Note: Descriptions are shown in the official language in which they were submitted.
CA 02037100 1999-O1-22
X-8163M -1-
TITLE
6-SUBSTITUTED-HEXAHYDROBENZ[CD)INDOLES
Field of the Invention
This invention relates to the fields of
synthetic organic chemistry and pharmaceutical chemistry
and involves hexahydrobenz[cd)indoles which are useful
in modifying the serotonin function in the body.
Background of the Invention
., In recent years it has become apparent that
the neurotransmitter serotonin (5-hydroxytryptamine, ie
5-HT) is associated with a number of physiological
phenomena such as acid secretion, anxiety, depression,
sexual dysfunction, emesis, memory, hypertension,
appetite, and sleep. [Glennon, R. A., J. Med. Chem.,
30, 1 (1987)). Multiple receptors have been found for
5-HT. These receptors have been classified as 5-HT,
5-HT1, 5-HT2, and 5-HT3 receptors with the former being
CA 02037100 1999-O1-22
X-8163M -2-
further classified as 5-HT1A, 5-HT1B, 5-HT1C and
5-HT1D. The binding activity of a compound to one or
more of these 5-FiT receptors has been recognized as
being predictive of physiological activity of the
compound.
Flaugh in U.S. Patent No. 4,576,959 (issued
1986) disclosed a family of 6-substituted-4-dialkylamino-
1,3,4,5-tetrahydrobenz[cd]indoles which show binding
affinity for 5-HT receptors and are described as central
serotonin agonists. Leander in U.S. Patent 4,745,126
(1988) disclosed a method for treating anxiety in humans
employing a 4-substituted-1,3,4,5-tetrahydrobenz[cdJindole-
6-carboxamide derivative.
Certain indolines have been reported, as in
U.S. Patent No. 4,110,339 of Bach et al. (1978), Flaugh
et al., J. Med. Chem., 31, pp 1746-1753 (1988), Flaugh
in U.S. Patent No. 4,576,959 and European Patent Ap-
plication 0153083 (published 1985). These were used
as intermediates in the preparation of the corresponding
indoles.
It has now been found that certain 4- and 6-
substituted hexahydrobenz[cd]indoles (indolines), and
particularly certain stereoisomers of such indolines,
are useful in treating conditions requiring alteration
of the 5-HT1A receptor function in the body. The 2aS,
4R isomer has been found to be particularly useful.
CA 02037100 1999-O1-22
X-8163M -3-
Summary of the Invention
This invention relates to a compound of the
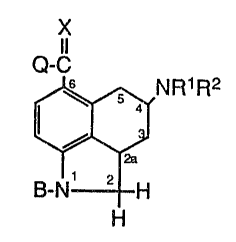
Formula IA
Q-C~ X
5 4 NR~ R2
/ IA
to B-N~ 2 H
H
wherein:
R1 is hydrogen, C1-C4 alkyl, C3-C4 alkenyl,
phenyl-substituted C1-C4 alkyl, cyclopropylmethyl,
O
-C-R4, -(CHZ)nS(C1-C4 alkyl) or -(CHZ)n C(O)I~TR9R1°;
RZ is hydrogen, C1-C4 alkyl, C3-CQ alkenyl, or
cyclopropylmethyl;
Q is OR3, SR3, NRSR6 or hydrogen;
R3 is C1-C8 alkyl, substituted C1-C$ alkyl,
aryl, substituted aryl, aryl (C1-C4 alkyl), substituted
aryl (C1-C4 alkyl), or C3-C~ cycloalkyl;
R4 is hydrogen, C1-C4 alkyl, C1-C4 haloalkyl,
C1-C4 alkoxy or phenyl;
RS and Rs are independently hydrogen, C1-C4
alkyl, phenyl-substituted C1-C4 alkyl, phenyl, or
together form a C3-CS heterocyclic ring with the proviso
that R$ and R6 are not both hydrogen;
CA 02037100 1999-O1-22
X-8163M -4-
R9 and R1° are independently hydrogen, a
Ci-C4 alkyl, or a CS-C8 cycloalkyl;
n is 1 to 4;
B is hydrogen, C1-C4 alkyl or an amino-
blocking group; and
X is oxygen or sulfur; and
a pharmaceutically acceptable salt thereof.
This invention further relates to a
substantially pure stereoisomer of a compound of the
Formula IB
A
R~R2
IB
B_N
H
wherein:
X
A is CI-
Q, hydrogen, halogen, CN, NO2, ~SRs~
NHC(O)Rs, -NHS02Rs, CONH2, X(C1-C8 alkyl), OH, O-acyl,
O-benzyl or CF3;
and Q, B, X, R1, R2, R3 R5 and Rs are as
defined hereinabove; and positions 2a and 4 have a
configuration of S and R respectively; and
a pharmaceutically acceptable salt thereof.
»
.
X-8163M -5-
Another embodiment of the invention is
characterized by a pharmaceutical formulation comprising
a compound of Formula IA or IB in combination with a
pharmaceutically acceptable carrier, excipient or
diluent therefor.
A further embodiment of the invention is a
method for effecting a biological response at the
5-HT1A receptor by administering to a patient a
pharmaceutically effective amount of a compound of
14 Formula IA or IB as defined above or a pharmaceutically
acceptable salt thereof. More particularly, further
embodiments involve treating a variety of conditions
which require regulation of serotonin function in the
body by administering a pharmaceutically effective
amount of a compound of Formula IA or IB as defined
above or a pharmaceutically acceptable salt thereof.
The present invention is characterized in a
further embodiment by a process for preparing a compound
of the formula
1R2
or a pharmaceutically acceptable salt thereof which is
characterized by
CA 02037100 1999-O1-22
X-8163M -6-
a) reacting a compound of formula
I
R~R2
s
wherein:
R1 is hydrogen, C1-C4 alkyl, C3-C4 alkenyl,
cyclopropylmethyl, phenyl-substituted C1-C4 alkyl,
-COR4, -(CH)nS(C1-C4 alkyl) or -(CHZ)nCONR9Rlo;
RZ is hydrogen, C1-C4 alkyl, C3-C4 alkenyl,
or cyclopropylmethyl;
n is 1-4;
R4 is hydrogen, C1-C4 alkyl, C1-C4 haloalkyl,
C1-C4 alkoxy or phenyl;
R9 and R1° are independently hydrogen, C1-C4
alkyl, or CS-C8 cycloalkyl; and
B is an amino-blocking group,
with CO and Q-H in the presence of a palladium catalyst
to provide a compound of the formula
Q_C~O
R~R2
wherein:
R1, RZ and B are as defined above and Q is
R30- or RSRsN-
~~ ~a ~ .A' r
X-8163M -7-
wherein:
R3 is C1-C$ alkyl, substituted C1-C$ alkyl,
aryl, substituted aryl, aryl (C1-C4 alkyl), substituted
aryl (G1-C4 alkyl), or C3-C~ cycloalkyl; and
R5 and R6 are independently hydrogen, C1-C4
alkyl, C1-C4 alkyl substituted with a phenyl group,
phenyl, or together form a C3-C5 heterocyclic ring,
with the proviso that RS and R6 are not both hydrogen;
b) reacting a compound of the formula
O
HO-C
R~R2
with R3X8 to provide a compound of the formula
O
R3X-C
NR~ R2
/
B-N-~-H
H
wherein:
X is oxygen or sulfur; and
R1, R2, R3 and B are as defined above;
X-8163M -8-
c) reacting a compound of the formula
a
R2
wherein:
R1 and RZ are as defined above
O
I I
with Y-C-OR3
wherein:
R3 is as defined above and Y is a leaving
group,
to provide a compound of the formula
O
R3o-c'
NR~R2
/
~CsHs)sC-N.-~-H
H
wherein
R1, R2, and R3 are as defined above,
O
I
or with Y-C-NR5R6.
a
X-8163M -9-
to provide a compound of the formula
NR~ R2
wherein:
H
R1, R2, R5, Rg and Y are as defined above;
d) replacing the 1-nitrogen blocking group B of a
compound of the formula
Q-C~~
s NR~R2
4
as
B-N~ 2 H
H
with hydrogen to provide a compound of the formula
Q-C~X
NR~ R2
4
H-N~ 2 H
H
wherein:
R1, R2, X and Q are as defined above and B is
an amino blocking group.
X-8163M -10-
In another embodiment the invention is
characterized by a process for preparing a compound of
the formula
H
wherein:
X
R2
A is hydrogen, Q-C-, NR5R6, NHC(O)Rs, NFiS02Rs,
O-acyl, O-benzyl, or CF3;
R1 is hydrogen, C1-C4 alkyl, C3-C4 alkenyl,
cyclopropylmethyl, phenyl-substituted C1-C4 alkyl,
-COR4 -(CHZ)nS(C1-C4 alkyl), or -(CH2)nCONR9R1°;
RZ is hydrogen, C1-C4 alkyl, C3-C4 alkenyl,
or cyclopropylmethyl;
Q is OR3, SR3, NR5R6 or hydrogen;
R~ is C1-C$ alkyl, substituted C1-C8 alkyl,
aryl, substituted aryl, aryl (C1-C4 alkyl), substituted
aryl (C1-C4 alkyl), or C3-C~ cycloalkyl;
n is 1-4;
R4 is hydrogen, C1-C4 alkyl, C1-C4 haloalkyl,
C1-C4 alkoxy or phenyl;
R5 and Rs are independently hydrogen, a C1-C4
alkyl, a C1-C4 alkyl substituted with a phenyl group,
phenyl, or together form a C3-C5 heterocyclic ring;
CA 02037100 1999-O1-22
X-8163M -11-
R9 and R1° are independently hydrogen,
C1-C4 alkyl, or C5-C8 cycloalkyl with the proviso
that when one of R9 or R1° is a cycloalkyl the other is
hydrogen;
X is oxygen or sulfur; and
the configuration at position 2a is S and at
position 4 is R,by replacing with hydrogen the amino-
blocking group B of a compound of the formula
to R'R2
wherein:
A, R1, and R2 are as defined above.
Detailed Descri tion of the Invention
As used herein, the term "alkyl" represents a
straight or branched alkyl chain having the indicated
number of carbon atoms. For example, "C1-C4 alkyl"
groups are methyl, ethyl, n-propyl, isopropyl, n-butyl,
sec.-butyl, isobutyl and tert-butyl. "C1-C$ alkyl"
groups include those listed for C1-C4 alkyl as well as
n-pentyl, 2-methylbutyl, 3-methylbutyl, n-hexyl, 4-
methylpentyl, n-heptyl, 3-ethylpentyl, 2-methylhexyl,
2,3-dimethylpentyl, n-octyl, 3-propylpentyl, 6-methyl-
heptyl, and the like.
~o~~~oo
X-8163M -12-
The term "C3-C4 alkenyl" refers to olefinically
unsaturated alkyl groups such as -CHZCH=CH2, -CHZCHZCH=CH2,
-CH(CH3)CH=CHZ and the like.
The term "aryl" means an aromatic carbocyclic
structure. Examples of such ring structures are phenyl,
naphthyl, and the like.
The term "cycloalkyl" means an aliphatic
carbocyclic structure having the indicated number of
carbon atoms in the ring. For example, the term
cycloalkyl" means cyclopropyl, cyclobutyl, cyclopentyl,
cyclohexyl, and cycloheptyl.
The term "aryl (C1-C4 alkyl)" means an
aromatic carbocyclic structure joined to a C1-C4 alkyl
group. Examples of such groups are benzyl, phenylethyl,
a-methylbenzyl, 3-phenylpropyl, a -naphthylmethyl,
~-naphthylmethyl, 4-phenylbutyl, and the like.
Similarly the term "aryl (C1-C3 alkyl)" means an
aromatic carbocyclic structure joined to a C1-C3 alkyl.
The C1-C8 alkyl, the aryl, the aryl (C1-C4
alkyl) groups, and aryl (C1-C3 alkyl) can be substituted
by one or two moieties. Typical aryl and/or alkyl
substituents are C1-C3 alkoxy, halo, hydroxy, C1-C3
thioalkyl, nitro, and the like. Moreover, the aryl,
aryl (C1-C4 alkyl) and aryl (C1-C3 alkyl) groups may
also be substituted by a C1-C3 alkyl or a trifluoro-
methyl group.
In the foregoing, the term "C1-C3 alkyl" means
any of methyl, ethyl, n-propyl, and isopropyl; the term
"C1-C3 alkoxy" means any of methoxy, ethoxy, n-propoxy,
CA 02037100 1999-O1-22
X-8163M -13-
and isopropoxy; the term "halo" means any of fluoro,
chloro, bromo, and iodo; and the term "C1-C3 thioalkyl"
means any of methylthio, ethylthio, n-propylthio, and
isopropylthio.
Examples of substituted C1-C$ alkyl are
methoxymethyl, trifluoromethyl, 6-chlorohexyl, 2-
bromopropyl, 2-ethoxy-4-iodobutyl, 3-hydroxypentyl,
methylthiomethyl, and the like.
Examples of substituted aryl are p-bromo-
phenyl, m-iodophenyl, p-tolyl, o-hydroxyphenyl, ~-
(4-hydroxy)naphthyl, p-(methylthio)phenyl, m_-trifluoro-
methylphenyl, 2-chloro-4-methoxyphenyl, a-(5-chloro)-
naphthyl, and the like.
Examples of the substituted aryl (C1-C4
alkyl) are p-chlorobenzyl, o-methoxybenzyl, m_-(methyl-
thio)-a-methyl-benzyl, 3-(4'-trifluoromethylphenyl)-
propyl, o-iodobenzyl, p-methylbenzyl, and the like.
The term "C3-C5 heterocyclic ring" includes
pyrrolidine, piperidine, morpholine and the like.
The term "amino-blocking group" is used as it
is frequently used in synthetic organic chemistry, to
refer to a group which will prevent an amino group from
participating in a reaction carried out on some other
functional group of the molecule, but which can be
removed from the amine when it is desired to do so.
Such groups are discussed by T. W. Greene in chapter 7
of Protective Grou s in Organic Synthesis, John Wiley
and Sons, New York, 1981, and by J. W. Barton in chapter
2 of Protective Groups in Organic Chemistry, J. F. W.
McOmie, ed., Plenum Press, New York, 1973,
CA 02037100 1999-O1-22
X-8163M -14-
Examples of such groups include those of the formula
-COOR where R includes such groups as methyl, ethyl,
propyl, isopropyl, 2,2,2-trichloroethyl, 1-methyl-1-
phenylethyl, isobutyl, t-butyl, _t-amyl, vinyl, allyl,
phenyl, benzyl, p-nitrobenzyl, _o-nitrobenzyl, and
2,4-dichlorobenzyl, benzyl and substituted benzyl such
as 3,4-dimethoxybenzyl, _o-nitrobenzyl, and triphenyl-
methyl; acyl and substituted acyl groups such as formyl,
acetyl, chloroacetyl, dichloroacetyl, trichloroacetyl,
trifluoroacetyl, benzoyl, and p-methoxybenzoyl; and
other groups such as methanesulfonyl, p-toluenesulfonyl,
p-bromobenzenesulfonyl, p-nitrophenylethyl, and p-
toluenesulfonylaminocarbonyl. Preferred amino-blocking
groups are benzyl (-CHZCsHS), acyl [C(O)R] or SiR3 where
R is C1-C4 alkyl, halomethyl 2-halo-substituted (CZ-C4
alkoxy), or phenyl.
The compounds of the instant invention have
at least 2 chiral centers and therefore at least four
stereoisomers can exist for each. If a substituent
group contains a chiral center, then additional stereo-
isomers can of course exist. The racemic mixtures of
the compounds of Formula 1A as well as the
substantially pure stereoisomers of Formula IB are
contemplated as within the scope of the present in-
vention. The term "substantially pure" refers to at
least about 90 mole percent, more preferably 95 mole
percent and most preferably at least 98 mole percent of
the desired stereoisomer being present compared to the
CA 02037100 1999-O1-22
X-8163M -15-
other stereoisomers present. Particularly preferred
stereoisomers are those in which the configuration of
the chiral centers at position 2a is S and at position 4
is R.
The terms "R" and "S" are used herein as
commonly used in organic chemistry to denote specific
configuration of chiral center. The term "R" refers to
"right" and refers that configuration of a chiral
center with a clockwise relationship of group
priorities (highest to second lowest) when viewed along
the bond toward the lowest priority group. The term
"S" or "left" refers to that configuration of a chiral
center with a counterclockwise relationship of group
priorities (highest to second lowest) when viewed along
the bond toward the lowest priority group. The priority
of groups is based upon their atomic number (heaviest
isotope first). A partial list of priorities and a
discussion of stereo chemistry is contained in the
book: The Vocabulary of Organic Chemistry, Orchin,
et al. John Wiley and Sons Inc., publishers, page 126, (1989),
While all of the compounds of the invention
are useful for the purposes taught herein, certain of
the present compounds are preferred for such uses.
Preferably X is oxygen or sulfur; R1 is hydrogen, C1-C4
alkyl, allyl or C(O)R4; RZ is hydrogen, C1-C4 alkyl, or
allyl; R3 is C1-C4 alkyl; R4 is hydrogen, methyl,
ethyl, propyl, trifluoromethyl or phenyl; and RS and Rs
are independently hydrogen, a C1-C4 alkyl, a phenyl
(C1-C4 alkyl), phenyl or together form a C3-C5
X-8163M -16-
heterocyclic ring with the proviso that Rs and Rg are
not both hydrogen. More preferably X is oxygen, Ri and
Rz are both C1-C4 alkyl, and especially n-propyl, and
R3 is hydrogen or C1-C3 alkoxy particularly methoxy or
ethoxy. Other preferred aspects of the present in-
vention are noted hereinafter.
As set forth above, this invention includes
the pharmaceutically-acceptable salts of the compounds
of Formula IA and IB. Since the compounds of this
invention are amines, they are basic in nature and
accordingly react with any number of inorganic and
organic acids to form pharmaceutically acceptable salts
such as hydrochloric acid, nitric acid, phosphoric acid,
sulfuric acid, hydrobromic acid, hydroiodic acid,
phosphorous acid and others, as well as salts derived
from non-toxic organic acids such as aliphatic mono and
dicarboxylic acids, amino acids, phenyl-substituted
alkanoic acids, hydroxyalkanoic and hydroxyalkandioic
acid, aromatic acids, aliphatic and aromatic sulfonic
acids. Such pharmaceutically-acceptable salts thus
include sulfate, pyrosulfate, bisulfate, sulfite,
bisulfite, nitrate, phosphate, monohydrogenphosphate,
dihydrogenphosphate, metaphosphate, pyrophosphate,
chloride, bromide, iodide, acetate, propionate, capry-
late, acrylate, formate, tartrate isobutyrate, caprate,
heptanoate, propiolate, oxalate, malonate, succinate,
suberate, sebacate, fumarate, maleate, mandelate,
butyne-1,4-dioate, hexyne-1,6-dioate, hippurate,
benzoate, chlorobenzoate, methylbenzoate, phthalate,
terephthalate, benzenesulfonate, toluenesulfonate,
203'~~.~0
X-8163M -17-
chlorobenzenesulfonate, xylenesulfonate, phenylacetate,
phenylpropionate, phenylbutyrate, citrate, lactate,
~-hydroxybutyrate, glycolate, malate, naphthalene-1-
sulfonate, naphthalene-2-sulfonate and mesylate.
The following list illustrates representative
compounds of the present invention:
4-(dimethylamino)-1,2,2x,3,4,5-hexahydro-
benz[c,d]indole-6-carbothioic acid, s-methyl ester;
4-(di-n-propylamino)-1,2,2x,3,4,5-hexa-
hydrobenz(c,d]indole-6-carbothioic acid, O-ethyl ester;
4-(methylethylamino)-1,2,2x,3,4,5-hexa-
hydrobenz[c,d]indole-6-carbodithioic acid, methyl ester;
4-(n-butylamino)-1,2,2x,3,4,5-hexahydro-
benz[c,d]indole-6-carboxylic acid, ethyl ester;
4-(di-n-propylamino)-1,2,2x,3,4,5-hexahydro-
benz[c,d]indole-6-carbothioic acid, S-methyl ester;
4-amino-1,2,2x,3,4,5-hexahydrobenz[c,d]-
indole-6-carbodithioic acid, n-propyl ester;
4-(methylamino)-1,2,2x,3,4,5-hexahydro-
benz[c,d]indole-6-carboxylic acid. n-propyl ester;
4-amino-1,2,2x,3,4,5-hexahydrobenz[c,d]-
indole-6-carboxylic acid, methyl ester;
4-(diethylamino)-1,2,2x,3,4,5-hexahydro-
benz[c,d]indole-b-carboxylic acid, n-propyl ester
maleate;
(2aS,4R)-4-(di-n-propylamino)-6-iodo-
1,2,2a,3,4,5-hexahydrobenz[cd]indole;
4-(dimethylamino)-1,2,2x,3,4,5-hexahydro-
benz[c,d]indole-6-carboxylic acid, methyl ester;
CA 02037100 1999-O1-22
X-8163M -lg-
4-(di-n-propylamino)-1,2,2a,3,4,5-hexa-
hydrobenz[c,d]indole-6-carboxaldehyde;
4-(methylethylamino)-1,2,2a,3,4,5-hexa-
hydrobenz[c,d]indole-6-carboxylic acid, ethyl ester;
4-(di-n-propylamino)-6-aminocarbonyl-1,2,2a,-
3,4,5-hexahydrobenz[c,d]indole;
(2aS, 4R)-4-(di-n-propylamino)-6-aminocarbonyl-
1,2,2a,3,4,5-hexahydrobenz[c,d]indole;
(2aS,4R)-4-(di-n-propylamino)-6-bromo-1,2,2a,-
3,4,5-hexahydrobenz[c,d]indole;
(2aS,4R)-4-(di-n-propylamino)-6-cyano-1,2,2a,-
3,4,5-hexahydrobenz[c,d]indole; and
(2aS,4R)-4-(di-n-propylamino)-6-methoxy-1,2,-
2a,3,4,5-hexahydrobenz[c,d]indole.
In a preferred method of preparation, 6-iodo-
1,2,2a,3,4,5-hexahydrobenz[cd]indole _2 is a useful inter-
mediate to the instant compounds in which the substituent
at the 6-position is aminocarbonyl, alkyl- or aryl-
substituted amides, or alkyl-or aryl-carboxylic acid
esters. The aminocarbonyl group can be introduced by
reacting the 6-iodo indoline with ammonia and carbon
monoxide in the presence of a palladium catalyst as
described by Schoenberg et al. J. Org. Chem., _39, p
3327, 1974 and Schoenberg et al. J. Org. Chem., _39, p
3318, 1974.
Substituted amides can be introduced at the 6-position
by using an amine instead of ammonia in the reaction.
Carboxylic acid esters substituted at the 6-position can
be prepared by using alcohols in place of ammonia. The
~..
X-8163M -19-
preferred palladium catalysts are bis(triphenylphos-
phine)palladium chloride, bis(triphenylphosphine)-
palladium bromide and tetrakis(triphenylphosphine)-
palladium. Inert solvents such as acetonitrile or
toluene are suitable. When ammonia is used, an approxi-
mately equimolar mixture of carbon monoxide and ammonia
is supplied to the reaction at approximately one to
approximately twenty atmospheres of pressure. When a
reactant such as an amine or an alcohol is used in place
of ammonia, the reagents are mixed in a reaction vessel
and the desired pressure of carbon monoxide is introduced.
The reaction mixture is stirred at a temperature between
about 25°C and about 150°C until the 6-iodo indoline is
substantially consumed, as determined, for example, by
thin layer chromatography or liquid chromatography.
This reaction can then be followed by additional steps
to remove any amino-protecting groups and add alkyl,
alkenyl, or other desired substituents to the amino
group at the 4-position. Of course, modifications to
this synthetic route may be desirable.
Preferably the 1-nitrogen is blocked with a
protecting group Z such as a tert-butoxycarbonyl
group before the carbonylation is initiated. Compounds
that contain reactive 6-substituents should also
contain a relatively labile 1-amino protecting group in
order for the protecting group to be selectively
removed. For example, when 6-alkoxycarbonyl derivatives
are prepared, it may be preferred to use a 1-amino
protective group such as the C13CCHZOCO- moiety instead
of tert-butoxycarbonyl and particularly instead of a
CA 02037100 1999-O1-22
X-8163M -20-
benzoyl group. Depending upon the desired final product,
the 4-amino group can be protected with a readily
removable blocking group such as benzoyl when R1 and/or
RZ is hydrogen. Amino blocking groups including acyl
groups such as formyl, acetyl, trifluoroacetyl and the
like can be introduced at the 4-amino position using
methods disclosed by T. W. Greene in Chapter 7 of
Protective Groups in Organic Synthesis, John Wiley and
Sons, New York, 1981, and by J. W. Barton in Chapter 2
of Protective Groups in Organic Chemistry, J. F. W.
McOmie, ed., Plenum Press, New York, 1973. When R1 or
RZ is alkyl or alkenyl in the desired compound of
Formula I, it is preferred that the 4-amino group be
alkylated before the carbonylation is accomplished.
In another method of preparation the 6-ester,
6-thioester and 6-amide compounds of formula IA can be
prepared from the 6-carboxylic acid derivative. For
example, the 6-carboxylic acid can be reacted with a
reagent RTH (where R is of the desired carbon-containing
substituent and T is oxygen, sulfur or nitrogen) and a
coupling reagent. Any of the coupling reagents
commonly employed in the synthesis of peptides and
esters can be used and the desired ester or amide
isolated. Examples of such coupling reagents include
carbodiimides, such as N,N'-dicyclohexylcarbodiimide,
N,N'-diisopropylcarbodiimide or N,N'-diethylcarbodiimide;
the imidazoles such as carbonyl diimidazole as well as
reagents such as N-ethoxycarbonyl-2-ethoxy-1,2-dihydro-
quinoline.
CA 02037100 1999-O1-22
X-8163M -21-
An alternative method of preparation is
depicted in Scheme I in which R1 and RZ are as defined
above and Z is an appropriate amino-blocking group. The
1-nitrogen of the 6-bromo compound _3 is protected with
an appropriate blocking group which should be relatively
nonreactive to butyllithium. A preferred blocking
group is the benzyl group which can be affixed to the
1-nitrogen by the reaction of compound 3 with benzyl
chloride. The 1-benzyl-6-bromo derivative _4 is con-
tacted with a lithiating reagent such as n-butyllithium
or t-butyllithium. The reagents are combined at a
temperature in the range of from about -100°C to about -20°C,
more preferably from about -60°C,to about -40°C. The 6-
lithium derivative 5 can then be converted to the
1,6-disubstituted-4-aminohexahydrobenz-[c,d]indole _6
upon reaction with an appropriate electrophile such as
QC(=X)Y, wherein X and Q are defined above and Y is a
good leaving group such as cyano. Typically a solution
of the compound 5 at a temperature in the range of from about
-100°C to about -60°C, preferably at about -80°C, is
added to a solution of the electrophile in a mutual
solvent. The desired compound 6 is purified by quench-
ing the reaction mixture with, for example, ice water.
The mixture is washed with a water-immiscible organic
solvent. The organic phase is extracted with acid, and
the aqueous phases are combined, made basic and the
desired compound extracted with a water immiscible
organic solvent. The organic solvent is removed,
typically under vacuum, and the desired compound _6 is
further purified if necessary by standard procedures. A
~~3~~aa
X-8163M -22-
disadvantage of this procedure is that some dehalo-
genation may occur resulting in a hydrogen in the
6-position which can require additional purification
steps to obtain substantially pure compound 6.
Scheme I
B~ NR~R2 Br NR~R2
HN ~ Z-'~
X
Q-C NR~RZ Li NR~R2
2 0 Z-N ~ Z-
The 6-lithium derivative 5 can be used to
prepare the corresponding 6-carboxylic acid derivative
by contacting the 6-lithium derivative with carbon
dioxide. The 6-carboxylic acid can be used as an
CA 02037100 1999-O1-22
X-8163M -23-
intermediate to prepare the 6-amides and 6-esters of the
instant invention by standard amidation and esterifica-
tion methods.
Thiocarboxylic acid esters defined by Formula
IA wherein X is sulfur, form another important group
of compounds that are a further embodiment of this
invention. The thiocarboxylic acid esters of the
invention can be prepared by thiating the corresponding
carboxylic acid ester or thioester. Any of several
thiating agents can be employed in this reaction in-
cluding phosphorus pentasulfide. Another thiating
agent is Lawesson's Reagent, which is 2,4-bis(4-
methoxyphenyl)-1,3-dithia-2,4-diphosphetane-2,4-
disulfide. This thiating agent and its general uses
are described in detail in Tetrahedron Letters, _21,
4061 (1980). The thiation reation is preferably carried
out by combining approximately equimolar quantities of
the carboxylic acid ester and thiating agent in a mutual
organic solvent such as toluene or dioxane. The reaction
is generally complete within about 1 hour to about 10
hours when carried out at a temperature of from about 50°C to
about 150°C. The thiocarboxylic acid esters thus formed
can be isolated and purified by normal methods such as
crystallization and the like.
The compounds of Formula IA where X is sulfur
can also be prepared by reacting the 4-amino-6-lithium-
tetrahydrobenz[c,d)indole 5, prepared as described
above, (or the corresponding Grignard reagent) sequentially
CA 02037100 1999-O1-22
X-8163M -24-
with carbon disulfide and a suitable electrophile or
with thiocarbonyl-1,1'-diimidazole and a suitable
nucleophile.
Compounds of Formula IB in which A is -NRSRs,
ie 6-amino and substituted 6-amino compounds, can be
prepared from the corresponding 6-nitro indoline. The
nitro is reduced to the corresponding 6-amine using
common reducing agents such as aluminum hydride. The
resulting amine can then be alkylated with the desired
groups using methods well known to those skilled in the
arts, such as contacting the amine with an alkyl halide
in the presence of sodium carbonate or contacting the
amine with an alcohol in the presence of a catalytic
amount of aluminum t-butoxide and Raney Nickel.
Compounds of Formula IB in which A is O(C1-C4
alkyl) or S(C1-C4 alkyl) can be prepared from the
corresponding 6-bromoindoline by displacement with the
appropriate alkoxide or thioalkoxide in the presence of
cuprous iodide. For example, the 6-methoxy derivative
can be prepared by contacting the 6-bromo indoline with
sodium methoxide in the presence of CuI.
Compounds of Formula IB in which A is OH can
be prepared by ether cleavage of a corresponding 6-
alkoxyindoline. This ether cleavage can be effected by
using standard reagents such as boron tribromide or
boron trichloride.
The 6-hydroxyindoline can be used as an inter-
mediate in the preparation of the corresponding O-acyl
or O-benzyl compounds of Formula IB. These O-acyl
compounds can be prepared using standard acylation
~~D3 ~ ~.~~
X-8163M -25-
reactions with the 6-hydroxy indoline. For example, the
appropriate acyl chloride or appropriate anhydride can
be contacted with the 6-hydroxyindoline. The O-benzyl
compounds can be prepared by contacting the appropriate
6-hydroxindoline with a benzylhalide.
The compounds of Formula IB in which A is CF3
can be prepared by contacting the corresponding 6-
carboxylic acid with SF4. Alternatively, these
compounds can be prepared by heating a mixture of the
corresponding 6-bromo compounds, CuI, CF3COZNa, and
N-methyl-2-pyrrolidone at 100° to 180°C.
Scheme 2 illustrates the preparation of
intermediates useful in preparing the compounds of the
instant invention. As is readily apparent, when
compounds of structure 7 are available in which A is
the desired 6-substituent, the desired compounds of
Formula 1B can be prepared directly. However, the
preferred route is to use the readily available
starting ketone of formula 7 in which A is hydrogen and
B is a benzoyl group.
Epoxides of formula 8 are known in the art or
can be prepared from compounds known to the art using
common reagents and techniques. For example, Flaugh,
et al., J. Med. Chem. , 31, 1746 (1988); Nichols et al.,
~~~ ~'~ ~
X-8163M -26-
SCHEME 2
A O A NHR~
A O OH
N~ -~ N~ --~r
s/ Z s/ $ e/N
R~
A A A
N
NH2 NHR~
B/N ~ 8/N 11 /N 1Q
B
Orq. Prep. and Proc., Int., 9, 2?? (1977); and Leanna
et al., Tet. Lett., 30, No. 30, 3935 (1989), teach
methods of preparation of various embodiments of com-
pounds of formula 8. Those skilled in the art of
organic chemistry will recognize that there are four
stereoisomers of formula 8:
X-8163M -27-
$a ~ ,
formulae 8a and 8b are herein referred to collectively
as the exo-isomers; similarly, formulae 8c and 8d are
the endo-isomers. Leanna et al., supra, teach the
preparation of epoxides of formula 8 which are sub-
stantially exo or substantially endo, as desired. The
preferred starting material is the compound of formula 8
wherein B is benzoyl and A is hydrogen with the most
preferred starting material being a mixture of the
exo-isomers thereof.
Amino alcohols of formula 9 are formed by
reacting an epoxide of formula 9 with an amine of
formula R~NH2. Such amines are readily available.
Opening of the epoxide ring proceeds substantially
regiospecifically with the amino group at the 5-position
and the hydroxyl group at the 4-position. The reaction
is also stereospecific in the sense that stereoisomers
of formulae 9a-d are predictably formed from, re-
spectively, stereoisomers of formulae 8a-d'
CA 02037100 1999-O1-22
X-8163M -2g-
NHR~
NHR~
,, ~~~OH -
IN ..,,.~H
s 8a
- ~c
A stereoselective synthesis of the amino alcohol
of formula 9, and hence of all the subsequent inter-
mediates and products of Scheme 2, can be effected by
using a substantially pure enantiomer of an amine of
the formula R'NFi2 wherein R' contains at least one
chiral center. The diastereomers of the resulting amino
alcohol can then be separated by a number of means known
in the art, for example by chromatography or crystal-
lization. Suitable solvents for recrystallization
include those such as diethyl ether, _n-butanol, and
mixtures of hexane and ethyl acetate. An alternative
method of achieving a stereospecific synthesis comprises
conversion of all the diastereomers of formula _9 to
corresponding diastereomers of formula _10, followed by
the separation of the diastereomers; that alternative
method is discussed below. If a stereoselective syn-
thesis is not desired, then no separation of the stereo-
isomers of the amino alcohol of formula _8 is required
and the amine R'NH2 need not be optically active. In
this case, R' could be the same as R1 and formula _11
could be used to prepare the desired compound.
X-8163M -29-
A particularly efficient stereoselective pro-
cess for a highly preferred compound of formula 9, 1-
benzoyl-4-hydroxy-5-(1-phenylethyl)amino-1,2,2a,3,4,5-
hexahydrobenz[cd]indole, comprises the reaction of a
mixture of substantially the exo-isomers of the corres-
ponding epoxide of formula 8, or a mixture of sub-
stantially the endo-isomers of the corresponding epoxide
of formula 8, with a substantially pure enantiomer of
1-phenethylamine in a suitable solvent such as n-butanol
and the subsequent selective crystallization of one of
the two isomers of the amino alcohol. The temperature
of the reaction can be from about 50° to about 150°C,
preferably about 80° to about 100°C.
After the reaction is complete, as determined
for example by thin layer chromatography or liquid
chromatography, the desired amino alcohol is crystal-
lized at about -20° to about 40°C with the preferred
temperature being between about 0° and 15°C. This
process has the valuable attribute that the reaction and
the separation of stereoisomers occur efficiently in a
single step. By the proper selection of the epoxide
isomers, exo or endo, and the enantiomer of 1-phenyl-
ethylamine, R or S, one can determine which of the
stereoisomers of the compound of formula 9 precipitates
from the reaction mixture. For example, a preferred
stereoisomer of 1-benzoyl-4-hydroxy-5-(1-phenylethyl)-
amino-1,2,2a,3,4,5-hexahydrobenz[cd]indole, the (2a-
S,4-S,5-S)-isomer (structure 9b), can be selectively
prepared by reacting the exo-epoxides with S-1-phenyl-
ethylamine.
.r~
X-8163M -30-
A number of methods of forming aziridines such
as those of formula 10 from amino alcohols such as those
of fornnula 9 are known to the art. Two examples are
the use of diethyl azodicarboxylate and triphenylphosphine
(O. Mitsunobu, Synthesis, January, 1981, page 1), and
the use of bromine and triphenylphosphine (J. P. Freemer
and P. J. Mondron, Synthesis, December, 1974, page 894).
A particularly efficient alternative to the
above methods involving treating a compound of formula
9 with a tertiary amine in an inert solvent followed
by the addition of methanesulfonyl chloride. The
following stereoisomers of the aziridine of formula
10, l0a-d arise respectively from the stereoisomers of
formula 9a-d with retention of configuration at any
chiral center in the substituents A, B or R' as well
as at position 2a:
A N/R~ A N/R~
N '.,y~H N H
B
i0a 1Qb 1Qc . 1Qd
Suitable tertiary amines are those of the formula
(R8)3N, where the R8 groups are independently C1-C4
alkyl. Suitable solvents are chlorinated hydrocarbons
such as methylene chloride, chloroform, carbon tetra-
chloride, and dichloroethane; aromatic hydrocarbons
such as benzene, toluene, and the xylenes; and ethers
such as tetrahydrofuran, diethyl ether, and methyl t-
~03'~1~0
X-8163M -31-
butyl ether. The reaction can be conducted at a
temperature from about -35° to about 45°C. In a
preferred embodiment, the amino alcohol is treated with
triethylamine in methylene chloride at about -20° to
about 0°C, then the reaction mixture is warmed to about
15° to about 35°C for the completion of the reaction. If
desired, the product, an aziridine of formula 10, can be
crystallized from an appropriate solvent such as
acetonitrile or isopropanol after an aqueous workup. In
the event that R' contains at least one chiral center in
substantially a single stereoconfiguration and that the
aziridine of formula 10 is prepared as a mixture of
stereoisomers, said stereoisomers can be separated by
methods such as chromatography and crystallization,
thereby providing a stereospecific synthesis of the
aziridine of formula 10 and subsequent products.
The aziridine ring can be opened to form an
intermediate secondary amine of formula 11. A number
of methods of opening aziridines are commonly known.
It is, however, crucial that the method used for opening
the aziridine to form a secondary amine of formula 11 be
substantially regiospecific; the aziridine must be
opened to form substantially the 4-amino compound rather
than the 5-amino compound. One such method is catalytic
hydrogenolysis as taught by Y. Sugi and S. Mitsui,
Bull. Chem. Soc. Jap., 43, pp. 1489-1496 (1970).
Catalysts which are suitable are the usual hydrogenation
and hydrogenolysis catalysts, such as the noble metal
catalysts; the preferred catalyst is palladium. Suit-
able solvents include hydrocarbons such as hexanes
X-8163M -32-
and heptanes; aromatic hydrocarbons such as benzene,
toluene, xylenes, ethylbenzene, and t-butylbenzene;
alcohols such as methanol, ethanol, and isopropanol; and
mixtures of solvents such as acetic acid mixed with said
alcohols. Preferred solvents for preparing the com
pound of formula 11, wherein B is benzoyl, A is hy-
drogen, and R' is 1-phenylethyl, include glacial acetic
acid or a mixture of methanol and phosphoric acid.
The source of hydrogen can be an atmosphere of hydrogen
supplied at a pressure of about 1 atmosphere or higher,
or the source of hydrogen can be compounds which are
suitable to serve as hydrogen donors in a catalytic
transfer hydrogenolysis reaction, such as formic acid,
cyclohexene, or hydrazine. The preferred hydrogen
source is an atmosphere of hydrogen gas supplied at
about 1 to about 10 atmospheres pressure. The tempera-
ture of the reaction may be from about -20° to about
80°C; the preferred temperature for the hydrogenolysis
of the aziridine wherein B is benzoyl, A is hydrogen,
and R' is 1-phenylethyl is about -20° to about 0°C.
The conversion of compounds of formula 10 to
compounds of formula 11 proceeds without disturbing the
stereochemical configuration of the chiral centers at
the 2a- or 4- positions of the formula 11 or of the
chiral centers that may be present in any of the
substituents.
If desired, the compound of formula 11 can be
isolated by the usual methods such as crystallization.
The secondary amine of formula 11 can be converted to a
primary amine of formula 12 by a number of methods known
2037~Q~
X-8163M -33-
to the art of organic chemistry, or alternatively the
secondary amine itself can be isolated. However, a
preferred method is to convert the secondary amine of
formula 11 to the primary amine of formula 12 without
isolating the secondary amine, but rather by simply
continuing, without interruption, the hydrogenolysis
reaction that produced the compound of formula 11.
Therefore, the preferred solvent and catalyst are the
same as those for the preparation of the secondary amine
of formula 11. It may be desirable to conduct the
hydrogenolysis of the secondary amine of formula 11 at
a different temperature or a different pressure or dif-
ferent temperature and pressure than the hydrogenolysis
of the aziridine of formula 10. For the hydrogenolysis
of the preferred compound of formula 11 wherein B is
benzoyl, A is hydrogen, and R~ is 1-phenylethyl, the
preferred temperature and pressure are about 50° to
about 60°C and about 1 to about 20 atmospheres.
The hydrogenolysis of compounds of formula 11
to compounds of formula 12 proceeds without disturbing
the stereochemical configuration of the chiral centers
at the 2a- or 4- positions.
The isolation of the compound of formula 12
can be accomplished by the usual methods such as crystal-
lization. If desired, the compound of formula 12 can be
further purified, for example by recrystallization.
Of course, as those skilled in the art will re-
cognize, variations of Scheme 2 may be desirable or
necessary for certain embodiments of the invention.
For example, it may be undesirable to subject a compound
~~~~~ ~fl
X-8163M -34-
in which A is halo to the catalytic hydrogenolysis
steps of Scheme 2 because the undesired displacement of
the halogen may compete with the desired hydrogenolysis
of the carbon-nitrogen bonds. Typically it is preferred
to postpone the halogenation until after the hydro-
genolysis. Another alternative strategy is to use
a milder means of reduction that would leave the halogen
in place. A third alternative is to perform the desired
displacement of halogen before the hydrogenolysis step
although care must be exercised if the new group at the
6-position is sensitive to hydrogenation.
Compounds of Formula I can be prepared from
the compounds of formula 12, whether they exist as a
mixture of stereoisomers or as substantially pure
enantiomers, using common reagents and methods well
known in the art. The 6-bromo indoline is a preferred
intermediate in the preparation of many of the
compounds of Formulae IA and IB. The 6-bromo derivative
can be prepared by standard phenyl bromination
reactions such as with bromine in acetic acid or with N-
bromosuccinimide.
In addition to the 6-bromo derivative, another
preferred intermediate to the compounds of the instant
invention is the 6-iodo derivative 2 as discussed
hereinabove. Preferably B is an amino-blocking group
such as benzoyl or p-nitrophenylethyl. A preferred
method of introducing iodine at the 6-position is by
reaction of the 6-hydro indoline with iodine and
orthoperiodic acid in the presence of an acid such
as trifluoroacetic acid or sulfuric acid, in a solvent
CA 02037100 1999-O1-22
X-8163M -35-
such as acetic acid. Another preferred method of
iodination is by the use of N-iodosuccinimide in the
presence of trifluoroacetic acid. Amino blocking groups
can be added, if desired, to the 4-amino substituent
using such methods as those disclosed by Greene, su ra,
and Barton, su ra. Alkyl groups can be added, if
desired, to the 4-amino substituent using such common
methods as reaction with the appropriate halide as
discussed on pages 734 and 735 of Morrison and Boyd,
Chapter 22, Organic Chemistry, Third Edition,
Allyn and Bacon, Boston, 1973.
A particularly preferred intermediate is
(2a-S,4-R)-1-benzoyl-4-(di-_n-propyl)amino-6-iodo-1,2,-
2a,3,4,5-hexahydrobenz[cd]indole, formula _13.
(C3H7)2
25 This can be prepared from the compound of formula 12
where B is benzoyl and A is hydrogen by iodination as
described above followed by alkylation of the 4-amino
group with n-propyl iodide in the presence of a base
such as potassium carbonate in a solvent such as aceto-
nitrile.
CA 02037100 1999-O1-22
X-8163M -36-
The 6-nitrile indoline can be prepared from
the corresponding 6-bromo derivative by contacting the
bromo compound with cuprous cyanide at an elevated
temperature such as 200°C. Other known methods can be
used such as contacting the 6-bromo indoline with
sodium cyanide in the presence of alumina.
The 6-nitrile indoline compounds can be
hydrolyzed by known methods such as aqueous acid or
base at elevated temperatures to provide the 6-
carboxylic acid derivative. Hydrolysis of the 6-
nitrile with polyphosphoric acid can conveniently
provide the 6-carboxamide derivative.
The 6-vitro derivatives can be prepared from
compounds of formula 12 by nitration using standard
methods such as with a mixture of sulfuric acid and nitric acid.
The vitro group can be reduced, for example by catalytic
hydrogenation, to provide the 6-amino derivative. The
6-amino indoline can be alkylated to provide 6-
substituted-amino indolines.
The following examples further illustrate the
preparation of compounds of this invention. The examples
are provided for purposes of illustration only and are
not to be construed as limiting the scope of the instant
invention in any way.
The terms and abbreviations used in the
instant examples have their normal meaning unless
otherwise designated, for example, "°C" refers to degrees
Celsius; "N" refers to normal or normality; "mmole"
CA 02037100 1999-O1-22
X-8163M -37-
refers to millimole; "g" refers to gram; "ml" means
milliliter; "M" refers to molar; "NMR" refers to
nuclear magnetic resonance; "IR" refers to infrared
spectroscopy; "U. V." refers to ultraviolet
spectroseopy; and "m. s." refers to mass spectrometry.
Example 1
Preparation of (2aR,4S)-1-benzoyl-4-amino-6-bromo-
1,2,2a,3,4,5-hexahydrobenz[cd]indole
(2aR,4S)-1-Benzoyl-4-amino-1,2,2a,3,4,5-
hexahydrobenz[cd]indole (29.4 g, 0.106 mole) was placed
into a 500 ml three neck round bottom flask equipped
with a mechanical stirrer, a nitrogen inlet and a
constant addition funnel. The substrate was dissolved
in glacial acetic acid (250 ml), and sodium acetate
(34.7 g, 0.423 mole, 4 mol equiv) was then added. A
solution of bromine (21.8 ml, 0.424 mole) in acetic acid
was then added dropwise over a period of one hour with
vigorous stirring and the reaction mixture was then stirred at room
temperature overnight. The resulting thick slurry was diluted with
ethyl ether, filtered and washed with ethyl ether. The
material thus obtained was slurried in H20 and the pH
adjusted to 11-12 with 5N NaOH. The solid was filtered,
washed well with H20 and dried in vacuo to provide 33.6
g (88.8%) of the title compound. An analytical sample
was prepared by recrystallization from isopropyl alcohol.
m.p.:169-173°C
2~~'~~QO
X-8163M -38-
IR: 3010, 2934, 1640, 1580, 1468, 1454, 1384 cm 1.
NMR: (1H, ppm, CDC13): 7.42-7.58 (m, 7H), 4.27 (br s,
1H), 3.68 (t, 1H, j = 11.1 Hz), 3.33 (m, 2H), 3.16
(dd, 1H, J = 6.3, 17.3 Hz), 2.28 (dd, 1H, J = 9.6,
17.3 Hz), 2.17 (m, 1H), 1.44 (br s, 2H), 1.32 (q,
1H, J = 11.6 Hz).
(13C~ ppm, CD30D): 170.6, 141.9, 137.3, 136.4,
134.1, 132.1, 132.0, 129.8, 128.1, 118.8, 116.2,
59.5, 49.3, 37.8, 37.1, 25.4.
M.S.:m/e = 356, 358, 339, 341, 105, 77.
U.V.:~max = 272 (E=14400) in ethanol.
Analysis:
Theory: C, 60.52; H, 4.80; N, 7.82
Found . C, 60.33; H, 4.89; N, 7.72
[a]D = +20.73 (589 nm).
Example 2
Preparation of (2aS, 4R)-1-benzoyl-4-amino-6-bromo-
1,2,2a,3,4,5-hexahydrobenz[c,d]indole.
The procedure of Example 1 was followed using
(2aS, 4R)-1-benzoyl-4-amino-1,2,2a,3,4,5-hexahydrobenz-
[c,d]indole to provide the named compound.
CA 02037100 1999-O1-22
X-8163M _3g-
Example 3
Preparation of (2aR, 4S)-1-Benzoyl-4-(di-n-propyl)-
amino-6-bromo-1,2,2a,3,4,5-hexahydrobenz[cd]indole
(2aR, 4S)-1-Benzoyl-4-amino-6-bromo-1,2,2a,3,-
4,5-hexahydrobenz[cd]indole (9.82 g, .0275 mol) was
placed in a 500 ml round bottom flask equipped with a
mechanical stirrer, condenser topped with a nitrogen
inlet, and a thermocouple. Acetonitrile (175 ml) and
KZC03 (0.275 mol) were added, followed by the addition
of propyl iodide (13.2 ml, 0.137 mol) with vigorous
stirring. The reaction mixture was stirred at 75°t5°C
under nitrogen overnight. After cooling to room
temperature, the reaction mixture was diluted with
CHZC12 (200 ml) and washed successively with H20,
NaHC03 solution, H20, brine and dried over Na2S04.
After filtration, the volatiles were removed in vacuo
to provide 11.5 g (94%) crude product. This material
was then recrystallized from 95% ethanol to provide the
desired product as colorless needles 9.7 g (80.0%).
m.p.:93-93°C.
IR: 2958, 1655, 1464, 1453, 1381 cm-1.
NMR: (1H, ppm, CDC13): 7.41-7.58 (m, 7H), 4.27 (m,
1I3), 3.34 (m, 1H), 3.19 (m, 1H), 2.92 (dd, 1H,
J = 5.6, 18.1 Hz), 2.48 (m, 5H), 2.16 (m, 1H),
1.47 (m, 4H), 1.40 (m, 1H), 0.90 (t, 6H, J = 7.3
Hz).
~3~a ~0~
X-8163M -40-
(13C, ppm, CDC13): 168.9, 140.9, 134.7, 131.3,
130.0, 128.9, 127.7, 118.6, 57.8, 53.1, 30.6,
29.2, 22.9, 12.1.
M.S:m/e = 440/442.
U.V.:~m~ = 272 (E=15600) in ethanol
Analysis:
Theory: C, 65.31; H, 6.62; N, 6.35; Br, 18.10
Found : C, 65.15; H, 6.70; N, 6.36; Br, 18.31
[a]588 = 11.6° (ethanol)
Example 4
Preparation of (2aS, 4R)-1-benzoyl-4-(di-n-propyl)-
amino-6-bromo-1,2,2a,3,4,5-hexahydrobenz[c,d]indole.
The same procedure as in Example 3 was
followed using (2a5, 4R)-1-benzoyl-amino-6-bromo-
1,2,2a,3,4,5-hexahydrobenz[c,d]indole to provide the
novel compound.
Example 5
Preparation of (2aR,4S)-1-benzoyl-4-(di-n-propyl)amino-
6-cyano-1,2,2a,3,4,5-hexahydrobenz[cd]indole
A. (2aR,4S)-1-Benzoyl-4-(di-n-propyl)amino-
6-bromo-1,2,2a,3,4,5-hexahydrobenz[cd]indole (I54.48 g,
0.35 mol) was dissolved in N-methylpyrrolidinone (NMP,
850 ml) to which CuCN (37.6 g, 0.42 mol, 1.2 mole equiv)
CA 02037100 1999-O1-22
X-8163M -41-
was added. The flask was equipped with a condenser
topped with a Firestone valve, a thermocouple, and a
mechanical stirrer. The mixture was degassed five
times (vacuum/NZ purge via Firestone valve) and slowly
brought to 200°C t 5°C (internal temp). After 1 hour,
TLC indicated that the reaction was nearly complete.
After a total of 2.5 hours, TLC showed no starting
material present. The resulting dark reaction mixture
had precipitated Cu on the flask walls, and was then
cooled to room temperature. The mixture was diluted
with CHZC12 (1 1) and washed with 15% NH40H (=500 ml
water + 500 ml concentrated reagent). The layers were
separated and the aqueous phase was extracted with
CHZC12 (500 ml). The combined organic layers were
washed with H20 (4 x 1 L), brine (1 L) and dried over
Na2S04. The desiccant was removed by filtration, and
the filtrate concentrated to dryness. The crude residue
was chromatographed in several small portions over
silica gel with a hexane/ethyl acetate gradient to
provide 102.7 g of the nitrile (75.7%). This material
was used in the subsequent deprotection step without
crystallization. A portion of this material was
recrystallized from 50% aqueous ethanol for analysis.
m.p.:109-111°C.
IR: 2959, 2213, 1661, 1616, 1470, 1453, 1368, 1355
Cm 1.
~o~~~aa
X-8163M -42-
NMR: (1H. PPm. CDC13): 7.34-7.58 (m, 7H), 4.35 (m,
1H), 3.72 (t, 1H, J - 11.2 Hz), 3.30 (m, 2H), 3.13
(m, 1H), 2.72 (m, 1H), 2.45 (m, 4H), 2.27 (m, 1H),
1.46 (m, 5H), 0.90 (t, 6H, J = 7.3 Hz).
(13C, ppm, CDC13: 169.0, 145.0, 138.2, 135.8,
134.1, 133.2, 131.0, 128.6, 127.3, 117.5, 113.9,
106.3, 58.4, 56.9, 52.7, 37.7, 29.3, 27.9, 22.5,
11.7.
M.S.:m/e = 387.
U.V.:~lmaX = 304 (~=19600), 287 (~=19800), 225 (E=23000)
in EtOH.
Analysis:
Theory: C, 77.47; H, 7.55; N, 10.85
Found : C, 77.09; H, 7.65; N, 10.74
[a]D - +1.59 (589 nm).
B. Alternative Procedure:
The bromide starting material (441 mg,
1 mmole), KCN (100 mg, 1.5 mmole), triphenylphosphine
(52 mg, 0.2 mmole), Zn dust (20 mg, 0.3 mmole) and
NiBr2[P(CsHS)z]z (74 mg, 0.1 mmole) was combined in a
dry three neck 25 ml round bottom flask equipped with a
condenser topped with a nitrogen inlet, and rubber
septa on the other necks. The reaction vessel was then
degassed several times by repeated vacuum/nitrogen
purge cycles. Freshly distilled THF (5 ml) was then
added via syringe and the flask was stirred at 60°C
CA 02037100 1999-O1-22
X-8163M -43-
(oil bath temp). The initially green solution became
orange/brown over a 30 minute period. The reaction
progress was monitored by HPLC and TLC. After 7 hours,
HPLC incated only 2% starting material remained. After
a total of 9 hours, the reaction mixture was allowed to
cool to room temperature overnight. The reaction mixture
became nearly colorless. The insoluble material was
removed by filtration through diatomaceous earth
("Hy-flo*~~)(1 g) and washed thoroughly with tetra-
hydrofuran (THF) (4 x 5 ml). The THF solution was
transferred to a three-necked flask and treated dropwise
with n-butyllithium as described in Example 7.
Example 6
Preparation of (2aS,4R)-1-benzoyl-4-(di-_n-propyl)amino-
6-cyano-1,2,2a,3,4,5-hexahydrobenz[c,d]indole.
The procedure of Example 5A was followed
using (2aS,4R)-1-benzoyl-4-(di-n-propyl)amino-6-bromo-
1,2,2a,3,4,5-hexahydrobenz[c,d]indole to provide the
named compound.
* Trade-mark
X-8163M -44-
Example 7
Preparation of (2aR,4S)-4-(di-n-propyl)amino-6-cyano-
1,2,2a,3,4,5-hexahydrobenz[cd]indole
(2aR,4S)-1-Benzoyl-4-(di-n-propyl)amino-6-
cyano-1,2,2a,3,4,5-hexahydrobenz[cd]indole (41.02 g,
0.106 mol) was dissolved in freshly distilled THF (375
ml) and cooled to -78°C with dry ice-acetone under
nitrogen. n-Butyllithium (59.3 ml, 0.148 mole, 1.6 mole
equivalent, 2.5 M) was then added dropwise at a rate to
maintain the temperature below -65°C. When thin layer
chromatography analysis indicated complete reaction,
glacial acetic acid (10 ml) was added carefully and the
reaction mixture was warmed to room temperature. Ethyl
ether (250 ml) and 1N HC1 (250 ml) were added and the
layers separated. The organic phase was extracted with
additional 1N HC1 (2 x 100 ml), and the combined aqueous
phase washed with ethyl ether (2 x 250 ml). 5N NaOH
(90-100 ml) was added dropwise with stirring followed by
extraction with CHZC12 (250 + 2 x 150 ml). The combined
organic phase was washed with brine, dried over Na2S04
and concentrated to dryness. The resulting light tan,
highly crystalline material was dried in vacuo to a
constant weight (28.4 g, 94.5%). This material was
recrystallized from hot aqueous ethanol (ethano1:H20 =
75:25), cooled, filtered and washed with ice cold
solvent.
X-8163M -45-
m.p.:113-114°C
IR: 3336, 2934, 2210, 1625, 1586, 805 cm 1.
NMR: (1H, PPm. CDC13): 7.27 (1H, d, J - 9.0 Hz), 6.39
(1H, d, J = 9.0 Hz), 4.12 (1H, br s), 3.75 (1H, m)
3.20 (1H, m), 3.03 (1H, dd, J - 18, 6.0 Hz), 2.63
(1H, ddd, J = 18, 12, 2.0 Hz), 2.45 (4H, t,
J = 9.0 Hz), 2.19 (1H, dt, J = 6.0, 3.0 Hz), 1.45
(5H, m), 0.89 (6H, t, J = 9.0 Hz).
(13C, ppm, CDC13): 154.0, 137.4, 134.0, 130.7,
119.2, 105.7, 99.6, 57.4, 55.7, 52.8, 38.9, 29.7,
27.6, 22.6, 11.8.
M.S.:m/e = 283, 254, 240, 183, 156, 128, 98, 72.
U.V.:~.m~ = 296 (E=16500), 231 (s=14100, 205 (s16300)
in EtOH.
Analysis:
Theory: C, 76.28; H, 8.89; N, 14.83
Found : C, 76.56; H, 8.85; N, 14.71
[a]D - -34.0 (589 nm), THF, c = 0.01.
[a]D - -217.7 (365 nm).
Example 8
Preparation of (2aS,4R)-4-(di-n-propyl)amino-6-cyano-
1,2,2a,3,4,5-hexahydrobenz[c,d]indole.
The procedure of Example 7 was followed using
(2aS,4R)-1-benzoyl-4-(di-n-propyl)amino-6-cyano-1,2,2a,3-
4,5-hexahydrobenz[c,d]indole to provide the title
compound.
CA 02037100 1999-O1-22
X-8163M -46-
Example 9
Preparation of (2aR,4S)-4-(di-n-propylamino)-6-amino-
carbonyl-1,2,2a,3,4,5-hexahydrobenz[c,d]indole.
Polyphosphoric acid (PPA, 300 ml) was placed
into a 500 ml three neck flask equipped with a mechanical
stirrer, a stopper and a condenser topped with a nitrogen
inlet. The reaction vessel was degassed by vacuum/purge
cycles (5x). The flask was then heated to 85-90°C
(internal temp) and (2aR,4S)-4-(di-n-propyl)amino-6-
cyano-1,2,2a,3,4,5-hexahydrobenz[cd]indole (22.65 g,
. 0.080 mole) was added portionwise. The reaction mixture
became homogeneous as the hydrolysis occurred. After
all of the nitrile had been added, the mixture was
stirred at this temperature for an additional 2.0 hours
to ensure complete hydrolysis. The reaction mixture was
then carefully poured onto crushed ice and stirred
vigorously. After the ice had melted, the pH was
adjusted with 5N NaOH to 11-12 and the mixture was extracted
with several portions of CHzCl2. The organic phase was
dried over sodium sulfate, filtered and concentrated to
afford 23.53 g of the amide as a foam.
m.p. - 161-164 °C
IR: (KBr): 3392 (br), 3180 (br), 2957 (m), 2934 (m),
2870 (w), 2810 (w), 1654 (s), 1584 (s), 1457 (s),
1380 (s), 1350 (s) cm 1.
CA 02037100 1999-O1-22
X-8163M -47-
NMR: (1H, ppm, CDCI3): 7.30 (d, 1H), 6.40 (d, 1H), 5.7
(brs, 2H), 3.9 (m, 1H), 3.70 (m, 1H), 3.05-3.30
(m, 4H), 2.85 (dd, 1H), 2.45 (m, 4H), 2.15 (m,
1H), 1.45 (m, 4H), 0.90 (t, 6H).
IR: 3381 (s), 3377 (s), 2956 (m), 2932 (m), 1645 (s),
1616 (s), 1585 (m), 1379 (s) cm-1.
M.S.:m/e = 301 (fd).
U.V.: Amax=273 (E=15400), 214 (E=22300) in ethanol.
Analysis:
Theory: C, 71.72; H, 9.02; N, 13.94
Found . C, 68.40; H, 8.78; N, 13.73
[a]D = -70.46 (589 nm) (CH30H, C=1.02)..
Example 10
Preparation of (2aS, 4R)-4-(di-n-propyl)-6-aminocaronyl-
1,2,2a,3,4,5-hexahydrobenz[c,d]indole.
The procedure of Example 1 was followed using
(2aS, 4R)-4-(di-n-propyl) amino-6-cyano-1,2,2a,3,4,5-
hexahydrobenz[c,d]indole to provide the above-titled
product.
CA 02037100 1999-O1-22
x-81s3M _4$_
Example 11
Preparation of Methyl (2aS,4R)-4-(Di-_n-propylamino)-
1,2,2a,3,4,5-hexahydrobenz[c,d]indole-6-carboxylate
A. (2aS,4R)-1-(t-Butyloxycarbonyl)-s-iodo-4-
(di-n-propylamino)-1,2,2a,3,4,5-hexahydrobenz[c,d]indole
A mixture of 10.0 g (20 mmol) of (2aS,4R)-1-
benzoyl-s-iodo-4-(di-n-propylamino)-1,2,2a,3,4,5-hexa-
hydrobenz[c,d]indole and 100 ml of 3 M HZS04 was
refluxed under nitrogen for 2.5 hours. After cooling
the mixture was filtered and the solid was washed with
1M H2S04. The filtrate and washings were combined,
washed with CHZC12, and basified with 10 N NaOH. The
oil that separated was extracted into CH2C12. After
drying over Na2S04, the solvent was evaporated leaving 6
g of brown oil. Chromatography over 100 g of "Florisil*"
using ethyl acetate afforded 4.82 g of an oil which by
NMR was a 1:2 mixture of (2aS,4R)-s-iodo-4-(di-_n-propyl-
amino)-1,2,2a,3,4,5-hexahydrobenz[c,d]indole and the
corresponding des-iodo compound. This mixture was
dissolved in 25 ml of CHZC12 and treated with 4.0 ml of
di-t-butyl dicarbonate. After stirring overnight, the
volatile materials were removed under vacuum. The
residual oil was dissolved in a small amount of CHZC12
and warmed briefly in the presence of a few ml of
Na2C03 solution. The CH2C12 solution was separated and
dried over Na2S04. The solvent was then evaporated, and
the product mixture was chromatographed over silica gel
~ Trade-mark
X-8163M -49-
using ethyl acetate/toluene (1:9). The crystalline
(2aS,4R)-1-(t-butyloxycarbonyl)-6-iodo-4-(di-n-propyl-
amino)-1,2,2a,3,4,5-hexahydrobenz[c,d]indole was re-
crystallized from isooctane to provide 1.89 g of product,
mp 124-128°C.
B. Methyl (2aS,4R)-1-(t-butyloxycarbonyl)-4-(di-n-
propylamino)-1,2,2a,3,4,5-hexahydrobenz[c,d]indole-6-
carboxylate
A solution of 0.50 g (1.03 mmol) of (2aS,4R)-
1-(t-butyloxycarbonyl)-6-iodo-4-(di-n-propylamino)-1,2,-
2a,3,4,5-hexahydrobenz[c,d]indole, 0.5 ml of triethyl-
amine, and SO mg of (Ph3P)4Pd in 100 ml of methanol was
heated at 55-60°C. under an atmosphere of CO for 20
hours. After allowing to cool, the solvent was evapo-
rated under reduced pressure. The residual oil was
dissolved in CHZC12 containing 5% methanol. This solu-
tion was then washed with NaCl solution and the CHZC12
evaporated. A solution of the residue in 25 ml of
methanol was treated with 3% H202 solution. After 30
minutes, a fine black precipitate was filtered off. The
filtrate was diluted with water and extracted with
CHZCI2. The extract was dried over Na2S04, then evapo-
rated. The residual oil was chromatographed over 15 g
of silica gel using first 1:9 ethyl acetate/toluene
(1:9) mixture than a (1:4) mixture. 1:4 EtOAc/toluene.
A few of the product-containing fractions from the
column were contaminated with Ph3P. These fractions were
further purified by partitioning between dilute tartaric
X-8163M -50-
acid and CHZC12, basifying the aqueous layer with 1 N
NaOH, and extracting with CHZC12. The total yield of
methyl (2aS,4R)-1-(t-butyloxycarbonyl)-4-(di-n-propyl-
amino)-1,2,2a,3,4,5-hexahydrobenz[c,d]indole-6-
carboxylate, a viscous oil, was 0.415 g (97%).
A solution of 0.284 g (0.68 mmol) of methyl
(2aS,4R)-1-(t-butyloxycarbonyl)-4-(di-n-propylamino)-
1,2,2a,3,4,5-hexahydrobenz[c,d]indole-6-carboxylate in 3
ml of trifluoroacetic acid was allowed to stand for 1
hour. The excess acid was evaporated under vacuum. The
residual oil was dissolved in CHZC12. After washing
this solution with 1 N NaOH, the product was extracted
in dilute tartaric acid (3 portions). This aqueous
solution was basified with 1 N NaOH, and the product
was extracted into CH2C12. Evaporation of the NaZS04
dried extract gave 0.214 g (95% yield) of methyl
(2aS,4R)-4-(di-n-propylamino)-1,2,2a,3,4,5-hexahydro-
benz[c,d]indole-6-carboxylate as a viscous oil.
Analysis (ClsHzsNzOz)
Theory: C, 72.12; H, 8.92; N, 8.85
Found . C, 72.30; H, 9.09; N, 8.94
NMR: (300 MHz, CDC13) 8 0.89 (t, 6H, CCH3), 1.41 (dd, 1H,
3a-H), 1.48 (sextet, 4H, CH2Me), 2.17 (br d, 1H,
3~-H), 2.49 (mult, 4H, CHZEt), 2.85 (dd, 1H, Sa-H),
3.14 (mult, 1H, 2aH), 3.19 (mult, 2H, 2a-H & 2~-H),
3.41 (dd, 1H, 5~-H), 3.72 (mult, 1H, 4-H), 3.82 (s,
3H, OCH3), 3.98 (br s, 1H, 1-H), 6.43 (d, 1H, 8-H),
7.80 (d, 1H, 7-H).
X-8163M -51-
Example 12
Preparation of (2aS,4R)-N,N-Dimethyl-4-(di-n-propyl-
amino)-1,2,2a,3,4,5-hexahydrobenz[cd]indole-6-
carboxamide
A. (2aS,4R)-N,N-Dimethyl-1-(t-butyloxycarbonyl)-
4-(di-n-propylamino)-1,2,2a,3,4,5-hexahydrobenz[cd]-
indole-6-carboxamide
A solution of 0.50 g (1.03 mmol) of (2aS,4R)-1-
(t-butyloxycarbonyl)-6-iodo-4-(di-n-propylamino)-1,2,-
2a,3,4,5-hexahydrobenz[c,d]indole (see previous example),
5 g of dimethylamine, and 50 mg of (Ph3P)4Pd in 100
ml of toluene was heated in an autoclave under CO at
100 psi for 8 hours at 100°C. When the resulting clear,
yellow solution was washed with NaCl solution a color-
less precipitate separated. This precipitate was
collected on a filter and thoroughly washed with ethyl-
acetate containing 5% methanol. These washings were
combined with the original toluene solution. The
solvents were evaporated under reduced pressure. The
residual oil was dissolved in 25 ml of methanol and
treated with a few ml of 3% HZO2. After 30 minutes the
solution was filtered, diluted with water, and extracted
with CHZC12. The extract was dried over Na2S04 then
evaporated under reduced pressure. The residue was
chromatographed over 15 g of silica gel using succes-
sively 1:9 ethyl acetate/toluene, 1:4 ethyl acetate/-
2a3,7~~~
X-8163M -52-
toluene, 2:3 ethyl acetate/toluene, and 100% ethyl
acetate. As in the previous example a few of the
product-containing fractions required further purifica-
tion by partitioning between CHQC12 and aqueous tartaric
acid. The total yield of (2aS,4R)-N,N-dimethyl-1-(t-
butyloxycarbonyl)-4-(di-n-propylamino)-1,2,2a,3,4,5-
hexahydrobenz[cd]indole-6-carboxamide was 0.184 g (42%).
B. A solution of 0.162 g (0.38 mmol) of (2aS,4R)-
N,N-dimethyl-1-(t-butyloxycarbonyl)-4-(di-n-propyl-
amino)-1,2,2a,3,4,5-hexahydrobenz[c,d]indole-6-carbox-
amide in 3 ml of trifluoroacetic acid was allowed to
stand for 1 hour. The excess acid was evaporated under
vacuum. The residual oil was dissolved in CFi2C12.
After washing this solution with 1 N NaOH, the product
was extracted into dilute tartaric acid (3 portions).
This aqueous solution was basified with 1 N NaOH, and
the product was extracted into CHZC12. Evaporation of
the Na2S04 dried extract gave 0.110 g (89% yield) of
(2aS,4R)-N,N-dimethyl-4-(di-n-propylamino)-1,2,2a,3,4,5-
hexahydrobenz[c,d]indole-6-carboxamide as a viscous oil.
Analysis (CZpHgiNgO) for:
Theory: C, 72.91; H, 9.48; N, 12.75
Found . C, 73.02; H, 9.47; N, 12.88
NMR: (300 MHz, CDC13) 8 0.88 (t, 6H, GCH3), 1.40
(dd, 1H, 3a-H), 1.46 (sextet, 4H, CH2Me), 2.18 (br d,
1H, 3S-H), 2.45 (octet, 4H, CHZEt), 2.63 (dd, 1H, 5a-H),
~~3'~1~~
X-8163M -53-
2.?7 (dd, 1H, 5~-H), 2.94 (br s, 3H, NCH3), 3.07 (br s,
3H, NCH3), 3.15 (mult, 3H, 2a-H & 2ø-H & 2a-H), 3.68
(mult, 1H, 4-H), 6.43 (d, 1H, 8-H), 6.86 (d, 1H, 7-H).
The compounds have been found to have selective
affinity for 5-HT1A receptors with much less affinity for
other receptors. Because of their ability to selectively
interact with 5-HT1A receptors, the compounds of Formula
(I) are useful in treating disease states which require
alteration of 5-AT1A function but without the side
effects which may be associated with less selective
compounds. This alteration may involve reproducing (an
agonist) or inhibiting (an antagonist) the function of
serotonin. These disease states include anxiety, depres-
sion, hypertension, acid secretion, sexual dysfunction,
motion sickness, nausea, senile dementia (cognition),
and consumptive disorders such as obesity, alcoholism,
drug abuse and smoking. A pharmaceutically effective
amount of a compound of Formula (I) is required to treat
the foregoing conditions.
The term "pharmaceutically effective amount",
as used herein, represents an amount of a compound of
the invention which is capable of diminishing the
adverse symptoms of the particular disease. The particular
dose of compound administered according to this invention
will of course be determined by the particular circumstances
surrounding the case, including the compound adminis-
CA 02037100 1999-O1-22
X-8163M -54-
tered, the route of administration, the particular
condition being treated, and similar considerations.
The compounds can be administered by a variety of routes
including the oral, rectal, transdermal, subcutaneous,
intravenous, intramuscular or intranasai routes. A
typical single dose for prophylactic treatment, however,
will contain from about 0.01 mg/kg to about 20 mg/kg of
the active compound of this invention when administered
orally. Preferred oral doses will be about 0.5 to about
10 mg/kg, ideally about 1.0 to about 5 mg/kg. When a
present compound is given orally it may be necessary to
administer the compound more than once each day, for
example about every eight hours. For IV administration
' by bolus, the dose will be from about 1.0 Ng/kg to about
3000 ~g/kg, preferably about 50 Ng/kg to about 500
N g/kg -
The following experiment was conducted to
demonstrate the ability of the compounds of the present
invention to interact with the serotonin la receptors.
This general procedure is set forth in Wong et al., _J.
Neural Transm. 71:207-218 (1988).
Male Sprague-Dawley rats (110-150 g) from
Harlan Industries (Cumberland, IN) were fed a~~Purina
Chow*~~ adlibitum for at least 3 days before being used
in the studies. Rats were killed by decapitation. The
brains were rapidly removed, and the cerebral cortices
were dissected out at 4°C.
Brain tissues were homogenized in 0.32 M
sucrose. After centrifugation at 1000 x g for 10 min
* Trade-mark
'~=~ .'
X-8163M -55-
and then at 17000 x g for 20 min, a crude synaptosomal
fraction was sedimented. The pellet was suspended in
100 vol of 50 mM Tris-HC1, pH 7.4, incubated at 37°C
for 10 min, and centrifuged at 50000 x g for 10 min.
The process was repeated and the final pellet was
suspended in ice-chilled 50 mM Tris-HC1, pH 7.4. By
the radioligand binding method, sites specifically
labeled by tritiated 8-hydroxy-2-dipropylamino-
1,2,3,4-tetrahydronaphthalene (3H-8-OH-DPAT) have been
identified as 5-HTlA receptors.
Binding of (3H-8-OH-DPAT) was performed
according to the previously described method [wong
et al., J. Neural Transm. 64:251-269 (1985)]. Briefly,
synaptosomal membranes isolated from cerebral cortex
were incubated at 37°C for 10 min. in 2 ml of 50 mM
Tris-HC1, pH 7.4; 10 NM pargyline; 0.6 mM ascorbic
acid; 0.4 nM 3H-8-OH-DPAT; and from 1 to 1000 mM of
test compound. Binding was terminated by filtering
samples under reduced pressure through glass fiber
(GFB) filters. The filters were washed twice with 5 ml
of ice cold buffer and placed in scintillation vials
with 10 ml of PCS (Amersham/Searle) scintillation
fluid. Radioactivity was measured with a liquid
scintillation spectrometer. Unlabeled 8-OH-DPAT at
10 NM was also included in separate samples to
establish non-specific binding. Specific binding of 3H-
8-OH-DPAT is defined as the difference of radioactivity
bound in the absence and in the presence of 10 NM
unlabeled 8-OH-DPAT.
X-8163M -56-
The results of the evaluation of various
compounds of the present invention are set forth below
in Table I. In Table I, the first column provides the
Example Number of the compound evaluated; and the
second column provides the amount of the test compound
expressed in nanomolar concentration required to
inhibit the binding of 3H-8-OH-DPAT) by 50%, and is
indicated in Table I as IC5o. For these compounds
which inhibited the binding of sH-8-OH-DPAT by less
than 50%, the percent of inhibition is given in
parenthesis.
Table I
Example ICso(a)
1 (6%)(b)
3 (5%)(b)
5 (11%)(b)
7 (21%)(b)
9 11 nM
10 2.1 nM
11 5.7 nM
12 5.1 nM
(a) concentration in nanomoles which inhibited binding
of 8-OH-DPAT by 50~
(b) percent of inhibition of binding of 8-OH-DPAT at
100 nanomoles if less than 50%.
X-8163M -57-
The compounds of the present invention are
preferably formulated prior to administration. There-
fore, yet another embodiment of the present invention is
a pharmaceutical formulation comprising a compound of
the invention and a pharmaceutically acceptable carrier,
diluent or excipient therefor.
The present pharmaceutical formulations are
prepared by known procedures using well known and
readily available ingredients. In making the composi-
tions of the present invention, the active ingredient
will usually be mixed with a carrier, or diluted by a
carrier, or enclosed within a carrier which may be in
the form of a capsule, sachet, paper or other container.
When the carrier serves as a diluent, it may be a solid,
semi-solid or liquid material which acts as a vehicle,
excipient or medium for the active ingredient. Thus,
the compositions can be in the form of tablets, pills,
powders, lozenges, sachets, cachets, elixirs, suspen-
sions, emulsions, solutions, syrups, aerosols (as a
solid or in a liquid medium), ointments containing for
example up to 10°~ by weight of the active compound, soft
and hard gelatin capsules, suppositories, sterile
injectable solutions and sterile packaged powders.
Some examples of suitable carriers, excipi-
ents, and diluents include lactose, dextrose, sucrose,
sorbitol, mannitol, starches, gum acacia, calcium
phosphate, alginates, tragacanth, gelatin, calcium
~~~~~.Q~
X-8163M -58-
silicate, microcrystalline cellulose, polyvinylpyrroli-
done, cellulose, water, syrup, methyl cellulose, methyl-
and propylhydroxybenzoates, talc, magnesium stearate and
mineral oil. The formulations can additionally include
lubricating agents, wetting agents, emulsifying and
suspending agents, preserving agents, sweetening agents
or flavoring agents. The compositions of the invention
may be formulated so as to provide quick, sustained or
delayed release of the active ingredient after adminis-
tration to the patient by employing procedures well
known in the art.
The compositions are preferably formulated in
a unit dosage form, each dosage containing from about 5
to about 500 mg, more usually about 1 to about 10 mg,
of the active ingredient. The term "unit dosage form"
refers to physically discrete units suitable as unitary
dosages for human subjects and other mammals, each unit
containing a predetermined quantity of active material
calculated to produce the desired therapeutic effect, in
association with a suitable pharmaceutical carrier.
The following formulation examples are illus-
trative only and are not intended to limit the scope of
the invention in any way.
X-8163M -59-
Fornuulation 1
Hard gelatin capsules are prepared using the
following ingredients:
Quantity (mg/capsule)
methyl ester 25
Starch, dried 425
Magnesium stearate 10
Total 460 mg
The above ingredients are mixed and filled
into hard gelatin capsules in 460 mg quantities.
Formulation 2
A tablet formula is prepared using the in-
gredients below:
Quantity (mg/tablet)
4-(di-n-propylamino)-6-amino-
carbonyl-1,2,2a,3,4,5-
hexahydrobenz[c,d]indole 25
Cellulose, microcrystalline 625
Colloidal Silicon dioxide 10
Stearic acid 5
The components are blended and compressed to
form tablets each weighing 665 mg.
X-8163M -60-
Formulation 3
A dry powder inhaler formulation is prepared
containing the following components:
Weight
4-(diethylamino)-1,2,2a,3,4,5-
hexahydrobenz[cd]-indole-6-
carboxylic acid, ethyl ester 5
Lactose g5
The active compound is mixed with the lactose
and the mixture added to a dry powder inhaling ap-
plicance.
Formulation 4
Tablets each containing 60 mg of active
ingredient are made up as follows:
(2aS,4R)-N,N,-Dimethyl-4-
(di-n-propylamino)-1,2,2a,3,4,5-
hexahydrobenz[c,d]indole-6-
carboxamide 60 mg
Starch 45 mg
Microcrystalline cellulose 35 mg
Polyvinylpyrrolidone (as 10%
solution in water) 4 mg
Sodium carboxymethyl starch 4.5 mg
Magnesium stearate 0.5 mg
Talc 1 mg
Total 150 mg
The active ingredient, starch and cellulose
are passed through a No. 20 mesh U.S. sieve and mixed
thoroughly. The solution of polyvinylpyrrolidone is
~~~~.a~~
X-8163M -61-
mixed with the resultant powders which are then passed
through a No. 4 mesh U.S. sieve. The granules so
produced are dried at 50-60°C and passed through a No.
16 mesh U.S. sieve. The sodium carboxymethyl starch,
magnesium stearate and talc, previously passed through
a No. 30 mesh U.S. sieve, are then added to the granules
which, after mixing, are compressed on a tablet machine
to yield tablets each weighing 150 mg.
Formulation 5
Capsules each containing 20 mg of medicament
are made as follows:
(2aS,4R)-4-(di-_n-propylamino)-
6-aminocarbonyl-1,2,2a,3,4,5-
hexahydrobenz-[c,d]indole 20 mg
Starch 169 mg
Magnesiwn stearate _ 1 mg
Total ' 190 mg
The active ingredient, cellulose, starch and
magnesium stearate are blended, passed through a No. 20
mesh U.S. sieve, and filled into hard gelatin capsules
in 190 mg quantities.
.,.,...
X-8163M -62-
Formulation 6
Suppositories each containing 225 mg of active
ingredient are made as follows:
(2aS,4R)-(Di-_n-propylamino)-
1,2,2a,3,4,5-hexahydrobenz[c,d]-
indole-6-carboxylic acid, methyl
ester 225 mg
Saturated fatty acid
glycerides to 2,000 mg
The active ingredient is passed through a No.
60 mesh U.S. sieve and suspended in the saturated fatty
acid glycerides previously melted using the minimum
heat necessary. The mixture is then poured into a
suppository mold of nominal 2 g capacity and allowed to
cool.
Formulation 7
Suspensions each containing 50 mg of medicament
per 5 ml dose are made as follows:
4-(di-_n-propylamino)-1,2,2a,3,4,5-
hexahydrobenz[c,d]indole-6-
carbothioic acid, S-methyl ester 50 mg
Xanthan Gum 4 mg
Sodium carboxymethyl cellulose (11~)
Microcrystalline Cellulose (89y) 50 mg
Sucrose 1.75 g
Sodium Benzoate 10 mg
Flavor q.v.
Color q.v.
Purified water to 5 ml
~~f~p~~U~
X-8163M -63-
The medicament, sucrose and xanthan gum are
blended, passed through a No. 10 mesh U.S. sieve, and
then mixed with a previously made solution of the
microcrystalline cellulose and sodium carboxymethyl-
cellulose in water. The sodium benzoate, flavor and
color are diluted with some of the water and added with
stirring. Sufficient water i.s then added to produce
the required volume.
Formulation 8
Capsules each containing 150 mg of medicament
are made as follows:
4-(methylamino)-1,2,2a,3,4,5-
hexahydrobenz[cd]-indole-6-
carboxylic acid, methyl ester 50 mg
Starch 507 mg
Magnesium stearate _ 3 mg
Total 560 mg
The active ingredient, cellulose, starch and
magnesium stearate are blended, passed through a No. 20
mesh U.S. sieve, and filled into hard gelatin capsules
in 560 mg quantities.