Note: Descriptions are shown in the official language in which they were submitted.
~ 5
The invention relates to new sulphonylated pyrimidine-
carboxamides, to processes for their preparation, and to
their use as herbicides.
It is already known that certain he~erocyclic N-acylsul-
phonamides, for example, N-(2-methoxy-phenylsulphonyl)-
4,6-dimethyl-pyrimidine-2-carboxamide and N-(2-chloro-
phenylsulphonyl)-4,6-dimethoxy-pyrimidine-2-carboxamide,
have herbicidal properties (cf. EP-A 353,640).
However, the herbicidal activity of these compounds is
not always entirely satisfactory.
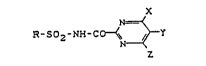
The new sulphonylated pyrimidinecarboxamides of the
general formula (I)
X
R-S02-NH-CO~;~Y t I )
in which
5 R represents in each case optionally substituted aryl,
aralkyl or heteroaryl,
X represents hydrogen, halogen, alkyl, halogenoalkyl,
alkoxyalkyl, alkoxy or halogenoalkoxy,
Y represents halogen, alkyl, halogenoalkyl,
Le A 27 532
' ' ' ' ' ' -- ' ' ~ '. '
,, - ~ :
2 ~
alkoxyalkyl, alkoxy or halogenoalkoxy and
Z represents hydrogen, halogen, alkyl, halogenoalkyl,
alkoxy ox halogenoalkoxy,
and salts of compounds of the formula (I),
have now been found.
The new sulphonylated pyrimidi:necarboxamides of the
general formula (I) are obtained
when
(a) pyrimidinecarboxamides of the general formula (II)
N==~
H2N-CO~ />--Y
N--~ (II)
in which
X, Y and Z have the abovementioned meanings
are reacted with sulphonyl halides or sulphonic
anhydrides of the general formula (III)
R-so2-Q (III)
in which
R has the abovementioned meaning and
Q represents fluorine, chlorine, bromine or the group
Le A 27 532 - 2 -
... _
' ' ` ' :
.
'`
2 0 ~ i ~ 3 ~3
-O-SO2-R where R has the abovementioned meaning,
if appropriate in the presence of an acid acceptor and if
appropriate in the presence of a diluent, or when
(b~ pyrimidinecarboxylic acids of the general formula
(IV) X
HOOC ~ /~-Y
N~ IV)
in which
X, Y and Z have the abovemantioned meanings,
or reactive derivatives of compounds of the formula
(IV),
are reacted with sulphonamides of the general
formula (V)
R-SO2-NH2 tv)
in which
R has the abovementioned meaning,
or with reactive derivatives of compounds of the
formula (V),
if appropriate in the presence of reaction auxili-
aries and if appropriate in the presence of
diluents,
Le A 27 532 - 3 _ -
.- : . : - :
. , . : - ~
~3~ ~q 3 ~,
and, if desired, the products obtained by processes
(a) or (b) are converted into salts by customary
methods.
The new sulphonylated pyrimidinecarboxamides of the
general formula (I) are distinguished by a powerful
herbicidal activity.
Surprisingly, the sulphonylated pyrimidinecarboxamides of
the formula (I~ according to the invention have a con-
siderably more powerful herbicidal action than the
heterocyclic N-acylsulphonamides which are known from the
prior art such as, for example, N-(2-methoxy-phenylsul-
phonyl)-4,6-dimethyl-pyrimidine-2-carboxamide and N-(2-
chloro-phenylsulphonyl)-4,6-dimethoxy-pyrimidine-2-
carboxamide.
The invention preferably relates to compounds of theformula (I~ in which
Rl
R represents the radical ~ where
R2
R1 and R2 are identical or different and represent
hydrogen, fluorine, chlorine, bromine,
iodine, cyano, nitro, carboxyl, Cl-C6-alkyl
(which is optionally substituted by
fluorine, chlorine, bromine, cyano, car-
boxyl,Cl-C4-alkoxycarbonyl,Cl-C4-alkylamino-
Le A 27 532 - 4 -
. . ~ .
- ,,'
.
.. : .
2 ~ ~ rgl i 3 ~
carbonyl, di-(Cl-C4-alkyl)-amino-carbonyl,
hydroxyl, Cl-C4-alkoxy, formyloxy, Cl-C4-
alkyl-carbonyloxy, C,-C 4 - alkoxy-
carbonyloxy, Cl-C4-alkylaminocarbonyloxy,
C1-C4-alkylthio, Cl-C4-alkylsulfinylt Cl-C4-
alkylsulphonyl, di-~C~-C4-alkyl)-amino-
sulphonyl, C3-C5-cycloalkyl or phenyl), or
represent C2-C6-alkenyl (which is option-
ally substituted by fluorine, chlorine,
bromine, cyano, Cl-C4-alkoxycarbonyl,
carboxyl or phenyl), or represent C2-C6-
alkinyl (which is optionally substituted
by fluorine, chlorine, bromine, cyano, Cl-
C4-alkoxy-carbonyl,carboxyl orphenyl), or
represent Cl-C4-alkoxy (which is optionally
substitutedby fluorine,chlorine,bromine,
cyano, carboxyl, Cl-C4-alkoxy-carbonyl,
C~-C4-alkoxy, Cl-C4-alkylthio, Cl-C4-alkyl-
sulphinyl or C1-C4-alkylsulphonyl), or
represent Cl-C4-alkylthio (which is option-
ally substituted by fluorine, chlorine,
bromine, cyano, carboxyl, C1-C4-alkoxy-
carbonyl, Cl-C4-alkylthio, C1-C4-alkyl-
sulphinyl or C1-C4-alkylsulphonyl), or
represent C3-C6-alkenyloxy (which is
optionally substituted by fluorine,
chlorine, bromine, cyano or Cl-C4-alkoxy-
carbonyl)l or represent C2-C6-alkenylthio
~which is optionally substituted by . :
fluorine, chlorine, bromine, cyano, nitro, ~ --
:
.
Le A 27 532 - 5 ~ ;~
.`
, ' ~ ~' '
2 $ 3 r~ 3
Cl-C3-alkylthio or Cl-C4-alkoxycarbonyl),
C3-C6-alkinyloxy, C3-C6-alkinylthio, or
represent the radical -S(o)p-R3 where
p represents numbers 1 or 2 and
R3 represents Cl-C4--alkyl (which is option-
ally substituteclby fluorine, chlorine,
bromine, cyano or Cl-C4-alkoxy-car-
bonyl), C3-C6-alkenyl, C3-C6-alkinyl, Cl-
C4-alkoxy, Cl-C4-alkoxy-Cl-C4-alkylamino,
Cl-C4-alXylamino, di-(Cl-C4-alkyl)-amino,
or represents the radical -NHoR4 where
R4 represents Cl-C6-alkyl (which is
optionally substituted by fluorine,
chlorine, cyano, Cl-C4-alkoxy, Cl-C4-
alkylthio, Cl-C4-alkylsulphinyl, Cl-
C4-alkylsulphonyl, Cl-C4-alkyl-
carbonyl, Cl-C4-alkoxy-carbonyl, Cl-
C4-alkylamino-carbonyl or di-(Cl-C4-
alkyl)-amino-carbonyl)l or repre-
sents C3-C6-alkenyl (which is option-
ally substituted by fluorine,
chlorine or bromine), C3-C6-alkinyl,
C3-C6-cycloalkyl, C3-C6-cycloalkyl-
Cl-C2-alkyl, phenyl-Cl-C2-alkyl (which
isoptionallysubstitutedbyfluorine,
chlorine, nitro, cyano, Cl-C4-alkyl,
Cl-C4-alkoxy or C~-C4-alkoxy-
Le A 2~ 532 - 6 ~
'
` ' ' `
.
'~
2 ~ 3 ~
carbonyl), or represents benzhydryl,
or represents phenyl (which is
optionally substituted by fluorine,
chlorine, nitro, cyano, Cl-C4-alkyl,
trifluoromethyl, Cl-C4-alkoxy, Cl-C2-
fluoroalkoxy, Cl-C4-alkylthio,
trifluoromethylthio or Cl-C4-alkoxy-
carbonyl),
Rl and R2 furthermore represent phenyl or phenoxy,
or represent Cl-C4-alkylcarbonylamino,
Cl-C4-alkoxy-carbonylamino, Cl-C4-alkyl-
amino-carbonylamino, di-(Cl-C4-alkyl)amino-
carbonylamino or represent the radical
-Co-R5 where
R5 represents Cl-C6-alkyl, C1-C6-alkoxy
(which is optionally substituted by
fluorine, chlorine, methoxy or ethoxy),
C3-C8-cycloalkoxy, C3-C6-alkenyloxy, Cl-
C4-alkylkthio, amino, Cl-C4-alkylamino,
Cl-C4-alkoxyamino, C~-C4-alkoxy-Cl-C4-
alkyl-amino or di-(Cl-C4-alkyl)-amino :
(which are optionally substituted by
fluorine and/or chlorine~,
Rl and R2 furthermore represent Cl-C4-alkylsulphonyl
oxy,di-(Cl-C4-alkyl)-aminosulphonylamino,
or represent the radical -CH=N-R6 where
Le A 27 532 _ 7 _
.. .. . . .
2 ~ 3 r~
R6 represents Cl-C6-alkyl which is option-
ally substituted by fluorine, chlorine,
cyano, carboxyl, Cl-C4-alkoxy, Cl-C4-
alkylthio, Cl-C4-alkylsulphinyl or Cl-
C4-alkylsulphonyl, or represents benzyl
which is optionally substituted by
fluorine or chlorine, or represents C3-
C6-alkenyl or C3-C6-alkinyl, each of
which is optionally substituted by
fluorine or chlorine, or represents
phenyl which is optionally substituted
by fluorine, chlorine, bromine, Cl-C4-
alkyl, Cl-C4-alkoxy, trifluoromethyl,
trifluoromethoxy or trifluoromethyl-
thio, or represents Cl-C6-alkoxy, C3-C6-
alkenoxy, C3-C6-alkinoxy or benzyloxy
each of which is optionally substituted
by fluorine and/or chlorine, or repre-
sents amino, Cl-C4-alkylamino, di-(C1-
C4-alkyl~-amino, phenylamino, Cl-C4-
alkyl-carbonyl-amino, Cl-C4-alkoxy-
carbonylamino, Cl-C4-alkyl-sulphonyl-
amino, or represents phenylsulphonyl-
amino which is optionally substituted
by fluorine, chlorine, bromine or
methyl,
where furthermore
Le A 27 532 - 8 -
,
2~3~
R represents the radical -CH ~ where
R7 represents hydrogen or Cl-C4-alkyl,
R8 and R9 are identical or different and represent
hydrogen, fluorine, chlorine, bromine,
nitro, cyano, carboxyl, Cl-C4-alkyl (which
is optionally substituted by fluorine
and/or chlorine), Cl~C4-alkoxy (which is
optionally substituted by fluorine and/or
chlorine), Cl-C4-alkoxy-carbonyl, Cl-C4-
alkylsulphonyl or di-(Cl-C4-alkyl)-amino-
sulphonyl; where furthermore
R represents the radical R10 ~ R11 where
Rl~ and R~lare identical or different and represent
hydrogen, fluorine, chlorine, bromine,
nitro, cyano, Cl-C4-alkyl ~which is option-
ally substituted by fluorine and/or
chlorine) or Cl-C4-alkoxy (which is option-
ally substituted by fluorine and/or
chlorine); where furthermore
Le A 27 532 - 9 -
. . . ~
' ' ~ ' ~ '
~3 -J~3 ~'J
R13
R represents the radical ~ where
~N~
R 1 2
Rl2 and R13are identical or different and represent
hydrogen, fluori:ne, chlorine, bromine,
nitro, cyano, Cl-C4-alkyl (which is option-
ally substitutecl by fluorine and/or
chlorine) or C2-C4-alkenyl (which is
optionally substitu~ed by fluorine and/or
chlorine), Cl-C4-alkoxy (which is
optionally substituted by fluorine and/or
chlorine)~ or represent Cl-C4-alkylthio,
Cl-C4-alkylsulphinyl or Cl-C4-alkylsulphonyl
(which are optionally substituted by
fluorine and/or chlorine), and represent
di-(Cl-C4-alkyl)-aminosulphonyl, Cl~C4-
alkoxy-carbonyl, dimethylaminocarbonyl or
dioxolanyl; where furthermore
R represents the radical R1 4~R15 where
Rl4 and R15are identical or different and represent
hydrogen, fluorine, chlorine, bromine,
Cl C4-alkyl (which is optionally substitu-
ted by fluorine and/or bromine), C1-C4-
alkoxy (which is optionally substi~uted by
fluorine and/or chlorine), or represent
Le A 27 532 - 10 - :
- ~ :
.
.
- : - . . .
. .. . ~, . - : -
.
2~r~3~i
C1-C4-alkylthio, Cl-C4-alkylsulphinyl or
C1-C4-alkylsulphonyl (which are optionally
substituted by fluorine and/or chlorine),
or represents di-tCl-C~-alkyl)-amino-
sulphonyl; where furthermore
R16
R represents the radical ~ ~ ~1 ? where .
Rl5 and R17are identical or different and represent
hydroqen, fluorine, c~lorine, bromine,
cyano, nitro, Cl-C4-alkyl (which is option-
ally substituted by fluorine, chlorine,
Cl-C4-alkoxy and~or Cl-C4-halogenoalkoxy),
Cl-C4-alkoxy (which is optionally sub-
stituted by fluorine and/or chlorine),
Cl-C4-alkylthio, Cl-C4-alkylsulphinyl or
Cl-C4-alkylsulphonyl (which is optional.ly
substituted by fluorine and/or chlorine~,
di-(Cl-C4-alkyl)-amino-sulphonyl or C,-C4-
alkoxy-carbonyl, and
Al represents oxygen, sulphur or the group N-Z1,
where
zl represents hydrogen, Cl-C4-alkyl (which is
optionally substituted by fluorin~,
chlorine, bromine or cyano), C3-C6-cyclo-
alkyl, benzyl, phenyl ~which is optionally:
Le A 2~ 532 - 11 -
:. ' ' - ~ . ....................... ' : .
- :
2~37~
substituted by fluorine, chlorine, bromine
or nitro), Cl-C4-alkylcarbonyl, C1-C4-
alkoxy-carbonyl or di-(Cl-C4-alkyl)-amino-
carbonyl; where furthermore
R18
R represents the radical ~ ~ ~ Rl9 where
Rl8 and Rl9are identical or different and represent
hydrogen, C,-C4-alkyl, halogen, Cl-C4-
alkoxycarbonyl, Cl-C4-alkoxy or Cl-C4-
halogenoalkoxy,
yl represents sulphur or the group N-R20 where
R20 represents hydrogen or Cl-C4-alkyl; where
furthermore
R represents the radical ~ . where
~7 RZ2
R21
R2l represents hydrogen, Cl-C4-alkyl, phenyl or
(iso)quinolinyl,
R22 represen~s hydrogen, halogen, cyano, nitro, Cl C4-
alkyl (which is optionally substituted by
Le A 27 532 - 12 -
.
: --
~. . : . . , :
2 ~ 3 ~
fluorine and/or chlorine), Cl-C4-alkoxy (which is
optionally subs~ituted by fluorine and/or chlo-
rine), dioxolanyl or Cl-L'4-alkoxy-carbonyl and
R23 represents hydrogen, halogen or Cl-C4-alXyl; where
S furthermore
R represents the radical ~ ~ R24 where
N~S
R24 represents hydrogen, halogen, Cl-C4-alkyl or C1-C4-
alko~y-carbonyl; where furthermore
R represents the radical ~ 2 where
R260~ N-R
R25 represents Cl-C4-alkyl and
R26 represents Cl-C4-alkyl, where furthermore
~}
R represents the radical ~ ~ where
~27
R27 represents hydrogen or methyl;
in which furthermore
Le A 27 532 - 13 -
, :. :. - .:
$
X represents hydrogen, halogen, Cl-C4-alkyl, Cl-C4-
halogenoalkyl, C1-C2-alkoxy-C1-C2-alkYl, c1-C4-~lkoxy
or C1-C4-halogenoalkoxy,
Y represents halogen, Cl-C4-alkyl, Cl-C4-halogenoalkyl,
C~-C2-alkoxy-Cl-C2-alkyl, Cl--C4-alkoxy, or Cl-C4-halo-
genoalkoxy and
Z represents hydrogen, halogen, Cl-C4-alkyl, Cl-C4-
halogenoalkyl, Cl-C4-alkoxy or Cl-C4-halogenoalkoxy.
-The invention furthermore preferably relates to salts of
compounds of the formula (I)
~) with protonic acids such as, for example, hydro-
chloric acid, sulphuric acid, phosphoric acid,
methanesulphonic acid, benzene- or p-toluenesulpho-
nic acid, or naphthalene mono- or -di-sulphonic
acids, or
~) with bases such as, for example, the hydroxides,
hydrides, amides or carbonates of sodium, potassium
or calcium, or sodium Cl-C4-alkanolates or potassium
C1-C4 alkanolates, ammonia, C1-C4-alkylamines, di-
(C1-C4-alkyl)-amines or tri-(C1-C4-alkyl)-amines.
In particular, the invention relates to compounds of the
formula (I) in which
Le A 27 532 - 14 -
2 ~ 3 ~
R represents the radical ~ where
Rl represents hydrogen, fluurine, chlorine, bromine,
cyano, nitro, carbogyl, methyl, trifluoromethyl,
methoxyr ethoxy, difluoromethoxy, trifluorometh-
oxy, Cl-C3-alXylthio, Cl-C3-alkylsulphinyl, Cl-C3-
alkylsulphonyl, dimethylaminosulphonyl diethyl-
aminosulphonyl, N-methoxy-N-methylaminosulphonyl,
phenyl, phenoxy or Cl-C3-alkoxy-carbonyl and
R2 represents hydrogen, fluorine, chlorine, bromine,
methyl, trifluoromethyl or methoxy; where fur-
thermore
R represents the radical -IH ~ where
R7 R8
R7 represents hydrogen,
Ra represents fluorine, chlorine, bromine, cyano,
carboxyl, methyl, trifluoromethyl, methoxy,
difluoromethoxy, trifluoromethoxy, ethoxy,
methoxycarbonyl, ethoxycarbonyl, methylsulphonyl
or dimethylaminosulphonyl and
R9 represents hydrogen, fluorine or chlorine; where
Le A 27 532 - 15 -
.
furtherm~re
R13
R represents the radical R12 ~ where
Rl2 represents dimethylaminocarbonyl or c,-C4-alkyl~
sulphonyl and
Rl3 represents hydrogen, fluorine or chlorine;
where ~urthermore
~ _,
R represencs the radic~l R280_ ~ s~ where
R23 represents methyl or ethyl, or
R R represents the radical
RZ 90 - c
~ where
N-N
~3
R29 represents methyl or ethyl, :~
in which furthermore
X represents hydrogen, fluorine, chlorine, bromine,
Le A 27 532 - 16 -
2~7~ 3~i
methyl, ethyl, trifluoromethyl, methoxymethyl,
methoxy, ethoxy or difluoromethoxy,
Y represent~ fluorine, chlorine, bromine, methyl,
ethyl, fluoromethyl, chloromethyl, difluoromethyl,
dichloromethyl, trifluoromethyl, trichloromethyl,
chlorodifluoromethyl,fluorodichloromethyl,methoxy,
ethoxy, difluoromethoxy or trifluoromethoxy and
Z xepresents hydrogen, fluorine, chlorine, bromine,
methyl, ethyl, trifluoromethyl, methoxy, ethoxy or
difluoromethoxy.
Very particularly preferred are compounds of the formula
(I) are those in which
R has the meaning given above as being particularly
preferred,
X represents hydrogen,
Y represents methoxy or methyl and
Z represents hydrogen.
If, for example, 2-fluoro-benzenesulphonyl chloride and
5-methoxy-pyrimidine-2-carboxamide are used as starting
substances in process (a) according to the invention, the
course of the reaction can be outlined by the following
equation;
e A 27 532 - 17 -
'~
3 ~1
~52 Cl , N CH3
/~< N =~
-HCl ~S02-NH-CO--~ ~OCH3
If, for example, 2-trifluoromethoxy-benzylsulphonamide
and methyl 4,5,6-trimethyl-pyrLmidine-2-carboxylate are
used as starting substances in process (b) according to
the invention, the cours~ of the reaction can be outlined
by the following equation:
OCF3 ~ CH3
-502-N~2 t 113C-O-CO~ ~CH~
CH3
OCF3 CH3
-HOCH3 ~H2-S02-NH-CO~H3
Formula tII) provides a general definition of the pyrimi-
dine carboxamides to be used a~ starting substances in
process (a) according to the invention for the
preparation of compounds of the formula (I).
In formula (II), X, Y and Z preferably, or in particular,
have those meanings which have already bPen mentioned
above in connection with the description of the compounds
of the formula (I) according to the invention as being
Le A 27 532 - 18 -
~- .
3 1:~
preferred, or particularly preferred, for ~, Y and Z.
Examples of the ~tarting substances of the formula (II)
which may be mentioned are:
5-fluoro-, 5-chloro-, 5-bromo-, S-methyl-, 5-ethyl-,
5-methoxy- and 5-ethoxy-pyrimidine-2-carboxamide, and
5-fluoro-4,6-dimethyl-, 5-chloro-4,6-dime~hyl-, 5-bromo-
4,6-dimethyl- and 4,5,6-trimethyl-pyrimidine-2-carbox-
amide.
The starting substances of the formula (II) are known
and/or can be prepared by processes known per se ~cf.
British Patent Specification 777,465; Compt. Rend. 248
(1959), 250-252 - cited in Chem. Abstracts 53, 18049i;
Ann. Chim. (Paris) 5 (1960), 351-379 - cited in Chem.
Abstracts 56, 5961g; Collect. C~ech. Chem. Commun. 37
(1972), 1721-1733 - cited in Chem. Abstracts 77, 101522s;
Org. React. (Tartu) 20 (1983), 85-95 - cited in Chem.
Abstracts 99, 157694w).
Formula (III) provides a general definition of the
sulphonyl halides or sulphonic anhydrides furthermore to
be used as starting substances in process (a) according
to the invention for the prepara~ion of the new compounds
of the formula (I). In formula (III), R preferably, or in
particular, has the meaning which has been mentioned
above in connection with the description of the compounds
of the formula (I) according to the invention as being
preferred, or particularly preferred, for R, and Q
preferably represents chlorine.
Le A 2? 532 - 19 -
:
Examples of starting substances of the formula (III)
which may be mentioned are:
benzenesulphonyl chloride, 2-chloro-, 3-chloro-,
4-chloro-, 2,5-dichloro-, 2-fluoxo-, 4-fluoro-, 2-bromo-,
4-bromo-, 2-cyano-, 2-nitro-, 4-nitro-, 2-methyl-,
4-methyl-, 2-chloroemthyl-, 2-tri~luoromethyl-, 2-meth-
oxy-, 4-methoxy-, 2-methylthio-, 2-trifluoromethylthio-,
2-difluoromethylthio_,2-cyclopropyloxycarbonyl-,2-phen-
oxy-, 2-difluoromethoxy-, 2-trifluorome~hoxy-, 2-(2-
chloroethoxy)-~ 2-methylthiomethyl-, 2-dimethylamino-
sulphonyl-, 2-phenyl-, 2-methoxycarbonyl-, 2-ethoxy-
carbonyl-, 2-dimethylaminocarbonyl-lphonyl-,
carbonyl-ben~enesulphonyl chloride and (2-chloro-phenyl)-,
(2-cyano-phenyl)-, (2-methoxycarbonyl-phenyl)- and
(2-trifluoromethoxy-phenyl)-methanesulphonyl chloride,
2-chloro-6-methyl-benzenesulphonyl chloride and 2,6-
dichloro-benzenesulphonyl chloride.
The sulphonyl halides or sulphonic anhydrides of the
formula (III) are known and/or can be prepared by pro-
cesses known per se (cf. J. Org. Chem. 33 (1968), 2104;
J. Org. Chem. 25 (1960), 1824; DE-AS (Germar Published
Specification) 2,308,262; EP-OS (European Published
Specifications) 23,140, 23,141, 23,422, 35,893, 48,143,
51,466, 64,322, 70,041, 44,808 and 44,809; VS Patent
Specifications 2,929,820, 4,282,242; 4,348,220 and
4,372,778, and Angew. Chem. 93 (1981), 151).
Process (a) according ~o the invention for the prepara-
tionof the new compounds of the formula (I) is preferably
Le A 27 532 - 20 -
- .
. ~
' ,. ~ '- -.. ,: , , .
'
carried out using diluents. Diluents which are suitable
for this purpose are virtually all inert organic sol-
Yents. These preferably include aliphatic and aromatic,
optionally halogenated hydrocarbons such as pentane,
hexane, heptane, cyclohexane, petroleum ether, benzine,
ligroin, benzene, toluene, xylene, methylene chloride,
ethylene chloride, chloroform, carbon tetrachloride,
chlorobenzene and o-dichlorobenzene, ethers such as
diethyl ether and dibutyl ether, glycol dimethyl ether
and diglycol dimethyl ether, tetrahydrofuran and dioxane,
ketones such as acetone, methyl ethyl ketone, methyl
isopropyl ketone and methyl isobutyl ketone, esters such
as methyl acetate and ethyl acetate, nitriles such as,
for example, acetonitrile and propionitrile, amides such
as, for example, dimethylformamide, dimethylacetamide and
N-methyl-pyrrolidone, and also dimethyl sulphoxide,
tetramethylene sulphone and hexamethylphosphoric triamide
and pyridine.
Acid acceptors which can be employed in process (a)
according to the invention are all acid-binding agents
which can customarily be employed for reactions of this
type. The following are preferably suitable: alkali metal
hydroxides such as, for example, sodium hydroxide and
potassium hydroxide, alkaline earth metal hydroxides such
as, for example, calcium hydroxide, alkali metal hydrides
such as, for example, sodium hydride and potassium
hydride, alkaline earth metal hydrides such as, for
example, calcium hydride, alkali metal carbonates and
alkali metal alcoholates such as sodium carbonate,
Le A 27 532 - 21 -
3 !~i
potassium carbonate, sodium tert.-butylate, potassium
tert.-butylate, furthermore aliphatic, aromatic or
heterocyclic amines, for example triethylaminel tri-
methylamine, dimethylaniline, dimethylbenzylamine,
pyridine, picoline, 1,5-diazabicyclo-[4.3.0~-non-5-ene
(DBN), 1,8-diazabicyclo-[5.4.0]-undec-7-ene (D~U) and
1,4-diazabicyclo-[2.2.2]-octane (DABCO).
When carrying out process (a) according to the invention,
the reaction temperatures can be varied within a substan-
tial range. In general, the process is carried out at
temperatures between -20C and ~100C, preferably at
temperatures between O~C and +80C.
For carrying out process (a) according to the invention,
between 1 and 5 moles, preferably between 1 and 3 moles,
of sulphonyl halide or sulphonic ~nhydride of the formula
(III) are generally employed per mole of pyrimidine-
carboxamide of the formula (II).
The reactants can be combined in any desired sequence.
In a preferred embodiment of process (a) according to the
invention, a starting compound of the formula (II) and an
acid acceptor are stirred with a diluent, and a starting
compound of the formula (III) is slowly metered into this
mixture (if appropriate in portions). The reaction
mixture is then stirred until the reaction is complete.
Working-up can be carried out by customary methods (cf.
the Preparation Examples).
Le A 27 532 - 22 -
'~
~3~ 3
Formula (IV) provides a general definition of the pyrimi-
dinecarboxylic acids to be used as starting substances in
process (b) according to the invention for the prepara-
tion of compounds of the formula (I).
In formula (IV), X, Y and Z preferably, or in particular,
ha~e those meanings which have already been mentioned
above in connection with the description of the compounds
of the formula (I) according to the invention as being
preferred, or as being particularly preferred, for X, Y
and Z.
Examples of the starting substances of the formula (IV)
which may be mentioned are:
5-fluoro-, 5-chloro-, 5-bromo-, 5-methyl-, 5-ethyl-,
5-methoxy- and 5-ethoxy-pyrimidine-2-carboxylic acid, and
also 5-fluoro-4,6-dimethyl-, 5-chloro-4,6-dimethyl-,
5-bromo-4,6-dimethyl- and 4,5,6-trimethyl-pyrimidine-2-
carboxylic acid.
The starting substances of the formula (IV) are known
and/or can be prepared by process known per se (cf.
British Patent Specification 777,465; Compt. Rend~ 248
(1959), 250-252 - cited in Chem. Abstracts 53, 18049i;
Ann. Chim. (Paris) 5 (1960), 351-379 - cited in Chem.
Abstracts 56, 5961g; Collect. Czech. Chem. Commun. 37
(1972), 1721-1733 - cited in Chem. Abstracts 77, 101522s;
Org. React. (Tartu) 20 (1983), 85-95 - cited in Chem.
Abstracts 99, 157694w; Chem. Pharm. Bull. 28 (1980),
1408-1414 - cited in Chem. Abstracts 94, 47251p).
Le A 27 532 - 23 -
2 ~ . 3 ~
In process (b) according to the ;Lnvention, the carboxylic
acid chlorides or alkyl esters (preferably methyl esters
or ethyl esters), aralkyl esters (preferably benzyl
ester) or aryl esters (preferably phenyl esters, option-
ally substituted in the phenyl group by cyano, nitro,chlorine, fluorine, bromine and/or methyl) which are
derived from the pyrLmidinecarboxylic acids of the
formula (IV) are optionally employed as starting substan-
ces ("reactive derivatives") in place of these pyrimi-
dinecarboxylic acids.
The carboxylic acid chlorides which are derived from th~compounds of the formula ~IV) are obtained from the
corresponding carboxylic acids by customary methods, for
example by rea~tion with acid chlorides which are custo-
marily used as ~chlorination agents" such as, forexample, phosgene, oxalyl chloride or thionyl chloride,
if appropriate in the presence of reaction auxiliaries
such as, for example, pyridine, and if appropriate in the
presence of diluents such as, for example, chloroform or
tetrachloromethane, at temperatures between 0C and
100C.
The esters, which are furthermore mentioned as reactive
derivatives of the compounds of the formula (IV), can be
obtained from the corresponding carboxylic acid chlorides
and suitable alcohols or phenol~ using customary methods,
in general by reaction in the presence of an acid accep-
tor such as, for example, triethylamine or pyridine, and
in the presence of a diluent such as, for example,
Le A 27 532 - 24 -
2 ~ 3 ii.~
acetonitrile, at ~emperatures between 0C and 100C.
However, the abovementioned esters can also be obtained
directly from the carboxylic acids of the formula (IV) in
the presence of condensation auxiliaries such as, for
example, dicyclohexylcarbodiimicle, if appropriate in the
presence of reaction auxiliaries such as, for example,
4-dimethylamino-pyridine, and if appropriate in the
presence of diluents such as, for example, methylene
chloride or chloroform, at temperatures between 0C and
100C.
Formula (V) provides a general definition of the sul-
phonamides furthermore to be used as starting substances
in process (b) according to the invention for the pre-
paration of compounds of the formula (I).
In formula (V) R preferably, or in particular, has the
meaning which has already been mentioned above in connec-
tion with the description of the compounds of the formula
(I) according to the invention as being preferred, or as
being particularly preferred, for R.
Examples of the starting substances of the formula (V)
which may be mentioned are:
benzenesulphonamide, 2-chloro-, 3-chloro-. 4-chloro-,
2,5-chloro-, 2-fluoro-, 4-fluoro-, 2-bromo-, 4-bromo,
2-cyano-, 2-nitro-, 4-nitro-, 2-methyl-, 4-methyl-,
2-chloromethyl-, 2-trifluoromethyl-, 2-methoxy-,
4-methoxy-, 2-methylthio-, 2-trifluoromethylthio-,
Le A 27 532 - 25 -
,
2 ~ ?~
2-difluoromethylthio-, 2-cyclopropyloxycarbonyl-,
2-phenoxy-, 2-difluoromethoxy-, 2-~rifluoromethoxy-,
2-(2-chloroethoxy)-, 2-methylt!hiomethyl-, 2-dimethyl~
aminosulphonyl-, 2-phenyl-, 2-mel;hoxycarbonyl-, 2-ethoxy-
carbonyl-, 2-dimethylaminocarbonyl- and 2-diethylamino-
carbonyl-benzenesulphonamide, and also (2-chloro-phenyl)-,
(2-cyano-phenyl)-, (2-methoxycarbonyl-phenyl)- and
(2-trifluoromethoxy-phenyl)--methanesulphonamide,
2-chloro-6-methyl-benzenesulphonyl chloride and 2,6-
dichloro-benzenesulphonamide.
The sulphonamides of the formula (V) are known and/or can
be prepared by processes known per se (cf. J. Org. Chem.
33 (1968), 2104; J. Org. Chem. 25 (1960), 1824; DE-AS
(German Published Specification) 2,308,262; EP-A 23,140,
23,141, 23,422, 35,893, 48,143, 51,466, 64,322, 70,041,
44,808 and 44,809; US Patent Specifications 2,929,820,
4,282,242; 4,348,220 and 4,372,778, and Angew. Chem. 93
(1981), 151).
In process (b) accordi.ny to the invention, the sulphonyl
isocyanates which are derived from the sulphon2mides of
the formula (V) ("reactive derivatives") are optionally
employed as starting substances in place of these sul-
phonamides. These compounds are known and/or can be
prepared by processes known per se (cf. J. Org. Chem. 31
(1966), 2658-2661; EP-A 7,687; EP-A 46,626; EP-A 21,641;
EP-A 23,140; EP-A 23,141; EP-A 70,041; EP-A 23,422;
EP-A 64,322; EP-A 34,431; EP-A 35,893; EP-A 51,466;
EP-A 44,808; EP-A 173,312; DE-OS (German Published
Le_A 27 532 - 26 -
: .. . . ..
-
: '
31l~
Specification~ 3,132,944; EP-A ~7,780; EP-A 271,780~.
Process (b) according to the invention for the prepara-
tion of the new compounds of the formula (I) is pre-
ferably carried ou~ using diluents. Diluents which are
suitable for this purpose are preferably the same
diluents which are mentioned above in the case of process
(a) according to the invention.
If appropriate, process (b) according to the invention~is
car~ied out in the presence of a reaction auxiliary.
Suitable reaction auxiliaries for this purpose are
compounds with which carboxylic acids are converted into
reactive intermediates which are then reacted in situ
with nucleophilic compounds such as, for example, the
sulphonamides of the formula (V), to give corresponding
carboxylic acid derivatives. Examples of reaction auxili-
aries of this type which may be mentioned are carbonyl
diimidazole and 2-chloro-1-methyl-pyridinium iodide.
Other suitable reaction auxiliaries for the reaction of
the esters and carboxylic acid chlorides mentioned as
reactive derivatives of compounds of the formula (IV)
with sulphonamides of the formula (V) are acid acceptors
as they are mentioned above in the case of process (a)
according to the invention.
When carrying out process (b) according to the invention,
the reaction temperatures can be varied within a substan-
tial range. In general, the process is carried out at
Le A 27 532 - 27 -
:- ' .' . " : . :
temperatures between O~C and 150~, preferably at tem-
peratures between 10C and 100C.
Process (b) according to the invention is generally
carried out under atmospheric pressure. However, it is
also possible to carry out the process under increased or
reduced pressure.
For carrying out process (b) according to the invention,
the starting substances required in each case are
generally employed in approximately equimolar amounts.
However, it is also possible to use one of the two
components employed in each case in a larger excess. In
general, the reactions are carried out in a suitable
diluent, if appropriate in the presence of a reaction
auxiliary, and the reaction mixture is stirred for
several hours at the particular temperature required.
Working-up in process (b~ according to the invention is
carried out in each case by customary methods (cf. the
Preparation Examples).
If appropriate, salts can be prepared from the compounds
of the general formula (I) according to the invention.
Such salts are obtained in a simple manner by customary
salt formation methods, for example by dissolving or
dispersing a compound of the formula (I) in a suitable
solvent such as, for example, water, methanol, ethanol,
methylene chloride or acetone, and adding a suitable acid
or base. The salts can then be isolated by concentration
or filtration with suction, if appropriate after pro-
Le A 27 532 - 28 -
- - - ~ ' , :
- ~ .
-: ' ':
. -
.
2 ~
longed stirring.
The active compounds according to the invention can beused as defoliants, desiccants, agents for destroying
broad-leaved plants and, especially, as weed-killers. By
weeds, in the broadest sense, ~here are ~o be understood
all plants which grow in locations where they are
undesired. Whether the substances according to the inven-
tion act as total or selective herbicides depends essen-
tially on the ~mount used.
The active compounds according to the invention can be
used, for example, in connection with the following
plants:
Dicotyledon weeds of the genera: Sinapis, Lepidium,
Galium, Stellaria, Matricaria, Anthemis, Galinsoga,
Chenopodium, Urtica, Senecio, Amaranthus, Portulaca,
Xanthium, Convolvulus, Ipomoea, Polygonum, Sesbania,
Ambrosia, Cirsium, Carduus, Sonchus, Solanum, Rorippa,
Rotala, Lindernia, ~amium, Veronica, Abutilon, Emex,
Datura, Viola, Galeopsis, Papaver, Centaurea, Trifolium,
Ranunculus and Taraxacum.
Dicotyledon cultures of the qenera: Gossypium, Glycine,
Beta, Daucusl Phaseolus, Pisum, Solanum, Linum, Ipomoea,
Vicia, Nicotiana, Lycopersicon, Arachis, Brassica,
Lactuca, Cucumis and Cucurbita.
Monocotyledon weeds of the genera: Echinochloa, Setaria,
Le A 27 532 - 29 -
2 ~
Panicum, Digitaria, PhleumJ Poa, Festuca, Eleusine,
Brachiaria, Lolium, Bromus, A~ena, Cyperus, Sorghum,
Agropyron, Cynodon, Monochoria, Fimbristylis, Sagittaria,
Eleocharis, Scirpus, Paspalum, Ischaemum, Sphenoclea,
Dactyloctenium, Agrostis, Alopecurus and Apera.
Monocot~ledon cultures of the qenera: Oryza, Zea,
Triticum~ Hordeum, Avena, Secale, Sorghum, Panicum,
Saccharum, Ananas, Asparagus and Allium.
However, the use of th~ active compounds according to the
invention is in no way restricted to these genera, but
also extends in the same manner to other plants.
The compounds are suitable, depending on the concentra-
tion, for the- total combating of weeds, for example on
industrial terrain and rail tracks, and on paths and
squares with or without tree plantings. Equally, the
compounds can be employed ~or combating weeds in peren-
nial cultures, for example afforestations, decorative
tree plantings, orchards, vineyards, citrus groves, nut
orchards, banana plantations, coffee plantations, tea
plantations, rubber plantations, oil palm plantations,
cocoa plantations, soft fruit plantings and hopfields,
on lawns, turf and pasture-land, and for the selective
combating of weeds in annual cultures.
The compounds according to the invention are very suit-
able for selectively combating monocotyledon and dicoty-
ledon weeds in monocotyledon and diootyledon crops, both
Le A 27 532 - 30 -
-. : :-
2 ~ 3 ~
pre- and post-emergence.
The active compounds can be converted into the customary
formulations, such as solutio:ns, emulsions, wettable
powders, suspensions, powders, dusting agents, pastes,
soluble powders, granules, sus;pension-emulsion concen-
trates, natural and synthetic materials impregnated with
active compound, and very fine capsules in polymeric
substances.
These formulations are produced in a known manner, for
example by mixing the active compounds with extenders r
that is liquid solvents and/or solid carriers, optionally
with the use of surface-active agents, that is emulsi-
fying agents and/or dispersing agents and/or foam-formins
agents.
In the case of the use of water as an extender, organic
solvents can, for example, also be used as auxiliary
solvents. As liquid solvents, there are suitable in the
main: aromatics, such as xylene, toluene, or alkyl-
naphthalenes, chlorinated aromatics and chlorinated
aliphatic hydrocarbons, such as chlorobenzenes, chloro-
ethylenes or methylene chloride, aliphatic hydrocarbons,
such as cyclohexane or paraffins, for example petroleum
fractions, mineral and vegetable oils, alcohols, such as
butanol or glycol as well as their ethers and esters,
ketones, such as acetone, methyl ethyl ketone, methyl
isobutyl ketone or cyclohexanone, strongly polar sol-
vents, such as dimethylformamide and dimethyl sulphoxide,
Le A 27 532 - 31 -
', ' ' '
~. .
- ' . .:
- , ,
2~7:~3~
as well as water.
As solid carriers there are suitable:
for example ammonium salts and ~round natural minerals,
such as kaolins, clays, talc, chalk, quartz, attapulgite,
montmorillonite or diatomaceous earth, and ground syn-
thetic minerals, such as highly disperse silica, alumina
and silicates, as solid carriers for granules there are
suitable: for example crushed and frac~ionated natural
rocks such as calcite, marble, pumice, sepiolite and
dolomite, as well as synthetic granules of inorganic and
organic meals, and granules of or~anic material such as
sawdust, coconut shells, maize cobs and tobacco stalks;
as emulsifying and/or foam-forming agents ~here are
suitable: for example non-ionic and anionic emulsifiers,
such as polyoxyethylene fatty acid esters, polyoxyethy-
lene fatty alcohol ethers, for example alkylaryl poly-
glycol ethers, alkylsulphonates, alkyl sulphates, aryl-
sulphonates as well as albumen hydrolysis products; as
dispersing agents there are suitable: for example lignin-
sulphite waste liquors and methylcellulose.
Adhesives such as carboxymethylcellulose and natural andsynthetic polymers in the form of powders, granules or
latexes, such as gum arabic, polyvinyl alcohol and
polyvinyl acetate, as well as natural phospholipids, such
as cephalins and lecithins, and s~nthetic phospholipids,
can be used in the formulations. Further additives can be
mineral and vegetable oils.
Le A 27 532 - 32 -
.
. . :
.
21~3rl ~
It is possible to use colorants such as inorganic pig-
ments, for example iron oxide, titanium oxide and
Prussian Blue, and organic dyestuffs, such as alizarin
dyestuffs, azo dyestuffs and metal phthalocyanine dye-
stuffs, and trace nutrients such as salts of iron,
manganese, boron, copper, cobalt, molybdenum and zinc.
The formulations in general contain between 0.1 and 95
per cent by weight of active compound, preferably between
0.5 and 90%.
For combating weeds, the active compounds according to
the invention, as such or in the form of their formula-
tions, can also be used as mixtures with known herbi-
cides, finished formulations or tank mixes being
possible.
Suitable herbicides for the mixtures are known herbi-
cides, such as, for example, 1-amino-6-ethylthio-3-(2,2-
dimethylpropyl)-1,3,5-triazine-2,4(lH,3H)-dione (AMETHY-
DIONE) or N-(2-benzothiazolyl)-N,N'-dimethylurea (META-
BENZTHIAZURON) for combating weeds in cereals; 4-amino-
3-methyl-6-phenyl-1,2,4-triazin-5(4H)-one (METAMITRON)
for combating weeds in sugar beet and 4-amino-6-(l,1-
dimethylethyl)-3-methylthio-1,2,4-triazin-5(4H)-one
(METRIBVZIN) for combating weeds in soya beans, further-
more also 2,4-dichlorophenoxyacetic acid (2,4-D); 4-(2,4-
dichlorophenoxy)-butric acid (2,4-DB); 2,4-dichloro-
phenoxypropion~c acid (2,4-DP); 5-(2-chloro-4-trifluoro-
methyl-phenoxy)-2-nitro-benzoic acid (ACIFLUORFEN);
Le A 27 532 - 33 -
` ~ ,
. '
2 ~ 3
2-chloro-2',6'-diethyl-N-methoxy-methylacetanilide
(ALACHLOR); methyl-6,6-dimethyl-2,4-dioxo-3-[1-(2-
propenyloxyamino)-butylidene]-cyclohexanecarboxylic acid
(ALLOXYDIM); 4-amino-benzenesu:lphonyl methylcarbamate
(ASULAM); 2-chloro-4-e~hylamino-6-isopropylamino-1,3,5-
triazine (ATRAZINE); methyl 2-[[[[[(4,6-dLmethoxypyrimi-
dine-2-yl)-amino]-carbonyl]-ami.no]-sulphonyl]-methyl]-
benzoate (BENSULFURON); 3-isopropyl-2,1,3-benzo-
thiadiazin-4-one 2,2-dioxide (BENTA20NE); methyl 5-(2,4-
dichlorophenoxy)-2-nitrobenzoate (BIFENOX); 3,5-dibromo-
4-hydroxy-benzonitril; (BROMOXYNIL); N-(butoxymethyl)-2-
chloro-N-(2,6-diethylphenyl)-acetamide (BUTACHLOR);
5-amino-4-chloro-2-phenyl-2,3-dihydro-3-oxypyridazine
(CHLORIDAZONE);ethyl2-{[(4-chloro-6-methoxy-2-pyrimidi-
nyl)-aminocarbonyl]-aminosulphonyl}-benzoate (CHLORI-
MURON); N-(3-chlorophenyl)-isopropyl carbamate (CHLOR-
PROPHAM); 2-chloro-N-{[(4-methoxy-6-methyl-1,3,5-triazin-
2-yl)-amino]-carbonyl}-benzenesulphonamide (CHLOR-
SULFURON); N,N-dimethyl-N'-(3-chloro-4-methylphenyl)-urea
(CHLORTOLURON); exo-l-methyl-4-(1-methylethyl)-2-t2-
methylphenyl-methoxy)-7-oxabicyclo-(2~2/l)-heptane
(CINMETHYLIN); 3,6-dichloro-2-pyridinecarboxylic acid
(CLOPYRALID); 2-chloro-4-ethylamino-6-(3-cyanopropyl-
amino)-1,3,5-triazine (CYANAZINE); N,S-diethyl N-cyclo-
hexylthiocarbamate (CYCLOATE); 2-[1-(ethoximino)-butyl]-
3-hydroxy-5-[tetrahydro-(2H)-thiopyran-3-yl]-2-cyclo-
hexen-l-one (CYCLOXYDIM); 2-[4-(2,4-dichlorophenoxy)-
phenoxy]-propionic acid, its methyl ester or its ethyl
ester (DICLOFOP); 2-[(2-chlorophenyl)-methyl]-4,4-di-
methylisoxazolidin-3-one (DIMETHAZONE); S-ethyl N,N-di-
Le A 27 532 - 34 -
2 ~ $
n-propyl-thiocarbamidate (EPTAME); 4-amino-6-t-butyl-3-
ethylthio~ ,4-triazin-5(4H)-one (ETHIOZIN); 2-{~-[(6-
chloro-2-benzoxazolyl)-oxy]-phenoxy}-propanoic acid, its
methyl ester or its ethyl ester (FENOXAPROP); 2-[4-(5-
trifluoromethyl-2-pyridyloxy)-phenoxy]-propanoic acid or
its butyl ester (~LUAZIFOP); N,N-dimethyl-N~-(3-tri-
fluoromethylphenyl)-urea (FL~OMETURON); l-methyl-3-
phenyl-5-(3-trifluoromethylphenyl)-4-pyridone (FLURI-
DONE); [(~-amino-3,5-dichloro-6-fluoro-2-pyridinyl)-oxy]-
acetic acid or its l-methylheptyl ester (FLUROXYPYR); 5-
(2-chloro-4-trifluoromethyl-phenoxy)-N-methylsulphonyl-
2-nitrobenzamide (FOMESAFEN); 2-{4-[(3-chloro-5-(tri-
fluoromethyl)-2-pyridinyl)-oxy]-phenoxy} propanoic acid
or its ethyl ester (HALOXYFOP); 3-cyclohexy1-6-dimethyl-
amino-1-methyl-1,3,5-triazin-2,4-dione (HEXAZINONE);
methyl 2-[4,5-dihydro-4-methyl-4-(1-methylethyl)-5-oxo-
lH-imidazol-2-yl]-4(5)-methylbenzoate (IMAZAMETHABENZ);
2-[5-methyl-5-(1-methylethyl)-~-oxo-2-imidazolin-2-yl]-
3-quinolinecarboxylic acid (IMAZAQUIN); 2-[4,5-dihydro-
4-methyl-4-isopropyl-5-oxo-(lH)-imidazol-2-yl]-5-ethyl-
pyridin-3-carboxylic acid (IMAZETHAPYR); 3,5-diiodo-4-
hydroxybenzonitril (IOXYNIL); N,N-dimethyl-N'-(4-iso-
propylphenyl)-urea (ISOPROTURON); (2-ethoxy-1-methyl-2-
oxo-ethyl 5-t2-chloro-4-(trifluoromethyl)-phenoxy]-2-
nitrobenzoate (LACTOFEN); (2-methyl-4-chlorophenoxy)-
acetic acid (MCPA); (4-chloro-2-methylphenoxy)-propioni~
acid (MCPP); N-methyl-2-(1,3-benzothiazol-2-yloxy)-
acetanilide(MEFENACET);2-chloro-N-(2,6-dimethylphenyl)-
N-~(lH)-pyrazol-1-yl-methyl]-acetamide (METAZA~HIOR); 2-
ethyl-6-methyl-N-(l-methyl-2-methoxyethyl)-chloro-
Le A 27 532 - 35 -
S'id rJ ~
acetanilide (METOLACHLOR); 2--{[[((4-methoxy-6-methyl-
1,3,5-triazin-2-yl)-amino)-carbonyl]-amino]-sulphonyl}-
benzoic acid or its methyl ester (METSULFURON); S-ethyl
N,N-hexamethylenethiocarbamate (MOLIN~TE); 1-(3-tri-
fluoromethyl-phenyl)-4-methylamino-5-chloro-6-pyridazone
(NORFLURA3ON); 4-(di-n-propylamino)-3,5-dinitrobenzene-
sulphonamide(ORYZALIN);2-chloro-4-trifluoromethylphenyl
3-ethoxy-4-nitro-phenyl ether (OXYFLUORFÆN); N-(l-ethyl-
propyl)-3,4-dimethyl-2,6-dinitroaniline (PENDIMETHALIN),
3-(ethoxycarbonylaminophenyl) N-(3'-methylphenyl)-car-
bamate (PHENMEDIPHAM); 2-chloro-N-isopropylacetanilide
(PROPACHLOR); isopropyl N-phenyl-carbamate (PROPHAM);
O-(6-chloro-3-phenyl-pyridazin-4-yl) S-octyl thiocar-
bonate (PYRIDATE); ethyl 2-[4-(6-chloro-quinoxalin-2-yl-
oxy)-phenoxy]-propionate (QUIZALOFOP-ETHYL); 2-[1-
(ethoxamino)-butylidene]-5-(2-ethylthiopropyl)-1,3-
cyclohexadione (SETHOXYDIM); 2-chloro-4,6-bis-(ethyl-
amino)-1,3,5-triazine(SIMAZINE);2,4 bis-[N-ethylamino]-
6~methylthio-1,3,5-triazine (SIMETRYNE); 4-ethylamino-2-
t-butylamino-6-methylthio-s-triazine(TERBUTRYNE);methyl
3-~[[[(4-methoxy-6-methyl-1,3,5-triazin-2-yl)-amino]-
carbonyl]-amino]-sulphonyl]-thiophene-2-carboxylate
(THIANETURON); S-[(4-chlorophenyl)-methyl]- N,N-diethyl-
thiocarbamate (THIOBENCARB); S-(2,3,3-trichloroallyl)
N,N-diisopropylthiocarbamate (TRI-ALLATE); 2,6-dinitro-
4-trifluoromethyl-N,N-dipropylaniline (TRIFLURALIN).
Surprisingly, some mixtures also show a synergistic
action.
Mixtures with other known active compounds, such as
Le A 27 532 - 36 -
2 ~?~
fungicides, insecticides, acaricides, nematicides, bird
repellants, plant nutrients and agents which improve soil
structure, are also possible.
The active compounds can be used as such, in the form of
their formulations or in the use forms prepared therefrom
by fuxther dilution~ such as ready-to-use solutions,
suspensions, emulsions, powders, pastes and granules.
They ar~ used in the customary mannerr for example by
watering, spraying, atomizing or scattering.
The active compounds according to the invention can be
applied either before or after emergence of the plants.
They can also be incorporated into the soil before
sowing.
The amount of active compound used can vary within a
lS substantial range. It depends essentially on the nature
of the desired effect. In general r the amounts used are
between 0.1 g and 2,000 g of active compound per hectare
of soil surface, preferably between 1 g and 1,000 g per
ha.
The preparation and use of the active compounds according
to the invention can be seen from the following examples.
Le A 27 532 - 37 -
: ~ .
:
2 ~
Preparation Examples:
Example 1
OCF3
~ O2-NH-C ~ ~ C~3
(Process (a))
A mixture of 3.1 g (0.02 mol) of 5-methoxy-pyrimidine-2-
carboxamide, 3.4 g (0.06 mol) of potassium hydroxide
(powder) and 50 ml of dioxane is heated to 80C And
stirred at this temperature for 10 minutes. The mixture
is cooled to 20C, and 10.4 g (0.04 mol) of 2-trifluoro-
methoxy-benzenesulphonyl chloride are then added, and the
reaction mixture is stirred for 20 hours at 20C. It is
then concentrated, the residue is stirred with 100 ml of
10% strength potassium carbonate solution, the mixture is
filtered, and the filtrate is acidified with 2N hydro-
chloric acid. The product which is separated in oil form
is taken up in methylene chloride, and this solution is
dried with sodium sulphate and filtered. The filtrate is
concentrated, the residue is triturated with diethyl
ether, and the product which is obtained in crystalline
form during this process is isolated by filtration with
suction.
O.7 g (9~ of theory) of N-(2-trifluoromethoxyphenyl-
sulphonyl)-5-methoxy-pyrimidine-2-carboxamide of melting
point 187C is obtained.
Le A 27 532 - 38 -
. ::
' ' .
,,
.
- . - - , ~ .
.. . . .
Example 2
OCF3
~ SO2-NH-C ~ ~ ~3
(Process (b))
A mixture of 1.46 g (6.8 mmol) of phenyl 5-methyl-
pyrimidine-2-carboxylate, 1.64 g (6.8 mmol) of 2-tri-
fluoromethoxy-benzenesulphonamide, 1.04 g (6.8 mmol) of
1,8-diazabicyclo-t5,4,0]-undec-7-ene (DBU) and 40 ml of
acetonitrile is stirred for 7 hours at 70C and sub-
sequently concentrated. The residue is stirred with 2N
hydrochloric acid, and the product which has remained
undissolved is isolated by filtration with suction.
1.74 g (71% of theory) of N-(2-trifluoromethoxy-phenyl-
sulphonyl)-5-methyl-pyrimidine-2-carboxamide of melting
point 123C are obtained.
Other examples of the compounds of the formula (I) which
can be prepared analogously to Examples 1 and 2 and
following the general description of the preparation
processes according to the invention are listed in Table
1 below.
Le A 27 532 - 39 -
2 ~ 'J~ ~ ~
R 502-N~-Co~ ~r ( I )
z
Table 1: Examples of the compounds of the formula ( I )
Ex. R X Y z Melting
No. point ( ~)
. .
502NtCH3)2
3 ~ H OCH3 H 226
OCHF2
4 ~ H CH3 H 141
OCF3
CH3 CH3 CH3 114
OCHF2
6 ~ CH3 CH3 CH3 130
COOCH3
7 ~ H OCH3 H 106
Le A 27 532 - 4D -
:: . ... ~ .. .
- ` . `
2 ~ 3 ~
Example for the preparation of startinq substances:
/~=~\ N=c~
~ -C~-~ ~ H3
4 g (0.029 mol) of 5-methyl-pyr:Lmidine-2-carboxylic acid
and 3 g (0.032 mol) of phenol ar~ initially introduced
into 50 ml of methylene chloride, and 0.36 g (0.003 mol)
of 4-dimethylamino-pyridine and 6.67 g (0.032 mol) of
N,N'-dicyclohexylcarbodiimide are added in succession.
The reaction mixture is stirred for 12 hours at room
temperature and filtered. The filtrate is washed with 5%
strength acetic acid and water, the organic phase is
separated off, dried over sodium sulphate and filtered,
and the filtrate is concentrated.
3.5 g (56% of theory) of phenyl 5-methylpyrimidine-2-
carboxylate remain as a solid of melting point 115C.
Le A 27 532 - 41 - ~-
- .
Use Examples:
In the use examples, the compounds tA) and (B) listed
below are used as comparison substances:
OCH3 CH3
SO2-NH-CO ~ ~ (A)
CH~
N-(2-Methoxy-phenylsulphonyl)-4,6-dimethyl-pyrimidine-2-
carboxamide
(disclosed in EP-A 353,640)
Cl OCH3
r==~N ~
sO2-NH-C~~~ ~ (B)
OCH3
N-(2-Chloro-phenylsulphonyl)-4,6-dimethoxy-pyrimidine-2-
carboxamide
(disclosed in EP-A 353,640).
Le A 27 532 - 42 -
s~
Example A
Post-emergence test
Solvent: 5 parts by weight of acetone
Emulsifier: 1 part by weight of alkylaryl polyglycol
ether
To produce a suitable preparation of active compound,
1 part by weight of active compound is mixed with the
stated amount of solvent, the stated amount of emulsifier
is added and the concentrate is diluted with water to the
desired concentration.
Test plants which have a height of 5 - 15 cm are sprayed
with the preparation of the active compound in such a way
as to apply the particular amounts of active compound
desired per unit area. The concentration of the spray
liquor is so chosen that the particular amounts of active
compound desired are applied in 1,000 1 of water/ha.
After three weeks, the degree of damage to the plants is
rated in % damage in comparison to the development of the
untreated control. The figures denote:
0~ = no action (like untreated control)
100~ = total destruction
In this test, a clearly superior activity compared with
the prior art is shown, for example, by the compounds of
Preparation Examples 1 and 3.
Le A_27 532 - 43 -
, ;- .,,
- - ~
~ ~v ~
Example B
Pre-emergence test
Solvent: 5 parts by weight of acetone
Emulsifier: 1 part by weight of alkylaryl polyglycol
ether
To produce a suitable preparation of active compound,
1 part by weight of active compound is mixed with the
stated amount of solvent, the stated amount of emulsifier
is added and the concentrate is diluted with water to the
desired concentration.
Seeds of the test plants are sown in normal soil and,
after 24 hours, watered with the preparation of the
active compound. It is expedient to keep constant the
amount of water per unit area. The concentration of the
active compound in the preparation is of no importance,
only the amount of active compound applied per unit area
being decisive. After three weeks, the degree of damage
to the plants is rated in ~ damage in comparison to the
development of the untreated control. The figures denote:
0% = no action (like untreated control)
100~ = total destruction
In this test, a clearly superior activity compared with
the prior art is shown, for example, by the compound of
Preparation ~xample 1.
Le A 27 532 - 44 -