Note: Descriptions are shown in the official language in which they were submitted.
UB-8453
- 1 - ;~039~19
PERVAPORATION PROCESS FOR SEPARATING A LOWER A~COHOL
COMPOUND FROM A MIXTURE OF A LOWER ALCOHOL
COMPOUND AND AN ETHER COMPOUND
BACKGROUND OF THE INVENTION
1) Field of the Invention
The present invention relates to a pervapora-
tion process for separating a lower alcohol compound
from a mixture of a lower alcohol compound and an ether
compound through an aromatic imide polymer asymmetric
separating membrane.
More particularly, the present invention
relates to a pervaporation process for separating a
lower alcohol compound from a mixture of a lower alcohol
compound and an ether compound through an aromatic imide
polymer asymmetric separating membrane through which the
liquid lower alcohol compound is selectively permeated
and separated from the ether compound, at a high5 selectivity and at a high permeation rate.
2) Description of the Related Arts
It is known that a liquid mixture of two or
more types of organic compounds can be separated into
individual organic components by a distillation method,
but in the distillation method, some of the organic
compounds form an azeotropic mixture, or have boiling
points close to each other, or are chemically modified
at the distillation temperature, and therefore, the
separation must be carried out by a complicated process,
for example, a combination of the distillation procedure
with ar. addition of a additional component or with a
distillation-extraction procedure. It is very difficult
to smoothly carry out this complicated separation
procedure, and a large amount of energy is consumed
thereby.
To avoid the above difficulty, a method of
separating the liquid organic compound mixture by using
- 2 - ~ ~39419
a semipermeable membrane has been attempted. In this
method, wherein a semipermeable membrane is used to
separate or concentrate an organic compound aqueous
solution, a diluted aqueous solution of organic
compounds is brought into contact with a face of a
semipermeable membrane, to allow a specific liquid
organic component to selectively permeate through the
membrane due to a differential osmotic pressure. This
method is referred to as a reverse osmosis method.
Usually, in the reverse osmosis method, a
higher pressure than the osmosis pressure of the aqueous
solution must be applied to the aqueous solution.
Therefore, the reverse osmosis method cannot be applied
to a concentrated organic compound aqueous solution
which exhibits a high osmotic pressure, and accordingly,
the reverse osmosis method can be applied only to
organic compound aqueous solutions having a limited
concentration.
Recently, as a new type of separating method
different from the conventional semipermeable membrane
method, in which a separating membrane is used, a
pervaporation method has been developed for a liquid
organic compound mixture and is now under serious
consideration in this field.
In the pervaporation method, an organic
compound mixture in the state of a liquid is brought
into direct contact with a feed side face of a
separating membrane capable of selectively allowing a
specific organic compound to permeate therethrough, and
the opposite delivery side face of the membrane is
exposed to a vacuum or a reduced pressure. The specific
compound is allowed to selectively permeate through the
membrane and is collected in the state of a vapor at the
opposite delivery side of the membrane. This method is
useful for selectively separating or concentrating an
individual organic compound from a liquid organic
compound mixture.
_ 3 _ ~0~941~
Many proposed pervaporation methods have been
reported.
For example, Japanese Unexamined Patent
Publication No. 52-11188~ discloses a separation of a
benzene-cyclohexane mixture solution or benzene-hexane
mixture solution by using an ionomer type polymer; and
Japanese Unexamined Patent Publication No. 59-30441
discloses a pervaporation separation of the above-
mentioned mixture solu~ion by using a polyamide
membrane. Also, Japanese Unexamined Patent Publication
No. 2-35921 discloses a pervaporation separation of an
organic compound aqueous solution through an aromatic
imide polymer membrane.
Nevertheless, the conventional separating
membranes for the pervaporation method are disadvanta-
geous in that;
(1) the permeation rate of the meI~brane for
individual organic compounds to be separated or concen-
trated is unsatisfactorily low;
(2) the selectivity of the membrane for
separating the individual organic compounds is
unsatisfactory;
(3) the heat resistance and solvent
resistance of the membrane are unsatisfactory; and
(4) the membrane has a low durability, and
thus cannot be employed continuously over a long time;
e.g., when separating various individual organic
compounds from a mixture.
Accordingly, the conventional separating
membrane for the pervaporation separation method is
practically useful only for limited aqueous solutions or
mixtures of specific organic compounds, and only data on
the separating properties of the above-mentioned
specific compounds is disclosed. Accordingly, the
conventional pervaporation separation method cannot be
industrially utilized, and therefore, substantially no
example of industrial utilization of the conventional
- 4 - 2039~19
pervaporation separation has been reported.
Particularly, a separation of lower alcohol
compound from a liquid mixture of the organic ether
compound with a lower alcohol compound can be effected
only by an improved distillation method. This method,
however, is disadvantageous in that it involves a large
consumption of energy, and therefore, it is not useful
as an industrial separation process.
Vnder the above-mentioned circumstances, there
is an urgent need for the provision of an improved
pervaporation process for separating a lower alcohol
compound from a mixture of a lower alcohol compound with
an ether compound through a separating membrane, with a
small energy consumption and at a high efficiency.
SUMMARY OF THE INVENTION
An object of the present invention is to provide a
pervaporation method of separating a lower alcohol
compound from a mixture of a lower alcohol compound with
an ether compound through a separating membrane, at a
high selectivity and a high permeation rate.
Another object of the present invention is to
provide a pervaporation method of industrially
separating a lower alcohol compound from a mixture o~ a
lower alcohol compound with an ether compound through a
specific aromatic imide polymer asymmetric separating
membrane at a high efficiency.
The above-mentioned objects can be attained by the
pervaporation method of the present invention for
separating a lower alcohol compound from a mixture of a
lower alcohol compound with an ether compound, which
method comprises the steps of bringing a feed comprising
a liquid aliphatic ether compound having 2 to 8 carbon
atoms and a liquid aliphatic alcohol compound having l
to 4 carbon atoms into direct contact with a feed face
of an asymmetric separating membrane comprising a heat
resistant aromatic imide polymer; exposing a delivery
face opposite to the feed face of the separating
~39~19
membrane to an atmosphere under a reduced pressure to
cause the lower aliphatic alcohol compound to
selectively penetra~e and permeate through the
separating membrane and then to be vaporized in the
delivery face side of the asymmetric separating
membrane, and collecting the lower alcohol compound at
the delivery face side of the separating membrane.
DESCRIPTION OF THE PREFERRED EMBODIME~TS
In the first step of the pervaporation method of
the present invention for separating a lower alcohol
compound from a mixture of a lower alcohol compound with
an ether compound, a feed comprising a liquid aliphatic
ether compound having 2 to 8 carbon atoms and a liquid
lower aliphatic alcohol compound having l to 4 carbon
atoms is brought into direct contact with a feed face of
an asymmetric separating membrane comprising a heat
resistant aromatic imide polymer.
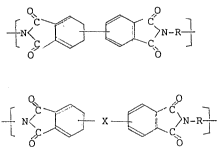
The aromatic imide polymer usable for forming the
asymmetric separating membrane preferably contains 70 to
l00 molar%, more preferably 80 to l00 molar%, still more
preferably 90 to l00 molar%, of at least one type of
recurring units selected from those of the formulae (I)
and(II~:
~ 6C ~ ~ >N-R ~ (I)
and
~ ,C ~ X ~ (II)
wherein R represents a divalent aromatic group having at
least a two benzene ring structure, and X represents a
member selected from the group consisting of -S-, -~2-'
-CO-~ -O-~ -C(CH3)2-~ -CH2- and -C(CF3)2-- The benzene
2039~9
rin~ structure represented by R in the formulae (I) and
(II) is derived from an aromatic diamine having at least
a two benzene ring structure, of the formula:
H2N R-NH2.
The above-mentioned aromatic imide polymer can be
prepared by dissolving a mixture of:
(A) an aromatic tetracarboxylic acid component
comprising:
(a) preferably 70 to l00 molar%, more
preferably 80 to l00 molar%, still. more preferably 90 to
l00 molar%, of at least one principal member selected
from aromatic tetracarboxylic acids of the formulae
(III) and (IV):
HOOC ~ f~ COOH (III)
HOOC COOH
and
HOOC ~ X ~ COOH (IV)
HOOC COOH
wherein X is as defined above, and dianhydrides, esters
and salts of the above-mentioned principal acids, and
(b) preferably 0 to 30 molar~, more pref-
erably 0 to 20 molar%, still more preferably 0 to
l0 molar%, of at least one additional member selected
from arornatic tetracarboxylic acids and dianhydrides,
esters and salts of those acids which are different from
the above-mentioned compound for the principal member;
with
(B) an aromatic diamine component comprising:
(c) preferably 70 to l00 molar%, more
preferably 80 to l00 molar%, still more preferably 90 to
l00 molar% of at least one principal member selected
from aromatic diamine having at least two, preferably 2
to 4, benzene ring structures, and
(d) preferably 0 to 30 molar%, more pref-
erably 0 to 20 molar%, still more preferably 0 to
2039419
lO molar%, of at least one additional member selected
from aromatic diamines different from the above-
mentioned compounds for the principal member, in an
organic solvent comprising at least one phenol compound,
and then by subjecting the solution to a polymeriza-
tion-imidization procedure a~ a high temperature of
150C to 250C or at a low temperature of about 10C to
100C in the presence of a imidizing agent, for example,
acetic anhydride or pyridine.
The phenol compound usable for the organic
solvent is preferably selected from phenol, 2-chloro-
phenol, 4-chlorophenol, 4-bromophenol, and cresol. The
solvent may contain N,N-dimethyl acetamide or dimethyl-
sulfoxide.
The aromatic tetracarboxylic acid compound of
the formula (III) for the principal acid member (a) is
preferably selected from the group consisting of
2,3,3',4~-biphenyltetracarboxylic acid, 3,3~,4,4~-
biphenyltetracarboxylic acid, and dianhydrides, lower
alkyl esters, preferably having l to 3 carbon atoms and
salts of the above-mentioned acids.
Also, the aromatic tetracarboxylic acid
compound of the formula (IV) for the principal acid
member (a) is preferably selected from the group
consisting of diphenylether tetracarboxylic acids, for
example, 3,3',4,4'-diphenylether tetracarboxylic acid;
benzophenone tetracarboxylic acids, for example,
3,3~,4,4'-benzophenone tetracarboxylic acid; diphenyl-
sulfone tetracarboxylic acids, for example, 3,3',4,4'-
diphenylsulfone tetracarboxylic acid; and 2,2-diphenyl-
propane- or hexafluoropropane-tetracarboxylic acids, for
example, 2,2-bis(3,4-carboxyphenyl) propane or 2,2-
bis(3,4-carboxyphenyl) hexafluoropropane, and dian-
hydrides, lower alkyl esters, preferably having l to 3
carbon atoms, and salts of the above-mentioned acids.
Among the above-mentioned aromatic tetra-
carboxylic acid compounds usable for the principal
- 8 - ~039419
member (a), 3,3',4,4~-biphenyl tetracarboxylic
dianhydride and 3,3',4,4~-diphenylether tetracarboxylic
dianhydride exhibit an excellent polyimide-forming
property and greatly contribute to the obtaining of an
aromatic imide polymer with a superior film-forming
property and the forming of an asymmetric separating
membrane having a high separating property for the
aliphatic ether-alcohol mixtures, and to a satisfactory
durability in practical use and a high mechanical
strength and heat resistance.
The aromatic tetracarboxylic acid compound for
the additional member (b) is preferably selected from
the group consisting of pyromellitic acid, and
dianhydride, lower alkyl esters, preferably having l to
3 carbon atoms, and salts of the above-mentioned acld.
In the aromatic tetracarboxylic acid component
(A) when the content of the principal aromatic tetra-
carboxylic acid component (a) is less than 70 molar%, or
the content of the additional aromatic tetracarboxylic
acid compound (b) is more than 30 molar%, the resultant
aromatic imide polymer sometimes exhibits a poor
solubility in phenolic solvents, and thus it becomes
difficult to produce an asymmetric separating membrane
having a uniform quality or a satisfactory pervapora-
tion-separation property for organic compounds.
The aromatic diamine compound having two or
more benzene ring structures and usable for the
principal member (c) of the aromatic diamine component
(B) are preferably selected from the group consisting of
diaminodiphenylethers, diaminodiphenylthioethers,
diaminodiphenylsulfons, diaminodiphenylmethanes,
diaminodiphenylpropanes, diaminodibenzothiophenes,
diaminodiphenylenesulfon!" diaminothioxanthones, and
diaminothioxanthenes which have -two benzene ring
structures; bis(aminophenoxy) benzenes and di(amino-
phenyl) benzenes, which have three benzene ring
structures; and di[(aminophenoxy)phenyl]alkanes,
- 9 - 2039419
di[(aminophenoxy)phenyl]sulfons and di(aminophenoxy)bi-
phenyls, which have four benzene ring structures.
The aromatic diamine compound having two
benzene ring structures is preferably selected from
4,4~-diaminodiphenylether, 3,4'-diaminodiphenylether,
4,4'-diaminodipheny].methane, 3,4'-diaminodiphenyl-
methane, 3,3'-dimethyl-4,4~-diaminodiphenylmethane,
2,2-bis(4-aminophenyl)propane, 2,2-bis(3-amino-
phenyl)propane, 3,4'-diamino-(2,2-di-phenylpropane),
4,4'-diaminodiphenylsulfone, 3,3'-diaminodiphenylsul-
fone, o- and m-dianisidine, and diamine compounds of the
formulae (V) and (VI):
Rl ~ 2
'1 (V'
H2N Sn NH2
and
H~N S~r ~ ~H2 (VI)
wherein R1 , R2 r R3 and R4 respectively and indepen-
dently from each other represent a member selected from
a hydrogen atom and methyl and ethyl radicals and n
represents zero or 2.
The diamine compounds of the formula (V)
include diaminobenzothiophene compounds, for example,
3,7-diaminodibenzothiophene, 2,8-dimethyl-3,7-diamino-
dibenzothiophene, 2,6-dimethyl-3,7-diaminodibenzo-
thiophene, 2,8-diethyl-3,7-diaminodibenzothiophene,
2,6-diethyl-3,7-diaminodibenzothiophene, and
4,6-diethyl-3,7-diaminodibenzothiophene, and
diaminodibenzothiophene-5,5-dioxide(diaminodiphenylene-
sulfon) compounds, for example,
3,7-diaminodibenzo-thiophene-5,5-dioxide, 2,8-dimethyl-
3,7-diaminodibenzothiophene-5,5-dioxide, 2,6-dimethyl-
3,7-diamino-dibenzothiophene-5,5-dioxide, 4,6-dimethyl-
- 1 o Z0394~9
3,7-diaminodibenzothiophene-5,5-dioxide, 2,8-diethyl-
3,7-diaminodibenzothiophene-5,5-dioxide, 2,6-diethyl-
3,7-diaminodibenzothiophene-5,5-dioxide, and 4,6-
diethyi-3,7-diaminodibenzothiophene-5,5-dioxide.
The diamine compounds of the formula (VI)
include diaminothioxanthene compounds, for example,
3,7-diaminothioxanthene, 2,8-dimethyl-3,7-diaminothio-
xanthene, 2,6-dimethyl-3,7-diaminothioxanthene, and
4,6-dimethyl-3,7-diaminothioxanthene; and diaminothio-
xanthene-5,5-dioxide compounds, for example, 3,7-di-
aminothioxanthene-5,5-dioxide, 2,8-dimethyl-3,7-diamino-
thioxanthene-5,5-dioxide, 2,6-dimethyl-3,7-diaminothio-
xanthene-5,5-dioxide, and 4,6-dimethyl-3,7-diaminothio-
xanthene-5,5-dioxide.
The aromatic diamine compounds having three
benzene structures include bis(aminophenoxy)benzene
compounds, for example, l,4-bis(4-aminophenoxy)benzene,
l,4-bis(3-aminophenoxy)benzene, and l,3-bis(4-amino-
phenoxy)benzene.
The aromatic diamine compounds usable for the
additional member of the aromatic diamine component
include phenylene diamine compounds, for example,
m-phenylene diamine p-phenylene diamine, which are used
preferably in a content of lO molar% or less, and
diaminobenzoic acid compounds and alkylphenyl diamine
compounds which are used preferably in a content of
30 molar% or less.
The above-mentioned type of aromatic imide
polymer is soluble in the afore-mentioned organic
solvent, and thus can be converted to an asymmetric
separating membrane.
The asymmetric separating membrane is
preferably in the form of a hollow filament or a film.
The asymmetric membrane usable for the method
of the present invention can be prepared by dissolving
the solvent soluble polymerization product of the
aromatic tetracarboxylic acid component (A) with the
- 1 1 - z~3~3~19
aromatic diamine component (B), namely, a solvent
soluble specific aromatic imide polymer, in a solvent
comprising at least one phenolic compound to provide a
dope solution; shaping the dope solution into a hollow
filament-formed stream or a film-formed layer; bringing
the shaped dope solution into contact witn a coagulating
bath to provide a solidified membrane; washing the
solidi~ied membrane with an organic solvent not capable
of dissolving the solidified membrane; drying the washed
membrane; and aging the dried membrane.
In the asymmetric membrane-preparing process,
the aromatic imide polymer is dissolved in the above-
mentioned solvent to provide a dope solution.
Preferably, in the dope solution, the aromatic
imide polymer is in a concentration of 5 to 30% by
weight, more preferably lO to 25% by weight.
The dope solution is shaped in a hollow
filamentary stream thereof by extruding through a
spinning nozzle for hollow filaments or in a flat filmy
Z layer by extruding through a slit for film, or by
spreading on a film-forming surface, for example, a
horizontal surface of a film-forming plate or a peri-
pheral surface of a rotating film-forming drum. The
resultant shaped dope solution is brought into contact
with a -oagulating liquid to provide a solidified
membrane. The coagulating liquid is compatible with the
solvent in the dope solution but cannot dissolve therein
the aromatic imide polymer in the dope solution. The
coagulated aromatic imide polymer asymmetric membrane is
washed with an organic solvent not capable of dissolving
the solidified membrane. The washing organic solvent
comprises at least one member selected from, for
example, lower aliphatic alcohols, for example, methyl
alcohol, ethyl alcohol, propyl alcohols and butyl
alcohols and aliphatic and cycloaliphatic hydrocarbons,
for example, n-hexane, n-heptane n-octane and
cyclohexane.
Z~)39~ 9
- 12 -
Then, the washed membrane is dried and aged or
heat treated at a temperature of 150C to 400C,
preferably 160C to 350C for 1 second to 20 hours.
When the aging or heat treatment is thoroughly
carried out at a high temperature of 250C or more, the
aromatic imide polymer in the asymmetric separating
membrane is partially cross-linked, and thus the
resultant aged membrane becomes insoluble in or
resistant to swelling in the organic sol~ent, and
exhibits an enhanced chemical resistance and durability
in practical use.
The method of producing a polymeric asymmetric
membrane is disclosed, for example, in Japanese
Unexamined Patent Publication Nos. 56-21602 and
56-157435-
The asymmetric separating membrane usable for
the present invention is composed of a dense layer
having a thickness of about 0.001 to 5 ~m and a porous
layer continuously incorporated with the dense layer and
having a thickness of about 10 to 2000 ~Im.
Generally, when the aromatic imide polymer
asymmetric membrane is used for the pervaporation method
of the present invention to selectively separate a
specific alcohol compound x having a highest permeation
rate; from a liquid organic compound mixture containing
the compound x and the ether compound y, the asymmetric
membrane preferably allows the specific alcohol compound
x to permeate therethrough at a permeation rate Q of
about 0.1 kg/m2.hr or more, more preferably 0.2 to
7 kg/m2.hr, and exhibits a separating coefficient ~ for
the specific compounds x and y, of 20 or more, more
preferably from 30 to 10,000.
The permeation rate Q of a specific compound
fraction through a separating membrane is defined by the
equation:
Q = A/B
wherein A represents an amount (in kg) of the specific
l3 - 2~39~19
compound fraction permeated through the membrane per
hour and B represents a permeation area in m2 of the
membrane through which the specific organic compound
fraction permeates.
The separation coefficient ~ of a separating
membrane for the specific compounds x and y is defined
by the equation:
C2/Cl
wherein Cl represents a proportion in weight of the
specific compound x to the weight of organic compound y
in the organic liquid mixture to be fed and separated
and C2 represents a proportion in weight of the specific
compound x permeated through the membrane to the weight
of the compound y permeated through the membrane.
In the pervaporation method of the present
invention a mixture of two or more types of organic
compound in the state of a liquid is brought into
contact with one surface of the aromatic imide polymer
separating membrane; the opposite face of the aromatic
imide polymer sepaxating, membrane is exposed to an
a~mosphere under a reduced pressure, to cause a fraction
consisting of at least one type of the organic compound
in the liquid mixture to selectively penetrate into and
permeate through the separating membrane while leaving
the other fraction consisting of at least one type of
the organic compound in the feed face side of the
separating membrane; the permeated fraction is collected
in the state of a vapor in the delivery face side of the
separating membrane; and the left fraction is collected
in the state of a liquid in the feed face side of the
separating membrane.
Practically, the pervaporation separating
process of the present invention is carried out in the
following steps.
(a) A liquid mixture comprising an aliphatic
ether compound having 2 to 8 carbon atoms and an
aliphatic alcohol compound having l to 4 carbon atoms i8
- l4 -- ~0394~9
fed to a feed side of a separating membrane module
containing a number of aromatic imide polymer asymmPtric
membranes (in the form of a hollow filament or a flat
film) so that the fed liquid mixture comes into direct
contact with one face of each membrane.
(b) A delivery side opposite to the feed side
of the separating membrane module is exposed to a
reduced pressure by connecting the delivery side to a
pressure-reducing or vacuum pump placed outside of the
separating membrane module, if necessary while flowing a
carrier gas (sweeping gas), for example, helium,
nitrogen, and argon gases and air, through the delivery
side, to selectively allow the alcohol compound to
penetrate into and permeate through the separating
membranes and to be withdrawn as a vapor at the delivery
side of the module.
(c) Finally, the remaining non-permeated
portion of the liquid mixture is recovered from the feed
side of the module, and the permeated portion in the
state of a vapor is collected from the delivery side of
the module, and if necessary, condensed by cooling.
The non-permeated portion of the liquid
mixture comprises the ether compound in an increased
concentration and the permeated portion of the liquid
mi~ture comprises the alcohol compound in an increased
conceniration.
Usually, the liquid organic compound mixture
is fed into the separating membrane module preferably at
a temperature of from about 0C to 120C, more pref-
erably from 20C to lO0C.
In the method of the present invention, the
feed side of the separating membrane module is under a
gauge pressure of from 0 to 60 kg/cm2G, preferably from
0 to 30 kg/cm2G.
Also, the pressure at the delivery side of the
separating membrane module is lower than the atmospheric
pressure, and is preferably about 200 Torr or less, more
;~)39~
- 15 -
preferably lO0 Torr or less. If necessary, a sweeping
gas is made to flow through the delivery side of the
module, to promote the permeation of the specific
organic compound.
In the method of the present invention, the
feed liquid comprises preferably at least 70% by weight
or more, more preferably 80% by weight or more, of a
mixture of a liquid aliphatic ether compound having 2 to
8 carbon atoms and a liquid aliphatic alcohol compound
having 1 to 4 carbon atoms.
The aliphatic alcohol compound is preferably
selected from methyl alcohol, ethyl alcohol, n-propyl
alcohol, isopropyl alcohol and butyl alcohol, more
preferably from methyl alcohol and ethyl alcohol.
The aliphatic ether compound is selected from
preferably dimethylether, diethylether, di-n-propyl-
ether, di-n-butylether, methyl-tert-butylether,
ethyl-tert-butyl-ether, methyl-tert-amylether.
The liquid feed preferably contain 30% by
weight or less, more preferably 20% by weight or less,
of at least one organic compound different from the
aliphatic ether and alcohol compounds, for example,
lower alkane compounds such as ethane, propane and
butane, and lower alkane compounds such as propylene and
isobut-^ne.
In the li~uid feed, the aliphatic ether
compound and the aliphatic alcohol compound are not
limited to a specific mixing ratio in weight and can be
mixed in any proportions.
There is no restriction on the structure,
type, and size of the separating membrane module to be
subjected to the pervaporation method of the present
invention, but preferably the separating membrane module
is a plate and frame type module, spiral type module or
hollow filament type module.
EXAMPLES
The present invention will be further illustrated
- 16 - 2 O 39 ~1 9
by way of specific examples, which are merely represen-
tative and do not restrict the scope of the present
invention in any way.
In the examples, the permeating rate Q and
separating coefficient ~ were determined in the
following manner.
When a liquid organic compound mixture was
subjected to a pervaporation me~hod, a fraction was
permeated through a separating membrane, liquefied by
cooling, and then collected, and the weight of the
liquefied fraction was measured. Then an internal
standard liquid was added to the liquefied fraction, and
the whole subjected to TCD-gas chromatography to
determine the proportions in weight of organic compounds
in the fraction to the total weight of the fraction.
The permeating rate Q and the separating coeffi-
cient ~ were determined in accordance with the
equatio..s:
Q = A/B
~ C2/C
as defined above.
In the examples, the compounds are represented by
the following abbreviations.
(A) Aromatic tetracarboxylic dianhydride
s-BPDA: 3,3',4,4'-biphenyltetracarboxylic
dianhydride
a-BPDA: 2,3,3',4'-biphenyltetracarboxylic
dianhydride
ETDA: 3,3',4,4'-diphenylether
tetracarboxylic dianhydride
DSDA: 3,3',4,4'-diphenylsulfone
tetracarboxylic dianhydride
6-FDA: 2,2-bis(3,4-carboxyphenyl)hexa-
fluoropropane dianhydride
PMDA: Pyromellitic dianhydride
BTDA: 3,3',4,4~-benzophenonetetra-
carboxylic dianhydride
- l7 - Z039419
(B) Aromatic diamine
TSN: Isomeric mixture of 2,8-dimethyl-
3,7-diaminodibenzothiophene-5,5-
dioxide, 2,6-dimethyl-3,7-diamino-
dibenzothiophene-5,5-dioxide and
4,6-dimethyl-3,7-diaminodibenzo-
thiophene-5,5-dioxide
DADE: 4,4'-diaminodiphenylether
DADM: 4,4'-diaminodiphenylmethane
DABA: 3,5-diaminobenzoic acid
TPEQ: l,4-bis(4-aminophenoxy)benzene
BAPB: 4,4'-di(4-aminophenoxy)biphenyl
DMMB: 4,6-dimethyl-m-phenylenediamine
Referential Examples l to 13
In each of Referential Examples l to 13, an
aromatic imide polymer solution was prepared by polym-
erizing and imidizing the aromatic tetracarhoxylic acid
component and the aromatic diamine component having the
composi~ions as shown in Table l, in substantially equal
molar amounts, in the organic polar solvent consisting
of p-chlorophenol at a polymerization temperature of
180C cr the polymerization time as shown in Table l.
The resultant aromatic imide polymer solution had
the polymer concentration and the solution viscosity,
which is a rotation viscosity (poise) at a temperature
of 100~, as shown in Table l.
The aromatic imide polymer solution was extruded,
as a spinning dope solution, through a hollow filament-
spinning nozzles, the resultant hollow filamentary
streams of the dope solution were travelled through
atmospheric air and then introduced into a coagulating
liquid consisting of an aqueous solution of 60% by
volume of ethyl alcohol at a temperature of 0C, the
resultant solidified hollow filaments were withdrawn
from the coagulating liquid at a take-up speed of
lO m/min in accordance with a semi dry-wet membrane-
forming method, and the hollow filaments were washed
- 18 - ~039~19
with ethyl. alcohol and then with an aliphatic hydro-
carbon, and dried and aged under the conditions as
indicated in Table 1, for 30 minutes.
The resultant hollow filaments had an asymmetric
layered structure and the dimensions (outside diameter
and membrane thickness of the hollow filament) as shown
in Table 1.
The type of resultant hollow filament will be
represented hereinafter by the number of the Referential
Example in which the hollow filament was prepared.
-- 19 - Z~)3~4~
,. , l I
O a ¦ u ~ I Oo ~0 ~0 ~,~ CO~ 0O~ r~ O ,~
" ~ I ~ C
3 1 ^ I C~` ~ ~ ~ `~
..1 ~1 ~
,a O'CIQ
a ~ I~ ~ ~ "` ~ c~ ~ ~ ~ c~ ~ ~ co c~ ¦
.~ 3 ~ _ I
¢ ~
, o ~ o o o o o o o o o o o o o o o o
a r~ ~ ~J ~ ~ o 0 0 ~ ~ o o o ~ C~ O
X ~ P '' '~ ~ ~ ~ ~ ~ ~ '`' ''' ~ ~ ~
o o ~ U~ C~ C~ Cl~ O ~
or5
m co o o co ccl ~ ,~ o 0 0 o Vl O O O c.
~I N
I I ~ ! ~
~ I ~
Ic c ,,,,,,,,,,,, ~
I Q ~a
I I I I I I I I I I I I I I
U ~ I Q
o I o o I I I I 1 ~ 1 1 1 1 I
I ~ I I I I o o I I o o o o I I I I I I
I o I Fl I Z I o o o I I I I I I I I o o o I I I
Q ~
!~1 } W lol_~oO~oO~oO~oO _~I
a ¦ I ¢ ¦ , , ,, ,, ~ o l l I
o I ~ ¦ q
Oa ¦ O I ¢ I I I I I I I I o
O
~ ¦ _l I Q ¦ ~ O
I I ~1 ¢ I o ~
Q l ¢
U I Q l l ~ O l l ,,
I 'LJ I I I
¢ I O h 17 0 0 0 0 0 1 1 1
l ~ l
Q I I _
1.~ C ~ CO ~ ~ O -- r~
Z039~19
- 20 -
Examples 1 to 21
In each of Examples 1 to 21, 4 hollow filaments of
the type shown in Table 2 and having a length of 7.5 cm
were arranged in parallel to each other to form a hollow
filament bundle, and at one end of the bundle, the ends
of the hollow filaments were sealed with an epoxy resin
to provide a hollow filament bundle element.
The hollow filament bundle element was placed in a
container having an inlet for feeding a liquid feed, an
outlet for recovering a non-permeated fraction, and an
outlet for collecting a permeated fraction, to provide a
separating membrane module.
A liquid feed comprising methyl-tert-butylether in
an amount as shown in Table 2 and the balance consisting
of methyl alcohol was fed into a feed face side of the
separating membrane module so that the feed came into
contact with the outside peripheral surfaces of the
hollow filaments. The hollow spaces of the hollow
filaments were connected to a pressure-reducing or
vacuum apparatus, and the pressure in the hollow spaces
was reduced to 3 Torr or less, to cause methyl alcohol
fraction to be selectively permeated through the hollow
filaments. The permeated fraction in the state of a
vapor was cooled, and the resultant liquefied methyl
alcohol was collected.
The permeating rate Q of the permeated fraction
through the hollow filaments and the separating coeffi-
cient ~ of the hollow filaments for methyl alcohol and
methyl-tert-butylether are shown in Table 2.
Example 22 and 23
In Examples 22 and 23, the asymmetric separating
hollow filaments made in Referential Examples 4(2) and
5(1) were converted to hollow filament bundle elements
in the same manner as in Examples 8 and 9, respectively.
Each of the above-mentioned hollow filament bundle
elements was immersed in a treating liquid consisting of
1 part by weight of methyl alcohol and 9 parts by weight
2039~'19
of methyl-tert-butylether at a temperature of 80C for
20 hours. The treated hollow filament bundle element
was placed, without drying, in a container having an
inlet for feeding a liquid feed, an outlet for dis-
charging a non-permeated fraction of the feed liquid and
an outlet for discharging a permeated fraction of the
feed liquid, to provide a separating ~embrane module.
A liquid feed having the composition as shown in
Table 2 was fed into the separating membrane module in
the same manner as in Example 1.
The results are shown in Table 2.
Examples 24 and 25
In each of Examples 24 and 25, the same procedures
as in Example 1 were carried out with the following
exceptions.
In Examples 24 and 25, the same hollow filament
bundle elements as in Examples 22 and 23 were dried at a
temperature of 30C for 20 hours, and then converted to
separating hollow filament modules in the same manner as
in Example, respectively.
A liquid feed having the composition as indicated
in Table 2 were fed into the separating module to
separate the methyl alcohol and methyl-tert-butylether
from each other. The results are shown in Table 2.
7~39419
-- 22 --
~ ~ ~ O O O ~ O ~ o u~ O o
4~ v ~ ~ ~ 1~ 0 ~ ~ ~ o ~1 u~ ~ ~ O ~(
~ _ t` I~ ~ o~ ~
o ~
n~ O ~ _
u~ .,~ ~ O' u~ o a~ ~ ~ ~D ~ c~ v~ ~ ~ ,~ I~ ~ I~ u~ O d` ~ L~ ~D C`l
n~ _~ ~ ~ ~ u~ o r~ ~ O ~D ~ ~ U~ ~ ~ ~ 0~ U~ O ~ I~ ~
~ ~ o o o o ~ ~ ,, o ,, ,~ o ~ ~ ~ o o o ~ ,i ~ U~ ,~ ,- o ,i
4~ ~ V
0 d O ~1 o ~ o~ ~ o ~ ~ ~ o c~ ~ ~D u~
6 o v o ~ o ~ 0 a~ o 0 ~ ~D
~ d 0 ~ t~ ~ a~ cr. a~
P~ C~ ~0
d d ~ ~ o ~ v~ ~ 1~ 1~ o o o~ o ~ ~1 o ~ ~ 1~ ~ ,~ a~ ~D C~l u 7
3 o~ o o o a~ o o ,1 ~ ~ o o~ ~i ,i ~ ~ o o ~ ~ ~ ~ o~ o o
~0 oo o~ In a) oo o~ a~
E~ ~ ~ _
a) C~ o o o o o o o o o o o o o o o o o o o o o o o o o
~ 0 o~ oo o~ c~
E~0
_ ___ ~ ~ ~
. C~l ~ ~ ~ ~ ~I C`l ~ ~ ~ ~1 C~ ~1 ~ ~1 O~ __ __
~ ~ ~ a~
,1 6 0
3 ~3 ~ ~1
o . 0 0
o ~ d d
:~ ~ P~
~1 ~ ~ ~ ~ ~D 1~ ~ a~ o ~ c~ ~ ~ 00 ~ o ,t ~ c~
,~ _~ ~
~ æ 6X ~:
;~03~19
- 23 -
Table 2 clearly shows that the aliphatic ether
compounds having 2 to 8 carbon atoms and the aliphatic
alcohol compounds having 1 to 4 carbon atoms can be
separated from each other at a high separation coeffi-
S cient by the pervaporation separation method of thepresent invention in which a specific aromatic imide
polymer asymmetric separating membrane is employed.
Accordingly, the aliphatic ether compounds and the
aliphatic alcohol compounds can be industrially isolated
and recovered from mixtures thereof under a stable
condition over a long period.