Note: Descriptions are shown in the official language in which they were submitted.
- 2041250
-- 2
The invention relates to the transdermal admini-
stration of a protein or peptide drug and an administra-
tion device therefor, and more particularly to the trans-
dermal administration of the ionized drug by electric
force after dissolving and ionizing a protein or peptide
drug with an ionizing solvent, also to the transdermal
administration device designed for applying such admini-
stration efficiently and conveniently.
Generally, in delivering a protein or peptide
drug, for example, insulin as physiological metabolism
modulator, interferon as anticancer agent, and captopril
as cardiovascular modulator, into the human body, some
administration methods such as oral administration,
injectables, mucosa delivery agent and pump transplant
have been commonly used, but such methods cause side
effects and their use is inconvenient, and may cause
psychological dlscomfort to patients.
In response to this problem, some researchers
have recently studied the transdermal administration
method of delivering a protein or peptide drug into skin.
Among other things, a most attractive method comprises
transferring a protein or peptide drug having electric
charge into the epidermis by an electric current.
For instance, R.L. Stephen et al, Biomed.
Biochem. Acta 43(1984)5, 553-558, have attempted to
deliver insulin into a pig's skin through the transmission
of constant electric current but the result was unsuccess-
ful due to the insufficient electric charge of insulin and
to failure in making its monomer.
Thereafter, another method to deliver insulin
into the human body by removing the stratum corneum was
made, but this method has been associated with some
problems including infections incurred as a result of
removing epidermis and the undesirable transformation/-
treatment of the skin. Also, the method of delivering
- 3 ~ 2 0 4 1 2~0
insulin without any skin bruise using pulse current has
been studied. However, this proved to be inefficient
because of the insufficient dose of the drug delivered,
and the short duration of the drug effect in the body.
In the meantime, R.R. Burnette and D. Marerro,
J. Pharm. sci., 75: 738(1986), experimented to trans-
dermally administer TRH (Thyrotropin Releasing Hormone), a
hormone for extending by iontophoresis the pregnancy and
lactation of women. Further, J.E. Marchand and N. Hagino,
J. Urol., 97:874(1982) have exemplified in animal experi-
ments the possibility of administering vasopressins into
the skin.
B. Robert Meyer et al, Clin. Pharm & Therape,
44, 6, 607 (1988) describe attempts to deliver LH (Lutein-
izing Hormone) across the skin by a direct current.
The aforementioned methods have recognized
several disadvantages as follows: when a protein or
peptide drug is delivered transdermally, its molecular
chains are susceptible to being destroyed by electric
current and depression of biological activities occur.
Moreover, since the delivery of a required drug amount is
not available at one time, because of the high permeation
resistance in skin, only a small percentage of the drugs
could be transdermally delivered in several divided times.
Besides, the transdermal delivery might raise other
specific problems such as skin irritation or impairment,
and more potent use of enhancers might also induce the
deformation of the human skin system.
Therefore, the present invention seeks to
provide an efficient method, wherein by means of a skin
stimulate needle (hereinafter "skin needle"), a sufficient
amount of protein or peptide drug can be transdermally
delivered into the skin on a continual basis with reduced
side effects.
~ 4 ~ 2041250
The present invention also seeks to provide a
transdermal administration device comprising a storage
reservoir of ionizing solvent, a drug reservoir and a skin
needle through which the sustained administration of a
protein or peptide drug may be available for three to four
days by a simple one-time treatment.
The present invention further seeks to provide a
-composition of ionizing solvent which functions to dis-
solve a protein or peptide drug having more than three
peptide units of amino acid into the monomer, increase the
ionizing degree, and enable the drug to efficiently pass
through the epidermis layer.
The detailed description of this invention is as
set forth hereunder.
The invention relates to the transdermal admini-
stration of a protein or peptide drug being characterized
by the following procedure: contacting and ionizing a
protein or peptide drug immersed in polyelectrolyte with a
composition of ionizing solvent comprising solvent,
polyelectrolyte and enhancer, forming a drug delivery
pathway into the skin, and penetrating the above ionized
drug into the skin by electric force.
The formation of the drug delivery pathway may
be achieved, for example, by an electric razor.
The invention is illustrated by reference to the
accompanying drawings in which:
FIG. 1 is a vertical section of an integration-
type transdermal administration device
as one embodiment of this invention;
FIG. 2 is a vertical section of a separation-
type transdermal administration device
as another embodiment of this invention,
and
FIG. 3 is a graph showing the change of blood
glucose level with the lapse of time when
2041250
5 --
insulin is administered transdermally in
accordance with Example 4 of this inven-
tion.
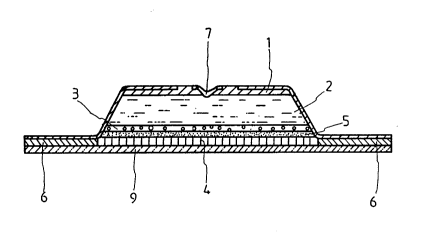
With further reference to Fig. 1, there is shown
a transdermal administration device according to this
invention in the form of an integration-type transdermal
administration device of patch-type used by attaching to
the skin, in which an upper side of the device has a
reservoir 2 for ionizing solvent and an electrode 1 open
to the outside, a lower part is formed by a drug-immersed
hydrophilic polymer drug reservoir 3.
A water swelling polymer skin needle supporter 5
is disposed below reservoir 3 and a pluralit.y of skin
needles 4 is vertically disyosed in a fixed state. A
stacking structure adhesive layer 6 is formed around the
skin needle supporter 5.
A solvent inlet 7 communicates centrally with
storage reservoir 2.
A release paper 9 engages adhesive 6 and skin
needles 4.
With further reference to Fig. 2, another
embodiment of the transdermal administration device of
this invention is a separation-type transdermal admini-
stration device of patch-type used by attaching to the
skin, in which the device has a reservoir 12 for ionizing
solvent and an electrode 11 open to the outside on an
upper side. A semi-permeable membrane 18 having a mole-
cular cut-off ranging from 200 to 20,000 forms a lower
side of reservoir 12. A drug-immersed hydrophilic polymer
drug reservoir 13, is disposed adjacent membrane 18. A
patch body 30 composed of an adhesive layer is formed
around reservoir 13. The device includes a skin needle
plate 15 comprising skin needles 14 on a water-swelling
polymer sheet, the needles 14 being vertically disposed in
a fixed state.
- 6 - 20412S0
The device further includes a release paper 19
and adhesive 16.
Also, this invention relates to the composition
of ionizing solvent for the solvent dissolving and ioniz-
ing protein or peptide drugs, which comprises in propor-
tion to water 100%, by volume, 1 to 50%, by volume, of a
solvent, for example any one or more solutions selected
from sodium acetate, sodium-EDTA, sodium salicylate, salt
buffer solution of phenol derivatives, acetic acid,
hydrochloric acid, ammonia water, and caustic soda; 1 to
30%, by volume, of polyelectrolyte; 1 to 30%, by volume,
of enhancer.
This invention can be described in more detail
as set forth hereunder.
In general, a protein or peptide drug being used
in the treatment of various human diseases has an innate
isoelectric point wherein its dissolution is made avail-
able only in the case of exceeding the isoelectric-point
or as a result of a lower pH. Since such drug has a
constant dipole polarity, the ionization of monomeric drug
having a positive or negative electric charge depends on
the pH of the solvent.
Therefore, in the case of a target drug having a
cation (less than the isoelectric point of the drug) an
anode is used and a cathode is used in an anionic drug
(more than the isoelectric point). Thus, a drug may be
transferrable by an electric repulsion force.
By applying such a principle, this invention
relates to the method of administering a protein or
peptide drug across human skin; in this invention, the
ionizing solvent used in dissolving a protein or peptide
drug into monomer having either cation or anion, uses a
composition of ionizing solvent comprising the following:
in proportion to water 100%, by volume, 1 to 50%, by
- 7 - 2041250
volume, of solvent, 1 to 30%, by volume, of polyelectro-
lyte, and 1 to 30%, by volume of enhancer.
The solvent may be selected from organic and
inorganic acids and organic and inorganic bases, for
example sodium acetate, sodium-EDTA, sodium salicylate,
and salt buffer solutions of phenol derivatives, acetic
acid, hydrochloric acid, ammonia water, and caustic soda.
However, in the case of using a cathode. a solvent such as
inorganic or organic base or salt buffer solution is
appropriate, and in the case of using an anode, a solvent
such as inorganic or organic acid is appropriate.
If the electrode of polyelectrolyte which plays
a role in preventing the pH change of solvent by elec-
trolysis is an anode, any one or more soluble or insoluble
polymers may be used, polyacrylamide (more than M.W.
10,000), polyvinylamine (more than M.W. 10,000), polymers
comprising quadrivalent ammonium, or pyridinium. If the
electrode is a cathode, any one or more solvents may be
used as the pH-controlling polyelectrolyte including
polyacrylic acid, carboxymethylcellulose (C.M.C.), and
alginic acid.
When the above polyelectrolyte is added, the pH
change of electrodes is described in Table 1.
Table 1 - pH Resistance of 1% polyelectrolyte solution
after applying lmA current for 2 hrs.
Polyelectrolyte pH Change of Cathode pH Change of Anode
Polyacrylamide - 7.5 -~ 9.1
Polyvinylamine - 7 5 -, 7 9
Polyacrylic acid7.0 - 6.6
C.M.C. 7.0 -~ 4.3
Alginic acid 7.0 -~ 4.4
D.I. Water 7.0 -~ 1.8 7.5 -~ 12.5
- 8 - 2041250
In order to facilitate the drug delivery, any
one or more enhancers may be used, and may be selected
from the following:
(a) EDTA as chelating agent, citric acid, N-alkyl
derivative,
(b) bile salts (sodium dioxychelate, sodium
tarocholate),
(c) fatty acids (oleic acid mono-olein, saponin).
The electric current may suitably have a density
ranging from 0.01 to 1 mA/cm. A pulsating current is more
preferable than a direct current because the occurrence of
impedance by the current leads to a great increase of
resistance and voltage, thus providing vulnerability to
skin burn, while the pulsating current source eliminates
the impedance wherein high currents may be available under
the low voltage.
By way of example to make the operation of the
invention more clear, reference is again made to the
accompanying drawings with reference to a specific embodi-
ment.
Fig. 1 is an integration-type transdermal
administration device embodying the concept of the present
invention comprising a solvent reservoir 2 made of plastic
film, for example polyethylene or polyethylene terephtha-
late, supporting the frame of the device and having no
solvent permeability and on the upper side, electrode 1
and solvent inlet 7 are open to the outside. The elec-
trode 1 consists of metal sheet comprising silver, lead or
tin and the solvent inlet is made of "V" type rubber, so
that under an air-tight condition from the outside, the
composition of ionizing solvent may be injected into the
solvent storage reservoir 2 by a syringe.
In the lower part of the solvent reservoir 2,
drug reservoir 3 comprising a drug-immersed (in powder)
hydrophilic polymer layer is formed. The soluble polymers
20412~0
g
usable as the drug reservoir 3 include polyacrylamide,
carboxymethylcellulose, polyvinylimine, polyacrylate,
alginate, karaya gum, and gelatin. The major functions of
the water soluble polymer are to support the drug, and
heighten the drug permeability by hydrating the human
skin.
In the skin needle supporter 5 stacked at the
lower part of the drug supporter 3, 1 to 15 pieces of skin
needle 4 per unit area (cm) are disposed in a fixed state,
of which the length protruding to the outside of the drug
supporter should be 0.2 to 2mm.
The skin needles 4 suitably have a diameter
ranging from 50 to 400 ~m and may be of sterilized stain-
less steel.
If the diameter of the skin needles 4 exceeds
400 ,um, it is very difficult to permeate the skin and if
smaller, the manufacture of the skin needles is not easy.
However, it appears that the thickness of the skin needles
does not greatly affect the permeable amount of drug.
Also, a protrusion of skin needle 4 of more than 2mm leads
to bruising of the capillary vessel in the corium layer,
thus causing coagulation. If the length of skin needle is
less than 0.2mm, the needle cannot permeate the skin
adequately, thus resulting in a drastic decrease in
delivery of the drug.
Should the distribution of skin needles 4 per
unit area of skin needle supporter 5 be overly high, an
excess of drug might be delivered and the skin's infection
might not be neglected after treatment. However, if
small, a drug delivery effect of substantial amount cannot
be expected. The skin needle supporter 5 is made of
water-swelling polymers and can be of a polymer of the
same class as indicated for the drug reservoir 3.
The skin needle supporter 5 is surrounded by the
adhesive layer 6 which allows the attachment of the
- 10 - 20~12~0
administration device to skin, and the skin needle
supporter 5 and adhesive layer 6 are covered with a
release paper 9.
The use of an integration-type transdermal
administration device is as follows: injecting the ioniz-
ing solvent into solvent reservoir 2 through solvent inlet
7 by a syringe, removing the release paper 9 from the
device, and compressing the device on the skin and obtain-
ing adhesion to the skin by adhesive layer 6.
Then, connecting an anode or cathode to the
electrode 1 protruding on the upper side, according to the
kind of drug or solvent, and having the opposite electrode
connected to a conductive pad, e.g. karaya gum, etc. (not
illustrated), attached to the side of the device, or
connected to another device, then, using two devices
simultaneously.
In this way, the composition of ionizing solvent
stored in solvent reservoir 2 dissolves the drug contained
in drug reservoir 3 and ionizes the drug into cations or
anions; the ionized drug moves to the skin under the
electric force generated with the electrode 1.
In the meantime, the skin needle 4 attached to
skin needle supporter 5 may penetrate the epidermis layer
by compression power, when the administration device is
attached to the skin, thus forming the pathway for the
drug delivery. With the lapse of time, when solvent
permeates -from solvent reservoir 2, the skin needle
supporter 5 becomes swelled by a water swelling effect and
the skin needle comes out from the skin.
If no electric current is applied to the pathway
formed by skin needle 4, the drug cannot penetrate across
the skin mainly because the hydrophobic epidermic layer
does not allow the permeation of hydrophilic drug and the
pathway formed by skin needle 4 is temporarily closed by
the swelling of the skin.
- ll- 20412S0
Therefore, the electric current applied in the
device makes the ionized drug and solvent move towards the
opposite electrode and then the hydrophilic protein and
polypeptide of the skin become arranged in equilibrium
towards the cathode, which causes a "contraction" of the
skin. By the above phenomenon, the pathway of epidermis
layer becomes open and the drug in the pathway may pene-
trate into the corium layer.
Fig. 2, as described above, shows a separation-
type transdermal administration device embodying another
concept of the present invention in which a patch body and
skin needle plate 15 are separated.
The patch body is made of the solvent reservoir
12, where ionization solvent is stored, semipermeable
membrane 18, drug reservoir 13, where the drug is dis-
persed, adhesive layer 16, and release paper 19. In
addition, several skin needles 14 are fixed vertically in
the skin needle plate 15 separated from the body frame.
The characteristics of a separation-type device
are that since the ionizing solvent is already contained
in the device, the administration of another ionizing
solvent is unnecessaryi there is a semipermeable membrane
18 between ionization solvent reservoir 12 and drug
reservoir 13; there is a skin needle plate separated from
the patch body.
A semipermeable membrane 18 whose molecular
cut-off is in the range from 200 to 20,000 is preferred
because the selection of a semipermeable membrane 18,
having a molecular cut-off lower than that of the delivery
drug, prevents the reduction of drug activity as the drug
is not mixed-in solvent reservoir 12.
The semipermeable membrane to be used in this
invention is suitably selected from the following: poly-
propylene, cellulose, and ethylene vinylacetate. By
making the molecular cut-off of the semipermeable membrane
- 12 - 20 41250
smaller than that of the drug and polyelectrolyte con-
tained in the ionizing solvent, the latter cannot permeate
the membrane. Then, the pH of the ionizing solvent
remains unchanged and skin irritation can be eliminated by
preventing contact between polyelectrolyte and skin.
Thus, the solvent molecule and enhancer only can pass
through the semipermeable membrane 18.
The formation of skin needle plate 15 should be
the same as that of a skin needle placed in an integra-
tion-type administration device and the common type of
textile fiber is suitable.
The method of using a separation-type admini-
stration device is as follows: Lightly compressing the
skin needle plate 15 on the skin, and forming the drug
delivery pathway on the skin, removing the skin needle
plate 15, and, on that skin, compressing the patch body 30
after removing release paper 19. The operation and
principle of other parts are the same as those of the
integration-type device.
The method of using the separation-type admini-
stration device may suitably include lightly shaving the
skin by a common type of razor without using the skin
needle plate 15, and alleviating the permeation resistance
of epidermis layer, and on that skin, compressing the
patch body 30 after removing release paper 19.
Protein or peptide drugs applicable to this
invention including the following: as the drugs having
more than three peptide bond units in amino acid, for
example, cardiovascular modulator (captopril, bradykinin,
atriopeptin, calcitonin gene factor, C.N.S. chole-
cystokinin (CCK-8, 32) as C.N.S. active peptide, ~-endor-
phin, nerve growth factor, melanocyte inhibitor-I, gastric
modulator (gastrin antagonist, neuro-tension, somato-
statin), antibiotics and anticancer drugs (interferon,
cyclosporin, encephalins), and biological metabolism
- 13 - 20412~o
modulators (albumin, insulin, vasopressins, oxytocin,
growth hormone, LH (Leutinizing Hormone) and TRH (Thyro-
tropin Releasing Hormone).
Referring to the influx mass of a protein or
peptide drug in this invention, the ionophoresis of the
ionized drug can be described as follows:
dc D2. Z. e. E. C
J = -D -- + --------______
dx KT
Where J is the mass of the drug delivered, D is
the diffusion coefficient of nonionized class, D2 is the
diffusion coefficient of ionized class, Z is the number of
electric charges in the molecule, e is the ionized degree,
E is the potential difference, C is the concentration of
ionized class, K is Planck's constant, and T is the
absolute temperature.
As shown in the above formula, a higher electric
conductivity of solution makes the ionized classes
dominate the diffusion in a more competitive manner. As a
result, the diffusion of nonionized class can be neglected
because of "dc/dx = O".
Thus, the above formula is expressed as follows:
D2. Z e. E. C
J _______
KT
In general, there is a method of increasing the
number of electric charge (Z) to heighten the electric
current of ionized drug: in case the drug having a limited
number of electric charges is bound with a functional
group such as sulfate base having plentiful numbers of
electric charge, the drug derivatives of increased numbers
-
- 14 - 2041250
of electric charge may be obtained. Such drug derivatives
may be more ionized than the general drugs which have a
relatively lower number of electric charges among
solvents, and further, their competitive movement by being
highly sensitive to the electric current results in
increasing the mass of the drug delivered.
With reference to the ionized degree "e" as
described in the foregoing, the ionization rate will
increase in accordance with the selection of ionizing
solvent. Therefore, as the ionization solvent, organic
and inorganic acids and organic and inorganic salt bases
may be used; as the case may be, it may be used as the
type of salt such as sodium chloride, phosphate or organic
acid salts.
The invention will now be illustrated by the
following examples.
EXAMPLE 1
MANUFACTURE OF INTEGRATION-TYPE ADMINISTRATION DEVICE
An aqueous solution of 0.5M sodium salicylate
containing 1% polyacrylic acid salts (trade-mark Carbopol)
is added to 100 IU/ml insulin and the mixture is dispersed
evenly. 3mg/ml Phenol and 16mg/ml glycerin are added into
this mixture and mixed sufficiently at less than 10C to
manufacture a gel mixture for the drug supporter.
On a woven sheet of polypropylene fibre, 100 ~m
diameter T-type skin needle is fixed in a range of 10
pieces/cm toward the bottom from the upside, evenly
coating the opposite sheet face of protruding skin needle
with the gel mixture of the drug reservoir, and drying it
by a freezing dryer, thus manufacturing a drug reservoir
and skin needle supporter.
The drug supporter and skin needle supporter may
be heat-sealed to the lower side of an already manu-
factured solvent storage reservoir with polyethylene
terephthalate, and applying an adhesive layer and release
- 15 - 20412S0
paper to the fringe and thus, manufacturing an integra-
tion-type administration device.
The composition of ionizing solvent usable in
the integration-type administration device suitably
contains 0.5M sodium salicylate salt buffer solution
containing 3% polyacrylamide, 3mg/ml phenol, 16mg/ml
glycerin, and 1% saponin. Such ionizing solvent is added
into the solvent storage tank prior to using the integra-
tion-type device.
EXAMPLE 2
MANUFACTURE OF SEPARATION-TYPE ADMINISTRATION DEVICE (1)
The composition of ionizing solvent is added
into a polyethylene solvent reservoir having a silver
electrode attached, and heat-sealing in a row a cellulose
membrane having a molecular cut-off of 3500; an already
manufactured drug reservoir is placed in the lower side.
The ionizing composition contains citric acid at pH 3,
0.2% polyacryl acid (M.W. 150,000), 3.5mg/ml phenol,
16mg/ml glycerin, and a small amount of polyoxyethylene
ether.
The manufacture of drug supporter is as follows:
completely dissolving 100 IU/ml insulin and citric acid at
pH 3 to porous polyurethane foam and mixing it with 10%
polyacrylate salts (trade-mark Carbopol), and drying in a
vacuum.
On a flexible aluminium foil, T-shape skin
needles are vertically fixed in a range of 10 pieces/cm
and a woven polyester sheet is formed on top. By com-
pletely fixing the needle with adhesives, a skin needle
plate is manufactured. The manufacture of other parts is
the same as that of Example 1.
EXAMPLE 3
MANUFACTURE OF SEPARATION-TYPE ADMINISTRATION DEVICE
A composition of ionizing solvent contains lM
sodium acetate, 0.1% polyethyleneimine (M.W. 200,000),
- 16 - 2~ 41250
3mg/ml phenol, 16 mg/ml glycerin, and a small amount of
mono-olein.
The ~emipermeable membrane is a cellulose
membrane having a molecular cut-off of 1,000. The manu-
facture of the drug supporter is as follows: 35% alginate
gel containing lM sodium acetate is added to 100 IU/ml
drug and a small amount of phenol and glycerin, this
mixture is mixed and dryed by freezing.
EXAMPLE 4
USE OF INTEGRATION-TYPE ADMINISTRATION DEVICE
The back hair of a white New Zealand-origin
rabbit is removed by shaving and its blood glucose is
measured, an integration-type administration device, as
manufactured in accordance with Example 1, is attached to
the shaved area. A cathode is attached to the electrode,
and an anode is connected to an E.C.G. electrode (trade-
mark Biolect) made from karaya gum, a current of electri-
city of 0.5 mA, 2 KHz is transmitted for 20 mins.
As a result of conducting the said procedure on
20 experimental animals, it was noted that the average
blood glucose level was decreased from 100 mg/dl to 60
mg/dl within 6 hours, and such effect was maintained for
12 hours. Fig. 3 herein illustrates the result of this
example.
EXAMPLE 5
USE OF SEPARATION-TYPE ADMINISTRATION DEVICE
The back hair of a white New Zealand-origin
rabbit was shaved and a skin needle plate of a separa-
tion-type administration device, as manufactured in
accordance with Example 2, was lightly compressed on the
shaved area, the plate was removed and a patch body was
attached.
A cathode was attached to the electrode, and an
anode was connected to an E.C.G. electrode, and a current
~ - 17 ~ 2 0 41250
of electricity of 0.5 mA, 2 KHz was transmitted for 20
mins.
The average blood glucose level of 20 experi-
mental animals was measured and was found to decrease from
100 mg/dl to 50 mg/dl within 4 hours, and such effect was
maintained for 12 hours.
EXAMPLE 6
SIMULTANEOUS USE OF BOTH INTEGRATION- AND SEPARATION-TYPE
ADMINISTRATION DEVICES
By attaching a cathode to an integration-type
administration device of Example 1 and having an anode
connected to a separation-type administration device, an
experiment was conducted in the same way as in Examples 4
and 5.
The average blood glucose level of 20 experi-
mental animals was measured and was found to decrease from
100 mg/dl to 30 mg/dl within 4 hours, 17 animals among
them were dead due to hypoglycemia within 8 hours.
EXAMPLE 7
USE OF ELECTRIC RAZOR
This Example was conducted in the same way as
Example 5 using a separation-type administration device of
Example 2. Shaving the back hair of a rabbit by an
electric razor instead of the skin needle plate, and
attaching to it a patch body of a separation-type admini-
stration device.
The average blood glucose level of 20 experi-
mental animals was measured and was found to decrease from
100 mg/dl to 50 mg/dl within 4 hours, 15 animals among
them showed less than 20 mg/dl hypoglycemia within 12
hours.
EXAMPLE 8-11
This Example was conducted in vitro with a
separation-type administration device of Example 2 by
changing the administered drug to insulin (Example 8),
- 18 - 20412S0
T.R.H. (Example 9), L.H. (Example 10), and cacitonin
(Example 11), respectively.
The outer surface of mouse skin was adhesively
fixed to the adhesive part of the administration device, a
diffusion cell adding saline solution was fixed in the
inside, and treated with an electric current of 0.1 mA for
20 mins. while connecting an administration device and
diffusion gel to the electrode.
The drug in the diffusion cell delivered through
the mouse skin was separated and assayed by HPLC and the
result was described in Table 2.
Table 2. Drug delivery in diffusion cell through the
mouse skin
Hour Example 8 Example 9 Example 10 Example 11
(Insulin/IU) (TRH/mM) (LH/mM) (Calcitonin/IU)
2 3.0 22 8 4
4 4.4 31 12 10.5
8 9.8 45 15 15.1
12 12.4 70 16 26.7
18 14.7 83 21 32.1
A transdermal administration method for protein
or peptide drug according to this invention has the
following merits: a) may prevent the transformation of
drug, by separating the ionizlng solvent from the drug and
avoiding contact between the drug and electrode, b) may
prevent the reduction of drug activity owing to the pH
change of solvent by using a pH-controlling poly-
electrolyte.
Further, since the drug is immersed in a water-
soluble polymer, the skin-administration effect of the
drug can be enhanced by contacting a highly concentrated
drug with the skin and hydrophilizing the skin. Further-
more, the use of a skin needle and/or razor makes it
possible to form a drug delivery pathway on epidermis,
thereby solving the following specific problems as shown
in the transdermal administration of protein or peptide
r 2 0 4 1 2 5 0
-- 19 --
drug, i.e. insufficiency of drug delivered, the trans-
formation of skin owing to chemical enhancer or electric
irritation, and the reduction of drug activity in the
skin. Along these lines, the sustained transdermal
administration of the said drug may be available for three
to four days by one-time use.
The comparison between the skin treatment by
razor and drug administration by skin needle according to
this invention is shown below.
Data on the delivery effect of insulin based
upon the skin treatment by using a skin needle and razor,
respectively:
Blood Glucose Level of Rabbit(mg/dl)
HourControl Skin Needle Razor
(No insulin)
0 115 104 104
2 115 54 64
4 110 66 53
6 99 52 47
8 103 60 40
102 65 11
Remarks:
a) Condition of electric current: 0.5 mA. on/off =l
b) Treatment time: 20 mins.
c) Skin needle: 3 pieces/cm
d) Razor: Treated by an electric razor for 20 secs.
The change of blood glucose in rabbit by the treatment
of electric razor:
Blood Glucose Level of Rabbit (mg/dl)
Hour2 secs. 10 secs.20 secs. 40 secs. 60 secs.
0 107 125 104 119 101
2 88 105 64 70 86
4 100 91 53 55 71
6 92 86 47 17 41
8 87 88 40 low 24
113 72 11 dead dead
The delivery effect by the razor has not been
fully established in its mechanism. It would seem that
like that of the skin needle, the mechanism by the razor
~ - 20 - 2041250
might be as follows: reducing the resistance to drug
permeability into the skin, facilitating the electric
current and thus, easily permeating the drug into the
skin.
The thickness of epidermis is, even if variable,
said to be in the range of 0.2 to 0.01 mm. By treating
the epidermis by a razor, the upper part is partially
removed and results in increasing the permeability of drug
and electric conductance. In other words, similar case
can be seen that an alcoholic lotion treatment after
shaving gives a feeble irritation.
In case of a skin needle, although there is
little skin resistance in the permeation pathway across
the skin, the whole delivery effect is negligibly small
because of a limitation of usable skin needles. The blood
glucose level of rabbit treated by an electric razor is
lower than that of skin needle, which may be due to the
fact that an electric razor gives a large surface area of
treated skin compared to that of skin needles.