Note: Descriptions are shown in the official language in which they were submitted.
~6~
--1--
POLY(OXYDIPHENYLA~INES)
Background of the Invention
The present invention relates to
poly(oxydiphenylamines) which are useful as rubber
additive.
Summary of the Invention
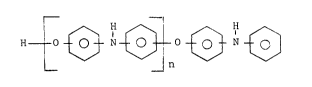
The present invention relates to
poly(oxydiphenylamines) of the formula:
~ 3 H ~ ~ H ~ (I)
wherein n is an integer ranging from about 1 to 100.
Detailed Description of the referred Invention
There is disclosed a poly(oxydiphenylamine) of the
formula:
~ ~ H ~ O ~ ~ ~ (I)
wherein n is an integer ranging from about 1 to 100.
Preferably, the poly(oxydiphenylamine) is of the
formula:
2 ~ 3
~ ~ H ~ ~ I ~ (II)
n
The term poly(oxydiphenylamine) is used herein to
describe dimers (where n is 1), oligomers (where n is
from about 2 to about 4) and polymers (where n is an
integer of from 5 to 100).
The molecular weight of the poly(oxydiphenylamines)
may range from about 370 to about 10,000. Preferably,
the molecular weight will range from about 370 to about
1000. As can be appreciated after having read the
present application, there may be dimers, oligomers and
polymers in the crude reaction mixture which all will
have different molecular weights within the above
ranges.
There is also disclosed a rubber composition which
comprises (1) a rubber selected from the group
consisting of natural rubber, homopolymers of
conjugated diolefins, copolymers of conjugated
diolefins and ethylenically unsaturated monomers or
mixtures thereof and a poly(oxydiphenylamine) of the
formula:
~ ~ n (I)
wherein n is an integer from about 1 to 100.
The poly(oxydiphenylamines) may be prepared by a
number of methods. For example, the
2~3~
poly(oxydiphenylamines) may be prepared by the air
oxidation of hydroxy diphenylamines. For example, the
poly(oxydiphenylamines) may be prepared by reacting
hydroxy diphenylamine with an oxygen containing gas
such as air for a period of from about 1 day to about
30 days a~ a temperature ranging from about room
temperature to about 200C. In the alternative, the
poly(oxydiphenylamines) may be prepared by heating a
hydroxy diphenylamine in the presence of a catalyst to
speed up the reaction. Examples of catalysts that may
be used include acid catalysts such as sulfuric acid,
hydrochloric acid and toluenesulfonic acid. The amount
the catalyst that will be used will vary depending on
the particular catalyst that is selected. For example,
when an acid catalyst is used from about 1 to about 20
percent by weight of the hydroxydiphenylamine is
recommended.
The reaction may be conducted over wide
temperatures. For example, the temperature may range
from about room temperature to an elevated temperature
depending on the particular type of reaction that is
selected. In general, the reaction may be conducted at
a temperature of between about room temperature to
about 220C. The preferred temperature range is from
about 100C to about 200C, while the most preferred
temperature range is from about 140C to about 180~C.
If all reaction variables remain constant except the
reaction temperature, it is believed that the reaction
product will have varying reactivities in the rubber.
Examples of hydroxy diphenylamines which may be
used include 4-hydroxydiphenylamine,
3-hydroxydiphenylamine and 2-hydroxydiphenylamine
Preferably, the hydroxydiphenylamine is
4-hydroxydiphenylamine, and mixtures of the above.
2~36~3
An organic solvent may be used to dissolve the
hydroxydiphenylamine. The solvent is preferably inert
to the reaction. Illustrative of solvents suitable for
use in the present invention include saturated and
aromatic hydrocarbons, e.g., hexane, octane, dodecane,
naphtha, decaline, tetrahydronaph~haleneS kerosene,
mineral oil, cyclohexane, cycloheptane,
alkylcycloalkane, benzene, toluene, xylene,
alkyl-naphthalene and the like; ethers such as
tetrahydrofuran, tetrahydropyran, diethylene ether,
1,2-dimethoxybenzene, 1,2-diethoxybenzene, the mono-
and dialkyl ethers of ethylene glycol, propylene
glycol, butylene glycol, diethylene glycol, dipropylene
glycol, oxyethylene oxypropylene glycol, and the like;
fluorinated hydrocarbons that are inert under the
reaction conditions such as perfluoroethane,
monofluorobenzene and the like. Another class of
solvents are sulfones such as dimethyl sulfone, diethyl
sulfone, diphenol sulfone, sulfolane, and the like.
Mixtures of the aforementioned solvents may be employed
so long as they are compatible with each other under
the conditions of the reaction and will adequately
dissolve the hydroxydiphenylamine and not interfere
with the reaction.
The process for the preparation of the
poly(oxydiphenylamines) may be carried out in a batch,
semi-continuous or continuous manner. The reaction may
be conducted in a single reaction zone or in a
plurality of reaction zones, in series or in parallel,
the reaction may be conducted intermittently or
continuously in an elongated tubular zone or in a
series of such zones. The material construction of the
equipment should be such as to be inert during the
reaction. The equipment should also be able to
2 ~
withstand the reaction temperatures and pressures. The
reaction zone can be fitted with internal and/or
external heat exchanges to control temperature
fluctuations. Preferably, an agitation means is
available to ensure uniform reaction. Mixing induced
by vibration, shaker, stirrer, rotating, oscillation,
etc. are all illustrative of the types of agitation
means which are contemplated for use in preparing the
composition of the present invention. Such agitation
means are available and well known to those skilled in
the art.
The reaction product will contain various
poly(oxydiphenylamines) of various molecular weights.
For example, it is contemplated that with respect to
the above formulae, various weight percentages of the
reaction mixture may range from about lO~ to about
100%. Use of the various mixtures of
poly(oxydiphenylamine) in rubber compounds is
contemplated herein.
The poly(oxydiphenylamine) may be added to sulfur
vulcanizable elastomers as an additive. The term
"rubber" or "elastomer" as used herein embraces both
natural and all its various raw and reclaimed forms as
well as various synthetic rubbers. Representative
synthetic elastomers are the homopolymerization
products of butadiene and its homologues and
derivatives, as for example, methylbutadiene,
dimethylbutadiene, chloroprene (neoprene synthetic
rubber) and pentadiene as well as copolymers such as
~0 those formed from butadiene or its homologues or
derivatives with other unsaturated organic compounds.
Among the latter are acetylenes, e.g., vinyl acetylene;
olefins, for example, isobutylene, which copolymerizes
with isoprene to form butyl rubber; vinyl compounds,
2~3~6~
--6--
for example, vinyl chloride, acrylic acid,
acrylonitrile (which polymerizes with butadiene to form
NBR), methacrylic acid and stvrene, the latter compound
polymerizes with butadiene to form SBR, as well as
vinyl esters and various unsaturated aldehydes, ketones
and ethers, e.g., acrolein, methyl isopropenyl ketone
and vinyl ethyl ether. Also included are the various
synthetic rubbers prepared by the homopolymerization of
isoprene and the copolymerization of isoprene with
other diolefins and various unsaturated organic
compounds. Additionally, included are the synthetic
rubbers such as 1,4-cis polybutadiene and 1,4-cis
polyisoprene and similar synthetic rubbers such as
EPDM. The preferred rubbers for use with the
poly(oxydiphenylamine) are SBR and polybutadiene or
mixtures thereof.
The rubber vulcanizates containing the
poly(oxydiphenylamine) may be used in the preparation
of tires, motor mounts, rubber bushings, power belts,
printing rolls, rubber shoe heels and soles, rubber
floor tiles, caster wheels, elastomer seals and
gaskets, conveyor belt covers, wringers, hard rubber
battery cases, automobile floor mats, mud flaps for
trucks, tires, ball mill liners and the like.
The poly(oxydiphenylamine) may be added to the
w lcanizable elastomer in a variety of levels.
Generally speaking, the concentration of the
polytoxydiphenylamine) ranges from about 0.1 parts per
hundred rubber (phr) to 10 phr and is in intimate
mixture with the elastomer. Preferably, the
poly(oxydiphenylamine) is at a concentration ranging
from about 0.5 phr to about 5 phr.
The poly(oxydiphenylamine) may be compounded in
either the productive or nonproductive stock.
Preferably, the poly(oxydiphenylamine) is compounded in
the nonproductive because uniform mixing is achieved.
Incorporation of the poly(oxydiphenylamine) into the
sulfur vulcanizable rubber may be accomplished by
conventional means of mixing such by the use of a
Banbury o-; Brabender.
Cure properties were determined using the Monsanto
Oscillating Disc Rheometer which was operated at a
temperature of 150 and at a frequency of 100 CPM. A
description of Oscillating Disc Rheometers can be found
in the Vanderbilt Handbook, edited by Robert O.
Babbitt, Norwalk, Connecticut, R. T. Vanderbilt
Company, Inc., 1978 (pages 583-591). The use of this
cure meter and standardized values read from the curve
are specified in ASTM D-2084. A typical cure curve
obtained on an oscillating disc rheometer is shown on
page 588 of the 1978 edition of the Vanderbilt Rubber
Handbook.
In such an oscillating disc rheometer, compounded
rubber samples are subjected to an oscillating shearing
action of constant amplitude. The torque of the
oscillating disc embedded in the stock that is being
tested is required to oscillate the rotor at the
w lcanization temperature. The values obtained using
this cure test are very significant since changes in
the rubber or the compounded recipe are very readily
detected. It is obvious that it is normally
advantageous to have a very fast cure rate. Some of
the following tables report cure properties that were
determined from cure curves that were obtained for the
various rubber formulations that were prepared. The
properties include minutes to 90% of torque increase
(t90 min.).
-8- ~ ~ 3 ~
Peel adhesion testing was done to determine the
interfacial adhesion between various rubber
formulations that were preparecl. The interfacial
adhesion was determined by pulling one compound away
from another at a right angle to the untorn test
specimen with the two ends being pulled apart at 180
angle to each other using an Instron machine. The area
of contact was determined from placement of a Mylar
sheet between the compounds during cure. A window in
the Mylar allowed the two materials to come into
contact with each other during curing and subsequent
testing.
Example 1
Preparation of Poly(oxy-4,4'-diphenylamine)
Poly(oxy-4,4'-diphenylamine) was prepared by
charging a one-liter 3-neck round bottom flask with 93
grams (0.5 mole) of 4-hydroxydiphenylamine, 11 grams of
p-toluene sulfonic acid and 97 ml of m-xylene. The
mixture was heated to 205C for 24 hours, wherein 6 ml
of water were removed. HPLC analysis showed the
formation of oligomers. The crude product was a black
crystalline solid melting at 67C with an acid number
of 46. The molecular weight range was from about 370
to several thousand.
Example 2
Four batches of poly(oxy-4,4'-diphenylamine) were
prepared by this same procedure except different
reaction temperatures were used. Each batch was
prepared by charging a one-liter, 3-neck round bottom
flask with 186 grams (1.0 mole) of
4-hydroxydiphenylamine, 22 grams of p-toluene sulfonic
acid and 200 ml of m-xylene. The mixtures were heated
2~s~a
_9_
to 160C (first batch), 180C (second batch~, 200C
(third batch), or 220C (fourth batch) for five hours.
HPLC analysis showed formation of dimers and oligomers.
The crude products were mostly dimers for the 160C
(first batch) and up to about 10,000 molecular ~eight
polymers in the 220C (fourth batch). The second and
third batches contained crude mixtures of
poly(oxydiphenylamine) oligomers.
Example 3
Table I below shows the basic rubber compound that
was used in this example. The rubber compound was
prepared in a one stage Banbury mix. All parts and
percentages are by weight unless otherwise noted.
15The control had no poly(oxy-4,4'-diphenylamine)
whereas, sample 1 contained 2 phr of
poly(oxy-4,4'diphenylamine). The
poly(oxydiphenylamine) was prepared by the procedure of
Example l. The physical data for each sample is shown
in Table II.
~33~4~
-10-
Table I
WeightBanbury
Material PartsStage
SBR 50.00
Polybutadiene 50.00
Carbon Black 64.50
Antiozonant/Antioxidant 1.75
Processing Materials34.90
10 Zinc Oxide 3.00
Poly(oxydiphenylamine) (1) 2.00
Sulfur/Accelerator 3.75
(1) Not used in control.
Table II
Control plus 2 phr
Poly(oxy-4,4'-
Controldiphenylamine
t90 (min.) 29.3 25.6
Tensile Strength 11.2 13.6
% Elongation @ Break 385 460
Modulus 300% 9.0 8.8
Peel Adhesion 63.5 83.8
to Self 61.9 70.6
The addition of 2 phr of
poly(oxy-4,4'-diphenylamine) results in a substantial
increase in peel adhesion, tensile strength, percent
elongation at break.
~3~
Example 4
Table III below shows the basic rubber compound
that was used in this example. The rubber compound was
prepared in a two stage Banbury mix. All parts and
percentages are by weight unless otherwise noted.
The two controls had no
poly(oxy-4,4'-diphenylamine) wherein the sample was
prepared in accordance with Example 2 (namely Sample 1)
at 160C, Sample 2 at 180C, Sample 3 at 200C and
Sample 4 at 220C. The physical data for the two
controls and each sample are shown in Table IV.
Table III
Weight Banbury
Parts Sta~e
Natural Rubber 50
SBR 50
Filler 63.6
Processing Oil 19.5
Stearic Acid 2.0
Zinc Oxide 3.5
Antioxidant 2.95
Poly(oxy-4,4'-diphenylamine) Varied
Sulfur, Accelerator 2.93 2
2~36~
-12-
O ~ ~ ~ ~
O u~ O~ I ~O ~ I~ ~ 00 ~ ~ C~l
~ U~
o
J-
~ o ~:
C~ U~ ~ I~ ~~ ~ ) ~ ~ o
o. . . . .. . . . C,)
~ I~ ~-- ~D ~ u~ ~ 00 C`l
O c~ ~ ~ ~u~ ~~ ~D C~
~ C~ ~ ,~
Q ~
.
t~
a~ ta
O ~ o
~,i ~ o~ u~ o 1~ ~n
U~ 00 C~l ~1 ~ 1~U~ ~~ ~ ~ a
U~
o U~
U~ ~ I
C~ ~ C~ ~ ~ oo o
O o o . . . . . .. . . o
h u~ o ~1 'D ~ ~ o ~ o
C~ ~ ~1 ~1U~ ~~t U) U~ C~
~D
>~ O rC ~
~ ~ ~ U~
OJ ~ O O C~
,D td h ~ JJ
td ~ ~ C~
~b ~ ~ I_ I~ U~~ a~~ ~ ~ ~
h ~ O ~ o ~
~ O ~ tn
a) P~
h e ~ ~ ~
O
~o ~ ~ u~ oo o ~
~ ~ ~~ C, "
X ;o ~ =Ob u~ 1~ 0~O ~ ~ ~ 00
O ~ O C~ ~ O
~ h C~ ~ ,C ,~
~ ~ e
S~ S~
a) o a~
e ~ h
~ u~ 3
E~ ,~
S~ h
e
~ o ~n
O ~ u e e ~ c~ ~ O
JJ ~ a)~ ~ ~o O c~ ~: o
rl o ~ O O
h Et ~ O e " e ~O~ a~
Q) e ,, ~ O O O O ~ _
J-l t~ O O ~ O O ~ ~r~
~ ~ ~ ~ ~ ~ e
e ~ O ~ o c ~ a) ..
o o cr~ ~ ~ ~ o ~ ~ ? oo ~
a~ ~ ~ ~ ~ o ~ ~ ~ o ~ ~ ~ ¢ ~ E~
.~ o a) ~ ~ ~ a) o
~ ~ E~ ~ X ~ ~ æ