Note: Descriptions are shown in the official language in which they were submitted.
2~Q0~7
URINE TESTING MEMBRANE MODULE AND
METHOD OF CONDUCTING SAME
BACKGROUND OF THE INVENTION
The present invention is directed to medical and
laboratorylspecimen collecting and testing equipment, and
more specifically to an apparatus for collecting biological
fluids such as blood for use in testing.
It is generally necessary in diagnosing and
testing for many diseases to collect biological fluids from
a patient, e.g., sputum, blood, pleural and peritoneal
cavity fluids, urine, etc. for analysis. It is important
during the collection handling of biological fluid specimens
that the potential of specimen contamination and the spread
- of any infection from the specimen be minimized. In
addition there is also the potential for specimen damage
during the collection and/or shipment process as well as the
potential for destruction of certain components of the fluid
specimen.
One of the problems in collecting biological fluid
specimens occurs not only during the collection of the
specimens but also in the transport or shipment of the
specimens after collection to the laboratory for analysis.
A typical specimen collecting apparatus is shown
by U.S. Patent 4,741,346. This apparatus includes a
base stand which supports the specimen vial in an upright
position. A funnel is inserted in the open end of the
specimen vial and surrounds and encloses the upper portion
of the vial. The base stand has an upwardly extending
20~00~'7
tubular wall which at least partially surrounds the vial
which in connection with the cap allows the user to remove
the vial without touching the surface or coming in contact
with the specimen. Examples of various types of liquid
containers for collecting and transporting biological fluids
are shown by U.S. Patents 3,777,739; 3,881,465; 4,042,337;
4,084,937; 4,244,920; 4,492,258 and 4,700,714.
One such specimen collection device shown by U.S.
Patent 4,040,791 discloses a collection receptacle having a
nipple upon which is mounted a specimen container which
receives a predetermined amount of the specimen in a sealed
condition. The specimen container is provided with an
integrally formed cap which is placed over the opening in
which the collector nipple is- inserted. U.S. Patent
4,557,274 discloses a midstream urine collector having a
funnel which transmits urine into a cup member which is
covered by a membrane cover.
A combined strip testing device and collection
apparatus is shown by U.S. Patent 4,473,530 and is directed
to an apparatus which integrates testing and collection by
having chemical reagent test strips present within the tube
together with specific gravity reading means allowing
immediate testing of the urine. U. S. Patent 4,573,983
is directed towards a liquid collection system having an
antiseptic member on the discharge section which uses a
filter of air and bacteria impervious material to filter the
urine.
o g y~
It is therefore desirable to provide an easy to
handle apparatus which obtains fluid samples such as blood
while separating various biological components of the blood.
In addition to antigen and antibodies in the blood cells,
cell debris and particulates contained in the blood have a
valuable medical use so that concentrating the same is
beneficial. In using the present invention blood sample
testing can be performed quickly and accurately with minimum
time.
For some testing, it is desirable to remove the
antigens from the blood so that various test procedures can
be run. It is also desirable to do so with minimal exposure
of laboratory personnel to the sample being tested.
Previously this type of testing has been accomplished by a
series of tests involving a number of different containers
and expensive laboratory equipment. Mass testing using such
a series of tests is expensive, time consuming, and often
unsatisfactory.
SUMMARY OF THE INVENTION
There is provided in the practice of the invention
according to the present preferred embodiment, a blood
collection and testing device. This device is in the form
of a removable sealable blood antigen sample container
having a sample chamber with antibody covalently bound to a
membrane which is removably held therein. The blood is
pumped through the container where it engages and passes
through a membrane filter having a 5 micron filter particle
2 ~ ,n, 7
size which screens out cells and cell debris but allows
passage of filtered blood plasma while collecting specific
antigens on an antibody matrix bed secured to a surface of
the membrane. The membrane bed or surface has specific
antibodies, covalently bound thereto to capture specific
antigen carried by the blood. The blood is pumped into a
chamber where it mixes with an anti-coagulate so that it
flows through the membrane. If there is an absence of the
antigen in the specimen sample the membrane antibodies will
remain unoccupied and render a negative test result. This
device is in the form of a tubular device having a
transportable sample compartment in the housing with
optional coloration membrane.
In the accompanying drawings, there is shown an
illustrative embodiment of the invention from which these
and other objectives, novel features and advantages will be
readily apparent.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 is an exploded cross sectional view of
the collection unit and storage unit of the invention ;
Figure 2 is a cross sectional view of the
assembled collection unit and storage unit shown in Figure
l;
Figure 3 is an exploded cross sectional view of
the plunger and test assembly;
Figure 4 is a cross sectional view of assembled
plunger and test assembly shown in Figure 3;
~4~087
Figure 5 is a cross sectional view of the test
apparatus showing the storage unit with anti-coagulent
contained therein and the plunger assembly being removed
from the collection unit,
- Figure 6 is a cross sectional view of the test
apparatus with direction of movement of the plunger assembly
and flow of the blood shown by arrow A;
Figure 7 is a cross sectional view of the test
apparatus with the plunger depressed and leur lock of the
storage unit capped; and
Figure 8 is an enlarged exploded cross sectional
view of the test assembly of Figure 7 with storage container
removed.
DETAILED DESCRIPTION OF THE INVENTION
The preferred embodiment and best mode of the
invention is seen in Figures 1 through 8. The invention
shown therein comprises a modular separable fluid testing
device. While the invention can be used for any body fluid
such as sputum, body fluids, urine or blood, it is primarily
designed for use in collecting blood samples for use in
testing for the presence of various kinds of cancer in the
body.
As shown in Figures 5-8, a sample testing
apparatus 20 is constructed of polystyrene and comprises a
tubular collection unit 22, a storage unit 30 and a plunger
unit 50 with associated test assembly 70.
a ~ il
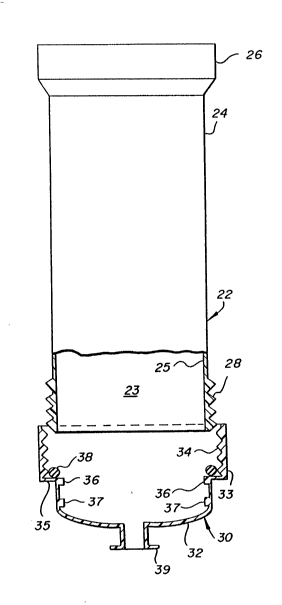
The tubular collection unit 22 shown in Figures 1
and 2 is constructed with a tubular open ended cylindrical
body 24 defining a chamber 23 with an open flared end
portion 26 formed on one end and an external thread 28
formed on the exterior surface of the other or distal end.
The flared end portion 26 has a wide mouth to more easily
receive the plunger unit 50 which is loaded into the unit.
The storage unit 30 is removably secured to the collection
unit 22 by virtue of a female internal thread 34 which
threadably engages cylindrical body external thread 28. The
storage unit 30 comprises a cylindrical cup shaped body 32
with a stepped open end 33, internal thread 34 and shoulder
35. A stop member 38 such as a circular "O" ring is formed
or seated on the upper inner surface of shoulder 35. This
stop member 38 serves as a stop for the threaded distal end
of cylindrical body 24 as shown in Figure 2. Two circular
seating ribs 36 and 37 are formed on the inner surface of
body 32 to hold and seat test assembly 70.
A leur lock 39 is integrally formed in the bottom
of the storage unit 30 and provides fluid communication into
the chamber 31 formed by the cup shaped body 32 and is
intended for the use of withdrawing blood or other body
fluid samples directly from the patient through an attached
syringe (shown in phantom schematic in Figure 5) well known
in the art. The storage unit 30 can be closed with a cap 40
as shown in Figures 7 and 8 to hold the test sample within
storage unit 30.
o g ~
The plunger unit or assembly 50 as shown in
Figures 3 and 4 is designed to fit within cylindrical body
24 and slideably move along the interior wall surface 25 in
a sealed relationship holding a test assembly 70 for
deposit within the seating ribs 36 and 37 of storage body
32. The plunger assembly 50 is constructed of a transparent
plastic comprising a hollow cylindrical plunger body 52
provided with a thumb cover 54 with a downwardly projecting
skirt 54a on one end and a bottom end membrane 55 mounted on
the other end. The filter membrane 55 preferably has a
filter particle size of 5 microns but can range from 1-5
microns or any size which is suitable to allow fluid flow
therethrough while providing fluid engagement of the
filtered blood plasma against the immobilized antibodies on
the matrix surface of the test assembly. The plasma
membrane can be a disposable sterile single use filter
assembly manufactured by Gelman Sciences under the trademark
ACRODISC with a 5 UM filter. However, any suitable filter
can be used such as the aqueous glass microfiber filter
manufactured by Xydex, a subsidiary of Genex Corporation or
a membrane member manufactured by Millipore Corporation or a
membrane member manufactured by Nuclepore Corporation. The
Nuclepore membranes will be discussed in greater detail
later in the specification.
An air release aperture 56 is formed in the
plunger body 52 so that there is communication between the
interior chamber 53 of the plunger body into the outside
atmosphere. The membrane 55 screens cell debris from
~o~o~
entering into the chamber 53 and provides a fluid flow
turbulence allowing maximum fluid engagement against the
immobilized antibody surface of the testing assembly.
Mounted around the plunger body in annular channels 61 and
63 cut into the exterior surface of the body 52 are
respectively an upper O-ring 62 and lower O-ring 64. These
O-rings slideably engage and form a fluid seal against the
interior surface 25 of collection unit 22. A test assembly
70 is adapted to be mounted to outer surface of wall 52 of
the plunger body through a friction fit of its s~irt member
- 86 against the outer surface.
The test assembly 70 is constructed with a
cylindrical base member 80 provided with a plurality of pass
through ports or openings 82. A cylindrical skirt 86 is
integrally formed on the base member 80 which has an inner
diameter substantially the same as the outer diameter of the
circular plunger wall 52 to provide a tight friction fit for
attachment of the test assembly. An "0" ring 88 abuts the
inner surface of the skirt 86 and rests on the top surface
of base member 80 to provide connection rigidity and a fluid
tight seal between the skirt 86 and base of body 52.
It should be noted that the air contained in
chamber 53 is pushed out by the anti-coagulent fluid 98
contained in chamber 31. Blood 100 pumped through the port
and passage syringe leur lock 39 enters chamber 31 and from
there through membrane 81 mounted to base body 80 through
membrane 55 into chamber 53. The air in chamber 53 is
discharged through air release aperture 56 into a chamber
3 i
formed by the concentric outer surface of the plunger body
52 and the inner surface 25 of sample collection unit 22.
In operation, the plunger and associated test
assembly is pulled up along the collection unit or driven
upward depending upon the pressure of the entering blood
until a suitable amount of blood mixed with the anti-
coagulent has been collected in the unit. The leur lock 39
is removed from the syringe end and capped. The test
assembly 70 is pushed down by the plunger body until it
enters into the body cavity 31 of storage unit 30 past the
seating rib 36. The base body 80 engages the seating rib 36
allowing the user to know the test assembly is seated once
it clears the seating rib 36. At this time the lower
surface of body 80 engages seating stop rib 37 thus seating
the test assembly 70 in a predetermined position so that it
cannot break or damage the storage unit 30. This will
discontinue the communication between the body cavity 53 and
the atmosphere through the air release aperture 56 as "0"
ring 62 provides an air lock. Consequently the fluid which
has entered the body chamber 53 through membrane 55 will be
trapped inside it even after removal of the collection unit
and plunger unit 50 from the storage unit 30.
The storage unit 30 is threaded off of the
tubular collection unit 22 with the test assembly 70
contained therein, the plunger assembly 50 remaining with
the tubular collection unit 22 as is seen in Figure 8.
While the invention can be used for any body fluid
it is primarily designed for use in collecting concentrated
20~00~ s
blood antigen samples for use in testing for the presence of
various kinds of cancer in the body to determine the
presence and stage of the cancer.
The membrane filter 81 on top of body 80
preferably~has a filter size ranging from .5 to 5 microns to
keep the blood cells and cell debris positioned on the
opposite side of body 80 within chamber 33.
Antibodies are immobilized (covalently bound) on
the membrane 81 as is well known in the art and are designed
to have binding sites which have a high affinity for the
epitopes of the designated cancer antigens carried in the
blood.
The principle of affinity chromatography requires
that a successful separation of a biospecific ligand is
available and that it can be chemically immobilized to a
chromatographic bed material, the matrix or coating of
membrane 81. Numbers of methods well known in the art have
been used to couple or immobilize antibodies to a variety of
activated resins. Rxamples of immobilization techniques
which exhibit variable linkage are those formed by the
reaction of the reactive groups on the support with amino,
thiol, hydroxyl, and carboxyl groups on the protein ligand.
The selection of the ligand is influenced by two factors.
First, the ligand should exhibit specific and reversible
binding affinity for the substance to be purified and
secondly it should have chemically modifiable groups which
allow it to be attached to the matrix without destroying its
binding activity. (Examples of such are Protein G Sepharose
~OQ3~-~
manufactured by Pharmacia, Hydrazide AvidGel Ax
manufactured by BioProbe International, and Actigel-ALD
manufactured by Sterogene Bioseparation Inc.)
An advantage to the use of Actigel-ALD is that it
does not c~oss link proteins therefore allowing proteins to
retain high bioactivity after their immobilization.
Actigel-ALO SUPER FLOW also available from Sterogene
Bioseparation Inc. permits a linear flow rate of up to 3000
cmJh which would fit nicely with the flow rates in the
apparatus (approx 10-100 cm/min).
The matrix surface or membrane 81 and primary
ligand (in this case immobilized antibody) having had flow
contact with the filtered blood captures through antigen-
antibody reaction with or immune reaction the specific
ligand component carried by the blood, namely, the non
complexed antigen. It should be noted that an anti-
coagulent solution 98 can be added to the container chamber
31 by directly adding it from a syringe into chamber 31
prior to withdrawing the blood or simply adding it from
another container. When the specific antigen is present in
the blood sample 100 the antigen reacts with the antibody to
form antigen-antibody complexes. The complexed antigen-
antibody remains affixed to the immobilized antibody carried
on the matrix surface of membrane 81 on body 80. If there
is an absence of the antigen in the specimen sample 100 ~he
antibody will remain unoccupied.
The membrane surface which is provided with
immobilized antibodies having had flow contact with the
11
2 ~
blood 100 captures the specific component of the blood which
is to be tested; in this example, antigens caused by cancer
cells. The storage unit 30 is then pulled off of the
tubular collection unit 22 with the test assembly 70
contained therein, the plunger assembly 50 remaining with
the tubular collection unit 22, and membrane 81 is tested
with a color developing solution.
Alternately the membrane surface contains primary
labelled antibodies having a binding site contoured to the
epitope structure and chemistry of antigen are added to
compartment 31 of storage unit 30 or to the blood itself.
- This antigen has been previously determined as being a
marker for a specific type of cancer. The antibodies are
labelled with HRP (horseradish péroxidase), an enzyme that
detoxifies hydrogen peroxide, H2O2, by converting it to
water. HRP initiates this transformation when it gives
hydrogen peroxide a pair of electrons. The enzyme
subsequently collects these electrons from suitable donors.
Thus the total color generated by peroxidase depends upon
the relative rates of color generation and product
inactivation of the enzyme. Membrane 81 mounted on body 80
contains antibodies immobilized (covalently bound) thereto
for reception of the complexed antibodies and can be
provided with an area which acts as a control. The antigen
has epitopes which have a high affinity for the binding
sites of the primary labelled antibody and immobilized
antibody on the membrane surface. The principle of affinity
chromatography requires that a successful separation of a
o g ;.~
biospecific ligand is available and that it can be
chemically immobilized to a chromatographic bed material,
the matrix. Numbers of methods well known in the art have
been used to couple or immobilize the antibodies to a
variety of'matrixes. Examples of immobilization techniques
which exhibit variable linkage are those formed by the
reaction of the reactive groups on the support with amino,
thiol, hydroxyl, and carboxyl groups on the protein ligand.
The selection of the ligand is influenced by two factors.
First, the ligand should exhibit specific and reversible
binding affinity for the substance to be purified and
secondly it should have chemically modifiable groups which
allow it to be attached to the matrix without destroying its
binding activity. (Examples of such are Protein G Sepharose
manufactured by Pharmacia, Hydrazide AvidGel Ax manufactured
by BioProbe International, and Actigel-ALD manufactured by
Sterogene Bioseparation Inc.)
Another advantage to the use of Actigel-ALD is
that it does not cross link proteins therefore allowing
proteins to retain high bioactivity after their
immobilization. Actigel-ALO SUPER FLOW also available from
Sterogene Bioseparation, Inc. permits a linear flow rate of
up to 3000 cm/h which would fit nicely with the flow rates
in the apparatus (approx. lO-100 cm/min).
After the blood has passed over the membrane 81
and deposited complexed ligands on the immobilized
antibodies, the membrane 81 is preferably soaked with ABTS
solution. A hydrogen peroxide H2O2 solution may be
alternately placed on the membrane when OPD or TMB or other
dual substrate systems are used.
The color solution used on the membrane is
preferably a substrate manufactured by Kirkegaard & Perry
Labs under one of several acronyms namely: ABTS (2,2'-
azino-di-[3-ethylbenzthiazoline sulfonate (6)]; OPD (ortho-
phenylene diamine); or TMB (tetramethylkbenzidine). In
choosing the substrate, the sensitivity of the immunoassay
is determined by the discrimination of the antibody
reagents. When this occurs, the use of a more sensitive
substrate serves only to proportionately increase the signal
and the background. The result is more color but the same
signal-to-noise ratio. Should the more sensitive substrate
push the absorbency over the cut-off of the reader, the
faster substrate may in fact reduce the signal-to-noise
ratio.
The preferred color solution ABTS used is a one-
component substrate. The HRP label on the primary antibody
is turned by the ABTS to a blue-green color and there is no
change in color or absorbency when the reaction is stopped
with SDS (sodium dodecyl sulfate). If the assay
optimization indicates the sensitivity of the immunoassay is
limited by the color generated by the HRP substrate, then
the more sensitive TMB substrate would give more color
development without a corresponding increase in the
background. Another advantage of the TMB substrate is that
it often lowers the amount of antibody and antigen reagents
required for the immunoassay. TMB substrate is a two
14
o ~ .i
component liquid substrate and requires hydrogen peroxide.
HRP converts TMB to a blue product. When the reaction is
stopped by acidification, the TMB product becomes yellow.
OPD is generally provided as a tablet that is dissolved in
buffer at the time of use. HRP converts OPD to a yellow
product which continues to oxidize into a brown precipitate.
Upon acidification the OPD product becomes orange.
The membrane material with matrix and immobilized
ligand (in this case immobilized antibody) having had flow
contact with the blood, captures through antigen-antibody
reaction or immune reaction the specific ligand component
carried by the blood, namely, the complexed primary labelled
antibody and antigen which was formerly contained in the
blood. This antibody as previously noted was provided
prelabelled with coloring enzyme HRP. When the specific
antigen is present in the testing sample which is added to
the container, the antigen reacts with the antibody to form
antigen-antibody complexes. The complexed antigen-antibody
carried by the fluid passes through ports or apertures 82 in
the disc body into a chamber formed by disc body 80,
membrane 81 and bottom membrane 55.
This testing apparatus will result in 500x fold
increase in the amount of antigen being captured by the
membrane.
The luer lock 39 port is closed with screw or snap
fit cap 40 to provide a container filled with concentrated
specimen sample.
2~0~7
It should be noted that membrane 55 and membran~
81 can be any one of a number of appropriate polycarbonate,
polyester, polytetrafluoroethylene and polypropyl-~ne
membranes currently being manufactured by ~luclepor~
Corporatio'n and set out in a publication titled Nucleporz
Corporation Products for Laboratory and Process Filtration,
copyright Nuclepore Corporation 1988, which is i~corporated
into this Application by reference. The specification of
these ilters are reproduced from said publication as
follows:
l`able 1
Nuclepore Pctlycarbonate ~nd Polyester l~tembranes
Nominal Speclflcatforrs.
~ ~ ~ --~ Typlcel ~low Re1ea
Flole~d~ated Homln~l Nominal
Pore Sl~e~ e ~enYIiy W~ Thick Bubble Poin(~
(llm)_ _ (Por~!~'c~ t o nA6a Ip~i) (b~r) ~ml/mlr~.~m~ Umlhr~l~c :~
:~ 12.0 1 x to~ ~ 1 6 < 1~0.072500 ¦ 6sr
~.o ~ 10~ ~_ 1.0 7 1 3 0.21 20~ ! ~ 1
~2~10! I'D ~I-t. 096 :~ ~
¦ 1 Y~ 10 ~ ~ 12~
0.2 1 3 x !' ~ ! ~ I 82 --5.65 20 1 i O -
l ! 3 >: 10' 0.6 1 6 1_~- 100: 6~90 - ~ 4.0 _ i 1 5
0.08 1 6 Y 10~ ¦ 06 _ 6 -- ~ ioo ~6.90 2.0 I Z10
. Typical nOw ale ~-sinS ~aie~ l ai"l 10 psi lo 7 b~-) 6 ~ =
16
~ 0 ~ ;7
Table 2
Nu~lr~porer' ~icrofiltratio~ le~nbralle C~ara~terlstics.
~UCbEPOnEt' I .
11~sls3~ANEs ¦ MEslEllA~ FlLlHEs~ COIIMEH7
. __ .~ -- .
_PROPERn _ _ Polycarbonlt!cP I jsllxstO;E6lor~ PlFE
Ihickness(llm) 6-11 'i-lI j 100-200 ~;D
sl 56onDli~ (psi¦ _ ~O-C ' .10 t~l ¦4.0 5 6 ~3-- ! 1 cm Unsu;ioorlr~ 7vl A cs ¦
Sp5cific Gravll~ uik _
Malerial (D/crlt~) '.20 1.39 11~ 2.3
H~a~ Ssaling Raros ( C~ Zo0-27$ ?50-2ao _ j 175-20b >375 D11iwll ID Ss
Max ServiceTomP.iC? 140 ¦ 150 1 120 _
Flamm_bilily .Slow Burrt Slot~ Bvrn j Hi8b NDI Flammable
kh Weiehl (u~-km ) 0.9Z 1.0 ¦ 2-3
PolKih Ir~ -- 2-12 ?-12 1 72-au 70-as _
Pore Oonsily (ltorr~c~rr`) 10~-6 ~:10 10 ~ ID l0t
Pore Si~a (rlrn) 0.015-14 0.1-t2 _ ! D '-5 _ 02-10
Surlace Texlure Flal and SrnDDI~ Flal and Srnoolh ¦ RouQh i~ouoh
Op~ical ~Trenslucenl Tranrlucenl ! OpequeOpaqus Transp2re.nl ~on aearino
Rslrac(ive Index 1.58~ 1.625 1~55 1~66 1.51 1 3B
.__ (~ileIrin8enl) l2/~e lringonl) --._ _ ,
i-iydrophilic __Yds res Yes No I --
HydrDphoblc _ Yes ¦Yes _ ! Yes _
':ibor Rel9asln~r No INo _ I ND No
Aulodavablo __Yes ¦Yes j Yes Yes _ j 121~C
~ale~Adsorplion~llls/.~ D.24 O.5 ! 8 0D i 2~hrs~inwaler
i3jDIO~iC Cùmpr31i3di1) Insr! ~_ Inerl ¦ Insil Irwl ! -- --
P~lymer Slruc!ure S~elohed (r.-rysl211rno) ~ S~relched (crysl81iine)
Loschables 1/.) .~ Insignilicam ¦ In3ign 1icanl ¦ 2.0 0.0
USP XXI Cl8ss Yl I Passas I ¦ Passes Passes I __
USPXXlSslaly _ ! _Pssses __ I _ Pessus - ...
(L9?gMouss FbroL~bsb) i Nonlo~k ¦ Nonloxic Nonlo~.ic
MRC-S Human Nonloxic I . _ . _
Agar Crvsrla) l onloxrc .. _ j' .
(L929 Mouse Fibroolæ!) Passes ¦ Pesses
I Hemolysis Tss( 1 ~
Passes I Par~3esPassff
¦ USP XXI Pyro~en _ j Passes j Passes
USP XXI Physb-Chrrrllco tesl ¦ PrAssr s ¦ _ Pa ises _ ¦
Thus the physician or laboratory technician now
has available the capability of quick testing for cancer
patients after surgery or for the testing of patients with
suspected cancer.
63 ~
It can thus be seen that the present invention
provides a unique modular usage for collecting, filtering,
concentrating, transporting, and purifying the ligand sample
for use in testing for disease or other analysis.
In the foregoing description, the invention has
been described with reference to a particular preferred
embodiment, although it is to be understood that specific
details shown are merely illustrative, and the invention may
be carried out in other ways without departing from the true
spirit and scope of the following claims:
18