Note: Descriptions are shown in the official language in which they were submitted.
2fl~~~~2
z,IQuID ~va~GE,v~NTIZ~TION ~~ THE
PULPiONARY SYST~I
This invention was made with government support
under Small Business Innovation Research Program Grant No.
1 R43 CA48611-O1 awarded by the Public Health Service,
Department of Health and Human Services. The government
has certain rights in the invention.
Technical Field
The invention relates to methods arid means for
introducing liquids into the pulmonary system of patients
for the treatment of pulmonary and/or systemic disease,
conditions and/or abnormalities such as, far example, to
effect hyperthermic treatment and augmented radiotherapy
and chemotherapy of lung cancer. This invention also
relates to the employment of liquid as a means of deliver-
ing, through the pulmonary air passages of a patient,
biologically active agents.
Backcxxound of the Invention
In the United States there has been a steady rise
in the age-adl~usted national death rate from pulmonary
related diseases. The overwhelmingly predominant con
tributor to this trend is lung cancer. Currently about 8~
of all deaths in the industrialized world are attributed to
lung cancer. In the United States, an estimated 155,000
new cases of lung cancer are currently diagnosed each year,
and about 142,000 will die of the disease, about 1 death
every 4 minutes! Only about 10~ of the patients currently
diagnosed with lung cancer will survive beyond 5 years.
6056-91(CIP)1.CN -1-
'aC
Luna cancer, or bronchial carcinoma, refers strictly to
tumors arising from the major airways (bronchi) and pul-
monary parenchyma (bronchioles, alveoli, and supporting
tissue), as opposed to those metastasizing from other
sites. The four major forms of lung cancer, squamous cell
carcinoma (SCC), adenocarcinoma (AC), large cell anaplastic
carcinoma (LCAC), and small cell anaplastic carcinoma
(SCAC), account for 98% of pulmonary malignancies. Al-
though lung cancer can occur anywhere in the lungs, about
three-quarters of primary lung cancers occur in and/or on
the bronchial walls within the first three bronchial
generations, i.e., near or proximal to the hilus, the
region where the airways and major vessels enter and leave
each lung. A smaller percentage occur in more distal areas
of the parenchyma. Many tumors occur near the carina, at
the junction of the right and left bronchi with the trach-
ea, presumedly due to increased deposition of inhaled
carcinogens. Squamous cell carcinoma tumors, the most
common histological type, making up 30-40% of lung tumors,
arise inside tl~~e surface layer of the bronchial wall and
then invade the wall and. adjacent structures. Squamous
cell carcinomas tend to be relatively localized with less
tendency than the other lung cancer tumors to metastasize.
Adenocarcinoma tumors, also comprising 30-40% of lung
cancers, occur in the mid- to outer third of the lung in
about three--quarters of the cases. Adenocarcinomas tend to
metastasize widely and frequently to other lung sites, the
liver, bone, kidney, and brain. Small cell cancer,
accounting for about 20~ of all lung cancer, is the most
aggressively metastatic and rapidly growing, and can begin
near the hilus or in the lung periphery. Large cell tumors
account for only a few percent of lung cancer and can occur
anywhere in the lung. "Local failure," where primary
tumors spread to mediastinal lymph nodes, pleura, adrenal
glands, bone, and brain, is common with adenocarcinoma,
6056-91(CTP)1.CN -2-
\ac
2Q3~4~
small cell anaplastic carcinoma, and large cell anaplastic
carcinoma, and less so in squamous cell carcinoma.
The current "curative" treatment for lung cancer is
surgery, but the option for such a cure is given to very
few. Only about 20% of lung cancer is resectable, and out
of this number less than half will survive five years.
Radiation therapy (RT) is the standard treatment for
inoperable non-small cell cancer, and chemotherapy (alone
or with radiation therapy) is the treatment of choice for
small cell and other lung cancer with wide metastasis.
Patients with clinically localized but technically unresec-
table tumors represent a major problem for the radiothera-
pist, accounting for an estimated 40% of all lung cancer
cases.
Adjunctive hyperthermia, the use of deep heating
modalities to treat tumors, is being used increasingly to
augment the therapeutic efficacy of radiotherapy and
chemotherapy in. cancer treatment. It has been estimated
that eventually "hyperthermia will be indispensable for 20
to 25% of all cancer patients" [1: see the appended listing
of literature citations]. Hyperthermia clinical research
is increasingly showing the importance of using specialized
heating equipment to treat specific anatomical locations
and sites rather than devices with more general-purpose
heating capabilities. Unfortunately, current hyperthermia
devices are il:L-suited to providing deep, localized heating
of lung cancer. Because of this limitation, very few
applications of localized lung hyperthermia have been
recorded in the literature [2].
Kapp [8] has shown that, in terms of absolute
numbers of patients (15,000 in 1987), more lung cancer
patients would benefit from effective local hyperthermia
than in any other cancer category, with the possible
exception of prostate carcinoma. Because of the present
difficulty of heating tumors locally in a controlled
fashion in the center of the thorax, the techniques most
6056-91(CIP)1.CN -3-
\ac
203~4~~
commonly attempted for lung cancer hyperthermia to date
have been whole-body hyperthermia (WBH), and radio-frequen-
cy (RF) heating of locoregional lung areas [2,9]. While
whole-body hyperthermia has produced some encouraging
results in combination with chemotherapy, the technique is
unsatisfactory since it produces significant systemic
toxicity and mortality, and because the thermal dose is
limited due to long induction times (warmup) and the need
to maintain core temperatures below 42°C. The electromag-
netic (EM) approaches to lung heating have also been
disappointing, due to the unpredictability of the heating
patterns produced, the difficulty of measuring intratumoral
temperatures in electromagnetic fields, the propensity of
radio-frequency heating to preferentially heat superficial
fat, and because of the physical inability of electromag
netic modalities to produce small focal volumes. The
modern microwave body-surrounding array systems also suffer
from difficulties associated with localization and predict
ability of heating, thermometry artifacts, and heat spikes
at fat muscle interfaces:
Because ,of its characteristically small wave-
lengths, therapeutic ultrasound has the best capability for
providing local heating in the body of all the convention-
ally used hyperthermia modalities. Focused and unfocused
ultrasound beams are routinely used clinically to success-
fully provide localized hyperthermia to many tumors resid-
ing in soft tissues and organs. However, the presence of
air in the lung has precluded this valuable energy source
from being applied to lung hyperthermia.
Thus, the need for a means of delivering safe,
effective, and well-tolerated localized heating to lung
tumors is clear. The invention solves this problem, in the
preferred embodiment, by an unconventional use of "breath
able liquids" (e. g., perfluorocarbon liquids) and thera
peutic ultrasound.
6056-91(CIP)1.CN -4-
\ac
CA 02035492 2000-O1-27
As used herein, the phrase "breathable liquids"
refers to liquids which have the ability to deliver oxygen
into, and to remove carbon dioxide from, the pulmonary
system (i.e., the lungs) of patients. Examples of breath-
s able liquids include, but are not limited to, saline,
silicone and vegetable oils, perfluorochemicals, and the
like. One of the presently-preferred breathable liquids is
perfluorocarbon liquids.
Perfluorocarbon (also referred to herein as "PFC" a
liquids are derived from common organic compounds by the
replacement of all carbon-bound hydrogen atoms with fluor
ine atoms. They are clear, colorless, odorless, nonflam
mable, and essentially insoluble in water. They have
extremely high dielectric strength and resistivity. They
are denser than water and soft tissue, have low surface
tension and, for the most part, low viscosity. Perfluoro-
carbon liquids appear to have the lowest sound speeds of
all liquids and are also unique in their high affinity for
gases, dissolving up to 20 times as much 02 and over three
times as much C02 as water. Like other highly inert
carbon-fluorine materials which are widely used in medicine
(e. g., in drugs, Teflon implants, blood oxygenator mem-
branes, etc.), perfluorocarbon liquids are extremely
nontoxic and biocompatible. For a review, see: Biro, P.B.,
and P. Blais, Perfluorocarbon blood substitutes, in CRC
Critical Reviews in Oncology/Hematology, Vol. 6, No. 4, pp.
311-374, 1987.
To date, about 300 liquid compounds have been
investigated for blood-gas exchange applications [4].
Those liquids which have evolved as artificial blood
substitutes are complex perfluorocarbon liquid-based
aqueous emulsions containing various chemical stabilizers
and viscosity modifiers, along with conventional parenteral
additives (glucose, electrolytes, starch, and buffers).
Compatibility with blood and a surprising lack of major
adverse effects have been demonstrated in several animal
6056-91(CIP)1.CN -5-
\ac
CA 02035492 2000-O1-27
species. The first administration of perfluorocarbon
liquid blood substitute (Fluosol~-DA, one of four commer-
cial blood substitutes now available) to human volunteers
occurred in 1978 [10], with the first clinical use taking
place shortly after in 1979 [11,12]. Subsequently, num-
erous other studies have been carried out in Japan, the
United States, Canada, and Europe that have confirmed the
comparatively benign impact of infusing significant amounts
(some tests used liters) of the perfluorocarbon/water emul-
sions directly into the systemic blood circulation
[13,14,15]. The blood substitutes are not yet ready for
general clinical systemic use for two reasons: a) the
requirement to form an emulsion to suspend the perfluoro-
carbon particles significantly reduces the volume fraction
of the gas carrier (the perfluorocarbon), thus large
volumes must be infused, and b) the emulsion gradually
coalesces as it circulates, leading to premature removal
of many of the synthetic constituents from the blood.
However, studies are currently ongoing in a number of
clinically related therapeutic perfluorocarbon applications
primarily taking advantage of the oxygen carrying capacity
of blood substitute emulsions [16,17,18,19].
It was first demonstrated that mammals submerged in
hyperoxygenated saline could breathe liquid and success
fully resume gas breathing in 1962 [20]. However, this
approach to liquid ventilation (LV) was eventually aban-
doned, due to the practical difficulties of dissolving
sufficient quantities of OZ in saline (done under high
pressure), and because saline rinses away much of the
surfactant lining the lung alveoli [21]. These problems
were overcome in 1966, by Dr. Leland Clark [22], who was
the first. to use perfluorocarbon liquids (now oxygenated at
atmospheric pressure) to support the respiration of mice,
cats, and puppies. The extreme biocompatability and
suitable properties of certain perfluorocarbon liquids has
* Trade mark
6056-91(CIP)1.CN -6-
\ac
n
~~~~:~~ ~:i~a
subsequently led t:o a significant body of ongoing research
yielding promising clinical applications.
To date it has been clearly established that
mammals can breathe (total ventilation support) oxygenated
perfluorocarbon liquids for long periods (> 3 hours) arid
return to gas breathing without untoward long-term effects
[23, 24]. In addition, studies have also shown that no
adverse morphological, biochemical, or histological effects
are seen after perfluorocarbon ventilation [24, 25, 26].
Perfluorocarbon .liquids have also beers investigated
for lung lavage (washing) [27], and have been found to be
effective for rinsing out congestive materials associated
with Respiratory Distress syndrome (RDS) in adult humans
[28]. While total respiratory support of both lungs via
perfluorocarbon liquids is not without side effects, they
are minor and transient (mild acidosis, lower blood p02,
and increased pulmonary vascular resistance and decreased
lung compliance) [3,29,30,31]. Other biomedical appli-
cations of perfluorocarbon liquid ventilation have also
received serious research effort [32,33].
Pertinent to connective lung- hyperthermia, i.e.,
lung heating by the repetitious infusion and removal of hot
liquids to anei from the lung, studies of the physiological
heat exchange occurring from high- and low-temperature
perfluorocarbon ventilation of animals have also been
performed [30,41,42]. These studies have involved
complete-lung liquid heating and cooling, and have been
done at only moderate temperatures, but have illuminated
and quantified many relevant physiological responses and
3o systemic temperature effects. A very recent study [43]
reporting hyperthermic (to 45°C) convection heating of
lungs involved sustained heating of surgically isolated dog
lung lobes via heated blood perfusion, i.e., heating
induced from the blood side rather than the airway side.
Taking measurements of lung edema, compliance, perfusion
pressure, and serotonin uptake during 2-hour sustained
6056-91(CIP)1.CN -7-
\ac
2Q~~4~~
hyperthermia (done at 37.6°, 40.7°, and 44.5°C, time-aver-
aged lung temperatures), no significant changes in lung
parameters were found other than expected increases in
perfusion pressure with temperature. The authors conclude
that a normal lung appears to tolerate well the sustained
heating regimens appropriate for cancer hyperthermia
applications.
However, the problem of how to effect controlled
and sufficiently localized hyperthermia of malignant lung
tissue has, until now, remained unsolved.
As stated earlier, one way of treating pulmonary-
related diseases, conditions and/or abnormalities is by the
implementation of chemotherapeutic agents, either alone or
in conjunction with other therapeutic techniques (e. g.,
radiotherapy). However, there are many problems existing
when employing conventional techniques of chemotherapy.
For example, in the presence of lung disease and intrapul-
monary shunting, systemically administered drugs are inef-
fectually delivered to the diseased portion of the lung.
One conventional method of introducing such agents
into a patient's pulmonary system .consists of interrupting
ventilatory support and exposing the delicate lung tissues
of the pulmonary system to higher, and potentially traumat-
izing, pressures needed for manually delivering the agents.
When practicing many of the conventional chemotherapeutic
techniques, the final distribution of the agents, through-
out the patient's pulmonary system, is generally non-
uniform and typically "patchy".
Another problem associated with the presently
practiced methods of chemotherapeutic treatment of pul
monary-related diseases, conditions and/or abnormalities
is often encountered during intensive care life support
procedures. During such procedures, conventional gas
ventilation is employed to maintain lung stability and to
prevent lung collapse. However, the deleterious conse-
quences of such life support procedures often precludes
6056-91(CIP)1.CN -g-
\ac
SLICCeSSful weaning from the particular life support system
back to pulmonary gas exchange. As such, the practice of
chemotherapeutic treatment, in conjunction with such
conventional life support systems and/or procedures, is
severely hampered.
As exemplified above, there are significant
problems which: exist with conventional chemotherapeutic
techniques of treating pulmonary--related diseases, con
ditions and/or abnormalities. Until this invention, these
problems were unsolved.
Summary of the Invention
The invention provides, in one embodiment, a
hyperthermic treatment of lung cancer, which includes the
steps of: temporarily filling with a liquid medium pre-
selected pulmonary air passages adjoining pulmonary tissues
containing malignant cells, circulating exogenously heated
liquid medium having a temperature in the range of from
about 41° to about 50°C (preferably from about 42° to
about
45°C) through the liquid-filled pulmonary air passages for
a predetermined period of time, and thereafter removing the
liquid medium from the pulmonary air passages of the
patient. The liquid medium may be a perfluorocarbon liquid
or physiological saline solution. Suitable perfluorocarbon
liquids having the requisite physical and thermal proper-
ties are charaicterized by an average molecular weight in
the range of from about 350 to about 560 and by having: a
viscosity less than about 5 CP at 25°C, a density less than
about 2.0 g/cm~' at 25°C, a boiling point greater than about
55°C, a vapor pressure in the range of from about 20 Torr
to about 200 Torr, and a Prandtl number less than about 10
at 25°C. Representatives of such perfluorocarbon liquids
are FC-84, FC-72, RM-82, FC-75, RM-101, anc~ perfluoro-
decalin. The preferred group of perfluorocarbon liquids is
characterized by having an average molecular weight in the
range of from about 420 to about 460, a vapor pressure less
6056-91(CIP)l.CN --g-
\ac
~~3~4~?
than about 100 Torr at 25°C, and a surface tension less
than about 17 dynes/cm at 25°C.
The invention provides in another embodiment a
hyperthermic treatment of lung cancer using ultrasound,
including the steps of: temporarily filling with a liquid
medium preselected pulmonary air passages adjoining pul-
monary tissues comprising malignant cells, heating the
adjoining pulmonary tissues comprising the malignant cells
to a temperature in the range of from about 41° to about
l0 5o°C (preferably from about 42° to about 45°C) for a
predetermined period of time by transmitting ultrasound
through the liquid-filled pulmonary air passages, and
thereafter removing the liquid medium from the pulmonary
air passages of the patient. Perfluorocarbon liquids
having the requisite physical, thermal, and acoustic
properties for this ultrasound treatment are characterized
by an average molecular weight in the range of from about
400 to about 560. Such perfluorocarbon liquids are also
characterized by having: viscosity less than about 5 CP at
25°C, density less than about 2.0 g/cmg at 25°C, boiling
point greater than about 75°C, vapor pressure in the range
of from about 25 Torr to about 100 Torr, surface tension
below about 17 dynes/cm at 25°C, acoustic impedance in the
range of from about 0.8 to about 1.6 MegaRayls at 37°C, and
acoustic attenuation less than about 1.2 dB/cm (at 1.0 MHz,
45°C, and acoustic intensity of about 3 W/cm2). The
preferred group of perfluorocarbon liquids far this purpose
is characterized by an average molecular weight in the
range of from about 420 to about 460, and representative of
these are FC-75, RM-101, and perfluorodecalin. Operable
and preferred ultrasound frequency ranges are also dis-
closed, for use with different liquid-filled regions of the
pulmonary air passages. The ultrasound may be produced by
a transducer disposed within the liquid-filled pulmonary
air passages, or the transducer may be disposed exogenous
to the liquid-filled pulmonary air passages. For example,
6056-91(CIP)1.CN -10-
\ac
2U3~~9~
the ultrasound may be transmitted through an intercostal
space of the patient, or it may be transmitted from an
exposed surface of the lung into the volume of same during
an intra-operative application involving an ~~acoustic
window~~ into the lung created by surgical means.
In yet another embodiment, the invention provides
liquid infusion and isolation catheters, intracavitary
ultrasound applicators, and intercostal ultrasound
applicators for practicing the disclosed convection and/or
ultrasound hyperthermia treatments of lung cancer.
In even another embodiment, the invention provides
a means for delivering biologically active agents directly
to at least a portion of the pulmonary system via liquid-
born agents which are either recirculated in and out of the
pulmonary system (e. g., by liquid lavage or liquid ventila-
tion) or maintained static (i.e., non-recirculatedj for
extended periods of time. Breathable liquids are capable
of providing, simultaneously, ventilation during drug
delivery.
In still another embodiment, the invention provides
a means to directly access cardiac output for drug infu-
sion of biologically active agents, when systemic collapse
precludes intravascular administration of such agents.
Other objects, aspects and embodiments of the
invention will become apparent to those skilled in the art
upon reading the following detailed description, when
considered in conjunction with the accompanying drawings
and the appended claims.
Brief Describtion of the Drawings
Figure d depicts a representative liquid infusion
and isolation catheter according to the invention:
Figure 2 depicts a pair of representative inter-
costal ultrasound applicators;
Figure 3 shows a representative intracavitary
ultrasound applicator, and also an optional cuff plug;
6056-91(CIP)1.CN -11-
\ac
~~at
Figure 4 shows 'the construction of a representative
intracavitarytransducer assembly;
figure 5 shows another representative intracavitary
ultrasound applicator;
Figure 6 illustrates in greater detail the repre-
sentative transducer assembly shown in Figure 5;
Figure 7 is a graph indicating the molecular
weights of representative perfluorocarbon liquids;
Figure 8 is a graph indicating the surface tension
(dynes/cm) of representative perfluorocarbon liquids;
Figure 9 is a graph indicating the viscosity at
25°C (CP) of representative perfluorocarbon liquids;
Figure 10 is a graph indicating the density at 25°C
(g/cm3 of representative perfluorocarbon liquids;
Figure 11 is a graph indicating the oxygen solubil-
ity (ml/100m1) of representative perfluorocarbon liquids;
Figure 12 is a graph indicating the boiling point
(°C) of representative perfluorocarbon liquids;
Figure 13 is a graph indicating the vapor pressure
(Tory) of representative perfluorocarbon liquids;
Figure 14 is a schematic depiction of a representa-
tive acoustical test system;
Figure 15 is a graph indicating the velocity of
sound (km/sec) in representative perfluorocarbon lic~ids;
Figure 16 is a graph indicating the acoustic
impedance (Mec~aRayls) of selected tissues and_ representa-
tive perfluorocarbon liquids at 37°C;
Figure 17 is a graph indicating the acoustic
impedance (Rayls x 10&) of representative perfluorocarbon
liquids as compared with water;
Figure 18 is a graph indicating the relationship
between perfluorocarbon cavitation threshold (W/cm) and
temperature (°C) as a function of gas saturation;
Figure 19 is a graph depicting acoustic losses in
perfluorocarbon liquids by plotting the relationship
6056-91(CIP)1>CN -12-
\ac
a~~~~:t'~
~w
between perfluorocarbon acoustic intensity (W/em2) and
electrical intensity (W/cm2) at 1.0 MHz and 25°C;
Figure 20 is a graph depicting acoustic losses in
perfluorocarbon Liquids by plotting the relationship
between perfluorocarbon acoustic intensity (W/cm2) and
electrical intensity (W/cm2) at 0.5 MHz and 25°C;
Figure 21 is a graph depicting acoustic losses in
perfluorocarbon liquids by plotting the relationship
between perfluoracarbon acoustic intensity (W/cm2) and
electrical intensity (W/cml) at 0.25 MHz and 25°C;
Figure 22 is a graph indicating ttze relationship
between perfluorocarbon attenuation (dB/cm) and acoustic
intensity (W/cm2) as functions of temperature (25° or 45°C)
and frequency (MHz);
Figure 23 is a graph indicating the relationship
between acoustic intensity (W/cm2) and electrical intensity
(W/cm2) for FC-75 at 0.25 MHz and 45°C;
Figure 24 is a graph of acoustic intensity (W/cm2
versus electrical intensity (W/cm2), indicating the atten
uatian range of various perfluorocarbons at 1.0 MHz and
25° C;
Figure 25 is a graph of acoustic intensity (W/cm2)
versus electrical intensity (W/cm2), indicating the atten
uating effects of gas saturation in perfluorocarbon FC-75
at 1.0 MHz and 25° or 45°C;
Figure 26 is a graph of attenuation coefficient
(dB/cm) versus frequency (MHz), showing in vitro perfluoro-
carbon-filled lung attenuation at various frequencies
(MHz);
Figure 27 is a graph of sound speed (m/sec) versus
temperature (°C), indicating the predominance of perfluoro-
carbon FC-75 in establishing the sound speed in
liquid-filled lungs; these properties are compared with
blood and muscle;
Figure 28 is a graph of lung temperature (°C)
versus treatment time (min), demonstrating ultrasound
6056-91(CIP)1.CN -13-
\ac
1
~ar:~~:~,
hyperthermi_a of perfluorocarbon-filled pulmonary air
passages;
Figure 29 is a schematic diagram of the Large
Animal Liquid Ventilation System at Temple University;
Figure 30 is a graph of tissue temperature (°C)
versus treatment time (min), demonstrating perfluorocarbon
convection lung hyperthermia as a function of tidal volume
and liquid inspiration temperature;
Figure 31 is a graphical depiction of ultrasound
l0 beam profiles from representative intracavitary applica
tors;
Figure 32 is a graphical depiction of intracavitary
phantom SAR profiles;
Figure 33 is a graphical depiction of intracavitary
applicator axial SAR profiles;
Figure 34 is a schematic diagram of a representa-
tive liquid-filled lung convection hyperthermia and liquid
infusion system, wherein the following abbreviations apply:
IP, insp. pump; EP, exp. pump; ITP, insp. temp. probe; ETP,
exp. temp. probe; IFM, insp. flow meter; EFM, exp. flow
meter; IR, insp. reservoir; ER, exp. reservoir; CV, check
valve; IRTP, insp. res. temp. probe; GCP, gas circular
pump; and FS, free surface;
Figure 35 is a graphical depiction of cardiopul
monary responses to the pulmonary administration of acetyl
choline;
Figure 36 is a graphical depiction of cardiopul-
monary responses to the pulmonary administration of
epinephrine; and
Figure 37 is a graphical depiction of cardiovas-
cular responses to the pulmonary administration of
priscoline.
6056-91(CIP)1.CN -14--
\ac
~~ ~~ r
,j
.~ J nd
Detailed Description_.of the Preferred Embodiments
The i.nventi.on provides, in one embodiment, a method
of treating lung cancer by convection hyperthermia.
Preselected pulmonary air passages that adjoin pulmonary
tissues containing malignant cells are temporarily filled
with a liquid medium such as physiological saline solution
or, preferably, a perfluorocarbon liquid. By "pulmonary
air passages" is meant the pulmonary channels, spaces or
volumes in the trachea, left and right bronchi, bronchi-
oles, and alveoli of the lungs that are normally occupied
by air. In the practice of the invention, only the pul-
monary air passages in contact with or near a patient's
tumor sites) are typically filled with the liquid medium,
and gaseous ventilation of the remaining pulmonary air
passages is maintained. Depending on the location of the
lung cancer, as determined by available diagnostic methods,
the fluid-filled pulmonary air passages may be localized in
a lung, lobe or lung segment, and/or the bronchial tree may
be selected for localized filling with, the liquid medium.
Localized filling of the pulmonary air passages in such a
preselected manner can be effected by means of the repre-
sentative infusion catheters described below. Diagnostic
ultrasonic imaging can be used to monitor the filling of
the pulmonary air passages, if either physiological saline
or a perfluorocarbon liquid serves as the liquid medium.
During the filling step, the perfluorocarbon liquid is
preferably degassed at least 50%, and is most preferably
substantially (almost totally) degassed.
To effect the localized convection hyperthermia
treatment, exogenously heated liquid medium having a
temperature in the range of from about 41° to about 50° C,
and preferably from about 42° to about 45°C, is circulated
through the liquid-filled pulmonary air passages for a
period of time that may be determined at the discretion of
the attending physician. During, prior to or subsequent to
this hyperthermic treatment, the malignant cells may be
6056-91(CIP)1.CN -15-
\ac
y~ ~~
°'' tip 2I' :.f
irradiated with ionizing radiation such as x-rays, electron
beams, neutron beams, etc. To potentiate the effects of
such radiation treatments, the liquid medium in the
fluid-filled pulmonary air spaces may be oxygenated. In
treatments where the preselected pulmonary air passages are
initially filled with substantially degassed perfluoro-
carbon liquid, exogenously heated oxygenated perfluoro-
carbon liquid may be circulated into the liquid-filled pul-
monary air passages after the filling process is complete,
prior to and/or during irradiation of the malignant cells
with the ionizing radiation.
The circulating liquid medium may also contain a
biologically active agent, e.g., a therapeutic agent such
as an anti-cancer drug (e. g., adriamycin), toxin,
antibody-linked radionuclide, etc. In treatments where the
adjunctive use o.f such water-soluble therapeutic agents is
desirable, the liquid medium may be an aqueous
perfluorocarbon liquid emulsion.
After the hyperthermic treatment period, which as
mentioned will vary in a patient-specific manner, depending
partly upon the tumor location and any adjunctive therapies
employed, the liquid medium is removed from the pulmonary
air passages of the patient.
A preferred liquid medium for this convection
hyperthermia treatment is a perfluorocarbon liquid of 'the
general type used for lung ventilation. Suitable per
fluorocarbon liquids having the requisite thermal as well
as physical properties for use in convection pulmonary
hyperthermia include perfluorocarbon liquids characterized
by an average molecular weight, of the perfluorocarbon
constituent(s), in the range of from about 350 to about
560. Such perfluorocarbon liquids are alternatively
characterized by having a viscosity less than about 5 CP at
25°C, a density less than about 2.0 g/cm3 at 25°C, a
boiling point greater than about 55°C, a vapor pressure
greater than about 20 Torr but less than about 200 Torr at
6056-91(CIP)1.CN -16-
\ac
25°C, a surface tension less than about 17 dyne/cm at 2.5°C,
and a Prandtl number less than about 7_0 at 25°C. To
provide some adjunctive respiratory support, and for use
with radiation therapy, and to provide efficient lung
filling in the degassed state, the perfluorocarbon liquid
should also have an oxygen solubility greater than about
40m1/100m1. Representative perfluorocarbon liquids that
meet the above criteria include FC-84, FC-72, RM-82, FC-75
(3M Company, Minneapolis, MN), RM-101 (MDI Corporation,
Bridgeport, CN), dimethyladamantane (Sun Tech, Inc.),
trimethylbicyclononane (Sun Tech, Inc.), and perfluoro-
decalin (Green Cross Corp., sapan). The preferred group of
perfluorocarbon liquids, in terms of optimizing the opera-
tive combination of physical and thermal properties, are
characterized by an average molecular weight in the range
of from about 400 to about 460. Such perfluorocarbon
liquids are characterized by having a vapor pressure less
than about 100 Torr. The most preferred perfluorocarbon
liquids have an average molecular weight in the range from
about 420 to about 460, and representative of this group
are FC-75, RM-101, and perfluorodecalin.
The invention also provides an ultrasonic hyper-
thermic treatment of lung cancer. In this embodiment,
after the pre~~elected pulmonary air passages adjoining the
patien't's malignant cells are filled with the liquid medium
such that an adequate and appropriate acoustic transmission
path has been established, the pulmonary tissues containing
the malignant cells are heated to a temperature in the
range of from about 41° to about 50°C by transmitting
ultrasound through the liquid-filled pulmonary air pas-
sages. In a preferred embodiment, the ultrasound is
produced by an intracavitary transducer that is positioned
within the liquid-filled pulmonary air passages. Alter-
natively, the transducer may be located exogenous to the
pulmonary air passages. For example, the ultrasound can be
transmitted through an intercostal space between the ribs
6056-91(CIP)1.CN -17-
~aG
of the patient, or the transducer can be applied to the
pulmonary pleura or lung surface overlying the fluid-filled
passages, following surgical displacement of ribs or other
interfering tissues.
In order to serve as a suitable acoustical propa-
gating medium in this ultrasonic hyperthermic treatment,
the perfluorocarbon liquid should have the following
physical., thermal, and acoustical properties: viscosity
less than about 5 CP at 25°C, density less than about 2.0
g/cmJ at 25°C, boiling point greater than about 75°C, vapor
pressure greater than about 25 Torr and less than about 100
Torr, acoustic impedance between about 0.8 to about 1.6
MegaRayls at 37°C, arid acoustic attenuation less than about
2.2 dB/cm (~20~) at 1.0 MHz, 45°C, and acoustic intensity
of about 3 W/cm2. The perfluorocarbon liquid is preferably
also characterized by an oxygen solubility greater than
about 40m1/100m1. Perfluorocarbon liquids having an
average molecular weight in the range of from about 400 to
about 500 generally satisfy the above criteria, with the
2o preferred group in terms of optimizing the thermal and
acoustical properties having an average molecular weight in
the range of from about 400 to about 460, and most prefer-
ably in the range of about 420 to about 460. Representa-
tive of this most preferred group of perfluorocarbon
liquids are FC-75, RM-101, and perfluorodeca7.in.
In tre:atmenta where the preselected liquid-filled
pulmonary air spaces axe localized in the bronchial tree,
the ultrasound from an intracavitary transducer preferably
has a frequency in the range of from about 250 KHz to about
3 MHz, and most preferably from about 500 KI-Iz to about 2
MHz. For peripheral lung treatments (i.e., in the mem-
branous airways and alveoli of the lung), where the sound
waves must necessarily traverse many more liquid-tissue
interfaces, a lower ultrasound frequency in the range of
from about 250 KFiz to about 1.5 MHz is necessary when
perfluorocarbon liquids serve as the liquid medium.
6056-91(CIP)1.CN -18-
\ac
2n~~~~2
Ultrasound frequencies in the latter range are also recom-
mended when the transducer is positioned exogenous to the
lung.
The desired frequency within these ranges is
established on the basis of the depth of heating sought.
Lower frequencies are attenuated less and, therefore, are
employed where deeper heating is preferred. Conversely,
higher frequencies are more readily absorbed, and thus are
more appropriate for more superficial heating. optimal
treatments may include a combination of the following
strategies. First, a single transducer may broadcast at
more than one frequency to effect a desired heating pat-
tern. The changes in frequency in this case may be done by
rapid incremental changes in frequency over a specified
bandwidth using frequency modulation (FM) methods, or they
may be done with serial changes over time whereby sound (in
FM mode or not) is generated in predetermined frequency
ranges for desired periods and then changed to other
frequencies for periods of time. Second, multiple trans-
ducers (focused, diverging, or unfocused) may be employed
to .operate in tandem at similar or different frequencie~...._ ..
(in FM mode or not) to effect desired heating patterns.
Where physiological saline serves as the liquid
propagating medium, the ultrasound can be in the frequency
range of from about 250 KHz to about 3 MHz from intracavi
tary transducers, and in the range of from about 500 KHz
(preferably about 750 IQiz) to about 3 MHz from exogenous
transducers.
While the perfluorocarbon liquid is preferably
degassed during the filling step, oxygenation of the liquid
may be desirable (e. g., for radiation treatment or respira
._ tory support) during the ultrasonic hyperthermic treatment,
However, in order to suppress cavitation, the dissolved
gas content (including oxygen, air, nitrogen, carbon
dioxide or other gases) of the perfluorocarbon liquid in
the liquid-filled pulmonary air passages should be held at
6056-91(CIP)1.CN -19-
\ac
2~3~~~~
no more than about 75% of saturation for ultrasonic treat-
ments in the 2-3 MHz range. No more than about 500 of
saturation should be permitted for ultrasonic treatments in
the 250 KHz to 1.5 MHz range. The requisite dissolved gas
content can be maintained by circulating the perfluorocar-
bon liquid into and out of the lung during the treatment
between the liquid-filled pulmonary air passages and an
extraneous source of gas-content processing, such as a
degassing chamber.
The invention also provides liquid infusion cathe-
ters, intracavitary ultrasound applicators, and exogenous
ultrasound transducers, representative embodiments of which
are shown in Figures 1-5. Prior bifurcated bronchial
catheters that have been used for delivering liquid into a
lung are not suitable for use in the subject convection and
ultrasonic hyperthermia treatments, for a number of rea-
sons. First, the subject treatments can be applied deeper
in the lung than heretofore possible, and prior commercial
devices lack sufficient flexibility and length to reach
many of the segmented bronchi. In addition, the inflatable
cuff material used in the prior devices...tends.ao lose its
structural integrity at the relatively high fluid tempera-
tures involved in the subject treatments. Furthermore, the
prior devices are in general too large in diameter to
penetrate several of the pertinent segmental bronchial
passageways in the lungs, and they also provide no instru-
mentation for monitoring local transient and steady state
temperature, and pressure, and are ill-suited for posi-
tional information.
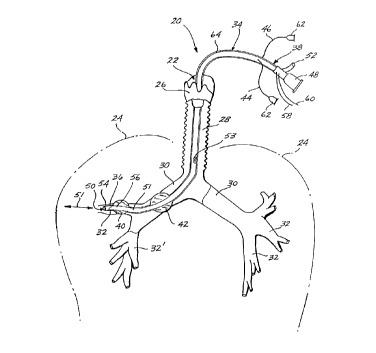
Referring initially to Figure 1, a representative
embodiment of the subject liquid infusion and isolation
catheter 20 is shown in conjunction with the pulmonary air
passages 22 that lead to and ramify throughout the lungs
24. More particularly, catheter 20 is shown passing
through the larynx 26 and trachea 28 and into a bronchus 30
and associated segmental bronchi 32.
6056-91(CIP)1.CN -20-
\ac
~~3~~:~Z
Catheter 20 includes a flexible conduit 34 having a
distal end 36 that is positioned, in this instance, within
segmented bronchus 32, and a proximal end 38 that is
positioned outside (or exogenous to) the patient. The
representative embodiment shown in FIGURE 1 has a pair of
inflatable cuffs 40 and 42 formed near the distal end 36
that are in fluid (liquid or gaseous) communication with
corresponding channels 44 and 46 that exit the conduit 34
near the proximal end 38. Also shown at the proximal end
38, a liquid inlet/outlet connector 48 is in fluid communi-
cation through a liquid passageway 51 with an opening 50 at
the distal end 36 of conduit 34. A gas ventilation channel
52 also is formed in the conduit 34 to be in fluid communi-
cation with a ventilation port 53 positioned so as to
ventilate the bronchial tree. A pressure sensor 54 and
temperature sensor 56 are positioned near the distal end
36, and have lead wires 58 and 60, respectively, passing
through the conduit 34 and exiting at the proximal end 38.
The temperature sensor 56 may take the form of a thermis-
tor, thermocouple, resistance-based temperature device,
etc. Suitable pressure sensors 54 include: solid-state
piezoresistive diaphragm-based sensors, semiconductor
strain gage sensors, etc.
The conduit 34 is typically formed from flexible
plastics, such as a Teflon'", silicon rubber, polyurethanes,
polyvinylchloride, Delrin'", or acetyl copolymers, or
combinations thereof, having an outer thermal insulation
layer 64 formed, for example, of a closed-cell plastic or
rubber, to reduce heat loss to the tissues in contact with
it, between the connector 48 and outlet 50 or at least the
most proximal cuff 42. Alternatively, effective thermal
,- insulation can be achieved by proper selection of the
catheter material itself and its channel wall thicknesses.
To minimize diameter and maximize flexibility, the conduit
34 is typically extruded to have the gas ventilation
channel 52, the fluid channels 44 and 46, and the liquid
6056-91(CIP)1.CN -21-
\ac
d
passageway 51 integrally formed therein. The above ele-
ments may alternatively be separately .formed and bound in a
common sheath (not shown), although this may disadvantage-
ously affect the diameter and flexibility of the conduit
20.
The cuffs 40 and 42 are preferably constructed of
polyurethane or other distensible material that will
maintain structural integrity when stretched and yet not
lose elasticity when subjected to high temperature liquids.
The cuffs 40 and 42 are concentrically formed about the
conduit 34 to be selectively inflated and deflated via
liquids such as physiological saline or perfluorocarbon
liquids, or gas such as air, through the channels 44 and
46. A suitable connector 62, such as a Leur lock fitting,
is located at the terminal end of each channel 44 and 46 to
provide attachment to a source of liquid or gas such as a
lockable syringe or a hand or mechanical pump. In the
circumstance rahereby liquid is the preferred cuff inflation
fluid, it is likely that some liquid will have been placed
in the cuff prior to use, to insure a gas-free volume
inside the cuff. When inflated, the cuffs 40 and 42 bear
against the encircling inner walls of the trachea 28,
bronchus 30, and/or lobar or segmented bronchus 32 (depend-
ing upon the positioned location of catheter 20 in the
pulmonary air passages 22), in order to locally seal the
lumen (3) of the airways) to prevent the passage of liquid
and gas during the hyperthermic treatment. Although a pair
of cuffs 4U and 42 are shown, one or both may be elim-
inated, e.g., if both lungs are to be filled with the
fluid. Additional cuffs may also be used to provide the
requisite sealing. The number of cuffs used will depend on
where the hyperthermic treatment is being directed in the
lung, the passageways to be isolated and those to be kept
gas ventilated, and the length of the catheter 20. In this
regard, the cuffs 40 and 42, when required, are sized
according to their application, i.e., whether they will be
6056-91(CIP)1.CN -22-
\ac
positioned in a large lobar bronchus (0.83 cm average diam-
eter) or in a smaller segmental bronchus (0.56 cm average
diameter). Cuffs sized to dam the main bronchi (1.2?. cm
average diameter) and trachea (1.8 cm average diameter) can
also be readily fabricated. The use of two cuffs 40 and 42
in Figure 1 is for illustration purposes only and is not
meant to imply that the untreated distal pulmonary segments
32 are to be unventilated by gas. In use, the catheter
configurations) will be selected to reflect the require-
ment to gas ventilate untreated, air-filled portions of the
lung.
The gas ventilation channel 52 is used to provide
respiratory gas exchange to the portions of the lungs 24
not sealed off by the cuffs 40 and 42 or filled with the
liquid. The channel 52 is preferably coupled to an appro-
priate machine, such as a mechanical ventilator, to supply
gas through the port 53 formed in the wall of the conduit
34. In the absence of such a connection air ventilation
may occur by the gas being drawn into channel 52 from room
air by the natural respiratory motion of the lung.
The liquid connector 48 is attached to a liquid
infusion system, such as described below. briefly, such a
system provides Liquid for the desired treatment at a
controlled but variable tidal volume and frequency, and at
a controlled temperature and gas content. The pressure
sensor 54 and the temperature sensor 56 positioned at the
delivery end 36 permit monitoring of the temperature and
pressure of the liquid within the liquid filled air pas-
sages. Additional sensors may be positioned at any point
along the conduit 34 to permit comparative measurements and
to permit flow rate information in the catheter to be
obtained from dynamic measurements.
In use, the catheter 20 may be fitted with a rod
(not shown) formed of bendable material, such as aluminum,
that is bent, prior to insertion in most cases, to a
configuration designed to guide the catheter 20 through the
6056-91(CIP)1.C1V -23-
\ac
S.I '..~ !.j ~r~ ~ : d
trachea 28 to the desired location in the pulmonary air
passageways 20. A fiber--optic assembly may be used either
alone or in conjunction with the rod to provide visual
confirmation of the positioning of the catheter 10. Such
a fiber-optic assembly, including an optical fiber having a
lens, may be integrated into or associated with the cath-
eter 20, and coupled to a light source and an eyepiece to
permit observation via video camera, still photographs, or
the eye. A fiber-optic bronchoscope may be alternatively
l0 inserted through liquid passageway 51 for the same purpose.
To assist in measuring distances to various parts of the
lung, the outer surface of catheter 20 may be provided with
distance indicator marks in spaced array.
Once the catheter 20 is in position, the various
connectors at the proximal end are connected to the appro
priate machines and monitoring devices. For instance, the
liquid inlet/outlet connector 48 is attached to a liquid
infusion system, and the fluid line connectors 62 are
attached to suitable sources of liquid or gas. The cuffs
40 and 42 are inflated as necessary to seal off the pul-
monary air passages adjoining the cancer cells while
maintaining ga.s communication to untreated lung volume.
The gas ventilation channel 52 is hooked to a mechanical
ventilator and a suitable gas mixture is supplied through
the port 53 to the unaffected air passageways. With
temperature and pressure being monitored, liquid from the
infusion system is sLbpplied through 'the liquid passageway
51 to the, in this instance, bronchiole 32 at a controlled
frequency and tidal volume (indicated by arrow 51).
Following the hyperthermic treatment, the liquid can be
removed from the pulmonary passages 20 by suction, by
gravity (i.e., placing the patient tilted with the head
down in the so-called "Trendelenburg" posture), and by
evaporation.
The liquid infusion and isolation catheter 20 may
also be used in conjunction with external intercostal
6056-91. (CIP) 1. CN -24-
\ac
ultrasound applicators to provide the means for liquid
filling and to provide additional heating and/or cooling to
the tumor site. For instance, as shown in FIGURE ?., a pair
of intercostal applicators 66 and 68 are placed externally
on the patient to direct sound waves between the ribs 70
and into the peripheral. portions and bronchial spaces of
the lung 24. These ultrasound power applicators 66 and 68
are composed of long aspect-ratio rectangular transducers
74, operated either singly or as a synchronous or asynchro-
nous pairs. These applicators 66 and 68 can have flat
(plane wave), broad-band unfocused transducers 74 or may
have curved, .focused transducers. Ideally these will be
operated in the range from 250 KHz to 1 MHz.
Such applicators 66 and 68 can be used in conjunc
tion with a liquid infusion and isolation catheter 20 to
apply heat both convectively and ultrasonically to a
specific portion of the lung 24. Although it would appear
that a venetian blind or striped pattern of heating would
result from this arrangement, it should be noted that the
targeted tissue can be °°scanned°° up and down in
front of
the transducer array by a cyclic variation of the inflation
pressure of the lung 24. This induced variation may be
large or small, according to the motion desired. Likewise,
the overall po~:ition of the tumor to be treated may be
located with respect to the applicators 66 and 68 by virtue
of inflation or deflation of the lung 24. Also, the
respiratory motion normally present in the lung 24 may be
suppressed by imposing a constant liquid infusion pressure
at the desired level. Although not shown, it is to be
understood that the applicators 66 and 68 may be in fixed
position relative to each other, such as by mounting to a
jag or frame.
Although not shown in this view, a transducer can
alternatively be applied directly to the body of the lung
following surgical resection of a rib or other interfering
tissues. The transducer for this application will typical-
6056-91(CIP)1.CN -25-
\ac
X03 ~4~2
ly be supplied with a bolus of degassed coupling liquid,
also serving the function of cooling the transducer and
tissue surfaces.
Another method of providing ultrasound hyperthermia
is to place an ultrasound applicator within the
fluid-filled pulmonary air passage near the tumor to be
treated. Figure 3 shows a representative embodiment of
such an intracavitary applicator 76 for delivering an
ultrasound transducer 78 to the treatment site. To facili-
tate the description, the reference numbers used in Figure
1 are correspondingly employed in Figure 3 (and in Figure
4, described below). The intracavitary applicator 76 of
Figure 3 includes a conduit 80 having a distal end 82
positionable within the pulmonary air passages 22 and a
proximal end 84 that remains outside the thoracic air
passage 22. The conduit 80 encases a ventilation passage-
way 86 passing through the transducer 78 in fluid communi-
cation with the pulmonary air passages 22 through a distal
opening 88. The passageway 86 terminates at the proximal
end 84 of the conduit 80 with a coupling 90 for attachment
to a respirator (not shown). The conduit 80 also houses a
liquid inlet port 92, typically positioned distal to the
transducer 78, and a liquid return port 94 positioned, in
this instance, proximal to the transducer 78. The liquid
inlet port 92 is in fluid communication with a liquid inlet
coupler 96, and a liquid return port 94 is in fluid commun-
ication with a liquid return coupler 98, both couplers 96
and 98 being located at the proximal end 84 of the conduit
80. Formed concentrically about the ventilation passageway
86 and positioned distal to the transducer 78 and liquid
ports 92 and 94 is an inflatable cuff 100. A fluid line
coupling 102 is in fluid communication with the cuff 100,
for connecting the cuff 100 to a suitable source of pres-
surized liquid or gas (e. g., air). Power cables 104 pass
through the conduit 80 to provide high frequency electrical
power to the transducer 78.
6056-91(CIP)1.CN -26-
\ac
This conduit 80 is constructed with similar materi-
als and by similar methods as the liquid infusion catheter
20 described above. Here, in Figure 3, the transducer
assembly 78 is positioned concentrically around the vent-
s ilation passageway 86. In this manner, the distal cuff
100, when inflated, serves to dam the proximal pulmonary
passages 30'. The distal. cuff 100 also anchors the distal
end 82, of the conduit 80, and thereby permits the trans-
ducer 78 to be manipulated into position in the center of
the bronchus 30 (or trachea 28) to avoid contact with the
bronchus wall 106 and the tumor 108. The cuff 100 is
otherwise substantially the same as the cuffs 40 and 42
described above with respect to Figure 1. When the cuff
100 is inflated, it seals off the bronchus 30 so that a
degassed liquid propagating medium 110 can be supplied to
and fill the bronchus 30 through the liquid inlet port 92,
to provide acoustic coupling and secondarily to cool the
transducer assembly 78. Circulation of the liquid 110 may
be accomplished by circulating liquid from the bronchus 30
through the liquid return port 94 to a liquid supply
system, such as described below.
In order to prevent filling of the other lung, if
that is desirable, an aptional cuff plug 112, which is
independent of the intracavitary transducer and its support
shaft and conduit 80, is inserted within the other bronchus
30', and its degree of distension is controlled with
pressurized liquid or gas supplied through a line 114.
Respiration is accomplished through the one lung by supply-
ing air through the ventilation passageway 86. Although
not shown, it is to be understood that the cuff assembly
112 and 114 may, and preferably should, be supplied with a
separate ventilation passageway (not shown) in order to
ventilate the pulmonary air passage 32 distal to cuff plug
122. Pressure and temperature sensors (not shown) may also
be disposed and used as desired, such as described above
with respect to figure 1. Installation of the intracavi-
6056-91(CIP)1.CN -27-
\ac
~9~ N. Pj ~ S'
~,
terry appl:icatar 76 can be accomplished substantially the
same way as described above with respect to the liquid
infusion and isolation catheter 20. Positioning of the
transducer 78 with respect to the 'tumor 108 is accomplished
by rotating tkae conduit. 80 as shown by the rotational arraw
116.
The construction of such a representative intra-
cavitary transducer assembly 78 is shown in greater detail
in Figure 4. Here, one approach to providing selective
directional heating patterns is illustrated. Figure 4
shows a thin-walled piezoelectric ceramic cylinder 180 that
is longitudinally and circumferentially sectioned into four
separate power transducers, with transducers 120 and 122
formed to have an arcuate cross-sectional shape of approx-
imately 120°, as indicated by angle B; and with transducers
124 and 126 formed to have an arcuate cross-sectional shape
with an included angle of approximately 240°, as repre-
sented by angle ø . Leads 128 supply power to the trans-
ducers, and the ventilation passageway 86 is shown, in this
instance, passing coaxially through the cylinder 180. This
multiple-transducer approach provides flexible heating
patterns. For instance, with transducers 120 and 122
driven in parallel, a 120° pattern can be achieved.
similarly, with transducers 124 and 126 driven in parallel,
a 240° heating pattern can be achieved. Finally, with all
of the transducers being driven 'together, a full 360° of
heating can be achieved along the length of the cylinder
180. Of course, full 360° heating patterns may also be
achieved by cylindrical piezoelectric cylinders that are
not sectioned.
Although the transducer assembly 78 is shown
mounted coaxial with the conduit 80, it is to be understood
that other positions and transducer configurations can be
used. For instance, transducers formed of flat plates may
be associated with or placed adjacent to the conduit 80 to
radiate sound waves in one or more directions. Likewise,
6056-91 (CIP) 1. C1V -2$-
\ac
2~~~49?
the transducers 124 and 126 may be eliminated to leave only
the transducers 120 and 122 mounted adjacent the conduit
80.
Figure 5 illustrates yet another representative
embodiment of an intracavitary ultrasound applicator 130,
in which a transducer assembly 132 positioned within a
self-contained liquid-filled sac 134 for acoustic coupling
and cooling. This applicator 130 includes a conduit 136
having a distal end 142 positioned within the bronchus 30
and a proximal end 144 positioned autside of the patient's
body. A ventilation passageway 138 is formed within or
associated with the conduit 130 having a ventilation port
140 formed approximately midway down the conduit 136 and an
air line coupling 146 located at the proximal end 144 for
attachment to a respirator (not shown). While not shown in
this view, a ventilation passageway can also be provided
to the distal end 142 if desired.
The conduit 136 also houses one or more liquid
passageways that supply liquid from a liquid inlet coupling
148 to the distensible sac 134, and circulate liquid back
to a liquid outlet coupling 150. The couplings 148 and 150
may be connected to a self-contained liquid supply system
or a larger system containing a separate power supply
circuitry and fluid flow module that circulates a degassed
liquid at a controlled temperature far cooling the trans-
ducer assembly 132 and providing an acoustic coupling
between the transducer assembly 132 and the pulmonary
tissues and tumor 152. It is also possible to derive the
coupling/cooling fluid from the liquid infusion system that
supplies liquids to the lung. The sac 134 is constructed
of a thin, pliable material, such as polyurethane, that
readily ,conforms to the shape of an abutting pulmonary
tissue or tumor to facilitate heating of the tumor. A
fiber-optic assembly is shown as part of the applicator 130
having one or more optical fibers (not shown) passing
through the liquid sac 134 and the transducer 132. The
6056-91(CIP)1.CN -29-
\ac
fiber-optic assembly includes a lens 156 positioned on the
distal end 14?. of the conduit 136, an optical coupler 158
at the proximal end 144 to facilitate viewing through the
lens 156 as previously described, and a light source that
is supplied through cables 160 that also include power
cables for the transducer assembly 132. 1~ cuff 162 typ-
ically is formed on the conduit 80 distal to the transducer
assembly 132, to be inflated and deflated through a cuff
fluid line coupling 164 that is connected to a source of
pressurized liquid or gas. This cuff 162 serves primarily
an anchoring function, to assist and maintain acoustical
positioning of the transducer 132 and liquid-filled sac 134
at the tumor site 152.
Both of the intracavitary applicators 76 and 130
described above can be positioned in the bronchial tree by
first locating the tumor target via a flexible bronchoscope
that indexes the lengths of the passageways and the posi
tion of the tumor. The applicator is then guided down the
airways with the aid of a bendable rod, as described above.
Such a rod is first bent slightly and then fed down one of
the inner passageways of the applicator. The bend of the
rod is sufficient to bend the distal end of the applicator
in the desired direction. Supplementing this steering
approach is a system of fiduciary marks taken from or
correlated with the bronchoscope traversal that establishes
the length required to descend down the airways. Finally,
the fiber-optic assembly 154 can be used alone or in
conjunction with the rod to accurately position the trans-
ducer assembly adjacent to the tumor to be treated.
The intracavitary applicator 130 may also be
configured for hyperthermic treatment in other body cavi-
ties, e.g., the mouth, esophagus, uterus, or rectum, in
which case a cuff may be provided for auxiliary anchoring
purposes.
Figure 6 illustrates in greater detail a represen-
tative transducer assembly 132 for use in conjunction with
6056-91(CIP)1.CN -30-
\ac
63 ~S 4"~ .... ~ f;
~d da _t . i :~ zy ~:,i
the distensible acoustic-coupling sac 134. EIere, the
conduit 136 is shown in cross section having a liquid inlet
passageway 166 centrally positioned within a concentric
liquid return passageway 168. The liquid 170 passes
through a manifold 172 into the lumen of the sac 234 to
distend the sac 134 and circulate around the transducers
174. The liquid 170 then passes through the manifold 172
and into the return passageway 168. The circulation of the
liquid 170, which is normally degassed water, aids in
cooling the transducers 174 and provides an acoustic
coupling for the ultrasound waves 176. Although not shown,
it is to be understood that cuff fluid lines and the
fiber-optic assembly lines can be constructed to pass
axially through the sac 134 and the transducers 174 to
distal positions along the conduit 136.
Another embodiment of the invention provides a
means for delivering biologically active agents, through
the pulmonary air passages of a patient, for treating,
controlling and/or diagnosing pulmonary and/or systemic,
diseases, conditions and/or abnormalities. In this
embodiment of the invention, the biologically active agents
are delivered into at least a portion of the pulmonary
system via the .implementation of liquid lavage/wentilation
of at least a portion of the patient's pulmonary air
passages. Specifically, in this embodiment of the
invention, biologically active agents are delivered into at
least a portion of the patien't's pulmonary system via
liquid-borne agents which are either recirculated in and
out of the preselected partion of the pulmonary air
passages in a liquid lavage fashion or maintained static
(non-recirculated) for extended periods of time. If
breathable liquids are used, pulmonary delivery of
biologically active agents can be performed with
simultaneous pulmonary ventilation.
As used herein, the phrase "biologically active
agents" refers not only to physiologically-active agents
6056-91(CIP)1.CN -31-
\ac
,~ e3 .
t p e:'w:r ~~-'. i~
(e. g., rhemotherapeutic agents), but also to agents which
may be physiologically inert but are nonetheless
biologically active in that they are useful as e.g.,
diagnostic agents.
As used herein, the phrases "liquid lavage",
"liquid ventilation", and/or "liquid lavage/ventilation"
individually and collectively refer to gravity-assisted
and/or mechanically-assisted passing of liquid mediums
through at least a portion of a patient's pulmonary air
passages. The liquid mediums being passed therethrough
need not, necessarily be "breathable'° (e. g., in those
instances when a liquid lavage process is employed solely
for washing/rinsing a portion of the lungs). However, when
employed in liquid ventilation, it is preferable that the
liquids have the ability of gas exchange.
This embodiment of the invention, pertaining to the
pulmonary administration of biologically active agents,
provides a method for treating, controlling and/or
diagnosing a patient's pulmonary-related diseases, condi-
tions and/or abnormalities. This new method is especially
useful when treating, controlling and/or diagnosing condi-
tions wherein blood is preferentially shunted away from
diseased pulmonary regions and, thereby, systemically
delivered agents are at least partially precluded from
reaching these regions. This embodiment is also useful as
a means for introducing agents such as surfactants,
steroids, antibiotic agents, chemotherapeutic agents,
chemotactic agents, diagnostic agents, and the like,
primarily, if not exclusively, into the pulmonary system,
when systemic absorption of such agents is undesirable.
The implementation of liquid lavage/ventilation
techniques, as a vehicle for delivering biologically active
agents to at least a portion of a patient's pulmonary
system, is of particular importance for many reasons.
examples of some of the advantages associated with the pul-
monary administration of biologically active agents
b056-91(CIP)1.CN -32-
\ac
~~3~~~?
includes, but are not limited to, the following: (a) it
results in the homogenous delivery of the agents throughout
the pulmonary system for treating, controlling and/or
diagnosing diffuse diseases, conditions and/or abnorm-
alities, while simultaneously supporting gas exchange, if
desired: and/or (2) it can be employed to selectively
deliver biologically active agents to desired areas of the
pulmonary system for treating controlling and/or diagnosing
local diseases, conditions and/or abnormalities. In each
of the aforementioned instances, the process of the
selective pulmonary administration of biologically active
agents minimizes normal, healthy, delicate pulmonary
tissues from being exposed to toxic agents, such as is
often encountered during conventional systemic chemothera-
peutic and/or diagnostic techniques.
When practicing the embodiment pertaining to the
pulmonary administration of biologically active agents, the
agents, passing through at least a portion of the patient's
pulmonary air passages, can react with, and/or diagnose,
the patient's biological system in a number of ways. For
example, the agents introduced in accordance with the
invention, by pulmonary administration, may be used in the
following ways: (a) to react directly on and/or diagnose
the patient's pulmonary system, (b) to react on and/or
diagnose both the patient's pulmonary and systemic system,
and/or (c) to differentially react on and/or diagnose
specified regions of the patient's pulmonary system.
Through research, it was discovered that there are
many advantages of delivering biologically active agents
directly to the surface of the pulmonary air passages (e. g.
lungs) via liquid lavage/ventilation. Some of the more
important advantages include, but are not limited to, the
following:
1. The delivery of the desired biologically
active agents directly through a patient's
pulmonary air passages is enhanced by several
6056-91(CIP)1.CN -33-
\ac
Y.A ~ <1 J
physiological principles, such as, for example,
(a)
the large exchange surface area of the lung (i.e.,
from about 50 to about 100 m2), (b) the entire
cardiac output passes through the pulmonary
capillary bed, (c) the thin barrier (i.e., alveolar
wall thickness) and the short diffusion distances
enhances absorption of the agent, and {d) the
uniform distribution of low surface tension liquids
throughout the pulmonary system.
2. In many cases, the action of the
biologically active agents (e.g., surfactants to
lower pulmonary surface tension, bronchodilators
to
relax airway smooth muscle, pulmonary vasodilators
to increase pulmonary blood flow, steroids for lung
inflammation, chemotactic agents, chemotherapeutic
agents and/or diagnostic agents for lung cancer,
and the like) are exclusively directed to a portion
of the patients pulmonary air passages (e. g.,
lungs) and would be undesirable in the rest of the
body.
3. In diseased and/or abnormal lungs, a
common problem is poor distribution of pulmonary
blood flow and ventilation. This problem is
obviated in the liquid-filled lung, in that liquid
and blood flow are uniformly distributed and
matched. This physiological principle enables
efficient exchange of biologically active agents
into a lung where exchange would otherwise be
impossible.
4. Liquids can be selectively directed to
specific regions of the patients pulmonary air
passages by a number of different conventional
means, such as a bronchoscope, a conventional
catheter or even a specialized catheter, similar
to that employed in the hyperthermia treatment
mentioned earlier. This capability of selectively
6056-91(CIP)1.CN~ -34-
\ac
~~~~~.~~?
directing liquids comprising biologically active
agents would be particularly useful when only a
specific region of the patient's pulmonary system
requires delivery of such agents (e. g.,
chemotherapeutic cancer drugs which may be harmful
to normal, healthy lung and body tissue in high
concentrations and agents which facilitate
pulmonary debridement).
5. In the case of systemic vascular com
promise or shock, intravascular administration of
agents is ineffective under conventional practices.
However, the passage of the necessary biologically
active agents through at least a portion of the
patient's pulmonary air passages by liquid
lavage/ventiJ.ation techniques provides a direct
route for agent administration.
When practicing the embodiment of the invention
pertaining to the pulmonary administration of biologically
active agents, the selected liquid is augmented with the
selected agents. These agents can be present in the liquid
medium in any suitable form (e. g., bulk farm, a suspension,
a dispersion, a liquid form, an emulsion form, encapsulized
and the lika~ and/or combinations thereof). 'fhe particular
form of the agent will depend upon many different vari-
ables (e. g., the specific agent being used, the area being
treated and/or diagnosed, the condition and/or abnormality
being treated, controlled and/or diagnosed, the parameters
under which the liquid lavage/ventilation process is
performed, etc.).
Moreover, the selected biologically active agents
can be incorporated into the liquid medium by any suitable
technique. Examples of suitable incorporation techniques
include, but are not limited to, injection, blending, dis-
solving, employing conventional incorporation procedures
and incorporation of specific incorporation procedures
(see. e.g., Figure 38).
6056-91(CIP)1.CN -35-
\ac
Any suitable biologically active agent can be
employed when practicing this embodiment of the invention.
Examples of suitable agents include, but are not limited
to, anti-cancer agents, vasoconstrictors, vasodilators,
bronchoconstrictors, bronchodilators, surfactants, ster-
oids, antibiotic agents, chemotactic agents, toxins, anti-
body-linked radionuclides, diagnostic agents, contrast
agents, and the like, and/or combinations thereof.
When employing vasoconstrictors, vasodilators,
bronchoconstrictors and/or bronchodilators (e. g., epineph
rine, acetylcholine, priscoline and sodium nitroprusside),
they can be used in any suitable amount necessary to
achieve the desired results, in view of the specific condi
tions, diseases and/or abnormalities present. For example,
the amount of these biologically active agents can range
from between about 0.001 to about 10.0 mg for each kilogram
of body weight of the patient whose physiological
conditions, diseases and/or abnormalities are being
controlled, diagnosed and/or treated in accordance with
this embodiment of the invention. In another instance, it
may be desirable to have the amount of these biologically
active agents range from between about 0.004 to about 7.0
mg/kg, or from between about 0.007 to about 4.0 mg/kg, or
from between about 0.01 to about 1.0 mg/kg.
As indicated above, the amount of biologically
active agent emp:Loyed depends, in part, on the specific set
of circumstances revolving around each individual case.
In addition to the above, this embodiment is
particularly use:Eul for delivering anti-cancer drugs (e. g.,
adriamycin), toxins, antibody-linked radionuclides, and the
like and/or combinations thereof, to at least a portion of
the patient's pulmonary system by being passed through the
patient's pulmonary air passages.
Any suitable liquid can be used as the liquid
carrier when practicing the embodiment pertaining to the
pulmonary administration of biologically active agents. As
6056-91(CIP)1.CN -36-
\ac
stated earlier, depending upon the speci.fi.c circumstances,
the. liquid carrier need not be breathable. In most
instances, however, the liquid carriers employed are
breathable.
Particularly useful breathable liquids which can be
used as the liquid carrier include, but are not limited to,
perfluorochemicals, saline, silicone and vegetable oils,
and the like. Of the aforementioned liquids perfluorochem
icals (e. g., perfluorocarbon) liquids are presently prefer
red.
Some of the reasons for preferring perfluorochemi-
cals include, but are not limited to, (a) they have a high
solubility for respiratory gases, thereby being able to
maintain ventilation during therapeutic and/or diagnostic
procedures; (b) they have a low surface tension which
facilitates the uniform distribution of the liquids and
the biologically active agents throughout the pulmonary
system; and/or (c) they are generally biologically inert,
thus preventing possible side-effects due to the liquid
carrier and the biologically active agent interacting. It
should be noted, however, that other liquids can be
preferred over perfluorochemicals, depending upon the
specific circums~Lances and the desired results.
There are a number of clinical conditions when
liquid lavage (washing) of the pulmonary system is neces-
sary to debr:ide the alveolar sur:Eaces of unwanted secre-
tions, particles, toxins, etc. (e. g., alveolar proteinosis,
cystic fibrosis, aspiration syndromes, and the like).
Conventional. :Lavage procedures generally employ the use of
isotonic saline as the washing media since it is relatively
non-damaging to the alveolar surface. However, because
saline does not carry a substantial amount of oxygen to
support respiration, only one lung can be washed at a time.
The other lung is maintained witra 1000 oxygen. This
imbalance usually results in hypoxia during and after
conventional the lavage procedures.
6056-91(CIP)1.CN -37-
\ac
In several research reports, it has been documented
that it is possible to wash both lungs, simultaneously, if
a breathable liquid (e.g., perfluorocarbon) is employed as
the washing media. In view of the embodiment of the
present invention pertaining to the pulmonary
administration of biologically active agents, an extension
of the concept which employs breathable liquids as the
washing media, in a liquid lavage procedure, is to augment
the breathable'liquid with an effective solvent appropriate
for the particular injury to the patient's pulmonary air
passages. For example in the case of Adult Respiratory
Distress Syndrome (also referred to herein as "ARDS"), the
breathable liquid may contain a suspension of antiproteases
to more effectively perform the following functions: (aj
inhibit protein leakage, (bj wash out alveolar debris and
(c) maintain gas exchange. Furthermore, in the case of
aspiration syndromes, the breathable liquid may contain an
agent to neutralize or buffer the action of the aspirant on
the lung surface. For example, if the aspirant is of an
acidic nature (e. g., gastric contents), the breathable
liquid may be buffered with bicarbonate to balance the pH
and minimize lung epithelial damage.
When practicing the embodiment of the invention,
wherein the biologically active agents are introduced via a
liquid lavage procedure, the liquid carrier (augmented with
the desired active agents, whether in bulk, suspension,
dispersion, emulsion and/or encapsulization form) can be
placed in an inspiratory reservoir (RI). This inspiratory
reservoir is generally suspended above the patient and is
in open communication with at least a portion of the
patient's pulmonary air passages. For example, two ends of
a Y-piece can be used to interconnect the RI with the
patient's endotracheal tube.
Gas ventilation is generally interrupted when
instilling the liquid functional residual capacity from the
RI~ This residual capacity may contain at least a portion
6056-91(CIP)1.CN _3g_
\ac
p ~.
~i r~D ~: 'B ~: ..~r
of the desired biologically active agents. Gas and/or
liquid ventilation is then resumed and/or initiated
depending upon whether the process is (a) a total or
partial ventilation, (b,~ a total or partial lavage with
breathable liquid, or (~) a total or partial lavage with a
non-breathable liquid. After the resumption and/or
initiation of gas and/or liquid ventilation, tidal volumes
of a liquid caashing medium are passed thraugh the pati.ent's
pulmonary ai.r passages. These tidal volumes o.f liquid
l0 medium may contain at least a portion of the desired
biologically active agents.
In liquid lavage techniques, the tidal volumes of
liquid can be passed through the desired portion of the
patient's pulmonary air passages via gravity assistance,
mechanical assistance or a combination of both. Similarly
during liquid lavage techniques, the liquids can be removed
from pulmonary air passages via gravity and/or mechanical
assistance. If a Y-piece is employed as described above,
the expired liquids can pass through its remaining port and
be deposited into an expiratory reservoir.
Although the residual capacity of liquid remains in
the pulmonary air passages throughout the entire Iavage/
ventilation procedure, each tidal volume of liquid is held
within the patient°s lungs for. a period of time necessary
to achieve the desired results, while maintaining the
necessary exchange of gases, if necessary. For example,
the liquid can be retained in the patient's pulmonary
system for a period of time ranging from between about 60
seconds to about 1 second. In most instances, however, it
will not be necessary to retain the tidal volume liquid in
the patient°s pulmonary system for more than about 30
seconds.
Similarly, the frequency of the tidal volumes of
liquid also depends upon the specific results desired.
On the other hand, when practicing the embodiment
of the invention, wherein biologically active agents are
6056-91 (CIP) 1 . CN --39-
\ac
W
. i.% c.~ %:-
' .; ~.J
introduced via a liquid ventilation procedure, the liquid
ve.nti.lation pt-ocess can also be achieved using a gravity-
assisted system and/or a znechanically-assisted system. In
this procedure, breathable liquid is generally oxygenated
to maintain the arterial oxygen tension (Pa02) constant;
and carbon dioxide (C02) is generally scrubbed from the
system. Thereafter, the p02 and pC02 of the liquid are
typically sampled, analyzed and/or controlled during the
ventilation process to ensure constant inspired gas
tensions and drug delivery levels. It should be noted that
the augmentation of the liquid carrier with the
biologically active agents can be performed either before,
during and/or after the liquid is oxygenated.
Once the patient is connected to the liquid ven
tilation system which is being used as the vehicle for the
pulmonary delivery of biologically active agents, tidal
volume (vT) and functional residual capacity (FRC) is
preferably monitored and/or controlled. Generally,
ventilation schemes will be initially adjusted fox effec
five carbon dioxide elimination and maintenance of physiol-
ogical arterial COZ tension (PaC02). In addition to the
above, breathing frequency (f), VT and FRC are also
generally monitored and/or adjusted to obtain physiological
Pa02 and PaC02.
Regardless of whether a liquid lavage and/or
ventilation technique is employed as the vehicle for
carrying biologically active agents to the patient°s
pulmonary system via the patient°s pulmonary air passages,
heart rate, arterial pressure, hemoglobin-oxygen satura-
tion, arterial blood gas tensions, and/or pulmonary
function are generally evaluated before and during the
process.
When practicing the embodiment of the invention
pertaining to the pulmonary administration of biologically
active agents via liquid lavage/ventilation, the liquid
medium can be heated or cooled to temperatures above or
6056-91 (CIP) I.CId -~0-
\ac
~~~t~acil~ ~j
below the patient.°s normal body temperature depending,
again, on the specific conditions and/or desired results.
For example, in addition to the liquid medium being at our
about the patient°s normal body temperature, it can also be
greater or less than that temperature.
For example, the temperature of the liquid medium,
either before and/or during the liquid lavage/ventilation
process, can range from between about the normal body
temperature of the patient whose physiological conditions,
diseases and/or abnormalities are being diagnosed, con-
trolled and/or treated to about 20% above the patient's
normal body temperature. Generally, if such a hyperthermic
treatment is desired, the temperature of the liquid medium
can range from about the patient's normal body temperature
to about 15% greater than the patient's normal body temp-
erature, or from about 'the patient's normal body tempera-
ture to a temperature about 10% greater than the patient's
normal body temperature, or from about the patient's normal
body temperature to a temperature about 5o greater than the
patient°s normal body temperature, or from about the
patient's normal body temperature to a temperature about
1% greater than the patient°s normal body temperature. Tn
each instance, the liquid medium's temperature will depend
upon the specific circumstances present and the results
desired.
Moreover, the temperature of the liquid medium,
either before and/or during the liquid lavage/ventilation
process, can range from between about the normal body
temperature of the patient whose physiological conditions,
diseases and/or abnormalities are being diagnosed, con-
trolled and/or treated to about 20~ below the patient's
normal body temperature. Generally, if such a hypothermic
treatment is desired, the temperature of the liquid medium
can range from about the patient's normal body temperature
to about 15% less than the patient's normal body tempera-
ture, or from about the patient's normal body temperature
6056-91(CTP)1.CN -41-
\ac
6 ~ ~ r,
a
~~r~'~l.
to a temperature about 10~ less than the patient°s normal
body temperature, or from about the patient°s normal body
temperature to a temperature about 5~ less than the pa-
tient's normal body temperature, or from about the pa-
s tient's normal body temperature to a temperature about 1%
less than the patient's normal body temperature. As above,
the temperature of the liquid medium ~,ail1 depend upon the
specific set of circumstances present and the results
desired.
In addition to the above, it is also within the
scope of this invention for the liquid medium's tempera-
ture, either before and/or during the liquid lavage/venti-
lation, to range from between about 10% below to about 10%
above the normal body temperature of the patient whose
physiological conditions, diseases and/or abnormalities are
being diagnosed, controlled and/or treated. In other
instances, however, it may be desirable to have the temp-
erature of the liquid medium range from between about 5%
below to about 5% above the patient's normal body tempera-
ture, or from between about 3% below to about 3o above the
patient's normal body temperature, or from between about 1%
below to about 1% above the patient's normal body tempera-
ture.
When practicing the embodiment pertaining to the
pulmonary delivery of biologically active agents, the
particular temps~rature range of the liquid medium, either
before and/or dluring the liquid lavage/ventilation, will
depend upon the desired results and specific circumstances
of each individual implementation.
As stated earlier, the embodiment of the invention,
pertaining to the pulmonary administration of biologically
active agents, is especially useful for treating certain
types of lung cancer. The phrase "lung cancer°' as used
herein, generally refers to tumors arising in major airways
and pulmonary parenchyma.
6056-93.(CIP)1.CN -42-
\ac
R3 c3 ,~' ;~ ~ ~l
a °< 7 r.~ ,~ '- a i~d
Therapeutic treatment o.f lung cancer with chemo-
therapeutic agents (e. g., adr.iamycin, toxins, antibody-
linked nuclides, etc.) may have devastating effects on
systemic tissues when delivered at the high levels which
are generally necessary for the treatment of many types of
lung cancer.
On the other hand, the embodiment of the present
invention, which employs liquid lavage/ventilation techni-
ques for the pulmonary administration of biologically
active agents, provides a successful means far delivering
therapeutically high levels of anti-cancer agents to the
lung surface (cancer site) relative to the systemic
tissues, therefore minimizing adverse side effects.
Another pulmonary abnormality, which can be chemo
therapeutically traated with the embodiment of the present
invention pertaining to the pulmonary administration of
biologically active agents, is respiratory distress
syndrome. Respiratory distress syndrome is characterized
in both neonate and adults by their inability to
effectively exchange oxygen and carbon dioxide as a result
of lung immaturity (infants only), damage, or a combination
of both.
Because breathable liquids, such as perfluoro-
carbons, have low surface tensions and high solubilities
for respiratory gases, when practicing the present inven-
tion, such liquids can be used to homogeneously expand the
lung with low pressures, while simultaneously supporting
gas exchange and delivering biologically active agents to
regions of the lung which are generally not accessible by
systemic circulation. In comparison to existing
conventionaa. procedures for treating respiratory distress
syndrome, the approach employed when practicing the present
invention significantly reduces 'the risk of pulmonary
damage.
Particular therapeutic agents which would be
applicable in the treatment of respiratory distress syn-
6056-91(CIP)1.CN -43-
\ac
~~3''tJrs ra..~:
drome (RDS) include, but are not limited too exogenous
surfactants, antibiotics, steroids, antz.oxidants, antipro-
teases, bicarbonate and the like. While all of these
agents have proven clinical applicability for treatment of
RDS, they have significant limitations associated with
their conventional means of delivery. However, the pul-
monary administration of these agents, in accordance with
the practices of the present invention, (a) provides a
means for overcoming most of the limitations encountered by
conventional administration techniques and (b) effectively
delivers the aforementioned agents to the injured/abnormal
regions of the patient's pulmonary system. Moreover, due
to the evaporative characteristics of many breathable li-
quids, practicing the present invention in this manner
provides a means for assured deposition of these agents
onto the lung surface, without residual interference due to
the liquid carrier.
Yet another process, wherein the embodiment of the
invention pertaining to the pulmonary administration of
biologically active agents can be employed, is in Airway
Smooth Muscle (ASM) Control. In addition to controlling
pulmonary vascular smooth muscle for pulmonary circulation,
by practicing the present invention, it is now possible to
augment certain breathable liquids (e. g., perfluorocarbons)
with therapeutic: agents which control ASM and, therefore,
airway resistance to flow.
In the case of severe asthma, the ASM contracts
such that respiration is impeded and hypoxia and hyper°
capnia generally results. We have demonstrated that the
addition of a ASM agonist and antagonist to a breathable
liquid can significantly alter ASM tone and, subsequently,
affect ventilating pressures. Specifically, in Example 12
of this invention (infra), acetylcholine was injected into
a perfluorocarbon liquid during inspiration. As that
Example demonstrates, there was a rapid increase in trach°
eal pressure due to airway constriction. Airway dilation,
5056-91(CIP)1.CN -4~-
\ac
2~~'~~~~
..~ ::~ ~J
on the other had, has also been demonstrated with the addi-
tion of other biologically active agents, such as
isoproterenol and epinephrine, to a perfluorocarbon
ventilation liquid.
The embodiment of the invention pertaining to the
pulmonary delivery of biologically active agents can also
be useful for diagnosing particular conditions, diseases
and/or abnormalities in the pulmonary system. For example,
contrast agents (e. g., radioopaque agents) can be augmented
into the liquid medium to enhance structural delineation of
the patient's pulmonary air passages: Moreover, agents
which can evaluate diffusional barriers, pulmonary blood
flow, and/or distribution of ventilation can also be
employed.
The embodiment of the invention pertaining to the
pulmonary delivery of biologically active agents can be
employed with any technique wherein liquids are passed
through, and/or retained in, at least a portion of a
patient's pulmonary air passages for a period of time.
Examples of such uses, wherein liquids are employed within
the pulmonary system to treat, diagnose and/or control
certain physiological conditions, diseases and/or
abnormalities, include, but are not limited to, the
following:
I. Mechanical effects of Ultrasound: This
category pertains to the use of ultrasound to produce
localized mechanical (non-thermal) effects of liquid-filled
pulmonary spaces. Ultrasound beams can be focused or
scanned on portions of pulmonary tissue (intercostally,
intracavitarily, or intra-operatively) to take advantage of
non-thermal effects. By employing appropriate powers and
frequencies (typically, lower than those used for hyper-
thermia) ultrasound can'be used to agitate and mechanically
stir (through a phenomenon termed "acoustic streaming'°)
local regions of the liquid in the pulmonary air spaces,
liquid which is in intimate contact with lung tissue, and
6056-91(CIP)1.CN -45-
\ac
~~,~~~F-i'~'Ji~
.< ~ x cl w
which may or may nwt be carrying the drugs. These mechani-
cal effects can, by inducing localized convective motion in
the fluid (on a scale varying from tracheal to alveolar
characteristic dimensions), a) enhance drug transport in
and to the lung, b) improve the flushing of substances out
of the lung when using lavage, c) breakup "plugged regions'°
of the airways by inducing high frequency oscillations in
the liquid and in movable substances (e. g., mucus,
proteinaceous fluids, inhaled particulates, etc.)
6056-91(CZP)I.CN _46_
\ac
~~~'-°..xr~:a'
<N ~ a
II. Non-cancer Hy~erthermi.a: This category
pertains to ultrasound hyperthermia (e.g., employing the
ultrasound methods and devices previously outlined for
cancer treatment) for non-lung cancer applications.
Examples of such uses, include, but are not limited to, (a)
the production of localized drug action caused by higher
local temperatures at desired locations, and/or (bj making
substances which are blocking airways less viscous by
heating, and thus enhancing their removal (e.g., by
lavage) with or without mechanical effects of ultrasound
being exploited.
When practicing any embodiment of the invention, it
is generally necessary to monitor and/or control certain
mechanical and/or physiological parameters. The particular
parameters which would fall into this category will depend
greatly upon specific circumstances surrounding the speci-
fic application. Examples of variables which are generally
taken into consideration when practicing the invention
include, but are not limited to: the technique being
employed for liquid ventilation/lavage, the particular
pulmonary condition being treated, diagnosed and/or con-
trolled the particular method of treating, diagnosing
and/or controlling the particular condition, and the likes
and/or any combination thereof.
Parameters which are most often monitored and/or
controlled generally can be divided into three categories.
The first category includes the monitoring and/or control
of parameters, such as breathing frequency, inspiratory and
expiratory times, volume, flow rate, and/or pressure. The
second category includes the monitoring and/or control of
parameters, such as the temperature of the inspired liquid.
The third category includes the monitoring and/or regula-
tion of parameters, such as oxygen and carbon dioxide
tensions of 'the inspired liquids. While the aforementioned
list of categories includes those parameters which are most
6056-91(CIP)l.CN -~7-
\ac
~~n;~~~~
J .~ x :~ ,.~
likely to be mor~itorecl and/or controlled, is not intended
to be an exhaustive li~;t.
As can be seen from the above disclosure, the
pulmonary administration o.f biologically active agents
through at least a portion of the patient's air passages is
also a means to directly access cardiac output for the
infusion of selected agents when systemic collapse
precludes delivery via intravascular administration.
As stated earlier, any suitable technique can be
employed to combine the selected biologically active agent
and the selected liquid carrier.
Furthermore, it should also be understood that the
embodiment of the invention pertaining to the pulmonary
administration of biologically active agents also includes
the administration of solid, insoluble agents. In these
instances, for example, a liquid-solid phase may be formed
by dispersing and/or suspending a fine powder of the
selected agent in the liquid carrier.
The examples which follow are intended to assist in
a further understanding of the invention. Particular
materials employed, species, and conditions are intended to
be illustrative of the invention and are not limitative of
the reasonable scope thereof.
EXAMPLES
Based on the well-established biocompatability of
perfluorocarbon liquids, the issues most central to deter-
mining the feasibility of the disclosed convection and
ultrasound hyperthermia techniques were those having to do
with the fluid, thermal, and acoustic characteristics of
perfluorocarbon liquids and lungs filled with perfluoro-
caz-bon liquids. Below, the general physical, thermal, and
acoustic properties at candidate liquids are quantified in
parameter ranges appropriate to lung heating, as confirmed
by isolated lung and in viwo experiments. By employing
perfluorocarbon liquids that meet the disclosed criteria,
6056-91(CZP)1.CN -48-
\ac
~~~~~~~_~ d
we have demonstrated sustained and controlled convectivc>.
anc9 ultzvasound hyperthermia in large animal lungs in vivo.
A thorough investigation of the requisite proper
ties of candidate perfluorocarbon liquids was undertaken.
From these studies, the most suitable class of liquids was
selected for use in confirming animal research. As de-
scribed below, perfluorocarbon liquids were found to
exhibit interesting acoustic properties leading to unex-
pected but, for the most part, favorable behavior for the
purpose of liquid-filled lung ultrasound hyperthermia
(LLUH). Chief among the findings are: a) pure perfluoro-
carbon liquids show measurable nonlinear acoustical be-
havior in intensity ranges suitable to LhUH (< 2 W/cm2 @ 1
MHz), i.e., attenuation increases with power as well as
frequency; and b) perfluorocarbons in the lung exhibit
significant acoustical scattering of the ultrasound beam.
The implications these observations have on the LLUH
devices include 1) the need for lower frequencies than are
used in conventional superficial ultrasound hyperthermia,
2) a natural advantage exists whereby inherent acoustic
beam profile "smoothing" (i.e., flattening of the nearfield
diffraction peaks) occurs due to augmented scattering, and
3) a potential benefit .favoring focused ultrasound devices
may exist in that preferential absorption in their focal
regions should result from the nonlinear properties of
these particular liquids. In addition, the physical
properties of perfluorocarbon liquids have yielded some
unexpected advantages. Chief among these are a) the
tremendous gas solubility of the liquids makes them unique
in their ability to quickly and completely fill lung
tissue, an advantage important for acoustic coupling, and
b) the high gas solubility can likely be exploited to
suppress cavitation in the liquid. In addition, the low
surface tensions of perfluorocarbon liquids, as shown in
Figure 8, enhance the liquids' ability to readily fill the
lung. Also, when a lung becomes filled with liquid, liquid
5056-91(CIP)1.CN -4~-
\ac
203~~~2
resides on both sides of the vascular spaces, that is, on
both the gas side and the blood side. By regulating the
amount of liquid infused into the lung space, the blood
flow can be controlled. This is because the more fluid
that is introduced, the more compressed the lung capil-
laries become. Reduced blood flow is an important mecha-
nism to reduce heat dissipation and therefore to further
localize the treatment to the desired target tissues.
Also, the liquid distribution in the lung can be used to
control the distribution of pulmonary blood flow.
A wide range of perfluorocarbon liquids were
initially considered in an evaluation of physical, thermal,
and acoustic properties for selecting the most apt liquids
for liquid-filled lung procedures. A summary of these
properties is described in detail below.
EXAMPLE 1
General Characteristics of Perfluorocarbon hic~uids
Physical properties: The candidate perfluorocarbon
liquids spanned a wide range of molecular weights, as
indicated in Figure 7. For reference purposes, the physi-
cal properties of the liquids are presented in the Figures
in order of molecular weight, with water properties in-
cluded for comparison, and, unless otherwise stated, are
measured at 25°C.
Fluid flow properties: The predominant force
involved in lung inflation is the surface force along
alveolar walls due to the action of surface tension effects
from the moist lining of the alveoli. The introduction of
bulk liquid into the lung significantly reduces these
forces since the gas/liquid interface is removed. Further
reducing, these forces is the fact that perfluorocarbon
liquids have some of the lowest surface tensions recorded
for liquids (Figure 8). These combined effects means that
the net pressure to maintain inflation in a PFC-filled lung
is roughly 20-30% of that required for air inflation [61].
6056-91(CIP)1.CN -50-
\ac
2035492
This fact is advantageous for providing cuff isolation of
lung lobes and segments since cuff sealing in the airways
can be accomplished with lower pressures than for normal
clinical bronchial intubation.
Perfluorocarbon liquids are generally poor sol-
vents, being essentially insoluble in water, alcohols, and
most biologically active materials. This is a primary key
as to why they are superior to saline as acoustic
coupling/heat transfer fluids for liquid-filled lung
ultrasound and convection hyperthermia treatments. This
immiscibility ensures that the phospholipid surfactant
(which maintains low surface tension in alveolar wall
. moisture) will not readily be washed out of the treated
lung. This in turn minimizes the respiratory difficulty
which might otherwise occur in a lung after returning to
gas ventilation [62].
To reduce liquid flow resistance into and out of
the lung it is important to minimize the effects of viscous
resistance. Figure 9 shows that some of the perfluoro-
carbon liquids considered are relatively high in absolute
-..-.. viscosity, compared to water. On this basis,. liquids with
molecular weights (see Figure 7 for molecular weights)
higher than F-Decalin (i.e., perfluorodecalin) become less
desirable. Strictly considered, flow resistance is more
closely related to the "kinematic viscosity" (absolute
viscosity/density) than absolute viscosity, usually as
expressed in the "Reynolds Number" [63]. Considering the
higher densities of the liquids (Figure 10), it is found
that those perfluorocarbon fluids with molecular weights
below F-Decalin have flow resistance characteristics
equivalent to or better than water.
Gas solubility: To illustrate the tremendous
capability of perfluorocarbon liquids to absorb dissolved
gases, Figure 11 shows the oxygen solubility of six per-
fluorocarbon liquids in comparison with water. From the
standpoint of exploiting this property to suppress cavita-
6056-91(CIP)1.CN -51-
~ac
~5f~~;r~
tion, to assist in lung filling, and, of course, to enable
simultaneous lung ventilation during liquid-fii.led lung
hyperthermic treatments (via ultrasound and/or convection),
the perfluorocarbon fluids are all roughly equivalent, with
a slight preference going to molecular weights below
F-Decalin.
EXAroIPLE 2
Thermal Prot~erties of Perfl.uorocarbon Licguids
Thermodynamic properties: In ultrasound lung
heating it caill be undesirable to induce boiling in the
coupling liquid since, at the very least, this will inter-
rupt acoustic coupling. As shown in Figure 12, this
criterion renders FC--72 a very poor liquid selection, and
RM-82 and FC-84 less than optimum as well. In this cate-
gory, RM-201 and FC-75 roughly match the boiling points of
tissues, so they are acceptable, though not as appealing as
the higher molecular weight fluids.
The efficient removal of perfluorocaz-bon liquid
from the lung after liquid-filled lung hyperthermia treat
ments must be a leading consideration in designing the
proposed therapies. The primary removal mechanisms for the
bulk liquid will be first pumping or suctioning the fluid
from the lung, permissibly followed by gravity-induced
drainage (enhanced by the high densities of perfluoro-
carbons). The remainder of the fluid is then removed by
evaporation. The facility with which a liquid evaporates
is expressed by its vapor pressure; the higher the value,
the more rapid the evaporatian. As Figure 13 demonstrates,
perfluorocarbon liquids with molecular weights above
F-Decalin are clearly unacceptable from this standpoint.
It is not surprising that the most favorable liquids in
this category (FC-72, RM-82, and FC-84) are the same ones
that were undesirable from a boiling point perspective,
since the physical phenomena are the same.
6056-91(CIP)1.CN -52-
\ac
203542
Heat transfer ~ro~erties: The ability to convec-
tively transfer heat to the lung will be governed by the
"Prandtl number" of the fluid, which defines the ratio of
viscous diffusivity to thermal diffusivity, i.e., the ratio
(specific heat) x (viscosity)/thermal conductivity [64].
Because the specific heat for perfluorocarbon liquids is
virtually constant (0.25 J/g-C) and since their thermal
conductivities only vary by about 20% (kave = 0.064 W/m-C),
a Prandtl number comparison is dominated by differences in
viscosity (see Figure 9). Thus, from this standpoint, all
fluids with molecular weights below F-Decalin are generally
preferred and are approximately the same.
The ability to sustain constant temperatures in the
lung is determined by the "thermal capacitance" of the
fluid, or (density) x (specific heat). Again, because
there is no variation in specific heat between the fluids,
the more dense fluids will be those with higher thermal
capacitances.
EXAMPLE 3
Laboratory acoustic measurements: .For the purpose
of obtaining attenuation data over the range of physical
parameters described, a special Perfluorocarbon Fluid
Conditioning and Acoustic Measurement Flow System was
constructed, as depicted schematically in Figure 14. This
system permitted low volumes of perfluorocarbon liquid (< 1
liter) to be conditioned to any desired temperature and gas
saturation level while exposing the liquid in a transparent
fluid sample cell to ultrasound. The fluid sample cell
featured thin membranes (l~mil Monokote) on the top and
bottom surfaces, allowing virtually loss-free coupling of
the sound to the cell via temperature-controlled degassed
water. Sound attenuation was measured via the force
balance method, which detects acoustic radiation pressure
[65]. The sound traversed the sample cell and was then
absorbed by an absorber plate suspended from a precision
6056-91(CIP)1.CN -53-
\ac
%~~~~~'~
;, r.,
load cell. The acoustic path length through the perfluoro-
carbon liquid was 5.0 cm. An adjustable-height base plate
below the absorber was adjusted to reduce oscil?_ations in
the absorber through the action of viscous fluid dampen-
s ing. The radiation forces were recorded by a digital
voltmeter connected to the load cel?., with the voltage
signals representing force automatically sent to a computer
where the data was converted to acoustic power in Watts and
stored for later analysis. To accommodate the need for
frequencies below 1.0 MHz, both 250 and 500 KHz power
transducers were constructed.
Cavitation is a complex function of temperature,
fluid properties, fluid purity and cleanliness, ambient
pressure, and gas content. For the purposes of this study,
clean perfluorocarbon liquid was used and measured over a
temperature range from 25° to 45°C and at gas saturations
from a completely degassed state to 100% saturation using
air, 02, and blood gas (70 02, 7% C02, balance N2).
Cavitation thresholds were determined primarily by
high-speed video camera recording, by still photography,
and by visual inspection for bubble formation through the
transparent sample cell walls. Sound speeds were measured
by frequency-matched ultrasound transmitter/receiver
transducer pairs separated by a known and fixed gap. A
single-path, time-of-flight method of velocity measurement
was employed using an oscilloscope.
Perfluor~acarbon liquids have sound speeds which are
among the lowest recorded for any liquids (Figure 15). To
obtain efficient coupling of the beam into the perfluoro-
carbon liquid from either water or tissue, the liquid's
acoustic impedance, (density) x (sound speed), should
approximately match that of water and tissue. Whereas the
sound velocities are indeed very low in perfluorocarbon
liquids, their higher densities favor a good acoustic
impedance match, as shown in Figure 16. Figure 17 shows
comparative values of perfluorocarbon acoustic impedances.
6056-91 (CIP) 1. CI3 -54-
\ac
~~~e3~~ ~ ~
Although Fc:-5311 shows an almost perfect match, this liquid
is ill-suited for use in liquid--filled lung hyperthermic
treatments on the basis of the physical properties dis-
cussed above. The remaining acoustic matching values,
while not ideal, can provide good coupling of sound between
water and tissue. For example, the transmission loss of
sound passing from water into FC-75 is only slightly over 3
percent.
FXAMPLE 4
Acoustic Properties of Perfluorocarbon Liquids
Acoustic measurement materials and methods:
Ultrasound transmission is primarily governed by attenua-
tion in the perfluorocarbon liquids and, at relatively
higher intensities, cavitation (i.e., creation of small
bubbles by gases liberated out of solution). Lab measure-
ments of sound speed, impedance, attenuation, and cavita-
tion were done in several perfluorocarbon fluids over a
temperature range from 25° to 45°C and at various gas
saturations representative of conditions anticipated in
liquid-filled lung hyperthermic treatments in a special
Perfluorocarbon Fluid Conditioning and Acoustic Measurement
Flow System (depicted schematically in Figure 18).
Blood perfusion in liquid-filled lungs is much
lower than under normal physiological conditions, partic
2,5 ularly when the lung 'tissue being treated is not simul
taneously ventilated. In addition to enhancing the local-
ization of the treatment, as discussed above, by virtue of
reducing blood perfusion dissipation of thermal energy, the
ultrasound power required for lung hyperthermia is surpris-
ingly lower than might be appropriate for other vascular-
ized tissue, e.g., muscle. The acoustic intensities
employed for the evaluation of perfluorocarbon acoustic
properties ranged from 0-3.5 W/cm2and are expected to
fully encompass the range appropriate for lung heating.
Somewhat higher output powers were used in the cavitation
6056-91(CIP)1,CN -55-
~aC
2d~~r~~?
evaluations. In addition, although properties were meas-
ured at frequencies of 0.25, 0.50, 0.90, 1.0, 1.1, and 2.25
MHz, only the range from 0.25 to 1.1 MHz was studied in
detail. due to the high attenuation associated with 2.25 MHz
sound. The following observations were made.
Acoustic impedance,: Perfluorocarbon liquids have
sound speeds which are among the lowest recorded for any
liquids. To obtain efficient coupling of the beam into the
perfluorocarbon liquid from either water (or tissue), the
liquid acoustic impedance, (density) x (sound speed),
should approximately match that of water and tissue.
Whereas the sound velocities are indeed very low in per-
fluorocarbon liquids, their high densities favor acoustic
impedance matching, as shown in Figure 16 (perfluorocarbons
listed according to molecular weight). For example, the
transmission loss of sound passing from water to FC-75 is
only slightly over 3 percent.
Acoustic attenuation: The most surprising acoustic
characteristic of perfluorocarbon liquids was found to be
their low threshold for exhibiting nonlinear behavior.
Figure 19 shows the attenuation behavioz~ of FC-75 by
comparing the acoustic power transmitted through 5 cm of
degassed water (virtually loss-free) versus that through
FC-75 at 1.0 riiHz. It can be seen that the attenuation
gradually increases with power (electrical power is normal-
ized by the transducer face area and expressed as
Electrical "Intensity'°), even over the moderate power
levels required for lung heating. The liquid attenuation
is, however, extremely sensitive to frequency. As shown in
Figures 20 and 21, the attenuation and the degree of non-
linearity fall dramatically at lower frequencies, showing
virtually loss-free behavior at 250 and 500 KHz (witrin the
limits of accuracy of the measurement method). The atten-
uation in perfluorocarbon liquids also increases as the
fluids are heated, as depicted in Figure 22. This is an
interesting nonlinear aspect as we3.l, for attenuation in
6056-91(CIP)1.CN -56-
\ac
20~~492
water and most fluids decrease with temperature, due to
the reduction of viscosity. Although perfluorocarbon
attenuation increases with temperature, the use of low
frequencies can compensate, resulting in very low losses,
as the 250 KHz, T=45°C data of Figure 23 show. Figure 24
compares the attenuation in three perfluorocarbon liquids
representing a significant range of molecular weights.
Cavitation: While the bio-effects of acoustic
cavitation are apparently tolerated in some therapeutic
applications [55], in principle it will be better to avoid
it in the liquid-filled lung. The data obtained in these
experiments have shown that cavitation is likely to occur
at ultrasound intensity levels only if the perfluorocarbon
liquid is at or very near its saturation point in terms of
dissolved gases (e. g., 02 or blood gases). Thus, it will
not be advisable to support respiration in the lung with
100% 02 saturated liquids while using ultrasound heating.
However, this does not preclude the use of incompletely
gassed liquids (e.g., ?5% saturation) for use in
simultaneous ventilation with ultrasound hyperthermia.
Also, it should.. be emphasized that simultaneous
100% 02 liquid ventilation support while convectively
heating the lung is feasible. Figure 25 shows the power
dissipation which occurs from cavitation in 100% saturated
FC-75 over the hyperthermic temperature range. This data
also indicates that no cavitation occurs in degassed
liquids. Some variation of the threshold for cavitation in
gas-saturated liquids was found as a function of frequency
as well as of temperature.
It is important to stress that even slight degas-
sing seems effective in suppressing cavitation at these
intensities. This,~is probably due to the tremendous
perfluorocarbon affinity for gases. Perfluorocarbons
appear less prone to cavitation at equivalent saturations
and intensities than water. The perfluorocarbon capacity
to dissolve gases is so high, in fact, it is difficult to
6056-91(CIPjI.CN -57-
\ac
~~ ~:~~~°>
cavitate liquids at the recommended ultrasound powers even
a few percent below saturation, as seen in Figure 1H (where
gas saturation is changed in small increments by changing
liquid temperature). Because of both decreased lung
perfusion during liquid-lung procedures and the large '°gas
sink" characteristics of perfluorocarbon liquids, it is
plausible that the pulmonary circulation would take several
minutes to saturate degassed perfluorocarbon liquids
introduced into the lung, particularly considering that the
partial pressure of H20 vapor may preclude sufficient
dissolved gas saturation conditions from occurring at all.
Completely degassed liquids were used in the animal experi
ments (described below). The liquids were cycled into and
out of the lung (several minutes apart) to maintain low
liquid gas levels.
Summary
On the basis of the foregoing experimental observa-
tions, the fluorocarbon liquids FC-~5 (a mixture of per
fluorobutyltetrahydrofuran and perfluoropropyltetrahydro
pyran; 3M Company, Minneapolis, MN) and RM-101 (a mixture
of Furan,2,2,3,3,4,4,5 heptafluorotetrahydro-5-(nonafluoro-
butyl) and 2H-Pyran,2,2,3,3,4,4,5,5,6-nonafluorotetra-
hydro-6-(nonafluorodecafluoro); MDI Corp., Bridgeport, CN)
were found equally suitable as ultrasound transmission and
heat transfer fluids in the lung. Reinforcing the selec-
tion of these fluids (which, in purified form, should be
substantially free of hydrogen) is the fact that both have
been used in animal liquid ventilation research and have
excellent records of biocompatibility [3]. FC-75 and
RM-101 are thus considered representative of the class of
perfluorocarbon liquids, most suitable for liquid-filled
lung convection and ultrasound procedures, having the most
preferred physical, thermal, and acoustical parameters.
Since FC-75 is representative of this class, the presenta-
6056-91 (C1P) 1.C:J -58-
\ac
tion of its properties caill be emphasized from this point
on.
EXAMPLE 5
Acoustic Pr~erties of Perfluorocarbon-Filled- i,unas
Animal model/study design: I3ue to their size and
structure, adult sheep lungs axe good pulmonary models. In
the following animal experiments, five adult sheep were
used in acute in vivo and in vitro studies. A narrow band,
1.0-MHz, 6-cm diameter piezo-ceramic disk transducer with
an integral temperature controlled coolant/coupling liquid
was utilized for these studies. Hoth thermal techniques
(measuring specific absorption rates (sAR) of power) and
acoustic methods (measuring acoustic pressures and inten-
sities) were employed. The animal preparation and experi-
mental methods employed for the an vivo studies are de-
scribed in detail below.
Animal Preparation: Following the methods normally
used in ongoing liquid ventilation research, the animals
were all initially given pentobarbital sodium (20 mg/kg) to
induce deep sedation. After a local infiltration of 1%
lidocaine in the neck, the right carotid artery and right
jugular vein were cannulated. A tracheotomy was performed
fox the placement of either an endotracheal tube or a
liquid infusion catheter (the catheter shown in Figure 1
could be used for this purpose). To maintain biological
stability, the sheep°s untreated lungs are ventilated on a
mechanical ventilator at a volume of 500 ml, at a frequency
of approximately 15-20 breaths per minute, under skeletal
muscle paralysis (pancuronium bromide; initial bolus of 0.1
mg/kg, followed by 0.1 mg/kg/hr). In addition, steady
state maintenance of the animals included an intravenous
crystalloid infusion (10~ dextrose with 10 mEq sodium
bicarbonate and 1 mg sodium pentobarbital/100 ml fluid)
administered at a rate of 3 ml/kg/hr. Physiological
monitoring was done via arterial blood gas tensions, pH,
6056-91(CIp)1.CN -59-
\ac
2~3~4~2
heart rate, and blood pressure measurements. Additional
surgical procedures during in vivo experiments included
double or triple rib resections, to expose an acoustic
window for the ultrasound applicator. Also, small needle
thermometry probes were inserted in deep muscle and in the
isolated region of the lungs (described below). All
animals were euthanized with magnesium chloride.
Liquid-filled lung procedures: To quantify acous
tic properties in perfluorocarbon-filled lungs in vitro, a
series of experiments were performed on isolated adult
sheep lungs. There is a striking visual difference between
a normal air-filled lung and one which is filled with
fluorocarbon liquid. The glistening dark red color charac-
teristic of the successfully filled ''liquid lung'' was one
measure of a lung reaching complete filling. In addition,
measurements of acoustic propagation were also used to
confirm the degree of filling. It was found, both in the
in vitro and in vivo cases, that the lung filling process
could be accomplished in about one-quarter of the time
previously required for perfluorocarbons if the liquid were
completely degassed prior to the initial infusion (only.l-3-
minutes). The enhanced filling process was due to the
perfluorocarbons~ ability to dissolve great quantities of
gas, rather than simply depend on displacing the trapped
alveolar air. It was found that saline filling required
much more time than for the fluorocarbons using partially
degassed liquids.
In vitro ultrasound exverimental materials and
methods: In vitro ultrasound characterizations were
performed with an applicator consisting of a narrow band
1.0-MHz, 6-cm piezo-ceramic disk transducer with tempera
,. ture controlled coolant/coupling liquid continuously .sur
rounding it. The system was capable of delivering 150
Watts of acoustic power, though these power levels were in
excess of that required for fast warmup and certainly much
6056-91(CIP)1.CN -60-
\ac
'e~'a,~~'~_
~ A '~.
more than was required for stable steady state lung hyper~-
thermia.
The isolated lungs were instrumented either with
thermocouple probes (29 gauge) or with ultrasound hydro-
phones for thermal or acoustic determinations of attenua-
tion, respectively. Acoustic gel was used to insure good
coupling into the tissue. The thermal technique used was
that of determining the Specific Absorption Rate (SARj from
the initial rate of temperature rise [66] at different
depths in the lung. Ratios of SAR at the various depths
yielded atternaat:ion. The hydrophone measurements recorded
dynamic pressure variations directly which were displayed
on an oscilloscope. Squaring of the pressure data resulted
in data proportional to intensity, which could then be
translated to attenuation values for known acoustic path
lengths.
In vivo li~juid-lung ultrasound hyperthermia materi-
als and methods: To provide efficient filling of the lung
lobe, completely degassed FC-75 was introduced through a
conventional clinical bifurcated bronchial catheter that
permitted infusion of the selected lung lobe while sustain-
ing gas ventilation in the remainder of. the lung. The
catheter was placed without benefit of a bronchoscope, so
the correct placement had to be determined by verification
of lung inflation motions in the desired lung segments.
The perfluorocarbon liquid was introduced at room tempera-
ture and only infrequently circL~lated in and out. In most
cases the cranial segment of the right apical lobe was
chosen for selective heating, both in the ultrasound and
the connective hyperthermia experiments.
These segments had inflated volumes of approximate-
ly ?50-300 ml. An "acoustic window" to the lung segment
was obtained by resection of portions of three ribs essen-
tially analogous to an intraoperative hyperthermia treat-
went. The treated lung segment was partially exteriorized
through the "window" to enable invasive thermometry of the
6056-91(CIP)1.CN -61-
\ac
treated lung at different depths. The sound was propagated
directly through coupling water and membrane into the lung.
In most cases, the lung surface was cooled with 3'I° C
coupling water.
In addition to heating data, in vivo acoustic
measurements were also performed via the hydrophone method
previously described. Continuous recording of relevant
physiological parameters were performed throughout the
experiments. These measurements included systolic and
diastolic blood pressure (reduced to Mean Arterial Pres
sure), core temperature, heart rate, and respiration rate.
Gas ventilation was maintained by a mechanical respirator.
Cardiopulmonary stability was confirmed throughout the
treatments by taking periodic blood samples for arterial
pH, p0?, and pCO2.
In vitro ultrasound results: Figure 26 presents
typical in vitro attenuation values for isolated lung of an
adult sheep. The attenuation shows a significant increase
with increasing frequency. Note also that the attenuation
levels are higher in the liquid-filled lung than for the
pure liquid. It is postulated that this augmented attenua-
tion is mostly attributable to scattering from the refrac-
tion effects of the sound speed mismatch between the
parenchymal tissue and the liquid (increased scattering is
supported by the ultrasound imaging results as well).
Because scattering increases the effective acoustic path
length, a wave traverses and spreads the beam slightly, the
near loss-free propagation for lower frequencies (e.g., 250
and 500 KHz: Figures 20, 21, and 23) in perfluorocarbon
3U liquids is no doubt preferred to frequencies above 1 MHz
for deeper hyperthermia. Le~wer frequency ultrasound should
also exhibit significantly reduced scattering since the
wavelength increases substantially (e.g., to 3-6 mm) in
relation to the main scattering structures (i.e., bronchi-
ales, diameters < 1 mm [56~).
6056-91(CIP)1.CN -62-
\ac
2~3~4~~
Figure 27 demonstrates that the liquid-filled lung
acoustic properties are dominated by the presence of the
liquid (this likely also holds true for the thermal proper-
ties). This data shows that the effective sound speeds
measured (both in vivo and in vitro) are close to those of
the pure liquid (dashed line). Note that connective tissue
sound speeds are usually higher than those of blood and
muscle.
EXAMPLE 6
In Vivo Acoustic Lunq Hyperthermia
Employing the methods and protocols described in
Example 5, sustained hyperthermia (42-45°C to about 4 cm
depth for 30 minutes) was successfully accomplished in the
two animals used for the tests. The temperature vs. depth
histories Which resulted are represented by Figure 28,
which depicts the experiment employing the greatest number
of temperature probes. In this case probes were located in
the interstitial tissue along the beam central axis at
depths of 0.5, 1.0, and 2.0 cm, and also at 3.0 cm but
20-- slightly off axis. In addition, an on-axis probe was
placed on the distal surface of the lung segment (approxi-
mately 6 cm from the treatment surface) between the lung
surface and a rubber mat (which also acted as an acoustic
absorber). As shown, lung temperatures exceeded 43°C to
approximately 3 cm depth, with acoustic penetration through
the lung segment indicated by the high, temperatures on the
distal surface (effectively 6 cm deep). The close tracking
of the 2 and 3 cm depth temperatures (again, not in line
with each other) may have been due to refractive effects,or
differences in local perfusion. The lower temperature at
the 0.5,cm site is due to the conductive cooling of the
coupling water (at 37°C). The "thoracic cavity" core
temperature probe was located near the treated segment in
the cavity. The steady state power requirements in this
6056-91(CIP)1.CN -63-
\ac
6 ~~
~.~ ~ e~ ~ ~~
case ranged between 12 to 15 watts, again indicative of low
pulmonary perfusions due to the liquid presence.
Ferfusion response of the liquid-filled lun~c: The
ultrasound power levels required for steady state hyper
thermia were unexpectedly low due to low blood flow levels
in the heated lung. An analysis of the physiological
mechanisms involved, however, indicates that the perfusion
is suppressed due to the combined effects of: 1) increased
pulmonary vascular resistance due to the presence of the
liquid compressing alveolar capillaries, 2) the shunting of
the pulmonary circulation to other areas of the lung from
locally low p02 (here from degassed liquids), and 3) to
shunting from a low pH buildup in the lobe [29,30,31].
EXArIPL'E 7
In Viva ~onvective Lung Hygerthermia
Convection hy~~erthermia materials and methods:
Using the large animal liquid ventilation (LALV) system of
Temple University (Figure 29), heated, temperature-con-
trolled FC-75 could be circulated in and out of lung lobes
and segments isolated via the bifurcated bronchial catheter
method as described above. The animal preparation was
essentially the same as for the ultrasound experiments. In
this way aonvective lung hyperthermia was successfully
administered to the cranial segment of the right apical
lung lobe. To instrument the lung so that temperature
probes could be easily placed at ~Cnown depths, the lung
segment was partially exteriorized through a "window°'
created in the same manner as was done for the ultrasound
experiments.
Figure 30 shows the temperature history data for
'the connective lung hyperthermia experiment. The setup
period was used for establishing proper placement and
sealing of the liquid delivery catheter, for proper temp-
erature probe placement, and to assess the response of the
lung to LLCH parameter changes. It was found that the very
6056-91(CIP)l.CN -64-
~aC
thin wall (~ 2 mil) vinyl air cuffs on the available
bifurcated catheters had very little structural integrity
at the elevated liquid temperatures required of the hyper-
thermic treatment. As such, the catheters provided ade-
quate, but not high quality, sealing. Although this had
very little physiological impact (since the gas ventilation
of the remaining lung was quite adequate), it did result in
diminished heat transfer rates. 'The development of a
suitable liquid delivery catheter was therefore mandated.
During the experiment, the heat transfer to the
lung segment was varied by changing both the inspiratory
liquid temperature (Tins) and the tidal volume (Vt) under
constant cycling (5 "breaths" per minute) conditions.
Beginning with a low Tins, low Vt condition (43°C, 40 ml),
it was found that temperatures in the therapeutic range
slowly fell below hyperthermic values. Lung perfusion
effectively cooled the lung under these conditions.
However, increases in Tins and Vt overcame this decline,
bringing temperatures back up above 45°C (t = 60 minutes).
Once the lung has reached the desired therapeutic tempera-
ture, the Tins and Vt settings were adjusted downward to
maintain good steady state hyperthermia (t > 60 min).
Notewort ~ trends: First, the temperature probes
in the center of the lung segment interstitium (spaced 2-3
cm apart) consistently were within 0.5°C of each other at
the higher tidal flows (t > 60 min), and were usually
within 1° C of each. other at the lower flows (t < 40 min) .
Therefore, spatially uniform heating can readily be achiev-
ed and controlled via the tidal flow. Secondly, the rates
of lung temperature increase shown during the experiment
(~ 0.25 C/min) are much more sluggish than rates which
should occur at similar Tins values in a properly designed
clinical device. This is due to the aforementioned com-
promised heat transfer from the leaky catheter cuff.
Indeed, much higher rates were found during the setup
period prior to cuff leakage (~ 1 C/min for t < 25 min) .
6056-9:1. (CIP) 1. CN -65-
\ac
g a~'~~ _
!J ?.; ~ ;~ C~ a ~
w
Lastly, it should be noted that steady state lung tempera-
tures closely tracked Tins, which was measured outside the
animal in the liquid circuit. By placing a temperature
sensor at the distal end of the catheter, at the entrance
to the heated lung lobe or segment, the lung temperatures
should be known with a high degree of certainty. This is
significant in that there should be no need for invasive
lung thermometry during the subject treatment.
EXAMPLi; 8
IDltrasound Imaging for Lic~txid-filled Lung Procedures
The presence of liquid in the lung theoretically
makes possible the use of ultrasound imaging, both for
viewing lung structures and for use in conjunction with the
ultrasound and convection treatments. Both in vitro and in
vivo ultrasound imaging experiments were performed on
perfluorocarbon-filled lungs as part of the animal studies
described above. The diagnostic imaging system was a
commercial clinical system (Diasonics) capable of
sector-scanned images at frequencies from 3 to 7 MHz.
B-scan images were obtained on exteriorized perfluoro-
carbon-filled lung lobes and on lung lobes viewed through
the rib cage and intrathoracically.
Consistent with the ultrasound results discussed
previously, it was found that the increased attenuation and
scattering of i~he very high diagnostic frequencies (3-7
MHz) rendered the diagnostic value of imaging deep lung
structures through liquid-filled lung parenchyma poor. The
imaging of structures through liquid-filled lung parenchyma
may be the one area where saline-filling of lungs provides
a distinct advantage (due to matched sound speeds).
The foremost advantages of diagnostic ultrasound
imaging in the present application are for monitoring the
lung-filling process and for confirming the integrity of
the acoustic path. This conclusion is based on the dis-
6056-91(CIP)1.CN -66-
\ac
~a ~~ ~:i '~ ~ f:~ ~~ '
tinct ultrasound images which were obtained when lung lobes
reached gas-free or near-gas-free states.
EXAMPLE 9
Liquid-Filled Lunq Hyperthermia and Chemotherapy
The prospects for using perfluorocarbon liquids as
drug delivery vehicles may be quite favorable since commer-
cial examples of fluoropharmaceuticals are many and diverse
[60]. In addition, simultaneous locally delivered anes-
thesia in the treated tissue should also be possible via
liquid delivery (though systemic anesthetic effects may
also result). Although anesthetic use is often contraindi-
cated in hyperthermia for safety reasons, because the
maximum temperature in the lung may be set by the clinician
with confidence in the subject (especially convection)
treatments, simultaneous anesthesia may be feasible in this
procedure. Coincidentally, fluorine-containing inhalation
anesthetics account for the largest volume of fluoro-
compounds sold for purposes that are nonindustrial [60].
EXAMPLE 10
ITltrasound Intracavitar~ Applicator ~ICA~ Experiments
Figure 4 shows a schematic of a representative
applicator head. A thin-walled piezoelectric ceramic
cylinder (~ 1.0 MHz :resonance) was longitudinally and
circumferentially sectioned into four separate power
transducers with 120° and 240° included angles, respective-
ly. The multiple--transducer approach provided flexible
heating patterns. In these studies transducers 120 and 122
were driven in parallel (forming a synchronous pair), as
were 124 and 126. Depending upon whether each pair or both
were driven, either 120°,, 240° , or a full 360° of
heating
could be achieved along the length of the cylinder. The
transducers were mounted in an applicator with self-con-
tained cooling and an integral water bolus for sound
coupling, as depicted in Figure 6. Z'he diameter of this
6056-91(CIP)l.CN -6?-
\ac
first engineering prototype ICA transducer was 16 mm.
Smaller cylinders more suitable for bronchial applications,
however, can be readily made.
The applicator was mounted on a long (1 meter),
flexible tubular shaft which housed the inlet and exit flow
channels to the coupling bolus, as well as the RF power
cables to the transducer. The water coolant flow dis
sipated heat to a maximum power of 100 Watts. The flow
system was also characterized for pressure drop vs. flow
rate to assure that acceptable pressure drops could be
maintained in the long, narrow coolant channels.
The ultrasound beam quality was mapped in an
acoustic test tank, while the thermal performance of the
device was evaluated in a specially constructed body cavity
phantom. Figure 31 shows the acoustic intensity mapped
(via hydrophone) in water along the axial direction (z) of
the transducer, 2 cm from the surface and in the middle of
the 240° arc of energized transducers 124 and 126. Figure
32 presents SAR patterns measured in tissue-equivalent
cavity phantoms by needle thermocouple probes at five axial
positions and several azimuthal angles (measured from the
center of the 240° arc of transducers 124 and 126. Figure
33 shows the depth (radially outward) heating patterns in
the phantom from the surface to 2 cm into the phantom
tissue. The 100-Watt maximum power employed is more than
will be needed for most applications.
(EXAMPLE 11
hiciuid-filled Lung Convection FTyperthex-raia ~~LLCH~ System
Figure 34 schematically shows a representative LLCH
system. The system is constructed under requirements
applicable to clinical use. It is designed to maintain
complete sterility of the liquids and catheters, and is
modular and portable for convenient use in either a surgi-
cal theater or hyperthermia/oncology suite. The LLCH
system provides heated, temperature-controlled perfluoro-
6056-91(CIP)1.CN -68-
\ac
4
carbon liquid to the patient in either degassed or oxygen-
ated form (partially degassed liquid states are also
possible). To impose controlled lung temperatures and heat
transfer. rates, the tidal volume and "ventilation" frequen-
cy (cycling rate of the fluid into and out of the lung),
and the input liquid temperature are controlled by the
operator. To insure sterility, the unit employs
roller-type peristaltic pumps which completely contain the
liquid in sterile tubing. Similarly, valves, fluid fit-
tings, and reservoirs are easily replaced and sterilizable,
or disposable. The inspiratory and expiratory flows,
system liquid temperatures and components status are
monitored and controlled by a central computer. The
computer serves as the operator console during treatment,
recording and displaying LLCH system parameters and invas-
ive temperature probe data, and is also a work station for
data playback and post-treatment analyses.
EXAAiPLE 12
Pulmonary Administration of Drucxs:
Cardiovascular and Airwav Smooth Aquscle Effect
This Example demonstrates a technique for directly
delivering biologically active agents (i.e., acetylcholine,
epinephrine, priscoline, sodium bicarbonate and sodium
nitroprusside) into a patient's cardiopulmonary system, via
the patient's pulmonary air passages.
Using previously developed perfluorochemical (PFC)
ventilation techniques, similar to those disclosed in
Shaffer, A Brief Review: Liquid Ventilation, and Wolfson,
et al., A Experimental At~t~roach for the Study of Cardiopul-
monary Physiolocly During Early Development, (see, notes 3
and 35, respectively, of literature citations, infra), pul-
monary gas exchange and acid-base balance were maintained
in anesthetized and tracheotomized young cats.
When testing the dose-dependency effect of the
pulmonary administration of acetylcholine [ACh], the effect
6056--91 ( CIP) 1 . CN -69-
\ac
after the administration of the drug (expressed as a
percentage of the baseline), as a function of the con-
centration of the drug, was monitored.
Here, the ACh was dispersed in the PFC liquid
medium. The initial amount of ACh dispersed in the PFC
liquid was 0.01 mg per each kilogram of the laboratory
animal's body weight.
The PFC/ACh liquid medium was then introduced
directly into the animal's pulmonary air passages, via the
endotracheal tube, during the inspiratory phase of PFC
liquid ventilation.
Each of the animals tested had at least one of the
following parameters recorded before, during and after the
pulmonary administration of the drug: (a) heart rate
(bpm), (b) mean arterial pressure (mm Hg), and (c) tracheal
pressure (cm water). The averages for each of these recor-
ded parameters were then calculated, depending upon the
number of cats tested. These calculated values are plotted
on the graph in Figure 35.
In addition to the above, the following parameters
were also monitored and/or controlled: tidal volume,
inspiratory and expiratory liquid flow rate, arterial
chemistry, pulmonary compliance and resistance, and/or
breathing frequency.
The dosage of ACh was then incrementally increased
from 0.01 mg/kg up to 1.0 mg/kg. For each incremental
increase, the animals tested had at least one of the
aforementioned parameters recorded before and after the
pulmonary administration of the drug.
Also for each incremental increase, the averages
for each recorded parameter were calculated and plotted on
the graph in Figure 35.
Referring to Figure 35, the data plotted therein
demonstrates that, as a function of increasing the corv
centration of ACh in the inspired PFC liquid, typical dose
dependent cholinergic responses to ACh showed progressive
6056-91 (CIP) 1. CIA -70-
\ac
~~1~~~~~
decreases in mean arterial pressure (i.e., reflecting
vasodilation) and heart rate, and a progressive increase
in the peak tracheal pressure (i.e., reflecting broncho-
constriction).
Another way in which the effect of the pulmonary
administration of ACh was tested was by monitoring carotid
pressure, as a function of time, before, during and after
the pulmonary administration of the drug. Here, 0.6
mg/kg of ACh was dispersed in the PFC liquid medium. The
PFC/ACh liquid medium was then introduced directly into the
animal's pulmonary air passages, via the endotracheal tube,
during the inspiratory phase of PFC liquid ventilation.
A tracing of the animal's carotid pressure, before,
during and after the pulmonary administration of ACh,
demonstrates, among other things, that (a) the carotid
pressure decreased by about 40 mm Hg after the drug was
administered; (b) the decrease in carotid pressure began
almost instantaneously after the drug was administered; and
(c) the total time necessary to decrease the carotid
pressure by about 40 mm Hg was about 10 seconds.
Yet another way in which the effect of the pul-
monary administration of ACh was tested was by monitoring
tracheal pressure, as a function of time, before, during
and after the pulmonary administration of the drug.
Here, 0.6 mg/kg of ACh was dispersed in the PFC
liquid medium. The PFC/ACh liquid medium was then intro-
duced directly into the animal's pulmonary air passages,
via the endotracheal tube, during the inspiratory phase of
PFC liquid ventilation.
An observation of the animal's tracheal pressure,
before, during and after the pulmonary administration of
ACh, demonstrates, among other things, that (a) the
tracheal pressure (cm water) increased by about 6 mm Hg
after the drug was administered: (b) the increase in
tracheal pressure began almost instantaneously after the
drug was administered; and (c) the total time necessary to
6056-91(CIP)1.CN -71-
\ac
G '> ''~ ?" ; ey
~~ ~J. ~'<~ hJ
increase the tracheal pressure (cm water) by about 5 mm Hg
was about 20 seconds.
When testing the dose-dependency effect of the
pulmonary administration of epinephrine [Epi], the effect
after 'the administration of the drug (expressed as a
percentage of the baseline), as a function of the con-
centration of the drug, was monitored.
Here, the Epi was dispersed in the PFC liquid
medium. The initial amount of Epi dispersed in the PFC
liquid was 0.01 mg er each kilogram of the laboratory
animal's body weight.
The PFC/Epi liquid medium was then introduced
directly into the animal°s pulmonary air passages, via the
endotracheal tube, during the inspiratory phase of PFC
liquid ventilation.
Each of the animals tested had at least one of the
following parameters recorded before and after the pul-
monary administration of the drug: (a) heart rate (bpm),
(b) mean arterial pressure (mm Hg), and (~) tracheal
~0 pressure (cm water), The averages for each of these recor-
ded parameters were then calculated, depending upon the
number of cats tested. These calculated values are plotted
on the graph in Figure 36.
The dosage of Epi was then incrementally increased
from 0.01 mg/kg up to 2.0 mg/kg. For each incremental
increase, the animals tested had at least one of the
aforementioned parameters recorded before and after the
pulmonary administration of the drug.
Also for each incremental increase, the averages
for each recorded parameter were calculated and plotted on
the graph in Figure 36.
Referring to Figure 36, 'the data plotted therein
demonstrates that, as a function of increasing the con-
centration of Epi in the inspired PFC liquid, typical dose-
dependent sympathomimetic responses to Epi showed increases
in mean arterial pressure (i.e., reflecting vasoconstric-
6056-91(CIP)1.CN -'72-
\ac
~3~.:;..b~;./~~~:i
~a t.~
tion) and heart rate, and a decrease in peak tracheal
pressure (i.e., reflecting bronchodilation).
Another way in which the effect of the pulmonary
administration of_ Epi was tested was by monitoring the
change in mean arterial pressure and heart rate resulting
from drug delivery.
Here, 0.50 mg/kg of Epi was dispersed in the PFC
liquid medium. The PFC/Epi liquid medium was then intro-
duced directly into the animal's pulmonary air passages,
via the endotracheal tube, during the inspiratory phase of
PFC liquid ventilation.
An observation of the animal's tracheal pressure,
before, during and after the pulmonary administration of
Epi, demonstrates, among other things, a 31o decrease after
the drug was administered. Moreover, an observation of the
animal's heart rate, before, during and after the pulmonary
administration of Epi, demonstrates, among other things, a
33o increase after the drug was administered.
When testing the dose°dependency effect of the
pulmonary administration of priscoline [P], the effect
after the administration of the drug (expressed as a
percentage of the baseline), as a function of the con
centration of the drug, was monitored.
Here, the P was dispersed in the PFC liquid medium.
The initial amount of P dispersed in the PFC liquid was 4
mg.
The PFC/P liquid medium was 'then introduced direct
ly into the animal's pulmonary air passages, via the
endotracheal tube, during the inspiratory phase of PFC
liquid ventilation.
Each of the animals tested weighed approximately
3kg. Each of these test animals had at least one of the
following parameters recorded before and after the pul-
monary administration of the drug: (a) heart rate (bpm),
(b) mean arterial. pressure (mm Hg), and (c) right ventricu-
lar pressure.
6056-91(CIP)1.CN -73-
\ac
c ~ .,.
z. 77 .
(a ~.Y r..S ,~ ~.X .
The averages for each of the aforementioned
recorded parameters were then calculated, depending upon
the number of cats tested. These calculated values are
plotted on the graph in Figure 37.
The dosage of P was then incrementally increased
from 4 mg up to 12 mg. For each incremental increase, the
animals tested had at least one of the aforementioned
parameters recorded before, during and after the pulmonary
administration of the drug.
Also for each incremental increase, the averages
for each recorded parameter were calculated and plotted on
the graph in Figure 37.
Referring to Figure 37, the data plotted therein
demonstrates that, as function of increasing the concentra
tion of P in the inspired PFC liquid, typical dose-depen
dent responses to P showed decreases in mean arterial
pressure (i.e., reflecting vasodilation) and right ventric-
ular pressure (i.e., reflecting systemic and pulmonary
vasodilation). The heart rate remained fairly constant.
Another way in which the effect of the pulmonary
administration of P was tested was by monitoring carotid
pressure, as a function of time, before, during and after
the pulmonary administration of the drug.
Here, 12 mg of P was dispersed in the PFC liquid
medium. The PFC/P liquid medium was then introduced
directly into the animal's pulmonary air passages, via the
endotracheal tube, during the inspiratory phase of PFC
liquid ventilation.
A tracing of the animal's carotid pressure, before,
during and after the pulmonary administration of P, demons
trates, among other things, that (a) the carotid pressure
decreased by about 30 mm Hg after the drug was administer
ed; (b) the decrease in carotid pressure began almost
instantaneously after the drug was administered; and (c)
the total time necessary to decrease the carotid pressure
by about 30 mm Hg was about 10 seconds.
6056-91(CIP)1.CN -74-
\ac
203492
When testing the absorption effect of the pulmonary
administration of sodium bicarbonate [SBi], the concentra-
tion of SBi in the animal's blood, as a function of time,
was monitored.
Here, 10 mEq of SBi was dispersed in the PFC liquid
medium. The PFC/SBi liquid medium was then introduced
directly into the animal's pulmonary air passages, via the
endotracheal tube, during the inspiratory phase of PFC
liquid ventilation.
An observation of the concentration of SBi present
in the animal's blood (monitored as a function of mEq/L)
30 and 120 seconds after the pulmonary administration of
SBi, demonstrates, among other things, a respective 4.7%
and 20.9% absorption level.
When testing the physiological effect of the
pulmonary administration of sodium nitroprusside [SNi], the
physiological change in the animal's arterial pressure and
heart rate, after the administration of the drug, was
monitored.
Here, 6 mg/kg of SNi was dispersed in the PFC
liquid medium. The PFC/SNi liquid medium was then intro-
duced directly into the animal's pulmonary air passages,
via the endotracheal tube, during the inspiratory phase of
PFC liquid ventilation.
An observation of the animal's heart rate, before,
during and after the pulmonary administration of SNi,
demonstrates, among other things, that it did not sig-
nificantly change, increase after the drug was adminis-
tered. Moreover, an observation of the animal's arterial
pressure, before, during and after the pulmonary
administration of SNi, demonstrates, among other things, a
24% decrease after the drug was administered.
It was observed from the above that the pulmonary
administration of drugs by liquid ventilation is an effec
tive approach for directly delivering therapeutic agents to
the pulmonary and/or systemic systems.
6056-91(CIP)1.CN -~5-
\ac
~. " i : ,...o
r> ~" ;~ N
COncluSlanS
The faregoi_ng research was highlighted by the first
in vivo demonstrations of both acoustic and connective
hyperthermia of the lung, here i.n a suitably large animal
model. Controlled and sustained therapeutic temperatures
were maintained with relatively few complications. These
experiments, complemented by laboratory bench and in vitro
acoustic measurements with perfluorocarbon liquids, identi
fied the important clinical requirements for liquid-filled
lung ultrasound and convection hyperthermia. Among these
are a) lower ultrasound frequencies than traditionally used
for soft tissue heating are required, b) traditional
bifurcated bronchial catheters are inadequate, mainly due
to their thin-walled air cuffs and lack of temperature and
pressure instrumentation, and c) the use of degassed
perfluorocarbon liquids greatly facilitates the filling of
lungs. Of tremendous practical significance are the
observations that d) diagnostic ultrasound imaging can be
very helpful in assessing the lung filling and the acoustic
path available, and e) invasive thermometry will likely not
be required for the convection hyperthermia treatments.
Additionally, the fundamental fluid and thermal design
ranges appropriate to the ultrasound treatment, including
the range of inflation pressures, temperatures and tidal
volumes, were determined.
Perfluorocarbon liquids have several unique proper-
ties. Measurable nonlinear acoustical behavior and scat-
tering in the range of powers suitable to hyperthermia were
found in laboratory and animal tests. While dictating the
use of lower ultrasound frequencies, these characteristics
can be advantageous for spatial smoothing of near field
beam patterns and may be able to be exploited for their
potential to produce localized enhanced absorption with
focused ultrasound beams. In addition, the high gas
solubility of perfluorocarbons should serve to suppress
6056-91~CIP)1.CN -76-
\ac
G ~ : s-)
a, a s~,~ ,u .k a~ r~
acoustic cavitation in the liquid by retarding rapid gas
saturat ion .
The salient design requiremewts for clinical
devices for 1) fluid processing and delivery systems
suitable for liquid-filled lung hyperthermia procedures, 2)
intracavitary ultrasound applicators for broncho-tracheal
tumors, and 3) low-frequency external ultrasound applica-
tors were also determined.
In addition to the above, the foregoing research
also demonstrates the operabi7.ity and significant utility
of pulmonary administered therapeutic agents through a
liquid lavage/ventilation process.
While representative and preferred embodiments of
the invention have been described and illustrated, it is to
be understood that, within the scope of the appended
c7.aims, various changes can be made therein. Hence, the
invention can be practiced in ways other than those
specifically described herein.
6056-91(CIP)2.CN -77-
\ac
LTTERATURT; CITATIONS
1. Petrovich, Zbigniew: "Advances: Using Heat
in Treatment of Some Cancers," article by
Thomas C. Haves, New York Times, Dec. 2, 1987.
2. Bleehan NM: Role of Hyperthermia in Treatment
of Lung Cancer. Cancer Treatment Symposia,
Vol. 2, Bleehan (Edj, p. 75, 1985.
3. Shaffer TH: A Brief Review: Liquid Ventila-
tion, Undersea Biomedical Research, Vol. 14,
No. 2, pp. 169-179, 198'7.
4. Biro PB and Blais P: Perfluorocarbon Blood
Substitutes. CRC Critical Reviews in
Oncology/Hematology, Vol. 6, No. 4, pp.
311-374, 1987.
5a. Silverberg E and Lubera JA: Cancer Statis-
tics, 1989. Ca - A Cancer Journal for Clini-
cians, Vol. 39, No. 1, pp. 3-20, 1989.
5b. Cancer Facts and Figures, 1987, p. 25. Ameri
can Cancer Society, New York, N.Y., 1987.
6. Timothy AR: The Role of Radiotherapy in Car-
cinoma of the Bronchus. Bronchial Carcinoma,
An Integrated Approach to Diagnosis and
Management,p. 232-254, Springer-Verlag, New
York, 1984.
7. Crawford S and Pierson D: Lung Cancer in "The
Respiratory System," syllabus from University
of Washington School of Medicine, pp. 240-249,
1985.
8. Kapp DS: Site and Disease Selection for
Hyperthermia Trials. International Journal of
Hyperthermia, Vol. 2, No. 2, pp. 139-156,
1986.
9. Karasawa K, Muta N, Takahashi N, Aoki Y,
Nakagawa K, Sakata K, Akanuma A, Iia M: RF
Capacitive Hyperthermia and Radiotherapy of
Lung Cancer, presented at the 5th
International Symposiuan on Hyperthermic
Oncology, Kyoto, Japan, Aug. 29 - Sept. 3,
1988.
6056-91(CIP)1.CN -78-
~aC
~'~ ~p P' n d ~ s
~$~:e~~~r~~'
10. r:ako~ski H, Tentchev P, Frey R, Necek S, Berg-
mann H and Blauhut B: Tolerance of an
Oxygen-carrying Colloidal Plasma: Substitute
in Human Beings. Fourth International Sym-
posium on Perfluorochemical Whole Blood
Substitutes, :Kyoto Mitsuno T and Naito R,
Excerpta Medico, Amsterdam, p. 47, 1979.
11. Maugh TH: Blood Substitute Passes its First
Test, Science, Vol. 206, p. 205, 1979.
12. Mitsuno T, Tabuchi Y, Ohyanagi H and Sugiyama
T: Further Studies on Intake and Retension of
Fluosol-DA in RES in Humans. Adeances in
Blood Substitute Research, Bolin, Geyer and
Nemo (Eds), Alan R. Liss, New York, p. 257,
1983.
13. Nishimura N and Sugi T: Changes of Hemody-
namics and 02 Transport associated with the
Perfluorochemical Blood Substitute Fluosol-DA.
Critical Care Medicine, Vol. 12, p. 36, 1983.
14. Tremper KK and Cullen BF: U.S. Clinical
Studies on the Treatment of Anemia with
Fluosol-DA (20%). Artificial Organs, Vol. 8,
p. 19, 1984.
15. Mitsuno T, Ohyanagi H and Naito R: Clinical
Studies of a Perfluorochemical Whole Blood
Substitute (Fluosol-DA): Summary of 186
Cases. Annals of Surgery, Vol. 195, p. 60,
1982.
16. Rude RE, Glogar D, Khuri SF, Kloner RA,
Karaffa S, Muller JE, Clark LC and Braunwald
E: Effects of Intravenous Fluorocarbons
during and wi~triout Oxygen Enhancement on Acute
Myocardial Ischemic Injury Assessed by Meas-
ure.rnent of Intramyocardial Gas Tensions.
American Heart Journal, Vol. 103, 986-995,
1982.
17. Ricci JL, Sloviter HA and Ziegler MM: Intes-
tinal Ischemia: Reduction of Mortality
Utilizing Intraluminal Perfluorochemical,
American Journal of Surgery, Vol. 149, p. 84
1985.
18. Usui M, Sakata H and Ishii S: Effect of
Fluorocarbon Perfusion upon the Preservation
of Amputated Limbs, British Journal of Bone
and Joint Surgery, Vol. 67, p. 473, 1985.
6056-91(CIP)1.CN -79-
\ac
203~~~~
19. Clark LC: Theoretical and Practical Consid-
erations of Fluorocarbon Emulsions in the
Treatment of Shock. Chap. 37 in Pathophysi-
ology of Shock, Anoxia and Ischemia, Cowley
and Trump (Eds), Williams and Wilkins, pp.
507°521, 1982.
20. Klystra JA, Tissing MO, Ban der Maen A: Of
Mice and Fish. Transactions of the American
Society for Artificial Internal Organs, Vol.
8, pp. 378-383, 1962.
21. Reufer R: Surfactant and Alveolar Surface
Forces after Breathing of an Inert Fluorinated
Liquid, Federal Proceedings., Vol. 29, No. 5,
pp. 1813-1815, 1970.
22. Clark LC and Gollan F: Survival of Mammals
Breathing Organic Liquids Equilibrated with
Oxygen at Atmospheric Pressure. Science, Vol.
152, pp. 1755-1756, 1966.
23. Modell JH, Newby EJ, and Ruiz BC: Long-term
Survival of Dogs after Breathing Oxygenated
Fluorocarbon Liquid, Federation Proceedings,
Vol. 29, NO. 5, pp. 1731-1739, 1970.
24. Modell JH, Calderwood HW, Ruiz BC, Tham MK and
Hood CI: Liquid Ventilation of Primates.
Chest, Vol. 69, pp. 79-81, 1976.
25. Calderwood HW, Ruiz BC, Tham MK, Modell JH and
Hood CI: Residual Levels and Biochemical
Changes after Ventilation with Perfluorinated
Liquid. Journal of Applied Physiology, Vol.
139, pp. 603-607, 1975.
26. Forman D, Bhutani VK, Hilfer SR and Shaffer
TH: A Fine Structure Study of the
Liquid-Ventilated New Rabbit. Federation
Proceedings Vol. 43, p. 647, 1984.
27. Rufer R and Spitzer L: Liquid Ventilation in
the Respiratory Distress Syndrome. Chest,
Vol. 66, July 1974 Supplement, pp. 29S-305,
1974.
28. Puchetti V, Maffezzoli GF, Costa~F, Montresor
E, Sgro M, and Carolo F: Liquid Ventilation in
Man: First Clinical Experiences on Pulmonary
Unilateral Washing using Fluorocarbon Liquid.
Fourth World Congress for Bronchology
(Abstracts), p. 115, 1984.
6056-91(CIP)1.CN -80-
\ac
~,{ '''i J
'..J t~ PJ ~ eJ N
29. Shaffer TH, Douglas PR, Lowe CA and Bhutani
VK: The Effects of Liquiri Ventilation on
Cardiopulmonary Function in Preterm Lambs.
Pediatric Research, Vol. 17, pp. 303-306,
1983.
30. Shaffer TH, Forman DL and Wilfson MR: Physio
logical Effects of Ventilation with Liquid
Fluorocarbon at Controlled Temperatures.
Undersea Biomedical Research, Vol. 11, No. 3,
pp. 287-298, 1984.
31. Lowe CA and Shaffer TH: Increased Pulmonary
Vascular Resistance during Liquid Ventilation.
Undersea Biomedical Research, Vol. 8, No. 4,
pp. 229-238, 1981.
32. Gollan F and Clark LC: Prevention of Bends
by
Breathing and Organic Liquid. Transactions
of
the Association American Physicians, Vol.
29,
pp. 102-109, 1967.
33. Sass DJ, Ritman EL, Caskey PE, Bancers
N, Wood
EH: Liquid Breathing: Prevention of Pulmonary
Artex~io-Venous Shunting during Acceleration.
Journal of Applied Physiology, Vol. 32,
pp.
451-455, 1972.
34. Shaffer TH, Rubenstein D, Moskowitz GD,
and
Delivoria-Papadopoulos: Gaseous Exchange
and
Acid-base Balance in Premature Lambs During
Liquid Ventilation Since Birth, Pediatric
Research, Vol. 10, p. 227-231, 1976.
35. Wolfson MR, Tran N, Bhutani VK, and Shaffer
TH: A New Experimental Approach for the
Study
of Cardiopulmonary Physiology During Early
Development, Journal of Applied Physiology,
Vol. 65, No. 3, pp. 1436-1443, 1988.
36. Shaffer TH, Tran HN, Bhutani VK, and Sivieri
EM: Cardiopulmonary Function in Very Preterm
Lambs During Liquid Ventilation. Pediatric
Research, Vol. 17, pp. 680-684, 1983.
37. Shaffer TH, Delivoria-Papadopoulos M, Arcinue
E, Paez P, and Dubois AB: Pulmonary Mechanics
in Premature Lambs During the First Few
Hours
of Life. Respiratory Physiology, Vol. 28,
pp. 179-188, 1976.
6056-91(CIP)1.CN -81-
\ac
t.~ ~ ~.' ~~ ~ ~
38. Shaffer TH, Douglas PR, Lowe CA, Bhutani VK:
Liquid Ventilation: Improved Gas Exchange and
Lung Compliance in Preterm Lambs. Pediatric
Research, Vol. 17, pp. 303-306, 1983.
39. Shaffer TH: Personal communicat~.on, June 1989.
40.Neus E: Premature Infant Held to Life by
Breathing Liquid. The Cincinnati Enquirer, p.
D1, August 3, 1989.
41. Moskowitz GD and Greiner TJ: Heat Transfer
During Liquid Breathing. Proceedings of 28th
Conference on Engineering in Medicine and
Biology, p~ 17, 1975.
42. Forman DL, Bhutani VK, Tran N, and Shaffer TH:
A New Approach to Tnduced Hypothermia.
Journal of Surgical Research, Vol. 40, pp.
36--42, 1986.
43. Dawson CA, Johnston MR, Rickaby DA, and
Fehring JF: The Influence of Hyperthermia on
Isolated Perfused Lung. FASEB Journal, Vol.
3, NO. 3, p. A546, 1989.
44. Teicher BA and Rose CM: Perfluorochemical
Emulsion Can Increase Tumor Radiosensitivity,
Science, Vol. 223, p. 934, 1984.
45. Teicher BA and Rose CM: Effects of Dose and
Scheduling on Growth Delay of the Lewis Lung
Carcinoma Produced by the Perfluorochemical
Emulsion, Fluosol-DA, International Journal of
Radiation Biology and Physics, Vol. 12, pp.
1311-1313, 1986.
46. Russell GE, Brass-Marlow EL, Nunno MP, Holst
RJ, Waldow SM, Lustig RA, and Wallner PE:
Response of Primary and Metastatic Lewis Lung
Tumors to Fluosol-DA 20~, Oxygen and X-Radia-
tion, presented at the Ninth Annual Meeting of
the North American Hyperthermia Group, Seat-
tle, Washington, March 18, 1989.
47. Rose CM, Lustig R, McIntosh N, and Teicher BA:
A Clinical Trial of Fluosol DA 20~ in Advanced
Squamous CeII Carcinoma of the Head and Neck,
International Journal of Radiation Biology and
Physics, Vol. 12, pp. 1325-1327, 1986.
6056--91 (CIP) 1. CN -82-
\ac
4 ~_~ C,3 L°_~ ~~ y ; .
~' :~..e ~, 2 !,~ Ma
48. Ohyanagi H, Nishiijima M, Usami M, and
Nishimatsu S: Experimental Studies on the
Possible Combined Chemotherapy to Neoplasms
with Fluosol-DA Infusion, in Advances in Blood
Substitute Research, Bolin, Geyer, and Nemo
(Eds.), Alan R. Liss, New York, p. 315, 2983.
49. Ref. 4 (Biro and Blais), p. 334.
50. Saunders MT: Hypoxic Cell Radiosensitization
in Carcinoma of the Bronchus, in Cancer
Treatment Symposia, Vol. 2, pp. 69-73, 1985.
51. Clark LC, Ackerman JL, Thomas SR, Millard RW,
Hoffman RE, Pratt RG, Ragle-Cole H, Kinsey RA,
and Janakiraman R: Perfluorinated Organic
Liquids and Emulsions as Biocompatible NMR
Imaging Agents for 19F and Dissolved Oxygen
from Oxygen Transport to Tissue -- VI, Bruley,
Bicker. and Reneau, Plenum, pp. 835-845, 1985.
52. Ackerman JL, Clark LC, Thomas SR, Pratt RG,
Kinsey RA, Becattini F: NMR Thermal. Imaging,
Proceedings of 3rd Annual Conference of the
Society of Magnetic Resonance in Medicine,
August 13, 1984, New York, New York.
53. Thomas SR, Clark LC, Ackerman JL, Pratt RG,
Hoffman RE, Busse LJ, Kinsey RA, and Sumara-
tunga RC: MR Tmaging of the Lung Using Liquid
Perfluorocarbons. Journal of Computer
Assisted Tomography, Vol. 10, No. 1, pp. 1-9,
1986.
54, Long DM, Mai-shian L, Szanto PS, Alrenga DP,
Patel MM, Rios ifil, and Nyhus LM: Efficacy and
Toxicity Studies with Radiopaque Perfluoro-
carbon. Radiology, Vol. 105, pp. 323-332,
1972.
55. Church CC: A Theoretical Study of Cavitation
Generated by An Extracorporeal Shock Wave
Lithotripter. Journal of the Acoustical
Society of America, Vol. 86, No. 1, pp.
2,15-227, 1989.
56. Comroe JH: P~siology of Respiration, 2nd Ed.,
Year Book Medical Publishers, pp. 118-119,
1974.
6056-91(CIP)1.CN -83-
\ac
&~~'~~~~,°
57. Sekins KM: An Oscillating Helix Artificial.
Lung Employing Oxygen-Perfused Inner Capil-
laries. Proceedings of the 30th Annual
Conference on Engineering in Medicine and
Biology, paper 35-9, 1977.
58. Kapp DS, Fessenden P, Cox RS, Bagshaw MA, Hahn
GM, Fei-Fei L, Lee E, Prionas SD, and Lohrback
A: Prognostic Factors Predicting Tumor
Response (TR), Local Control (LC), and
Complications (C) in Patients Treated on
Randomized Protocols with Radiation Therapy
(XRT) and Either 2 or 6 Hyperthermia T~eat-
ments (HT). Abstracts of Eighth Annual
Meeting of the North American Hyperthermia
Group, p. 20, 1988.
59. Haveman, J: "Enhancement of Radiation Effects
by Hyperthermia,°' in Hyperthermia in Cancer
Treatment, Vol. I, Anghileri and Robert
(Eds.), pp. 171-172, CRC Press, Boca Raton,
Florida, 1986.
60. Chase GD, Deno RA, Gennaro AR, Gibson MR,
Stewart CK, King RE, Martin AN, Swinyard EA,
VanMeter CT, Osol A, Witlin B, and Hoover JE:
Remington's Pharmaceutical Sciences 14th
Edition, Mack Publishing, Easton, PA, 1970.
61. Gollan F, McDermott J, Johnson AE, and Namon
R: Compliance and Diffusion During Respiration
with Fluorocarbon Fluid. Federal Proceedings,
Vol. 29, No. 5, pp. 1725-1730, 1970.
62. Reu.fer R: Surfactant and Alveolar Surface
Forces after Breathing of an Inert Fluorinated
Liquid, Federal Proceedings, Vol. 29, No. 5,
pp. 1813-2815, 1970.2. Olson RM: Essentials
of Engineering Fluid Mechanics, 3rd Ed.,
Intext, New York, p. 336, 1973.
63. Olson RM: Essentials of Engineering Fluid
Mechanics, 3rd Ed., Intext, New York, NY, p.
336, 1973.
64. Holman, JP: Heat Transfer, 4th Ed.
McGraw-Hill, New York, p. 170, 1976.
65. Carson PL and Banjavec RA: Radiation Force
Balance Systems for Precise Acoustic Power
Measurement. ALPS document PAPS JASMA-70,
American Institute of Physics, New York, NY,
1980.
6056-91(CIP)1.CN -84-
\aC
~~~~4~w
66. Guy AW, Lehmann JF, and Stonebridge JB: Thera-
peutic Applications of Electromagnetic Power,
IEEE Vol. 62, No. l, pp. 59-60, 1974.
67. Keilman GK: New Ultrasound Hyperthermia
Applicators with Improved Bandwidth and
Spatial Uniformity. Abstracts of the 9th
Annual Meeting of the North American Hyper-
thermia Group, p. 32, 1989.