 3,5-dichlorobenzyl
1.137 2,3-dimethylbenzyl
3~ 1.138 2,4-dimethylbenzyl
1.139 2,5-di~ethylbenzyl
1.140 2,6-dimethylbenzyl
1.141 3,4-dimethylbenzyl
1.142 3,5-dimethylbenzyl
35 1.143 4-chloro-2-methylbenzyl
t.144 2-methoxycarbonyl-benzyl
1.145 2-ethoxycarbonyl-benzyl
1.146 2-butoxycarbonyl-benzyl
1.147 2-cyano-benzyl
40 1.148 3-cyano-benzyl
1.149 4-cyano-benzyl
1.150 2-pyridyl
1.151 3-pyridyl
2 ~ 1 5 ~
890828
o.z. 0050/41358
Table I (contd.)
No. A-X-(CH2)n mp. IR: v(cm~l)
5 1.152 4-pyridyl
1.153 6-methyt-2-pyridyl
1.154 6-ethyl-2-pyridyl
1.155 6-n-propyl-2-pyridyl
1.156 6-n-butyl-2-pyridyl
10 1.157 6-tert.-butyl-2-pyridyl
1.158 6-n-hexyl-2-pyridyl
1.159 6-phenyl-2-pyridyl
1.160 6-benzyl-2-pyridyl
1.161 6-trifluoromethyl-2-pyridyl
15 1.162 6-methoxy-2-pyridyl
1.163 6-chloro-2-pyridyl
1.164 3,6-dimethyl-2-pyridyl
1.165 4,6-dimethyl-2-pyridyl
1.166 5,6-dimethyl-2-pyridyl
20 1.167 4-phenyl-6-methyl-2-pyridyl
1.168 3,4-dichloro-6-methyl-2-pyridyl
1.169 3,4,5-trichloro-6-methyl-2-pyridyl
1.170 4-trifluoromethyl-6-methyl-2-pyridyl
1.171 3-acetyl-4,6-dimethyl-2-pyridyl
25 1.172 3-cyano-6-~ethyl-2-pyridyl
1.173 3-cyano-6-phenyl-2-pyridyl
1.174 3-methyloxycarbonyl-2-pyridyl
1.175 3-methyloxycarbonyl-6-iso-propyl-2-pyridyl
1.176 5-trifluoromethyl-2-pyridyl
30 1.177 2-quinolyl
1.178 3-methyl-2-quinolyl
1.179 4-phenyl-2-quinolyl
1.180 8-chloro-2-quinolyl
1.181 4-methyl-8-methoxy-2-quinolyt
35 1.182 4-quinolyl
1.183 2,6-dimethyl-4-quinolyl
1.184 8-quinolyl
1.185 5,7-dichloro-8-quinolyl
1.186 2-pyrimidinyl
40 1.187 4-trifluoromethyl-2-pyrimidinyl
1.188 4,6-dimethyl-5-chloro-2-pyrimidinyl
1.189 3,5-dimethyl-4-pyrimidinyl
1.190 2-pyrazinyl
2~t3~
890828
ll o.Z~ 0050/41358
Table I (contd.)
No. A-X-(CH2)n mp. 1~: v(cm~1)
5 1 . 191 2-thienyl
1.192 3-thienyl
1.193 3-methyl-2-quinoxalinyl
1.194 3-phenyl-5-isoxazolyl
1.195 2-benzoxazolyl
10 1.196 2-benzthiazolyl
1.197 4-chloro-2-benzothiazolyl
1.198 5-chloro~2-benzothiazolyl
1.199 6-chloro-2-benzothiazolyl
1.200 6-methyl-2-benzothiazolyl
15 1.201 6-methoxy-2-benzothiazolyl
203l~ L~6
890828
12 O.Z. 0050/41358
Table 2
A-X--(CH2)n`S~
H3CO2C NOCH3
No. A-X-(CH2)n mp. IR: v(cm~1)
5 2. 1 H
2. 2 methyl
2. 3 ethyl
2. 4 isopropyl
2. 5 butyl
10 2. 6 sec.-butyl
2. 7 t-butyl
2. 8 isobutyl
2. 9 pentyl
2.10 pivaloyl
15 2.11 hexyl
2.12 octyl
2.13 decyl
2.14 phenyl oi1 2935,1738,1722,1152,1043
2.15 benzyl oil 2935,1726,1151,1042
20 2.16 phenylethyl
2.17 phenylpropyl
2~18 phenylpentyl
2.19 I-naphthyl
2.20 2-naphthyl
25 2.21 2-bromophenyl
2.22 3-bromophenyl
2.23 4-bromophenyl
2.24 2-chlorophenyl
2.25 3-chlorophenyl
30 2.26 4-chlorophenyl
2.27 2-fluorophenyl
2.28 3-fluorophenyl
2.29 4-~luorophenyl
2.30 2-methylphenyl
35 2.31 3-methylphenyl
2.32 4-methylphenyl
2.33 2-ethyl-phenyl
2.34 3-ethyl-phenyl
2.35 4-ethyl-phenyl
. .
.
2~3~
~90828
13 o.Z. 0050/~1358
Table 2 (contd.)
No. A-%-lCH2)n mp. IR: v(cm~l)
5 2.36 2-iso-propyl-phenyl
2.37 3-iso-propyl-phenyl
2.38 4-iso-propyl-phenyl
2.39 2-tert.-butyl-phenyl
2.40 3-tert.-butyl-phenyl
10 2.41 4-tert.-butyl-phenyl
2.42 4-butyl-phenyl
2.43 4-hexyl-phenyl
2.44 4-nonyl-pheny1
2.45 4-decyl-phenyl
15 2.46 2-methoxy-phenyl
2.47 3-methoxy-phenyl
2.48 4-methoxy-phenyl
2.49 2-trifluoromethyl-phenyl
2.50 3-trifluoromethyl-phenyl
20 2.51 4-trifluoromethyl-phenyl
2.52 2-formylpheryl
2.53 3-formylphenyl
2.54 4-formylphenyl
2.55 2-alIyl-O-N=C~phenyl
25 2.56 3-alIyl-O-N=CH-phenyl
2.57 4-alIyl-O-N-CH-phenyl
2.58 2-nitro-phenyl
2.59 3-nitro-phenyl
2.60 2,4-dichlorophenyl
30 2.61 2,5-dichlorophenyl
2.62 2,6-dichlorophenyl
2.63 3,4-dichlorophenyl
2.64 2,3-dichlorophenyl
2.65 3,5-dichlorophenyl
35 2.66 2,3,4-trichlorophenyl
2.67 2,4,5-trichlorophenyl
2.68 2,4,6-trichlorophenyl
2. 69 2,3,4,6-tetrachlorophenyl
2. 70 2,3,4,5,6-pentachlorophenyl
40 2. 71 2,3,4,5-tetrafluorophenyl
2. 72 2,3,5,6-tetrafluorophenyl
2. 73 2,3,4,5,6-pentafluorophenyl
2. 74 2-chloro, 4-fluorophenyl
"` 2~3~
890828
14 O.z. 0050/41358
Table 2 tcontd.)
No. A-X-~C~2)n mp. IR: v(cm~l)
5 2. 75 3-chloro, 4-fluorophenyl
2. 76 2-chloro, 6-methyl-phenyl
2. 77 4-chloro, 2-methyl-phenyl
2. 78 2,4-dichloro, 5-methyl-phenyl
2. 79 4-chloro, 2,5-dimethyl-phenyl
10 2. 80 4-bromo, 2-methyl-phenyl
2. 81 3,5-bistrifluoromethyl-phenyl
2. 82 2,3-dimethyl-phenyl
2. 83 2,4-dimethyl-phenyl
2. 84 2,5-dimethyl-phenyl
15 2. 85 2,6-dimethyl-phenyl
2. 86 3,4-dimethyl-phenyl
2. 87 3,5-dimethyl-phenyl
2. 88 2,4,5-trimethyl-phenyl
2. 89 2,6-diethyl-phenyl
20 2. 90 2,4-di-tert.-butyl-phenyl
2. 91 2,5-dimethoxy-phenyl
2. 92 3,4-dimethoxy-phenyl
2. 93 2-methyl, 4-tert.-butyl-phenyl
2. 94 2-methoxycarbonyl-phenyl
25 2. 95 2-ethoxycarbonyl-phenyl
2. 96 2-propoxycarbonyl-phenyl
2. 97 2-butoxycarbonyl-phenyl
2. 98 2-cyano-phenyl
2. 99 3-cyano-phenyl
30 2.100 4-cyano-phenyl
2. 101 I-naphthylmethyl
2.102 2-naphthylmethyl
2.103 2-bromobenzyl
2.104 3-bromobenzyl
35 2.105 4-bromobenzyl
2.106 2-chlorobenzyl
2.107 3-chlorobenzyl
2.108 4-chlorobenzyl
2.109 2-fluorobenzyl
40 2.110 3-fluorobenzyl
2.111 4-fluorobenzyl
2.112 2-methylbenzyl
2.113 3-methylbenzyl
.
~3~
890828
O.Z. 0050/41358
Table 2 (contd.)
No. A-X-(CH2)n mp. IR: v(cm~l)
5 2.114 4-methylbenzyl
2.115 2-methoxybenyzl
2.116 3-methoxybenzyl
2. 117 4-methoxybenzyl
2.118 4-ethoxybenzyl
10 2.119 4-butyloxybenzyl
2.120 4-benzyloxybenzyl
2.121 2-trifluoromethyl-benzyl
2.122 3-trifluoromethyl-benzyl
2.123 4-trifluoromethyl-benzyl
15 2.124 2-formylbenzyl
2.125 3-formylbenzyl
2.126 4-formylbenzyl
2.12 7 2-allyl-O-N=CH-benzyl
2.128 3-allyl-O-N=CH-benzyl
20 2.129 4-allyl-O-N=CH-benzyl
2.130 2-nitrobenzyl
2.131 3-nitrobenzyl
2.132 2,4-dichlorobenzyl
2.133 2,6-dichlorobenzyl
25 2.134 3,4-dichlorobenzyl
2.135 2,3-dichlorobenzyl
2.136 3,5-dichlorobenzyl
2.137 2,3-dimethylbenzyl
2.138 2,4-dimethylbenzyl
30 2.139 2,5-dimethylbenzyl
2.140 2,6-dimethylbenzyl
2.141 3,4-dimethylbenzyl
2.142 3,5-dimethylbenzyl
2.143 4-chloro-2-methylbenzyl
35 2.144 2-methoxycarbonyl-benzyl
2.145 2-ethoxycarbonyl-benzyl
2.146 2-butoxycarbonyl-benzyl
2.147 2-cyano-benzyl
2.148 3-cyano-benzyl
40 2.149 4-cyano-benzyl
2~150 2-pyridyl
2.151 3-pyridyl
2.152 4-pyridyl
2~3'~15~
890828
16 O.Z. 0050/41358
Table 2 (contd.)
No. A-X-(CH2)n mp. IR: v(cm~l)
5 2.153 6-methyl-2-pyridyl
2.154 6-ethyl-2-pyridyl
2.155 6-n-propyl-2-pyridyl
2.156 6-n-butyl-2-pyridyl
2.157 6-tert.-buty1-2 pyridyl
10 2.158 6-n-hexyl-2-pyridyl
2.159 6-phenyl-2-pyridyl
2.160 6-benzyl-2-pyridyl
2.161 6-trifluoromethyl-2-pyridyl
2.162 6-metho~y-2-pyridyl
15 2.163 6-chloro-2-pyridyl
2.164 3,6-dimethyl-2-pyridyl
2.165 4,6-dimethyl-2-pyridyl
2.166 5,6-dimethyl-2-pyridyl
2.167 4-phenyl-6-methyl-2-pyridyl
20 2.168 ~,4-dichloro-6-methyl-2-pyridyl
2.169 3,4,5-trichloro-6-methyl-2-pyridyl
2.170 4-trifluoromethyl-6-methyl-2-pyridyl
2.171 3-acetyl-4,6-dimethyl 2-pyridyl
2.172 3-cyano-6-methyl-2-pyridyl
25 2.173 3-cyano-6-phenyl-2-pyridyl
2.174 3-methyloxycarbonyl-2-pyridyl
2.175 3-methyloxycarbonyl-6-iso-propyl-2-pyridyl
2.176 5-trifluoromethyl-2-pyridyl
2.177 2-quinolyl
30 2.178 3-methyl-2-quin~lyl
2.179 4-phenyl-2-quinolyl
2.180 8-chloro-2-quinolyl
2.181 4-methyl-8-methoxy-2-quinolyl
2.182 4-quinolyl
35 2.183 2,6-dimethyl-4-quinolyl
2.184 8-quinolyl
2.185 5,7-dichloro-8-quinolyl
2.186 2-pyrimidinyl
2.187 4-trifluoromethyl-2-pyrimidinyl
40 2.188 4,6-dimethyl-5-chloro-2-pyrimidinyl
2.189 3,5-dimethyl-4-pyrimidinyl
2.190 2-pyra7inyl
2. 191 2-thienyl
~03~
890828
17 O.Z. 0050/41358
Table 2 (contd.)
No. A-X-(CH2)n mp. IR: v(cm~l)
5 2.192 3-thienyl
2.193 3-methyl-2-quinoxalinyl
2.194 3-phenyl-5-isoxazolyl
2.195 2-benzoxazolyl
2.196 2-benzthiazolyl
10 2.197 4-chloro-2-benzothiazolyl
2.198 5-chloro-2-benzothiazolyl
2~199 6-chloro-2-benzothiazolyl
2.200 6-methyl-2-benzothiazolyl
2.201 6-methoxy-2-benzothiazolyl
203~
890828
18 o.Z. 0050/41358
Table 3
f~
A--X--(CH2)n`$~
H3C02C-l~CHOCH3
No. A-X-(CH2)n mp. IR: v~cm~1)
5 3. I n-butyl
3. 2 phenyl
3. 3 2-methoxyphenyl
3. 4 3-methoxyphenyl
3. 5 4-methoxyphenyl
10 3. 6 2-chlorophenyl
3. 7 3-chlorophenyl
3. 8 4-chlorophenyl
3. 9 2-methylphenyl
3.10 3-methylphenyl
15 3.11 4-methylphenyl
3.12 2,3-dimethylphenyl
3.13 2,4-dimethylphenyl
3.14 2,5-dimethylphenyl
3.15 2,6-dimethylphenyl
20 3.16 3,4-dimethylphçnyl
3.17 3,5 dimethylphenyl
3.18 4-chloro-2-methylphenyl
3.19 4-methoxycarbonylphenyl
3.20 4-ethoxycarbonylphenyl
25 3.21 4-butoxycarbonylphenyl
3.22 2-alIyl-0-N=CH-phenyl
3.23 3-alIyl-0-N=CH-phenyl
3.24 4-allyl-0-N=CH-phenyl
3.25 2-pyridyl
30 3.26 6-methyl-2-pyridyl
3.27 6-chloro-2-pyridyl
3.28 4-chloro-2-benzothiazolyl
3.29 5-chloro-2-benzothiazolyl
3.30 6-chloro-2-benzothiazolyl
35 3.31 2-methoxybenzyl
3.32 3-methoxybenzyl
3.33 4-methoxybenzyl
3.34 2-chlorobenzene
3.35 3-chlorobenzene
2~3~
890828
19 0.7. 0050~41358
Table 3 (contd.)
No. A-x-lc~i2)n mp. IR: v(cm~1)
5 3.36 4-chlorobenzyl
3.37 2-methylbenzyl
3.38 3-methylbenzyl
3.39 4-methylbenzyl
3.40 2,3-dimethylbenzyl
10 3.41 2,4-dimethylbenzyl
3.42 2,5-dimethylbenzyl
3~43 2,6-dimethylbenzyl
3.44 3,4-dimethylbenzyl
3.45 3,5-dimethyIbenzyl
15 3.46 4-chloro-2-methylbenzyl
3.47 4-methoxycarbonylb~nzyl
3.48 4-ethoxycarbonylbenzyl
3.49 4-butoxycarbonylbenzyl
3.50 2-allyl-O-N=CH-phenyl
20 3.51 3-allyl-O-N=CH-phenyl
3.52 4-allyl-O-N=CH-phenyl
2~3~S
890828
O.Z. 0050J41358
Table 4
A-X-(CH2)n`S ~
H 3C0 2C CH-CH 3
No. A-X-(CH2)n mp. IR: v(cm~1)
5 4. 1 n-butyl
4. 2 phenyl
4. 3 2-methoxyphenyl
4. 4 3-methoxyphenyl
4. 5 4-methoxyphenyl
10 4. 6 2-chlorophenyl
4. 7 3-chlorophenyl
4. 8 4-chlorophenyl
4. 9 2-methylphenyl
4.10 3-methylphenyl
15 4.11 4-methylphenyl
4.12 2,3-dimethylphenyl
4.13 2,4-dimethylphenyl
4.14 2,5-dimethylphenyl
4.15 2,6-dimethylphenyl
2~ 4.16 3,4-dimethylphenyl
4.17 3,5-dimethylphenyl
4.18 4-chloro-2-methylphenyl
4.19 4-methoxycarbonylphenyl
4.20 4-ethoxycarbonylphenyl
25 4.21 4-butoxycarbonylphenyl
4.22 2-alIyl-O-N=CH-phenyl
4.23 3-allyl-O-N=CH-phenyl
4.24 4-allyl-O-N=CH-phenyl
4.25 2-pyridyl
30 4.26 6-methyl-2-pyridyl
4.27 6-chloro-2-pyridyl
4.28 4-chloro-2-benzothiazolyl
4.29 5-chloro-2-benzothiazolyl
4.30 6-chloro-2-benzothiazolyl
35 4.31 2-methoxybenzyl
4.32 3-methoxybenzyl
4.33 4-methoxybenzyl
4.34 2-chlorobenzene
4.35 3-chlorobenzene
~3~
890828
21 O.Z. 0050/41358
Table 4 (contd.)
No. A-X-¦CH2)n mp. IR: v(cm~1)
5 4.36 4-chlorobenzyl
4.37 ~-methylbenzyl
4.38 3-methylbenzyl
4.39 4-methylbenzyl
4.40 2,3-dimethylbenzyl
10 4.41 2,4-dimethylbenzyl
4.42 2,5-dimethylbenzyl
4.43 2,6-dimethylbenzyl
4.44 3,4-dimethylbenzyl
4.45 3,5-dimethylbenzyl
15 4.46 4-chloro-2-methylbenzyl
4.47 4-methoxycarbonylbenzyl
4.48 4-ethoxycarbonylbenzyl
4.49 4-butoxycarbonylbenzyl
4.50 2-allyl-O-N=CH-phenyl
20 4.51 3-allyl-O-N=CH-phenyl
4.52 4-alIyl-O-N=CH-phenyl
~031~5~
89~828
22 O.Z. 0050/41358
Table 5
f~ .
A-x-(cH2)n`s~
H3CO2C~U
No. A-X-(CH2)n U mp. I~: Y/cm 1)
5 5. 1 2-methylphenyl CH2
5. 2 3-methoxyphenyl CH2
5. 3 4-chlorophenyl CH2
5. 4 2-allyl-O-N=CH-phenyl CH2
5. 5 3-methoxycarbonylphenyl CH2
10 5. 6 6-chloro-2-pyridyl CH2
5. 7 6-chloro-2-benzothiazolyl CH2
5. 8 2-methylbenzyl CH2
5. 9 3-methoxybenzyl CH2
5.10 4-chlorobenzyl CH2
15 5.11 2-alIyl-O-N=CH-benzyl CH2
5.12 3-methoxycarbonyl-benzyl CH2
5.13 2-methylphenyl CH-CH2-CH3
5.14 3-methoxyphenyl CH-CH2-CH3
5.15 4-chlorophenyl CH-CH2-CHl3
20 5.16 2-allyl-O-N=CH-phenyl CH-CH2-CHl3
5.17 3-methoxycarbonylphenyl CH-CH2-CHl3
5.18 6-chloro-2-pyridyl CH-CH2-C~ll3
5.19 6-chloro-2-benzothiazolyl CH-CH2-CH3
5.20 2-methylbenzyl cH-cH2-c~l3
z5 5.21 3-methoxybenzyl CH-CH2-C~l3
5.22 4-chlorobenzyl CH-CH2-CH3
5.23 2-allyl-O-N=CH-benzyl CH-CH2-CH3
5.24 3-methoxycarbonyl-benzyl CH-CH2-CH3
5.25 2-methylphenyl N-NH-CH3
30 5~26 3-methoxyph~nyl N-NH-CH3
5.27 4-chlorophenyl N-NH-CH3
5.28 2-allyl-O-N=CH-phenyl N-NH-CH3
5.29 3-methoxycarbonylphenyl N-NH-CH3
5.30 6-chloro-2-pyridyl N-NH-CH3
35 5.31 6-chloro-2-benzothiazolyl N-NH-CH3
5.32 2-methylbenzyl N-NH-CH3
5.33 3-methoxybenzyl N-NH-CH3
5.34 4-chlorobenzyl N-NH-CH3
5.35 2-allyl-O-N=CH-benzyl N-NH-CH3
2 ~ 3 ~ 6
890828
23 O.Z. 0050/41358
Table 5 (contd.)
No. A-X-(CH2)n U mp. IR: v/cm~1)
5 5.36 3-methoxycarbonyl-benzyl N-NH-CH3
5.37 2-methylphenyl CH-S-CH3
5.38 3-methoxyphenyl CH-S-CH3
5.39 4-chlorophenyl CH-S-CH3
5.40 2-allyl-0-N=CH-phenyl CH-S-CH3
10 5.41 3-methoxycarbonylphenyl CH-S-CH3
5.42 6-chloro-2-pyridyl CH-S-CH3
5.43 6-chloro-2-benzothiazolyl CH-S-CH3
5.44 2-methylbenzyl CH-S-CH3
5.45 3-methoxybenzyl CH-S-CH3
15 5.46 4-chlorobenzyl CH-S-CH3
5.47 2-allyl-0-N=CH-benzyl CH-S-CH3
5.48 3-methoxycarbonyl-benzyl CH-S-CH3
2 ~
24 O.z. 0050/41358
In general terms, the novel compounds are extremely effective on a broad
spectrum of phytopathogenic fungi, in particular those from the class
consisting of the Ascomycetes and Basidiomycetes. Some of them have a
systemic action and can be used as foliar and soil fungicides.
The fungicidal compounds are of particular interest for controlling a
large number of fungi in various crops or their seeds, especially wheat,
rye, barley, oats, rice, Indian corn, lawns, cotton, soybeans, coffee,
sugar cane, fruit and ornamentals in horticulture and viticulture, and in
10 vegetables such as cucumbers, beans and cucurbits.
The novel compounds are particularly useful for controlling the following
plant diseases:
15 Erysiphe graminis in cereals,
Erysiphe cichoracearum and Sphaerotheca fuliginea in cucurbits,
Podosphaera leucotricha in apples,
Uncinula neçator in vines,
Puccinia species in cereals,
20 Rhizoctonia solani in cotton,
Ustilago species in cereals and sugar cane,
Venturia inaequalis (scab) in apples,
Helminthosporium species in cereals,
Septoria nodorum in wheat,
25 Botrytis cinerea (gray mold) in strawberries and grapes,
t:ercospora arachidicola in groundnuts,
Pseudocercosporella herpotrichoides in ~heat and barley,
Pyricularia oryzae in rice,
Phytophthora infestans in potatoes and tomatoes,
30 Fusarium and Verticillium species in various plants,
Plasmopara viticola in grapes,
Alternaria species in fruit and vegetables.
The compounds are applied by spraying or dusting the plants with the
35 active ingredients, or treating the seeds of the plants with the active
ingredients. They may be applied before or after infection of the plants
or seeds by the fungi.
The novel substances can be converted into conventional formulations such
40 as solutions, emulsions, suspensions, dusts, powders, pastes and granules.
The application forms depend entirely on the purposes for which they are
intended; they should at all events ensure a fine and uniform distribution
of the active ingredient. The formulations are produced in known manner,
for example by extending the active ingredient with solvents and/or
carriers, with or without the use of emulsifiers and dispersants; if water
~3~t~
O.Z. 0050/41358
is used as solvent, it is also possible to employ other organic solvents
as auxiliary solvents. Suitable auxiliaries for this purpose are solvents
such as aromatics (e.g., xylene), chlorinated aromatics (e.g., chloro-
benzenes), paraffins (e.g., crude oil -fractions), alcohols (e.g.,
5 methanol, butanol), ketones (e.g., cyclohexanone), amines ~e.g.,
ethanolamine, dimethylformamide), and water; carriers such as ground
natural minerals (e.g., kaolins, aluminas, talc and chalk) and ground
synthetic minerals (e.g., highly disperse silica and silicates);
emulsifiers such as nonionic and anionic emulsifiers (e.g., polyoxyethyl-
lO ene fatty alcohol ethers, alkyl sulfonates and aryl sulfonates); anddispersants such as lignin, sulfite waste liquors and methylcellulose.
The fungicides generally contain from 0.1 to 95, and preferably from 0.5
to 90, wt% of active ingredient. The application rates are from 0.02 to 3
15 kg or more of active ingredient per hectare, depending on the type of
effect desired. The novel compounds may also be used for protecting
- materials (timber), e.g., against Paecilomyces variotii. When the active
ingredients are used for treating seed, amounts of from 0.001 to 50, and
preferably from 0.01 to 20, g per kg of seed are generally required.
The agents and the ready-to-use formulations prepared from them, such as
solutions, emulsions, suspensions, powders, dusts, pastes and granules,
are applied in conventional manner, for example by spraying, atomizing,
dusting, scattering, dressing or watering.
Exarnples of formulations are given below.
I. A solution of 90 parts by weight of compound no. 2.15 and 10 parts by
weight of N-methyl--pyrrolidone, which is suitable for application in the
30 form of very fine drops.
II. A mixture of 20 parts by weight of compound no. 2.15, 80 parts by
weight of xylene, 10 parts by weight of the adduct of 8 to 10 moles of
ethylene oxide and I mole of oleic acid-N-monoethanolamide, 5 parts by
35 weight of the calcium salt of dodecy1benzenesulfonic acid, and 5 parts by
weight of the adduct of 40 moles of ethylene oxide and I mole of castor
oil. By finely dispersing the mixture in water, an aqueous dispersion is
obtained.
40 III. An aqueous dispersion of 20 parts hy weight of compound no. 2.15,
40 parts by weight of cyclohexanone, 30 parts by weight of isobutanol,
20 parts by weight of the adduct of 40 moles of ethylene oxide and 1 mole
of castor oil. By pouring the solution into water and finely dispersing it
therein, an aqueous dispersion is obtained.
2n3~
26 O.Z. 0050/41358
IV. An aqueous dispersion of 20 parts by weight of compound no 2.15, 25
parts by weight of cyclohexanol, 65 parts by weight of a mineral oil
fraction having a boiling point between ~10 and 280C, and 10 parts by
weight of the adduct of 40 moles of ethylene oxide and 1 mole of castor
5 oil. By pouring the solution into water and finely dispersing it therein,
an aqueous dispersion is obtained.
V. A hammer-milled mixture of 80 parts by weight of compound no. 2.15,
3 parts by weight of the sodium salt of diisobutylnaphthalene--sulfonic
10 acid, 10 parts by weight of the sodium salt of a lignin-sulfonic acid
obtained from a sulfite waste liquor, and 7 parts by weight of powdered
silica gel. By finely dispersing the mixture in water, a spray liquor is
obtained.
15 Vl . An intimate mixture of 3 parts by weight of compound no. 2.15 and
97 parts by weight of particulate kaolin. The dust contains 3wt70 of the
active ingredient.
VII. An intimate mixture of 30 parts by weight of compound no. 2.15, 920 parts by weight of powdered silica gel and 8 parts by weight of paraffin
oil sprayed onto the surface of this silica gel. This formulation of the
active ingredient exhibits good adherence.
Vlll. A stable aqueous dispersion of 40 parts by weight of compound no.
25 2.15, 10 parts of the sodium salt of a phenolsulfonic acid-urea-
formaldehyde condensate, 2 parts o-f silica gel and 48 parts of water,
which dispersion can be further diluted.
IX. A stable oily dispersion of 20 parts by weight of compound no. 2.15,
30 2 parts by weight of the calcium salt of dodecylbenzenesulfonic acid,
8 parts by weight of a fatty alcohol polyglycol ether, 2 parts by weight
of the sodium salt of a phenolsulfonic acid-urea-formaldehyde condensate
and 68 parts by weight of a paraffinic mineral oil.
35 In these application forms, the agents according to the invention may also
be present together with other active ingredients, for example herbicides,
insecticides, growth regulators, and fungicides, and may furthermore be
mixed and applied together with fertili ers. Admixture with other fun-
gicides frequently results in a greater fungicidal action spectrum.
4~
2 8 3 '~
27 O.Z. 0050J41358
The following list of fungicides with which the novel compounds may be
combined is intended to illustrate possible combinations but not to impose
any restrictions.
5 Examples of fungicides which may be combined with the novel compounds are:
sulfur,
dithiocarbamates and their derivatives, such as
ferric dimethyldithiocarbamate,
10 zinc dimethyldithiocarbamate,
zinc ethylenebisdithiocarbamate,
manganese ethylenebisdithiocarbamate,
manganese zinc ethylenediaminebisdithiocarbamate,
tetramethylthiuram disulfides,
15 ammonia complex of zinc N,N'-ethylenebisdithiocarbamate,
ammonia complex of zinc N,N -propylenebisdithiocarbamate,
zinc N,N -propylenebisdithioccarbamate and
N,N -polypropylenebis(thiocarbamyl) disulfide;
20 nitro derivatives, such as
dinitro(l-methylheptyl)-phenyl crotonate,
2-sec-but~yl-4,6-dinitrophenyl 3,3-dimethylacrylate,
2-sec-butyl-4,6-dinitrophenyl isopropylcarbollate and
diisopropyl 5-nitroisophthalate;
heterocyclic substances, such as
2-heptadecylimidazol-2-yl acetate,
2,4-dichloro-6-(o-chloroanilino)-s-triazine,
O,O-diethyl phthalimidophosphonothioate,
3~ 5-amino-1-[-bis-(dimethylamino)-phosphinyl]-3-phenyl-1,2,4-triazole,
2,3-dicyano-1,4-dithioanthraquinone,
2-thio-1,3-dithio~4,5-b]quinoxaline,
methyl 1-(butylcarbamyl)-2-benzimidazolecarbamate,
2-methoxycarbonylaminobenzimidazole,
35 2-(fur-2-yl)-benzimidazole,
2-(thiazol-4-yl)benzimidazole,
N-(1,1,2,2-tetrachloroethylthio)-tetrahydrophthalimide,
N-trichloromethylthiotetrahydrophthalimide,
N-trichloromethylthiophthalimide,
4~
N-dichlorofluoromethylthio-N',N'-dimethyl-N-phenylsulfuric acid diamide,
5-ethoxy-3-trichloromethyl-1,2,3-thiadiazole,
2-thiocyanatomethylthiobenzothia~ole,
l,4-dichloro-2,5-dimethoxyben7ene,
2~3~
28 O.Z. 0050/41358
4-(2-chlorophenylhydrazono)-3-methyl-5-isoxazolone,
2-thiopyridine l-oxide,
8-hydroxyquinoline and its copper salt,
2,3-dihydro-5-carboxanilido-6-methyl-1,4-oxathiyne,
5 2,3-dihydro-5-carboxanilido-6-methyl-1,4-oxathiyne 4,4-dioxide,
2-methyl-5,6-dihydro-4H-pyran-3-carboxanilide,
2-methylfuran-3-carboxanilide,
2,5-dimethylfuran-3-carboxanilide,
2,4,5-trimethylfuran-3-carboxanilide,
10 2,5-dimethyl-N-cyclohexylfuran-3-carboxamide,
N-cyclohexyl-N-methoxy-2,5-diethyl~uran-3-carboxamide,
2-methylbenzanilide,
2-iodobenzanilide,
N-formyl-N-morpholine-2,2,2-trichloroethylacetal,
15 piperazine-1,4-diylbis-(1-(2,2,2-trichloroethyl)-formamide),
1-(3,4-dichloroanilino)-1-formylamino-2,2,2-trichloroethane,
2,6-dimethyl-N-tridecyImorpholine and its salts,
2,6-dimethyl-N-cyclododecylmorpholine and its salts,
N-~3-(p-tert.-butylphenyl)-2-methylpropyl]-cis-2,6-dimethylmorpholine,
20 N-[3-(p-tert.-butylphenyl)-2-methylpropyl]-piperidine,
1-[2-(2,4-dichlorophenyl)-4-ethyl-1,3-dioxolan-2-ylethyl]-lH-1,2,4-
-triazole,
1-[2-(2,4-dichlorophenyl)-4-n-propyl-1,3-dioxolan-2-ylethyl]-lH-1,2,4-
-triazole,
25 N-(n-propyl)-N-(2,4,6-trichlorophenoxyethyl)-N'-imidazolyl-urea,
1-(4-chlorophenoxy)-3,3-dimethyl-1-(lH-1,2,4-triazol-1-yl)-butan-2-one,
1-(4-chlorophenoxy)-3,3-dimethyl-1-(1~1-1,2,4-triazol-1-yl)-butan-2-ol,
a-(2-chlorophenyl)-~-(4-chlorophenyl)-5-pyrimidinemethanol,
5-butyl-(2-dimethylamino-4-hydroxy-6-methylpyrimidine,
30 bis-(p-chlorophenyl)-3-pyridinemethanol,
1,2-bis~(3-ethoxycarbonyl-2-thioureido)-benzene,
1,2-bis-(3-methoxycarbonyl-2-thioureido)-benzene,
and various fungicides, such as
3~ dodecylguanidine acetate,
3-[3-(3,5-dimethyl-2-oxycyclohexyl)-2-hydroxyethyl]-glutaramide,
hexachlorobenzene,
DL-methyl-N-(2,6-dimethylphenyl)-N-fur-2-yl alanate,
methyl DL-N-(2,6-dimethylphenyl)-N-(2 -methoxyacetyl)-alanate,
40 N-(2,6-dimethylphenyl)-N-chloroacetyl-DL-2-aminobutyrolactone,
methyl DL-N-(2,6-dimethylphenyl)-N-(phenylacetyl)-alanate,
5-methyl-5-vinyl-3-(3,5-dichlorophenyl)-2,4-dioxo-1,3-oxazolidine,
3-[3,5-dichlorophenyl]-5-methyl-5-methoxymethyl-1,3-oxazolidine-2,4-dione,
3-(3,5-dichlorophenyl)-1-isopropylcarbamylhydantoin,
~ ~3 3 '~
29 O.Z. 0050/41358
N-(3,5-dichlorophenyl)-1,2-dimethylcyclcpropane-1,2-dicarboximide,
2--cyano-[N-(ethylaminocarbonyl)-2-methoximino]-acetamide,
l-[2-(2~4-dich!orophenyl)-pentyl]-lH-l~2~4-triazole~
2,4-difluoro--(lH-1,2,4-triazol-1-ylmethyl)-benzhydryl alcohol,
5 N-(3-chloro-2,6-dinitro-4 trifluoromethylphenyl)-5-trifluoromethyl-3-
chloro-2-aminopyridine, and
l-((bis-(4-fluorophenyl)-methylsilyl)-methyl)-lH-1,2,4-triazole.
use Example
lO Action on brown rust of wheat
Leaves of pot-grown wheat seedlings of the "Kanzler" variety were dusted
with spores of brown rust (Puccinia recondita). The pots were then placed
for 24 hours at 20 to 22C in a high-humidity (90 - 95%) chamber. During
15 this period the spores germinated and the germ tubes penetrated the leaf
tissue. The infected plants were then sprayed to runoff with aqueous
liquors containing (dry basis) 80~o of active ingredient and 20% of
emulsifier. After the sprayed-on layer had dried, the plants were set up
in the greenhouse at 20 to 22C and a relative humidity of 65 to 70%. The
20 extent of rust fungus spread on the leaves was assessed after 8 days.
The results show that active ingredient 2.15, applied as a 0.025wt% spray
liquor, has a good fungicidal action (100%).
3~