Note: Descriptions are shown in the official language in which they were submitted.
~317~;~3
Substituted ethylamines their process of ~reparation their
use as a medicament and their sYnthesis intermediates
The present invention relates to novel substituted
ethylamines, to a process ~or prsparing them, to pharmaceutical
composition containing them and to their used in medecine, as
well as to intermediates useful in their synthesis.
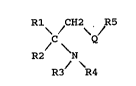
These ethylamines correspond to the general formula
(I) :
Rl / H2 / R5
C Q
R2 N~ (I)
R3 R4
in which :
Q represents an ethylene-1,2-diyl group (-CH=CH-) or5 a cyclopropane-1,2-diyl group ~-C~-CH-),
C~2
Rl is an aromatic heterocyclic ring of 5 to 7 members,
in which the sole hetero-atom is nitrogen, oxygen or sulphur,
or, with the proviso that Q represents a cyclopropane-
1,2-diyl group or with the proviso that R5 represents an
aromatic heterocyclic ring as defined below, Rl is a phenyl
radical which is optionally monosubstituted, disubstituted or
trisubstituted by identical or different radicals which are
lower alkyl or lower alkoxy,
R2 is lower alkyl,
R3 and R4, which are identical or different, are hydrogen
or lower alkyl radiaals without, however, both R3 and R4
representing hydrogen, and
R5 is an aromatic heterocyclic ring of 5 to 7 members,
in which the sole hetero-atom is nitrogen, oxygen or sulphur,
or is a phenyl radical which is optionally
monosubstituted, disubstituted or trisubstituted by identical
or different radicals which are lower alkyl or lower alkoxy.
In the preceding definitions, the qualification "lower"
comprises the radicals having from 1 to 5 carbon atoms in a
linear or branched chain.
It is preferred that :
Rl is an aromatic heterocyclic ring of 5 memb~rs, in
. ', '
,
2 i~l37~3
which the sole hetero-atom is oxygen, as in a Z-furyl or 3-
furyl radical, or is sulphur in a 2-thienyl or 3-thienyl
radical or is nitrogen in a 2-pyridyl, 3-pyridyl or 4-pyridyl
radical,
or is a phenyl radical which can be monosubstituted,
disubstituted or trisubstituted by lower alkoxy radicals such
as methoxy, with the proviso that Q represents a cyclopropane-
1,2-diyl group or with the proYiso that R5 represents an
aromatic heterocyclic ring as de~ined above,
lQ R3 and R4 are hydrogen or lower alkyl, such as methyl,
without, however, both R3 and R4 representing hydrogen, and
R5 is an aromatic heterocyclic ring of 5 members, in
which the sole hetero-atom is oxygen, as in a 2-furyl or 3-
furyl radical, or is sulphur in a 2-thienyl or 3-thienyl
radical,
or is phenyl which is optionally monosubstituted,
disubstituted or trisubstituted by lower alkoxy radicals such
as methoxy.
The invention also compri~es the addition salts of the
ethylamines of the formula (I) as well as those of their
optical or geometrical isomers which form an integral part of
the present invention, with inorganic or organic acids. Among
the~e salts, those obtained by reaction of the ethylamines with
pharmaceutically acceptable acids are prPferred. For example,
preferred salts are those prepared with acetic,
benzenesulphonic, camphosulphonic, citricl ethanesulphonic,
fumaric, hydrobromic, hydrochloric, lactic, maleic, malic,
methanesulphonic, mucic, nitric, pamoic, phosphoric, salicylic,
stearic, succinic, sulphuric or tartaric acid.
The ethylamines (I) of the invention have low toxicity
in animals and e~hibit valuable psychotropic properties which
are responsi~le for their usefulness in therapy in the form of
medicaments.
For this application, the preferred compounds are those
in which R1 i~ a furyl or thienyl radical, R5 is phenyl, furyl
or thienyl, R2 is ethyl and R3 and R4 are identical and are
methyl. More especially, these preferred compounds are the
following ethylamines (I) :
3 ~37~23
- 4-N,N-Dimethylamino-l-phenyl-~-(3-thienyl)-hex-1-en and its
salts,
- trans 1-[2-N,N-Dimethylamino-2-(3-thienyl)-butyl]-2-phenyl-
cyclopropane and its salts,
- 4-N,N-Dimethylamino-4-(2-furyl)-1-phenyl-hex-1-en and its
salts,
- trans 1-[2-N,N-Dimethylamino~2-(2-furyl)-butyl]-2-phenyl-
cyclopropane and its salts.
The invention al50 provides a process for the preparation
of the ethylamines of the formula (I), characterised in that it
essentially consists, as shown in Scheme 1 below,
for obtaining an ethylamine of the formula (I) in which
R3 is methyl and R4 is hydrogen,
i) in reducing an isocyanate of the formula (II) or an
isonitrile of the formula (II')
Rl~ ~CH2\ R5
C\ Q (II)
R2 NCO
20Rl fCH2 R5
/C\ Q (III)
R2 NC
with a metal hydride or or~ano-metallic hydride (Hm) of
the general formula
Ml(t) M2 H(r) Rx(s) (Hm)
in which :
Ml represents an alkali metal which is preferably lithium
or sodium, and whose index (t) has a value of O or 1,
M2 is a metal of group III of the periodic classification
of the elements and preferably boron or aluminium,
(r) is the index representing the number of hydrogen
atoms of the hydride and has values of 1, 2, 3 or 4,
RX represents a carbonitrile group, or a lower alkyl or
lower alkoxy group whose index (s) has values of 0, 1, 2 or 3,
the indices (t), (r) and (s) defined above correspond to
the relation (r) + (5~ - (t) = 3 and
the hydrides (Hm.1) in which M2 is aluminium or is boron,
. : .: . ~ .
. . . . . ~ ; : ,:: : .
':
. , - ~ .
,
4 ~3~3
when (r) = 3, (t) and (s) having a value of 0, are preferred
for the reduction of the compounds (II) and (II'~
ii) or in hydrolysing an isocyanate of the formula (II)
or an isonitrile of the formula (II') to give an amine (III) of
the formula
R1 CH2 R5
\ / \~
~C Q (III)
R2 NH2
which is acylated wi~h formic acid in the presence of N,N'-
carbonyldiimidazole to give an int~rmediate N-formyl compound:
Rl CH2 R5
~C\ Q
15R2 NH-CH0
which is reduced with a hydride (~m.2) of formula (Hm) in which
M2 is aluminium or, if M2 represents boron, (r) has a value
of 3 and (t) has a value of 0,
and, to obtain an ethylamine of ~ormula (I) in which R3
is lower alkyl other than methyl and R4 is hydrogen,
i) in alkylating an amine (III) with a halide ZlR3 in
which R3 has the meanings stated above and Z1 is chlorine,
bromine or iodine, or
ii) in acylatiny an amine (III~ with a reagent
(R6-CO)n Z2 in which R6 is the carbon homologue immediately
lower than R3 (R3 = -CH2-R6) and n has a value of 1 if Z2 is a
halogen, such as chlorine or bromine, or if ~2 is a hydroxyl
radical, and n has a value of 2 if Z2 repres~nts an oxygen
atom, to give an intermediate carboxamide (IV) o~ the ~ormula
R1 CH2 R5
C Q (IV)
R2 NH
35C0
R6
which is then reduced with a hydride (Hm.2) defined above,
..
: .
~
,~, ,
Z~37~2~
S CHEME
Rl&H2 ~ 5 Rl CH2 ~R5
R2NC (II ~ ) R2 NCO (II~
Rl CH2 R5
R2 NX (R3=CH3 )
CO
~ R6 ~
Rl CH2 ~R5 Rl ~ H2~R5
2 0 C Q ~ ~ C
R2 NH2 (III) R2 NH (I~
R3
(R3=R4=CH3)
RI C~2 R5 Rl ~CH2 ~R5 Rl ~CH2~R5
R2 N~ (VI) R2 N ~ ( ~) R2 N ~
R3 R4 R3 R4 R3 C0
R7
~,,~" . ;. i.~.~
6 ~(~3~3
to obtain an ethylamine of formula (I), in which R3
and R4 are identical and are methyl, in dimethylating an
amine (III3 by reacting it with formaldehyde and with either
formic acid or a reducing metal hydride or reducing organo-
metallic hydride (Hm.3) of formula ~Hm), in which M2 is boron,and among which the preferred compounds are those where Rx
represents a carbonitrile group,
to obtain an ethylamine (I~ in which R3 and R4 are lower
alkyl and are different,
i) in acylating an ethylamine (I), in which R3 is lower
alkyl and R4 is hydrogen, with an agent R7 COZ5, in which R7 is
the homologue immediately below ~4 (R4 = -CH2-R7) and Z5
represents bromine or chlorine, to give the carboxamide (V)
Rl C~2 R5
\/C \Q (V)
R2 N\
R3 C0
R7
which is then reduced with a hydride (Hm.2) defined above,
ii) or in reacting an organo-magnesium reagent R2MgZ3,
in which R2 is lower alkyl and Z3 is a chlorine, bromine or
iodine atom, with an amino nitrile (VI)
Rl CH2 R5
C Q (VI)
NC N~
R3 R4
in which R3 and R4 wich are identical or different and are
lower alkyl, and
to obtain an ethylamine (I) in which R3 is lower alkyl
and R4 is methyl, in N-methylating an ethylamine (I), in which
R3 is lower alkyl and R4 is hydrogen, with formaldehyde and a
reducing agent such as a metal hydride or organo-metallic
hydride (Hm.3) de~ined above.
7 ~ 3
The invention also provides the intermediates (XX) of the
general formula
R11 CH2 R15
/ ~ Q~ (XX)
R12 R16
in which :
Q' represent~ an ethylene-1,2-diyl group (-CH=CH-~ or a0 cyclopropane-1,2-diyl group (-CH-C~-),
CH2
R11 is an aromatic heterocyclic ring of 5 to 7 members,
in which the sole hetero-atom is nitrogen, oxygen or sulphur,
or, if Q' represents a cyclopropane 1,2-diyl group or if
R15 represents an aromatic heterocyclic ring as de~ined below,
Rll is a phenyl radical which i~ optionally monosubstituted,
disubstituted or tri~ubstituted by identical or dif~erent
radicals which are lower alkyl or lower alkoxy,
R12 is lower alkyl or a carbonitrile radical -CN,
R15 is an aromatic heterocyclic ring of 5 to 7 members
in which the sole hetero-atom is nitrogen, oxygen or sulphur,
or is a .phenyl radical which is optionally
monosubstituted, disubstituted or trisubstituted by identical
or different radicals which are lower alkyl or lower alkoxy,
and
R16 represents an isoGyanate radical -NC0 or an
isonitrile radical -NC, or a radical -N(R13)R14 in which R13
and R14 are hydrogen, lower alkyl or radicals R~-CO- or R7-CO-,
in which R6 and R7 ar~ hydrogen or alkyl which is the carbon
homologue below R13 or R14 (R13 = -CH2-R6; R14 = -CH2-R7).
.
~: , . . .
'.' .:', : , ' , . . .
8 ;~3~23
5CHEME 2
Rl
CH2
W
Rl Rl Rl
1 0 CH r ~ (~H CHO
R2 CN R2 COOH ~VII )
Rl CH2 lR5 Rl CH2 R5 Rl
~C~ Q ~ C Q C~I
R2 CN R2 COOH ~I~) NC ~N (XI)
R3 R4
Rl~ CH2~ ~R5 Rl CH2~ ~5 Rl CH2 R5
lR2 NC ~II ' ) R2 NCO (II) NC N (VI)
R3 R4
1'
Rl~ Rl Rl~
CH ~ ~CH ~ C=O
R2 NC R2 NH-CHO R2
: . ~
. . ~
: . , , : . :
:, , , :
,, :. -` ::
: . . : ,,.. .~ , ,
. . ~ ' ~ ', ........... .: '
.
::
9 2~37~23
The process of preparation of the intermediate
compounds (XX), of formulae (II), (II'), (III), (IV), (V)
and (VI) consists, as shown in Schemes 1 and 2 :
to prepare the isocyanate compounds (II), in alkylating
a compound Rl-CH2~W, in which W is a carbonitrile radical ( CN)
or carboxyl radical (-COOH), with an alkyl halide of formula
R2Z6, Z6 being a halogen, to give,
if W = -COOH, an acid of formula (VII) Rl(R2~-CH-COOH,
and, if W = -CN, a nitrile of ~ormula Rl(R2)-CH-CN, which0 is hydrolysed to the acid (VXI),
and thereafter alkylating ~he acid (VII) with a reagent
~VIII) of formula Z7-C~2-Q-R5, Z7 being a halogen or an
alkylsulphonyloxy radical, to give an acid (IX)
Rl(R2)C~COOH)CH2-Q-R5, and therea~ter preparing the5 isocyanates (II) by the Curtius reaction, and
to prepare the isonitrile compounds (II'), in utilizing
a suitable process, for example that described in European
Patent Application No. 0,298,703, and which consists in
reacting a ketone Rl-CO-R2 with formamide and formic acid to0 give the N-substituted formamide o~ formula Rl(R2)C~~NH-CHO,
which is dehydrated with phosphorus oxychloride to convert it
to the corresponding isonitrile Rl(R2) CH-NC, which, when
alkylated with the reagent (VIII) already described, gives the
isonitrile (II'), and
to prepare an amine (III), in hydrolysing either an
isocyanate (II) or an isonitrile (II') and
to prepare an intermediate carboxamide (IV), in acylating
an amine (III) with a reagent (R6-CO)nZ2 already defined, and
to prepare an intermediate carboxamide (V), in acylating0 an ethylamine (I)
R1 CH2 R5
/C Q
R2 NH
R3
with a hali~e of ~ormula ~7-COZ5, in which R7 is lower alkyl
and Z5 is bromine or chlorine and
~,
~ 7~ 23
to prepare an aminonitrile (VI), in reacting an
aldehyde ~l-CHO with an amine of ~ormula R3-NH-R4 and an alkali
metal cyanide to give the intermediate aminonitrile (XI)
Rl(CN)CH-N(R3)R4, in which R3 and R4 are both lower alkyl, and
then alkylating it with a reagent (VIII~ already described.
The compounds (I) of kh~ invention differ from the
nearest known compounds in respect of their chemical structure
and also their application.
Thus, European Patent Application No. 0,29,703 describes
thiophene derivatives of the formula :
Rt ~(CH2)p-Rph
R~ N
R2 R3
in which, in the broadest sense,
Rt is a thienyl radical,
R1, R2 and R3 represent lower alkyl radicals,
Rph is an optionally substituted phenyl radical, and
p has a value of 1, 2 or 3,
and in which, for the pre~erred compounds,
R1 is an ethyl radical,
R2 and R3 are methyl radicals,
Rph is a phenyl, 3,4-dimethoxyphenyl, 3,4,5-
trimethoxyphenyl or 4-chlorophenyl radical and
p has a value of 1 or 3.
These compounds are presented as having low toxicity and
having a regulating effect on the motility of the
gastro-intestinal tract, characterised by a stimulant effect on
a tract of slowed activity and conversely by an inhibiting
e~ect on a hyperactive tract.
The compounds of the European Application are different
from the ethylamines (I) of the invention in re~pect of their
chemical structure, especially the nature of the carbon chain
linking the two aromatic sites, and also in respect of their
properties.
Essentially, a psychotropic-type activity has not been
11 ~C337~23
reported for the compounds of EP Application No. 0,298,703, and
it is such an activity which makes it possible to envisage the
usefulness o~ the ethylamines (I) in the treatment of
neuro-psychic disturbances.
Among the processes for the preparation of the
compounds (I) which have been described above and presented in
Scheme 1, it is preferred, if R3 and R4 are lower alkyl, to use
the method which consists in reacting an aminonitrile
intermediate (VI) with an organo- magnesium derivative R2MgZ3.
Explici~ly, this process consists :
i) in reacting an aldehyde of formula R1-CHO with a
secondary amine R3~ R~, ~o obtain the aminonitril~ (XI) of
the formula NC(Rl)-CH-N(R33(R4).
This reaction is frequ~ntly used for the preparation of
amino acids according to the Strecker method. It is applied to
the synthesis of compounds (XI) and consists in reacting 1 mole
of aldehyde with 0.8 to 3.0 mole of sodium cyanide or potassium
cyanide and with 0.8 to 3.0 mole o~ a salt of a secondary amine
of formula R3-NH-R4 in an alcoholic or aqueous-alcoholic
medium, at a temperature of between 5C and the reflux
temperature of the mixture, for from 1 to 24 hours.
The secondary amine salts used preferably are
water-soluble, as in the case of, inter alia, the hydro-
chloride, hydrobromide and sulphate.
The reaction solvent comprises a low molecular weight
alcohol which is miscible in all proportions with water, such
as methanol or ethanol. In the case of aqueous-alcoholic media,
the respective proportions are between 95 and 10 % of alcohol,
and water to make up to 100 %, this ratio making it possible to
obtain a homogeneous reaction medium in a favourable manner.
In a usual procedure, loO mole of aldehyde dissolved in
75 to 200 ml of methanol is added to a solution of 1.1 to
1.3 mole of sodium cyanide and of 1.1 to 1.3 mole of secondary
amine hydrochloxide dissolved in lS0 to 400 ml of water. The
mixture is stirred for 3 to 5 hours at a temperature of between
15 and 30C and is then treated to isolate the aminonitrile
(XI), which, if necessary, i8 purified by distillation.
,
,
.~ ,
.
12 ;~:~3~2~3
ii) and thereafter in alkylating this compound with ~
reagent (VIII) to obtain the intermediate (VI) of the
invention.
The reaction consists, in a first stage, in preparing the
anion of the aminonitrile by treatment with a strong base. For
this purpose, lithium N,N-diisopropylamide (L~A) is preferred;
it is prepared l'in situ" from equimolecular amounts of
diisopropylamine and butyl- lithium. Per mole of LDA thus
prepared, there is then added, in THF, from 1.0 to 0.6 mole of
intermediate (XI), so as to obtain the anion thereof. The
reagent (VIII) is then introduced at a temperature between -10
and 50C, after which the mixture is left to react for 2 to
48 hours, depending on the reactivity of the compounds.
Thus, preferentially there is added to 1 mole of
diisopropylamine in 500 ml of TH~, at about -20C, from 0.95 to
1 mole of butyl-lithium followed by from 0.8 to 1.0 mole of
intermediate (XI) dissolved in about 500 ml of THF. After
reacting for 1 to 2 hours at between 20 and 100C to form the
anion, the mixture is cooled to about 0C and 0.8 to 1.0 mole
of the reagent (VIII) is added thereto.
The reaction proceeds for 1 to 2 hours at ambient
temperature and the mixture is then treated to isolate and
purify the intermediate aminonitrile (VI) obtained,
iii) and thereafter in reacting the intermediate (VI)
with an organometallic reagent such as a Grignard organo-
magnesium derivative of the formula R2MgZ3, in which Z3 is a
halogen and more especially bromine or chlorine, in accordance
with a reaction described, for example, by N.J. Léonard et al.l
J. Am. Chem. Soc., 1956, 78, p. 1986 and 1957, 79, p. 5279.
This replacement of the nitrile radical of the compound (VI) by
the alkyl radical R2 of the organo-magnesium derivative i5
carried out in ethers such as diethyl ether, methyl t-butyl
ether, diisopropyl ether or dibutyl ether or in
tetrahydrofuran, which is the preferred solvent, and consists
in reacting l mole of compound (VI) with 1. 5 tG 6 mole of
organo-magnesium derivatiYe at a temperature of between 5 and
50C for 30 minutes to 12 houxs.
The preferred method consists in adding 1 mole of
.
,..
.
-
,
13 ~37~
compound (VI), optionally dissolved in THF, at a temperature ofbetween 10 and 20C, to 4-5 mole of the organomagnesium
compound, also dissolved in THF. The reaction is continued for
2 to 5 hours at th~ same temperature and the complex obtained
is then decomposed by adding an aqueous ammonium chloride
solution. After treatments, the ethylamine (I) is isolated and
purified.
The working methods which ~ollow illustrate, without
howevPr implying any limitation, the preparation of the
essential intermediate d~rivatives and the preparation of the
ethylamines ~I) o~ the invention.
Depending on the reactions carried out, the products are
obtained directly in a satisfactory state of purity or are
purified by appropriate techniques indicated in the examples,
thPse techniques generally being crystallisation, vacuum
distillation or column chromatography. In the latter case, it
is advantageous to use the so-called "chromatoflash" technique
on a silica carrier (trademark "Merck", product Kieselgel 60,
particle size 230 to 400 mesh).
Furthermore, the purity, identity and physicochemical
characteristics of the products prepared are reported and are
determined by :
their boiling point under the degree of vacuum prevailing
during their distillation (in Pascal),
their meltiny point, determined by the capillary tube
method, the value indicated being uncorrected, and
the thin layer chromatography (TLC) on silica (ready-
to-use plates : a "Merck" product ref. 60 F 254), in accordance
with a technique which will be briefly recorded : the products
to be studied are deposited on the plate in an amount of about
lO0 mcg and then subjected to ascending elution with the
solvents or their mixtures enumerated below, their respective
proportions being given in volume/volume in the list which
follows :
14 ~37~3
Ref. S.A - hexanes 100 / ethyl acetate 10
S.B - " 60 / " 10
S.C - ll 40 / ll 10
S.D - " 20 / 1- 10
S~E - " 10 / " 10
S.F methylene chloride 20 / hexanes 80
S.G - methylene chloride
S.H - 7l 90 / acetone 10
S.I - " 85 / " 15
S.~ _ l 80 / " 20
S.K - l 98 / methanol 2
S.L - " 95 / " 5
S.M - " 90 / '~ 10
S.N - " 85 / " 15
After development, the chromatograms are observed under
ultraviolet light of wavelength 254 nm and/or after colour
development by spraying with Dragendorff reagent or tolidine
reagent. The ~f value~ observed as well as the references of
the elution solvents used are indicated in the examples.
elementary percentage analysis, o~ which the results, in
accordance with accepted standards, are not reported
numerically but are indicated as having been carried out by
mentioning the element detexmined,
infrared spectrography of the compounds in pellets in KBr
or in the form of films between two NaCl windows or in
suspension in Nujol (R) or in solution in CC14; the most
intense absorptions are reported in terms of the value of their
wavelength in cm~1
proton nuclear magnetic resonance (MMR) i5 studied at 60
or 90 MHz, the products being solubilised in deuterochloroform.
The appearance of the signals and their chemical shift
expressed in ppm relative to the tetramethylsilane used as the
internal reference are indicated. The so-called "exchangeable"
protons after addition of deuterium oxide are also indicated.
..
: -
-, . ,.. -: ... . ...
. ~
;. :, : ': . .:
:~ , , . ~, . .
- , . ..
- ,
Z~3'7~3
PREPARATION OF THE INTER~EDIATES
Com~ounds of formula (XI~)
XI.l. ~-Dimethyla~ino-phenylacetonitrile
(Rl = C6H5; R3 = R4 = CH3~
In a reactor, a solution of benzaldehyde (0.200 mole) in
20 ml of methanol is added in the course of one hour, at a
temperature o~ between 30 and 40C, to a solution of 11.82 g
(0.~41 mole) of sodium cyanide and of 19.61 g t0.240 mole) of
dimethylamine hydrochloride in 40 ml of water. The mixture is
stirred ~or 4 hours at ambien~ temperature and is then
precipitated in 150 ml of iced water and extracted with ether.
The ether phases are washed successively with water, with
a 25 % sodium bisulphite solution and again with water. ~fter
evaporation of the ether, the residue is purified by
distillation. B.p/93 Pa = 74~79C.
Weight = 30.4 g Yield = 95 %
The intermediate ~-amino-acetonitriles XI.2 to XI.6 were
prepared in accordance with this method of working, starting
from the appropriate aldehydes and secondary amine salts.
XI.2. a-Dimethylamino-~-~3 ~.5-trimethoxyPhenvl~acetonitrile
(Rl = 3,4,5(CH30)3-C6H2; R3 = R4 = CH3)
yield = 54 % M.p = 77C (petroleum ether)
XI.3. ~-Dimethylamino-~-(2~pyridyl)-acetonitrile
(R1 = 2-pyridyl; R3 = R4 = CH3)
yield = 67 % B.p/66 Pa = 98-110C
XI.4. ~-Dimeth~lamino-~-(2-furyl)-acetonitrile L
~Rl = 2-furyl; R3 = R4 = CH3)
yield = 58 % B.p/13 Pa = 55-75C
XI.5. ~-Dimethylamino-~-(2-thienyl)-acetonitrile
(Rl = 2-thienyl; R3 = R4 = CH3)
yield = 84 ~ B.p/7 Pa = 75D-85C
XI.6. ~-Dimethylamino-~-(3-thienvl~cetonitrile
(R1 = 3-thienyl; R3 = R4 = CH3)
yield = 72 % B.p/200 Pa = 86C
~6 ~3~ 3
Compounds of formula (VIII)
VIII.l. 1-(2-Thienyl) 3-chloro-~roP-l~ene
(R5 = 2-thienyl; Q = -CH=CH-; Z7 = Cl)
First stage 104.06 g of malonic acid (1.0 mole),
56.07 g (0.50 mole) of ~-thiophene-carboxaldehyde, 250 ml of
pyridine and 5 ml of piperidine are ~eated on a water bath for
2 hours and then to ~he reflux temperature for 5 minutes. After
cooling, the solution is precipitated in water and treated with
an excess of hydrochloric acid (250 ml of 37 % concentrated
solution) to preci~itate the product, which i5 thereafter
filtered off and then recrystallised from an ethanol-water
mixture to give the purified 2-thienylacrylic acid.
Weight = 42.38 g yield = 58 % M.p = 143-144C
Second stage : 37.34 g (0.24 mole) of the preceding acid
and 30 ml (0.24 mole) of BF3-ether complex in 310 ml of
methanol are heated to the reflux temperature for 6 hours. The
cooled solution is precipitated in water and then extracted
with methylene chloride. The organic extraction phases are
combined, washed with a saturated NaHC03 solution and then with
a saturated NaCl solution, and thereafter dried over MgS04. The
solid brown-coloured residue which is obtained after removal of
the solvents by distillation is recrystallised from hexane to
give the purified methyl 2 thienylacrylate.
Weight = 32.65 g yield = 81 % M.p = 46-47 a C
Third stage : A suspension of 4.51 g (118.9 mmol~ of
lithium aluminium hydride in 150 ml of THF is added slowly,
with stirring, to a mixture of 5.28 g (39.6 mmol) of aluminium
chloride and 40 ml of diethyl ether cooled to 10C, under a
nitrogen atmosphere.
A solution of 10.0 g (59.45 mmol) of the preceding methyl
ester in 50 ml of THF is added slowly at -10C and the solution
is then stirred at the same temperature for one and a half
hours. The complexes of the solution are decomposed by adding
a 3M sulphuric acid solution and the mixture is extracted with
ether. The combined ether phases are washed with a saturated
NaHC03 solution and then with a saturated NaCl solution, and
dried ov~r MgS04. ~vaporation of the ether in vacuo gives 7.83
g (94 %) of residual product in the form of a brown oil. The
;:
17 2~3~23
crude 1-(2-thienyl)-prop-1-en-3-ol, which is unstable at
ambient temperature, is kept at a temperature below 0C.
Fourth stage : 21.74 ml (296 mmol~ of dimethyl sulphide
are added slowly, at a te~perature of 0C, to a mixture of
39.53 g (296 mmol) of N-chlorosuccinimide in 180 ml of
anhydrous methylene chloride. The mixture is cooled to -10C
and a solution of 11.86 g (84.6 mmol) of the preceding alcohol
in 50 ml of methylene chloride is added.
The temperature of the solution is brought back to 0C
and kept thereat for 2 hours. The mixture is diluted with
100 ml of hexanes and then precipitated in 200 ml of iced
water. The organic phase is separated off and the aqueous phase
i~ reextracted with ethex. The combined ether phases are washed
and then drie~. The ether is removed by vacuum distillation and
crude 1-(2-thienyl)-3-chloro-prop-1-ene is obtained in the form
of an unstable reddish brown oil which is used as it is.
Weight = 12.06 g yield = 94 %
VIII.2. 1-~3-Thienvl)-~-chl ro-prop-l-ene
(R5 = 3-thienyl; Q = -CH=CH-; Z7 - Cl)
The intermediate is obtained from 3-thiophenecar-
boxaldehyde in accordance with the process described in the
preceding example.
First stage : 3-thienylacrylic acid
M.p = 46~C (ethanol/water)
Second stage methyl 3-thienylacrylate
M.p = 49C (hexanes)
Third stage : 1-(3-thienyl)-prop-1-en-3-ol
unpurified oil
Fourth stage : 1-(3-thienyl)-3-chloro-prop-1-ene
unpurified amorphous white solid.
VIII.3. 1-(2-Furyl)-3-chloro-Pro~1-ene
(R5 = 2-furyl; Q = -CH=CH-; Z7 = Cl)
The intermediate is obtained from 2~furylcarboxaldehyde
in four stages, in accordance with the process described for
the preparation o~ the intermediate VIII.1.
VIII.4. trans-1-M sYloxvmeth~l-2-~henvl-cvclopro~ane
(R5 = C6H5; Z7 = ~H3-SO3; Q = cyclopropane-1,2-diyl)
" :.
.
- ,
' ' .
, ~ ,
~7~2~
18
First stage o A solution of 25.0 g (154 mmol) o~ trans-2-
phenyl-cyclopropanecarboxylic acid in 100 ml of THF is added
dropwise, under a nitrogen atmosphere, to a solution o~
borane-THF complex. The solution is heated to the reflux
temperature for 3 hours, after which 130 ml of 2N NaOH solution
are added slowly, and the mixture is stirred for 30 minutes.
The ether extracts are concentrated in vacuo to give 20.78 g o~
crude trans-1-hydroxymethyl-~-phenyl-cyclopropane which is
purified by distill~tion~
Weight Y ~8.19 g yield = 80 % ~.p/33 Pa = 90-97DC
Second stage : 9.18 g (66.2 mmol) of the alcohol obtained
above and 13.83 ml (99.25 mmol) of triethylamine are added to
100 ml of methylene chloride. 5.63 ml ~72.8 mmol) of
methanesulphonyl chloride are added dropwise under a nitrogen
atmosphere, at 1o1C. The mixture is stirred for 15 minutes at
-10C and then washed successively with iced water, with a 10
% strength HCl solution, with a saturated NaHC03 solution and
then with a saturated NaCl solution. After having been dried
over MgS04 at 0C, the solution is concentrated in vacuo to
give a yellow oil. The intermediate thus obtained is dissolved
in anhydrous THF and used as it is.
VIII.5. trans-1-Bromomethyl-2~phe~ yclopropane
(R5 = C6H5; Z7 = Br; Q = cyclopropane-1,2 diyl)
61.0 g (0.34 mol) o~ N-bromosuccinimide are added to
300 ml of methylene chloride and the mixture is cooled to 0C
under a nitrogen atmosphere, after which 23.4 ml (0.41 mol) of
dimethyl sulphide are added dropwise. The mixture is stirred at
0C for 30 minutes and then cooled to -5C; a solution of
33.6 g (0.23 mol) of trans-1-hydroxymethyl-2-phenyl-
cyclopropane obtained in preparation VIII.4, in 100 ml ofmethylene chloride, is then introduced dropwise. The mixture is
stirred for ~ hours at 0C and then for 16 hours at 25C;
thereafter it is diluted by addition o~ 250 ml of hexanes and
the mixture is pr~-cipitated in 250 ml of iced water. The
organic phase is washed with a saturated NaC1 solution and then
dried over MgS04. The solvents are removed by concentration in
vacuo and the residue is purified by distillation
Weight = 40.81 g yield = 85 % B.p/27 Pa = 72C
19 ~3~23
VIII.5. tran~-1 Mesyloxymethyl-2-~3,4 s-trimethoxyphenYl~-
cyclopropane
(R5 = 3,4,5(CH30)3-C6H2; Z7 = CH3-S03; Q = cyclopropane-1,2-
diyl).
First stage : 55.0 g (0.~3 mol) of 3,4,5-tri-
methoxycinnamic acid and 28.39 ml (0.23 mol) ~f BF3- ~ther
complex are added to 400 ml o~ methanol and the olution is
haated for 6 hours to the reflux temperature. The mixture is
cooled, then precipitated in water, and extracted with
methylene chloride. The combined organic phases are washed by
extraction with water and dried, and the solvent is then
removed by distillation in vacuo. The methyl 3,4,5-
trimethoxycinnamate is purified by recrystallisation from
methanol.
Weight = 46.17 g yield = 80 % M.p c 96-98C
Second stage : 106.83 ml (213.7 mmol) of n-butyl-lithium,
as a 2M solution in hexane, are added slowly, under a nitrogen
atmosphere, to a solution of 20.15 ml (21~ mmol) of 2-methyl-~-
propanol in 125 ml of anhydrous THF~ After 30 minutes, the
solution is treated by dropwise addition of a solution of
17.97 g (71.22 mmol) of the preceding methyl ester in 100 ml of
THF. The reaction mixture is heated to the reflux temperature
for 2 hours and a half and is then cooled by means of an ice
bath and hydrolysed by addition of 200 ml of water. The aqueous
phase is extracted with ethyl acetate and the organic fractions
are dried over MgS04. The ~olvents are evaporated in vacuo to
give a residue of t~rt-butyl 3,4,5-trimethoxycinnamate which is
purified by recrystallisation from hex~ne.
Weight = 16.27 g yield = 78 % M.p = 83-85C
Third stage : 3.0 g of sodium hydride as a 60 % strength
oily disper~ion (75 mmol) are added, under nitrogen, to a
stirred suspension of 15.47 g (70.3 mmol) of
trimethylsulphonium iodide in 150 ml of DMS0, which is kept at
25-30~C.
After the evolution of hydrogen as ceased (about
30 minutes), a solution of 15.9 g (54 mmol) of the preceding
tert-butyl ester in 100 ml of DMS0 is added, without exceeding
35C.
, ~ :
. .
~37~L23
The mixture is stirred for 30 minutes at 25-30C and then
for one and a half hours at 55-60C. It is thereafter
precipitated in 3~0 ml of water and extracted with ethyl
acetate. The combined extracts are dried and concentrated in
vacuo to give a residue of tertrbutyl trans-2-(3,4,5-
trimathoxy-phenyl)cyclopropanecarboxylate in the form of a
yellow oil which solidifies. The product is purified by
recrystallisation from hexane.
Weight = 9.69 g yield = 58 % ~.p = 68-70C
Fourth stage : 3.66 g (~6.5 mmol~ of lithium aluminium
hydride are dispersed in 150 ml of T~F under a nitrogen
atmosphere. 9.92 g (32.2 ~mol3 of the preceding tert-butyl
ester dissolved in 100 ml of THF are added slowly. The mixture
is heated to the reflux temperature for one hour and a half and
5.58 ml of 10 % strength NaOH solution ar~ added cautiously,
followed by 7.32 ml of water. The suspension obtained is
stirred overnight; the insoluble matter is ~iltered off and the
filtrate is concPntrated in vacuo to give crude trans-l-
hydroxymethyl-2-(3,4,5-trimethoxyphenyl~-cyclopropane, which is
purified by distillation in vacuo.
Weight = 6.65 g yield 87 ~ B.p/3 Pa = 145-165C
Fifth stage : Following the procedure described for
Stage 2 of the preceding intermediate VIII.4, but using the
derivative obtained above, trans-l-mesyloxymethyl-2-(3,4,5-
trimethoxyphenyl)-cyclopropane i5 obtained in the form of a
white solid which is used without further purification.
VIII.7. trans-1-Chloromethyl-2-(2-thienyl~-cyclopropane
(R5 = 2-thienyl; Z7 = Cl: Q = cyclopropane-1,2-diyl)
In accordance with the process described in the
preceding example (stages 2, 3 and 4) the trans-l-
hydroxymethyl-2-~2-thienyl~-cyclopropane is obtained from the
methyl 3-(2-thienyl)-propenoate. 15,50g (100 mmol) of the
prec~ding alcohol and 40,0g of dichlorotriphenylphos-phorane
(85% - 102 mmol) are added in 75,0 ml of anhydrous
triethylamineO The mixture is stirred at 25C during 24 hours
then precipitated in 500 ml of water. After adding 100 ml of
hexanes the mixture is filtred then the aqueous layer is
extracted twice with 100 ml of hexanes. The. organic phases are
.
.
.
21 ~37~3
combined and therea~ter dried over Na2S04.
After removal of the solvant by vacuum distillation the
crude product is puri~ied by distillation.
Weight = 9,7 g yield 56% ~.p/40 Pa = 65-70C
VIII.8. trans-1-Chloromethy~ L3-thienYl~-cYclopropane
(R5 = ~-thienyl; Z7 = Cl; ~ = cyclopropane 1,2 diyl)
With the same process described in the preceding
example, the product is obtenaid from the methyl 3-(3-thienyl)-
propenoate.
the crude product is puri~ied by vacuum distillation in the
~orm of yellowish oil B.p/400 Pa = 60 - 7~C
VIII.g. trans-l-Chloromethyl-2-(2~furYl)-cyclopro~ane
(R5 = 2~furyl; Z7 = Cl; Q = cyclopropane 1,2 diyl)
The compound is obtenaid from the methyl 3-(2-furyl)-
propenoate with the same process des cribed in the precedingexample. It is purified by vacuum distillation. B.p/1,3 Pa =
55-60C
~-Amino-acetonitriles of ~ormula ~VI)
The compounds are prepared by alkylation of the
acetonitriles (XI) with the reagents (VIII) described above.
After reaction, the compounds obtained, which are often
unstable, axe either purified by crystallisation or employed as
they are in the subsequent reactions.
The purity and identity of the products is confirmed by
thin layer chromatography and by NMR.
General workinq method
In a reactor protected from moisture and under a nitrogen
atmosphere, 1.025 moles of n-butyl-lithium (as a lOM solution
in hexanes) are added dropwise at -20C to a solution of
1.025 moles of diisopropylamine in 1 litre of anhydrous
tetrahydrofuran.
The mixture i kept at -20C for 15 minutes. At -72C,
1.0 mole of nitrile (XI) dissolved in 200 ml of THF is
introduced, stirring is continued for 1 hour 30 minutes at khis
temperature and 1.025 moles of reagent (VIII) dissolved in
500 ml of THF are then added. After 20 minutes at 72C, the
mixture is stirred ~or 1 hour at ambient temperature.
Thereafter, 1.5 1 o~ 10 % (weight/volume) NH4Cl solution
.
: .
,
~2 ~ 3
and 750 ml of a 1 :1 (volume/volume) mixture of hexanes and
ethyl acetate are added.
The organic phase is separated off and the aqueous phase
is reextracted with the same mixture of solvent~. The combined
organic phases are washed by extraction with a saturated sodium
chloride solution and then dried over MgS04. The solvents are
removed by distillation in vacuo and on a water bath. ~he oily
residue is, depending on circumstances, cry~tallized by
addition of hexanes or used as it is ~or the next stage.
The intermediates VI.l to VI.22 are prepared in
accordance with this method of working.
VI.l. trans~ 2-Cyano-2-phenyl-N.N-dimethylaminoethyl)-2-
phenvl-cyclo~ropane.
(Rl = R5 = C6H5; R3 = R4 = CH3; Q = cyclopropane-1,2-diyl)
From XI.l and VIII.4
Yield = 57 % (crude) TLC : 0.65-0.70; S.C.
NMR 0~60-Oo~O (m,3H); 1.28 (m, lH); 2.15 (m, 2H); 2030 (d~
6H); 6.78-7.78 (m, lOH).
VI.2. _ trans~ Cyano 2- ~3,~5-trimethoxYphenyl)-N,N-
dimethYlaminoethyll-2-~henyl-cYcloPro~ane.
(Rl = 3,4,5(CH30)-C6H2, R3 = R4 = CH3; R5 = C6H5; Q =
cyclopropane-1,2-diyl)
Fxom XI.2 and VIIIo5
Yield = 87 % (crude) TLC : 0050-0.55; S.D.
25 NMR : 0.60-0~80 (m, 2H); 1.10-1.30 (m, lH); 1.60-1.90 (m, lH);
2.28 (d, 8H); 3.86 (m, ~H); 6.78 (d, 2H); 6.89-7.39 (m, 5H)
VI.3. 1-Cy~ano-1-N N dimethylamino-l-phenyl-4-(2-thienyl~-but-3-
ene.
(R1 = C6H5; R3 = R4 = CH3; R5 = 2-thienyl; Q = -CH=CH-)
From XI.1 and VIII.l
Yield = 80 % (crude) TLC : 0.70; S.D.
NMR : 2.23 (s, 6H); 2.80 (d, 2H); 5.88-6.19 (dt, lH); 6.60 td,
lH); 6.87-7.38 (m, 8H)
VI.4._1-Cyano-1-N.N-dimethylamino-1-phenyl-4-(3-thienYl)-but-3-
ene.
(R1 = C6H5; R3 = R4 - CH3; R5 = 3-thi~nyl; Q = -CH=CH-)
From XI.l and VIII.2
Yield = 75 % (crude) TLC : 0.60; S.A.
.
23 2~3~23
NMR : 2.30 (~, 6H); 2.85 (m, 2H); 5.40 (m, lH); 6.30 ~d, lH~;
6.90-7.80 (m, 8H~
VI.5. trans-1-f2-Cyano-2-~he~l-N,N-dimethylaminoethYl]-2-
(3,4,5-trimethoxyphenylL~cyclopropane.
(Rl = C6H5 ; R3 = R4 = CH3; R5 = 3,4,5(CH30)-C6H2; Q =
cyclopropane-1,2-diyl)
From XI.1 and VIII.6
Yield = 78 % (crude) TLC : 0.35-0.40; S.D~
NMR : 0.55-1.45 (m, 4H~; 2.20 (d, 2H); 2.35 ~s, 6H) 3.85 (s,
9H); 6.28 (~, 2H); 7.50 (m, 5H)
VI.6. trans-1-r2- ~ano-2-~2-p~ridyl)-N,N-dimethYlaminoethyl]-2-
phenvl-cyclopropane.
(Rl = 2-pyridyl; R3 = R4 = CH3; R5 = C6H5; Q = cyclo- propane-
1,2-diyl)
From XI.3 and VIII.5
Yield = 79 % (crude) TLC : 0.25; S~Eo
NMR : 0.62-1.65 ~m, 4H); ~.35 (s, 6H~; 2.40 (d, 2H); 7.16 (m,
6H); 7.65 (m, 1~); 7.75 (m, lH); 8.78 (m, lH)
VI.7. l-Cyano-l-N,N-dimethYlamino-l-~ 2-fur~1)-4-phenyl-but-3-
ene.
(Rl = 2-furyl; R3 = R4 = CH3; R5 = phenyl; Q = -CH=CH-)
From XI.4 and cinnamyle chloride
Yield = 98 % (crude) TLC : 0.50; S.C.
NMR : 0.20-0.85 (m, 5H): 2.31 (m, 2H); 2.45 (s, 3H): 3.00 (d,
2H); 5.81 (dt, lH); 6.35-6.51 (m, 3H); 7.28 (s, 5H); 7.48 (m,
lH)
VI.8. trans-l- r 2-Cyano-2-(2-furYl~-N~N-dimethylaminoethyll-2
phenvl-cyclop~epaneO
(Rl = 2-furyl; R3 = R4 = CH3; R5 = C6H5: Q = cyclo- propane-
1,2-diyl)
Fro~ XI.4 and VIII.5
Yield = 91 ~ (crude) TLC : 0.25-0.30, S.C.
NMR : 0.46-1.43 (m, 4H); 2.17~2.30 (m, 8H); 6.35 (m, lH); 6.62
(m, lH); 7.20 (m, 5H); 7.45 (m, lH)
VI.9. 1-Cy_no-l-N,N-dim~thylamino-1-(2 furyl)-4-~2-thieny~)-
but-3-ene~.
(Rl = 2-furyl, R3 = R4 - CH3; R5 = 2-thienyl; Q =-CH=CH-)
From XI.4 and VIII.1
,
24 ;~-3'73L23
Yield a 96 % (crude) TLC : 0.40~0.45; S.C.
NMR : 2.31 (s, 6H); 2.98 (d, 2H~; 5.68 (dt, lH); 6.31-6~61 (m,
3H); 6.90-7.18 (m, 3H); 7.49 (m, lH)
VI.10. 1-Cx~o-1-N,N-d _ethYlamino-4-pheny~ (2-thienyl) -but-
3-ene.
(R1 = 2-thienyl; R3 = R4 = CH3; Rs = phenyl; Q = -CH=CH-)
From XI.5 and 3-phenyl-1 chloropropene
Yield - 91 % (crude) ~LC : 0.45-0.50; S.C.
NMR : 2.45 (s, 6H~; 2~88 (d, 2H); 5.82 (dt, lH); 6.43 (d, lH);
6.88-7.01 (m, lH); 7.28 (m, 7H)
VI.ll. trans-l- r 2-Cyano-2-(2-thienyl)-N~N-dimethylamino ethyll-
2-phenyl-cyclo~ropane.
(Rl = 2-thienyl; R3 - R4 = CH3; R5 = C6H5; Q = cyclo- propane-
1,2-diyl)
From XI.5 and VIII.5
Yield = 79 % (crude) TLC : 0.80-0.85; S.D.
NMR : 0.65 -1.40 (m, 4H); 2.10 - 2.25 (m, 2H); 2.37 (s, 6H);
6.73 -7~32 (m, 8H)
VI.12. 1-Cyano-l-N,N-dimethylamino-4-(2-furyl)-l-(2-thienvl~-
but-3-ene.
(Rl = 2-thienyl; R3 = R4 = CH3; R5 = 2-furyl; Q = CH=CH-)
From XI.5 and VIII.3
Yield = 79 % (crude) TLC : 0.50; S.CO
NMR : 2.38 (g, 6H); 2.90 (d, 2~); 5.78 (dt, lH); ~.15-6.33 (m,
3H); 6.89-7.05 (m, lH); 7.20-7.30 (m, 3H)
VI.13. l-CYano-l-N~N-dimethy~amino-l 4-di(2-thienylL-but-3-ene.
(Rl = R5 = 2-thienyl; R3 = R4 = CH3; Q = -CH=CH-)
From XI.5 and VIII.3
Yield = 84 % (crude) TLC : 0.50; S.C.
NMR : 2.38 (s, 6H); 2.80 (d~ 2H); 5.67 (dt, lH); 6.51 (d, lH~;
6.88-7.38 (m, 6H)
VI.14. 1-Cyano-l~N~N-dimeth~lamino-1-[2-thienY1)-4-(3-thienyl)-
but-3-ene.
(Rl = 2-thienyl; R3 - R4 = CH3; R5 = 3-thienyl; Q =
-CH=CH )
Fr~m XI.5 and VIII.2
Yield = 87 % (crude) TLC : 0.50; S.C.
NMR : 2.35 ~s, 6H); 2.9-3.2 (m, 2H); 5.7 ~dt, lH); 6.4.~ (d,
.
.
,
;
25 ~3~23
lH); 6.85-7.50 (m, 6H)
VI.15. 1-Cyano-l-N,N-dimethylamino-4-pheny~-1-(3-thien~l) -but-
3-ene.
(R1 = 3-thienyl; ~3 = R4 c CH3: R5 = phenyl; Q - -CH=CH-)
From XI.6 and 3-phenyl-1-chloropropene
Yield = 87 % ~crude) TLC : 0.40-0.45; S.C.
NMR : 2.35 (s, 6H); 2.5-3.2 (m, 2H); 5.5-6.0 (dt, lH~; 6.3 (d,
lH); 7.3 (~, 5H); 7.0-7.6 (m, 3H)
VI.16. trans~ 2-Cyano-2-(3-thienyl)~N,N-dimethylamino sthyl~ r
2-phenyl-cyclopro~ane.
(Rl = 3-thienyl; R3 = R4 = CH3; R5 = C6H5; Q = cyclo- propane-
1,2-diyl)
From XI.6 and VIII.4
Yield = 83 % (crude) TLC : 0.45-0.50; S.B.
15 NMR : 0.50-2.30 ~m, 6H); 2.27 (s, 6H); 6.7-7.5 (m, 8H)
VI.17. l-Cyano-l-N,N dimethylamino-1-(3-thienyl)-4-f2-thienyl)
but-3-ene.
(Rl = 3-thienyl; R3 = R4 = C~3; R5 = 2-thienyl; Q =
-CH=CH-)
From XI.6 and VIII.l
Yield = 85 % (crude) TLC : 0.50; S.C.
NMR : 2.35 (s, 6H); 2.9-3.2 (m, 2H); 5.7 (dt, lH); 6.45 (d,
lH); 6.85-7.50 (m, 6H)
VI.18. 1-Cyano-l N,N-dimethylamino-1.4-di(3-thienyl)-but-3-ene.
25 (Rl = R5 = 3-thienyl; R3 = R4 = CH3; ~ = -CH=CH-)
From XI.6 and VIII.2
Yield = 75 % (crude) T~C : 0.75; SOC.
NMR : 2.30 (s, 6H); 2.9 (m, 2H); 5.6 (dt, lH); 6.40 (d, lH);
7.0~7.45 ~m, 6~)
30 VI.l9. trans-1-(2-Cvano 2- ~enYl-N,N-dimethylaminoethyl~ -2-(2-
thienvl)-cYclopro~ane.,
(R1 = C6H5; R3 - R4 = CH3; R5 c 2-thienyl; Q = cyclo- propane-
1,2-diyl)
From XI.l and VIII.7
35 Yield = 64 % (crude) ~LC : 0.70; S.D.
NMR : 0.50-2.80 (m, 6H); 2.27 (s, 6H); 6.49-7.10 (m, 3H); 7.35
(m, 3H); 7.60 (m, 6H)
26 ~Gi37~23
VI.20. trans-l ~2-CYano=2-(2-thienyl-y~ N-dimethyl amino ethvl ~ -
2-(2-thienyl)-cyclo~ropane.
(Rl - R5 = 2-thienyi ; R3 - R4 = CH3 ; Q = cyclo- propane-1,2-
diyl)
From XI.5 and VIII.7
Yield - 58 % (crude) TLC : 0.50-0.55 ; S.C.
NMR : 0.60-2060 (m, 6H~; ~.35 (s~ 6~); 7.00 (m, 4H); 7.40
(m, 2H)
VI.21. trans-l- r 2-Cyano-2-~3-thienyl~-NI~-dimethYlamino ethyl~-
2-(2-furYl) cycloPro~ane.
(Rl = 3-thienyl ; R3 = R4 = CH3 ; R5 = 2-furyl ; Q =
cyclopropane-1,2-diyl)
From XI.6 and VIII.9
Yield = 98 ~ (crude) TLC : 0.60 ; S.C.
VI.22. trans-l- r 2-Cyano-2-(2-furyl)-N,N dimethylamino eth~l]-2-
(3-thienylL~cyclopro~ane.
(Rl = 2-furyl ; R3 = R4 = CH3 ; R5 = 3-thienyl ; Q =
cyclopropane-1,2-diyl)
From XI.4 and VIII~8
Yield = 95 % (crude) TLC : 0.45 ; S.c.
NMR : 0.40-2.40 (m, 6H); 2.25 (s, 6H); 6.20-6.60 (m, 6H)
ETHY~AMINES OF T~E INVENTION - ~XAMPLE5
General workina method :
2.26 l of a 2.0 M solution of ethyl-magnesium bromide
(4.52 mol) in THF are introduced into a reactor protected from
moisture, under a nitrogen atmosphere.
275.0 g (1.0 mol) of a-amino-acetonitrile (VI) dissolved
in 1.6 l of THF are introduced over 15 minutes, with stirring
and at ambient temperature.
The mixture is stirred at laboratory temperature for
3 hours and 4.5 l of an aqueous saturated ammonium chloride
solution are then added cautiously, without exceeding 20C.
The aqueous phase is decanted and extracted with twice
650 ml o~ a 1 :3 (volume/volume) mixture of hexanes and ethyl
acetate.
The combined organic pha~es are extracted with twice
600 ml of N HCl solution. The combined acid aqueous phases are
rendered alkaline with a concentrated sodium hydroxide solution
.,
27 ~37~3
and the mixture is then extracted with 3 times ~50 ml of
hexanes/ethyl acetate mixture.
The combined organic phases are washed with water, dried
over MgSO4 and then evapora~ed in vacuo. The residual product
is purified by crystallisation or by preparing and then
purifying one o~ its additio~ salts which most commonly is the
hydrochloride.
This experimental method is applied to the intermediates
VI.1 to VI.22, using ethyl-magnesium bromide, to obtain the
compounds of the invention (I)) of Examples 1 to 22.
Example 1 : trans 1-~2-N~N-Dimethylamino-2-phenvl-butyl)-2-
phenyl-cvclopropane.
(Rl - R5 = C6H5; R2 = C2H5; R3 = R4 = CH3; Q = cyclopropane 1,2
diyl)
Starting material : the intermediate compound VI.1
Yield = 47 % boiling point/13 Pa = 14~-152C TLC : 0.60; S.C
IR (film) : 3080, 3060, 3020, 2980, 2960, 2875, 2820, 2780,
1579, 1490, 1452, ~44~, 1352, 1220, 1180, 1085, 1025, ~9~, 750,
690 cm~l
NMR : 0.65-2.15 (m, llH); 2.26 (d, 6H); 6.87-7.50 (m, lOH)
Analysis (C21H27N) C, H, N
ExamPle 2 : trans 1~I2-N ! N-Dimethylamino-2-(3.4.5-
trimethoxyphenvl)-butyl~-2-phenyl cyclo~ropane.
(Rl = 3,4,5~CH30)3-C6H2; R2 = C2H5: R3 = R4 = CH3; R5 = C6H5;
Q = cyclopropane-1,2-diyl)
Starting material : the intermediate compound VIo2
Yield = 38 ~ TLC : 0.35; S.C
IR (film) : 3036, 2940, 2860, 2780, 1600, 1585, 1500, 1460,
1400, 1320, 1240, 1185, 1160, 1140, 1020, 750, 700 cm~1
NMR : 0.62-0.95 (m, 6H); 1.41-1061 (m, lH); 1.87-2.02 (m, 4H);
2.31 (d, 6H); 3.70-3.88 (t, 9H): 6.66 (s,2H); 6.90-7.22 (m, 5H)
Analysis (C24H33N03) C, H, N, O
Example 3 : 4-N N-Dimethylamino-4-phenyl-1-(2-thienyl~-hex-1_
ene.
(Rl = C6H5; R2 = C2H5; R3 - R4 = CH3; RS = 2-thienyl;
Q = -CH=CH-)
Starting material : the intermediate compound VI.3
Yield = 62 % melting point 77-78C l'LC : 0.60-0.65; S.C
28 ~al3~23
IR (~ilm~ : 3220, 2960-2880, 1465, 1380, 950, 760, 730,
700 cm~l
NMR : 0-72 (t, 3H); 1.90 (q, 2H); 2.25 (s, 6H); 2.80 (d, 2H);
5.90-6.22 (dt, lH); 6.58 (d, lH); 6.89-7.41 (m, 8H)
Analysis (C18H23NS) C, H, N, S
Exam~le 4 : 4-N.N-Dimethvlamino-4-phenYl-1-(3-thienYl)-hex-1-
ene.
(Rl = C6H5); R2 = C2H5; R3 = R4 = CH3; R5 = 3-thienyl;
Q = -CH=CH-)
Starting matexial . the intermediate compound VI.4
Yield = 58 % melting point = 95-96C TLC : 0.50
IR (sol. CC14) : 3080, 3040, 2980, 2930, 2850, 2820, 2775,
1500, 1470, 1460, 1450, 1250, 1190, 1140, llOOr 1040, 1020,
980 cm~l
NMR : 0.78 (t, 3H)f 1.95 (q~ 2H~; 2.26 (s, 6H); 2.87 (d~ 2H);
5.90-6.26 (dt, lH); 6.55 (d, lH~; 7.05-7.50 (m, 8H)
Analysis (C18H23NS) C, H, N, S
Example 5 : trans ~-(2-N N-Dimethylamino-2-phenYl-butYl)-2-
(3 4,5-trimethoxvphenyl)-cYclopropane.
(Rl = C6H5; R2 = C2H5; R3 = R4 - CH3; R5 = 3,4,5(CH30)3-C6H2;
Q = cyclopropane-1,2-diyl)
Starting material : the intermediate compound VI.5
Yield = 37 % TLC : 0.45; S~M
IR (KBr) : 3060, 2980, 2930, 2780, 2740, 2400, 1585, 1515,
1470, 142~, 1320, 1255, 1235, 1150, 1130, 1010, 910, 850, 810,
760, 705 cm~l
NMR : 0.95-1.15 (m, 6H); 1.88 (m,2H); 2.48 (m, lH); 2.71 ~s,
6H); 3.30 (m, 2H); 3.77 (d, 9H): 6.33 (s, 2H); 7.50-7.82
(m, 5H)
- Hydrochloride : melting point = 205-208C
Analysis (C24lH33N03.HCl) C, H, N, 0, Cl
Exame~Le 6 : trans 1- r 2-N.NIDimethy~ no-2-(2-pyridYl2 butYl1-
2-~henyl-cyclopro~ane.
(Rl = 2-pyridyl; R2 = C2H5; R3 - R4 - CH3; R5 = C6H5;
Q = cyclopropane 1,2 diyl)
Starting material : the intermediate compound VI.6
Yield = 52 % boiling point/13 Pa = 130C TLC : 0.30; S.E
IR (film) : 3060, 3020, 2970, 2940, 2875, 2820, 1610, 1590,
~, ~ ,. , -
,;
.
29 ~37~l23
1570, 1500, 1465, 1430/ 1150, 1100, 1050, 1000, 915, 785, 740,
700 cm~l
NMR : 0.66-0.91 (m, ~) 1.44-1.63 (m,2H); 1.80-2.24 (m, 4H);
2.30 (s, 6H~, 6.89-7.35 (m, 6H); 7.52-7.69 (m, 2H); 8.62
(d, lH)
Analysis (C2OH26N2) C, H, N
ExamPle7:4-N N-Dimethylamino-4-(2-~uryl~-1 phenyl-hex-l-ene.
(R1 = 2-furyl; R2 = C2~5; R3 ~ R~ - CH3; R5 = C6H5;
Q = -CH=CH-)
Starting material : the interm~diate compound VI.7
Yield = 37 % TLC : 0.35; S.C
IR (film) : 3020, 2980, 2940, 2880, 2820, 2780, 1600, 1575,
1495, 1470, 1450, 1160, 1040, 968, 800, 735, 690 cm~1
NMR : 1.81 (t, 3H); 1.86 (q,2H); 2.20 (5, 6H); 2.62-2.85 (m,
2H~; 5.98-6.58 (m, 4H~; 7.21-7.38 (m, 6H)
Analysis (C18H23NO) C, H, N, O
Exam~le 8 : trans 1-r2-N N-Dimethylamino-2-(2-furyl)-butyll~2-
~henyl-cyclgpropane.
(R1 = 2-furyl; R2 = C2H5; R3 = R4 = CH3; R5 = C6H5;
Q = cyclopropane~1,2-diyl)
Starting material : the intermediate compound VI.8
Yield = 65 % TLC : 0.40; S~A
IR (film) : 306Q, 3020, 2980, 2960, 2870, 2820, 2780, 1610,
1500, 1465, 1380, 1160, 1020, 940, 887, 805, 740, 700 cm~1
NMR : 0.80-0.97 (m, 6H); 1.23-1.70 (m, lH); 1.91-2003 (m, 4H);
2.16 ~8, 6H); 6.06 (d, lH); 6.26 (dd, lH); 6.80-7.20 (m, 5H);
7.32 (d, lH)
Analysis (C19H26NO) C, H, N, O
Example 9 : 4-N N-Dimethvlamino-4-(2-furvl)-1-(2-thienvl)-hex-
1-ene.
(R1 = 2-furyl; R2 = C2H5; R3 = R4 = CH3; R5 = 2-thienyl;
Q = -CH=CH-)
Starting material : the int~rmediate compound VI.9
Yield = 56 % melting point = 68-69C TLC : 0.50-0.55; S~C
IR (Nujol) : 2880-2940, 2840, 1460l 1370, 1150, 1040, 1020,
1000, 955, 850, 800, 740, 700, 695 cm~1
NMR : 0.85 (t, 3H~; 1.90 (q, 2H); 2.25 (s, 6H); 2.80 (m, 2H);
5.80-6.12 (dt, lH); 6.18 (m, lH); 6.34 (m, lH); 6.60 (d, lH);
,
:
21~37~Z3
6.90-7.12 (m, 3H); 7.40 (d, lH)
Analysis (C16H21NOS~ C, H, N, O, S
ExamPle 10 : 4-N,N-Dimethylamino-l-phenvl-4-(2-thienyl)-hex-1-
ene.
(R1 = 2 thienyl; R2 = C2H5; R3 = R4 = CH3; R5 = C6H5;
Q = -CH=CH-)
Starting material : the intermediate compound VI.10
Yield = 32 % TLC : 0.45-0.50; S.C
IR (film) : 3070, 3050, 3010, 2960, 2930, 2~60, 2810, 2770,
1590, 1580, 1490, 1440, 1240, 960, 820, 735, 685 cm~l
NMR : 1.87 (t, 3H); 1.94 (q,2H~; 2.24 (s, 6H); 2.86 (d, 2H);
6.05-6.63 (ddt, 2H); 6.87-7.07 (m, 2H); 7.17-7.37 (m, 6H)
Analysis (C18H23NS) C, H, N, S
Exam~le 11 :trans l- r 2-N N-Dimeth~ylamino-2-(2-thienyl)-butYl]-
2-phenYl-cycloPropane.
(R1 = 2-thienyl; R2 = C2~5; R3 = R4 = CH3; R5 = C6H5;
Q = cyclopropane-1,2-diyl)
Starting material : the intermediate compound VI.11
Yield = 58 % boiling point/0.1 = 155-~67~C TLC : 0.60, S.C
IR (film) : 3060, 3020, 2970, 2930, 2870, 2820, 2780, 1610,
1500, 1460, 1375, 1250, 1240, 1225, 1090, 1040, 1030, 850, 830,
750, 700 cm~1
NMR : 0.75-0.98 (m, 6H); 1.55-1.77 (m, lH); 2.00-2.10 (m, 4H);
2.23 (s, 6H); 6.83-7.26 (m, 8H)
Analysis (C19H26NS) C, H, N, S
Examplel2:4-N,N-Dimethylamino-1-(2-furyl~-4-(2-thien~l)-hex-
l-ene.
(Rl = 2-thienyl; R2 = C2H5; R3 = R4 =CH3; R5 = 2-furyl;
Q = -CH=CH~)
Starting material : the intermediate compound VI.12
Yield = 51 % melting point = 59-60C TLC : 0.50-0.55; S.C
IR (Nujol) : 2940-2900, 2840, 1470, 1450, 1380, 1150, 1110,
1060, 740, 730, 700 cm~1
NMR : 0.85 (t, 3H); 1.95 (q, 2H); 2.25 (s, 6H): 2.85 (m, 2H);
35 6.10-6.35 (m, 4H); 6.85-7.06 (m~ 2H); 7.23-7.32 (m, 2H)
Analysis (C16H2lNOS) C, H, N, O, S
Example 13 4-N.N-Dimeth~lamino-1 4-di(2-thienyl)-hex-1-ene.
(R1 = 2-thienyl ; R2 = C2H5 ; R3 = R4 = CH3 ; R5 = 2-thienyl ;
31 ~37~23
Q = -CH=CH-)
Starting matarial ~ the intermediate compound VI.13
Yield = 65 % melting point = 69-71C TLC : 0.60; 5.C
IR (Nujol) : 2820-2980, 1455, 1375, 1040, 995, 960, 850, 820,
720, 690 cm~l
NMR : 0.72 (t, 3H); 1~90 (q, 2H); 2.25 (s, 6H~ 2.80 (d, 2H);
6.08 (dt, lH); 6.60 (d, lH); 6.82-7.25 (m, 6H)
Analysis (C16H2lNS2) C, H, N, S
Example 14 : 4-N.N-Dimethylamino-4-f2-thienyl)-1-(3-thienvl)~
hex-l-ene.
(R1 = 2-thienyl; R2 - C2H5; R3 = R4 = CH3; R5 - 3-thienyl;
Q = -CH=CH-)
Starting material : the intermediate compound VI.14
Yield = 61 % melting point = 85.5-86C TLC : 0.40; S.M
IR (CC14) : 2970, 2930, 2860, 2820, 2780, 1550, 1250, 1225,
1010, 960 cm-~
NMR : 0.90 (t, 3H); 1.95 (q, 2H); 2.25 (s, 6H); 2.85 (d, 2H);
5.90-6.25 (m, lH); 6.60 (d, lH); 6.90-7~35 (m, 6H)
Analysis (C16H2lNS2) C, H, N, S
Example 15 : 4-N.N-Dimethylamino-1-phenyl-4-(3-thienyl)-hex-1-
ene.
(Rl - 3-thienyl; R2 = C2H5; R3 = R4 = CH3; R5 = C6H5;
Q = -CH=CH-)
Starting material : the intermediate compound VI.15
Yield ~ 45 ~ melting point = 91C TLC ~ 0.60-0.65; S.E
IR (KBr) : 3100, 3020, 2950, 2930, 2850, 2810, 2770, 1590,
1490, 1470, 1450, 1290, 1245, 1000, 962, 843, 780, 690,
665 cm l
NMR : 0.83 ~t, 3H); 1.92 (q,2H~; 2.20 (s, 6H); 2.75-3.00 (d,
2H); 6.00-6.65 (m, 2H); 6.90-7.45 (m, 8H)
Analysis (C18H23NS) C, H, N, S
Example 16: trans 1-L2-N.N-DimethYlamino-2-(3-thienyl)-butyll-
2-phenyl-cyalopropane.
(Rl = 3-thienyl; R2 = C2H5; R3 = R4 = CH3; R5 = C6H5;
Q = cyclopropane-1,2-diyl)
Starting mat~rial : the intermediate compound VI.16
Yield = 47 ~ boiling point/6,6 Pa = 140C TLC 0.30; S.B
IR (film) : 3050, 3010, 2960, 2930, 2860, 2810, 2770, 1600,
,, . .. . , : ~ : :
.
,
32 ~37~3
1492, 1455, 1087, ~028, 856, 838, 772, 7~5, 691, 668 cm~1
NMR : 0.~5-1.05 (m, 6H); 1.55 2.10 (m, sH); 2.2s (s, 6H); 6.90-
7.50 (m, 8~)
Analysis ~C19H26NS) C, H, N, S
Example 17 : 4-N N-Dimethylamino-l-L2-thienYl)-4-(3-thienyl)-
hex-l-ene.
(Rl = 3-thienyl; R2 = C2H5; R3 - R4 = CH3; R5 = ~-thienyl; Q =
-CH=CH-)
Starting material : the intermediate compound VI.17
Yield = 64 % melting point = 91-92C TLC : 0.50; S.E
IR (CC14) : 2960, 2920, 2810, 2775, 1545, 1245, 1000, 950 cm~
NMR : 0.85 (t, 3H); 1.95 (q, 2H) 2.2 (5, 6H); 2.8 (d, 2H):
5.9-6.25 (m, lH); 6.65 (d, lH); 6.9-7.35 (m, 6H)
Analysis (C16H21NS2) C, H, N, S
Example_18 : 4-N,N-Dimethylamino-1 4-di(3-thienylL-hex-l-ene.
(Rl = 3-thienyl; R2 = C2H5; R3 ~ R4 = CH3; R5 = 3-thienyl; Q =
-CH=CH-)
Starting material : the intermediate compound VI.18
Yield = 59 % melting point = 104-105C TLC : O.~o; S.E
IR (CC14) : 2970, 2930, 2870, 2820, 2780, 1550, 1255, 1225,
1020, 9~0, 775 cm~1
NMR : 0.85 (t, 3H); 1.95 (q, 2H); 2.2 (5, 6H); 2.85 (d, 2H);
5.9-6.6 (m, 2H); 7.05-7.40 (m, 6H)
Analysis (C16H21NS2 ? C, H, N, S
Exam~lel9:trans-1-(2-N N-pimethylamino-2-phenyl~butyl)-2~2
thienyl~-cyclopropane.
(Rl = C6H5; R2 = C2H5; R3 = R4 = CH3; R5 = 2-thienyl; Q =
cyclopropane-1,2-diyl)
Starting material : the intermediate compound VI.l9
Yield = 66 % TLC : 0.25; S.D.
IR (film) ~ 3060, 2980, 2960, 2820, 2780, 1450, 850, 760, 700
cm-l
NMR : 0.70-0.90 (m, 6H); 1.62-1.85 (m, lH); 1.91-2.0~ (m, 4H);
2.21 (s, 6H); 6.61 (t, lH); 6.81 (m, lH): 6.98 (d, lH); 7.21-
7.40 (m, 5H)
Analysis (C19H25NS) C, H, N, S
Example20 trans-1 r 2-N,N-Dimethvlamino-2-t2-thienYl)-butyll-
2-(2-thienyl)-cyclopro~ane.
.. . . .
.:
' ' ` `
33 ~37~Z3
(Rl = R5 = 2-thienyl; R~ = C2H5; R3 = R4 = CH3; Q =
cyclopropane-1,2 diyl)
Starting material : the intermediate compound VI.20
Yield = 45 % TLC : 0.30; S.D.
IR (film) : 3060, 2960, 2920, 2870, 2~60, 2810, 1660, 1530,
1450, 1235, 1040, 845, 820, 690 cm-1
NMR : 0.85-1.00 ~m, 6H); 1.71-2.10 (m, sH); 2.25 (s, 6H)
6.70-7.28 (m, 6H)
Analysis (C17H23NS2) C, H, N, S
Example21:trans~ 2-N,N-Dimethylamino-2-(3-thienyl?-butY11-
2-(2-furYl~-cyclopropane.
(Rl = 3-thienyl; R2 = C2H5; R3 = R4 = CH3; R5 = 2-furyl;
Q = cyclopropane-1,2-diyl)
Starting material : the intermediate compound VI.21
Yield = 26 % ~LC : 0.20; S.D.
IR (film) : 3060, 2960, 2930, 2865, 2810, 2765, 1595, 1510,
1375, 1250, 1090 cm-l
NMR : 0060-2.20 ~m, llH); 2.21 (s, 6H) ; 5.80-6.00 (m, lH);
6.20-6.40 (m, lH); 7.10-7.20 (m, 2H); 7.20-7.50 (m, 2H)
Analysis (C17H23NOS) C, H, N, O, S
Example22:trans-1-[2-N~N-DimethYlamino-2-(2-~uryl)-but~ 2-
(3-thienyl)-cyclop~r2Pane.
(Rl = 2-furyl; R2 = C2H5; R3 = R4 = CH3; R5 = 3-thienyl;
Q = cyclopropane-1,2-diyl)
Starting material : the intermediate compound VI.22
Yield = 30 % TLC : 0.30; S.D.
IR (film) : 3110, 3070, 2940, 2870, 2820, 2780, 1470, 1460,
1160, 1020, 775, 740 cm-l
NMR : 0.50-1.10 ~m, 6H); 1.30-2.10 (m, 5H); 2.20 (s, 6H)
6.10-6.20 (m, lH); 6.30-6.50 (m, lH); 6.60-6.80 (m, lH); 6.80-
6.90 (m, lH); 7.10-7.30 (m, lH); 7.40-7.50 (m, lH)
Analysis (C17H23NOS) C, H, N, O, S
The toxicological and pharmacological tests carried out
with the ethylamines o~ the invention, o~ the ~ormula (I),
described in Examples 1 to 22 above demonstrate their low
toxicity as well as valuable psychotropic properties which make
these compounds useful for the treatment of neuro-psychic
disorders.
,
~337~23
34
The study o~ the toxicity of the products of the
invention is carried out in the mouse by oral administration,
by approximate determination of kheir LD 50, which is the
lethal dose causing~ 50 % of deaths in the animals under the
conditions of the experiment. It is carried out on batches of
four male "Swiss" mice weighing about 20 g and made to fast on
the day before th~ test.
Each determination is carried out with four doses of
products corresponding respectively to an administration of
100, 300, 600 and 1000 mg of product, expressed in the foxm of
the base, per kg of animalO
It is found in this way that the products of the
invention in general have an acute toxicity of LD 50 greater
than or equal to 1,000 mg/kg. Exceptionally, some compounds
exhibit an LD 50 of about 600 mg/kg.
The psychotropic properties of the compounds were
determined by protection against convulsions induced by
picrotoxin in the mouse, the teæt being carried out in
accordance with a method based on that of Krall et al.,
"Epilepsiat', 1978, 19, p. 409-428.
The administration of picrotoxin causes a convulsive
crisis in the animal, characteri~ed by a myoclonic extension
syndrome followed by extension of the limbs, leading to the
death of the animal. Certain substance~, especially those which
act on the GABA/benzodiazepines/Cl-ionophore complex make it
possible to protect the animals against this convulsive crisis.
In practice, the study is carried out on batches of 10
male "Swiss" mice, weighing about 20 g, to which the product to
be studied is administered in agueous solution, either
intraperitoneally (i.p.) in a volume of 0.2 ml of solution per
animal, or orally (p.o) in a volume of ~-.0 ml of solution per
animal.
Thereafter, a picrotoxin solution is injected
intraperitoneally in an amount of 24 mg/kg, in a volume of
0.2 ml per animal, either 30 minutes after intraperitoneal
administration of the product or 60 minutes after oral
administration of the product. The dose of product injected
causes a clonic crisis which leads to the death of the
~03'71Z3
untreated animals. Under the test conditions, it is found that
the tonic extension phase is suppressed in the treated animals.
The results are expressed :
either in percentagas of animals protected against this
phase under the action of 50 mg/Xg of the compound studied,
administered i~p., or 100 mg~kg administered p.o.,
or a~ the ED50 for each of these methods of
administration, this being the effective dose of compound
tested, expressed in m~/kg, which protects 50 % of the animals
against this extension phase.
The significance of the results is generally indicated
as follows :
* result significant to p. c 0.05
** result significant to p. < o.o
~** result highly significant to p. < o.OOl.
The results of the study are reported in the table which
follows :
': :
36
Table: Resul1:B - inhibition of convulsions
.
Examplei.p. : % prot~ction p. o. : % protection
or ED50 or ED50
5-- -- _
70 % ** 90 % ***
2 70 % ** N.T.
10 3 70 % ** 70 % **
4 70 % ** 80 % **
6 70 ~ ** 80 % ***
7 90 % *** 100 % ***
8 80 96 *** 60 % *
20 9 50 % * NoT~
N.T. 80 % **
11 60 % * 50 % *
2 5 -- --- - _ . _ .
12 70 96 * 50 % *
. _ _ .
13 90 % *** 50 % *
3015 70 % * 100 % ***
16 100 % *** N . T .
17 90 % *** 80 % **
3 5
18 80 % *** 100 % ***
19 ED 50 = 42 ED 50 - 75
4022 ED 50 = 20 50 % *
N . T . = not tested
: -
.
~3~2~
37
These results show, for the studied products of theinventiorl and regardless of the administration route used, an
activity in the test for protection against convulsions induced
by picrotoxin in the mouse.
These pharmacological properties, coupled with the low toxicity
of the compounds of the invention, make it possible to envisage
their usefulness, in the form of medicaments, for preventive
and curative treatments o~ dis~urbances of a neurological type
and/or psychic type in general, such as, for example
convulsions, depressive s~ates, anxiety and/or psychotic
states.
When presented in pharmaceutical forms, the useful unit
doses are usually between 1 and 500 mg and more particularly
between 5 and 200 mg of product, depending on the nature and
gravity of the condition to be treatedO The daily therapeutic
doses can be divided into several administrations and are
generally between 5 and 2000 mg o~ product per day. In general,
a daily dosage of 5~ to 500 mg of product, divided into two to
four administrations, is satisfactory.
The products o~ the invention are administered to the
patients to be treated in the form of medicaments of
appropriate nature for the condition to be remedied. Depending
on circumstances, the medicinal preparations are, to quote
examples without implying any limitation, tablets, pills,
capsules, powders, solutions, suspensions, gels or
suppositories. These pharmaceutical forms are prepared from the
products in the form of the base or from their salts, and in
accordance with the methods usually employed in this industry.
Generally, in the solid medicinal forms, the active
principle represents from 5 to 90 % by weight of the total of
the final form, while the pharmaceutically suitable excipients
represent from 95 to 10 %. For the liquid forms, or for forms
which may be considered a liquid, the amount o~ active
principle is between 0.1 and 10 % by weight of the final ~orm,
while the pharmaceutically suitable excipients can represent
from 99.9 to 90 % by weight of this form.
The ~ormulation and the preparation of tablets is shown
by way of illustration.
' '
38 ~37~:3
Formulation
Compound of Example 10 lo.o to 50.0 mg
Polyvinylpyrrolidone 20.0 mg
Carboxymethyl-starch 8.0 mg
5 Magnesium stearate 2.0 mg
Colloidal silica 0.4 mg
Lactose sufficient to make up to 200.0 mg
Preparation
The active principle is mixed with the lactose and then
granulated with the polyvinylpyrrolidone in solution. The
particles are dried and sieved on a 1 mm mesh. The
carboxymethyl-starch is mixed with the colloidal silica and
then added to the granules. Thereafter the magnesium stearate
is thoroughly mixed in, and the mixture is then oompressed in
an amount of 200.0 mg per tablet.
'
. ~ .
: