Note: Descriptions are shown in the official language in which they were submitted.
203~
Herbicidal comPound~ havinq a triazolopyridine qrou~
The present invention thus concerns novel
herbicidal compounds from the family of the 1,2,4-
triazolol4,3-a]pyridine~, a~ well as processe3 for
their preparation, the compo~itions which contain them,
and their u~e for combating weeds.
It is therefore an ob~ect of the pre~ent
invention to propose compounds which can ~e used as
herbicide~ both before emergence (in pre-emergence) and
after emergence (in po~t-emergence~.
It is another ob~ect of the present inventicn
to propose compound~ which can be used both against
weeds of the monocotyledon type and those of the
dicotyledon type.
It is another ob~ect of the present invention .
to propo~e compounds which can be u~ed before and~or
after emergence as selective herbicide~ in
monocotyledon crops (in particular wheat, maize and
rice) and dicotyledon crop~ (in particular ~oya, cotton
or ~unflower).
It has now been found that all, or some, of
the~e aims can be achie~ed by means of the novel
compounds according to the i~vention.
In the pra~ent exposition, the expre3~ion
lower, qualifying a radical, is under~tood as meaning
that this radical can have not more than 4 carbon
atom~.
The compounds according to the invention are
-
.
,
2035144
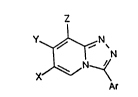
characterised by the fact that they have, a~ their
formula, the formula (I) which i8 given at the end of
. the description, in which:
: * X, Y and Z repre3ent a hydrogen atom or a
halogen atom or an alkyl or haloalkyl or alkoxy group,
at least one of th~ radical~ X, Y or Z having a méaning
other than the hydrogen atom,
* Ar repre~ents a phenyl group which is
; optionally mono- or polysubstituted (pref~rably
`~ 10 monosubstituted) by a lower alkyl group or lower alkoxy
group or lower alkylthio group or phenyl group or
phenoxy group or a halogen atom, preferably chlorine or
fluorine; or a heterocycle Het,
* Het represents a heterocysle having 5 or 6
15 ring Members and containing one or more hetero atom(s)
such as sulphur, nitrogen or oxygen, thi~ heterocycle
being optionally mono- or polysubstituted (preferably
monosubstituted) by a lower alkyl group or lower alkoxy
group or lower alkylthio group or a halogen atom,
: 20 preferably chlorine or fluorine.
:.- . - :
: .
~ . ". ' ' - ' ' ' ' '
2~ 2035~
with the exclusion of compounds wherein,
~ # simultaneously Y represents a methyl
group, X and Z represent a hydrogen atom, Ar represents a 3-
chloro phenyl or 4-chlorophenyl or 3-pyridyl or 3,4,5-
5 trimetho~y phenyl group; or
~ simultaneously X represents a chlorine
atom, Y and Z represent a hydrogen atom, Ar represents a 2-
pyndyl or 4-pyridyl group.
The invention ~ncludes also the agriculturally
10 acceptable salts, especially acid addition-salts; such acids
may be organic or inorganic, for examl)le the chlorhydric
sulphuric, acetic, arylsulphonic, and ~e like.
Preferred compounds among~t the compounds
accordin~ to the invention are those ha~ing one or the
other of the following characteristics:
Only one or two of the three radical~ X, Y or
Z represent a radi~al other than the hydrogen atom,
when ~, Y or Z are a halogen atom, the atom
in que~tion is chlorine or bromine, -:
~ 20 when X, Y or Z are a radical which is at
.,. . :
, .
- ~ ,
. . -. : . .. . ~ . -
2~3~4~
least partially hydrocarbon in nature, this radical ha
preferably 1 to 4 carbon atoms, 1 carbon atom being
even more ~referred.
X is other than a chlonne atom,
Z is other than a hydrogen atom, e.g. chlorine or ~ -
methyl, .
- X and Y are a hydrogen atom (and Z is other than a
hydrogen atom) .
when Ar represents a subs~tituted phenyl group, the
substituent(s) is(are) in ortho or para position.
The following Het groups can be mentlonned as
especially use~ul in the invention: thienyl (preferably 2- or 3-
thienyl), thiazolyl (preferably 2- or 4- thiazolyl), pyridyl
(preferably 2-pyridyl), pyITolyl (preferably 2-pyrrolyl),
thiadiazolyl (preferably 5-thiadiazolvl).
The following compounds can be mentioned as
particular compounds which can be produced according to
the invention (for the numbering of the atoms, see note
at the end of tho description):
a-ChlOro-3-(3~-methylthien-2~-yl)-s-triazolo[4~3-a]-
pyridine,
8-chloro-3-phenyl-s-triazolo[4~3-a]pyridine~
8-methyl-3-(3'-methylthien-2'-yl)-s-triazolot4,3-a]-
pyridine,
8-methyl-3-(thien-3~-yl)-~-triazolo[4~3-a]pyridine~
8-methyl-3-(pyrid-2~-yl)-s-triazolo[4~3-a]pyridine~
8-methyl-3-(4'-methylphenyl)-8 -triazolo~4,3-a]pyridine,
8-methyl-3-(4'-fluorophenyl)-s-triazolot4,3-a]pyridine,
8~methyl-3-(2~-chlorophenyl)-s-triazolo[4~3-a]pyridine~
6-methyl-3-phenyl-~-triazolo[4,3-a]pyridine,
... . . . . . .
:: . : , . . . . .
, . -,
,
.-
,: . - , . :-
,~ .
3a
2 ~) 3
8-trifluoromethyl-3-phenyl-s-triazolot4,3-a]pyridine,
6-chloro-3-phenyl-~-triazolo[4~3-a]pyridine~
8-methyl-3-~pyrid-3'-yl)-8 -triazolo[4,3-a]pyridine,
8-methyl-3-(pyrid-4'-yl)-s-triazolot4,3-a]p~ridine,
8-methyl-3-(furan-2'-yl)-~-triazolot4,3-a]pyridine,
8-methyl 3-(4'-methoxyphenyl)-s-triazolo[4,3-a]-
pyridine,
7-methyl-3-phenyl-~-triazolo~4,3-a]pyridine,
8-methyl-3-(3'-methylphenyl)-8 -triazolot4,3-a]pyridine,
8-methyl-3-(2',4'-dichlorophenyl)-~-triazolo[4,3-a]-
~. ., .:
~- ' ' ' ' ~ '~
.
4 203~
pyridine
8-methyl-3-(4~-chlorophenyl)-s-triazolo[4,3-a]pyridine,
8-methyl-3-(4'-phenylphenyl)-s-triazolot4,3-a]pyridine,
8-methoxy-3-phenyl- 8 -tri~zolo~4,3-a]pyridine,
8-me~hyl-3-~thien-2~-yl)-s-triazolo[4~3-a]pyridine~
8-methyl-3-(4'-thiomethylphenyl)-s-triazolo[4,3-a]-
pyridine,
8-methyl-3-(2'-fluorophenyl)-s-triazolo[4,3-a]pyridLne,
8-methyl-3-(2'-methylphenyl)-~-trlazolo[4,3-a]pyridine,
10 8-methyl-3-~2~,4~-dimethylphenyl~-~-triazolo[4,3-a]-
pyridine,
8-mQthyl-3-phenyl-s-triazolo[4~3-a]pyridin2,
8-promo-3-t3'-methylthien-2'-yl]-~-triazolot4,3-a]-
pyridine,
8-trifluoromethyl-3-t3~-methylthien-2~-yl]-s-triazolo-
: [4,3-a]pyridine,
8-methyl-3-t2~-methylthien-3~-yl]-s-triazolot4~3-a]
pyridine,
8-bromo-3-phenyl-s-triazolo~4,3-a]pyridine,
20 8-chloro-3-t2'-methylthien-3'-yl]-s-triazolot4,3-a]-
pyridine,
8-chloro-3-tthien-3'-yl]-s-triazolot4,3-a]pyridine,
8-methyl-3-tl'-methylpyrrol-2'-yl]-s-triazolot4,3-a]-
pyridine,
25 8-~hloro-3-t3',5~-dimethylthien-2'-yl]-~-triazolo-
[4,3a]pyridine,
7,8-dimethyl-3-phenyl-~-triazolot4,3-a]pyridine~
8-methyl-3-[3',5'-dimethylthien-2'-yl]-s-triazolo-
' ' . ' ' '~ ' .' ' - ~ , ;. :
;' '~ ' ~ . ' , ''
" .
~ . ...
` 5 203~14'~
[4l3-a]pyridine~
8-ethyl-3-l3~-methylthien-2~-yl]-s-triazolo[4~3-a]
pyridine,
8-ethyl-3-phenyl-s-trLazolo r 4,3-a]pyridLne,
8-ethyl-3-tl'-methylpyrrol-2'-yl]-s-triazolo[4,3-a~-
pyridine,
8-ethyl-3-[thien-3~-yl]-s-triazolo[4,3-a]pyridine,
8-trifluoromethyl-3-[1~-methylpyrrol-2~-yl]-s-triazolo-
[4,3-a]pyridine,
8-trifluoromethyl-3-[pyrid-2'-yl]-s-triazolo[4,3-a]-
pyridine,
8-chloro-3-[4~-methylthien-2~-yl~-s-triazolo[4~3-a~-
pyridine,
7,8-dimethyl-3-t3'-methylthien-2'-yl]-~-triazolo-
[4,3-a~pyridine,
8-methyl-3-t4~-methylthien-2~-yl]-s-triazolot4~3-a]
pyridine,
8-trifluoromethyl-3-tthien-3~-yl]-y-triazolo[4~3-a]
pyridine,
8-msthyl-3-[4~-isopropylphenyl]-s-triazolot4~3-a]-
pyridine,
8-methyl-3-[4~-bromothien-2~-yl~-s-triazolot4~3-a]
pyridin~,
8-chloro-3-t4'-bromothien-2'-yl]-s-triazolo[4,3-a~-
pyridine.
Th~ compounds according to the invention can
be prepared by moans of various processes.
According to a first process for the
.. . ~, ', ~
: - ,.
203~
preparation of compounds of the formula (I), a compound
of the arylidene-2-~pyrid-2'-yl)hydrazine type which
has as its formula the formula (II), in which the
various substituents have the meanings given above, i8
reacted with an oxidant according to a cycl~ing
oxidation reaction. Substance~ which may be mentioned
as oxidants are metal cations derived from metal~ which
have various degrees of oxidation and which are in the
statQ of a higher degree of oxidation, such a~, for
example, lead tetraacetate or ferric chloride;
atmospheric oxygen can al80 be u~ed as an oxidant. The
reaction i~ advantageou~ly carried out in a liquid
organic ~olvent madium, the solvent preferably being
~elected in ~uch a way a~ ~o dissolva tha reactants and
the final products as much a~ pos~ible. Suitable
solvents are hydrocarbons, halogenated hydrocarbons,
acids and alcohol~; solvents of the nitrated aromatic
hydrocarbon type can 2180 be used, specifically in
tho~e case~ where the oxidant is atmospheric oxygen.
The molar ratio of the amount of oxidant used in
relation to the compound of the formula (II) is
generAlly between 1 and 5.
According to a first variant, the oxidation
reaction for the compound of the formula (II) can also
be effected by adding a halogen (preferably in
approximately ~toichiometric amount), such a~ bromine,
followed by a dehalogenation reaction. These oxidation
reactions (according to the general method or according
.... .
7 2 0 3 v~
to the variant) are generally effected between 10 and
210C (preferably between 10 and 50C in the case of
the variant).
Cyclisinq oxidation is achieved by following
this oxidation by means of halogenation by a
dehalogenation reaction which is generally effected in
the pre~ence of an alkaline agent, for example in the
presence of an alkali metal salt of a carboxylic acid
such as sodium scetate, in an acetic acid medium. The
molar ratio of the amount of alkaline agent used in
relation to the halogenated compound of the formula
(II) i~ generally between 1 and 5.
According to a second variant, the oxidation
reaction for the compound of the formula (II) can al~o
be effected by adding an N-halo-N-metallo~ulphonamidate
of the formula (IV~:
RSO2~X M where ~ represent~ an alkyl group, or
preferably phenyl which i~ optionally monosubstituted
in the para position by an alkyl group (for example a
p-tolyl group), X represent~ a halogen atom (preferably
chlorine or bromine) and M a alkali metal atom
(preferably ~odium). A preferred example of an N-halo- :
N-metallo~ulphonamidate i3 chloramine T, of the formula
(IVa):
CN~ ~ S0~ N¢l Na-
; ' - .. ~;' . ' ' ~
.
.
.
.
8 203~44
The u-~e of an N-halo-N-metallo~ulphonamidate
of the preceding formula a~ an oxidant which cycli~e~
a~ylidene-2-(pyrid-2'-yl)hydrazines, which have a~
their formula the general formula (II~ original and
S thu~ con~t~tutes a novel preparation proce~ for
compound~ of the formula (I).
This cycli~ing oxidation reaction i8
generally effected between 10 and lSO-C (preferably
betwaen 20 and 50-C) and is Ædvantageou~ly effected in
a liquid organic ~olvent medium, preferably alcohol.
~ he molar ratio of the amount of oxidant used
in relation to the compound of the formula (II) i8
preferably approximately stoichiometric.
According to a second preparation method for
compounds of the formula (I), a compound of the
2-(aioylhydrazine)pyridine type, which has, as its
formula, the formula (I~I) in which the variou~
substituents have the ~ame meaning a~ in formula (I),
is dehydrated (cyclising dehydration).
The cyclising dehydration reaction of the
compound of the formula (III) is usually carried out by
heating at between 100 and 250-C, with el~mination of
the water formed as the reaction proceeds. ~he water
formed can bs el~minated by simple distillation or,
alternatively, by azeotropic distillation, if the
heating i8 effected in the presence of an aromatic
solvent which i8 capable of dissolving the compound of
the formula (III) and forming an azeotrope with water.
. . .
- .. . .. .
.~': ~ . . ' ,
- .
. .
9 203~144
Azeotropic solvent~ which are suitable for this
dehydration method and which may be mentioned are the
halogenated or unhalogenated aromatic hydrocarbon , a~
well as the phenols, for example xylene, phenol and
1,2,4-trichlorobenzene.
According to a first ~ariant of the cyclising
dehydration of the compound of the formula (III), the
proces~ is carried out in the pre~ence of a dehydrating
agent, preferably in the presence of an inert solvent,
for example an aromatic hydrocarbon such a~ benzene,
toluene and xylene. The dehydrating agent i8
advantageously a water scavsnger known per 8e, such as
POCl3 or concentrated acetic acid or polypho~phoric
acid, the amount of dehydrating agent used being
advantageously between 1 and 50 times the molar amount
of the compou~d of the formula (III).
According to a second variant, the
dehydrating cyclisation of the compounds of the formula
(III) i~ effected via a transitory pas~age through an
intermediate of the formula ~XI) or ~XII), in which the
~ubstituents have the ~ame meaningQ as in formula (I)~
The intermediates of the formula ~XI) are
formed by reac~ion of SOCl2 with a compound of the
formula (III) tin an amount of, for exampla, between 1
and 3 moles per mole of compound of the formula (III)],
at a temperature between -5-C and 50C, preferably at
ambient temperature; the reaction is advantageously
effected in the pre~ence of a solvent and/or an acid
.7 ,, ,, ,', '' ' ' ' . " ' ', ,. '' ' ' , ., ' ' " " '' ' ' ' ~ ' ' ' ~
''''' ' ' ' ' " . . . .
' ~ ' ~ . ' ' ' ' ' ' ~.
~ ' ' .
.~. ' ' .
' '
. .
lo 2 0 3 ~; 1 4 4
scavenger, in particular pyridine or dimethylformamide
(DMF) in the pre~ence of tri~thylamine.
Thermoly~is of the compounds of the formula
(XI) to give compounds of the formula (I) i8
accompanied by a liberation of SO2. It i8 preferred for
thi~ thermoly~is to be carried out in the pre~e~ce of a
solvent such a~ nitrile~ or aromatic hydrocarbons, in
particular acetonitrile, toluene, xylenes or
naphthalenes, which are optionally alkylated
tPreferably methylated).
The cyclising dehydration of compounds of the
formula (III) to give compounds of the formula ~I), via
intermediates of the formula (XII), is advantaqeously
effected by reacting compound~ of the
dichlorotriarylphosphorane type with a compound of the
formula (III), in the pre~ence of a tertiary base ~uch
as triethylamine, and of a ~olvent of the nitrile type.
According to a third preparation method for
compoundR of the formula (I3, a cyclising deamination
is carried out by heating a compound of the amidrazone
type, which hss, a~ it~ formula, (IV) in which the
~arious sub~tituent~ have the same meaning as in
formula (I).
The reaction is advantageou~ly effected in
the presenca of agents (liquid~) which ~cavenge a onia
moleGules, for example in the pre~ence of acids,
anhydrides or halides of acids, these acid~ preferably
being carboxylic acids. The temperature is generally
.
11 203~
between 25 and 220C, preferably between S0 and 180C.
The amount of agent which scaven~es ammonia molecule4
i~ generally between 1 and 30 times the amount (in
moles) of the product of the formula (IV).
The deamination can al~o be carried out by
simple heating in a liquid medium (thermal
deamination), preferably in an inert organic ~olvent
medium, for example a halogenated or unhalogenated
aromatic hydrocarbon.
According to a fourth preparation proce~ for
products of the formula (I), a compound of the
hydrazine type of the formula (V) i8 reacted with a
compound of the formula ~VI): U~-C(=Wl)-Ar according to
the reaction:
~!1
(V) + (VI) _ (I) + UlH + NlH2
the various radicals of the~e formulae (V)
and (VI) having the same meanings a~ in the formula (I)
and, in addition:
W1 represents an oxygen atom or an NH group,
when Wl represents the oxyqen atom, then Ul
represents a hydroxyl group [(VI) is thu~ an acid] or
alkoxy group t(VI) iB thus an ester] or aroyloxy group
t(VI) is thu~ an acid anhydride], or a halogen atom
t(VI) i8 thu~ an acid halide], preferably chlorine,
when Wl represents an NH group, then U
represents an alkoxy group or alkylthio group or
: . . . ~. .: .
- . :
-
~5 ' .;
12 ~03~14~
arylalkylthio group.
The preceding reaction i~ effected at a
temperature of generally between 20 and 200C,
preferably between 50 and 180-C, over a period of 1 to
24 hours. The reactants (V) and (VI) are used in
respective molar proportions between 0.8 and 1.2,
preferably equal to, or near, 1. The reaction can be
effected in the presence or absence of a solvent.
Solvents which can be used, as shown hereinafter in
oertain cases, are qolvents which lead to the formation
of azeotropic mixtures. Solvents of the alcohol type
can also be u~ed when ~he compound of the formula (VI)
i8 an imidate, or an aromatic solvent such as a
halogenated or unhalogenated hy~rocarbon, or pyridine,
when the compound of the formula (VI) is a thioimidate
or an acid halide.
~ he light reaction products (U1H and WlH2) are
advantageously eliminated a~ the reaction proceeds,
generally by distillation when the product in question
20 i8 water or alcohols (U1 thus represent~ a hydroxyl or
alkoxy group), and al~o by scavenging wi~h the aid of a
tertiary base such a~ triethylamine, when Ul is a
halogen ato~, and al80 by scavenging of the ammonia
molecule, when Wl i~ NH and Ul is alkoxy (the product of
the formula (VI~ is thu an imidate).
The abovementioned di~tillation of the light
products can be an azeotropic distillation in the
presence of aromatic halogenated or unhalogenated
, :~::
" 13 203514~
solvents such a~ pyridine, or the chlorobenzenes, in
particular 1,2,4-trichlorobenzene.
The abovementioned ~cavenging of ammonia iR
advantageously effected in the presence of acid~
(preferably carboxylic acids) or of their derivatives
such as acid anhydrides or acid halides. These acids or
their derivatives are used in a molar amount of
generally between 1 and 30 times tha amount of product
of the formula (VI) used. These proportions are in the
same order of magnitude (mutatis mutandis) when a base
i8 used when Ul is a halogen atom.
According to a fifth preparation process for
compounds of the formula (I~, a pyridine derivative of
the formula (VIII) is reacted with a tetrazole compound
of the formula (IX), in which formulae X, Y, Z and Ar
have the same meaning as in formula (I) and T
represents a halogen atom, preferably chlorine. The
reaction i8 advantageously effected between 20 and
l50-C, prsferably in an organic 801vent~ for ex~mple a
halogenated or unhalogenated aromatic hydrocarbon such
as xylenes, chlorobenzenes or tetralin, or a soivent` of
the heterocyclic type such as pyridine.
The invention also relates to novel products
which can opt~onelly be u3ed as intermediate~ in the
processes according to the invention, characterised in
that they have, as their formula, one of th~ formulae
(II~ ,- (IV), (VIII), (XI) or (XII) in
which the 8ymb01 X~ ~ Z and ~r have the meanings
.. . . . . .
: ..... : -
i . ... -
~ ' ~: ,:- , .,
14 203~
given in the cs~e of the formula (I).
Another ob~ect of the invention is the use of
the compound~ of the formu~a (II) as herbiclde~. In
what follows, but al~o in the preceding text, the
reaction~ which are effected under hot condition~ in a
solvent, unless specifically indicated to the contrary,
are advantageoucly carried out at the boiling point of
the solvent in question.
The preparation of the tetrazoles of the
formula (IX) is conveniently effected by reacting an
aromatic nitrile of the formula Ar-CN with an alXali
metal azide, for example ~odium azide or ammonium
azide, at temperature~ between 80 and 130C. Ths molar
amount of azide is advantageou~ly between 1 and 3 times
(preferably 1.5 and 2 time~) the amount of nitrile.
This reaction is generally effected in a polar solvent
such as ~MF or acetic acid or the alcohol~ or their
mixtures. Such a reaction i3 described in Advances in
Heterocyclic Chemistry, ~ol. 21, page~ 323-435,
published in 1977 by Academic Pre~ in an articla by
R.N. Butler with the title: Recent advances in
tetrazole chemi~try.
The preparation of the arylidene-2-(pyrid-2'-
yl)hydrazine~ of the formula (II) is conveniently
effected by reacting 2-hydrazinopyridines of the
formula (V) with aldehyde~ of the formula Ar-CHO in
which the symbol8 X, Y, Z and Ar have the meanings
given in the case of the formula (I). The reaction is
,~:'- ' .. ,'
.: :
. .
. -
:
203~1~4
generally effected at a temperature between 50 and
l50~C, preferably in a ~olvent. A lower alcohol such as
methanol or ethanol i~ advantagsously used as the
solvent. The reaction is promoted by the prei~ence of
catalytic amounts of a mineral inorganic or organic
acid, for example hydrochloric acid, sulphuric acid,
acetic acid, trichloroacetic acid or perchloric acid.
Such a reaction i~ described in Quarterly review,
Chemical Society, vol. 23, page~ 37-56, 1969, in an
article by J. Buckingham a~ wall a~ in Houben-Weyl,
Methoden der organischen Chemie, 4th edition, 1967,
vol. X-~, pages 410-487.
The preparation of the 2-(aroylhydrazino)-
p~ridine~ of the formula (III) i~ conveni~ntly effected
by reacting 2-hydrazinopyridines of the formula (V)
with compounds of the formula Ar-CO-U2 in which the
~ymbols X, Y, Z and Ar have the meanings given in the
case of the formula (I), and U2 has on~ of the meaning~
given above in the case of Ul. The reaction proceeds in
accordance with the equation:
(V~ + Ar-CO-U2 ~ --~(III) + U2H
The reaction i~ generally effected by mixing
reactants at a temperature between 0 and 180C, in the
pres2nce or absence of a ~olvent. A polar ~olvent i~
advantageously used ai~ solvent.
The following can therefore be cited a~
~- - .. . . ,
,
.
.
.
'~
16 203~
~olvents which can more particularly be u~ed: alcohols
when Ar-CO-U2 is not an acid halide; ethers or aliphatic
chlorinated or unchlorinated hydrocarbon~ such as
methylene chloride, chloroform; ~ol~ents which have
acid-scavenging properties, 3uch as pyr~dine, can
likewise be used wh~n Ar-CO-U2 repreqent~ an acid
halide.
The proportion of the two reactants [that of
the formula (V) and that of the formula Ar-CO-U2] can be
varied within wide limits around ~toichiometric. When
Ar-CO-U2 is an acid ~U2 i~ OH), an excess of the latter
derivative i~ u~ed ~n relation to the compound of the
formula (Y), for example from 2 to 8 moles per mole of
(v). When Ar-CO-U2 i8 an ester (U2 is alkoxy), an
approximately sto~chfometric amount of the latter
derivative i8 u~ed in relation to the compound of the
formula (V), for example from 0.8 to 1.1 moles per mole
of (V). When Ar-CO-U2 is an acid halide (U2 is a halogen
atom), an exces~ of the compound of the formula (V) i8
used in relation to the compound of the formula
Ar-CO-~2, for example from 1 to 5 mole~ per mole of
Ar-CO-U2 .
Proces~es which allow tha compounds of the
formula (III) to be obtained in accordance with what
has ~ust been described are described in the work by
Pata~, The chemi~try of carboxylic acids and ester~,
~ol. 5, chapter 9, pages 425-428, publi~hed in 1969 by
Interscience/Wiley in an article by Satchell, and also
.
- . ' ' .: ~ '
,~
` ~" 17 203S14~
in Houben-Weyl, Methoden der organischen Chemie, 1952,
Vol. VIII, chapter 5, pages 676-680.
The 2-(aroylhydrazino)pyridine~ of the
formula (III) can also be prepared conveniently by
reacting a pyridine derivative of the formula (VIII)
with an aryl hydrazide of the formula Ar-C0-NH-NH2 in
which the symbols T, X, Y, Z and Ar have the meanings
already given in the case of the formulae (I) and
(VIII). The reaction proceed~ in accordance with the
e~uation:
(VIII) I Ar-CO-NH-NH2 > (III
,.
This reaction i~ advantageou~ly carried out
at a temperature betwoen 50 and 150C, in solvents such
a~ alcohols or aromatic solvent~ t for e~ample pyridine
or hydrocarbon~ ~uch aY toluen~. The reactants ar~
advantageously in an approximately stoichiometric
proportion, for example in a molar ratio ranging from
0.8 to 1.2. The reaction can ~e promoted by the
presence o~ a likewi~s approximately stoi~hiometric
amount of a basic agent uch aY an alcoholat~ or an
alkali metal bicarbonate.
The aryl hydrazide~ of the formula
Ar-C0-NH-NH2 can be obtained by reacting hydrazine
hydrate with an acid or one of it~ derivative~ such as
the e3ters, halide~ or anhydride~. The reaction is
advantageou~ly effected at a temperature between 0 and
,, . : ' '''' .; '
, - ,
. . - , .
18 203S~L4
150~C, preferably in the presence of a ~olvent such as
a lower alcohol, the molar proportion of hydrazLne
hydrate in relation to the other reactant generally
being between 1.01 and 1.5.
Processe~ which allow the aryl hydraz~de~ to
be obtained in accordance with what has ~U8t been
described are described in the work by Pata~, Th~
chemi~try of amides, vol. 11, chapter 10, pages 515-
600, published in 1970 by Interscience/Wiley, in an
article by Paulsen and Stoye, and also in Organic
Reactions, the Curtius reaction, 1962, Vol. III,
chapter 9, pages 366-369 publi~hed by Wiley.
The amidrazone~ of the formula (IV) can be
prepared uYing 2-hydrazinopyridin~s of the formula (V)
according to the following reaction equations
(V) + Ar-C(=NH)-U3 ~ ~ (IV) + U3H~
the various radicals of these reactants and reaction
products having the ~ame meaning as in the preceding
formulae, and U3 represents a preferably lower alkoxy or
alkylthio radical.
~ he reaction is advan~ageously effected a~ a
temperature between O and 30~C, preferably in an
alcohol-type solvent.
Proce~ses which allow tho amidrazones of the
formula (IV) to be obtainsd in accordance with what ha~
~ust been described are described in the work by Pata~,
~' : , . . . .
- : :
-- , . .
.
. . :
.
.~ .. . .
19 203~
The chemi~try of amidines and Lmidates, vol. 20,
chapter 10, pages 491-545, published in 1975 by
Interscience/Wiley in an article by Watson, a~ well as
in an article by Neilson et al., Chemical Review, 1970,
Vol. 70, pages 151-170.
The iminoethers of the formula Ar-C(=NH)-U3 in
which U3 represents an alkoxy group can be prepared by
reacting an aromatic nitrile Ar-CN with a lower
alcohol, preferably an alkanol, between -20 and +30C,
10 advantageously in an ether-type solvent, for example
ethyl ethsr or 1,2-dimethoxyethane or dioxane, or a
halogenated, preferably aliphatic, hydrocarbon such as
chloroform. More precisely, the reaction i8 effected in
an anhydrous medium in the presence of (gaseous) HCl.
15 Processes which allow these iminoethers of the formula n
Ar-C(=NH)-U3 to be obtained are de~cribed in the work by
Pata~, The chemistry of amidines and imidates, vol. 20,
chapter 9~ page~ 385-489, published in 1975 by
Interscience/Wiley in an article by Neilson, as well as
: 20 in an article by Roger and ~eilson, Chemical Review,
; 1961, vol. 61, pages 179-211.
~he thioimidates of the formula Ar-Ct=NH)-U3
in which U3 represents an alkylthio group can be
prepared by alkylation of arylthiobenzamides by a
25 proc~s such a~ de3cribed by Doyle et al. in Synthesis,
583 (1974). These arylth$obenzamides ~hem~elves can be
obtained for example a~ described in the work by Patai,
The chemistry of amides, vol. 11, chapter 8, page~
.~ .. . . . .
.
- - ~ ~ .- .. , -
. : ~
2r~3s~.~L~ .,
383-475, published in 1970 by Interscience/Wiley, in an
article by Walta and Vo~.
The 2-hydrazinopyridine~ of the formula (V)
can be obtained by reacting hydrazine hydrate with a 2-
halopyridine of the formula (VIII) at a temperaturebetween 0 and 120C, in the presence or absence of a
solvent. Solvent~ which may be cited are, in
particular, polar ~olvents such as alcohols, pyridine,
or dimethyl sulphoxide. The molar amount of hydrazine
hydrate is generally between 3 and 15 times the amount
of product of the formula (VIII~ used. Processes which
allow the compounds of the formula (V) to be obtained
in accordance with what has ~u~t been described are
described by Enders in Houben-Weyl, Methoden der
organischen Chemie, 4th edition, 1967, vol. X-2,
chapter 5, pages 252-287.
In particular, the 2-halopyridine~ of the
formula (VIII) can be prepared by a proces~ similar to
those described by ~linsberg in The Chemistry of
Heterocyclic Compounds, Pyridine and its derivative~,
part~ I - I~, publi~hed in 1974-1975 by
Interscience/Wiley, and by Abramovitch, in The
chemistry of ~eterocyclic compounds, Pyridine and its
derivatives, supplQments 1 - 5, published in 1960-1964
by Interscience/Wiley.
The following examples, which are given
without implied lLmitation, illustrate the invention
and show how it can be implemented. In these example~,
.~' ' .
2, 203~144
the symbol Ac designates the radical CH3-CO-, the ~ymbol
DMF designates dimethylformamide. The word amidrazone
de3i~nates compounds which have a group
-NH-N= C
NH2
Examples 1 to 14 illustrate the preparation
of herbic~dal compound~ according to the invan~ion.
Examples I-l to I-10 illustrate the preparation of
intermediates of the preceding products.
*Example 1: -
Lead tetraacetate (6.7 g; 0.015 mol) are
added to phenyl(3'-chloropyrid-2'-yl)hydr~zon~ (3.5 g;
0.015 mol), prepared a~ in Example I-l, in qlacial
acetic acid (300 ml) at ambient temp~ratur~ and with
stirring. Stirring i~ continued for on~ hour at 60-C.
The medium iB concentrated to dryne~s under reduced
presaure. The residua is triturated with water
(100 ml), and the mix~ure is filtered, washed with
water and dried. 8-Chloro-3-phenyl-s-triazolot4,3-
~pyridine ~3.1 g; 0.0135 mol), wh$ch melts at 153-C,
iB obtained (yield 90%).
*~xample 2:
A mixture of 3-formylthiophene (2.8 q;
0.025 mol) and 2-hydrazino-3-methylpyridine (3.1 g;
0.025 mol), prepared a~ in Example I-8, in ethanol
- : -: . , . ~
22 203~1~4
(30 ml) containing a few drop~ of concentrated HCl, is
kept at boiling point for two hours. An ethanolic
solution of FeCl3 . 6HzO (70 ml; 0.125 mol) is added
gradually, and heating at boiling point is con~i~ued
for two hours. The reaction medium i~ concentrated to
dryness ur.der reduced pre~sure, the residue is taken up
in water (100 ml), the mixture i~ rendered neutral with
the aid of gaseou~ NH3 (pH between 8 and 9), and
extracted with CH2C12. After the organic phass~ have
been concentrated and the residue ha~ been dried and
recrystallised u~ing aqueous ethanol, 8-methyl-3-
(thien-3'-yl)-~-triazolo[4,3-a]pyridine (6.05 g;
0.02180 mol), which melt~ at 165C, i~ obtained (yield
87%).
*Example 3s
Bromine (1.25 ml) in glacial acetic acid
(5 ml) i8 added at ambient temperature to a suspen~ion
of anhydrou~ sodium acetat2 (6.15 q; 0.075 mol) in
: glacial acetic ac$d (50 ml) containing phenyl (3'-
20 trifluoromethylpyrid-2'-yl)hydrazone (6.65 g; 0.025
mol) prepared a3 in ~xample I-2. The reaction medium i~
stirred for one hour and poured into an aqueou~
solution of 2N NaOH (300 ml). The precipitate is
filtered, wa~hsd with water and dried. 3-Phenyl-8-
25 trifluoromethyl-~-triazolot4,3-a]pyridine (4.75 g;
0.018 mol)~ which melts at 197C, is obtained (yield
72%).
,, ' . ~
23 2 o 3 5 1 4 ,~
~ExamPle 4s
2-Formyl-3-methylthiophene (3.15 g;
0.025 mol)~ dissolved in ethanol (20 ml), is added at
ambient temperature to a stirred solution of 3-chloro-
2-hydrazinopyridine (3.6 g; 0.0~5 mol), prep~red a~ in
Example I-7, in ethanol (30 ml) which has been
acidified with a little HCl. The mixture i~ heated at
boiling point for 2 hour~ and concentrated to dryne~s.
3-Methylthien-2-yl(3'-chloropyrid-2'-yl)hydrazone i8
obtained. This substance i 8 dis301ved in nitrobenzene
(50 ml) and oxidi~sd in air by heating at boiling point
for 4 hours. The solution i8 concentrated under reduced
preQsure. The solid, which ha~ been collected by
cooling, i3 solubilised in N HCl (20 ml), and then
reprecipitated by neutrali~ation with an ammoniacal
solution. 3-(3~-Methylthien-2'-yl)-8-chloro-3-
triazolot4,3-a]pyridine (4.45 g; 0.017B mol), which
melts at 135DC, is obtained (yield 71%).
*Exam~le 5:
A mixture of 3-methyl-2-(3'-methylthien-2'-
oylhydrazino)pyridine (1.25 g; 0.005 mol), prepared as
in ~ample I-5, and POC13 (7.65 g) in toluene (25 ml) i~
refluxed for two hours. The reaction medium i8
concentrated, the concentrate i~ taken up in iced water
(50 ml), the mixture i~ rendered alkaline (pH betw~en 8
and 9) with thQ aid of an aquQous KHC03 solution, and
then extracted with CH2C12. The organic solution is
dried o~er NgS0~ and then concentrated. The residue i8
:: ' ,~ ' , . ' ' . ' ': " ' ,~- .
:: . .
~ ~ 3 ~
24
washed with isopropanol and then dried. 8-Methyl-3-(3'-
methylthien-2'-yl)-s-triazolo[4,3-a]pyridino (0.8 g;
0.0035 mol~, which melts at 94C, iR obtained (yield
70%).
*Example_6:
SOCl2 (4.2 g; 0.0425 mol) i8 added dropwise at
0C to a solution of 2-(2'-chlorobenzoylhydrazino)-3-
methylpyridine (13.1 ~; 0.05 mol), prepared a~ in
Example I-3, in anhydrous pyridine (50 ml). The
reaction mixture i~ stirred for one hour at 0UC and
thén fil$ered. The in~oluble portion is heated at
boiling point for 3 hours in acetonitrile (50 ml~. The
301ution i9 concentrated and the re~idue i~
recryQtallised. 8-Methyl-3-(2'-chlorophenyl)-8-
triazolo[4,3-a]pyridine (5.75 g; 0.0235 mol), which
melts at 72C, i~ obtained (yield 47%).
*Example 7:
2-(2',4'-DLmethylbenzoylhydrazino)-3-
methylpyridine (3.85 g; 0.015 mol), prepared as in
Example I-4, is dissolved at ambient temperature in
30 ml of glacial acetic acid.
The reaction mixture i8 kept at boiling point
for 12 hours and then evaporated to dryne~s. The
residua i~ washed with water and then with pentane and
recrystallised from absolut~ ethanol. 8-Nethyl-3-
(2',4' dLmethylphenyl)-s-triazolo[4,3-a]pyridine (1.65
g; 0.007 mol), which melts at 106C, is obtained (yield
47%).
' ~ ' - . . , :
- ~
;
25 203~4
~Example 8:
Nl-(5~-methylpyrid-2~-yl)phenylamidrazone
(6.8 ~; 0.03 mol), prepared as in Example I-6, i~
dissolved in formic acid (25 ml). The batch i~ refluxed
5 for one hour, cooled and poured into water (200 ml).
The precipitate is recovered by filtration, washed with
water and dried. 3-Phenyl-6-methyl-~-triazolo~4,3-a]-
pyridine (4.7 g; 0.0225 mol), which melts at 160CC, is
obtained (yield 75%).
10 *Exam~le 9:
A mixture of 2-hydrazino-3-methylpyridine
(3.1 g; 0.025 mol), prepared as in Example I-8, and 4-
fluorobenzoic acid (3.5 g; 0.025 mol) i~ heated at
180C for 3 hour~ and then cooled and mixed with
15 boiling chloroform (80 ml). The solution is filtered t
and the filtrate is concentrated to dryne~. The
residue i~ tr~ated with an aqueous ~olution of Na2CO3,
filtered, washed with wat~r, dried and recrystallised
from methanol. 3-(4'-Fluorophenyl)-8-methyl-~-triazolo-
t4,3-a]pyridine (2.3 g; 0.01 mol), which melt~ at
166-C, i~ obtained (yield 40%).
*~xample 10: `
A mixture of 2-hydrazino-3-methylpyTidine
(3.1 g; 0.025 mol), prepared as in Example I-8, and -~
picolinic acid (3.1 g; 0.025 mol~ is heated for 4 hours
at 180-C, cooled, diluted with the aid of aqueouY
ethanol (100 ml) and rendered alkaline (pH
approximately 8-9) with a solution of dilute NaOH. The
.
' .
~ , .
26 203~
precipitate i~ recovered by filtration, wa~hed with
water, dried and recry~tallised uslng aqueous ethanol.
8-Methyl-3-(pyrid-2'-yl)-~-triazolo[4,3-a]pyridine
(1.0 g; 0.00475 mol), which melt~ at 141C, is obtained
(yield 19%).
*Exam~ple 11:
Example 9 i~ repeated but 4-fluorobenzoic
acid i~ r~placed by 2-fluorobenzoic acid. 3-(2'-
Fluorophenyl)-8-methyl-s-triazolot4,3-a]pyridine
(4.1 g; 0.018 mol), which melt~ at 130C, is obtained
(yield 72~).
*Example 12s
Triethylamine (5.05 g; 0.05 mol) and 2-
hydrazino-3-methylpyridine (3.1 g; 0.025 mol), prepared
as in Exa~ple I-8, ara added ~ucc2ssively at ambient
temperature to a su~p~nsion of ethyl 4-methylbenzimidate
chlorohydrate (5 g; 0.025 mol) in ethanol (50 ml). The
reaction medium i~ kept at boiling point for one hour
and then concentrated, cooled and poured into cold
water (250 ml). The mixture i8 extracted with the aid
of HCCl3. The organic solution i~ dried and concentrated
to dryness. 3-(4'-Ethylphenyl)-8-methyl-s-triazolo[4,3-
a]pyridine (3 g; 0.0135 mol), which melts at 145C, i8
obtained (yield 54%).
*Example 13:
Example 2 i~ repeated but the 3-formylthiophene
~2.8 g) is replaced by 4-phenylbenzaldehyde (4.6 g;
0.025 mol). 8-Methyl-3-(4'-phenylphenyl)-s-triazolo-
, .
- .;
. ' '-
'' ' '
203~
27
[4,3-a]pyridine (2.3 g; 0.008 mol), which melts at
156-C, is obtained (yield 32%).
*Example 14:
Example 12 i8 repeated, replacing the ethyl
4-methylbenzimidate chlorohydrate by methyl benzimidate
chlorohydrate. 3-Phenyl-8-methyl-s-triazolo[4,3-a]-
pyridine (3.5 g; 0.0168 mol), which melts at 118C, is
obtained (yield 67%).
*Example 15:
Example 4 i8 repeated but replacings
1) the 2-formyl-3-methylthiophene (3.15 g) by 4-
methylthiobenzaldehyde (4.00 g; 0.025 mol), and
2) 3-chloro-2-hydrazinopyridine (3.6 g) by 2-hydrazino-
3-methylpyridine (3.1 g; 0.0~5 mol ) . 4-Methylthiophenyl
(3'-methylpyrid-2'-yl)hydrazone ~8 obtained and
dissolved in nitrobenzene (50 ml), and then treated as
in Example 4. 3'-(4'-Methylth~ophenyl)-8-methyl ~-
triazolot4,3-a]pyridine (4.60 g; 0.018 mol), which
melts at 137C, i~ obtzined (yield~ 72%).
*Ex~mpl~ 16~
2-Foxmylthiazole (2.0 g; 0.018 mol) di~301ved
; in ethanol (20 ml) is added with stirring at ambient
temperature to a solution of 3-chloro~2-
hydrazinopyridine (2.4 g; 0.018 mol), prepared as in
~xamplo I-~, in ethanol (30 ml) which contdins p-
toluenesulphonic acid (0.2 g). The mixture iB heated at
boiling point for 2 hours and evaporated to drynes~.
Thiazol-2-yl(3'-chloropyrld-2'-yl)hydrazone is
....... .
. : ~
- :. .
- - ' '
:'
.
203~
28
obtained. This substance is dixsolved in ethanol
(50 ml). Chloramine T (5.1 g; 0.018 mol) i~ added
rapidly at ambient temperature to the preceding
ethanolic solution. The reaction medium i8 stirred for
30 minute~. After the ~olvent ha~ been evaporated, the
re~idue i~ chromatographed (eluent heptane/ethyl
acetste 40J60). 3-(Thiazol-2~-yl)-8-chloro-s-
triaz~lo[4,3-a]pyridine (1.50 g; 0.0063 mol), which
melts at 218C, i3 obtained (yield 36~).
~Example 17:
Example 16 i8 repeated but the 3-chloro-2-
hydrazinopyridine i~ replaced by 2-hydrazino-3-
methylpyridine (prepared a~ in Example I-8).
3-(Thiazol-2'-yl)-8-methyl-s-triazolo~4,3-a]pyridine
(1.26 g; 0.0058 mol), which melts at 140C, i8 obtained
(yield 32%).
*Example 18:
Example 17 i3 repeated but the 2-
formylthiaZOlQ i8 replaced by 4-phenoxybenzaldehyde.
3-(4'-Phenoxyphenyl)-8-methyl-~-triazolo[4,3-a]pyridine
(4.7 g; 0.0156 mol), which melts at 132~C, i~ obtained
~yield 87%).
*Example 19:
Example 16 i8 repeated but the 2-
formylthiazole i~ replaced by 4-phenoxybenzaldehyde. 3-
(4'-Phenoxyphenyl)-8-chloro-s-triazolo[4,3-a]pyridine
(5.0 g; 0.0155 mol), which melts at 145-C, is obtained
(yield 86%).
. . ~ ,. ................................... .
- '
` 203~144
29
~Example 20:
Example 5 i~ repeated but the 3-methyl-2-(3'-
methylthien~2~-oylhydrazino)pyridine i8 replaced by 3-
methyl-2-(4~-methy~ 2~3~-thiadiazol-5~-
oylhydrazino)pyridine. 3-(4'-Methyl-1',2',3'-
thiadiazol-5-yl)-8-methyl-~-triazolot4,3-a]pyridine
(0.3 g; 0.0013 mol), which melt~ at lBO~C, i~ obtained
(yield 26%).
To obtain the compounds of the following
sxample~, one of Examples 4 or 5 i8 repeateds
*Example ?l
8-Bromo-3-(3'-methylthien-2'-yl)-8-
triazolo[4,3-a]pyridine, ~ :
*Example 22
8-trifluoromethyl-3-~3'-methylthien-2'-yl)-s- ~ `
triazolot4,3-a]pyridine,
xample ?3
8-methyl-3-(2'-methylthien-2'-yl~
triazolol4,3-a]pyridine~
*Ex2mple_23
8-methyl-3-(2-methylthien-2'-yl) 8-
triazolo[4,3,-a~pyridine,
*Example 24
8-bromo-3-phenyl-s-triazolo[4,3-a]pyridine, .
*Example 25
B-chloro-3-(2'-methylthien-3'-yl)-~-
triazolot4,3-a]pyridine,
- ~ .
203~
Example 26
8-chloro-3-(thien-3'-yl)-s-triazolo[4,3-a]-
pyridine,
*Example 27
8-methyl-3-(1'-methylpyrrol-2~-yl)-8-
triazolo[4,3-a]pyridine,
Example 28
8-chloro-3-(3~,5'-dLmethylthien-2'-yl)-~-
triazolo[4,3-a]pyridine,
~Example 29
7,8-dimethyl-3-phenyl-s-triazolo[4r3-a]
pyridino,
Example 30
8-methyl-3-(3',5'-dimethylthièn-2~-yl)-s-
triazolot4,3-a]pyridine,
*Example 31
8-ethyl-3-(3~-methylthien-2~-yl)-s-
triazolot4,3-a]pyridine, .-
*Example 32
8-ethyl-3-phenyl-s-triazolo[4,3-a]pyridine,
*Example 33
8-ethyl-3-(1'-methylpyrrol-2~-yl) -8-
triazolot4,3-a]pyridine,
*~xampla.34
8-ethyl-3-(thien-3~-yl)-~-triazolo[4~3-a]
pyridine,
*Example 35
8-trifluoromethyl-3~ -methylpyrrol-2~-yl)-
.:., . :., .; ,:: . ~
.~ . i
., . : . - ~ .
_ 31 ~0 3 ~14 ~
s-triazolo[4,3-a]pyridine,
Example 36
8-trifluoromethyl-3-(pyrrol-2'-yl~-8-
triazolo[4,3-a~pyridine,
*Example 37
8-chloro-3-(4~-methylthien-2~-yl)-s-
triazolo[4,3-a]pyridine,
*Example 38
7,8-dLmethyl-3-(3'-methylthien-2'-yl)-s-
triazolot4,3-a]pyridine, ,i
*Example ~9 ;
8-methyl-3-(4'-methylthien-2'-yl)~
triazolot4,3-a]pyridine, ~: -
*ExamplQ 40
8-trifluoromethyl-3-(thien-3'-yl)-s- t
triazolot4,3-a~pyridine~
*Example 41
8-methyl-3-(4~ opropylphenyl)
triazolot4,3-a]pyridine,
*Example 42
~ 8-methyl-3-(4'-bromothien-2'-yl)-8-
triazolot4,3-a]pyridine,
*Example 43
8-chloro-3-(4~-bromothien-2~-yl)-~-
triazolot4,3-a]pyridine,
Example 44
8-methyl-3-(3'-chlorothien-2'-yl)-8-
triazolot4,3-alpyridine,
.
- .
' ' ' ~ ,
' ~ . ' :. "'" ' : -
: - -.
: . .
32 203~
~_xample 45
8-chloro-3-(3~-chlorothien-2'-yl)-8-
triazolo[4,3-a]pyridine,
~Example 46
8-methyl-3-~3'-bromothien-2'yl)-~-
triazolo[4,3-a]pyridine,
xample 4?
8-chloro-3-(3'-bromothien-2'-yl)-s-
triazolo[4,3-alpyridine,
The above products are obtained in accordance
with the methods described in ~xamples 4 and 5.
Example I-1:
Banzaldehyde (5.35 g; 0.05 mol) in ethanol
(29 ml) is added with stirring and at ambient
temperature to a solut~on of 3-chloro-2-
hydrazinopyridine (7.2 g; 0.05 mol~ in ethanol (30 ml
which contains a few drops of concentrated HCl. The
batch is refluxed for two hours and then concentrated.
After the mixturs ha cooled, the solid re~idue i8
recovered by filtxation, dried and recrystalli~ed from
~hanol. Phenyl (3'-chloropyrid-2'-yl)hydrazone
(10.45 g; 0.045 mol), which malt~ at l51~C, i~ obtained
(yield 91%~.
*Example I-2s
Benzaldehyde (5.35 g; 0.05 mol) in ethanol
(20 ml) i8 added to a 301ution of 3rtrifluoromethyl-2-
hydrazinopyridine (13.25 g; 0.05 mol~, prepared as in
Example I-9, in ethanol (30 ml) which contain~ acetic
. . .
.: ~ . - ''
. . , . - ~ . .
. .
203~
33
acid (0.3 ml). The mixtur~ i~ refluxed for 2 hours,
concentrated, cooled and diluted with water (S ml). The
precipitste i~ recovered by filtration and dried.
Phenyl (3'-trifluoromethylpyrid-2'-yl)-
hydrazone (11.15 g; 0.04~ mol), which metl~ at 119C,
is obtained (yield 84%).
*Example I-3:
2-Chlorobenzoyl chloride (8.75 g; 0.05 mol)
are added 810wly and at the temperature of the ico-bath
to 3-methyl-2-hydrazinopyridine (6.15 g; 0.05 mol),
prepared a3 in Example 1-8, in anhydrous pyridine (50
ml). The reaction mixture is stirred for 4 hours at
ambient temperature and then poured into iced-water
(100 ml). After ths mixture ha~ been allowed to stand
15 for 12 hours at O~C, it is filtered and the precipitate `~
is washed with water, dried and recrystallised using
aqueous ethanol. 2-(2'-Chlorobenzoylhydrazino)-3-
methylpyr~dine tlO.45 g; 0.04 mol), which melts at
; 115C, is obtainQd (yield 80%).
*Example I-4s
2,4-Dimethylbenzoyl chloride (8.45 g;
0.05 mol) are added slowly at O-C to 3-methyl-2-
hydrazinopyridine (6.15 g; 0.05 mol), prepared as in
Example I-8, in CH2~12 (200 ml) containing triethylamine
(5.5 g; 0.055 mol). The reaction mixture is refluxed
for 3 hour~, cooled, and filtered, and the filtrate is
concentrated. Tha residu~ i~ wa~hed with water, dried
and recry~tallised u~ing aqueous ethanol. 2-(2',4'-
. . .
. :
34 20351~
and recrystalli~ed u~ing aqueou~ ethanol. 2-(2~,4~-
Dimethylbenzoylhydrazino)-3-methylpyridine (9.45 g;
0.037 mol~, which melts at 120C, i8 ohtained (yield
74%).
*Example_I-5:
A mixture of methyl 3-methylthien-2-yl
carboxylate t3.9 g; 0.025 mol) and 2-hydrazino-3-
methylpyridine (3.1 g; 0.025 mol), prepared as in
Example I-8, is heated in n-butanol ~30 ml) for 6 hours
at boiling point. ~he mixture is concentrated to
drynes~ and recrystallised from tolusne. 3-Methyl-2-
(3'-methylthien-2'-oylhydrazino)pyridine (5.7 g;
0.023 mol), which melts at 196-C, is obtained (yield
92
*Example I-6:
Methyl benzimidate chlorohydrate (12 g;
0.07 mol) i8 added at ambient temperature to a solution
of 2-hydrazino-5-methylpyridine (8.65 g; 0.07 mol),
prepared a in Example I-10, in a mixture of absolute
ethanol (150 ml) and triethylamine (7.1 g; 0.07 mol).
The batch i8 ~tirred for 24 hour~ at ambient
temperature. The precipitate is recovered by
f~ltration, washad with ethanol and ~hen dried. Nl-(5'-
methylpyrid-2'-yl)phenylamidrazone (9.95 g; 0.044 mol),
which melts at a temperaturs around 166C, i~ obtained
(yield 63%).
*Example I-7:
85% hydrazine (125 ml) is added 510wly and a~
., - , ~ , , , ,~
.~ . . : . . , .
203~44
ambient temperature to a solution of 2,3-dichloropyridine
(74 g; 0.5 mol) in ethanol (400 ml). The reaction
mixture i8 kept at boiling point for 24 hours and then
cooled to O~C. The ~olid precipitate i~ recovered by
filtration and recry~talli~ed us;ng ethanol.
2-Hydrazino-3-chloropyridine (66.05 g; 0.46 mol), which
melt~ at 16~C, i~ obtained (yield 92%).
Example I-8:
A mixtur~ of 2-bromo-3-methylpyridine
(51.6 g; 0.3 mol) and 85% hydrazine (110 ml) is kept at
boilin~ point for 20 hour~. After the mixture ha~
returned to ambient temperature, the precipitate i8
recovered by filtration, washed with i~opropyl ether
and solubilised in CH2Cl2. The organic ~olution i~
filtered and evaporated to dryne~. 2-Hydrazino-3-
methylpyridine (26.45 g; 0.215 mol), which melts at
; 122-C, i~ obtained (yield 71%J.
*Example I-5:
Example I-8 is repeated, but the 2-bromo-3-
methylpyridine (51.6 g) is replaced by 2-chloro-3
trifluoromethylpyridine (54.45 g), and the mixture i~
heated for 24 hours. 2-Hydrazino-3-trifluoromethyl-
pyridine (34.55 g; O.l9S mol), which melts at 63~C, i8
obtained (yield 65%).
*Example I-lOs
Exampla I-8 i~ repeated, but the 2-bromo-3-
methylpyridine i~ replaced by the ~ame amount of 2-
bromo-5-methylpyridine. 2-Hydrazino-5-methylpyridine
,. , , . . ~ . -
.
~ - . - ~. . ' ' :
~ , . . .
36 203~4
(yield 58~).
The example~ hereinafter, which are givan
without implied lLmitation, illu~trate the use of the
products according to the invention and their
application in weeding. In these examples, the
following abbreviations are used:
: . . . . . ~ . . ,
' . '
."' -' ;' ' '
20351~4
37
~ _
Abbrevia- Engli~h name of Latin name
tions weed~
~ .. _
5 AVE Wild oat Avena fatua
. _ _
ECH Japanese millet Echinochloa
cru~-galli
._ .
CHR Corn marigold Chrysanthemum
3egetum
10 POR Common purslane Portulaca oleracea
. _ . _ .
DIG Hairy finger gra~s Digitaria
sanguinali~
SIN Nhite mustard Sinapi3 alba
. _ . I
ALO Black grass Alopecuru~
~yo~uroide~
. , . .
IPO China ~ute Ipomea purpurea
.
ABU Black nightshade Abutilon
theophrasti
SOL Common chickweed Solanum nigrum
.. ,
20 STE Stitchwort Stellaria media,
.
CEN Cornflower Centaurea cyanu3
. _ .. .__ . -
SES Hemp ~esbania Sesbania exaltata
SET Faber's foxtail Setaria faberii
CHY - Corn marLgold Chrysanthemum
. segetum
,~ , ,
: - . .
'
203~14~
3~
. ____. ~ .. . _
Abbrevia- Engli~h name of Latin name
tions weeds
. _ _ _ ... ... __ . .,
S RZ Wheat Triti~um ae~tivum
ZEA Maize Zea May~
.. _ _ I
ORY Rice Oryza sativa
_ Soya Glycine maximum
GOS Cotton Gossypium hlr~utum
10 HE~ Sunflower Helianthu~ annuus
BRS Oilsaed rape Brassica napus
Example Vl:
Herbicidal application before emergence of the plant t
15 species.
A number of eeds selected as a function of the
plant specie~ and the ~ize of the seed is sown in 7 x 7
x 8 cm pots filled with light agricultural 80il.
The pot~ are treated by spraying them with a
20 mixture in an amount which corresponds to an
application rata of 500 l/ha and which contains the
active ingredient at the desired concentration.
The seeds which are not covered by 80il are
thus treated with the mixture tthe term mixture is used
25 to designate generally compositions which ar~ diluted
with water, such as are applied to the plants).
The mixture used for the treatment i5 a
,. . . . .
- :~
. . - ., . : ,. .
,.~ ;. :
.~ ,
203~
39
solution or suspension of the active in~redient in an
acetone/water mixture in the ratio of 50/50, in the
presencQ of 0.05% by weight of Cemul~ol NP 10
(surfactant) which is composed of a polyethoxyl~ted
S alkylphenol, in particular 8 polyethoxylated
nonylphenol) and 0.04% by weight of Tween 20
(surfactant composed of an oleate of a derivatiYe of a
polycondensate of ethylene oxide with sorbitol).
In the case of a suspen~ion~ the latter is
obtained by mixing and grinding the ingredients in a
microniser in such a way a~ to obtain an averags
particle ~ize of less than 40 micron~.
After the treatment, the ~eed~ are covered by a
layer of soil about 3 mm in thicknes3.
The pot8 are then placed in containers for
receiving the watering water, by way of ~ubirrigation,
and maintained for 24 hour~ at ambient temperature and
60% relative humidity.
After 24 days, a de3truction percentage (D) of
the plants in the treated pot is determined in relation
to the number of plants in the untreated pots
(controls). On the remaining treated plants, the size
reduction percentage (SR) i8 measured in relation to
the control plants.
The percentage of foliar volume destroyed and
reduced by the product i8 therefore given by the
formula
~ ~ 3 ~
D + SR (100 - D) = A
100
This value A i~ transformed into a key from O
to 5 ac~ording to the following gradations
Rey
O ~ A < 10 0 no effect
10 < A < 30
30 ~ A ~ 50 2
50 < A < 70 3
70 ~ A < 90 4
90 < A ~ 100 5 complete de~truction.
.
~ : : ?
.:
.. : ~ .
... : , . .. .
:: ~ , , ': ..
~. ~ : . - -: :
20351~
41
The result~ obtained are given hereinafter:
Compound accor- 6 2 10 3 5 1
ding to Ex.No. . ¦ .
5 (applied at a .
dosage rate of
4 kg/ha)
~ ~ - _ _ _
Pre-emergence .
activity A
_ _ _ _ _
AYE 0 0 2 0 5 5 1 4 3 5
ECH 3 0 2 1 5 4 3 5 5 5
ALO 2 1 4 3 5 5 4 5 5 5
DIG 5 5 5 3 5 5 5 5 5 5 ff~-
IPO 4 3 4 5 5 3 1 5 2 5
SIN 5 5 5 3 5 5 2 5 5 5
_ _ __
A~U 4 5 5 5 5 4 3 5 5 5
SOL 5 5 0 5 5 5 5 5 15 5
,. . .
,
^" 203S1 ~4
42
PRE-EMERGENCE ACTIVITY
EXAMPLES ¦ ECH ¦ ALO ¦ DIG SIN ABU j SOL ¦ STE ¦
._-- 1- l _ _
~i 1 1 65 - 5 ---- -5 5 -- 5 5 5
171 4 5 5 2 5 1 5
~ .... . _
20 4 5 5_ 5 5 5 5
22 4 4 5 5 1 0 1
I
23 3 4 5 1 _5_ __5
24 4 4 4 2 0 5 5
-25 3 5 5 1 0 5 5
27 4 5 5 2 4 4
31 5 5 5 5 5 5 5
32 -5 5 5 5 5 5 5
_
l~i 33 5 5 5 5 5 5 5
34 5 5 ' 5 5 5
. _
37 5 5 5 5 0 5
. _
38 5 4 5 O 3 5 5
. .
39 3 5 5 1 0 5 ~i
, -I
4 0 3 5 5
..... _
41 4 4 5 2 1 5 4
1- -' i -- ,
42 1 3 5 1 5 1 5 1 0 1 5 1 5
1 43 1 5 5 1 5 1 5 1 5 1 5
2a
. .
'' , . :- . ~ : ..
~ ~' ' . . . -
.
.
.:. . . : : . .
203~
43
_ .. _
PRE-EMERGENCE ACTIVITIES
CROPS
EXAY- DOS- _ . _ _ _
PLES ARGEE
Xg/ha E~RS GOS GLX S SL TRZ Z SA ORY
10 17 2 0 0 1 0 0 0 0
27 2 ~ 0 0 1 0 0
31 1 0 0 0 0 0 0 0
36 2 5 0 0 4 1 0 1
15 38 1 5 0 0 0 0 0 0
.
. .:
- : -
, ~ -, .:: ,
2 0 3 ~ ~ 4 '~
44
_~ PRE-EMERGENCE ACTIVITIES
WEEDS
S E~ DOS-
PLES ARAEE
kg/h~ STE CHY SOL DIG SET
17 2 5 5 5 5 5
27 2 5 5 1 5 5
31 1 2 5 4 4
36 2 5 5 5 5 5
33 1 5 5 5 5 5
~. . -, :
. ' ~' . ~ ' '
20351~
_ . .
PRE-EMERGENCE ACTIVITIES
CROPS WEEDS
, . .. .. . __
EXAM- DOS- i
5 ¦ PL S ¦ RA rE
kg~ha GLX TRZ ZEA STE AE~U SOL DIG
. _ l _
32 2 O t) O O 5 1 4 4
1039 1 O O O 5 5 5 5
1 O 2 O 4 5 5 5
33 2 O O O 4 1 4 5
16 2 O 2 O 5 4 51 5
. 43 4 1 O 1 _ 5 5 5I 5
- - . - . . :
~: . - .:- ....... - , - :
-: - - ~
:
203~144
46
Compound according to Ex.No 5 1 12
Applied at a dosage rate of
5 (kg/ha) 2 1 4
Pre-emergence activity A
_
DIG 5 5 4
_
ECN 5 4 1
SET 4 4 4
SOL 4 5 5
CEN 5 4 3
GOS 0 _
HE~ 0 3 2
15 GLX 1 O 1
__ ,
TRZ 2 3 0
..
ZEA 0 2 1
ORY 1 0 0 ;
E amples which follow:
These examples show that, pre-emergence, the
products according to the invention have a good general
activity against the weed~ and a good ~electivity
toward~ one or more crops ~uch as wheat, maize, rice,
cotton, ~unflower, ~oya and oilseed rape.
,
.~ . . .~ .
:. . .
47 20351~
Examp~~ U2
Herbiçidal apDlication after emer ence of the ~lant
species
A number of seeds selected as a function of the
plant specie~ and the size of the seed i8 sown in 7 x 7
x 8 cm pots filled w~th light agricultural soil.
The seeds are then covered by a layer of 80il
of about 3 mm in thickness and the seed i8 allowed to
germinate until it give~ ri~e to a plantlet in a
suitable stage. In the case of tha Gramineae, the
treatment st~ge is the ~formation of second leaf~
stags. In the case of the dicotyledon3, the treatment
StagQ i8 the "cotyledons unfolded, first true leaf
being developed~ stage.
The pota are then treated by spraying ~hem with
a mixture in an amount which corresponds to an
application rate cf 500 l~h~ and which contain~ the
active ingredient at the desired ~oncentration.
The mixtuxe used for the treatment is prepared
a~ in Example Ul.
After the treatment, the seeds are covered by a
soil layer of about 3 mm in thickness.
The potA are then placed in containers for
receiving the watering water, by way of subirrigation,
and maintained for 24 days at ambient tempera~ure and
60% relative humidity.
A (pexcentage of destroyed foliage volume) is
measured as in Example U~.
. ,: - ,. : : -
48 2V351~4
The resul~s obta:Lned are given hereinafters
_ 6 1~ 4~ 3 1 5~ 13--
Compound in
accordance
5 with Ex.No.
(applied at a
dosage rate of 1 ,
4 kg/ha) . 1 ~ ¦
. . _ . _ _ . i i
10 Post-emergence
activity
AVE 2 3 ] ~ - 4 2 5 _ 3 2
. 1 ECN 3 4 c ~ ' 4 3 5 c _ 5
, ALO 2 4 4 5 2 4 5 4 .
. _ _ _ _ _ ,
. DIG 2 3 ~ q -c 4 4 3 5 4 4 5
IPO 2 4 4 . 4 3 5 5 5
, _ _ _
SIN 5 5 c c c c 5 5 5 c 5 5
ABU 3 4 ~ q ~ ~ 4 l 5 . 3 5
SOL 4 4 c i c ~ 5 5 ~ 6 _ 3 5
,~
.
~. ': , : ` - ~ : ,
: . ,
`:
2~35144
49
_
~XAMPLES j POST-EMERGENCE ACTIVITIES
, ,
- I ECI~ ALO ¦ DIC ¦ SIN ABU j SOL ! STE
. _ l _j
16 4 5 4 5 3 5 ~ I
_ . ... . ~
17 5 1 2 5 3 5 5
18 3 _ 5 5 5 _
2 4 2 4 2 2 5
_
22 4 2 3 5 O 4 3
24 2 3 2 5 1 2 4
_ _
4 5 4 1 O 2 5
~ 26 4 1 4 4 5 5 4 ! _ 5
28- 3 3 - 4 5 4 5 5
.
1~ 295 2 ~ 5 5 4 4
315 5 5 5 5 5 5
. , .
325 54- 1 5 4 5 1 6
335 55 1 5 ~ 1 5 L 5
34 1 55 1 5 5 1 5
l . I I ~ I .
~0 355 1 5 5 1 5 5 1 -4 1 5
37 15 4 1 5 5 1 5 1 -
38 ~ 13 4 1 2 5 1 5 1 5
. 39 1 5 15 j 5 1 5 1 3 1 4 1 5
1 5 14 1 5 1 5 1 5 1 5 1 5
~5 ' - 41 ~ 5! 5 1 5 1 5 1 5 1 5 1 5
42 1 4 13 1 4 1 5 1 5 1 5 1 5
----1 1 l I I
43 1 5 j5 1 1 1 5 1 5 1 5
, . , .: , ~ .
: ` `
:' . -. ' ' .:
: ~, . '
.
20351~
Compound accor~
ding to Ex.No. l
lj 2 l 10 12 9
Applied at a
dosage rate of
(kg/ha) l l l ¦ ¦
-- '1~ 21 1 21 1 ---~ 2
Post-emergence l l i
activity A ~ l l ¦
! I l ~
weeds ,;
IPO 3 4 3 4 3 4
ECR 5 4 5 5 2 5 5
POR 5 5~ 5 41 Z ¦ 3 1 4
crops ¦ ¦ .
T~Z 0 2 0 1 0 0 2
-ZEA 0 1 1 0 0 0 0
. _ 1 1 1 1 . O 1 ~ .
. ,. ~
'
,: , . , . .. . : ' . ~ '
51 203~4
POST-E~ERGENCE ACTIVITIE:S
¦ WEEDS
1: ~C~ Rl~TE ~ n I
PLE:S ¦ Kg/h~- GOS j TRZ ! ZEA ! ORY GLX
38 l ~ 2 O l O
:~1 0,5 O I 1 1 1 0
37 2 0 O O O
36 1 1 O O
10 28 2 l O l
32 4 1 O 1 1
1~ 16 1 2 O O 1
211 2 O 0 ~ O
~>o 26 2 O O
333 0;5 3 0
~ 1 O O I
¦ WEEDS
EXAN- DOSaG~
PLES Kg /h STE CHY SES CEN SET
38 1 4 5 1 4 4
. , ._.
1 31 0,5 5 5 1 3 - 5 3
: -, . : . -
.
~ ~, , , '
52 203~
WSAGE I POST-EME~GENCE ACTIVITIE5
PLES j ~g/ha WEEDS
ABU ¦ IPO ¦ CHY ¦ SOL ¦ POL ¦ STE
37 1 2 1 3 1 4 1 5 ll 4
43~ 5 1 ~ 5
j i ¦POST-~MERGENCE ~CTIVITIES
WEEDS
1 005 GE I i ! l
PLES ~/ha STE ~ I SOL ! SET
29 4 4 5 4 4
21 22 55 3 4 44 , :
17_ 2 5 5 3 5
. ,: - :, .: . , . , : -
, . . . .
203all~
53
POST-EMERGENCE ACTIVITIES
WEEDS
DOSAGE; ! I _
EXAM~ 'rE ln l I l
¦ PLES ! Kg~h~ STE A}IU IPO SOL ECH ¦ DIG ~ SET
39 1, 2! 5 1 5 1 4, 5 ~ 5~' ~i5
25 ! 2 ~1 5 ! 4 1 5 5 ! 5 1 ~ j 5
262 ~ 4 1 5 ' 4 4 1 5 1 4 4
lC 350.5~ 5 ! 5 1 4 ~ l 51 3 5
33ll 5 5 5 5 l 51 3 5
28l 1 5 5 5 5 1 l 1 5 1 4
- Moreover, the compound of Example 14 has shown
the following activities A at a dosag~ rate of 2 kg/ha:
IPC¦ 501~ SE~ STE¦ CHY¦ GL~ NEI¦ TRZ¦ ZEA OBY
4 1 5 1 3 1 4 ~ O
These example~ ~how that, post-emer~ence, the
compound~ according to the invention have a good
general activity against waed~ and a good selectivity
in one or more crop~, such a~ wheat, maize, rice,
sunflower, cotton and soya.
~ he trials which hav~ been carried out show
therefore the advantageou~ propertie~ of the compounds
according to the invention as herbicide3, in particular
herbicides which have a broad spectrum of activity and
., ,~ . . , ~ ,
203~i4~
54
which can be active pre-emergence and post-emergence.
For their use in practice, the compounds
according to the invention are rarely utilised by
themselves. Most frequently, these compounds are part
S of compositions. These compositions, which can be used
as herbicidal agent~, contain, a4 the active
ingredient, a compound according to the invention such
as has been de~cribed above, in a mixture with at least
one ~olid or liquid carrier which iB agriculturally
acceptable and, if appropriate, at least one agent with
surface activity i~ likewiso agriculturally acceptable.
By agent with surface activity, there is to bs
under~tood, in the present ~tatement, an agent known in
the English language under the term ~surfactant n ~ which
embraces principally the surfa~e-active agents, wetting
agent or di~persants.
These compo~itions are likewise part of the
invention. ~hey can also contain a large range of other
ingredients such as, for example, protective colloids,
adhesives, thickeners, thixotropic agent~, penetrants,
~tabili~ers, sequestering agents, etc. More generally,
the compounds used in the invention can be combined
with all solid or liquid additives which ~orrespond to
techniques cu~tomary in the art of formulation.
Generally ~peaking, the compounds according to
the invention cu~tomarily contain approximately from
0.05 to 95% (by weight) of a compound according to the
invention, one or more ~olid or liquid carriers and, if
,. - , . ~ , . .
-: .: . . .
203514~
desired, one or mor~ surface-active agent
~ he term 'carrier~ designate~, in the pre~ent
statement, an organic or inorganic natural or 6ynthetic
material, with which the compound is combined far
S facilitating it~ application to the plant, to the seeds
or ~o the soil. This carrier is thus generally inert
and it must be agriculturally acceptable, in particular
to the treated plant.
The carrier can be of any customary type. In
particular, it can be solid (clays, natural or
synthetic silicate~, ~ilica, re~ins, waxes, ~olid
fertilizers, etc....) or liguid (water; alcohol~,
especially butanol etc...).
The agent with surface activity, or the
~urface-active a~ent, can al~o be of any customary
type. It can be an emul~ifier, di~persant or wetting
agent of the ionic or non-ionic type, or a mixture of
such surface-active agent~. Examples which may be
mentioned are 6alts of polyacrylic acids, ~alts of
lignosulfonic acid~, salt~ of phenolsulfonic acids or
naphthalenesulfonic acids, or polycondensates of
ethylene oxide with fatty Alcohols or with fatty acid~
or with fatty amines, or substituted phenols
~especially alkylphenols or arylphenols), ~alt~ of
esters of ~ulfosuccinic acids, derivatiYes of taurine
(especially alkyltaurates), phosphoric e~ters of
alcohol~ or of polycondensates of ethylene oxide with
phenols, of fatty acid esters and of polyols, or
~, . .
,,
'
'
56 20351~
derivstivQ~ of the preceding compounds which have
sulfate, qulfonate and phoqphste functions.
The presence of at least one surface-active
agent is generally indispensable when the compound
and/or inert carrier are not water-soluble and the
veetor agent for the application i8 water.
Thus, the compounds for agricultural use
aeeording to th~ invention can contain the active
ingredients aeeording to the invention within very wide
limits, from 0.05% to 95% (by weight). Their content of
~urface-acti~e agent is advantageouYly between 0.1% and
50~ by weight.
As regards the eomposition~ which are adapted
to storage and transport, they contain more
advantageously from 0.5 to 95~-(by weight) of active
substance, the eompositions as they are applied to the
plant~ being generally markedly more diluted than the
eompositions which are adapted to storage and
transport, which are more eoncentrated.
The compo3itions aceording to the invention
themselves eome in a very wide range of forms, solid or
liquid.
Solid e~mposition forms whieh may be mentioned
are the dusts for dusting (having a compound content
whieh ean reaeh 100~); the wettable powders; the
granules, specially those obtained by extrusion, by
compacting, by impregnating a granulated ~upport, by
granulation using a dust (the eompound content in these
...... . . .
- , .. . . . .
.- . ~
.. . .
- , , ,
,, , , , , :: : . . .
. : , ' . : . ' . ,' . ' ' ~ - :
57 203~
granules being between 0.5 and 80% in the la~t-
mentioned ca~es).
The wettable powders (or sprayable powder) are
customarily prepared in such a way that they contain 20
to 95~ of active compound, and they cu~tomarily
contain, in addition to the solid carrier, from 0 to
30% of a wetting agent, from 3 to 20~ of a disperYant
and, if neces~ary, from 0 to 10% of one or more
stabilisers and/or other additlves, such as penetrant~,
adhesive~, or anticaking agents, colourants, QtC.
To obtain the ~prayable powders, or wettable
powders, the active substances are mixed intimately in
suitable mixer~ with the additional substances, and the
mixture is ground by mea~s of mills or other suitable
grinders. In thi~ way, powder~ for spraying are
obtained whose wettabil~ty and su~pension properties
are advantageous; they can be suspended in water at any
desired concentration, and the suspension3 can ba used
very advantageously, in particular for application to
the leaves of the plants.
In place of the wettable powders, pastes can be
rQalised. The conditions and detail~ of production and
utili~ation of th~se pa~te~ are similar to tho~e in the
case of the wettable powdars or sprayable powder~.
What follow~ now by way of example are various
compositions of wettable powders ~or sprayable
powders):
:
; .
,
..,
58 20351~4
Example F 1:
Active compound (compound No.1) 50
~ Ethoxylated fatty
alcohol (wetting agent) 2.5
5 ~ Condensate of ethylene oxide with phenyl-
ethylphenol (dispersant) 5%
~ Chalk (inert carr~er) 42.5%
Example F 2:
* Active compound (compound No.l) 10%
10 * Synthetic oxo Cl3 alcohol of the branched
type, ethoxylated with 8 to 10 ethylene oxide unit~
(wetting agent)
0.75%
* Neutral calcium ligno~ulfonate (dispersant) 12%
* Calcium carbonate (inert filler) q.s. 100
Example F 3: this wettable powder contains the ~ame
ingredients as in the preceding example, in the
following proportions: -
* Active compound 75
20 * Wetting agent 1.50%
* Disper~ant 8%
* Calcium carbonate (inert filler~ q.s. 100%
Example F 4: .
* Active compound (compound No.1) 90%
25 * Ethoxylated fat~y alcohol (wetting agent) 4%
* Ethoxylated phenylethylphenol (di~persant) 6%
. ' -
,:' .. -- , '' .
,:~ . -. . . :
` -`` 203~i44
59
Exam~e F5s
* Active compound (compound No.1) 50%
~ Mixture of anionic and non-ionic curfactants ~wetting
agent) 2.5%
~ Sodium lignosulfonate (di~persant) 5~
* Raolin-type clay (inert carrier) 42.5%
The compounds according to the invention which
can be formulated in the form of water-dispexsible
granule~ sre likewi~e comprised in the Rcope of the
invention.
These di~persible granule~, whose apparent
density is generally between about 0.3 and 0.6, have a
particle s~ze of generally between about 0.15 and 2 mm
and preferably between 0.3 and 1.5 mm.
The active ~ubstance eontent of the3e granules
is generally between about 1% and 90%, and preferably
between 25~ and 90%.
The remainder of the granule is es entially
composed of a solid filler and, if appropriate, of
surface-active ad~uvants which impart to the granule
properties of water dispersibility. ~hese granules can
esaentially be of two type which are distinguished
a~cording to whether the filler carried i8 water-
soluble o~ not. When the filler i5 water-soluble, it
can be inorganic or, preferably, organic. Excellent
re~ulta have been obtained using urea. In the casQ of ~
an insoluble filler, the latter is preferably
inorganic, ~uch as, for example, kaolin or bentonite.
; , :.
. ~
-., : . . ' :
. .; ' ~: ,
2 f3 3 ~
It i8 therefore advantageou~ly accompanied by surface-
active agents (ln a ratio of 2 to 20~ by weight of the
granule) of which more than half iB, for example,
composed by least one, e~sentially anionic, dispersant
such as an alkali metal polynaphthalene~ulfonate or
alkaline earth metal polynaphthalenesulfonate or an
alkali metal lignosulfonatQ or an alkaline earth metal
lignosulfonate, the remainder being composed of non-
ionic or anionic wettinq agent~ ~uch a~ an alkali metal
alkylnaphthalenesulfonate or alkaline earth metal
alkylnaphthalenesulfonate.
Moreover, other ad~uvants such as antifoam
agents can be added, even though this is not
indispensable.
The ~ranules accordin~ to the invention can be
prepared by mixing the necessary ingredient~ followed
by granulation according to various techniques known
per se (coating apparatus, fluidised bed, atomiser,
extrus~on, etc.). The proces~ i8 generally fini~hed by
20 a cxushing step followed by a sieving step at the ~;.
particle d~men~ion selected within the above lLmits
mentioned.
It ~ preferably obtained by extrusion,
proceeding a8 shown in the example~ hereinafter.
xam~le F 6: Disper~ible aranules
90% by weight of active ~ubstance tcompound
No.l) and 10% of urea in bead form are mixed in a
' ~ -
- 2 0 3 ~
61
mixer. The mixture i~ ~ub~equently ground in a pin
mill. Thi~ giVQ8 8 powder which i~ moistened wlth about
8% by weight of water. The moi~t powder i~ extruded ln
a perforated-roll extruder. This g~ves granule~ which
are dried, then cru~hed and ~eved, in such a way as to
retain only the granules of a size between 0.15 and
2 mm.
Exam~le F 7: Disper~ible granules
The following components are mlxed in a mixer:
* Active sub~tance (compound No.l) 75~
* Wetting agent ~sodium alkylnaphthalene~ulfonate)
2%
* Disper~ant (sodium polynaphthalene~ulfonats) 8
~ Inert water-insoluble filler (kaolin) 15~
Thi~ mixture is granul~ted in a fluidised bed
in the presence of water, and then dried, cru~hed and
sie~ed in such a way as to obtain granules of a
size between 0.15 and 0.80 mm.
The~e granule~ can be used on their own, or in
solution or dispersion in water in ~uch a way as to
obtain the de~ired dosage rate. They ~an also be u~ed
for preparing combination~ with other active
substances, e~pec~ally herbicides, the latter being in
th~ form of wettable pcwders or of granules or aqeuou~
~uspensions.
~ he compoundY of the formula (I) can also be
used in the form of dusts for dusting; it is also
possible to use a composition containing 50 g of actiYe
:, . ~ . . ~.
' ~" - ~' ' ' .
203~
62
substanc~ and 9S0 g of talc; it i~ also pos~ible to use
a composition containing 20 g of active substance, 10 g
of finely-divided silica and 970 g of talc; the
components are mixed and ground, and the mixture i5
applied by dusting.
Liquid forms of compo~itions, or forms of
compositions intended for making up liquid composit~ons
at the time of application, which may be mentioned are
~olutions, in particular wa~er-soluble concantrate~,
emulsifiable concRntrates, emulsion~, flowable~ and
aerosols; wettabl~ powders (or sprayable powder) and
pastes are solid compo~itions but intended for making
up liquid compo3itions at the time of application.
~he emulsifiable concentrates or soluble
concentrates most frequently c~mprise 10 to 80% of
active substance, while the ready-to-use emulsion~ or
solutions, for their partl contain 0.001 to 20% of
active substance.
Besides th~ solvent, the emulsifiable
concentrates can contain, if necessary, 2 to 20% of
suitabla additive~ such as ~tabilisers, surface-active
agent~, penGtrants, corrosion inhibitors, colourants or
adhesives, a3 mentioned above.
Using these concentrate~, d~lution with water
can givo emulsions of any desired concentration, which
i~ particulsrly convenient for application to crops.
What now follow~ by way of example is the
composition of some emulsifiable concentrate~:
. . .. .
..
: -
.: , .~ .
203~
63
Exam~le F 8:
~ Active substance 400 g/l
* Alkali metal dodecylbenzene sulfonate 24 g~l
* Nonylphenol with oxyethylated 10 molecules
5 of ethylene oxide 16 g/l
* Cyclohexan~ne 200 g/l
Aromatic solvent q.s. 1 litre According to another formula for an
emulsifiable concentrate, the following are used:
10 Example F 9:
* Active ~ubstance 250 g
* Epoxidised vegetable oil 25 g
* Nlxture of alkylarylsulfonate and an ether
of polyglycol with fatty alcohols100 g
15 * DLmethylformamide - 50 g
* Xylene 575 g
The flowables, which can likewi~e be applied by
spraying, are prepared in 3uch a way as to obtain a
fluid stable produc~ which does not sattle out, and
they customarily contain from 10 to 75% of active
substance, from 0.5 to 15% of surface-active agents,
from 0.1 to 10% of thixotropi~ agents, from 0 to 10~ of
suitable additive~ such as antifo2ms~ corrosion
inhibiters, ~tabiliser3, penetrants and adhesive , and,
as a carrier, water or an organic l~quid in which the
active substance i~ sparingly soluble, or insolubles
certain ~olid organic material~ or mineral ~alt~ can be
:
203~14~
64
dissolved in the carrier for helping prevent
sedimentation, or as antigels in the casQ of water.
What now follows by way of example is a
composition of a flowable:
Example F 102
Compound 500 g
* Polyconden~ate of ethylene oxide with tri~tyrylphenol
phosphate 50 g
* Polyethoxylated alkylphenol 50 g
10 ~ Sodium polycarboxylate 20 g
~ ~thylene glycol 50 g
* Organopoly~iloxane-type oil (antifoam) 1 g
* Polysaccharide 1.5 g :~
* Water 327.5 g
The aqueous di~persion~ and emulsions, for
example the compositions obtained by dilutin~ a
wettable powder or an emul~ifiabla concentrate
according to the invention with the aid of water, are
Qncompassed within the general ~cope of th~ present
invention. The emulsions can be of ths water-in-oil
type or oil-in-water type, and they can have a thick
consistency like that of a ~mayonnaise.
The pre3ent invention also relates to a weeding
method (e~pecially in area~ of dicotyledon or
monocotyledon crop~), which consists of applying, to
the area to be weeded, and/or on the plants to be
destroyed, an effective amount of a compound according
,
20 3 51 ~1
to the invention, especially a compound of the formula
(I), it being possible for the plants (or weeds) which
are to be destroyed, or whose growth it is desired to
prevent, to bs of the monocotyledon or dicotyledon
type.
The product~ and compositions according to the
invention are conveniently applied to tha vegetation
and especially to the weed~ to be eliminated when they
have a green foliage (post-emergence application).
It is also po~ible to use a weeding method
which consists of applying an effective quantity of a
compound of the formula (I) to the areas or terrains
where it i~ desired to prevent the germination or the
development of plant~ which have not yet germinated
~pre-emergence application). In thi~ case, it i5
possible for which the crop to be sown before or after
the treatment.
The application rate of active sub~tance i~
generally between 0.05 and 8 kg!ha, preferably between
0.4 and 4 kg/ha.
.
66 203~
~umbe r i ng
~NFormul a (1) 7 ~N
N ~ ` 6
Ar
Y~,NH-N-CH-Ar Y~5,T
Forrr~.~J~ a (Il) Fr.~m~la ~\1111)
Y~NH-NH-CO-Ar ~ ~--C//
N ~ ;
Formula (111), Forrnula (IX)
X~ Ar ~
For~ a (IV) Formub (Xl)
,
Z Z
Y~NH-NH2 Y~N--N=C-At
X~N X N
Fom~la (V) Fom~la(XII)
, ~ : . : , . ~
- . . .. :